Abstract
Endothelial cells line the innermost layer of arterial, venous, and lymphatic vascular tree and accordingly are subject to hemodynamic, stretch, and stiffness mechanical forces. Normally quiescent, endothelial cells have a hemodynamic set point and become “activated” in response to disturbed hemodynamics, which may signal impending nutrient or gas depletion. Endothelial cells in the majority of tissue beds are normally inactivated and maintain vessel barrier functions, are anti-inflammatory, anti-coagulant, and anti-thrombotic. However, under aberrant mechanical forces, endothelial signaling transforms in response, resulting cellular changes that herald pathological diseases. Endothelial cell metabolism is now recognized as the primary intermediate pathway that undergirds cellular transformation. In this review, we discuss the various mechanical forces endothelial cells sense in the large vessels, microvasculature, and lymphatics, and how changes in environmental mechanical forces result in changes in metabolism, which ultimately influence cell physiology, cellular memory, and ultimately disease initiation and progression.
Keywords: endothelial cell metabolism, glycolysis, shear stress, oxidative phosphorylation, stiffness, barrier, inflammation, contraction
Introduction
The plasticity of cell types is a fundamental concern in biology, from differentiation of stem cells to tissue and organ specialization. Each of these processes relies on a combination of pre-programmed differentiation timing, paracrine chemical gradients, or membrane receptor clustering due to differential tension. This review is focused on the mechanical forces involved in metabolic signaling in endothelial cells especially as they relate to vascular disease and endothelial plasticity. Mechanical forces are involved in development but also in disease progression. In general, mechanical forces can be divided based on the tissue bed or the type of force, such as hemodynamic, stretch, and stiffness forces. As endothelial cells contract, migrate, signal inflammation, and possibly transdifferentiate into other cell types, they usually do so as a response to changes in mechanical forces, and coordinate their metabolism to meet this plasticity need (Table 1). This underscores the significance of cellular metabolism to coordinate genetic changes with phenotypic changes.
Table 1.
Summary of studies on mechanical forces cooperating with metabolic changes in driving endothelial phenotypes.
| Mechanical Force | Vasculature (cell type) | Regulation Mechanism | Metabolism | EC Phenotype | Disease | Ref |
|---|---|---|---|---|---|---|
| Shear stress (high/static, UF/DF) | HUVEC, ApoE-KO mouse, LDLR-KO mouse | ↑KLF2 (↓PFKFB3, ↓HK2) |
↓Glucose uptake, ↓Glycolysis |
↓Proliferation, ↓migration, ↓inflammation, ↓monocyte adhesion |
Pulmonary hypertension, atherosclerosis, thrombosis, pathological angiogenesis | 1–6 |
| Shear stress (UF/DF and long/short) | HUVEC, BAEC, RFPEC, ApoE-KO mouse | ↑KLF2 (↑HAS2 for glycocalyx) |
↓Glycolysis (↑Hexosamine and glucuronic acid biosynthesis) |
↓Permeability, ↓monocyte adhesion |
Atherosclerosis | 7–16 |
| Shear stress (DF/UF) | HAEC, HUVEC, mouse, ApoE-KO mouse | ↑HIF-1a (↑SLC2A1, ↑HK2, ↑PFKFB3, ↑LDHA, ↑PDK1, ↑NDUFA4L2) |
↑Glycolysis, ↓OXPHOS |
↑Proliferation, ↑inflammation |
Atherosclerosis | 6, 17–19 |
| Shear stress (DF/UF) | HUVEC, HAEC, mouse brain EC, mouse, ApoE-KO mouse | ↑YAP/TAZ (↑JNK, ↑MYC, ↑PGC1α) |
↑Glycolysis, ↑OXPHOS, ↑Mitochondria biogenesis |
↑Proliferation, ↑inflammation, ↑migration, ↑monocyte adhesion |
Atherosclerosis | 20–25 |
| Shear stress (DF/UF) | HAEC, mouse | ↑SLC2A1/3 (possibly by YAP/TAZ) | ↑Glucose uptake and glycolysis | ↑Migration | Aorta leakiness | 26 |
| N/A | Mouse bone EC | ↑YAP/TAZ (↓HIF-1a) |
↓Glycolysis | ↓Proliferation | Angiogenesis, osteogenesis | 27 |
| Shear stress (DF/UF) | HUVEC, MAEC, ApoE-KO mouse | ↑PRKAA1 | ↑Glycolysis | ↑Proliferation | Atherosclerosis | 28 |
| Shear stress (high/static and UF/DF) | HAEC, HUVEC, mouse | ↑SIRT1 (↑PGC1α) |
↑Mitochondria biogenesis | ↑NO bioavailability | 29–31 | |
| Shear stress (high/static) | HPAEC | ↓Plasma membrane cholesterol | ↑OXPHOS | ↑Ca2+ signaling | 32 | |
| Shear stress (UF/DF) | HUVEC, RAEC, mouse, LDLR-KO mouse, ApoE-KO mouse | ↑OPA1, ↑MFN2, ↓DRP1, ↓FIS1 |
↑Mitochondria fusion, ↓Mitochondria fission |
↓Inflammation, ↓monocyte adhesion |
Atherosclerosis | 33–36 |
| Transient shear stress (high/static) | HUVEC, BAEC | ↑DRP1, ↑[Ca2+]i |
↑Mitochondria fission, ↓OXPHOS, ↑mitochondria ROS |
37 | ||
| Shear stress (high/low) | HPAEC, HUVEC | ↑Mitochondria ATP (↑Purinergic receptors) |
↑Ca2+ influx | ↑Vasodilation, ↓inflammation |
Hypertension | 38–40 |
| Shear stress (high/low) | HUVEC | ↑Mitochondria Ca2+ release/uptake | ↑ER Ca2+ uptake/release | 41 | ||
| Shear stress (DF/UF) | HUVEC | unknown | ↓Lipid metabolism, ↓LDLR |
42 | ||
| Shear stress (high/static) | HPAEC | unknown | ↑ether-containing lipids | ↓Inflammation | 43 | |
| Shear stress (UF/DF) | HUVEC | ↑NOTCH1 (↑CPT1A) |
↑FAO | Possibly ↓EndMT, ↑dNTP synthesis |
Angiogenesis | 44–46 |
| Shear stress (DF/UF) | BAEC | ↑SREBP1 | ↑FA synthesis ↑Lipid accumulation |
47–48 | ||
| Shear stress (high/static) | HUVEC, BAEC | ↑ASS1 | ↑L-arginine synthesis | ↑NO production, ↑viability |
49–51 | |
| Shear stress (capillary-like/static) | HBMEC | ↑TCA enzymes such as PDH, ↓LDHA |
↑OXPHOS, ↓Glycolysis |
↓Proliferation | 52 | |
| Shear stress (high/static) | HGEnC | ↑ENOS | unknown | ↓Permeability | 53 | |
| Hypoxia/normoxia (unknown in mechanotransduction) | HMVEC, mouse | SIRT3 (↑HIF-2a, ↑PFKFB3) |
↑Glycolysis, ↓OXPHOS |
↑Proliferation | Diastolic dysfunction | 54–55 |
| Hypoxia/normoxia (unknown in mechanotransduction) | HPAEC | ↑FA synthase (↑HIF-1a) |
↑Glycolysis | ↑Proliferation, ↓eNOS |
Pulmonary hypertension | 56 |
| Hypoxia/normoxia (unknown in mechanotransduction) | HMVEC | ↑HIF-2a (↑Arginase II) |
↓L-arginine for eNOS | ↓NO production | Pulmonary hypertension | 57 |
| Long-term shear stress (high/static) | HRMEC, BRMEC | ↑ENOS, ↑TM, ↓ET-1 |
unknown | ↑Vasodilation, ↑Antithrombotic |
58–59 | |
| Shear stress (low/static) | HRMEC | ↑E-selectin, ↑ICAM-1, ↑Cytokine/ chemokine, ↑Procoagulant factors |
unknown | ↑Inflammation | 60 | |
| Shear stress (DF/UF) | HLEC, mouse | ↑FOXC2-PROX1 (↑Connexin37, ↑calcineurin) |
unknown | ↑Lymphatic identity | lymphatic-valve morphogenesis | 61–62 |
| Shear stress (low/static) | HDMLEC | ↑GATA2-FOXC2 | unknown | lymphatic vessel maturation, lymphedema | 63–64 | |
| Shear stress (high/static) | HLEC | ↓PROX1 (↓CPT1) |
↓FAO (↓dNTP synthesis) |
↓Lymphatic identity | Lymphangiogenesis | 65–66 |
| Shear stress (chronic low/static) | LLEC | ↑HIF-1a | Altered metabolomics | ↑Proliferation | 67 | |
| Stretch | BPAEC, HUVEC | Actin filaments | ↑Mitochondria ROS | Possibly ↑focal adhesion kinase signaling; ↑Inflammation |
68–69 | |
| Stretch | HUVEC | unknown | ↓Glucose utilization | 5 | ||
| Stretch | MLEC, mouse | ↑YAP/TAZ (↓VE-PTP) |
unknown | ↓Permeability, ↓inflammation |
Ventilator-induced lung injury | 71 |
| Stiffness | RPAEC, rat | ↑YAP/TAZ (↑GLS1, ↑LDHA) |
↑Glutamate (↑TCA intermediates), ↑glycolysis |
↑Proliferation, ↑migration |
Pulmonary hypertension | 26 |
| Cytoskeleton | HUVEC | ↑PFKFB3 | ↑Glycolysis | ↑Filopodia formation (↑Migration) |
Vessel sprouting | 72–73 |
| Cytoskeleton | HPAEC | ↑HDAC6 (↓Microtubule) |
Acetyl-CoA | ↑Permeability | Acute lung injury | 74–76 |
| Cytoskeleton | HAEC | ↑RhoA (↑SLC2A3) |
↑Glucose uptake | ↑Migration | 77 |
Abbreviation list of Table 1:
BAEC: bovine aortic endothelial cell
BRMEC: bovine retinal microvascular endothelial cells
HAEC: human aortic endothelial cell
HBMEC: human brain microvascular endothelial cell
HDMLEC: human dermal microvascular lymphatic endothelial cell
HGEnC: human glomerular endothelial cell
HLEC: human lymphatic endothelial cell
HPAEC: human pulmonary arterial endothelial cells
HRMEC: human retinal microvascular endothelial cell
HUVEC: human umbilical endothelial cell
LLEC: lamb lymphatic endothelial cell
MLEC: murine lung endothelial cell
NDUFA4L2: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex, 4-like 2
RAEC: rat aortic endothelial cell
RFPEC: the rat fat pad endothelial cell
RPAEC: rat pulmonary arterial endothelial cells
Reference directory of Table 1:
(D. Wu et al., 2017, p. 1)
(B. Kim et al., 2014, p.)
Mechanotransduction in large vessels: blood flow and stiffness in the aorta
Macrovascular flow (vessels > 10 μm in diameter) is complex, varying in both space and time as vessel boundary conditions continuously change and heart pump strength is modulated dynamically by both neural and volumetric fluid inputs in a beat to beat fashion. Endothelial cells are subjected to shear stress from 10 to 50 dyne/cm2 in large arteries (Paszkowiak & Dardik, 2003). Arterial flow can be classified into two classes: “atheroprotective” and “atheroprone” (Davies, 1995). Atheroprotective flow is unidirectional (“unidirectional flow”, UF) whereas atheroprone has no time-averaged direction but is instead chaotic and reminiscent of eddy currents or vortexes (“disturbed flow”, DF).
Aortic hemodynamics have been the most studied in the context of development (Combs & Yutzey, 2009; O’Donnell & Yutzey, 2020; Vermot et al., 2009) and atherosclerosis, as arterial branch points and vessel curvature result in disturbed flow, leading to coronary artery disease, aortic atherosclerosis, and carotid artery disease (Chiu et al., 2009; Davies et al., 2013) (Figure 1). Aortic and carotid hemodynamics have been modeled based on magnetic resonance imaging studies of flow, which are used in clinical practice (Ferdian et al., 2020), making in vitro study of flow-related changes in endothelial cell biology possible (Dai et al., 2004; Krause et al., 2018; Maurya et al., 2021; Parmar, 2005; C. Wu et al., 2015). In general, unidirectional flow results in elaboration of nitric oxide, barrier protection, and is protective against inflammation and thrombosis, whereas disturbed flow results in vessel constriction, permeable barriers, thrombotic pathways, and inflammatory signaling – hallmarks in the development of atherosclerosis. Although not discussed in detail here, vascular endothelial cells are also subjected to significant circumferential cyclic stretch (Fang et al., 2019). For instance, heart propulsions result in cyclic stretch in arterial endothelium and spontaneous respiration (or mechanical ventilation in critically ill patients) causes mechanical stretch of lung microvascular endothelium. Mechanotransduction through hemodynamics is mediated by transcriptional, posttranscriptional and epigenetic mechanisms and flow-sensitive transcription factors are instrumental to the endothelial responses to blood flow (Andueza et al., 2020; Krause et al., 2018; Ku et al., 2019; Nagel et al., 1999; Partridge et al., 2007; Peghaire et al., 2019; Zhou et al., 2014).
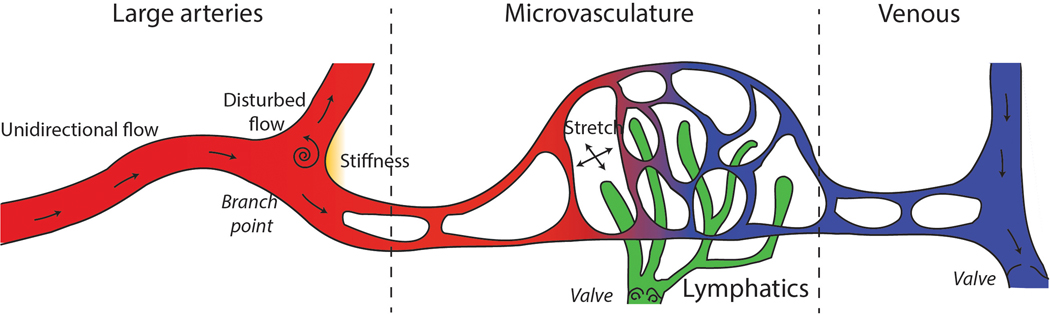
Figure 1: Major mechanical forces in vasculature.
(Left) In large arteries, endothelial cells in the straight vessels are subjected to unidirectional flow, whereas endothelial cells in the branch points and curvatures are subjected to disturbed flow and stiffness, leading to atherosclerosis formation. (Middle) Microvascular flow occurs in vessels, capillaries, and lymphatics. Shear stress is critical for lymphatic valve development. Stretch in the lung modulates capillary barrier function. (Right) Veins have valves which are under the control of lymphangiogenesis genes, but how metabolism plays a role is unclear.
Microenviroemntal stiffness also plays a fundamental role in cell differentiation. Matrix stiffness properties per se can cause mesenchymal stem cell differentiation (Engler et al., 2006)- neuronal differentiation programs are activated on soft surfaces (0.1–1 kPa), whereas muscle or bone differentiation programs are activated by hard surfaces (10–100 kPa). Tissue culture plastic is in the ~106 kPa range. Thus, if not properly considered stiffness may cause experimental artifacts. Endothelial cells produce fold-changes in actin with increasing substrate stiffness (Byfield et al., 2009) and affects, for instance, leukocyte transmigration in in vitro studies (Stroka & Aranda-Espinoza, 2011). Like flow, mechanotransduction by stiffness sensors causes nuclear translocation of transcription factors.
Matrix stiffness and disturbed flow work in tandem, amplifying disease processes. Stiffer vessels could lead to increased flow and/or reduced pulsatility, which is particularly harmful to endothelial cells in the brain vascular bed. Vascular stiffening is by itself sufficient to explain primary hypertension (Pettersen et al., 2014). In the systemic arterial circulation, increased vascular stiffness is associated with and precedes systemic hypertension (Beltran, 2001; Pettersen et al., 2014), and is a predictor of cardiovascular morbidity (Benetos et al., 2012; Smulyan et al., 2016) and mortality (Laurent et al., 2001).
Vascular stiffening is also pathological in the pulmonary circulation and microvasculature. Increased macrovascular stiffness promotes microvascular damage (Cardoso & Salles, 2016; Cooper et al., 2018; Mitchell, 2008) and therefore end-organ damage through dysregulated transmission of hemodynamics from large vessels and stiffness-dependent control of RhoA GTPase activity, permeability, and inflammation (Birukova et al., 2013; Mambetsariev et al., 2014; Meng et al., 2015). Besides arterial hypertension, microvascular stiffness in the pulmonary circulation has been identified as an independent cause of mortality in pulmonary hypertension (Campo et al., 2010; Gan et al., 2007; Hunter et al., 2008; Mahapatra et al., 2006; Thenappan et al., 2016). Current standard of care antihypertensive treatment is thought to have an anti-stiffness component (Y. Chen et al., 2017) and improves mortality (Brunström & Carlberg, 2018).
Mechanotransduction in microvasculature and lymphatics.
The microvasculature is composed of capillaries. The lung has the most microvasculature (vessel diameter 10 μm or less) in the body, as its estimated capillary surface area is roughly 50–70 m2, which is 20-times that of all other vessels (Albertine, 2016; Weibel, 1973). Lung microvascular ECs are subjected to shear stress, which has profound consequences to microvascular barrier function (Adamson et al., 2013; R.-T. Huang et al., 2017; Ostrowski et al., 2014) and production of reactive oxygen species (Milovanova et al., 2006). Shear stress in the microvasculature can theoretically be higher than in large vessels since shear stress is inversely proportional to the 3rd power of vessel radius and only linearly dependent on flow rate. However, shear stress is not a precisely measured quantity in the microvasculature since vessel diameter can be equal to or even smaller than the diameter of a red blood cell. Besides subjected to shear stress, ECs in the lungs are also exposed to copious – and possibly injurious stretch during mechanical ventilation – due to the respiratory cycle.
Lymphatic flow is even slower than microvascular flow, averaging 0.64 dyn/cm2 with peaks of 4–12 dyn/cm2 (Dixon et al., 2006). Mechanotransduction and mechanosensation are less well-developed in understanding compared to the microvasculature and large vessels. However, mechanotransduction is important in lymphatic development especially valve formation and lymphatic plexus development (Sweet et al., 2015).
Lung endothelial cells are also subject to regulation of substrate stiffness. Traction forces are estimated to be much higher – typically ~5 kPa (Balaban et al., 2001) and artery wall strains ~100 kPa (Humphrey et al., 2014). This is important in pulmonary hypertension and chronic fibrotic lung diseases.
Macrovascular flow and metabolism
Cross-talk between mechanotransduction, metabolism, and disease has been most studied using in vitro models of large vessel hemodynamics and animal model correlates. Endothelial cells are mostly glycolytic with a relatively small contribution of ATP generation from oxidative phosphorylation, and exhibit the Warburg effect, or, utilization of glycolysis in the presence of high concentrations of oxygen (De Bock et al., 2013; Doddaballapur et al., 2015; B. Kim et al., 2017; D. Wu et al., 2017). However, mechanical forces including shear stress and surface stiffness are able to dynamically modulate metabolism by changing the proportion of ATP produced by oxidative phosphorylation (Feng et al., 2017; D. Wu et al., 2017). The metabolic activity and throughput of glycolysis and the TCA cycle have profound effects on endothelial cell physiology. In this section, we will mainly focus on atherosclerosis as the disease phenotype to summarize the metabolic regulation due to blood flow and shear stress in arterial beds. We will divide these metabolism pathways/substrates into glycolysis, mitochondria, fatty acids, and amino acids. Veins and valve formation are less well studied, especially in the context of endothelial metabolism; however, venous valve formation is under the control of lymphangiogenesis genes (Bazigou et al., 2011).
Glycolysis
Blood flow/shear stress regulates glycolysis through, at least partially transcription factors Krüppel-like factor-2 (KLF2), hypoxia inducible factor-1α (HIF-1α) and Yes-associated protein (YAP) with PDZ-binding motif (TAZ). KLF2 expression is significantly up-regulated by arterial-levels of shear stress and is anti-angiogenic, barrier protective, and anti-inflammatory and protective against atherosclerosis and acute lung injury (Atkins & Jain, 2007; Dekker et al., 2002; R.-T. Huang et al., 2017; Lin et al., 2005, 2010). HIF family of transcription factors are regulated by targeting for degradation via hydroxylation by prolyl hydroxylases (PHDs), which are sensitive to oxygen concentration (Prabhakar & Semenza, 2012; Semenza, 2012) as well as the shear stress (D. Wu et al., 2017). The Hippo pathway involving transcriptional co-activators YAP/TAZ are also flow responsive (K.-C. Wang et al., 2016; L. Wang et al., 2016) and stimulate metabolism (refs). Glycolysis is reduced by unidirectional flow in a KLF2-dependent manner, while induced by disturbed flow mainly in a HIF-1α (Doddaballapur et al., 2015; Feng et al., 2017; D. Wu et al., 2017) and YAP/TAZ -dependent manner (K.-C. Wang et al., 2016; L. Wang et al., 2016). Microvascular endothelial KLF2 expression was markedly reduced in critically ill acute respiratory distress syndrome (ARDS) patients infected with SARS-CoV-2 (Lee et al., 2021, p.). COVID-19-induced inflammation is also reported to suppress endothelial KLF2 expression, possibility contributing to the endothelialitis (S. Xu et al., 2021).
KLF2 reduces endothelial glycolysis through transcriptional repression of HK2 and PFKFB3
KLF2, induced by unidirectional flow, inhibits endothelial glucose uptake and glycolysis (Doddaballapur et al., 2015; Parmar, 2005; D. Wu et al., 2017). Both RNA silencing of KLF2 in cultured human umbilical vein endothelial cells (HUVECs) and endothelial-specific KLF2 deletion in mouse increased glycolysis as measured by extracellular acidification rate, whereas KLF2 overexpression reduced extracellular acidification rate (Doddaballapur et al., 2015) and recapitulated the inhibitory effect of unidirectional flow on EC glycolysis (D. Wu et al., 2017). Glycolytic-suppression due to KLF2 is mainly through transcriptional suppression of key glycolytic enzymes including hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-3 (PFKFB3) (Doddaballapur et al., 2015). HK2 catalyzes the phosphorylation of glucose, the rate-limiting and first committed step in glycolysis. PFKFB3 is an allosteric activator of phosphofructokinase (PFK) in the second rate-limiting reaction of glycolysis. Both of these steps are the ATP consuming reactions constituting the “investment” phase of glycolysis prior to the ATP producing steps. Decreasing HK2 (P. Yu et al., 2017) and PFKFB3 (Schoors et al., 2014; Y. Xu et al., 2014) in endothelial cells both led to reduced glycolysis and impaired proliferation. In addition to reducing proliferation, endothelium-specific PFKFB3 knockout suppressed the development of pulmonary hypertension by reducing inflammation and leukocyte adhesion (Cao et al., 2019). Also, pharmacological inhibition of PFKFB3 in high fat diet-fed LDLR−/− mice attenuated atherosclerosis progression and increased plaque stability, indicating an atherogenic role of this DF-induced glycolytic gene (Poels et al., 2020). In addition to KLF2, KLF4 was recently reported to suppress glycolysis in vascular endothelium under pulsatile shear stress (Y. Han et al., 2021).
KLF2 shunts glycolytic intermediates for production of glycocalyx
Besides its transcription suppression on these glycolytic enzymes, KLF2 also reduces glycolysis by inducing the biosynthesis of hyaluronan, the major structural component of glycocalyx on endothelial cell surface (G. Wang et al., 2020). Glycocalyx serves as an important structure on the surface of endothelial cells for sensation of shear stress generated by blood flow (Fu & Tarbell, 2013; Pries et al., 2000; Tarbell & Ebong, 2008; Zeng & Tarbell, 2014). The thickness of endothelial glycocalyx is associated with local shear stress (Lewis et al., 1982; van den Berg et al., 2006). Using both cultured HUVECs and Apoe−/− mouse model, Wang et al., showed that laminar shear stress induces endothelial cells to produce thicker coating of glycocalyx on the luminal surface. Specifically, this shear stress-induced hyaluronan production is through KLF2-dependent hyaluronan synthase 2 (HAS2) expression and UDP-sugar availability. KLF2 not only directly induced the transcription of HAS2, but also indirectly shuttles the glycolysis pathway (by inhibition of PFKFB3) into hexosamine- and glucuronic acid biosynthesis pathways (G. Wang et al., 2020). Consequently, the biosynthesis of UDP-GlcA and UDP-GlcNAc was increased, serving as substrates for hyaluronan production (G. Wang et al., 2020). In addition to acting as a mechano-sensor, glycocalyx also prevents endothelial permeability (Curry & Adamson, 2012; Tarbell, 2010), suppresses leukocyte-endothelium adhesion (Lipowsky, 2011; Tarbell & Ebong, 2008), and maintains potassium channel activation by blood flow (Fancher et al., 2020).
Disturbed flow-induced HIF-1α increases endothelial glycolysis
HIF-1α is associated with atherosclerosis formation by enhancing endothelial cell inflammation, proliferation, and monocytes adhesion (Akhtar et al., 2015; Feng et al., 2017; D. Wu et al., 2017, p. 1). Disturbed flow (DF) activates HIF-1α to increase endothelial metabolism beyond its baseline high glycolysis (Feng et al., 2017; D. Wu et al., 2017). There are three mechanisms which have been demonstrated related to DF-induced HIF-1α activation: (1) transcriptionally, HIF-1α is upregulated by nuclear factor NF-κB (Feng et al., 2017); (2) post-translationally, HIF-1α protein is stabilized by deubiquitinating enzyme Cezanne (Feng et al., 2017), and also (3) post-translationally, HIF-1α is stabilized by reactive oxygen species (ROS) produced by NAD(P)H Oxidase-4 (NOX4) (D. Wu et al., 2017). HIF-1α promotes endothelial glycolysis by upregulating a cohort of glycolytic genes including lactate dehydrogenase A (LDHA), glucose transporter-1 (SLC2A1/GLUT1), HK2, PFKFB3, and probably additional glycolytic genes as the glycolysis and angiogenesis gene sets have a high degree of overlap (Feng et al., 2017; D. Wu et al., 2017). Furthermore, DF-induced HIF-1α can shift the fate of pyruvate towards glycolysis away from TCA cycle by enhancing the transcription of pyruvate dehydrogenase kinase-1 (PDK1) (J. Kim et al., 2006; D. Wu et al., 2017). HIF-1α-driven glycolytic reprogramming is required for the disturbed flow-induced endothelial inflammation and excessive proliferation, leading to atherosclerosis formation (Feng et al., 2017; D. Wu et al., 2017). Endothelial specific knockout of HIF-1α suppressed DF-induced atherosclerosis in Apoe−/− mice (Feng et al., 2017); similarly, knockdown of SLC2A1 reduced DF-induced HIF-1α expression and EC inflammation (D. Wu et al., 2017). Interestingly, HIF-1α and KLF2 are counter-regulated: KLF2 has been shown to disrupt binding between HIF-1α and its chaperone HSP90 (Kawanami et al., 2009). This suggests that there are possibly two exclusionary metabolic poles which define the metabolic state of aortic endothelial cells.
Disturbed flow-induced YAP/TAZ increases EC glycolysis
YAP/TAZ are transcriptional co-activators that bind primarily to enhancer elements by interacting with TEAD factors, effectors of the Hippo dependent pathway (or Hippo independent). YAP/TAZ plays a major role in transduction of mechanical signals from actin to the nucleus. YAP and TAZ have been shown to be activated in epithelial, fibroblast, endothelial cells, oncogenesis, neurons, and stem cells (Y.-A. Chen et al., 2019; Furukawa et al., 2017; Lian et al., 2010; F. Liu et al., 2015; Totaro et al., 2017).
YAP/TAZ are regulated by mechanical stimuli including shear stress (Halder et al., 2012; K.-C. Wang et al., 2016; L. Wang et al., 2016; Zhu et al., 2021). Disturbed flow increases while unidirectional flow reduces YAP/TAZ activity in endothelial cells (K.-C. Wang et al., 2016; L. Wang et al., 2016). High shear stress activates endothelial integrin and promotes integrin-Gα13 interaction, which inhibits RhoA, leading to YAP/TAZ phosphorylation thus inactivation (L. Wang et al., 2016, p.). Reduced endothelial YAP/TAZ activity has been further suggested to downregulate the expression of pro-inflammatory genes, reduce monocytes adhesion and infiltration (K.-C. Wang et al., 2016; L. Wang et al., 2016) and retard endothelial proliferation (K.-C. Wang et al., 2016), one mechanism of which is through suppression of Jun Kinase (JNK) activity (L. Wang et al., 2016). Furthermore, both in vivo blockade of YAP/TAZ activity either by CRISPR-Cas9-mediated endothelial-specific YAP knockdown (L. Wang et al., 2016) or by translational inhibition using morpholino oligo (K.-C. Wang et al., 2016) can reduce the atherosclerotic plaque size in hyperlipidemic Apoe−/− mice. These findings indicate that YAP/TAZ activity is partially responsible for the disturbed-flow induced atherosclerosis.
YAP/TAZ is also a key regulator of metabolism. YAP/TAZ plays an important role in the metabolism of many cell types, and acts both as an energy-sensor and energy-regulator (Koo & Guan, 2018). In brief, YAP/TAZ activity is activated when nutrient supply is sufficient, and YAP/TAZ activity will in turn promote glycolysis, glutaminolysis, anapleurosis, and lipogenesis to regulate cell growth and homeostasis (Koo & Guan, 2018). YAP/TAZ has also been shown to regulate endothelial metabolism. RNA interference of YAP/TAZ in HUVECs decreases both glycolysis and mitochondria oxidative phosphorylation. In addition, brain endothelial cells from YAP/TAZ endothelial cell-specific knockout mice showed downregulation of genes that are involved in glycolytic and the OXPHOS pathway (J. Kim et al., 2017). Furthermore, YAP/TAZ induced endothelial glycolysis is dependent on MYC, another potent glycolysis transcription factor (J. Kim et al., 2017). Moreover, in addition to disturbed flow, YAP/TAZ is activated by increased matrix stiffness (see below, section on pulmonary vasculature). Notably, atherosclerosis lesions are also very stiff (Kohn et al., 2015).
In bone endothelial cells, YAP1/TAZ was found to repress the pro-angiogenic activity of HIF-1α, suggesting that the relationship between YAP/TAZ and HIF-1α is tissue-specific or dependent on the local chemical/mechanical microenvironment (Sivaraj et al., 2020), as in cancer cells, YAP/TAZ helps stabilize HIF-1α, preventing the latter’s degradation (see below). Furthermore, whereas HIF-1α in HAECs was found to drive glycolysis and suppress oxidative phosphorylation (D. Wu et al., 2017), YAP/TAZ was found to stimulate both glycolysis and oxidative phosphorylation (Bertero et al., 2016), which suggests that HIF-1α may specifically reprogram endothelial cell metabolism for migration or inflammation, whereas perhaps YAP/TAZ stimulates endothelial cells for both migration and growth.
In cancer cell types, YAP/TAZ has been shown to increase glycolysis by binding to transcription factors and promoting glucose transporter expression. YAP/TAZ drives glycolysis by increasing lncRNA BCAR4 to increase Hedgehog signaling which promotes HK2 and PFKFB3 transcription (Zheng et al., 2017). YAP/TAZ also recruits HIF-1α at pyruvate kinase M2 (PKM2) gene promoter to enhance its transcription (Jia et al., 2019), and binds to HIF-1α to prevent HIF-1α degradation (X. Zhang et al., 2018). Transcriptomics data suggested that zebrafish embryos lacking YAP has reduced mRNA of glucose transporters SLC2A1 and SLC2A2, causing decreased glucose uptake to support nucleotide synthesis (Cox et al., 2018). In cancer cells, YAP/TAZ enhances SLC2A1 membrane translocation in an AKT-dependent manner (White et al., 2019). Additionally, YAP/TAZ/TEAD complex can promote HEK293A cells glycolysis via increasing the transcription of the SLC2A3. Knocking down SLC2A3 in cells with constitutively active YAP can partially reverses glucose uptake and lactate production (W. Wang et al., 2015).
It is noteworthy that SLC2A1 and SLC2A3 are the most highly transcribed glucose transporters in human aortic endothelial cells (D. Wu et al., 2021). SLC2A3 regulates thrombin-induced endothelial glycolysis burst and its endothelial-specific overexpression results in mouse aorta leakiness (D. Wu et al., 2021). Therefore, it would be reasonable to hypothesize and interesting to explore if disturbed flow-induced YAP/TAZ also acts as a transcriptional activator of SLC2A1/3 in endothelial cells, contributing to the glycolysis-driven atherosclerosis burden.
Endothelial cell glycolysis can play pro- and anti-atherogenic roles
Although many of the abovementioned studies revealed that disturbed flow-induced endothelial glycolysis is detrimental to vascular health, interestingly, Yang et al. demonstrated a beneficial role of a glycolytic regulator protein kinase AMP-activated (AMPK) in protection against atherosclerosis (Q. Yang et al., 2018). Disturbed flow increased the expression of AMPK in endothelial cells both in vitro and in vivo. Selectively deleting endothelial PRKAA1, the major catalytic subunit of AMPK in vascular cells, reduced endothelial cell glycolysis and proliferation, while aggravating atherosclerosis formation in hyperlipidemic mice. In addition, overexpressing SLC2A1 rescued the impaired glycolysis in PRKAA1-deleted endothelial cells and reversed the severity of atherosclerosis, suggesting that reduced endothelial glycolysis was partially responsible for promoting atherosclerosis (Q. Yang et al., 2018). However, excessive glycolysis also triggered atherosclerosis as evidenced by overexpression of SLC2A1 in PRKAA1-intact endothelium, which increased plaque size in the partial ligation mouse model (Q. Yang et al., 2018). Thus, disturbed flow-induced endothelial glycolysis can play a double-edged sword in atherogenesis, emphasizing the importance of metabolic tuning in endothelial phenotype and therapeutic targeting. It is also important to note that the completed deletion of a metabolic enzyme, although instrumental to demonstrate the causality in animal models, rarely occurs in humans during the pathophysiological processes.
Oxidative phosphorylation
Studies from our lab and others have collectively shown that disturbed flow reduces, whereas unidirectional flow increases oxidative phosphorylation in cultured HAECs (B. Kim et al., 2014; D. Wu et al., 2017, p. 1). This is in accordance with in vivo data which demonstrated that increased vascular shear stress boosted mitochondrial health in rodents (B. Kim et al., 2014; J.-S. Kim et al., 2015). The unidirectional flow-induced OXPHOS in endothelial cells could be dependent on transcription factors KLF2/4. KLFs may be responsible for the unidirectional flow-increased mitochondria biogenesis in endothelial cells and other cell types (B. Kim et al., 2014; Liao et al., 2015). In addition to KLFs, the expression and activity of deacetylase sirtuin 1 (SIRT1) is also enhanced by unidirectional flow and induces mitochondria biogenesis (Z. Chen et al., 2010; J.-S. Kim et al., 2015). Unidirectional flow may also induce endothelial OXPHOS through degrading HIF-1α, as HIF-1α has been shown to promote the transcription of PDK1, which phosphorylates and suppresses pyruvate dehydrogenase (PDH) to catalyze glucose-derived pyruvate into acetyl-CoA entering TCA cycle (J. Kim et al., 2006; D. Wu et al., 2017, p. 1). Furthermore, HIF-1α has also been shown to attenuate OXPHOS through inhibiting mitochondria complex 1 activity (Tello et al., 2011).
YAP signaling also induces OXPHOS in endothelial cells. Inhibition of YAP/TAZ in HUVECs markedly reduced OXPHOS along with decreased glycolysis (J. Kim et al., 2017). YAP/TEAD1 complex enhanced mitochondria biogenesis and oxygen consumption to support HUVECs angiogenesis, which is through promoting the transcription of peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1α) (Mammoto et al., 2018).
A recent paper reported that plasma membrane cholesterol also plays a role linking shear stress to OXPHOS in cultured human pulmonary aortic endothelial cells (Yamamoto et al., 2020). Shear stress reduced plasma membrane cholesterol both through efflux and internalization, leading to increased OXPHOS. Similarly, depleting membrane cholesterol using methyl-β-cyclodextrin (MβCD) mimicked shear stress-induced mitochondria ATP production, whereas addition of cholesterol to cells suppressed this shear stress-induced OXPHOS, suggesting a novel flow-activated OXPHOS mechanism mediated by membrane cholesterol (Yamamoto et al., 2020).
Nevertheless, cultured endothelial cells only generate ~15% ATP through oxidative phosphorylation, indicating that instead of as a major energy source, mitochondria in endothelial cells may play a more vital role as an organelle for signaling and metabolic intermediates production (Quintero et al., 2006). These signaling functions of endothelial mitochondria include maintaining Ca2+ homeostasis and regulating oxidative stress (X. Tang et al., 2014). It is worthwhile to note that multiple in vivo studies have suggested that increased shear stress in major vessels improves mitochondrial function (B. Kim et al., 2014; J.-S. Kim et al., 2015).
Shear stress regulates mitochondrial fusion and fission
Mitochondria are highly dynamic organelles that frequently undergo fusion and fission. Fusion and fission are important for their proper cellular distribution, inheritance of mtDNA, energy production and removal of their dysfunctional companions by mitophagy (Westermann, 2010), and are highly responsive to environmental stress (Youle & van der Bliek, 2012). In general, fusion is beneficial to complement partially dysfunctional mitochondria by mixing their contents to meet increased energy demands, whereas fission helps quality control by eliminating damaged mitochondria and promotes apoptosis (H. Chen & Chan, 2009; Y. J. Liu et al., 2020; Suárez-Rivero et al., 2016).
Shear stress has also been shown to regulate mitochondria dynamics in endothelial cells. Unidirectional flow promotes mitochondria health often by inducing mitochondria fusion, compared to disturbed flow (Chehaitly et al., 2021; L.-H. Wu et al., 2018). Unidirectional flow upregulates mitochondria fusion protein optic atrophy protein 1 (OPA1) and mitofusin 2 (MFN2) (Chehaitly et al., 2021; L.-H. Wu et al., 2018) while downregulates mitochondria fission dynamin-related protein 1 (DRP1) (Chehaitly et al., 2021) and mitochondria fission 1 (FIS1) protein (L.-H. Wu et al., 2018). Consistently, the translocation of fission protein DRP1 from cytosol to mitochondria is decreased in response to laminar shear stress. In vivo studies reported in an abstract seem to support this observation in mouse aorta; when compared to aortic area subjected to high shear stress, inner curvature exposed to disturbed flow had reduced OPA1 and increased DRP1 (Chehaitly et al., 2021). On the contrary, short-term laminar shear stress promoted mitochondria fission in both HUVECs and bovine aortic endothelial cells (BAECs) compared to static conditions (Bretón-Romero et al., 2014). This UF-induced mitochondria fission is dependent on enhanced translocation of DRP1 to the mitochondria membrane and the increased intracellular Ca2+ level (Bretón-Romero et al., 2014). Interestingly, this transient mitochondria fission induced by UF is also accompanied by reduced mitochondria bioenergetics and increased ROS production (Bretón-Romero et al., 2014). Whether the degree or time-dependence is a factor in these conflicting results is unclear.
DRP1 inhibition reduces endothelial inflammation. Interestingly, in the diabetic Apoe−/− mouse model, inhibiting DRP1 using mitochondria division inhibitor 1 improved endothelial function, reduced inflammatory makers (VCAM-1 and ICAM-1) expression and attenuated the development of diabetic-induced atherosclerosis, suggesting mitochondria fission contributes to atherogenesis in diabetes (Q. Wang et al., 2017). Moreover, inhibiting DRP1 in cultured rat aortic endothelial cells reduced TNFα-induced NF-κB activation, VCAM-1 expression and leukocyte adhesion (Forrester et al., 2020). Heterozygous DRP1-deficient mice and endothelial-specific DRP1 silencing showed reduction in inflammatory leukocyte adhesion (Forrester et al., 2020). Chehaitly et al also reported that in high fat diet-fed LDLR−/− mice with OPA1 or DRP1 heterozygous deletion, LDLR−/− OPA1−/+ mice aggravated while LDLR−/− DRP1−/+ attenuated atherosclerosis plaque formation when compared to wildtype mice (Chehaitly et al., 2021). Beyond global knockout model, it would be interesting to adopt mice with endothelium-specific mitochondria dysregulation, to further dissect the role of mitochondria fusion and fission in the context of atherogenesis in response to hemodynamic forces.
Ca2+ signaling
Intracellular calcium ion, or [Ca2+]i, acts as a key second messenger in endothelial cells in regulation of migration, proliferation, inflammation, vasodilation and cell survival (Antoniotti et al., 2003; Dalal et al., 2020; Fiorio Pla et al., 2012, p. 4; Sessa, 2005; Tsai et al., 2014; Yokota et al., 2015). An irregular endothelial [Ca2+]i could lead to a variety of pathological consequences including impaired angiogenesis, barrier integrity and vasodilation (Dalal et al., 2020; Seeley et al., 2013; Yokota et al., 2015). It has been widely accepted that shear stress regulates intracellular calcium level in endothelium (James et al., 1995; Scheitlin et al., 2016; Yamamoto et al., 2000, 2018). One contributing mechanism is mediated by shear-sensitive potassium channels. In endothelium, unidirectional shear stress significantly increased the activity of inwardly rectifying potassium channels (Kir) which maintain the endothelial membrane potential, the major driving force of endothelial Ca2+ influx (Fancher & Levitan, 2020, p.; Fang et al., 2005, p. 2, 2006; Hoger et al., 2002; Olesen et al., 1988). Impairments of endothelial Kir channels resulted in endothelial dysfunction and vascular pathology in vitro and in vivo (Ahn et al., 2017; Boriushkin et al., 2019; Fancher et al., 2020; Mohler et al., 2007).
Shear stress was shown to induce [Ca2+]i, which was mainly from the uptake of extracellular Ca2+(Mendoza et al., 2010, pp. 4-; Yamamoto et al., 2000), release from the endoplasmic reticulum (Jafarnejad et al., 2015; Melchior & Frangos, 2012), and release from mitochondria (Scheitlin et al., 2016), the second largest Ca2+ storage organelles in the cell. Besides their direct role in calcium release and uptake, mitochondria may also control endothelial calcium level through releasing ATP to induce the extracellular Ca2+ entry (Yamamoto et al., 2000) or ER-stored Ca2+ flux (C. Wilson et al., 2019).
The presence of ATP has been shown to be required for sustained induction of intracellular calcium in cultured BAECs in response to shear stress (James et al., 1995). When exposed to shear stress, cultured human pulmonary artery endothelial cells (HPAECs) induced mitochondria ATP production, which triggered caveolae ATP release, thus activating P2X4 and P2Y2 receptors-mediated extracellular calcium uptake (Yamamoto et al., 2000, 2018). This purinergic receptors-regulated calcium influx was shown to be required for shear stress-mediated eNOS activation, PECAM-1 and VEGFR-2 phosphorylation. Mice with endothelial-specific P2Y2 deficiency showed impaired flow-induced vasodilation and hypertension (S. Wang et al., 2015).
Endothelial mitochondria can mediate [Ca2+]i level through their own Ca2+ flux. In cultured HUVECs, mitochondria are important for shear stress-induced [Ca2+]i transients, and are essential to induced [Ca2+]i oscillation (Scheitlin et al., 2016). One proposed mechanism of mitochondria-controlled [Ca2+]i is due to the Ca2+ uptake/release by mitochondria would alter local Ca2+ level to either activate or deactivate the IP3 receptor-mediated ER Ca2+ release, leading to subsequent Ca2+ oscillation (Scheitlin et al., 2016). This regulation was further shown to be dependent on the mitochondria calcium uptake/release but not on ATP production, since knocking down of mitochondria Ca2+ uniporter or inhibiting electron transport chain using Antimycin A, but not with ATP synthase inhibitor, was able to prevent the shear stress-induced intracellular calcium transients/oscillation (Scheitlin et al., 2016). However, how this ATP-independent while mitochondria Ca2+-mediated endothelial [Ca2+]i homeostasis contributes to vascular dysfunction has not been studied, and it would be informative to further investigate the cell signaling role of mitochondria in regulating disturbed flow-induced atherosclerosis.
Fatty acid uptake and oxidation
Vascular endothelial cells are exposed to plasma free fatty acids, the metabolism of which is regulated by shear stress, and ultimately occurs in the mitochondria. Fatty acids play an important role regulating endothelial functions such as inflammation, NO production and insulin signaling, contributing to pathologies of CVDs including atherosclerosis (A. Ghosh et al., 2017; X. L. Wang et al., 2006). Endothelial cells rely on passive diffusion or fatty acid translocase to import fatty acid from plasma into the cells for fatty acid oxidation (FAO) (Harjes et al., 2016). Multiple unbiased -omics studies suggest shear stress regulates lipid metabolism in endothelial cells. Using proteomics approach in HUVECs, it was discovered that high shear stress induced proteins participating in lipid metabolism including lipid transport, oxidation, catabolism and biosynthesis (Venturini et al., 2019). Specifically, atheroprone low shear stress reduced the membrane fraction of LDLR in cultured HUVECs compared to high shear stress (Venturini et al., 2019). This reduced membrane localization of LDLR is due to its hypo-glycosylation modification (immaturity) induced by low shear stress, thus causing LDLR to accumulate around the nuclei (Venturini et al., 2019). In support of this, metabolomics data showed that HUVECs exposed to low shear stress compared to high shear stress had downregulation of lipids and lipid metabolites (Venturini et al., 2019). In parallel, untargeted lipidomics in cultured HPAECs demonstrated the alteration of global lipid profile induced by shear stress (Hirata et al., 2021). Specifically, compared to static conditions, high shear stress upregulated ether-containing lipids, which is responsible for attenuating the phorbol 12-myristate 13-acetate (PMA)-induced VCAM-1 expression (Hirata et al., 2021).
Furthermore, several flow-sensitive genes/pathways regulate fatty acid metabolism in endothelial cells. For instance, NOTCH1 signaling is activated by unidirectional flow (Mack et al., 2017), and NOTCH1 signaling has been shown to promote FAO through transcriptional activation of carnitine palmitoyltransferase 1A (CPT1A) (Kalucka et al., 2018). On the contrary, DF has been shown to induce endothelial fatty acid synthesis through sustained activation of sterol regulatory element binding transcription factor 1 (SREBP1), which enhances the transcription of HMG-CoA synthase and fatty acid synthase genes (Y. Liu et al., 2002). In concert, SREBP1 can also be increased by YAP/TAZ signaling to facilitate lipid accumulation (Aylon et al., 2016, p. 2). Expression of CD36, a scavenger receptor and fatty acid transporter, was markedly up-regulated in endothelium subjected to disturbed flow (Le Master et al., 2018), although the role of CD36 in flow-regulated fatty acid uptake remains to be determined.
In summary, these studies indicated that UF induced FAO while DF preferentially promoted lipid synthesis and accumulation in endothelial cells, suggesting a relevant role of lipid metabolism in mechanosensing and possibly mechanotransduction. Together, these data indicate that lipid metabolism is dynamically regulated in endothelial cells by different types of hemodynamic flows, which may contribute to the flow-regulated endothelial phenotypes.
FAO is dynamically modulated in endothelial cells to maintain their functions. Endothelial FAO is essential for supplementing dNTP synthesis for maintaining endothelial DNA replication and angiogenesis (Schoors et al., 2015). In addition, FAO has also been shown to benefit redox homeostasis through NADPH regeneration, thus protecting quiescent endothelial cells from oxidative-stress exposure (Kalucka et al., 2018). Inhibiting CPT1A has been shown to induce endothelial cell permeability in vitro and blood vessel leakage in vivo (Patella et al., 2015).
Another unconventional role of FAO is to regulate endothelial to mesenchymal transition (EndMT). EndMT has been considered as an atherogenic phenotype of endothelial cells and has been shown to drive atherogenesis (P.-Y. Chen et al., 2015; Evrard et al., 2016). It is widely accepted that disturbed flow induces EndMT (Andueza et al., 2020; P.-Y. Chen et al., 2015; Moonen et al., 2015), but the specific mechanism has not been well elucidated. FAO can restrain EndMT by maintaining the acetyl-CoA pool for the post-translational inhibition of the mesenchymal marker SMAD7 (Xiong et al., 2018). Inhibiting FAO in mice by endothelium-specific CPT2 deletion promoted EndMT through thickening of heart valves and increasing permeability in multiple vascular beds, suggesting the critical role of FAO in maintaining EC identity and vascular homeostasis (Xiong et al., 2018). Therefore, it is reasonable to hypothesize that FAO also acts as a metabolic link to regulate the DF-induced EndMT.
Fatty acid synthesis
In contrast to FAO, fatty acid synthesis is upregulated by DF (Y. Liu et al., 2002); however, it has not been elucidated how this DF-induced FA synthesis contributes to EC function and the atherogenic phenotype. Nevertheless, studies in other cell types have provided possible hypotheses linking FA synthesis to EC function. In cardiomyocytes, fatty acids prevented HIF-1α stabilization by decreasing succinate concentration thus enhancing HIF-1α hydroxylases (Dodd et al., 2018), suggesting that fatty acids synthesis may be beneficial to endothelium exposed to disturbed flow. On the other hand, in cancer cells, blocking monounsaturated fatty acids synthesis by inhibiting stearoyl-CoA-desaturase 1 (SCD1) reduced YAP/TAZ stabilization and nuclear localization (Noto et al., 2017), indicating fatty acid synthesis may also contribute to the hyperglycolytic phenotype of endothelium exposed to disturbed flow.
Amino acids
Glutamine is the most abundant amino acid in human plasma (Newsholme et al., 2003; Williamson & Brosnan, 1974). Glutamine serves as a major carbon source for TCA cycle in endothelial cells which contributes to EC proliferation both in vitro and in vivo (H. Huang et al., 2017; B. Kim et al., 2017). One mechanism on the glutamine-dependent EC proliferation is due to it serves as the precursor to synthesize asparagine, which is needed for protein synthesis in support of angiogenesis (H. Huang et al., 2017; Pavlova et al., 2018). Besides protein synthesis, glutamine is also important for maintenance of EC redox homeostasis by producing the antioxidant glutathione (DeBerardinis & Cheng, 2010). In addition, glutamate generated by glutamine is subsequently converted to ornithine for the synthesis of polyamine and nitric oxide, which is a critical regulator of vasodilation and angiogenesis (Kucharzewska et al., 2010; Tousoulis et al., 2012). Although glutamine is an important amino acid in ECs, its regulation by shear stress has not been thoroughly investigated. Interestingly, stiffness-activated YAP/TAZ has been shown to stimulate the transcription of glutaminase (GLS1), the first enzyme that catabolizes glutamine to glutamate and ammonia (Bertero et al., 2016). Whether a similar mechanism exists by disturbed flow-activated YAP/TAZ is unclear.
L-arginine can be converted by eNOS to synthesize NO and citrulline in vascular endothelial cells (Palmer et al., 1988). eNOS can be both transcriptionally activated (Davis et al., 2001, 2004; Tao et al., 2006), stabilized (Davis et al., 2001) and post-translationally phosphorylated by shear stress at various serine residues (Boo, Hwang, et al., 2002; Boo, Sorescu, et al., 2002), leading to increased NO production. Argininosuccinate synthetase 1 (ASS1) catalyzes the penultimate step of L-arginine synthesis, and ASS1 is transcriptionally upregulated in HUVECs subjected to high shear stress, compared to static conditions (McCormick et al., 2001; Mun et al., 2009). RNA interference of ASS1 impaired NO production in bovine aortic endothelial cells (Goodwin et al., 2004), suggesting that ASS1 takes on an essential mechano-sensitive role in key endothelial functions.
In summary, unidirectional flow is associated with an oxidative phosphorylation/ mitochondrial phenotype – promoting mitochondrial fusion, biogenesis, fatty acid uptake, oxidation, and glutamine uptake for anaplerosis and nitric oxide production. In contrast, disturbed flow is associated with a glycolytic phenotype, mitochondrial fission, and fatty acid synthesis. The metabolic switch in disturbed flow promotes atherosclerosis,
Microvasculature flow and metabolism
Capillary beds
In animal models of sepsis, microvascular flow becomes disturbed or oscillatory (De Backer et al., 2002). Microvascular flow is important in brain vascular and ear development (Q. Chen et al., 2012; D. Wu et al., 2011). Microvasculature-mitochondrial dysfunction is a well-known consequence of sepsis, resulting in dysregulated NO production, glycocalyx shedding, and barrier dysfunction (Miranda et al., 2016). Clinical therapies are focused on restoring appropriate blood flow to the capillary beds, but whether flow has a direct effect on microvascular metabolism remains poorly understood.
Brain and kidney endothelial cells
In addition to capillaries and lymphatics, different organs have distinct microvascular beds with their own metabolic specialization (Kalucka et al., 2020). Liver ECs have discontinuous basement membranes for passage of macromolecules whereas brain ECs have a tight blood brain barrier. Kidney ECs are specialized for blood filtration. Unsurprisingly, EC shear stress responsiveness in the various tissue beds has a different shear stress set point (Baeyens et al., 2015). In the brain microvasculature, physiologic shear (10–20 dyn/cm2) upregulates expression of tight junction markers such as ZO1 and Claudin-5. However, excessive shear (40 dyn/cm2) and/or pulsatility decreased their expression to basal levels and altered EC junctions morphology (Colgan et al., 2007; Garcia-Polite et al., 2017). This suggests a mechanism whereby atherosclerosis (increased stiff vessels) and hypertension predispose the microvasculature to endothelial dysfunction. In the brain, this would manifest as strokes. From a metabolic standpoint, measurements of glucose consumption versus lactate production showed that shear stress negatively modulated the glycolytic bioenergetic pathways of glucose metabolism in favor of the more efficient aerobic respiration and increased synthesis of TCA cycle genes (Cucullo et al., 2011). Thus, shear stress’ effect on brain endothelial cells metabolism seems similar to HAECs (Doddaballapur et al., 2015; D. Wu et al., 2017).
Renal endothelial cells are very metabolically heterogeneous depending on their proximity to arterial oxygen and water deprivation (Dumas et al., 2020). The metabolism of renal endothelial cells is likely dominated by limited delivery of oxygen. Much like other endothelial cell types, nitric oxide production and permeability of glomerular endothelial cells are regulated by laminar shear stress (Bevan et al., 2011). In contrast to lymphatic ECs (discussed below), renal endothelial cells may be subjected to true hypoxia with pO2 < 20 mm Hg (Neuhofer & Beck, 2005). Mixed venous pO2 never reaches below 75 mm Hg in normal situations; thus, venous blood is rarely truly hypoxic. Interestingly, whereas HAECs are to some degree HIF-1α dependent for increased glycolysis in response to hypoxia in large vessels (D. Wu et al., 2017), renal ECs (and perhaps other microvasculature) also are in part dependent on SIRT3-dependent HIF-2α, in addition to HIF-1α, for glycolysis (He et al., 2017; Nauta et al., 2017).
Pulmonary vasculature
Microvascular flow dysfunction is probably prevalent in the lung. Lung diseases such as pulmonary fibrosis, acute lung injury in addition to pulmonary hypertension all have microvascular dysfunction involving pressure changes in the pulmonary vasculature due to either chronic hypoxic vasoconstriction, chronic thromboembolism, vascular obliteration due to plexiform lesions, vascular apoptosis, or heart failure causing pressure back up.
In human pulmonary microvasculature, metabolism through the HIF-2α pathway plays a role in the development of pulmonary hypertension and resolution of acute lung injury (Cowburn et al., 2016; M. C. Ghosh et al., 2021; Gong et al., 2015; C.-J. Hu et al., 2019; H. Tang et al., 2017); however, the role of HIF-2α in pulmonary microvascular mechanotransduction is unknown (in HAECs, disturbed flow also induces HIF-2α, although the consequence of this has not been studied (D. Wu et al., 2017)). Activation of HIF-2α also leads to upregulation of arginase II and ultimately lowers arginine availability for NO production, which probably occurs in the kidney microvasculature (Krotova et al., 2010).
Impairment of fatty acid synthase leads to HIF-1α de-stabilization, a reduction in HIF-1α-mediated changes in glucose transport and metabolism, and eNOS function restoration, suggesting that the inhibition of fatty acid synthesis may be beneficial for EC function in hypoxia (Singh et al., 2017). Other impacts of hypoxia, such as activation of HIF-1α, dysregulated nitric oxide and pulmonary artery metabolism, and endothelial-mesenchymal transition can be found in this review (D. Wu & Birukov, 2019).
Other endothelial beds
Retinal endothelial cells also require some degree of shear stress for quiescence involving the nitric oxide pathway (Ishibazawa et al., 2011; Lakshminarayanan et al., 2000), with lower shear stress upregulating proinflammatory pathways (Ishibazawa et al., 2013). As retinal angiogenesis is a critical factor in diabetic retinopathy (and serves as a model for developmental angiogenesis), the role of metabolism and flow-limited delivery of oxygen in retinal endothelial cell biology is ongoing (X. Han et al., 2019); like in other endothelial beds, disturbed flow likely simulates pathways mimicking hypoxia. In liver, sinusoidal endothelial cells are known to be mechanically responsive to shear stress (Braet et al., 2004), and express many classical mechano-sensitive receptors and transcription factors, but whether there is a connection to metabolism is unknown (Soydemir et al., 2020).
Lymphatics
LECs have similar metabolism to arterial-derived endothelial cells and are highly glycolytic with low mitochondrial oxidative phosphorylation in culture (De Bock et al., 2013). In some respects, this is less surprising than arterial endothelial cells, as lymphatic vasculature has about 15–60 mm Hg pO2 compared with 80–110 mm Hg pO2 in arterial circulation(Barankay et al., 1976; Witte et al., 1967). Furthermore, lymphatic fluid has glucose concentrations that are similar or slightly higher than arterial blood (Hendrix & Sweet, 1917). However, pO2 limitations for mitochondria function are thought to be < 1 mm Hg and lactate production from end organs (as a sign of oxygen-limited ATP production) is thought to occur only below pO2 values of ~15 mm Hg (Koike et al., 1994; Richmond et al., 1997; Wasserman, 1999). Therefore, it is likely that in all cases oxygen supply to the lymphatic vasculature is enough to power oxidative phosphorylation, suggesting that the Warburg effect is indeed present in lymphatic ECs, as in arterial and venous.
LECs are mechano-sensitive to hemodynamics. Low, oscillatory shear stress is sufficient to induce GATA2-FOXC2, which is a key transcriptional pathway for lymphatic vessel maturation (Sweet et al., 2015) and valve formation (Sabine et al., 2012, 2015). PROX1, another transcription factor marker for lymphatic endothelial cells, can be abolished with high amounts of shear stress (C.-Y. Chen et al., 2012). Loss of GATA2 results in lymphedema (Emberger syndrome) (Kazenwadel et al., 2015, p. 2). In LECs, the mechanosensation mechanism is thought to be through PECAM1 sensation of shear stress, phosphorylation of VEGFR2/3 which activated PI3K/AKT signaling and are held together by VE-Cadherin in the plasma membrane. Deletion of any of these components leads to lymphatic valve loss and is dependent on mechanosensation (Hägerling et al., 2018; Tzima et al., 2005; Y. Wang et al., 2016; Y. Yang et al., 2019).
During development, LEC glycolysis is critical for vasculogenesis. HK2 is essential for glycolysis. Knockout of HK2 in an endothelial cell-specific manner leads to impaired EC proliferation and migration. FGF regulates c-MYC which in cooperates with HIF-1α and regulates HK2 (J. Kim et al., 2007; Mathupala et al., 2001; P. Yu et al., 2017).
Lymphangiogenesis is also dependent on fatty acid oxidation. FAO flux in LEC is higher than in other endothelial cell types (Wong et al., 2017). Besides for energy supply, FAO are used for nucleotide synthesis in ECs (Schoors et al., 2015). Interestingly, PROX1 regulates lymphatic cell identity by causing epigenetic changes through histone acetylation via upregulation of CPT1A expression, which increases acetyl coenzyme A production, which is dependent on fatty acid oxidation (Wong et al., 2017).
Study addressing the intersection of metabolism and mechanotransduction in LECs is in its infancy. LECs from lambs exposed to increased pulmonary lymph flow are hyperproliferative, have increased expression of HIF-1α and its target genes, and demonstrate altered central carbon metabolism in vitro (Boehme et al., 2021).
Stretch and metabolism
Stretch is an especially important mechanical factor in lung microvascular endothelial cells. Physiologic levels of cyclic stretch are essential for endothelial homeostasis (Lehoux & Tedgui, 1998); excessive stretch leads to apoptosis and expression of inflammatory factors (Fang et al., 2019). This is an especially important topic in the pandemic era as excessive lung distention is still thought to be responsible for excess mortality during mechanical ventilation (Li et al., 2011), despite current lung protective strategies (Amato et al., 2015; The Acute Respiratory Distress Syndrome Network, 2000). From a metabolic standpoint, mitochondria are especially affected by excess stretch. Mitochondria anchor to the cytoskeleton and release ROS in response to cytoskeletal strain (Ali et al., 2006). This suggests that in endothelial cells, mitochondria and cytoskeleton together serve as a united mechanosensor and mechanotransducer (Ali et al., 2004). In a novel cellular glucose sensor experiment, it was found that glucose utilization is reduced under stretched state in endothelial cells (Peng et al., 2021). New devices that can simultaneously visualize externally applied stretch (Poulin et al., 2018) and detect single-cell metabolism (D. Wu et al., 2021) can better clarify the relationship between stretch and metabolism.
Mechano-sensitive transcription factors YAP may be active in stretch in addition to stiffness (see below). Interestingly, YAP may act to prevent stretch induced cell injury of ECs. Surprisingly, YAP knockout exaggerated vascular endothelial (VE)-cadherin phosphorylation, downregulation of vascular endothelial protein tyrosine phosphatase (VE-PTP), and dissociation of VE-cadherin and catenins following mechanical ventilation, causing endothelial barrier failure (Su et al., 2021). Although stretch is a constant feature of endothelial cells in the vasculature, its effects on cell metabolism remain under-investigated.
Stiffness and endothelial cell metabolism
As the cytoskeleton is connected to focal adhesions, it is not surprising that they play a large role in sensing stiffness. The cytoskeleton transmits the stiffness signal through YAP and TAZ. Endothelial cells also demonstrate a strong cell shape dependence in their phenotype that is driven by surface stiffness and extracellular matrix contact (C. S. Chen, 1997). YAP/TAZ activates downstream pathways that are known to increase fibrotic pathways resulting in the synthesis of extracellular matrix (Totaro et al., 2018). The precise mechanosensing mechanism that transmits the signal from cell sensing of substrate stiffness to YAP and TAZ is poorly understood.
The cytoskeleton plays a critical role in force transmission as the nuclear translocation of YAP/TAZ was mitigated by inhibitors of non-muscle-myosin II in a study on matrix stiffness and its effects on the transcriptional output of epithelial cells (Dupont et al., 2011). Interestingly, osmotic forces affect intracellular crowding and stiffness, which in turn affects cell differentiation (Guo et al., 2017), and can cause both nuclear translocation (Hong et al., 2017) and phase separation of YAP, which when condensed are active sites of gene transcription in HEK293 cells (Cai et al., 2019). It remains to be seen if these affects play out in endothelial cells.
The cytoskeleton engages in mechanical-metabolic crosstalk through YAP/TAZ. Pulmonary vascular stiffness causes YAP to bind to GLS1 promoter sequence in response to matrix stiffening, and hence conversion of glutamine to glutamate in pulmonary artery endothelial cells. As glutamate enters the TCA cycle as alpha-ketoglutarate, YAP/TAZ therefore stimulates synthesis of biosynthetic growth and proliferation intermediates (Bertero et al., 2016). YAP also increases transcription of LDHA to promote glycolysis and cell migration (Bertero et al., 2016). YAP/TAZ accelerates lipid accumulation via activation or dysregulation of SREBP2 (Aylon et al., 2016; Jeong et al., 2018).These growth signals are thought to drive the plexiform lesions and thickening of the vasculature leading to the clinical phenotype found in pulmonary hypertension. Furthermore, YAP/TAZ coordinates EC proliferation and metabolic activity by upregulating MYC signaling (J. Kim et al., 2017).
In vascular development, YAP/TAZ promotes cell migration and barrier function by linking mechanical signals with bone morphogenetic protein (BMP) signaling to establish functional network formation of blood vessels during angiogenesis (Neto et al., 2018). BMP family regulates VEGFR2 and NOTCH signaling via TAZ-Hippo pathway (Pulkkinen et al., 2021). Furthermore, YAP/TAZ constrain HIF-1α target gene expression in vivo and in vitro. Surprisingly, YAP/TAZ suppresses bone angiogenesis in hypoxia (Sivaraj et al., 2020). Upstream kinase signaling components of YAP/TAZ (such as Wwc2, which activates LATS) are also critical for vascular development (Hermann et al., 2021, p. 2). YAP and TAZ are also important for lymphatic plexus patterning and postnatal lymphatic valve maintenance by negatively regulating PROX1 (Cho et al., 2019).
YAP/TAZ and stiffness are instrumental to endothelial-adjacent cells in the microvasculature. YAP is induced after injury and promotes wound healing (proliferation, migration) which provides evidence for tension sensing at wound fronts (Kimura et al., 2016; X. Wang et al., 2012). Stiffness-activated YAP/TAZ has also been found to regulate metabolism in fibroblasts (cancer-associated) by increasing glutamine metabolism in response (Bertero et al., 2019). Matrix sensing/remodeling is responsible for smooth muscle growth in pulmonary hypertension models (Bertero et al., 2015; Dieffenbach et al., 2017; Kudryashova et al., 2016) and in vitro models of idiopathic pulmonary fibrosis (F. Liu et al., 2015). Interestingly, YAP inhibition causes resolution of scars and prevent the formation of keloids, an excessive scarring process (Mascharak et al., 2021).
Cytoskeletal regulation of metabolism
In addition to external mechanical forces, metabolism is regulated cell-autonomously by internal mechanical cues largely in response to cytoskeletal remodeling. ATP and GTP hydrolysis drive actin and tubulin filamentation; ATP hydrolysis drives myosin-dependent formation of actin stress fibers in many cell types. Thus, it is not surprising that changes in cell internal mechanics through cytoskeletal reorganization can autonomously regulate metabolism and energy production.
The cytoskeleton can regulate energetic demand by sequestering enzymes that can regulate glycolysis. Tripartite motif containing-21 (TRIM21) is sequestered by F-actin, which hides a protease that degrades phosphofructokinase (PFK), making it more active (Park et al., 2020). Glycolytic enzymes aldolase A has a catalytic site right next to its actin binding site (J. Wang et al., 1996). Filamentation of actin is therefore thought to regulate aldolase A activity (J. Wang et al., 1997). Insulin-dependent activation of PI3K/PIP3 recruits and activates Rac which promotes actin fiber monomerization, causing release of aldolase A which increases glycolysis (H. Hu et al., 2016).
Cells exhibit structural and functional compartmentalization of ATP production
The cell has segregated pools of energy production and consumption and is far from equilibrium. The nucleus has its own pool of glycolytic enzymes (Y. Liu et al., 2018; Vega et al., 2016; H.-J. Wang et al., 2014, p. 5; Yalcin et al., 2009) (most of uncertain function (Boukouris et al., 2016)) and can perform glycolysis on its own (Rechsteiner & Catanzarite, 1974; Siebert & Humphrey, 2006). In dividing hepatocytes, glycolysis is more active in the nucleus than in the cytosol (Kuehl, 1967). Data suggest that plasma membrane Na/K ATPase is tightly coupled with cytoplasmic glycolysis and receives almost no ATP from mitochondria (Sepp et al., 2014). Structural barriers prevent free diffusion of ATP in muscle cells (Vendelin et al., 2004). Muscle cells exhibit regular patterns of glycolytic enzymes that align spatially and functionally with sarcomeres (Sullivan et al., 2003; Wojtas et al., 1997). Spermatozoa have glycolytic enzymes regularly organized along flagella and exhibit cytoplasmic droplets enriched with glycolytic enzymes which and are critical for sperm maturation (Yuan et al., 2013). Studies in red blood cells, which lack mitochondria, suggest that glycolytic proteins compartmentalize near the plasma membrane and that their subcellular organization is important for the regulation of cellular cation pumps (Chu et al., 2012; Mercer & Dunham, 1981).
Stress affects the spatial distribution of glycolytic enzymes. Hexokinase (HK) exists in both cytoplasmic and mitochondrial membrane bound phases(Roberts & Miyamoto, 2015; J. E. Wilson, 1978). HK transits to its membrane bound form during periods of ischemia (Knull et al., 1973, 1974). In this way, cells use spatial distribution to control enzyme function: diversion of critical glycolytic intermediates may be used to prevent intracellular competition for ATP (Ottaway & Mowbray, 1977) or to divert energy between anabolic or catabolic processes (John et al., 2011).
Several glycolytic enzymes were among the first actin-binding proteins identified (Masters, 1984), which suggests an intimate relationship between cytoskeletal organization and energy production (Pagliaro, 1995). Glycolytic enzyme aldolase has separate sites for actin binding and isomerase activity; reorganization of cytoskeleton is thought to activate glycolysis by freeing aldolase from its bound state (H. Hu et al., 2016; Lew & Tolan, 2013). Aldolase and other glycolytic enzymes glyceraldehyde 3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), and lactate dehydrogenase (LDH) also bind to tubulin; binding of these enzymes to tubulin cytoskeleton changes their activity in vitro (Kovács et al., 2003; Marmillot et al., 1994; Volker et al., 1995), suggesting active cytoskeletal regulation of glycolysis.
Teleologically, these observations fit a globally parsimonious view of cellular energy production/consumption as (some) cells do not have excess pools of freely diffusing ATP and in fact have developed sophisticated mechanisms to sense energy stress, demand, and anabolic requirements (Hardie et al., 2012; Herzig & Shaw, 2018; J. Kim & Guan, 2019; Saxton & Sabatini, 2017). Whether these observations can be applied to ECs is unknown.
Subcellular metabolic heterogeneity in migrating ECs
More recently, “glycolytic metabolons” have been suggested in ECs. An enrichment of glycolytic enzymes such as PFKFB3 in lamellipodial ruffles may drive increased ATP in the lamellapodia, which may in turn drive migration (De Bock et al., 2013; Eelen et al., 2018). In thrombin-stimulated endothelial cells, glycolysis is more active near the contracting lamella and co-localizes with actin turnover, which again suggests cell autonomous subcellular organization of energy production (D. Wu et al., 2021). Targeting local ATP supply with ATP/ADP exchange enzyme adenylate kinase-1 enhances cell migration in embryonic fibroblasts (van Horssen et al., 2009).
The cytoskeleton regulates glycolysis in response to increased energy demand and cell migration
Obeying basic laws of physics, there is a strong correlation between cellular work (distance migrated) and cellular energy production (Kondo et al., 2021; D. Wu et al., 2021). In breast cancer cells, cellular ATP/ADP ratio correlates with leader-cell invasion (J. Zhang et al., 2019). Furthermore, in cell migration, there is acute energy demand that should be heterogeneous within a cell, as some parts of a cell active extend while other part are stationary. It follows that there should be a way to upregulate energy intake at the subcellular level. Using single cell metabolism assays, it was recently discovered that RhoA in response to thrombin stimulus stimulates SLC2A3 to uptake more glucose in HAECs. The resultant glycolysis is spatially heterogeneous within a cell and only occurs in areas of the cell which are actively contracting (D. Wu et al., 2021). Interestingly, intracellular pH, which is largely determined by the subcellular glycolytic rate, is regulated by integrin-mediated cell spreading, and thus cellular tension may also regulate metabolism autonomously (Schwartz et al., 1989, 1990, 1991). SLC2A3, which has a lower Km for glucose than SLC2A1, is thus a promising candidate for rapid glucose uptake (Burant & Bell, 1992); whether SLC2A3 is spatially regulated within ECs is unknown.
Metabolic memory is encoded in epigenetic modifications and the cytoskeleton
Metabolic changes are critical for changing cell phenotype. This occurs functionally in the production of metabolites but also provides a basis for cellular differentiation or creation of memory in the form of epigenetic modifications. Fatty acid-derived acetyl-CoA was found to be critical for maintaining lymphatic differentiation through acetylation of histones (Wong et al., 2017). Acetylation requires the key intermediate metabolite acetyl-CoA, the sole donor of acetyl groups for acetylation (Choudhary et al., 2014). In addition to epigenetic modifications, the cytoskeleton is also acetylated: α-tubulin promotes microtubule stability (Szyk et al., 2014). Thus it can be said that the cytoskeleton also possesses some metabolic memory.
Acetylated tubulin improves EC barrier function. Histone deacetylase 6 (HDAC6) phosphorylation, which deacetylates microtubules (MTs) and reduces barrier function, exacerbates EC barrier dysfunction in a cigarette smoke/LPS animal and Staphylococcus aureus model and of lung injury (Borgas et al., 2016; Karki et al., 2019; Kratzer et al., 2012), whereas hyper-acetylated tubulin is protective against LPS-induced ALI (Y. Zhang et al., 2008). Selective HDAC6 inhibition by tubastatin A also reduced TNFα-induced lung endothelial cell hyperpermeability (J. Yu et al., 2016).
In acute lung injury models, multiple studies invoke a final common pathway of RhoA-induced activation of myosin light chain, causing EC contractions and pulmonary vascular leak. Microtubules modulate RhoA activity in an LPS-dependent manner via oxidative-stress induced release of GEF-H1(Kratzer et al., 2012). GEF-H1 bound to MTs is dependent on microtubule acetylation. Mechanically, stiffness can activate GEF-H1 expression and thereby exacerbate LPS-induced lung inflammation (Mambetsariev et al., 2014).
In addition to microtubules, acetylation is important for stable adherens junctions. β-catenin HDAC6-dependent deacetylation causes β-catenin nuclear translocation and disassembly of adherens junctions (J. Yu et al., 2016, p. 6); on the contrary, β-catenin acetylation promotes its membrane localization thus stabilizing adherens junctions (Iaconelli et al., 2015), although these studies are not in endothelial cells. Thus, acetyl-CoA, a key mitochondrial-derived metabolite, may be critical for vascular permeability in lung injury. As mitochondria activity of ECs is enhanced by unidirectional flow and suppressed by disturbed flow (D. Wu et al., 2017), it is now possible to speculate that shear stress-induced mitochondria function affects cytoskeleton and junctional stability and hence is protective against lung injury.
Discussion
Mechanical forces drive changes in endothelial cell phenotypes which can lead to disease states. Mechano-transduction mechanisms result in changes to endothelial phenotypes such as production of matrix, expression of inflammatory markers and TNFα signaling, changing barrier properties, and endothelial-mesenchymal transformation. Continued endothelial transformation at a large enough scale in tissue or organ beds leads eventually to pathological disease processes such as atherosclerosis, pulmonary hypertension, and capillary leak syndromes.
These changes require rewiring cellular biomass and energetics to support new cellular functions instead of maintaining cellular quiescence. All major metabolic pathways become altered including glucose, amino acids, fatty acid metabolism. Glucose is diverted from synthesis of glycocalyx into fueling cell migration. Amino acids and fatty acids are diverted for synthesis of nucleic acids in preparation for cell division.
Metabolic byproducts have a profound impact on cellular identity and act to preserve the state of the cell in accordance with the nutrient microenvironment. While transcription factors are central actors in promoting differential transcriptomic identities, metabolic products are central to preserving the epigenetic change of cells including methylation, acetylation, and lactylation. These changes occur via one-carbon metabolism, the production of acetyl-CoA from pyruvate, and through lactate dehydrogenase from pyruvate to lactate, respectively. Production of these metabolites thus changes the chromatin state and fundamental identity of the cell.
Not only are cellular level epi- and transcriptomic memories created from metabolic products, but also the structural fabric of endothelial cells. Microtubules and cell-cell junctions are acetylated, which stabilizes the cell integrity. This is especially important since the cytoskeleton is an integral and necessary component of mechanical signaling. Mechanical forces are often transient, whereas nutrient supply changes are much slower; buffering against sudden mechanical changes prevents rapid signaling changes which may be counter-productive. Acetylation of the cytoskeleton, as well as changes to chromatin, require significant integration of time-dependent mechanical forces prior to cellular decision making and cellular memory formation.
However, there are certain instances where quick sensation of nutrients is important, namely, such as during ischemia and reperfusion. While not discussed in depth in this current review, hypoxia can act digitally in an on-off manner through the action of HIF-1α. Degradation of HIF transcription factors through prolyl hydroxylases requires alpha-ketoglutarate and thus TCA metabolism plays a central role in regulation of the hypoxia response. Longer term, the reduction in acetyl-CoA via shutting down pyruvate dehydrogenase acts to reverse cytoskeletal and chromatin memory. Nevertheless, since O2 plays such an important role in ATP generation and ROS signaling in ECs, its role as a digital switch makes sense in that regard.
The interaction between mechanical forces and nutrient flux must be further clarified both in vitro and in vivo. One often unrecognized difficulty with mechanotransduction and metabolism experiments is that due to fundamental thermodynamic principles, mechanical forces and nutrient delivery are co-dependent variables. Much like the thermoelectric effect where current necessarily produces changes in temperature through Onsager reciprocal relations, changes in mechanical flux, be it shear stress, pressure, stiffness, or stretch, will necessarily change the inbound flux of nutrients and outbound flux of waste products. For instance, in disturbed flow there is no net shear stress and therefore no net flux; in this case, the delivery of O2 is only reliant on diffusion and not convective flow. Therefore, the rate of consumption of nutrients must also be considered. Mitochondrial oxygen consumption, for example, may outstrip its diffusion-limited supply in the absence of convectional flux. Thus, mechanical forces, nutrient flux and nutrient gradients are inexorably linked through basic mass transport mechanisms.
Acknowledgments
Funding Sources
This work was supported by AHA Predoctoral Fellowship 834861 (JL), NIH grants R01HL138223 (YF), 534 and R01HL136765 (YF), and R00HL145113 (DW).
References
- Adamson RH, Sarai RK, Altangerel A, Clark JF, Weinbaum S, & Curry FE (2013). Microvascular permeability to water is independent of shear stress, but dependent on flow direction. American Journal of Physiology-Heart and Circulatory Physiology, 304(8), H1077–H1084. 10.1152/ajpheart.00956.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Fancher IS, Bian J-T, Zhang CX, Schwab S, Gaffin R, Phillips SA, & Levitan I. (2017). Inwardly rectifying K+ channels are major contributors to flow-induced vasodilatation in resistance arteries. The Journal of Physiology, 595(7), 2339–2364. 10.1113/JP273255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S, Hartmann P, Karshovska E, Rinderknecht F-A, Subramanian P, Gremse F, Grommes J, Jacobs M, Kiessling F, Weber C, Steffens S, & Schober A. (2015). Endothelial Hypoxia-Inducible Factor-1α Promotes Atherosclerosis and Monocyte Recruitment by Upregulating MicroRNA-19a. Hypertension, 66(6), 1220–1226. 10.1161/HYPERTENSIONAHA.115.05886 [DOI] [PubMed] [Google Scholar]
- Albertine KH (2016). Anatomy of the lungs. In Murray and Nadel’s Textbook of Respiratory Medicine (pp. 3–21.e25). Elsevier Saunders. [Google Scholar]
- Ali MH, Mungai PT, & Schumacker PT (2006). Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: Role of mitochondrial oxidants. American Journal of Physiology-Lung Cellular and Molecular Physiology, 291(1), L38–L45. 10.1152/ajplung.00287.2004 [DOI] [PubMed] [Google Scholar]
- Ali MH, Pearlstein DP, Mathieu CE, & Schumacker PT (2004). Mitochondrial requirement for endothelial responses to cyclic strain: Implications for mechanotransduction. American Journal of Physiology-Lung Cellular and Molecular Physiology, 287(3), L486–L496. 10.1152/ajplung.00389.2003 [DOI] [PubMed] [Google Scholar]
- Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard J-CM, Carvalho CRR, & Brower RG (2015). Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. New England Journal of Medicine, 372(8), 747–755. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- Andueza A, Kumar S, Kim J, Kang D-W, Mumme HL, Perez JI, Villa-Roel N, & Jo H. (2020). Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Reports, 33(11), 108491. 10.1016/j.celrep.2020.108491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniotti S, Fiorio Pla A, Pregnolato S, Mottola A, Lovisolo D, & Munaron L. (2003). Control of endothelial cell proliferation by calcium influx and arachidonic acid metabolism: A pharmacological approach. Journal of Cellular Physiology, 197(3), 370–378. 10.1002/jcp.10359 [DOI] [PubMed] [Google Scholar]
- Atkins GB, & Jain MK (2007). Role of Krüppel-Like Transcription Factors in Endothelial Biology. Circulation Research, 100(12), 1686–1695. 10.1161/01.RES.0000267856.00713.0a [DOI] [PubMed] [Google Scholar]
- Aylon Y, Gershoni A, Rotkopf R, Biton IE, Porat Z, Koh AP, Sun X, Lee Y, Fiel M-I, Hoshida Y, Friedman SL, Johnson RL, & Oren M. (2016). The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes & Development, 30(7), 786–797. 10.1101/gad.274167.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas J-L, Humphrey JD, & Schwartz MA (2015). Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. ELife, 4, e04645. 10.7554/eLife.04645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, & Geiger B. (2001). Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nature Cell Biology, 3(5), 466–472. 10.1038/35074532 [DOI] [PubMed] [Google Scholar]
- Barankay T, Baumgärtl H, Lübbers DW, & Seidl E. (1976). Oxygen pressure in small lymphatics. Pflügers Archiv European Journal of Physiology, 366(1), 53–59. 10.1007/BF02486560 [DOI] [PubMed] [Google Scholar]
- Bazigou E, Lyons OTA, Smith A, Venn GE, Cope C, Brown NA, & Makinen T. (2011). Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. Journal of Clinical Investigation, 121(8), 2984–2992. 10.1172/JCI58050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran A. (2001). Arterial compliance abnormalities in isolated systolic hypertension. American Journal of Hypertension, 14(10), 1007–1011. 10.1016/S0895-7061(01)02160-4 [DOI] [PubMed] [Google Scholar]
- Benetos A, Gautier S, Labat C, Salvi P, Valbusa F, Marino F, Toulza O, Agnoletti D, Zamboni M, Dubail D, Manckoundia P, Rolland Y, Hanon O, Perret-Guillaume C, Lacolley P, Safar ME, & Guillemin F. (2012). Mortality and Cardiovascular Events Are Best Predicted by Low Central/Peripheral Pulse Pressure Amplification But Not by High Blood Pressure Levels in Elderly Nursing Home Subjects. Journal of the American College of Cardiology, 60(16), 1503–1511. 10.1016/j.jacc.2012.04.055 [DOI] [PubMed] [Google Scholar]
- Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, … Chan SY (2015). Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Reports, 13(5), 1016–1032. 10.1016/j.celrep.2015.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang Y-Y, Rehman S, Sugahara M, Qi Z, Gorcsan J, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, … Chan SY (2016). Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. Journal of Clinical Investigation, 126(9), 3313–3335. 10.1172/JCI86387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, Estrach S, Feral CC, Chan SY, Bozec A, & Gaggioli C. (2019). Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metabolism, 29(1), 124–140.e10. 10.1016/j.cmet.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan HS, Slater SC, Clarke H, Cahill PA, Mathieson PW, Welsh GI, & Satchell SC (2011). Acute laminar shear stress reversibly increases human glomerular endothelial cell permeability via activation of endothelial nitric oxide synthase. American Journal of Physiology-Renal Physiology, 301(4), F733–F742. 10.1152/ajprenal.00458.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Tian X, Cokic I, Beckham Y, Gardel ML, & Birukov KG (2013). Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvascular Research, 87, 50–57. 10.1016/j.mvr.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme JT, Morris CJ, Chiacchia SR, Gong W, Wu KY, Kameny RJ, Raff GW, Fineman JR, Maltepe E, & Datar SA (2021). HIF-1α promotes cellular growth in lymphatic endothelial cells exposed to chronically elevated pulmonary lymph flow. Scientific Reports, 11(1), 1468. 10.1038/s41598-020-80882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, & Jo H. (2002). Shear stress stimulates phosphorylation of eNOS at Ser 635 by a protein kinase A-dependent mechanism. American Journal of Physiology-Heart and Circulatory Physiology, 283(5), H1819–H1828. 10.1152/ajpheart.00214.2002 [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, & Jo H. (2002). Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. The Journal of Biological Chemistry, 277(5), 3388–3396. 10.1074/jbc.M108789200 [DOI] [PubMed] [Google Scholar]
- Borgas D, Chambers E, Newton J, Ko J, Rivera S, Rounds S, & Lu Q. (2016). Cigarette Smoke Disrupted Lung Endothelial Barrier Integrity and Increased Susceptibility to Acute Lung Injury via Histone Deacetylase 6. American Journal of Respiratory Cell and Molecular Biology, 54(5), 683–696. 10.1165/rcmb.2015-0149OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriushkin E, Fancher IS, & Levitan I. (2019). Shear-Stress Sensitive Inwardly-Rectifying K+ Channels Regulate Developmental Retinal Angiogenesis by Vessel Regression. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 52(6), 1569–1583. 10.33594/000000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukouris AE, Zervopoulos SD, & Michelakis ED (2016). Metabolic Enzymes Moonlighting in the Nucleus: Metabolic Regulation of Gene Transcription. Trends in Biochemical Sciences, 41(8), 712–730. 10.1016/j.tibs.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, & Spira G. (2004). [No title found]. Comparative Hepatology, 3(1), 7. 10.1186/1476-5926-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretón-Romero R, Acín-Perez R, Rodríguez-Pascual F, Martínez-Molledo M, Brandes RP, Rial E, Enríquez JA, & Lamas S. (2014). Laminar shear stress regulates mitochondrial dynamics, bioenergetics responses and PRX3 activation in endothelial cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1843(11), 2403–2413. 10.1016/j.bbamcr.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Brunström M, & Carlberg B. (2018). Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Internal Medicine, 178(1), 28. 10.1001/jamainternmed.2017.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant CF, & Bell GI (1992). Mammalian facilitative glucose transporters: Evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry, 31(42), 10414–10420. 10.1021/bi00157a032 [DOI] [PubMed] [Google Scholar]
- Byfield FJ, Reen RK, Shentu T-P, Levitan I, & Gooch KJ (2009). Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. Journal of Biomechanics, 42(8), 1114–1119. 10.1016/j.jbiomech.2009.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, & Lippincott-Schwartz J. (2019). Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nature Cell Biology, 21(12), 1578–1589. 10.1038/s41556-019-0433-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, & Hassoun PM (2010). Hemodynamic Predictors of Survival in Scleroderma-related Pulmonary Arterial Hypertension. American Journal of Respiratory and Critical Care Medicine, 182(2), 252–260. 10.1164/rccm.200912-1820OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhang X, Wang L, Yang Q, Ma Q, Xu J, Wang J, Kovacs L, Ayon RJ, Liu Z, Zhang M, Zhou Y, Zeng X, Xu Y, Wang Y, Fulton DJ, Weintraub NL, Lucas R, Dong Z, … Huo Y. (2019). PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension. Proceedings of the National Academy of Sciences of the United States of America, 116(27), 13394–13403. 10.1073/pnas.1821401116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, & Salles G. (2016). Aortic Stiffness as a Surrogate Endpoint to Micro- and Macrovascular Complications in Patients with Type 2 Diabetes. International Journal of Molecular Sciences, 17(12), 2044. 10.3390/ijms17122044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehaitly A, Guilbaud Guihot A, Grimaud L, Aurriere J, Proux C, Roy-Vessieres E, & Henrion D. (2021). Role of mitochondrial dynamics in the response of endothelial cells to shear stress during early phase of atherosclerosis. Archives of Cardiovascular Diseases Supplements, 13(2), 181. 10.1016/j.acvdsp.2021.04.088 [DOI] [Google Scholar]
- Chen CS (1997). Geometric Control of Cell Life and Death. Science, 276(5317), 1425–1428. 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vincente A, Mericko P, Levy RJ, Makinen T, Oliver G, & Kahn ML (2012). Blood flow reprograms lymphatic vessels to blood vessels. Journal of Clinical Investigation, 122(6), 2006–2017. 10.1172/JCI57513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, & Chan DC (2009). Mitochondrial dynamics—Fusion, fission, movement, and mitophagy—In neurodegenerative diseases. Human Molecular Genetics, 18(R2), R169–176. 10.1093/hmg/ddp326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-Y, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, & Simons M. (2015). Endothelial-to-mesenchymal transition drives atherosclerosis progression. Journal of Clinical Investigation, 125(12), 4514–4528. 10.1172/JCI82719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jiang L, Li C, Hu D, Bu J, Cai D, & Du J. (2012). Haemodynamics-Driven Developmental Pruning of Brain Vasculature in Zebrafish. PLoS Biology, 10(8), e1001374. 10.1371/journal.pbio.1001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shen F, Liu J, & Yang G-Y (2017). Arterial stiffness and stroke: De-stiffening strategy, a therapeutic target for stroke. Stroke and Vascular Neurology, 2(2), 65–72. 10.1136/svn-2016-000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-A, Lu C-Y, Cheng T-Y, Pan S-H, Chen H-F, & Chang N-S (2019). WW Domain-Containing Proteins YAP and TAZ in the Hippo Pathway as Key Regulators in Stemness Maintenance, Tissue Homeostasis, and Tumorigenesis. Frontiers in Oncology, 9, 60. 10.3389/fonc.2019.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Peng I-C, Cui X, Li Y-S, Chien S, & Shyy JY-J (2010). Shear stress, SIRT1, and vascular homeostasis. Proceedings of the National Academy of Sciences, 107(22), 10268–10273. 10.1073/pnas.1003833107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J-J, Usami S, & Chien S. (2009). Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Annals of Medicine, 41(1), 19–28. 10.1080/07853890802186921 [DOI] [PubMed] [Google Scholar]
- Cho H, Kim J, Ahn JH, Hong Y-K, Mäkinen T, Lim D-S, & Koh GY (2019). YAP and TAZ Negatively Regulate Prox1 During Developmental and Pathologic Lymphangiogenesis. Circulation Research, 124(2), 225–242. 10.1161/CIRCRESAHA.118.313707 [DOI] [PubMed] [Google Scholar]
- Choudhary C, Weinert BT, Nishida Y, Verdin E, & Mann M. (2014). The growing landscape of lysine acetylation links metabolism and cell signalling. Nature Reviews Molecular Cell Biology, 15(8), 536–550. 10.1038/nrm3841 [DOI] [PubMed] [Google Scholar]
- Chu H, Puchulu-Campanella E, Galan JA, Tao WA, Low PS, & Hoffman JF (2012). Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proceedings of the National Academy of Sciences, 109(31), 12794–12799. 10.1073/pnas.1209014109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, & Cummins PM (2007). Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. American Journal of Physiology-Heart and Circulatory Physiology, 292(6), H3190–H3197. 10.1152/ajpheart.01177.2006 [DOI] [PubMed] [Google Scholar]
- Combs MD, & Yutzey KE (2009). Heart Valve Development: Regulatory Networks in Development and Disease. Circulation Research, 105(5), 408–421. 10.1161/CIRCRESAHA.109.201566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LL, Musani SK, Washington F, Moore J, Tripathi A, Tsao CW, Hamburg NM, Benjamin EJ, Vasan RS, Mitchell GF, & Fox ER (2018). Relations of Microvascular Function, Cardiovascular Disease Risk Factors, and Aortic Stiffness in Blacks: The Jackson Heart Study. Journal of the American Heart Association, 7(20). 10.1161/JAHA.118.009515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowburn AS, Crosby A, Macias D, Branco C, Colaço RDDR, Southwood M, Toshner M, Crotty Alexander LE, Morrell NW, Chilvers ER, & Johnson RS (2016). HIF2α–arginase axis is essential for the development of pulmonary hypertension. Proceedings of the National Academy of Sciences, 113(31), 8801–8806. 10.1073/pnas.1602978113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Tsomides A, Yimlamai D, Hwang KL, Miesfeld J, Galli GG, Fowl BH, Fort M, Ma KY, Sullivan MR, Hosios AM, Snay E, Yuan M, Brown KK, Lien EC, Chhangawala S, Steinhauser ML, Asara JM, Houvras Y, … Goessling W. (2018). Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. The EMBO Journal, 37(22), e100294. 10.15252/embj.2018100294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Hossain M, Puvenna V, Marchi N, & Janigro D. (2011). The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neuroscience, 12(1), 40. 10.1186/1471-2202-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry FE, & Adamson RH (2012). Endothelial Glycocalyx: Permeability Barrier and Mechanosensor. Annals of Biomedical Engineering, 40(4), 828–839. 10.1007/s10439-011-0429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, & Gimbrone MA (2004). Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proceedings of the National Academy of Sciences, 101(41), 14871–14876. 10.1073/pnas.0406073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal PJ, Muller WA, & Sullivan DP (2020). Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. The American Journal of Pathology, 190(3), 535–542. 10.1016/j.ajpath.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF (1995). Flow-mediated endothelial mechanotransduction. Physiological Reviews, 75(3), 519–560. 10.1152/physrev.1995.75.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Civelek M, Fang Y, & Fleming I. (2013). The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovascular Research, 99(2), 315–327. 10.1093/cvr/cvt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Cai H, Drummond GR, & Harrison DG (2001). Shear Stress Regulates Endothelial Nitric Oxide Synthase Expression Through c-Src by Divergent Signaling Pathways. Circulation Research, 89(11), 1073–1080. 10.1161/hh2301.100806 [DOI] [PubMed] [Google Scholar]
- Davis ME, Grumbach IM, Fukai T, Cutchins A, & Harrison DG (2004). Shear Stress Regulates Endothelial Nitric-oxide Synthase Promoter Activity through Nuclear Factor κB Binding. Journal of Biological Chemistry, 279(1), 163–168. 10.1074/jbc.M307528200 [DOI] [PubMed] [Google Scholar]
- De Backer D, Creteur J, Preiser J-C, Dubois M-J, & Vincent J-L (2002). Microvascular Blood Flow Is Altered in Patients with Sepsis. American Journal of Respiratory and Critical Care Medicine, 166(1), 98–104. 10.1164/rccm.200109-016OC [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, & Cheng T. (2010). Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene, 29(3), 313–324. 10.1038/onc.2009.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng L-K, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, … Carmeliet P. (2013). Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell, 154(3), 651–663. 10.1016/j.cell.2013.06.037 [DOI] [PubMed] [Google Scholar]
- Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, & Horrevoets AJG (2002). Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood, 100(5), 1689–1698. 10.1182/blood-2002-01-0046 [DOI] [PubMed] [Google Scholar]
- Dieffenbach PB, Haeger CM, Coronata AMF, Choi KM, Varelas X, Tschumperlin DJ, & Fredenburgh LE (2017). Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. American Journal of Physiology-Lung Cellular and Molecular Physiology, 313(3), L628–L647. 10.1152/ajplung.00173.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, & Zawieja DC (2006). Lymph Flow, Shear Stress, and Lymphocyte Velocity in Rat Mesenteric Prenodal Lymphatics. Microcirculation, 13(7), 597–610. 10.1080/10739680600893909 [DOI] [PubMed] [Google Scholar]
- Dodd MS, Sousa Fialho M. da L., Montes Aparicio CN, Kerr, Timm KN, Griffin JL, Luiken JJFP, Glatz JFC, Tyler DJ, & Heather LC (2018). Fatty Acids Prevent Hypoxia-Inducible Factor-1α Signaling Through Decreased Succinate in Diabetes. JACC. Basic to Translational Science, 3(4), 485–498. 10.1016/j.jacbts.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M, Dimmeler S, & Boon RA (2015). Laminar Shear Stress Inhibits Endothelial Cell Metabolism via KLF2-Mediated Repression of PFKFB3. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(1), 137–145. 10.1161/ATVBAHA.114.304277 [DOI] [PubMed] [Google Scholar]
- Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV, Falkenberg K, Teuwen L-A, de Rooij L, Kalucka J, Chen R, Khan S, Taverna F, Lu W, Parys M, De Legher C, Vinckier S, Karakach TK, Schoonjans L, … Carmeliet P. (2020). Single-Cell RNA Sequencing Reveals Renal Endothelium Heterogeneity and Metabolic Adaptation to Water Deprivation. Journal of the American Society of Nephrology, 31(1), 118–138. 10.1681/ASN.2019080832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, & Piccolo S. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, & Carmeliet P. (2018). Endothelial Cell Metabolism. Physiological Reviews, 98(1), 3–58. 10.1152/physrev.00001.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, & Discher DE (2006). Matrix Elasticity Directs Stem Cell Lineage Specification. Cell, 126(4), 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman K-R, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, … Kovacic JC (2016). Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nature Communications, 7(1), 11853. 10.1038/ncomms11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancher IS, Le Master E, Ahn SJ, Adamos C, Lee JC, Berdyshev E, Dull RO, Phillips SA, & Levitan I. (2020). Impairment of Flow-Sensitive Inwardly Rectifying K+ Channels via Disruption of Glycocalyx Mediates Obesity-Induced Endothelial Dysfunction. Arteriosclerosis, Thrombosis, and Vascular Biology, 40(9), e240–e255. 10.1161/ATVBAHA.120.314935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancher IS, & Levitan I. (2020). Endothelial inwardly-rectifying K+ channels as a key component of shear stress-induced mechanotransduction. Current Topics in Membranes, 85, 59–88. 10.1016/bs.ctm.2020.02.002 [DOI] [PubMed] [Google Scholar]
- Fang Y, Mohler ER, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, & Levitan I. (2006). Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circulation Research, 98(8), 1064–1071. 10.1161/01.RES.0000218776.87842.43 [DOI] [PubMed] [Google Scholar]
- Fang Y, Schram G, Romanenko VG, Shi C, Conti L, Vandenberg CA, Davies PF, Nattel S, & Levitan I. (2005). Functional expression of Kir2.x in human aortic endothelial cells: The dominant role of Kir2.2. American Journal of Physiology. Cell Physiology, 289(5), C1134–1144. 10.1152/ajpcell.00077.2005 [DOI] [PubMed] [Google Scholar]
- Fang Y, Wu D, & Birukov KG (2019). Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Comprehensive Physiology, 9(2), 873–904. 10.1002/cphy.c180020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Bowden N, Fragiadaki M, Souilhol C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, Thompson AAR, Jo H, Weber C, Ridger V, Schober A, & Evans PC (2017). Mechanical Activation of Hypoxia-Inducible Factor 1α Drives Endothelial Dysfunction at Atheroprone Sites. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(11), 2087–2101. 10.1161/ATVBAHA.117.309249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdian E, Suinesiaputra A, Dubowitz DJ, Zhao D, Wang A, Cowan B, & Young AA (2020). 4DFlowNet: Super-Resolution 4D Flow MRI Using Deep Learning and Computational Fluid Dynamics. Frontiers in Physics, 8, 138. 10.3389/fphy.2020.00138 [DOI] [Google Scholar]
- Fiorio Pla A, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, Paria B, Munaron L, & Ambudkar IS (2012). TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene, 31(2), 200–212. 10.1038/onc.2011.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Preston KJ, Cooper HA, Boyer MJ, Escoto KM, Poltronetti AJ, Elliott KJ, Kuroda R, Miyao M, Sesaki H, Akiyama T, Kimura Y, Rizzo V, Scalia R, & Eguchi S. (2020). Mitochondrial Fission Mediates Endothelial Inflammation. Hypertension (Dallas, Tex.: 1979), 76(1), 267–276. 10.1161/HYPERTENSIONAHA.120.14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu BM, & Tarbell JM (2013). Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function: Mechano-sensing and transduction by endothelial surface glycocalyx. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 5(3), 381–390. 10.1002/wsbm.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa KT, Yamashita K, Sakurai N, & Ohno S. (2017). The Epithelial Circumferential Actin Belt Regulates YAP/TAZ through Nucleocytoplasmic Shuttling of Merlin. Cell Reports, 20(6), 1435–1447. 10.1016/j.celrep.2017.07.032 [DOI] [PubMed] [Google Scholar]
- Gan CT-J, Lankhaar J-W, Westerhof N, Marcus JT, Becker A, Twisk JWR, Boonstra A, Postmus PE, & Vonk-Noordegraaf A. (2007). Noninvasively Assessed Pulmonary Artery Stiffness Predicts Mortality in Pulmonary Arterial Hypertension. Chest, 132(6), 1906–1912. 10.1378/chest.07-1246 [DOI] [PubMed] [Google Scholar]
- Garcia-Polite F, Martorell J, Del Rey-Puech P, Melgar-Lesmes P, O’Brien CC, Roquer J, Ois A, Principe A, Edelman ER, & Balcells M. (2017). Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. Journal of Cerebral Blood Flow & Metabolism, 37(7), 2614–2625. 10.1177/0271678X16672482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Gao L, Thakur A, Siu PM, & Lai CWK (2017). Role of free fatty acids in endothelial dysfunction. Journal of Biomedical Science, 24(1), 50. 10.1186/s12929-017-0357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Zhang D-L, Ollivierre WH, Noguchi A, Springer DA, Linehan WM, & Rouault TA (2021). Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood, 137(18), 2509–2519. 10.1182/blood.2020009138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Rehman J, Tang H, Wary K, Mittal M, Chatturvedi P, Zhao Y, Komorova YA, Vogel SM, & Malik AB (2015). HIF2α signaling inhibits adherens junctional disruption in acute lung injury. Journal of Clinical Investigation, 125(2), 652–664. 10.1172/JCI77701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin BL, Solomonson LP, & Eichler DC (2004). Argininosuccinate Synthase Expression Is Required to Maintain Nitric Oxide Production and Cell Viability in Aortic Endothelial Cells. Journal of Biological Chemistry, 279(18), 18353–18360. 10.1074/jbc.M308160200 [DOI] [PubMed] [Google Scholar]
- Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, & Weitz DA (2017). Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proceedings of the National Academy of Sciences, 114(41), E8618–E8627. 10.1073/pnas.1705179114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerling R, Hoppe E, Dierkes C, Stehling M, Makinen T, Butz S, Vestweber D, & Kiefer F. (2018). Distinct roles of VE -cadherin for development and maintenance of specific lymph vessel beds. The EMBO Journal, 37(22). 10.15252/embj.201798271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Dupont S, & Piccolo S. (2012). Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature Reviews Molecular Cell Biology, 13(9), 591–600. 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- Han X, Kong J, Hartnett ME, & Wang H. (2019). Enhancing Retinal Endothelial Glycolysis by Inhibiting UCP2 Promotes Physiologic Retinal Vascular Development in a Model of Retinopathy of Prematurity. Investigative Opthalmology & Visual Science, 60(5), 1604. 10.1167/iovs.19-26553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, He M, Marin T, Shen H, Wang W-T, Lee T-Y, Hong H-C, Jiang Z-L, Garland T, Shyy JY-J, Gongol B, & Chien S. (2021). Roles of KLF4 and AMPK in the inhibition of glycolysis by pulsatile shear stress in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 118(21), e2103982118. 10.1073/pnas.2103982118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, & Hawley SA (2012). AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology, 13(4), 251–262. 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjes U, Kalucka J, & Carmeliet P. (2016). Targeting fatty acid metabolism in cancer and endothelial cells. Critical Reviews in Oncology/Hematology, 97, 15–21. 10.1016/j.critrevonc.2015.10.011 [DOI] [PubMed] [Google Scholar]
- He X, Zeng H, Chen ST, Roman RJ, Aschner JL, Didion S, & Chen J-X (2017). Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. Journal of Molecular and Cellular Cardiology, 112, 104–113. 10.1016/j.yjmcc.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix BM, & Sweet JE (1917). A STUDY OF AMINO NITROGEN AND GLUCOSE IN LYMPH AND BLOOD BEFORE AND AFTER THE INJECTION OF NUTRIENT SOLUTIONS IN THE INTESTINE. Journal of Biological Chemistry, 32(3), 299–307. 10.1016/S0021-9258(18)86617-0 [DOI] [Google Scholar]
- Hermann A, Wu G, Nedvetsky PI, Brücher VC, Egbring C, Bonse J, Höffken V, Wennmann DO, Marks M, Krahn MP, Schöler H, Heiduschka P, Pavenstädt H, & Kremerskothen J. (2021). The Hippo pathway component Wwc2 is a key regulator of embryonic development and angiogenesis in mice. Cell Death & Disease, 12(1), 117. 10.1038/s41419-021-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, & Shaw RJ (2018). AMPK: Guardian of metabolism and mitochondrial homeostasis. Nature Reviews. Molecular Cell Biology, 19(2), 121–135. 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Yamamoto K, Ikeda K, & Arita M. (2021). Functional lipidomics of vascular endothelial cells in response to laminar shear stress. The FASEB Journal, 35(2). 10.1096/fj.202002144R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoger JH, Ilyin VI, Forsyth S, & Hoger A. (2002). Shear stress regulates the endothelial Kir2.1 ion channel. Proceedings of the National Academy of Sciences of the United States of America, 99(11), 7780–7785. 10.1073/pnas.102184999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong AW, Meng Z, Yuan H, Plouffe SW, Moon S, Kim W, Jho E, & Guan K. (2017). Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Reports, 18(1), 72–86. 10.15252/embr.201642681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-J, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, McKeon B, Frid MG, Mouradian G, Li M, Riddle S, Pugliese SC, Brown RD, Wallace EM, Graham BB, & Stenmark KR (2019). Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. European Respiratory Journal, 1900378. 10.1183/13993003.00378-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, Oh D, Varma G, Hung YP, Ullas S, Lauring J, Seth P, Lundquist MR, Tolan DR, Grant AK, Needleman DJ, Asara JM, Cantley LC, & Wulf GM (2016). Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell, 164(3), 433–446. 10.1016/j.cell.2015.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Brüning U, Visnagri A, Yuldasheva N, Goveia J, Cruys B, Brepoels K, Wyns S, Rayport S, Ghesquière B, Vinckier S, Schoonjans L, Cubbon R, Dewerchin M, Eelen G, & Carmeliet P. (2017). Role of glutamine and interlinked asparagine metabolism in vessel formation. The EMBO Journal, 36(16), 2334–2352. 10.15252/embj.201695518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R-T, Wu D, Meliton A, Oh M-J, Krause M, Lloyd JA, Nigdelioglu R, Hamanaka RB, Jain MK, Birukova A, Kress JP, Birukov KG, Mutlu GM, & Fang Y. (2017). Experimental Lung Injury Reduces Krüppel-like Factor 2 to Increase Endothelial Permeability via Regulation of RAPGEF3–Rac1 Signaling. American Journal of Respiratory and Critical Care Medicine, 195(5), 639–651. 10.1164/rccm.201604-0668OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, & Schwartz MA (2014). Mechanotransduction and extracellular matrix homeostasis. Nature Reviews Molecular Cell Biology, 15(12), 802–812. 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter KS, Lee P-F, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, & Shandas R. (2008). Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. American Heart Journal, 155(1), 166–174. 10.1016/j.ahj.2007.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli J, Huang JH, Berkovitch SS, Chattopadhyay S, Mazitschek R, Schreiber SL, Haggarty SJ, & Karmacharya R. (2015). HDAC6 Inhibitors Modulate Lys49 Acetylation and Membrane Localization of β-Catenin in Human iPSC-Derived Neuronal Cells. ACS Chemical Biology, 10(3), 883–890. 10.1021/cb500838r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, Ando J, & Yoshida A. (2011). Effects of Shear Stress on the Gene Expressions of Endothelial Nitric Oxide Synthase, Endothelin-1, and Thrombomodulin in Human Retinal Microvascular Endothelial Cells. Investigative Opthalmology & Visual Science, 52(11), 8496. 10.1167/iovs.11-7686 [DOI] [PubMed] [Google Scholar]
- Ishibazawa A, Nagaoka T, Yokota H, Ono S, & Yoshida A. (2013). Low shear stress up-regulation of proinflammatory gene expression in human retinal microvascular endothelial cells. Experimental Eye Research, 116, 308–311. 10.1016/j.exer.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Jafarnejad M, Cromer WE, Kaunas RR, Zhang SL, Zawieja DC, & Moore JE (2015). Measurement of shear stress-mediated intracellular calcium dynamics in human dermal lymphatic endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology, 308(7), H697–H706. 10.1152/ajpheart.00744.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James NL, Harrison DG, & Nerem RM (1995). Effects of shear on endothelial cell calcium in the presence and absence of ATP. The FASEB Journal, 9(10), 968–973. 10.1096/fasebj.9.10.7615166 [DOI] [PubMed] [Google Scholar]
- Jeong S-H, Kim H-B, Kim M-C, Lee J, Lee JH, Kim J-H, Kim J-W, Park W-Y, Kim S-Y, Kim JB, Kim H, Kim J-M, Choi H-S, & Lim D-S (2018). Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. Journal of Clinical Investigation, 128(3), 1010–1025. 10.1172/JCI95802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Li H-Y, Wang J, Wang Y, Zhang P, Ma N, & Mo S-J (2019). Phosphorylation of 14–3-3ζ links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis, 8(5), 31. 10.1038/s41389-019-0143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Weiss JN, & Ribalet B. (2011). Subcellular Localization of Hexokinases I and II Directs the Metabolic Fate of Glucose. PLoS ONE, 6(3), e17674. 10.1371/journal.pone.0017674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Brüning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi L-C, Schoors S, Harjes U, … Carmeliet P. (2018). Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metabolism, 28(6), 881–894.e13. 10.1016/j.cmet.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen L-A, Veys K, García-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, … Carmeliet P. (2020). Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell, 180(4), 764–779.e20. 10.1016/j.cell.2020.01.015 [DOI] [PubMed] [Google Scholar]
- Karki P, Ke Y, Tian Y, Ohmura T, Sitikov A, Sarich N, Montgomery CP, & Birukova AA (2019). Staphylococcus aureus–induced endothelial permeability and inflammation are mediated by microtubule destabilization. Journal of Biological Chemistry, 294(10), 3369–3384. 10.1074/jbc.RA118.004030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami D, Mahabeleshwar GH, Lin Z, Atkins GB, Hamik A, Haldar SM, Maemura K, LaManna JC, & Jain MK (2009). Kruppel-like Factor 2 Inhibits Hypoxia-inducible Factor 1α Expression and Function in the Endothelium. Journal of Biological Chemistry, 284(31), 20522–20530. 10.1074/jbc.M109.025346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Betterman KL, Chong C-E, Stokes PH, Lee YK, Secker GA, Agalarov Y, Demir CS, Lawrence DM, Sutton DL, Tabruyn SP, Miura N, Salminen M, Petrova TV, Matthews JM, Hahn CN, Scott HS, & Harvey NL (2015). GATA2 is required for lymphatic vessel valve development and maintenance. Journal of Clinical Investigation, 125(8), 2979–2994. 10.1172/JCI78888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Lee H, Kawata K, & Park J-Y (2014). Exercise-Mediated Wall Shear Stress Increases Mitochondrial Biogenesis in Vascular Endothelium. PLoS ONE, 9(11), e111409. 10.1371/journal.pone.0111409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Li J, Jang C, & Arany Z. (2017). Glutamine fuels proliferation but not migration of endothelial cells. The EMBO Journal, 36(16), 2321–2333. 10.15252/embj.201796436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gao P, Liu Y-C, Semenza GL, & Dang CV (2007). Hypoxia-Inducible Factor 1 and Dysregulated c-Myc Cooperatively Induce Vascular Endothelial Growth Factor and Metabolic Switches Hexokinase 2 and Pyruvate Dehydrogenase Kinase 1. Molecular and Cellular Biology, 27(21), 7381–7393. 10.1128/MCB.00440-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Guan K-L (2019). MTOR as a central hub of nutrient signalling and cell growth. Nature Cell Biology, 21(1), 63–71. 10.1038/s41556-018-0205-1 [DOI] [PubMed] [Google Scholar]
- Kim J, Kim YH, Kim J, Park DY, Bae H, Lee D-H, Kim KH, Hong SP, Jang SP, Kubota Y, Kwon Y-G, Lim D-S, & Koh GY (2017). YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. Journal of Clinical Investigation, 127(9), 3441–3461. 10.1172/JCI93825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Tchernyshyov I, Semenza GL, & Dang CV (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3(3), 177–185. 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kim J-S, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, Brown MD, & Park J-Y (2015). Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology, 309(3), H425–H433. 10.1152/ajpheart.00438.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura TE, Duggirala A, Smith MC, White S, Sala-Newby GB, Newby AC, & Bond M. (2016). The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. Journal of Molecular and Cellular Cardiology, 90, 1–10. 10.1016/j.yjmcc.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knull HR, Taylor WF, & Wells WW (1973). Effects of energy metabolism on in vivo distribution of hexokinase in brain. The Journal of Biological Chemistry, 248(15), 5414–5417. [PubMed] [Google Scholar]
- Knull HR, Taylor WF, & Wells WW (1974). Insulin effects on brain energy metabolism and the related hexokinase distribution. The Journal of Biological Chemistry, 249(21), 6930–6935. [PubMed] [Google Scholar]
- Kohn JC, Lampi MC, & Reinhart-King CA (2015). Age-related vascular stiffening: Causes and consequences. Frontiers in Genetics, 06. 10.3389/fgene.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike A, Wasserman K, Taniguchi K, Hiroe M, & Marumo F. (1994). Critical capillary oxygen partial pressure and lactate threshold in patients with cardiovascular disease. Journal of the American College of Cardiology, 23(7), 1644–1650. 10.1016/0735-1097(94)90669-6 [DOI] [PubMed] [Google Scholar]
- Kondo H, Ratcliffe CDH, Hooper S, Ellis J, MacRae JI, Hennequart M, Dunsby CW, Anderson KI, & Sahai E. (2021). Single-cell resolved imaging reveals intra-tumor heterogeneity in glycolysis, transitions between metabolic states, and their regulatory mechanisms. Cell Reports, 34(7), 108750. 10.1016/j.celrep.2021.108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JH, & Guan K-L (2018). Interplay between YAP/TAZ and Metabolism. Cell Metabolism, 28(2), 196–206. 10.1016/j.cmet.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Kovács J, Löw P, Pácz A, Horváth I, Oláh J, & Ovádi J. (2003). Phosphoenolpyruvate-dependent Tubulin-Pyruvate Kinase Interaction at Different Organizational Levels. Journal of Biological Chemistry, 278(9), 7126–7130. 10.1074/jbc.M210244200 [DOI] [PubMed] [Google Scholar]
- Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, & Birukova AA (2012). Oxidative Stress Contributes to Lung Injury and Barrier Dysfunction via Microtubule Destabilization. American Journal of Respiratory Cell and Molecular Biology, 47(5), 688–697. 10.1165/rcmb.2012-0161OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause MD, Huang R-T, Wu D, Shentu T-P, Harrison DL, Whalen MB, Stolze LK, Di Rienzo A, Moskowitz IP, Civelek M, Romanoski CE, & Fang Y. (2018). Genetic variant at coronary artery disease and ischemic stroke locus 1p32.2 regulates endothelial responses to hemodynamics. Proceedings of the National Academy of Sciences of the United States of America, 115(48), E11349–E11358. 10.1073/pnas.1810568115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotova K, Patel JM, Block ER, & Zharikov S. (2010). Hypoxic upregulation of arginase II in human lung endothelial cells. American Journal of Physiology-Cell Physiology, 299(6), C1541–C1548. 10.1152/ajpcell.00068.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku KH, Subramaniam N, & Marsden PA (2019). Epigenetic Determinants of Flow-Mediated Vascular Endothelial Gene Expression. Hypertension, 74(3), 467–476. 10.1161/HYPERTENSIONAHA.119.13342 [DOI] [PubMed] [Google Scholar]
- Kucharzewska P, Welch JE, Svensson KJ, & Belting M. (2010). Ornithine decarboxylase and extracellular polyamines regulate microvascular sprouting and actin cytoskeleton dynamics in endothelial cells. Experimental Cell Research, 316(16), 2683–2691. 10.1016/j.yexcr.2010.05.033 [DOI] [PubMed] [Google Scholar]
- Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, Zhao J, Ihida-Stansbury K, Chang B, DeLisser H, Tuder RM, Kawut SM, Silljé HHW, Shapiro S, Zhao Y, & Goncharova EA (2016). HIPPO–Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine, 194(7), 866–877. 10.1164/rccm.201510-2003OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl LR (1967). Evidence for nuclear synthesis of lactic dehydrogenase in rat liver. The Journal of Biological Chemistry, 242(9), 2199–2206. [PubMed] [Google Scholar]
- Lakshminarayanan S, Gardner TW, & Tarbell JM (2000). Effect of shear stress on the hydraulic conductivity of cultured bovine retinal microvascular endothelial cell monolayers. Current Eye Research, 21(6), 944–951. 10.1076/ceyr.21.6.944.6985 [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, & Benetos A. (2001). Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension, 37(5), 1236–1241. 10.1161/01.HYP.37.5.1236 [DOI] [PubMed] [Google Scholar]
- Le Master E, Huang R-T, Zhang C, Bogachkov Y, Coles C, Shentu T-P, Sheng Y, Fancher IS, Ng C, Christoforidis T, Subbaiah PV, Berdyshev E, Qain Z, Eddington DT, Lee J, Cho M, Fang Y, Minshall RD, & Levitan I. (2018). Proatherogenic Flow Increases Endothelial Stiffness via Enhanced CD36-Mediated Uptake of Oxidized Low-Density Lipoproteins. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(1), 64–75. 10.1161/ATVBAHA.117.309907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-H, Wu D, Guzy R, Schoettler N, Adegunsoye A, Mueller J, Hussein A, Sperling A, Mutlu GM, & Fang Y. (2021). SARS-CoV-2 infection reduces Krüppel-Like Factor 2 in human lung autopsy. BioRxiv: The Preprint Server for Biology, 2021.01.15.426691. 10.1101/2021.01.15.426691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux S, & Tedgui A. (1998). Signal Transduction of Mechanical Stresses in the Vascular Wall. Hypertension, 32(2), 338–345. 10.1161/01.HYP.32.2.338 [DOI] [PubMed] [Google Scholar]
- Lew CR, & Tolan DR (2013). Aldolase sequesters WASP and affects WASP/Arp2/3-stimulated actin dynamics. Journal of Cellular Biochemistry, 114(8), 1928–1939. 10.1002/jcb.24538 [DOI] [PubMed] [Google Scholar]
- Lewis JC, Taylor RG, Jones ND, St Clair RW, & Cornhill JF (1982). Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Laboratory Investigation; a Journal of Technical Methods and Pathology, 46(2), 123–138. [PubMed] [Google Scholar]
- Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, & Gajic O. (2011). Eight-Year Trend of Acute Respiratory Distress Syndrome: A Population-based Study in Olmsted County, Minnesota. American Journal of Respiratory and Critical Care Medicine, 183(1), 59–66. 10.1164/rccm.201003-0436OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LSB, Abujarour R, Ding S, & Guan KL (2010). The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes & Development, 24(11), 1106–1118. 10.1101/gad.1903310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Zhang R, Lu Y, Prosdocimo DA, Sangwung P, Zhang L, Zhou G, Anand P, Lai L, Leone TC, Fujioka H, Ye F, Rosca MG, Hoppel CL, Schulze PC, Abel ED, Stamler JS, Kelly DP, & Jain MK (2015). Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis. Journal of Clinical Investigation, 125(9), 3461–3476. 10.1172/JCI79964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Balasubramanian V, García-Cardeña G, & Jain MK (2005). Kruppel-Like Factor 2 (KLF2) Regulates Endothelial Thrombotic Function. Circulation Research, 96(5). 10.1161/01.RES.0000159707.05637.a1 [DOI] [PubMed] [Google Scholar]
- Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, & Jain MK (2010). Kruppel-Like Factor 2 Regulates Endothelial Barrier Function. Arteriosclerosis, Thrombosis, and Vascular Biology, 30(10), 1952–1959. 10.1161/ATVBAHA.110.211474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky HH (2011). Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discovery Today: Disease Models, 8(1), 57–62. 10.1016/j.ddmod.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, & Tschumperlin DJ (2015). Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology, 308(4), L344–L357. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen BP-C, Lu M, Zhu Y, Stemerman MB, Chien S, & Shyy JY-J (2002). Shear Stress Activation of SREBP1 in Endothelial Cells Is Mediated by Integrins. Arteriosclerosis, Thrombosis, and Vascular Biology, 22(1), 76–81. 10.1161/hq0102.101822 [DOI] [PubMed] [Google Scholar]
- Liu Y, Guo J-Z, Liu Y, Wang K, Ding W, Wang H, Liu X, Zhou S, Lu X-C, Yang H-B, Xu C, Gao W, Zhou L, Wang Y-P, Hu W, Wei Y, Huang C, & Lei Q-Y (2018). Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth. Nature Communications, 9(1), 4429. 10.1038/s41467-018-06841-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, McIntyre RL, Janssens GE, & Houtkooper RH (2020). Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mechanisms of Ageing and Development, 186, 111212. 10.1016/j.mad.2020.111212 [DOI] [PubMed] [Google Scholar]
- Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragón RL, Su T, Romay MC, McDonald AI, Kuo C-H, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, … Iruela-Arispe ML (2017). NOTCH1 is a mechanosensor in adult arteries. Nature Communications, 8(1), 1620. 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S, Nishimura RA, Sorajja P, Cha S, & McGoon MD (2006). Relationship of Pulmonary Arterial Capacitance and Mortality in Idiopathic Pulmonary Arterial Hypertension. Journal of the American College of Cardiology, 47(4), 799–803. 10.1016/j.jacc.2005.09.054 [DOI] [PubMed] [Google Scholar]
- Mambetsariev I, Tian Y, Wu T, Lavoie T, Solway J, Birukov KG, & Birukova AA (2014). Stiffness-Activated GEF-H1 Expression Exacerbates LPS-Induced Lung Inflammation. PLoS ONE, 9(4), e92670. 10.1371/journal.pone.0092670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Muyleart M, Kadlec A, Gutterman D, & Mammoto T. (2018). YAP1-TEAD1 signaling controls angiogenesis and mitochondrial biogenesis through PGC1α. Microvascular Research, 119, 73–83. 10.1016/j.mvr.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmillot P, Keith T, Srivastava DK, & Knull HR (1994). Effect of Tubulin on the Activity of the Muscle Isoenzyme of Lactate-Dehydrogenase. Archives of Biochemistry and Biophysics, 315(2), 467–472. 10.1006/abbi.1994.1526 [DOI] [PubMed] [Google Scholar]
- Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, Shen AH, Januszyk M, Kwon SH, Wernig G, Wan DC, Lorenz HP, Gurtner GC, & Longaker MT (2021). Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science, 372(6540), eaba2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. (1984). Interactions between glycolytic enzymes and components of the cytomatrix. The Journal of Cell Biology, 99(1 Pt 2), 222s–225s. 10.1083/jcb.99.1.222s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathupala SP, Rempel A, & Pedersen PL (2001). Glucose Catabolism in Cancer Cells. Journal of Biological Chemistry, 276(46), 43407–43412. 10.1074/jbc.M108181200 [DOI] [PubMed] [Google Scholar]
- Maurya MR, Gupta S, Li JY-S, Ajami NE, Chen ZB, Shyy JY-J, Chien S, & Subramaniam S. (2021). Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proceedings of the National Academy of Sciences of the United States of America, 118(4), e2023236118. 10.1073/pnas.2023236118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu C-M, Russell CG, & Chittur KK (2001). DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proceedings of the National Academy of Sciences, 98(16), 8955–8960. 10.1073/pnas.171259298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior B, & Frangos JA (2012). Gα q/11 -mediated intracellular calcium responses to retrograde flow in endothelial cells. American Journal of Physiology-Cell Physiology, 303(4), C467–C473. 10.1152/ajpcell.00117.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, & Zhang DX (2010). TRPV4-mediated endothelial Ca 2+ influx and vasodilation in response to shear stress. American Journal of Physiology-Heart and Circulatory Physiology, 298(2), H466–H476. 10.1152/ajpheart.00854.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Mambetsariev I, Tian Y, Beckham Y, Meliton A, Leff A, Gardel ML, Allen MJ, Birukov KG, & Birukova AA (2015). Attenuation of Lipopolysaccharide-Induced Lung Vascular Stiffening by Lipoxin Reduces Lung Inflammation. American Journal of Respiratory Cell and Molecular Biology, 52(2), 152–161. 10.1165/rcmb.2013-0468OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RW, & Dunham PB (1981). Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. Journal of General Physiology, 78(5), 547–568. 10.1085/jgp.78.5.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, DeBolt K, Madesh M, Moore JS, & Fisher AB (2006). Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. American Journal of Physiology-Cell Physiology, 290(1), C66–C76. 10.1152/ajpcell.00094.2005 [DOI] [PubMed] [Google Scholar]
- Miranda M, Balarini M, Caixeta D, & Bouskela E. (2016). Microcirculatory dysfunction in sepsis: Pathophysiology, clinical monitoring, and potential therapies. American Journal of Physiology-Heart and Circulatory Physiology, 311(1), H24–H35. 10.1152/ajpheart.00034.2016 [DOI] [PubMed] [Google Scholar]
- Mitchell GF (2008). Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. Journal of Applied Physiology, 105(5), 1652–1660. 10.1152/japplphysiol.90549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler ER, Fang Y, Shaffer RG, Moore J, Wilensky RL, Parmacek M, & Levitan I. (2007). Hypercholesterolemia suppresses Kir channels in porcine bone marrow progenitor cells in vivo. Biochemical and Biophysical Research Communications, 358(1), 317–324. 10.1016/j.bbrc.2007.04.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen J-RAJ, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJA, Zeebregts CJ, Krenning G, & Harmsen MC (2015). Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovascular Research, 108(3), 377–386. 10.1093/cvr/cvv175 [DOI] [PubMed] [Google Scholar]
- Mun GI, Lee SJ, An SM, Kim IK, & Boo YC (2009). Differential gene expression in young and senescent endothelial cells under static and laminar shear stress conditions. Free Radical Biology and Medicine, 47(3), 291–299. 10.1016/j.freeradbiomed.2009.04.032 [DOI] [PubMed] [Google Scholar]
- Nagel T, Resnick N, Dewey CF, & Gimbrone MA (1999). Vascular Endothelial Cells Respond to Spatial Gradients in Fluid Shear Stress by Enhanced Activation of Transcription Factors. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(8), 1825–1834. 10.1161/01.ATV.19.8.1825 [DOI] [PubMed] [Google Scholar]
- Nauta TD, van den Broek M, Gibbs S, van der Pouw-Kraan TCTM, Oudejans CB, van Hinsbergh VWM, & Koolwijk P. (2017). Identification of HIF-2α-regulated genes that play a role in human microvascular endothelial sprouting during prolonged hypoxia in vitro. Angiogenesis, 20(1), 39–54. 10.1007/s10456-016-9527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto F, Klaus-Bergmann A, Ong YT, Alt S, Vion A-C, Szymborska A, Carvalho JR, Hollfinger I, Bartels-Klein E, Franco CA, Potente M, & Gerhardt H. (2018). YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. ELife, 7, e31037. 10.7554/eLife.31037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhofer W, & Beck F-X (2005). CELL SURVIVAL IN THE HOSTILE ENVIRONMENT OF THE RENAL MEDULLA. Annual Review of Physiology, 67(1), 531–555. 10.1146/annurev.physiol.67.031103.154456 [DOI] [PubMed] [Google Scholar]
- Newsholme P, Procopio J, Lima MMR, Pithon-Curi TC, & Curi R. (2003). Glutamine and glutamate?their central role in cell metabolism and function. Cell Biochemistry and Function, 21(1), 1–9. 10.1002/cbf.1003 [DOI] [PubMed] [Google Scholar]
- Noto A, De Vitis C, Pisanu ME, Roscilli G, Ricci G, Catizone A, Sorrentino G, Chianese G, Taglialatela-Scafati O, Trisciuoglio D, Del Bufalo D, Di Martile M, Di Napoli A, Ruco L, Costantini S, Jakopin Z, Budillon A, Melino G, Del Sal G, … Mancini R. (2017). Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene, 36(32), 4573–4584. 10.1038/onc.2017.75 [DOI] [PubMed] [Google Scholar]
- O’Donnell A, & Yutzey KE (2020). Mechanisms of heart valve development and disease. Development, 147(13), dev183020. 10.1242/dev.183020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, & Davies PF (1988). Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature, 331(6152), 168–170. 10.1038/331168a0 [DOI] [PubMed] [Google Scholar]
- Ostrowski MA, Huang NF, Walker TW, Verwijlen T, Poplawski C, Khoo AS, Cooke JP, Fuller GG, & Dunn AR (2014). Microvascular Endothelial Cells Migrate Upstream and Align Against the Shear Stress Field Created by Impinging Flow. Biophysical Journal, 106(2), 366–374. 10.1016/j.bpj.2013.11.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway JH, & Mowbray J. (1977). The role of compartmentation in the control of glycolysis. Current Topics in Cellular Regulation, 12, 107–208. 10.1016/b978-0-12-152812-6.50010-x [DOI] [PubMed] [Google Scholar]
- Pagliaro L. (1995). Glycolysis in Vivo: Fluorescence Microscopy as a Tool for Studying Enzyme Organization in Living Cells. In Advances in Molecular and Cell Biology (Vol. 11, pp. 93–123). Elsevier. 10.1016/S1569-2558(08)60249-0 [DOI] [Google Scholar]
- Palmer RMJ, Ashton DS, & Moncada S. (1988). Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature, 333(6174), 664–666. 10.1038/333664a0 [DOI] [PubMed] [Google Scholar]
- Park JS, Burckhardt CJ, Lazcano R, Solis LM, Isogai T, Li L, Chen CS, Gao B, Minna JD, Bachoo R, DeBerardinis RJ, & Danuser G. (2020). Mechanical regulation of glycolysis via cytoskeleton architecture. Nature, 578(7796), 621–626. 10.1038/s41586-020-1998-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar KM (2005). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. Journal of Clinical Investigation, 116(1), 49–58. 10.1172/JCI24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, & Evans PC (2007). Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 21(13), 3553–3561. 10.1096/fj.06-8059com [DOI] [PubMed] [Google Scholar]
- Paszkowiak JJ, & Dardik A. (2003). Arterial Wall Shear Stress: Observations from the Bench to the Bedside. Vascular and Endovascular Surgery, 37(1), 47–57. 10.1177/153857440303700107 [DOI] [PubMed] [Google Scholar]
- Patella F, Schug ZT, Persi E, Neilson LJ, Erami Z, Avanzato D, Maione F, Hernandez-Fernaud JR, Mackay G, Zheng L, Reid S, Frezza C, Giraudo E, Fiorio Pla A, Anderson K, Ruppin E, Gottlieb E, & Zanivan S. (2015). Proteomics-Based Metabolic Modeling Reveals That Fatty Acid Oxidation (FAO) Controls Endothelial Cell (EC) Permeability. Molecular & Cellular Proteomics, 14(3), 621–634. 10.1074/mcp.M114.045575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, Rabinowitz JD, Thompson CB, & Zhang J. (2018). As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metabolism, 27(2), 428–438.e5. 10.1016/j.cmet.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peghaire C, Dufton NP, Lang M, Salles-Crawley II, Ahnström J, Kalna V, Raimondi C, Pericleous C, Inuabasi L, Kiseleva R, Muzykantov VR, Mason JC, Birdsey GM, & Randi AM (2019). The transcription factor ERG regulates a low shear stress-induced anti-thrombotic pathway in the microvasculature. Nature Communications, 10(1), 5014. 10.1038/s41467-019-12897-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Zhao X, Wang C, Guan L, Li K, Gu C, & Lin Y. (2021). In Situ Observation of Glucose Metabolism Dynamics of Endothelial Cells in Hyperglycemia with a Stretchable Biosensor: Research Tool for Bridging Diabetes and Atherosclerosis. Analytical Chemistry, 93(2), 1043–1049. 10.1021/acs.analchem.0c03938 [DOI] [PubMed] [Google Scholar]
- Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, & Omholt SW (2014). Arterial Stiffening Provides Sufficient Explanation for Primary Hypertension. PLoS Computational Biology, 10(5), e1003634. 10.1371/journal.pcbi.1003634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels K, Schnitzler JG, Waissi F, Levels JHM, Stroes ESG, Daemen MJAP, Lutgens E, Pennekamp A-M, De Kleijn DPV, Seijkens TTP, & Kroon J. (2020). Inhibition of PFKFB3 Hampers the Progression of Atherosclerosis and Promotes Plaque Stability. Frontiers in Cell and Developmental Biology, 8, 581641. 10.3389/fcell.2020.581641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin A, Imboden M, Sorba F, Grazioli S, Martin-Olmos C, Rosset S, & Shea H. (2018). An ultra-fast mechanically active cell culture substrate. Scientific Reports, 8(1), 9895. 10.1038/s41598-018-27915-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, & Semenza GL (2012). Adaptive and Maladaptive Cardiorespiratory Responses to Continuous and Intermittent Hypoxia Mediated by Hypoxia-Inducible Factors 1 and 2. Physiological Reviews, 92(3), 967–1003. 10.1152/physrev.00030.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, & Gaehtgens P. (2000). The endothelial surface layer. Pflügers Archiv - European Journal of Physiology, 440(5), 653–666. 10.1007/s004240000307 [DOI] [PubMed] [Google Scholar]
- Pulkkinen HH, Kiema M, Lappalainen JP, Toropainen A, Beter M, Tirronen A, Holappa L, Niskanen H, Kaikkonen MU, Ylä-Herttuala S, & Laakkonen JP (2021). BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis, 24(1), 129–144. 10.1007/s10456-020-09748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero M, Colombo SL, Godfrey A, & Moncada S. (2006). Mitochondria as signaling organelles in the vascular endothelium. Proceedings of the National Academy of Sciences, 103(14), 5379–5384. 10.1073/pnas.0601026103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, & Catanzarite V. (1974). The biosynthesis and turnover of nicotinamide adenine dinucleotide in enucleated culture cells. Journal of Cellular Physiology, 84(3), 409–421. 10.1002/jcp.1040840309 [DOI] [PubMed] [Google Scholar]
- Richmond KN, Burnite S, & Lynch RM (1997). Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. American Journal of Physiology-Cell Physiology, 273(5), C1613–C1622. 10.1152/ajpcell.1997.273.5.C1613 [DOI] [PubMed] [Google Scholar]
- Roberts DJ, & Miyamoto S. (2015). Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death & Differentiation, 22(2), 248–257. 10.1038/cdd.2014.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes J-M, Adams RH, Mäkinen T, Kiefer F, Kwak BR, & Petrova TV (2012). Mechanotransduction, PROX1, and FOXC2 Cooperate to Control Connexin37 and Calcineurin during Lymphatic-Valve Formation. Developmental Cell, 22(2), 430–445. 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Mäkinen T, Martinez-Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N, & Petrova TV (2015). FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. Journal of Clinical Investigation, 125(10), 3861–3877. 10.1172/JCI80454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, & Sabatini DM (2017). MTOR Signaling in Growth, Metabolism, and Disease. Cell, 169(2), 361–371. 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Scheitlin CG, Julian JA, Shanmughapriya S, Madesh M, Tsoukias NM, & Alevriadou BR (2016). Endothelial mitochondria regulate the intracellular Ca 2+ response to fluid shear stress. American Journal of Physiology-Cell Physiology, 310(6), C479–C490. 10.1152/ajpcell.00171.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, Bruning U, Missiaen R, Queiroz KCS, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Goddé L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, … Carmeliet P. (2015). Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature, 520(7546), 192–197. 10.1038/nature14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouché A, Vinckier S, Diaz-Moralli S, Gerhardt H, … Carmeliet P. (2014). Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metabolism, 19(1), 37–48. 10.1016/j.cmet.2013.11.008 [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Both G, & Lechene C. (1989). Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proceedings of the National Academy of Sciences, 86(12), 4525–4529. 10.1073/pnas.86.12.4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Cragoe EJ, & Lechene CP (1990). PH regulation in spread cells and round cells. The Journal of Biological Chemistry, 265(3), 1327–1332. [PubMed] [Google Scholar]
- Schwartz MA, Lechene C, & Ingber DE (1991). Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proceedings of the National Academy of Sciences, 88(17), 7849–7853. 10.1073/pnas.88.17.7849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley EJ, Rosenberg P, & Matthay MA (2013). Calcium flux and endothelial dysfunction during acute lung injury: A STIMulating target for therapy. Journal of Clinical Investigation, 123(3), 1015–1018. 10.1172/JCI68093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2012). Hypoxia-Inducible Factors in Physiology and Medicine. Cell, 148(3), 399–408. 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp M, Sokolova N, Jugai S, Mandel M, Peterson P, & Vendelin M. (2014). Tight Coupling of Na+/K+-ATPase with Glycolysis Demonstrated in Permeabilized Rat Cardiomyocytes. PLoS ONE, 9(6), e99413. 10.1371/journal.pone.0099413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC (2005). Regulation of endothelial derived nitric oxide in health and disease. Memórias Do Instituto Oswaldo Cruz, 100(suppl 1), 15–18. 10.1590/S0074-02762005000900004 [DOI] [PubMed] [Google Scholar]
- Siebert G, & Humphrey GB (2006). Enzymology of the Nucleus. In Nord FF (Ed.), Advances in Enzymology—And Related Areas of Molecular Biology (pp. 239–288). John Wiley & Sons, Inc. 10.1002/9780470122723.ch5 [DOI] [PubMed] [Google Scholar]
- Singh N, Singh H, Jagavelu K, Wahajuddin M, & Hanif K. (2017). Fatty acid synthase modulates proliferation, metabolic functions and angiogenesis in hypoxic pulmonary artery endothelial cells. European Journal of Pharmacology, 815, 462–469. 10.1016/j.ejphar.2017.09.042 [DOI] [PubMed] [Google Scholar]
- Sivaraj KK, Dharmalingam B, Mohanakrishnan V, Jeong H-W, Kato K, Schröder S, Adams S, Koh GY, & Adams RH (2020). YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells. ELife, 9, e50770. 10.7554/eLife.50770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulyan H, Lieber A, & Safar ME (2016). Hypertension, Diabetes Type II, and Their Association: Role of Arterial Stiffness. American Journal of Hypertension, 29(1), 5–13. 10.1093/ajh/hpv107 [DOI] [PubMed] [Google Scholar]
- Soydemir S, Comella O, Abdelmottaleb D, & Pritchett J. (2020). Does Mechanocrine Signaling by Liver Sinusoidal Endothelial Cells Offer New Opportunities for the Development of Anti-fibrotics? Frontiers in Medicine, 6, 312. 10.3389/fmed.2019.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka KM, & Aranda-Espinoza H. (2011). Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood, 118(6), 1632–1640. 10.1182/blood-2010-11-321125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K, Wang J, Lv Y, Tian M, Zhao Y-Y, Minshall RD, & Hu G. (2021). YAP expression in endothelial cells prevents ventilator-induced lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology, 320(4), L568–L582. 10.1152/ajplung.00472.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Rivero JM, Villanueva-Paz M, de la Cruz-Ojeda P, de la Mata M, Cotán D, Oropesa-Ávila M, de Lavera I, Álvarez-Córdoba M, Luzón-Hidalgo R, & Sánchez-Alcázar JA (2016). Mitochondrial Dynamics in Mitochondrial Diseases. Diseases (Basel, Switzerland), 5(1), E1. 10.3390/diseases5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DT, MacIntyre R, Fuda N, Fiori J, Barrilla J, & Ramizel L. (2003). Analysis of glycolytic enzyme co-localization in Drosophila flight muscle. Journal of Experimental Biology, 206(12), 2031–2038. 10.1242/jeb.00367 [DOI] [PubMed] [Google Scholar]
- Sweet DT, Jiménez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, & Kahn ML (2015). Lymph flow regulates collecting lymphatic vessel maturation in vivo. Journal of Clinical Investigation, 125(8), 2995–3007. 10.1172/JCI79386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, & Roll-Mecak A. (2014). Molecular Basis for Age-Dependent Microtubule Acetylation by Tubulin Acetyltransferase. Cell, 157(6), 1405–1415. 10.1016/j.cell.2014.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, Wang Z, Gupta A, Zhou T, Sun X, Dash S, Wang Z, Balistrieri A, Zheng Q. yu, Cordery AG, Desai AA, Rischard F, Khalpey Z, Wang J, … Yuan JX-J (2017). Endothelial HIF-2α Contributes to Severe Pulmonary Hypertension by Inducing Endothelial-to-Mesenchymal Transition. American Journal of Physiology-Lung Cellular and Molecular Physiology, ajplung.00096.2. 10.1152/ajplung.00096.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Luo Y-X, Chen H-Z, & Liu D-P (2014). Mitochondria, endothelial cell function, and vascular diseases. Frontiers in Physiology, 5. 10.3389/fphys.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Yang Z, Wang J-M, Tu C, & Pan S-R (2006). Effects of Fluid Shear Stress on eNOS mRNA Expression and NO Production in Human Endothelial Progenitor Cells. Cardiology, 106(2), 82–88. 10.1159/000092636 [DOI] [PubMed] [Google Scholar]
- Tarbell JM (2010). Shear stress and the endothelial transport barrier. Cardiovascular Research, 87(2), 320–330. 10.1093/cvr/cvq146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell JM, & Ebong EE (2008). The Endothelial Glycocalyx: A Mechano-Sensor and -Transducer. Science Signaling, 1(40), pt8–pt8. 10.1126/scisignal.140pt8 [DOI] [PubMed] [Google Scholar]
- Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E, Martínez-Ruiz A, Enríquez JA, Aragonés J, Cadenas S, & Landázuri MO (2011). Induction of the Mitochondrial NDUFA4L2 Protein by HIF-1α Decreases Oxygen Consumption by Inhibiting Complex I Activity. Cell Metabolism, 14(6), 768–779. 10.1016/j.cmet.2011.10.008 [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network. (2000). Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine, 342(18), 1301–1308. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, & Weir EK (2016). The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Annals of the American Thoracic Society, AnnalsATS.201509–599FR. 10.1513/AnnalsATS.201509-599FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, & Piccolo S. (2017). YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nature Communications, 8(1), 15206. 10.1038/ncomms15206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro A, Panciera T, & Piccolo S. (2018). YAP/TAZ upstream signals and downstream responses. Nature Cell Biology, 20(8), 888–899. 10.1038/s41556-018-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Kampoli A-M, Tentolouris Nikolaos Papageorgiou C, & Stefanadis C. (2012). The Role of Nitric Oxide on Endothelial Function. Current Vascular Pharmacology, 10(1), 4–18. 10.2174/157016112798829760 [DOI] [PubMed] [Google Scholar]
- Tsai F-C, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjö S, & Meyer T. (2014). A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nature Cell Biology, 16(2), 133–144. 10.1038/ncb2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, & Schwartz MA (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature, 437(7057), 426–431. 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- van den Berg BM, Spaan JAE, Rolf TM, & Vink H. (2006). Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. American Journal of Physiology-Heart and Circulatory Physiology, 290(2), H915–H920. 10.1152/ajpheart.00051.2005 [DOI] [PubMed] [Google Scholar]
- van Horssen R, Janssen E, Peters W, van de Pasch L, Lindert M. M. te, van Dommelen MMT, Linssen PC, Hagen T. L. M. ten, Fransen JAM, & Wieringa B. (2009). Modulation of Cell Motility by Spatial Repositioning of Enzymatic ATP/ADP Exchange Capacity. Journal of Biological Chemistry, 284(3), 1620–1627. 10.1074/jbc.M806974200 [DOI] [PubMed] [Google Scholar]
- Vega M, Riera A, Fernández-Cid A, Herrero P, & Moreno F. (2016). Hexokinase 2 Is an Intracellular Glucose Sensor of Yeast Cells That Maintains the Structure and Activity of Mig1 Protein Repressor Complex. Journal of Biological Chemistry, 291(14), 7267–7285. 10.1074/jbc.M115.711408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelin M, Eimre M, Seppet E, Peet N, Andrienko T, Lemba M, Engelbrecht J, Seppet EK, & Saks VA (2004). Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Molecular and Cellular Biochemistry, 256(1/2), 229–241. 10.1023/B:MCBI.0000009871.04141.64 [DOI] [PubMed] [Google Scholar]
- Venturini G, Malagrino PA, Padilha K, Tanaka LY, Laurindo FR, Dariolli R, Carvalho VM, Cardozo KHM, Krieger JE, & Pereira A. da C. (2019). Integrated proteomics and metabolomics analysis reveals differential lipid metabolism in human umbilical vein endothelial cells under high and low shear stress. American Journal of Physiology-Cell Physiology, 317(2), C326–C338. 10.1152/ajpcell.00128.2018 [DOI] [PubMed] [Google Scholar]
- Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, & Fraser SE (2009). Reversing Blood Flows Act through klf2a to Ensure Normal Valvulogenesis in the Developing Heart. PLoS Biology, 7(11), e1000246. 10.1371/journal.pbio.1000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker KW, Reinitz CA, & Knull HR (1995). Glycolytic enzymes and assembly of microtubule networks. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 112(3), 503–514. 10.1016/0305-0491(95)00096-8 [DOI] [PubMed] [Google Scholar]
- Wang G, Kostidis S, Tiemeier GL, Sol WMPJ, de Vries MR, Giera M, Carmeliet P, van den Berg BM, & Rabelink TJ (2020). Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 40(2), 350–364. 10.1161/ATVBAHA.119.313399 [DOI] [PubMed] [Google Scholar]
- Wang H-J, Hsieh Y-J, Cheng W-C, Lin C-P, Lin Y. -s., Yang S-F, Chen C-C, Izumiya Y, Yu J-S, Kung H-J, & Wang (2014). JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1 -mediated glucose metabolism. Proceedings of the National Academy of Sciences, 111(1), 279–284. 10.1073/pnas.1311249111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Morris AJ, Tolan DR, & Pagliaro L. (1996). The molecular nature of the F-actin binding activity of aldolase revealed with site-directed mutants. The Journal of Biological Chemistry, 271(12), 6861–6865. [PubMed] [Google Scholar]
- Wang J, Tolan DR, & Pagliaro L. (1997). Metabolic Compartmentation in Living Cells: Structural Association of Aldolase. Experimental Cell Research, 237(2), 445–451. 10.1006/excr.1997.3811 [DOI] [PubMed] [Google Scholar]
- Wang K-C, Yeh Y-T, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan K-L, Li Y-SJ, & Chien S. (2016). Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proceedings of the National Academy of Sciences, 113(41), 11525–11530. 10.1073/pnas.1613121113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo J-Y, Li B, Tian XY, Chen L-J, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak K-LK, Tong KK, Kwan KM, Wang N, Chiu J-J, Zhu Y, & Huang Y. (2016). Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature, 540(7634), 579–582. 10.1038/nature20602 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, & Zou M-H (2017). Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes, 66(1), 193–205. 10.2337/db16-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, & Offermanns S. (2015). P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. Journal of Clinical Investigation, 125(8), 3077–3086. 10.1172/JCI81067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao Z-D, Li X, Aziz KE, Gan B, Johnson RL, & Chen J. (2015). AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nature Cell Biology, 17(4), 490–499. 10.1038/ncb3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu G, Gao X, Wang Y, Zhang W, Harmon EY, Zhi X, Xu Z, Lennartz MR, Barroso M, Trebak M, Chen C, & Zhou J. (2012). The Induction of Yes-Associated Protein Expression After Arterial Injury Is Crucial for Smooth Muscle Phenotypic Modulation and Neointima Formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(11), 2662–2669. 10.1161/ATVBAHA.112.254730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zhang L, Youker K, Zhang M-X, Wang J, LeMaire SA, Coselli JS, & Shen YH (2006). Free Fatty Acids Inhibit Insulin Signaling-Stimulated Endothelial Nitric Oxide Synthase Activation Through Upregulating PTEN or Inhibiting Akt Kinase. Diabetes, 55(8), 2301–2310. 10.2337/db05-1574 [DOI] [PubMed] [Google Scholar]
- Wang Y, Baeyens N, Corti F, Tanaka K, Fang JS, Zhang J, Jin Y, Coon B, Hirschi KK, Schwartz MA, & Simons M. (2016). Syndecan-4 controls lymphatic vasculature remodeling during embryonic development. Development, dev.140129. 10.1242/dev.140129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K. (1999). Critical Capillary PO2 and the Role of Lactate Production in Oxyhemoglobin Dissociation during Exercise. In Eke A. & Delpy DT (Eds.), Oxygen Transport to Tissue XXI (Vol. 471, pp. 321–333). Springer US. 10.1007/978-1-4615-4717-4_39 [DOI] [PubMed] [Google Scholar]
- Weibel ER (1973). Morphological basis of alveolar-capillary gas exchange. Physiological Reviews, 53(2), 419–495. 10.1152/physrev.1973.53.2.419 [DOI] [PubMed] [Google Scholar]
- Westermann B. (2010). Mitochondrial fusion and fission in cell life and death. Nature Reviews. Molecular Cell Biology, 11(12), 872–884. 10.1038/nrm3013 [DOI] [PubMed] [Google Scholar]
- White SM, Avantaggiati ML, Nemazanyy I, Di Poto C, Yang Y, Pende M, Gibney GT, Ressom HW, Field J, Atkins MB, & Yi C. (2019). YAP/TAZ Inhibition Induces Metabolic and Signaling Rewiring Resulting in Targetable Vulnerabilities in NF2-Deficient Tumor Cells. Developmental Cell, 49(3), 425–443.e9. 10.1016/j.devcel.2019.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DH, & Brosnan JT (1974). Concentrations of Metabolites in Animal Tissues. In Methods of Enzymatic Analysis (pp. 2266–2302). Elsevier. 10.1016/B978-0-12-091304-6.50093-8 [DOI] [Google Scholar]
- Wilson C, Lee MD, Heathcote HR, Zhang X, Buckley C, Girkin JM, Saunter CD, & McCarron JG (2019). Mitochondrial ATP production provides long-range control of endothelial inositol trisphosphate–evoked calcium signaling. Journal of Biological Chemistry, 294(3), 737–758. 10.1074/jbc.RA118.005913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE (1978). Ambiquitous enzymes: Variation in intracellular distribution as a regulatory mechanism. Trends in Biochemical Sciences, 3(2), 124–125. 10.1016/S0968-0004(78)80029-2 [DOI] [Google Scholar]
- Witte CL, Clauss RH, & Dumont AE (1967). Respiratory Gas Tensions Of Thoracic Duct Lymph: An Index Of Gas Exchange In Splanchnic Tissues. Annals of Surgery, 166(2), 254–262. 10.1097/00000658-196708000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtas K, Slepecky N, von Kalm L, & Sullivan D. (1997). Flight muscle function in Drosophila requires colocalization of glycolytic enzymes. Molecular Biology of the Cell, 8(9), 1665–1675. 10.1091/mbc.8.9.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, García-Caballero M, Missiaen R, Huang H, Brüning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hägerling R, Moral-Dardé V, … Carmeliet P. (2017). The role of fatty acid β-oxidation in lymphangiogenesis. Nature, 542(7639), 49–54. 10.1038/nature21028 [DOI] [PubMed] [Google Scholar]
- Wu C, Huang R-T, Kuo C-H, Kumar S, Kim CW, Lin Y-C, Chen Y-J, Birukova A, Birukov KG, Dulin NO, Civelek M, Lusis AJ, Loyer X, Tedgui A, Dai G, Jo H, & Fang Y. (2015). Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circulation Research, 117(4). 10.1161/CIRCRESAHA.117.306457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, & Birukov K. (2019). Endothelial Cell Mechano-Metabolomic Coupling to Disease States in the Lung Microvasculature. Frontiers in Bioengineering and Biotechnology, 7, 172. 10.3389/fbioe.2019.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Freund JB, Fraser SE, & Vermot J. (2011). Mechanistic Basis of Otolith Formation during Teleost Inner Ear Development. Developmental Cell, 20(2), 271–278. 10.1016/j.devcel.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Harrison DL, Szasz T, Yeh C-F, Shentu T-P, Meliton A, Huang R-T, Zhou Z, Mutlu GM, Huang J, & Fang Y. (2021). Single-cell metabolic imaging reveals a SLC2A3-dependent glycolytic burst in motile endothelial cells. Nature Metabolism, 3(5), 714–727. 10.1038/s42255-021-00390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Huang R-T, Hamanaka RB, Krause M, Oh M-J, Kuo C-H, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, & Mutlu GM (2017). HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. ELife, 6, e25217. 10.7554/eLife.25217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-H, Chang H-C, Ting P-C, & Wang DL (2018). Laminar shear stress promotes mitochondrial homeostasis in endothelial cells. Journal of Cellular Physiology, 233(6), 5058–5069. 10.1002/jcp.26375 [DOI] [PubMed] [Google Scholar]
- Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu Z-X, Rovira II, Brittain EL, Wolfgang MJ, Jurczak MJ, Fessel JP, & Finkel T. (2018). A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Molecular Cell, 69(4), 689–698.e7. 10.1016/j.molcel.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu Y, Ding Y, Luo S, Zheng X, Wu X, Liu Z, Ilyas I, Chen S, Han S, Little PJ, Jain MK, & Weng J. (2021). The zinc finger transcription factor, KLF2, protects against COVID-19 associated endothelial dysfunction. Signal Transduction and Targeted Therapy, 6(1), 266. 10.1038/s41392-021-00690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, Caldwell RB, Fulton DJ, Su Y, Hoda MN, Zhou G, Wu C, & Huo Y. (2014). Endothelial PFKFB3 plays a critical role in angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(6), 1231–1239. 10.1161/ATVBAHA.113.303041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem AL, Brock E, Siow D, Wattenberg B, Telang S, & Chesney J. (2009). Nuclear Targeting of 6-Phosphofructo-2-kinase (PFKFB3) Increases Proliferation via Cyclin-dependent Kinases. Journal of Biological Chemistry, 284(36), 24223–24232. 10.1074/jbc.M109.016816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Imamura H, & Ando J. (2018). Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells. American Journal of Physiology. Heart and Circulatory Physiology, 315(5), H1477–H1485. 10.1152/ajpheart.00204.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Korenaga R, Kamiya A, & Ando J. (2000). Fluid Shear Stress Activates Ca 2+ Influx Into Human Endothelial Cells via P2X4 Purinoceptors. Circulation Research, 87(5), 385–391. 10.1161/01.RES.87.5.385 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Nogimori Y, Imamura H, & Ando J. (2020). Shear stress activates mitochondrial oxidative phosphorylation by reducing plasma membrane cholesterol in vascular endothelial cells. Proceedings of the National Academy of Sciences, 117(52), 33660–33667. 10.1073/pnas.2014029117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Xu J, Ma Q, Liu Z, Sudhahar V, Cao Y, Wang L, Zeng X, Zhou Y, Zhang M, Xu Y, Wang Y, Weintraub NL, Zhang C, Fukai T, Wu C, Huang L, Han Z, Wang T, … Huo Y. (2018). PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nature Communications, 9(1), 4667. 10.1038/s41467-018-07132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cha B, Motawe ZY, Srinivasan RS, & Scallan JP (2019). VE-Cadherin Is Required for Lymphatic Valve Formation and Maintenance. Cell Reports, 28(9), 2397–2412.e4. 10.1016/j.celrep.2019.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Nakajima H, Wakayama Y, Muto A, Kawakami K, Fukuhara S, & Mochizuki N. (2015). Endothelial Ca2+ oscillations reflect VEGFR signaling-regulated angiogenic capacity in vivo. ELife, 4, e08817. 10.7554/eLife.08817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, & van der Bliek AM (2012). Mitochondrial fission, fusion, and stress. Science (New York, N.Y.), 337(6098), 1062–1065. 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ma Z, Shetty S, Ma M, & Fu J. (2016). Selective HDAC6 inhibition prevents TNF-α-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. American Journal of Physiology-Lung Cellular and Molecular Physiology, 311(1), L39–L47. 10.1152/ajplung.00051.2016 [DOI] [PubMed] [Google Scholar]
- Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS, Xie Y, Zhu J, Chen Z, De Smet F, Zhang J, Jin S-W, Sun L, Sun H, Kibbey RG, Hirschi KK, Hay N, Carmeliet P, Chittenden TW, … Simons M. (2017). FGF-dependent metabolic control of vascular development. Nature, 545(7653), 224–228. 10.1038/nature22322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Zheng H, Zheng Z, & Yan W. (2013). Proteomic Analyses Reveal a Role of Cytoplasmic Droplets as an Energy Source during Epididymal Sperm Maturation. PLoS ONE, 8(10), e77466. 10.1371/journal.pone.0077466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, & Tarbell JM (2014). The Adaptive Remodeling of Endothelial Glycocalyx in Response to Fluid Shear Stress. PLoS ONE, 9(1), e86249. 10.1371/journal.pone.0086249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Goliwas KF, Wang W, Taufalele PV, Bordeleau F, & Reinhart-King CA (2019). Energetic regulation of coordinated leader–follower dynamics during collective invasion of breast cancer cells. Proceedings of the National Academy of Sciences, 116(16), 7867–7872. 10.1073/pnas.1809964116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng X, Zong Z, Sun X, Hua X, & Li H. (2018). Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. Journal of Experimental & Clinical Cancer Research, 37(1), 216. 10.1186/s13046-018-0892-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng H-L, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, & Matthias P. (2008). Mice Lacking Histone Deacetylase 6 Have Hyperacetylated Tubulin but Are Viable and Develop Normally. Molecular and Cellular Biology, 28(5), 1688–1701. 10.1128/MCB.01154-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Han H, Liu G, Ma Y, Pan R, Sang L, Li R, Yang L, Marks JR, Wang W, & Lin A. (2017). Lnc RNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. The EMBO Journal, 36(22), 3325–3335. 10.15252/embj.201797609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li Y-S, & Chien S. (2014). Shear Stress–Initiated Signaling and Its Regulation of Endothelial Function. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(10), 2191–2198. 10.1161/ATVBAHA.114.303422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yeh C-F, Huang R-T, Lee T-H, Shentu T-P, Wu D, Yang K-C, & Fang Y. (2021). Endothelial restoration of CAD GWAS gene PLPP3 by nanomedicine suppresses YAP/TAZ activity and reduces atherosclerosis in vivo [Preprint]. Bioengineering. 10.1101/2021.05.06.443006 [DOI] [Google Scholar]