Abstract
The green-lipped mussel (GLM) contains novel omega-3 polyunsaturated fatty acids, which exhibit anti-inflammatory and joint-protecting properties. Osteoarthritis (OA) is a degenerative joint disease characterized by a progressive loss of cartilage; oxidative stress plays a role in the pathogenesis of OA. The objectives of this study were to investigate the in vivo effects of the GLM on pain severity and cartilage degeneration using an experimental rat OA model, and to explore the mode of action of GLM. OA was induced in rats by intra-articular injection of monosodium iodoacetate (MIA) into the knee. Oral GLM was initiated on the day after 3dyas of MIA injection. Limb nociception was assessed by measuring the paw withdrawal latency and threshold. Samples were analyzed both macroscopically and histologically. Immunohistochemistry was used to investigate the expression of interleukin-1β (IL-1β), IL-6, nitrotyrosine, and inducible nitric oxide synthase (iNOS) in knee joints. Also, the GLM was applied to OA chondrocyte, and the expression on catabolic marker and necroptosis factor were evaluated by real-time polymerase chain reaction. Administration of the GLM improved pain levels by preventing cartilage damage and inflammation. GLM significantly attenuated the expression levels of mRNAs encoding matrix metalloproteinase-3 (MMP-3), MMP-13, and ADAMTS5 in IL-1β-stimulated human OA chondrocytes. GLM decreased the expression levels of the necroptosis mediators RIPK1, RIPK3, and the mixed lineage kinase domain-like protein (MLKL) in IL-1β-stimulated human OA chondrocytes. Thus, GLM reduced pain and cartilage degeneration in rats with experimentally induced OA. The chondroprotective properties of GLM included suppression of oxidative damage and inhibition of catabolic factors implicated in the pathogenesis of OA cartilage damage. We suggest that GLM may usefully treat human OA.
Introduction
Osteoarthritis (OA) is the most widespread form of arthritis, which is a dynamic disease of the articular cartilage, the underlying bones, and the synovia of joints. The degenerative disease is characterized by articular cartilage destruction caused by an anabolic/catabolic imbalance imparted by mechanical stress [1,2]. OA features low-grade inflammation. Inflammatory cytokines, chemokines, reactive oxygen species (ROS), and matrix metalloproteinase (MMPs) are released by chondrocytes and related cells in the joints of patients with OA [3–5]. The proinflammatory cytokines and chemokines compromise the homeostasis of the cartilage matrix of OA joints, associated with increased production of interleukin-1β (IL-1β), which induces chondrocytes to produce inflammatory mediators such as nitrotyrosine and IL-6, which further increase the harmful cellular responses [6]. And previously studies have reported the nuclear factor-kappaB (NF-κB) transcription factors as over activated in OA and as a diseases-providing factor [7,8]
Necroptosis (programmed necrosis), a recently recognized form of cell death, triggers serious inflammation [9]. Necroptotic cells feature damage-associated molecular patterns that trigger robust inflammatory responses; necroptosis is markedly more immunogenic than apoptosis, associated with high-level inflammation and extracellular matrix (ECM) degradation [10]. Necroptosis is mediated principally by the receptor interacting protein kinase 1 (RIP1), the receptor interacting protein kinase-3 (RIP3), and the mixed lineage kinase domain-like protein (MLKL) [11,12]. RIP3 expression was increased in patients with OA. Necroptosis inhibitors could serve as OA therapeutics [13].
A green-lipped mussel (GLM) has been prepared from a significant commercial marine species of New Zealand. The GLM is a natural dietary supplement that reduces OA inflammation and arthritis. GLM contains abundant long-chain omega-3 polyunsaturated fatty acids, docosahexaenoic acid (DHA), and many minor fatty acids (including 5, 9, 12, 16-nonadecatetraenoic acid, 5, 9, 12, 15-octadecatetraenoic acid, and 5, 9, 12, 15, 18-heneicosapentaenoic acid) [14]. Many animal and clinical studies have shown that GLM exhibits anti-inflammatory, anti-arthritic, and gastro-protective properties [15–18]. Specifically, long-chain omega-3 polyunsaturated fatty acids such as EPA and DHA are known to exert anti-inflammatory effects. Also, previous study showed that osteoarthritis development is notably associated with oxidative stress and ROS [19,20]. There are many studies of its application in the oral administration of osteoarthritis through anti-inflammatory and antioxidant effects [21,22]. In particular, GLM have antioxidant activity. Bioactive peptides of GLM with the strongest radical scavenging activity and ACE inhibitory activity would supply a therapeutic effect for the osteoarthritis [23].
Given the possible significance of catabolic and necroptotic pathways in OA pathogenesis, and the anti-inflammatory and anti-oxidative effects of GLM, we explored the effects of GLM in an animal model of OA. GLM reduced pain, cartilage destruction, regional catabolism, inflammation, and injury in a rat model of OA. Apart from its chondroprotective effects, GLM inhibited catabolic factors and necroptotic markers involved in OA pathogenesis.
Materials and methods
Animals
Seven-week-old male Wistar rats weighing 180~250 g at the start of the experiment were purchased from Shizuoka Laboratory Center (Shizuoka, Japan). A maximum of three animals per cage were housed in a room featuring controlled temperature (20–26°C) and light (12-h/12-h light/dark cycle) conditions. The rats had free access to a gamma-ray-sterilized diet (Teklad Global 18% Protein Rodent Diet, Harlan Laboratories, Inc., Indianapolis, IN, USA) and autoclaved water. All animal research procedures were conducted in accordance with the Laboratory Animals Welfare Act (Korea), the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experiments of the Institutional Animal Care and Use Committee (IACUC) of the School of Medicine, the Catholic University of Korea. The IACUC and the Department of Laboratory Animals of the Catholic University of Korea, Songeui Campus, accredited the Korean Excellence Animal Laboratory Facility in co-operation with the Korean Food and Drug Administration in 2017 and full accreditation by AAALAC International followed in 2018. All experimental procedures were evaluated and conducted in accordance with the protocols approved by the Animal Research Ethics Committee at the Catholic University of Korea (Permit Number: CUMC 2020-0244-01). All procedures performed in this study followed the ethical guidelines for animal use.
MIA-induced OA
The rats were randomly assigned to treatment groups prior to the beginning of the study. Rats were divided into six groups (6 rats in each group) and were group housed in two cages. Each in vivo experiment was repeated a total 2 times. After anesthetization with isoflurane, the rats were injected with 3 mg monosodium iodoacetate (MIA) (Sigma, St. Louis, MO, USA) dissolved in 50 μL (60 mg/mL) volume using a 26.5-G needle inserted through the patellar ligament into the intra-articular space of the right knee. GLM at 100, 300mg/kg and celecoxib at 50 mg/kg, were administered orally to MIA-injected rats.
Osteoarthritic pain assessment
Nociception in MIA-treated rats was tested using a dynamic plantar aesthesiometer (Ugo Basile, Gemonio, Italy). This is an automated version of the von Frey hair assessment tool and is used to assess mechanical sensitivity. Assessment was conducted by placing each rat on a metal mesh in an acrylic chamber housed in a temperature-controlled room (20–26°C), where the rat rested for 10 min before the touch stimulator unit was positioned under the animal. An adjustable angled mirror was used to place the stimulating microfilament (0.5 mm in diameter) below the plantar surface of the hind paw. When the instrument was activated, the fine plastic monofilament advanced at a constant speed and touched the paw in the proximal metatarsal region. The filament exerted a gradually increasing force on the plantar surface, beginning below the threshold of detection, and increasing until the stimulus became painful, as indicated by the rat’s withdrawal of its paw. The force required to elicit a paw-withdrawal reflex was recorded automatically and measured in g. A maximum force of 50 g and a ramp speed of 25 s were used for all aesthesiometer tests.
Weight balance assessment
The weight balance of MIA-treated rats was analyzed using a capacitance meter (IITC Life Science, Victory Blvd, CA, USA). Each rat was allowed to acclimate for 5 min in an acrylic holder. Then, both feet were fixed to the pad and the weight balance measured over 5 s. This was repeated twice. The weights the unguided and guided legs were calculated and entered into a formula that yielded a percentage; this compared the legs with and without OA.
Histopathological analysis
Knee joints were collected 3 weeks after MIA induction. The tissues were fixed in 10% (v/v) formalin for 24 h, decalcified using Decalcifying Solution-Lite (Sigma) for 72 h, and embedded in paraffin. Sections 5-μm in thickness were cut, dewaxed using xylene, dehydrated through alcohol gradient baths, and stained with safranin O.
Immunohistochemistry
Paraffin-embedded sections were incubated at 4°C with the following primary monoclonal antibodies: Anti-cluster of differentiation (CD)4, anti-CD19, anti-interleukin (IL)-1β, anti-IL-6, anti-inducible nitric oxide synthase (iNOS), anti-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), anti-matrix metalloproteinase (MMP)-1, and anti-MMP3. The samples were then incubated with the appropriate, secondary biotinylated antibodies, followed by a 30-min incubation with a streptavidin-peroxidase complex. The reaction products were developed using the 3, 3-diaminobenzidine chromogen (Dako, Glostrup, Denmark). Immunohistochemistry evaluations were performed independently by two experienced researchers who were blinded to the study group. The positive cell percentage was analyzed with HDAB (Hematoxylin & DBA) by selecting color deconvolution in the plugin item in the image J program (NIH, MD USA).
Human chondrocyte isolation and differentiation
Human articular cartilage was obtained from five OA patients treated in Uijeongbu St. Mary’s Hospital (UC14CNSI0150) where they underwent knee arthroplasty or joint replacement surgery. All five patients gave fully informed written consent in accordance with the relevant ethical guideline. Chondrocytes were isolated as previously described [24]. Briefly, OA joints were chopped finely and digested with 5 mg/mL protease at 36°C for 1 h and then with 2 mg/mL collagenase and 0.5 mg/mL hyaluronidase (all from Sigma-Aldrich) for 3 h. After several subcultures, chondrocytes below passage 5 were seeded at 5x104 cells/well into 24-well plates. Following 24 h of starvation, the chondrocytes were stimulated with 20 ng/mL IL-1β or various concentrations of GLM (10, 100, 250 μg/mL) in serum-free Dulbecco’s Modified Eagle’s Medium (DMEM) for 48 h. The supernatants and cell layers were collected for further analyses.
Enzyme-linked immunosorbent assays (ELISAs)
The concentrations of IL-6 and MCP-1 in cell supernatants were measured using sandwich ELISAs (Duoset; R&D Systems, Minneapolis, MN, USA). After color development, the absorbance was measured at 405 nm on an ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Real-time Polymerase Chain Reaction (PCR)
Total RNA was isolated from human chondrocytes with the aid of the TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions and reverse-transcribed using a first-strand cDNA synthesis kit (Takara, Shiga, Japan). qPCR was performed with the aid of a LightCycler 2.0 instrument (Roche Diagnostics Indianapolis, IN, USA; software version 4.0) and the SensilFAST SYBR Kit (Bioline, Cincinnati, OH, USA). The housekeeping β-actin gene served as the normalization control. The primer sequences were as follows: β-actin, forward 5′-GGACTTCGAGCAAGAGATGG-3′, reverse 5′-TGTGTTGGGGTACAGGTC TTTG-3′; MMP3 forward: 5′-CTCACAGACCTGACTCGGTT-3′, reverse 5′-CACGCCTGAAGGAAGAGA TG-3′; MMP13 forward: 5′-CTATGGTCCAGGAGATGAAG-3′, reverse 5′-AGAGTCTTGCCTGTATCCTC-3′; MCP1 forward 5′-CAGCCAGATGCAATCAATGC-3′, reverse 5′-GTGGTCCATGGAATCCTGAA-3’, MLKL forward 5′-GCAAGCTGGTGGCTGTGAAGC-3′, reverse 5′-TTCATCCACAGAGGGCCGCA-3′; and RIPK1 forward 5′-GACGAAGCCAACTACCATCTT-3′, reverse 5′-TCTCCTTTCCTCCTCTCTGTT-3′; RIPK3 forward 5′-ATGTCGTGCGTCAAGTTATGG-3′, reverse 5′-CGTAGCCCCACTTCCTATGTTG-3′;
CCK8 assay
Chondrocyte proliferation was measured using the Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) as previously described [25]. Chondrocytes were seeded into 96-well plates at 1x104 cells/well. Cells were pre-incubated for 24 h in serum-free DMEM with the Insulin-Transferrin Selenium-A (ITSA) solution (ThermoFisher, Waltham, MA, USA) followed by stimulation with 20 ng/mL IL-1β or GLME (10, 100, 250 μg/mL) for 48 h. Cells were supplemented with the CCK-8 solution for another 3 h at 37°C and the OD450 measured using a microplate reader (Molecular Devices).
Statistical analysis
Statistical analyses were performed using the nonparametric Mann-Whitney U-test for comparisons between two groups, and one-way ANOVA with the Bonferroni post-hoc test for multiple comparisons. GraphPad Prism (ver. 5.01; GraphPad Software Inc., San Diego, CA, USA) was used for all analyses. The data are presented as means ± standard deviations (SDs). For all comparisons, P < 0.05 was taken to indicate statistical significance.
Results
GLM regulates the pain threshold
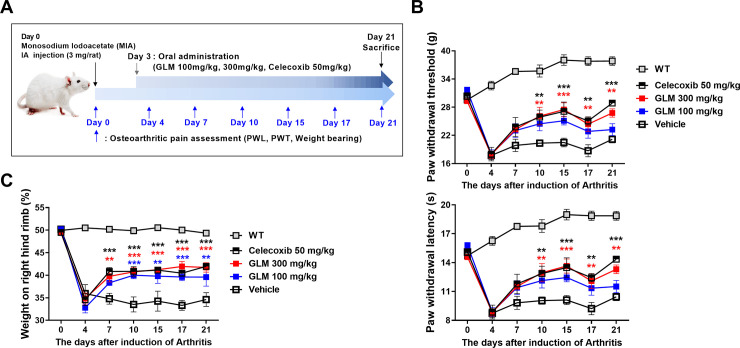
The pain-killing capacity of GLM was analyzed; pain was quantified using the electronic von Frey system. Weight bearing was assessed employing a capacitance meter. Three weeks after MIA injection into the right knees, we found that the paw withdrawal latency and withdrawal threshold were dose-dependently reduced by GLM compared to the vehicle (Fig 1A and 1B); GLM also improved the weight balance (Fig 1C).
Fig 1. Green-lipped mussel extract reduced pain in MIA-injected rats.
(A) Schedule of rat experimental procedure is shown by a diagram. (B) Pain was analyzed as the difference between the PWL (left) and PWT (right) in vehicle-treated MIA-induced OA rats, and those given GLME orally. Rats with MIA-induced OA (n = 6 per group) were evaluated to day 21. Nociceptive testing was performed using a dynamic plantar esthesiometer; this is an automated version of the von Frey hair assessment tool. (C) Weight-bearing was examined (n = 6 per group) today 28. Data are presented as the means ± SDs of those of three independent experiments. Significant differences were evident between the vehicle- and GLME-treated groups. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the vehicle-treated group.
GLM suppresses cartilage destruction and inflammation in the rat model of MIA-induced OA
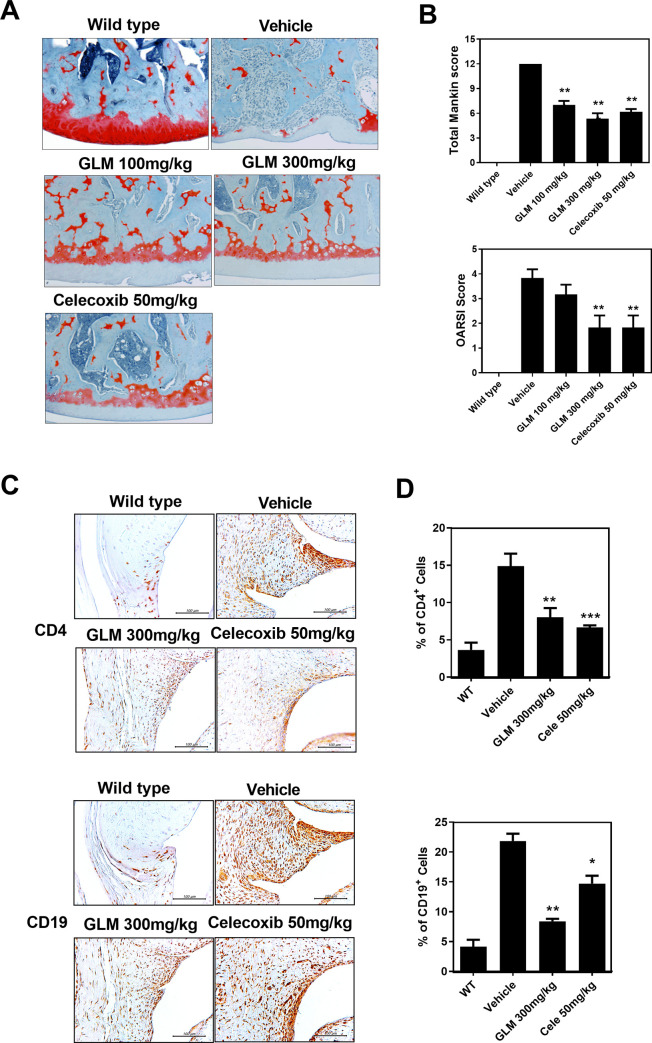
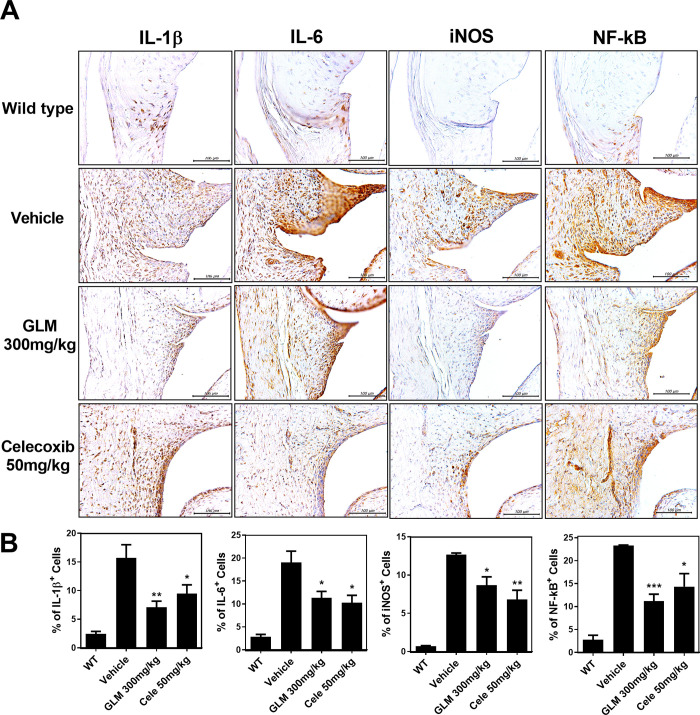
We collected the right knee joints 3 weeks after MIA injection and used safranin O staining for histopathological analysis. GLM dose-dependently reduced cartilage damage and proteoglycan depletion (Fig 2A) as assessed by OARSI and Mankin scoring (Fig 2B). Immunohistochemistry confirmed the presence of inflammatory mediators and the aging factor. Rheumatoid arthritis-like synovitis was observed after OA induction (Figs 2C and 3A). The T- and B-cell immune responses were reduced by GLM at 300 mg/kg compared to the vehicle, as were the levels of IL-1β, IL-6, iNOS, and NF-κB (Figs 2D and 3B).
Fig 2. Histological evaluation of joints after oral administration of GLME to rats with MIA-induced OA.
Rats were injected with 3 mg of monosodium iodoacetate (MIA) (into the right knee). GLME was given orally every day from day 3 after MIA injection. The joints were resected on day 21 after MIA injection. (A) Knee joints from the OA rats. Joint samples were acquired from the WT, vehicle, GLM (100 mg/kg, 300 mg/kg) and Celecoxib (50 mg/kg) groups and stained with hematoxylin and eosin (original magnification x 200). (B) The OA lesions were graded on a scale of 0–13 using the modified Mankin scoring system that evaluates structure, cellular abnormalities, and matrix staining. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the MIA-injection group. (C) CD4 and CD19 expression levels in the synovia of OA rats as revealed immunohistochemically 21 days after MIA injection. Immunohistochemistry was used to evaluate representative sections of joints from MIA-injected rats given GLM, celecoxib, or the vehicle. Positive cells stain brown; the nuclei were counterstained with hematoxylin. The bar graphs represent the means ± SDs of the numbers of stained cells. *P < 0.05, **P < 0.01 compared to the MIA-injection group.
Fig 3. Effects of GLME on the expression levels of IL-1β, IL-6, iNOS, and NF-kB in the synovia of OA joints.
(A) Immunohistochemical staining for IL-1β, IL-6, iNOS, and NF-kB after oral administration of GLME daily for 21 days after MIA injection. (B) The positive cells stain brown; the nuclei were counterstained with hematoxylin. Slides of representative sections showing expression of IL-1β, IL-6, iNOS, and NF-kB. *P < 0.05, **P < 0.01 compared to the MIA-injection group.
GLM regulates the expression of catabolic enzymes and pro-inflammatory cytokines in human OA chondrocytes
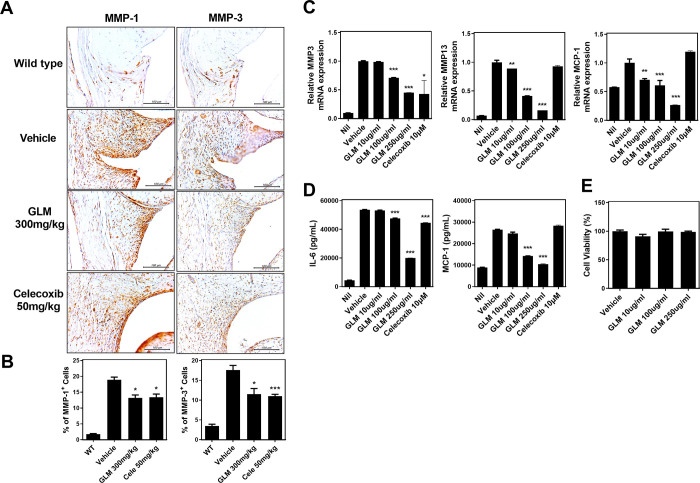
We used immunohistochemistry to explore the levels of catabolic factors. We previously found that GLM inhibited cartilage destruction. We confirmed that GLM regulated the levels of catabolic factors (Fig 4A). The MMP-1 and MMP-3 levels were reduced by treatment with GLM (300 mg/kg) (Fig 4B). We explored whether GLM regulated catabolic gene expression in OA chondrocytes. We found that the levels of mRNAs encoding MMP3 and MMP13 (ECM degradation proteins of articular cartilage) were significantly downregulated in the GLME compared to the control group (Fig 4C). In addition, the level of MCP-1, which chemoattracts macrophages, was also decreased by GLM treatment (Fig 4C). The concentrations of IL-6 and MCP-1 in culture supernatants were also reduced by GLM (Fig 4D). To control for possible GLM cytotoxicity, we performed the CCK-8 assay. GLM (10 μg/mL, 100 μg/mL) did not affect the proliferation of OA chondrocytes (Fig 4E).
Fig 4. Reductions in the levels of catabolic markers and inflammatory cytokines in OA rats and human chondrocytes.
(A, B) Immunohistochemical staining was used to identify MMP1 and MMP3. (C) qRT-PCR analysis of the levels of mRNAs encoding MMP3, MMP13 and MCP-1 in IL-1β (20 ng/mL)-stimulated OA chondrocytes in the presence or absence of (various concentrations of) GLME (10, 100, 250 μg/mL) and Celecoxib (10 μM). (D) IL-6 and MCP-1 levels in cell supernatants were measured via sandwich ELISAs. (E) OA chondrocyte cell viability in the presence or absence of GLME, as revealed by the CCK8 assay. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the vehicle-treated group.
GLM inhibits inflammatory cell death of human OA chondrocytes
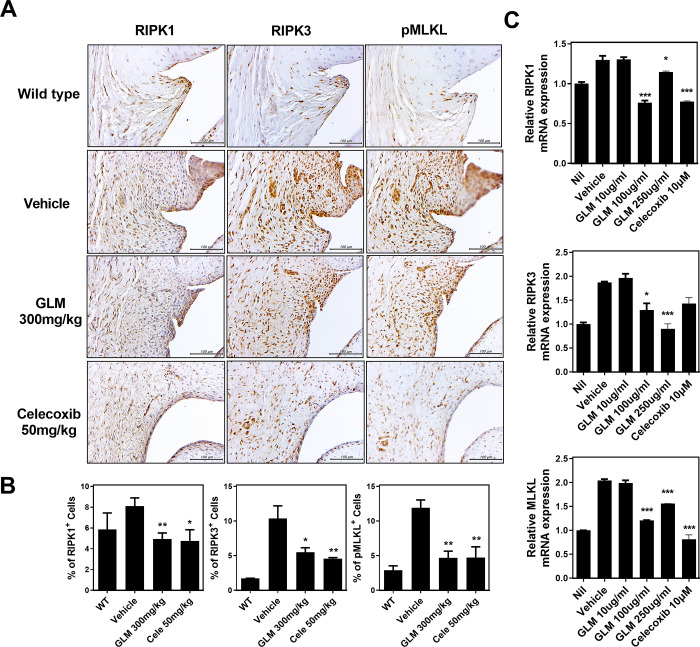
We next tested whether GLM modulated necroptosis of human OA chondrocytes, as was true of the cells in MIA-induced OA cartilage tissue. We immunohistochemically stained for RIP1, RIP3, and pMLKL (markers of necroptosis) to determine whether the anti-nociceptive effect of GLM reflected an anti-necroptosis activity, perhaps explaining the protective effect of GLM in terms of structural damage to knee joints. Synovial RIP1, RIP3 and pMLKL staining were reduced in the GLM group (Fig 5A and 5B). We next stimulated human OA chondrocytes with IL-1β, with or without GLM, and found that the transcriptional levels of RIPK1, RIPK3, and MLKL were substantially suppressed by GLME, consistent with the immunohistochemical data (Fig 5C).
Fig 5. Reduced levels of necroptosis-related markers in OA rats and human chondrocytes.
(A, B) Representative immunohistochemical staining of RIP1, RIP3, and pMLKL in the synovia of non-OA rats (WT), vehicle-treated MIA-induced OA rats, and GLME-treated MIA-induced OA rats. (C) The levels of mRNAs encoding necroptotic marker genes (as revealed by real-time PCR) in human OA chondrocytes treated with IL-1β (20 ng/mL) and then co-cultured with GLME or celecoxib. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the vehicle-treated group.
Discussion
Of the various dietary supplements, GLM is favored by patients with OA. Several clinical studies have reported good results [26]. We found that GLM reduced the clinical and histological scores of rats with MIA-induced OA. GLM significantly reduced pain, inflammatory cell infiltration, and the adverse histopathological changes. Thus, GLM simultaneously controlled OA pain and protected cartilage.
Articular cartilage degeneration is a major clinical feature of OA [27]. OA is not entirely a degenerative disease, being partly an inflammatory condition involving the actions of IL-1β, IL-6, and nitrotyrosine [28]. We found that GLM reduced cartilage injury and pain in the subchondral bone and the articular cartilage. IL-1β and IL-6 expression decreased significantly after GLM administration. The expression of iNOS was inhibited after GLME administration. The iNOS expression level reflects the NO content of OA joints. The role of NO in OA pathogenesis has recently been reported in detail [29]. In OA patients, control of oxidative stress is of therapeutic importance. This suggests that the reduced cartilage degradation in response to GLM was, in part, attributable to a lessening of oxidative damage and attenuated production of proinflammatory cytokines.
Persistent low-rate oxidative stress is a fundamental feature of OA pathology [30]. Increases in age-related oxidative stress render human chondrocytes susceptible to oxidant-mediated apoptosis via dysregulation of the antioxidant system [31]. Other forms of oxidative stress boost NO production, inducing aberrant apoptosis of human chondrocytes mediated via mitochondrial dysfunction [32]. MIA, a blocker of glyceraldehyde-3-phosphate dehydrogenase, generates OA-like lesions in rat joints because of cartilage degradation resembling that of human OA [33,34]. Turning to the significance of ROS and mitochondrial functional disorders in human OA, MIA-induced chondrocyte apoptosis has recently been reported to involve mitochondrial pathways including those responsible for ROS production and caspase activation [35]. These findings support the suggestion that the MIA model fairly reflects human OA; the cartilage breakdown mechanisms are similar. In our rat OA model, GLM reduced pain and eliminated the mitochondrial dysfunction caused by MIA.
Several recent reports have demonstrated the osteoarthritis therapeutic effectiveness of the administration of natural compound. Dietary supplementation with Palmitoyl-Glucosaine with curcumin decreased MIA-induced secondary allidynia at either threshold of latency level. Also, PGA-Cur with significantly decrease in serum levels of TNF-α, IL-1β, NGF as well as metalloproteases 1,3, and 9 [36]. In addition, there are studies on pain and inflammation ameliorate in osteoarthritis disease studies using natural compounds such as cashew nut, hyaluronic acid, and ALIAmide palmitoylglucosamine [21,22,37,38]. Also, in our studies GLM ameliorate pain and cartilage destruction in monosodium iodoacetate induced OA. The chondroprotective effects of GLM implicated inhibition of oxidative damage and suppression of catabolic factors involved in the pathogenesis of OA cartilage damage. But, further studies are need to further elucidate the relationship between natural compound and therapeutic effect of osteoarthritis.
The chondroprotective effects correlated strongly with catabolic inhibition of the articular cartilage matrix. The levels of specific markers of cartilage destruction, including MMP1, MMP3, and MMP13, were significantly decreased following GLM treatment of both rats and human OA chondrocytes. These valuable properties of GLM also delayed OA development, suggesting that the effects may be significant throughout the various stages of disease.
Many studies have explored whether necroptosis inhibitors suppress cell death [39]. Necroptosis is significant in terms of inflammatory disease development. To the best of our knowledge, this is the first study to investigate the anti-necroptotic properties of GLME using both an animal model and human OA chondrocytes. GLM was decrease the levels of necroptotic mediators in the synovium of rats. GLM may attenuate the inflammatory response by inhibiting necroptosis.
GLME reduced NF-κB expression in rat joints. GLM controlled the levels of upstream mediators that modulate the expression of inflammatory cytokines. Thus, the anti-inflammatory effects of GLM may reflect reduced NF-κB activation. Further molecular characterization of the relevant factors is required.
In conclusion, To the best of our knowledge, this study is the first to demonstrate the potential of GLM as an OA therapeutic agent. MIA-induced arthritis was decreased by GLM, which inhibited catabolism, and the expression of proinflammatory cytokines and mediators of necroptosis. GLM may usefully prevent OA. Our data will aid OA patients who seek valuable natural products. GLM may be a useful OA therapeutic.
Abbreviations
- GLM
The green-lipped mussel
- OA
Osteoarthritis
- MIA
monosodium iodoacetate
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- MLKL
mixed lineage kinase domain-like protein
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- RIP
receptor interacting protein kinase 1
- DHA
docosahexaenoic acid
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
Data Availability
All relevant data are within the manuscript.
Funding Statement
This research was supported by a grant of the Food Industry Promotional Agency of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–43. Epub 2014/02/18. doi: 10.1016/j.cell.2014.01.007 . [DOI] [PubMed] [Google Scholar]
- 2.Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840(8):2414–40. Epub 2014/03/13. doi: 10.1016/j.bbagen.2014.02.030 . [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. Epub 2012/12/01. doi: 10.1016/j.joca.2012.11.012 . [DOI] [PubMed] [Google Scholar]
- 4.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. Epub 2014/05/31. doi: 10.1155/2014/561459 ; PubMed Central PMCID: PMC4021678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto C, Giordano DM, Maroni L, Marzioni M. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1270–8. Epub 2017/07/30. doi: 10.1016/j.bbadis.2017.07.024 . [DOI] [PubMed] [Google Scholar]
- 6.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. Epub 2010/12/02. doi: 10.1038/nrrheum.2010.196 . [DOI] [PubMed] [Google Scholar]
- 7.Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. Epub 2010/03/05. doi: 10.2174/138945010791011938 ; PubMed Central PMCID: PMC3076145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi MC, Jo J, Park J, Kang HK, Park Y. NF-kappaB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells. 2019;8(7). Epub 2019/07/20. doi: 10.3390/cells8070734 ; PubMed Central PMCID: PMC6678954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–20. Epub 2015/01/17. doi: 10.1038/nature14191 . [DOI] [PubMed] [Google Scholar]
- 10.Cai Z, Zhang A, Choksi S, Li W, Li T, Zhang XM, et al. Activation of cell-surface proteases promotes necroptosis, inflammation and cell migration. Cell Res. 2016;26(8):886–900. Epub 2016/07/23. doi: 10.1038/cr.2016.87 ; PubMed Central PMCID: PMC4973336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24(7):1184–95. Epub 2017/05/13. doi: 10.1038/cdd.2017.65 ; PubMed Central PMCID: PMC5520172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 2016;26(10):721–32. Epub 2016/07/03. doi: 10.1016/j.tcb.2016.06.004 . [DOI] [PubMed] [Google Scholar]
- 13.Jeon J, Noh HJ, Lee H, Park HH, Ha YJ, Park SH, et al. TRIM24-RIP3 axis perturbation accelerates osteoarthritis pathogenesis. Ann Rheum Dis. 2020;79(12):1635–43. Epub 2020/09/09. doi: 10.1136/annrheumdis-2020-217904 ; PubMed Central PMCID: PMC7677493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treschow AP, Hodges LD, Wright PF, Wynne PM, Kalafatis N, Macrides TA. Novel anti-inflammatory omega-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp Biochem Physiol B Biochem Mol Biol. 2007;147(4):645–56. Epub 2007/06/05. doi: 10.1016/j.cbpb.2007.04.004 . [DOI] [PubMed] [Google Scholar]
- 15.Whitehouse MW, Macrides TA, Kalafatis N, Betts WH, Haynes DR, Broadbent J. Anti-inflammatory activity of a lipid fraction (lyprinol) from the NZ green-lipped mussel. Inflammopharmacology. 1997;5(3):237–46. Epub 1997/01/01. doi: 10.1007/s10787-997-0002-0 . [DOI] [PubMed] [Google Scholar]
- 16.Pollard B, Guilford WG, Ankenbauer-Perkins KL, Hedderley D. Clinical efficacy and tolerance of an extract of green-lipped mussel (Perna canaliculus) in dogs presumptively diagnosed with degenerative joint disease. N Z Vet J. 2006;54(3):114–8. Epub 2006/06/06. doi: 10.1080/00480169.2006.36622 . [DOI] [PubMed] [Google Scholar]
- 17.Stebbings S, Gray A, Schneiders AG, Sansom A. A randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of a novel green-lipped mussel extract -BioLex(R) -for managing pain in moderate to severe osteoarthritis of the hip and knee. BMC Complement Altern Med. 2017;17(1):416. Epub 2017/08/24. doi: 10.1186/s12906-017-1907-9 ; PubMed Central PMCID: PMC5568208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SH, Jung YB, Seong SC, Park HB, Byun KY, Lee DC, et al. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna Canaliculus) in patients with osteoarthritis of the hip and knee: a multicenter 2-month clinical trial. Eur Ann Allergy Clin Immunol. 2003;35(6):212–6. Epub 2003/07/23. . [PubMed] [Google Scholar]
- 19.Li D, Xie G, Wang W. Reactive oxygen species: the 2-edged sword of osteoarthritis. Am J Med Sci. 2012;344(6):486–90. Epub 2012/08/14. doi: 10.1097/MAJ.0b013e3182579dc6 . [DOI] [PubMed] [Google Scholar]
- 20.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13(8):643–54. Epub 2005/06/07. doi: 10.1016/j.joca.2005.04.002 . [DOI] [PubMed] [Google Scholar]
- 21.Fusco R, Siracusa R, Peritore AF, Gugliandolo E, Genovese T, D’Amico R, et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants (Basel). 2020;9(6). Epub 2020/06/14. doi: 10.3390/antiox9060511 ; PubMed Central PMCID: PMC7346149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordaro M, Siracusa R, Impellizzeri D, R DA, Peritore AF, Crupi R, et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res Ther. 2019;21(1):254. Epub 2019/11/30. doi: 10.1186/s13075-019-2048-y ; PubMed Central PMCID: PMC6883534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaprakash R, Perera CO. Partial Purification and Characterization of Bioactive Peptides from Cooked New Zealand Green-Lipped Mussel (Perna canaliculus) Protein Hydrolyzates. Foods. 2020;9(7). Epub 2020/07/09. doi: 10.3390/foods9070879 ; PubMed Central PMCID: PMC7404561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon JY, Lee SH, Na HS, Jung K, Choi J, Cho KH, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8(1):13832. Epub 2018/09/16. doi: 10.1038/s41598-018-32206-7 ; PubMed Central PMCID: PMC6138726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, Kwok SK, Son HJ, Ryu JG, Kim EK, Oh HJ, et al. IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res Ther. 2013;15(1):R31. Epub 2013/02/21. doi: 10.1186/ar4179 ; PubMed Central PMCID: PMC3672783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zochling J, March L, Lapsley H, Cross M, Tribe K, Brooks P. Use of complementary medicines for osteoarthritis—a prospective study. Ann Rheum Dis. 2004;63(5):549–54. Epub 2004/04/15. doi: 10.1136/ard.2003.010637 ; PubMed Central PMCID: PMC1754991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. Epub 1998/05/08. [PubMed] [Google Scholar]
- 28.Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol. 2006;2(6):304–12. Epub 2006/08/26. doi: 10.1038/ncprheum0193 . [DOI] [PubMed] [Google Scholar]
- 29.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008;16 Suppl 2:S15–20. Epub 2008/10/01. doi: 10.1016/S1063-4584(08)60008-4 . [DOI] [PubMed] [Google Scholar]
- 30.Alcaraz MJ, Megias J, Garcia-Arnandis I, Clerigues V, Guillen MI. New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol. 2010;80(1):13–21. Epub 2010/03/09. doi: 10.1016/j.bcp.2010.02.017 . [DOI] [PubMed] [Google Scholar]
- 31.Carlo MD Jr., Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–30. Epub 2003/12/16. doi: 10.1002/art.11338 . [DOI] [PubMed] [Google Scholar]
- 32.Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem. 2007;101(6):1520–31. Epub 2007/05/12. doi: 10.1002/jcb.21268 . [DOI] [PubMed] [Google Scholar]
- 33.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. Epub 2001/04/03. doi: 10.7326/0003-4819-134-7-200104030-00007 . [DOI] [PubMed] [Google Scholar]
- 34.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63(3):264–8. Epub 2004/02/14. doi: 10.1136/ard/2003.007666 ; PubMed Central PMCID: PMC1754924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, et al. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 2013;31(3):364–9. Epub 2012/11/06. doi: 10.1002/jor.22250 . [DOI] [PubMed] [Google Scholar]
- 36.Gugliandolo E, Peritore AF, Impellizzeri D, Cordaro M, Siracusa R, Fusco R, et al. Dietary Supplementation with Palmitoyl-Glucosamine Co-Micronized with Curcumin Relieves Osteoarthritis Pain and Benefits Joint Mobility. Animals (Basel). 2020;10(10). Epub 2020/10/15. doi: 10.3390/ani10101827 ; PubMed Central PMCID: PMC7601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Paola R, Fusco R, Impellizzeri D, Cordaro M, Britti D, Morittu VM, et al. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res Ther. 2016;18(1):291. Epub 2016/12/14. doi: 10.1186/s13075-016-1189-5 ; PubMed Central PMCID: PMC5153857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Lozano ML, Cesaro A, Mazor M, Esteve E, Berteina-Raboin S, Best TM, et al. Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants (Basel). 2021;10(2). Epub 2021/02/13. doi: 10.3390/antiox10020265 ; PubMed Central PMCID: PMC7914872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–9. Epub 2006/01/13. doi: 10.1038/nchembio711 . [DOI] [PubMed] [Google Scholar]