Abstract
The hypocretins/orexins were discovered in 1998. Within 2 years, this led to the discovery of the cause of human narcolepsy, a 90% loss of hypothalamic neurons containing these peptides. Further work demonstrated that these neurons were not simply linked to waking. Rather these neurons were active during pleasurable behaviors in waking and were silenced by aversive stimulation. This was seen in wild-type mice, rats, cats, and dogs. It was also evident in humans, with increased Hcrt release during pleasurable activities and decreased release, to the levels seen in sleep, during pain. We found that human heroin addicts have, on average, an increase of 54% in the number of detectable Hcrt neurons compared to “control” human brains and that these Hcrt neurons are substantially smaller than those in control brains. We found that in mice, chronic morphine administration induced the same changes in Hcrt neuron number and size. Our studies in the mouse allowed us to determine the specificity, dose response relations, time course of the change in the number of Hcrt neurons, and that the increased number of Hcrt neurons after opiates was not due to neurogenesis. Furthermore, we found that it took a month or longer for these anatomical changes in the mouse brain to return to baseline. Human narcoleptics, despite their prescribed use of several commonly addictive drugs, do not show significant evidence of dose escalation or substance use disorder. Similarly, mice in which the peptide has been eliminated are resistant to addiction. These findings are consistent with the concept that an increased number of Hcrt neurons may underlie and maintain opioid or cocaine use disorders.
ANATOMY
The hypocretin (Hcrt)/orexin peptides were discovered by two independent groups in 1998 (De Lecea et al., 1998; Sakurai et al., 1998; Siegel et al., 2001). The name hypocretin was created because of the hypothalamic localization of all somas containing the peptides and the resemblance of the peptides to secretin (De Lecea et al., 1998). The name orexin was selected because of the hypothesis that these peptides might drive appetite (Sakurai et al., 1998), since early work had shown that damage to the lateral hypothalamus produces anorexia, whereas damage to the medial hypothalamus produces hyperphagia and obesity (Anand and Brobeck, 1951; Teitelbaum and Epstein, 1962). Although the Hcrt peptides are often erroneously described as being in the “lateral” hypothalamus, these neurons are in fact present through the medial-lateral extent of the hypothalamus. An equal number of Hcrt neurons are present medial and lateral to the fornix, a structure used to define the boundary between the medial and lateral hypothalamus. Mice, rats, and humans all have Hcrt neurons throughout the medial-lateral extent of the hypothalamus (Peyron et al., 1998; Thannickal et al., 2000a,b; McGregor et al., 2011). Hcrt neurons are also present in the zona incerta in primates and other species (Bhagwandin et al., 2011; Dell et al., 2012, 2013, 2016a,b,c; Olateju et al., 2017; Pillay et al., 2017). In the rostro-caudal dimension, Hcrt neurons are present in the tuberal and mamillary regions of the hypothalamus, and though the majority of these neurons are located dorsally to the fornix there are some neuronal somas ventral to this structure. Throughout their distribution, Hcrt neurons are intermingled with many other cell types, not forming a dense homogeneous nucleus. From their hypothalamic location they send extensive projections within the hypothalamus and to the rest of the neuraxis, from the spinal cord to the cerebral cortex (Peyron et al., 1998; Chen et al., 1999; Date et al., 1999; Horvath et al., 1999; van den Pol, 1999). Hcrt signaling is conveyed through two G-protein-coupled receptors (HcrtR1 and, HcrtR2) with a range of distribution that overlaps that of Hcrt fibers (Marcus et al., 2001; Kukkonen and Leonard, 2014). Phylogenetic studies have shown a high degree of receptor homology between different species indicating that this system is evolutionary conserved (Ammoun et al., 2003). Activation of these receptors by Hcrts has short-term effects like depolarization and increase in neuronal firing rate and long-term effects including modulation of cell plasticity (Sakurai et al., 1998; Smart et al., 1999; Eriksson et al., 2001). Hcrt neurons respond to Hcrt peptides directly via the HcrtR2 or indirectly (via HcrtR1) through the release of glutamate (Li et al., 2002; Yamanaka et al., 2010).
Hcrt LINK TO NARCOLEPSY
The development of a Hcrt peptide knockout mouse, in which the neurons normally containing hypocretin are present (identified by the cotransmitters dynorphin and neuronal activity regulated pentraxin (Narp) (Chou et al., 2001; Blouin et al., 2005; Crocker et al., 2005), but the Hcrt peptide itself is not (Siegel, 2004; Blouin et al., 2005; Crocker et al., 2005), produced the disappointing observation that these animals were not anorexic, leading Chemelli et al. (1999) to use video observation to determine if there were any other abnormalities in their behavior. They made the striking observation that these mice showed sudden movement arrests. Their further work demonstrated that these were not seizures or losses of consciousness, but rather had electroencephalographic and electromyographic signs of waking, resembling those of cataplexy in human narcoleptics. This led to the discovery that there was a 90% loss of Hcrt neurons in human narcoleptics, amid signs of prior hypothalamic inflammation (Peyron et al., 2000; Thannickal et al., 2000a,b, 2003). This was the first indication of a neuroanatomical abnormality in human narcoleptics, although we had previously identified an abnormality in genetically narcoleptic dogs (Siegel et al., 1999). These dogs have a mutation that disrupts the function of the HcrtR2 (Lin et al., 1999). We found that they had elevated levels of axonal degeneration and reactive neuronal somata, an indicator of neuronal pathology, in a number of subcortical structures. These degenerative changes precede or coincide with symptom onset. In very rare cases, human narcolepsy can be caused by an Hcrt mutation, impairing peptide trafficking and processing (Peyron et al., 2000).
Nearly all human narcolepsy appears to be linked to an autoimmune process that causes destruction of Hcrt neurons (Scammell, 2006). This autoimmune hypothesis stems from the discovery that nearly all (~95%) of all human narcoleptics have an HLA immune subtype (DQB1*0602) present in only about 25% of the general population (Honda et al., 1984; Mignot et al., 2001). This hypothesis received further support from the finding that cases of narcolepsy increased during the H1N1 influenza epidemic in individuals immunized for the virus (Dauvilliers et al., 2010) and in those who contracted H1N1 without immunization (Han et al., 2011).
HYPOCRETIN, REWARD, AND OPIOIDS
We (Kiyashchenko et al., 2002; Mileykovskiy et al., 2005; McGregor et al., 2011; Wu et al., 2011a,b) and others (Nestler et al., 2002; Georgescu et al., 2003; Harris et al., 2005; Boutrel and De Lecea, 2008; Borgland et al., 2009; Aston-Jones et al., 2010; Nestler, 2013; Baimel et al., 2015; Hassani et al., 2016; James et al., 2017) have demonstrated that increased neuronal discharge in Hcrt neurons is linked to the performance of rewarded tasks in wild-type (WT) mice, rats, cats, and dogs.
Mice in which the Hcrt peptide is genetically knocked out (Hcrt-KO) learn to bar press for food or water as quickly as their WT littermates in the light phase and will respond as well as WT on fixed ratio tasks requiring relatively low effort. This indicates that they experience the rewarding properties of these natural reinforcers. However, when the effort to obtain these rewards is increased in a progressive ratio, the mice invariably stop bar pressing before the end of the 2 h test period, whereas their WT littermates continue until the end of the session (Fig. 22.1). The Hcrt-KO mice never showed cataplexy during the positive reinforcement-tests, but often fell asleep as the amount of work required to receive the reward (progressive ratio) increased. However, surprisingly, the KO mice were unimpaired relative to WT mice when working for a positive reward during the dark phase (Fig. 22.1E). This indicates that Hcrt peptides play a critical role in mediating motivated behaviors during the natural “sleep time” in these animals (McGregor et al., 2011).
Fig. 22.1.
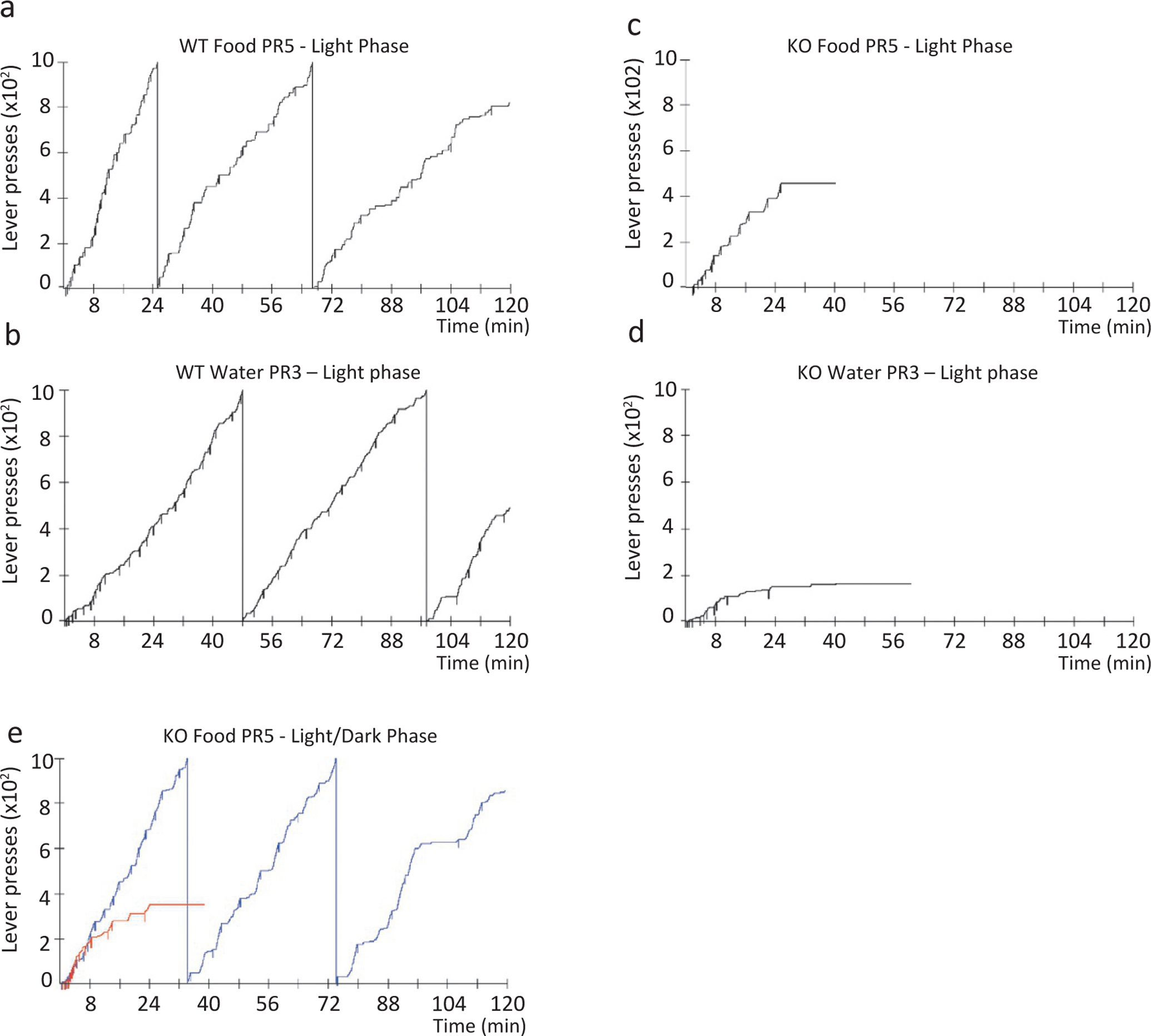
Operant performance of WT and KO mice on progressive ratio responding for food or water reinforcement paradigm. Hcrt-KO mice are unable to sustain bar pressing for food or water in the light phase, in contrast to littermate WT mice. Representative cumulative records of the performance of a WT animal (A, food, 2817 total presses; B, water, 2494 total presses) and an Hcrt-KO animal (C, food, 456 total presses; D, water, 164 total presses) responding for positive reinforcers. The downward pips on the cumulative record denote food or water deliveries. The Hcrt-KO mouse sessions were terminated when they ceased pressing the lever for 15min. Hcrt-KOs are unimpaired on the same task in the dark phase (E). Redrawn from McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM (2011). Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci 31: 15455–15467.
These behavioral results find striking parallels with the activity of Hcrt neurons. Mirroring the behavioral deficits seen in Hcrt-KO animals, we found that in WT mice, expression of the immediate early gene cFos in Hcrt neurons, an indirect indicator of neuronal activation, occurs only in the light phase when working for positive reinforcement in a progressive ratio task. In a second set of experiments, we observed that Hcrt-KO mice were unimpaired relative to WT when working to avoid a foot shock in a progressive ratio schedule during the light or dark phase. Analysis of Hcrt activation (cFos) under these conditions revealed that these neurons were not activated during the performance of this task. Furthermore, cFos was not expressed in Hcrt neurons above baseline when expected or unexpected rewards were presented, or when given or expecting an unavoidable foot shock, even though these conditions elicit maximal electroencephalogram (EEG) arousal (Figs. 22.2–22.3). Together these results from behavioral and anatomical studies point toward an emotional specificity in the recruitment of Hcrt neurons (McGregor et al., 2011; Blouin et al., 2013).
Fig. 22.2.
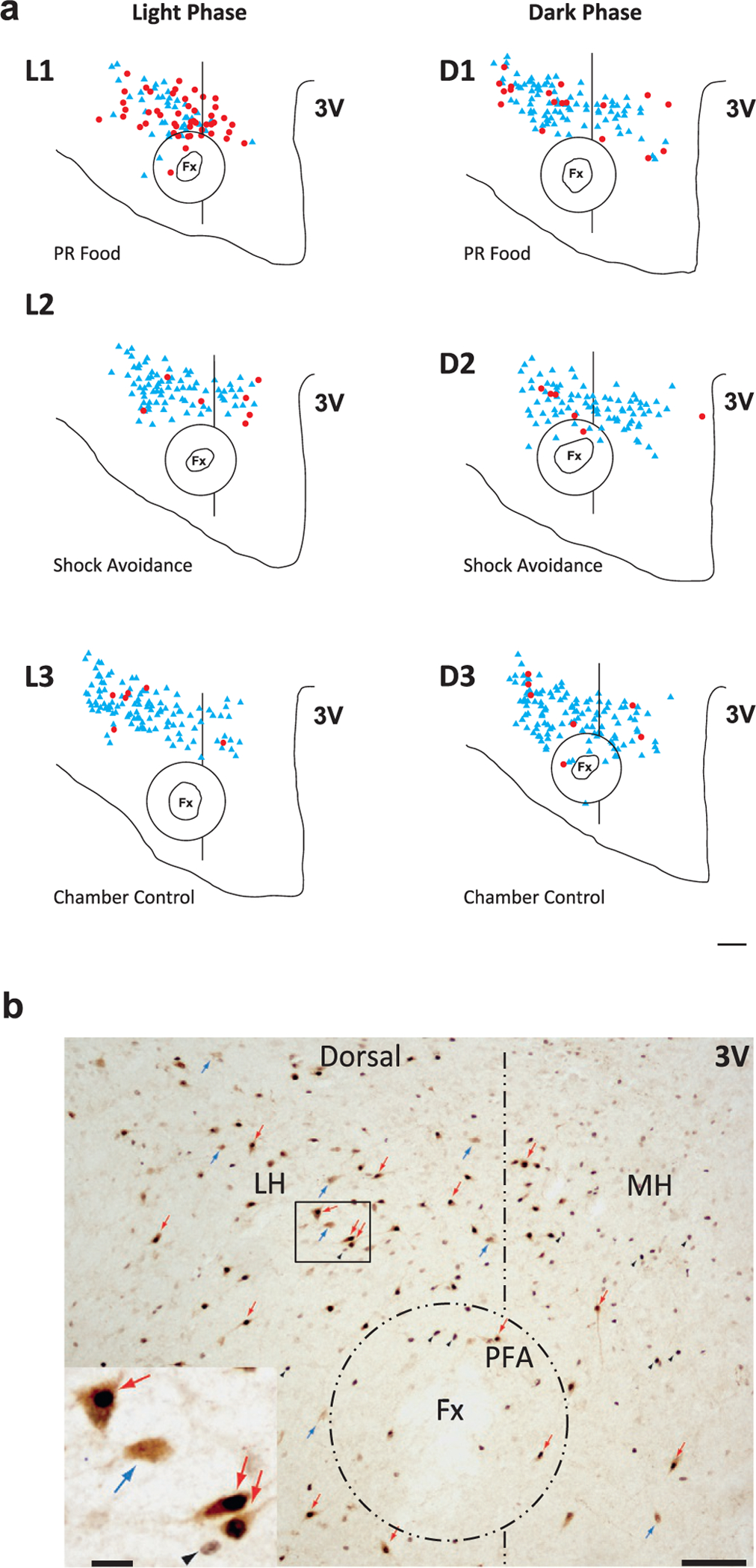
Distribution of Hcrt and cFos/Hcrt neurons in the hypothalamus of WT mice under different behavioral conditions. Hcrt neurons express cFos during a food motivated task in the light phase. Neither food nor shock avoidance tasks increase cFos expression in the dark phase. (A) Diagrams of coronal sections of the hypothalamus stained for Hcrt and cFos of six animals each under one of six different experimental conditions during the light and the dark phase: L1, PR food, light phase; L2, shock avoidance, light phase; L3, chamber control, light phase; D1, PR food, dark phase; D2, shock avoidance, dark phase; D3, chamber control, dark phase. Red dots indicate double-labeled cFos/Hcrt neurons; blue triangles correspond to Hcrt neurons. Fx, Fornix; 3V, third ventricle. Scale bar, 150 μm. (B) Photomicrographs of the same hypothalamic region in a section processed for Hcrt and cFos. LH, Lateral hypothalamus; MH, medial hypothalamus. Scale bar, 150 μm. The rectangular region in the LH is magnified in the insert at the lower left. Scale bar, 20 μm. The double-labeled neurons (red arrows) show the characteristic black nucleus due to the presence of cFos protein and a brown precipitate in the cytoplasm, indicating their hypocretinergic nature. These cells are easily distinguishable from single-labeled hypocretin neurons (blue arrows) and single-labeled cFos cells (black arrowheads). Redrawn from McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM (2011). Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci 31: 15455–15467.
Fig. 22.3.
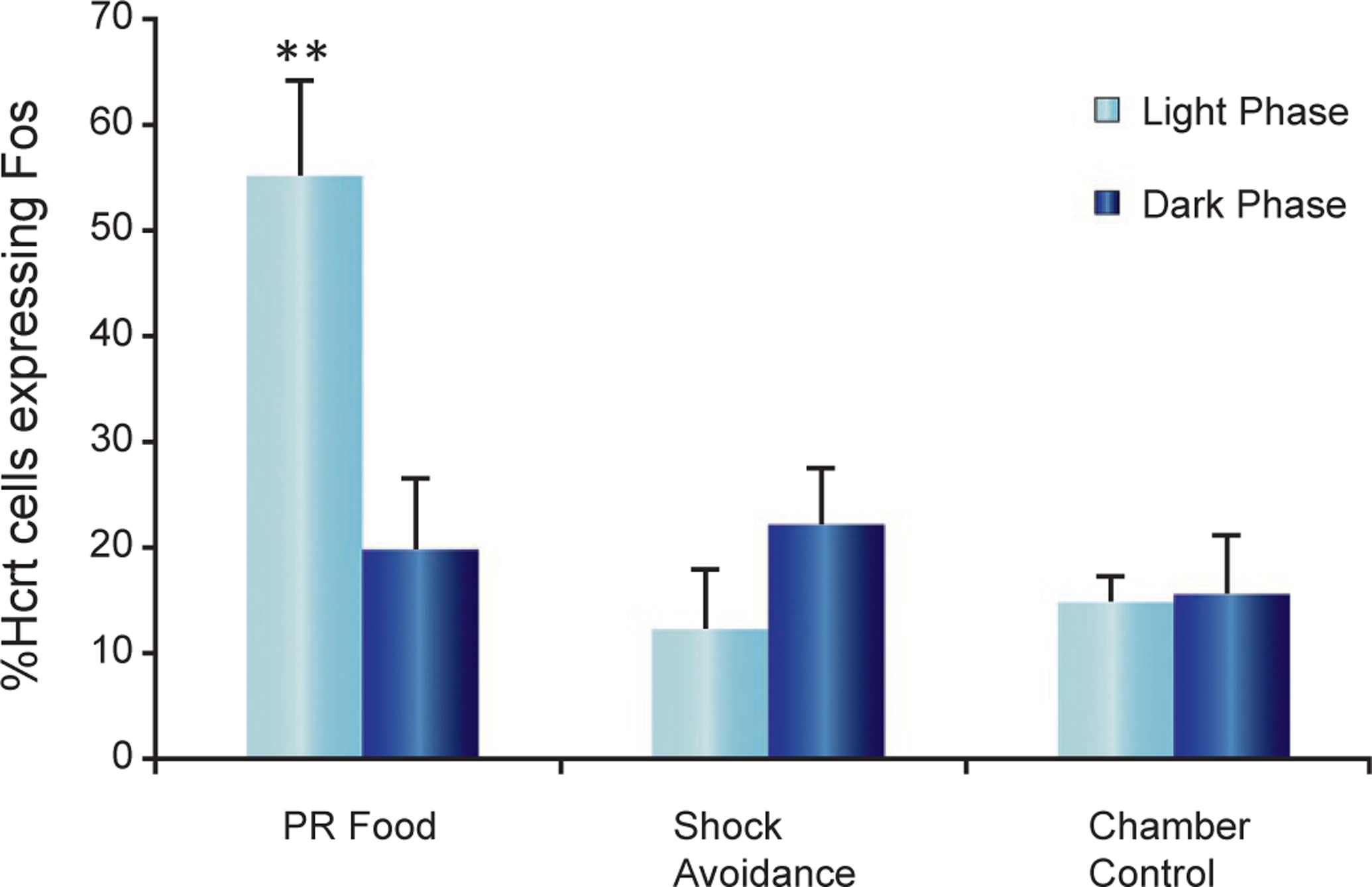
Percentage of Hcrt neurons expressing cFos in the hypothalamus of WT mice under different behavioral conditions. Activation of Hcrt neurons was maximal when bar pressing for food in the light phase, but not during shock avoidance. Comparison of the percentage of hypocretin neurons expressing cFos in the PR food, shock avoidance, and chamber control conditions during the light and dark phases (**P < 0.01, Newman–Keuls post hoc test comparing food task during the light phase with all other conditions; n = 4 in each condition). There is no significant difference between the light and dark phases in shock avoidance and chamber control conditions. Redrawn from McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM (2011). Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci 31: 15455–15467.
Interestingly when light was turned off, cFos was not expressed in Hcrt neurons beyond control levels in the light phase during positive reinforcement, indicating a very specific role of light in Hcrt’s involvement in reinforcement (McGregor et al., 2011; Blouin et al., 2013). This finding is consistent with the lack of light-induced arousal in human narcoleptics (Hajek et al., 1989), reported prior to the discovery of Hcrt, in contrast to the arousing effects of light in nonnarcoleptics.
It has been previously reported that there is a dichotomy in the functions of the Hcrt neuronal population, with the medial group related to arousal and the lateral group to reward (Harris and Aston-Jones, 2006). In our studies we did not observe a restricted distribution in the double-labeled Hcrt/cFos neurons. Rather they were seen homogeneously throughout the medial-lateral extent of the Hcrt field.
Recording of Hcrt neurons in freely moving rats showed that they discharged maximally during exploration, grooming, and eating, but ceased discharge during aversive stimulation in waking (Mileykovskiy et al., 2005); all changes consistent with our work on reinforcement in mice (McGregor et al., 2011). They reduced discharge in non-REM sleep with a low level of activity in REM sleep (Fig. 22.4).
Fig. 22.4.
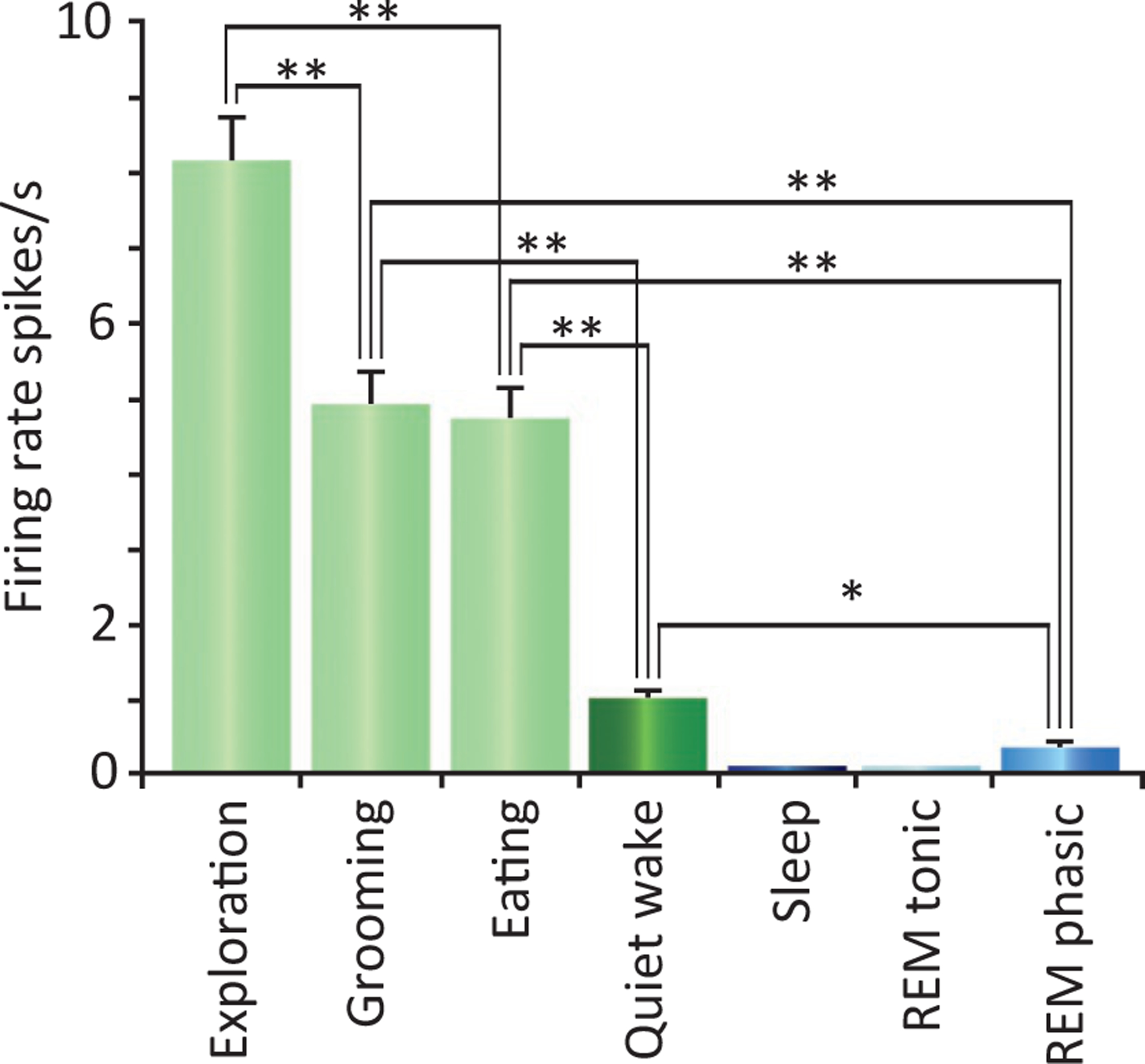
Firing rate of Hcrt neurons in waking and sleep behaviors in freely moving rats. Maximal discharge is seen during exploration-approach behavior. Group average of the discharge pattern of Hcrt neurons (n = 9) in different behavioral conditions (*P < 0.05, **P <=0.01 Bonferroni t-test). Error bars indicate SEM. Redrawn from Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005). Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46: 787–798.
HCRT, DOPAMINE, AND ADDICTION
Somewhat similar to Hcrt neurons, dopamine neurons, particularly those located in the ventral tegmental area (VTA), have long been implicated in reinforcement in general and addiction in particular (Beitner-Johnson et al., 1992, 1993; Mignot et al., 1995; Schilstrom et al., 1998; Sarti et al., 2002; Meye et al., 2012; Farahimanesh et al., 2017). Hcrt and dopamine are evolutionarily linked from both a neurochemical and anatomical perspective (Stefano and Kream, 2007). VTA plasticity associated with drug rewards requires functional Hcrt receptors (Baimel et al., 2015). The levels of dopamine and its major metabolites in the nucleus accumbens are markedly increased by the microinjection of Hcrts into the VTA. Hcrt neurons project strongly to the VTA, where the peptides appear to act via volume conduction (Del Cid-Pellitero and Garzon, 2014), and to the nucleus accumbens and paraventricular nucleus of the thalamus (Peyron et al., 1998). The paraventricular nucleus also projects directly to the nucleus accumbens (Zhu et al., 2016). Thus via its direct and indirect projections, Hcrt can strongly modulate circuits implicated in addiction (Peyron et al., 1998; Sim-Selley et al., 2011; Ho and Berridge, 2013; Zhu et al., 2016, Chen et al., 2006; Anderson et al., 2017).
Hcrt and opioids
An in vitro slice study found that opioids decrease the activity of Hcrt neurons and that blockade of μ-opioid receptors enhances the activity of Hcrt neurons. Morphine pretreatment inhibits subsequent excitatory responses to Hcrt in Hcrt neurons (Li and van den Pol, 2008). However, our current in vivo data (Fig. 22.5) (Thannickal et al., 2018) shows that systemic administration of morphine greatly increases Hcrt unit activity in intact rats. The effects of opioid agonists can be exerted not only in plasma membrane receptors and endosomes but also in the Golgi apparatus (Stoeber et al., 2018), suggesting a possible pathway for the alteration of Hcrt neuronal size after chronic opioid exposure that we have reported (Thannickal et al., 2000a,b, 2018) and for receptor expression (Cai et al., 2019). A large percentage of Hcrt cells also release glutamate (Torrealba et al., 2003), trigger glutamate release from adjacent cells. They also contain corelease dynorphin (Li and Van Den Pol, 2006;Muschamp et al., 2014), a member of the opioid peptide family that preferentially binds to the kappa opioid receptor (KOR) (Schwarzer, 2009). These two neuropeptides have opposing roles in reward related behaviors such as cocaine and alcohol self-administration, cocaine seeking, impulsivity, and brain stimulation reward (Matzeu and Martin-Fardon, 2018; Anderson et al., 2018). The VTA firing rate is increased by Hcrt and decreased by dynorphin, but bath coapplication of both peptides resulted in no net changes in neuronal firing (Muschamp et al., 2014). HcrtR1 and KOR can form receptor heterodimers, altering signal transduction and second messenger activation including increased protein kinase A activity and intracellular cAMP levels (Chen et al., 2015). Hcrt neurons also contain neuronal activity regulated pentraxin, involved in aggregating AMPA receptors and thought to have a role in addiction (Blouin et al., 2005; Crocker et al., 2005).
Fig. 22.5.
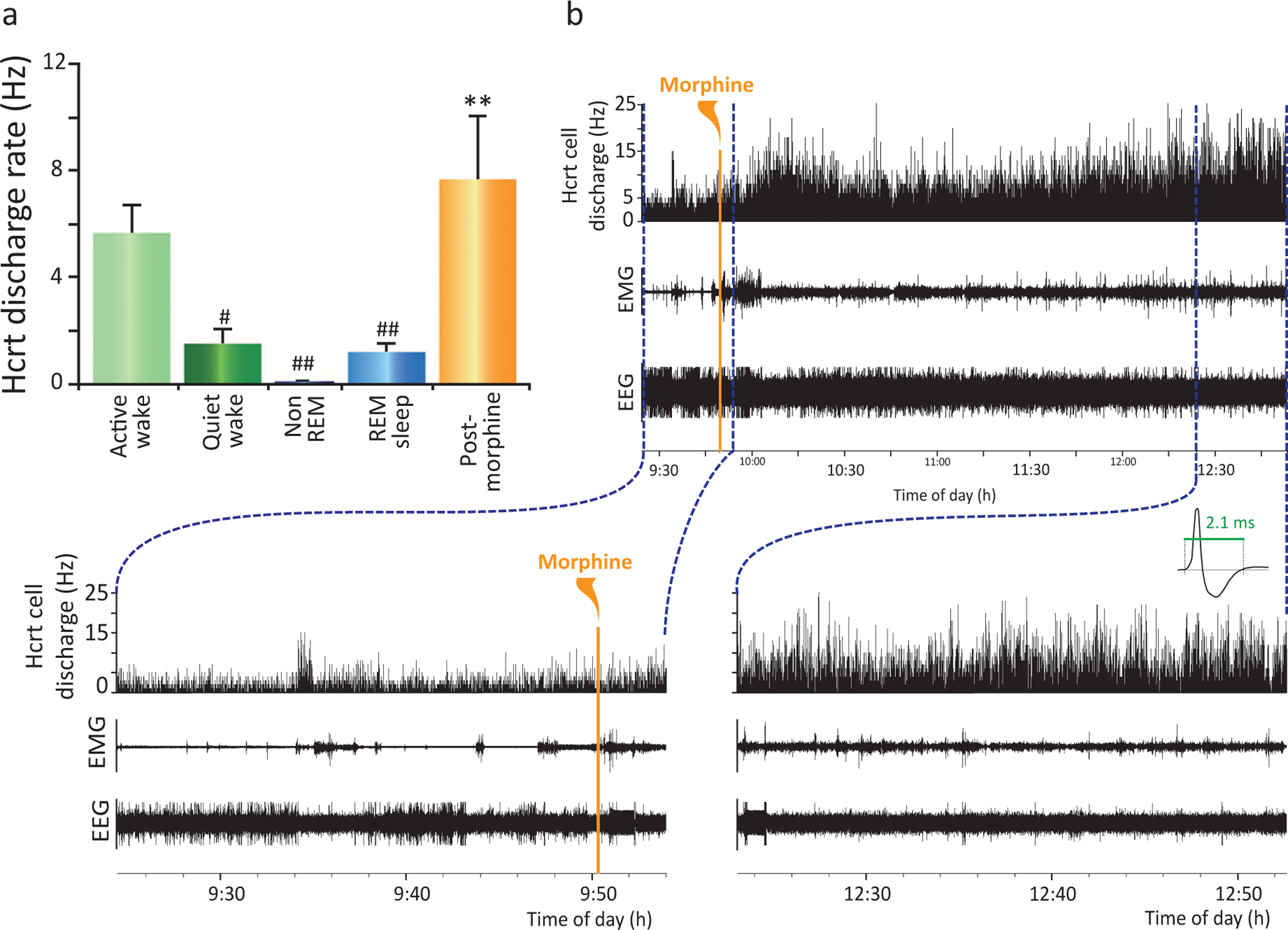
Effect of morphine administration on hypocretin cell activity in freely moving rats. A species-appropriate dose of morphine (15 mg/kg) injected into three freely moving rats resulted in an elevated neuronal discharge rate lasting for 3 h accompanied by an increase in EMG activity. (A) Sleep rates are averages of mean rate determined by five 10-s samples in each of five hypocretin neurons from three rats in each sleep state: active waking, quiet waking, non-REM sleep, and REM sleep. Postmorphine injection rate was based on five 10-s samples in each neuron taken 15 min after morphine injection. One-way ANOVA of hypocretin neurons (n = 5): F4,16=18.2, **P < 0.0001. Posthoc comparisons with Tukey/Kramer procedure: active waking/quiet waking, #P < 0.05; active waking/non-REM sleep, ##P < 0.01; active waking/REM, ##P < 0.01. (B) Discharge rate of rat hypocretin neurons after morphine administration. Bottom: Traces show EEG activation immediately after morphine injection (left) and 3 h after injection (right). Expanded trace shows the characteristic long average waveform of hypocretin neurons. Redrawn from Thannickal TC, John J, Shan L, Swaab DF, Wu M-F, Ramanathan L, McGregor R, Chew K-T, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM (2018). Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10: p. pii: eaao4953. doi: 10.1126/scitranslmed.aao4953.
Human studies
In another study, we found that Hcrt is released in the brain of nonaddict humans when they are engaged in enjoyable tasks, but not when they are aroused by pain or feeling sad (Fig. 22.6) (Blouin et al., 2013). Elevating Hcrt production by self-administration of opioids (Thannickal et al., 2018) creates a positive mental state. A negative affect is correlated with reduced administration of opioids and a diminishing rate of Hcrt production (C.D.C, 2017). Humans with narcolepsy have greatly elevated levels of depression (Ponz et al., 2010b; Lee et al., 2016; Nordstrand et al., 2019), with similar changes in animal models of narcolepsy (Lutter et al., 2008; James et al., 2018), i.e., both a low rate of Hcrt production (in narcoleptics) and a diminishing rate of Hcrt production (in addicts attempting withdrawal) (Thannickal et al., 2018) are correlated with depression. Similarly it has been shown that humans who have attempted suicide have lower levels of cerebrospinal Hcrt (Brundin et al., 2007, 2009). Circadian, sex-related differences, and brain region-specific changes in Hcrt system functioning have been reported in relation to human depression (Lu et al., 2017).
Fig. 22.6.
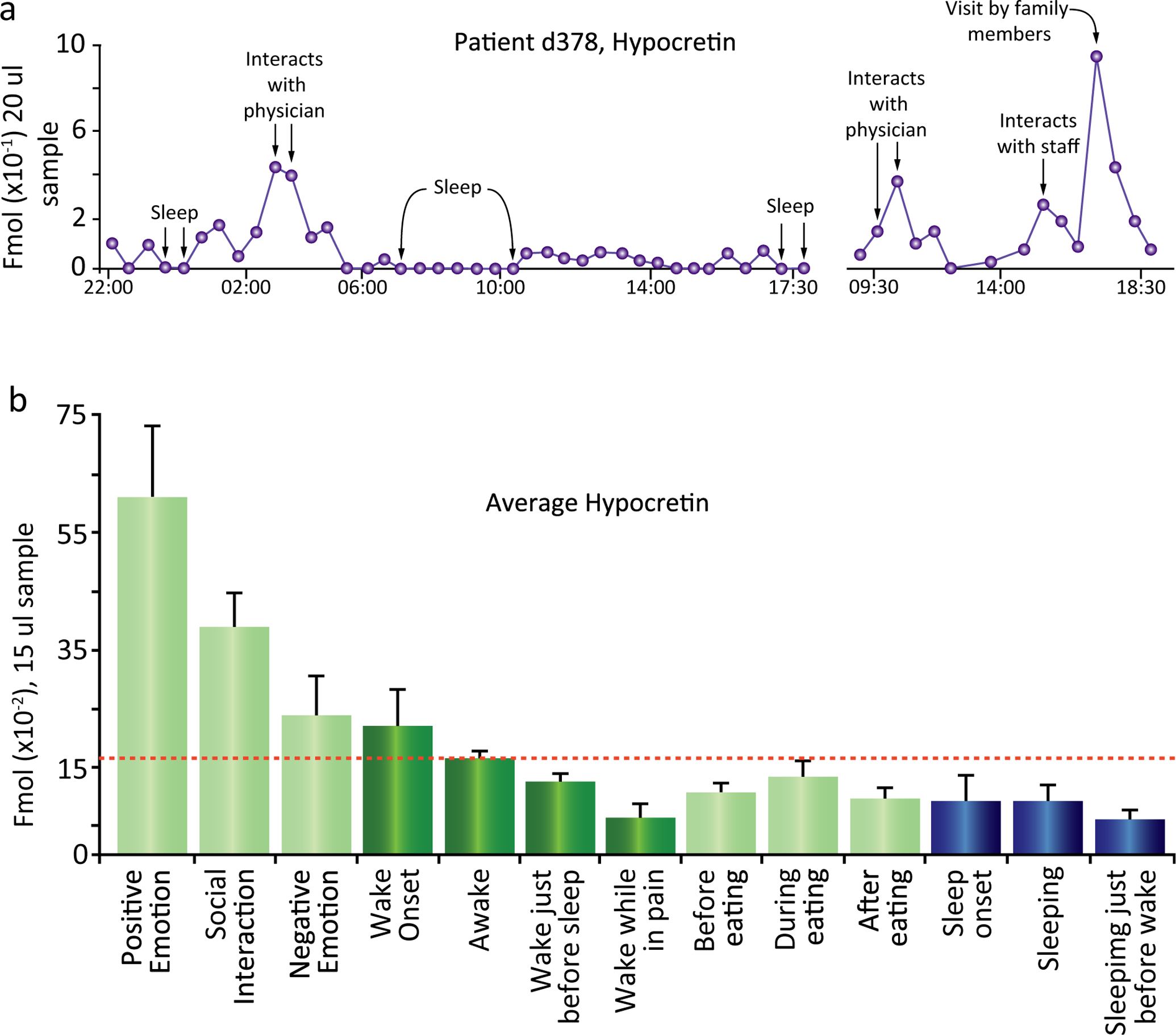
(A) Time course of Hcrt release over a 20-h period in patient d378. Maximal release occurred during interactions that the subject rated as pleasurable on a periodically administered questionnaire. Hcrt release was minimal during sleep and during pain. (B) Maximal Hcrt levels in waking are seen during positive emotions, social interactions, and awakening; minimal levels are seen before sleep and when reporting pain. Changes during and after eating are smaller than those during monitored non-eating-related activities. Waking values in shades of green, and sleep values in shades of blue. Awake indicates samples in which subjects were awake but were not exhibiting social interaction or reporting emotion. Redrawn from Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KAE, Lapierre JL, Siegel JM (2013). Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun 4: 1547.
We have shown that Parkinson’s disease patients have a considerable loss of Hcrt neurons (Fronczek et al., 2007; Thannickal et al., 2007, 2008), although not to the extent seen in narcoleptics. This loss may help explain the symptoms that Parkinson’s patients have in common with narcoleptics including daytime sleep attacks, nocturnal insomnia, hallucinations and depression, keeping in mind the much more extensive neuronal loss and symptoms in Parkinson’s.
From a medical standpoint, the most critical issue in opiate addicts is the inability of many addicts to successfully withdraw from opioid use (Li and van den Pol, 2008; Editors, 2016; C.D.C, 2017; Chang et al., 2017; Ostling et al., 2018). The difficulty of withdrawal for addicts is not principally caused by the seeking of a pleasurable “high.” Rather it is seeking relief from the symptoms induced by withdrawal. These include insomnia (Valentino and Volkow, 2020), anxiety, irritability, hot flashes/chills, sweating, restlessness, and hyperalgesia. Acute symptoms typically peak 24–48 h after withdrawing from short-acting opioids (e.g., heroin or oxycodone). These acute symptoms may be followed by anhedonia, fatigue, anorexia, depression, and insomnia (Christie, 2008; Shi et al., 2009; Del Bello et al., 2013; Lutz et al., 2014; Zhu et al., 2016), effects that persist for weeks to months or years in humans (Sigmon et al., 2012). These short- and long-term effects drive most subjects who have attempted withdrawal to relapse within 1 year (McLellan et al., 2000; C.D.C, 2017; Volkow et al., 2018), even after medically supervised detoxification and pharmacological intervention.
HUMAN NARCOLEPTICS RARELY GET ADDICTED
It has long been noted that narcoleptics, who have an average 90% loss of Hcrt neurons (Thannickal et al., 2000a,b), show little, if any, evidence of drug abuse, addiction or overdose (Borgland et al., 2009; Guilleminault and Cao, 2011; Brown and Guilleminault, 2011; James et al., 2017), despite their daily prescribed use of gamma hydroxybutyrate, methylphenidate, and amphetamine. These drugs reverse the sleepiness and cataplexy of narcolepsy and are frequently abused in the general population with considerable loss of life (Harris et al., 2007; Borgland et al., 2009; Nishino and Mignot, 2011; Dauvilliers et al., 2013, 2014; Barateau et al., 2016; Darke et al., 2019; Jalal et al., 2018; Turner et al., 2018). Yet dose escalation and overdose are virtually nonexistent in narcoleptics (Galloway et al., 1997; Aston-Jones et al., 2010; Bayard and Dauvilliers, 2013; Baimel et al., 2015). Human narcoleptics have been shown to have a greatly reduced reward activation of the VTA, amygdala, and accumbens (Ponz et al., 2010a,b) and altered processing of humor in the hypothalamus and amygdala (Schwartz et al., 2007). The lack of abuse in human narcoleptics is consistent with the greatly reduced addiction potential in mice and rats with reduced Hcrt function (Sharf et al., 2010; Tabaeizadeh et al., 2013; Zarepour et al., 2014; Bentzley and Aston-Jones, 2015; Bali et al., 2015; Sadeghi et al., 2016; Sadeghzadeh et al., 2016; Guo et al., 2016; Farahimanesh et al., 2017; Alizamini et al., 2017; Assar et al., 2019; Azizbeigi and Haghparast, 2019; Azizbeigi et al., 2019; Pourhamzeh et al., 2019; Farzinpour et al., 2019; Shirazy et al., 2020; Zarrabian et al., 2020). It is also consistent with our recent finding of the converse phenomenon, greatly increased Hcrt cell number in human heroin addicts (Fig. 22.7) (Thannickal et al., 2018). Whereas a reduced number of Hcrt cells in narcoleptics is correlated with a greatly reduced addiction susceptibility in human and mouse narcoleptics, a greatly increased number of detected Hcrt-producing cells is elicited by opioid administration in humans and mice (Thannickal et al., 2018).
Fig. 22.7.
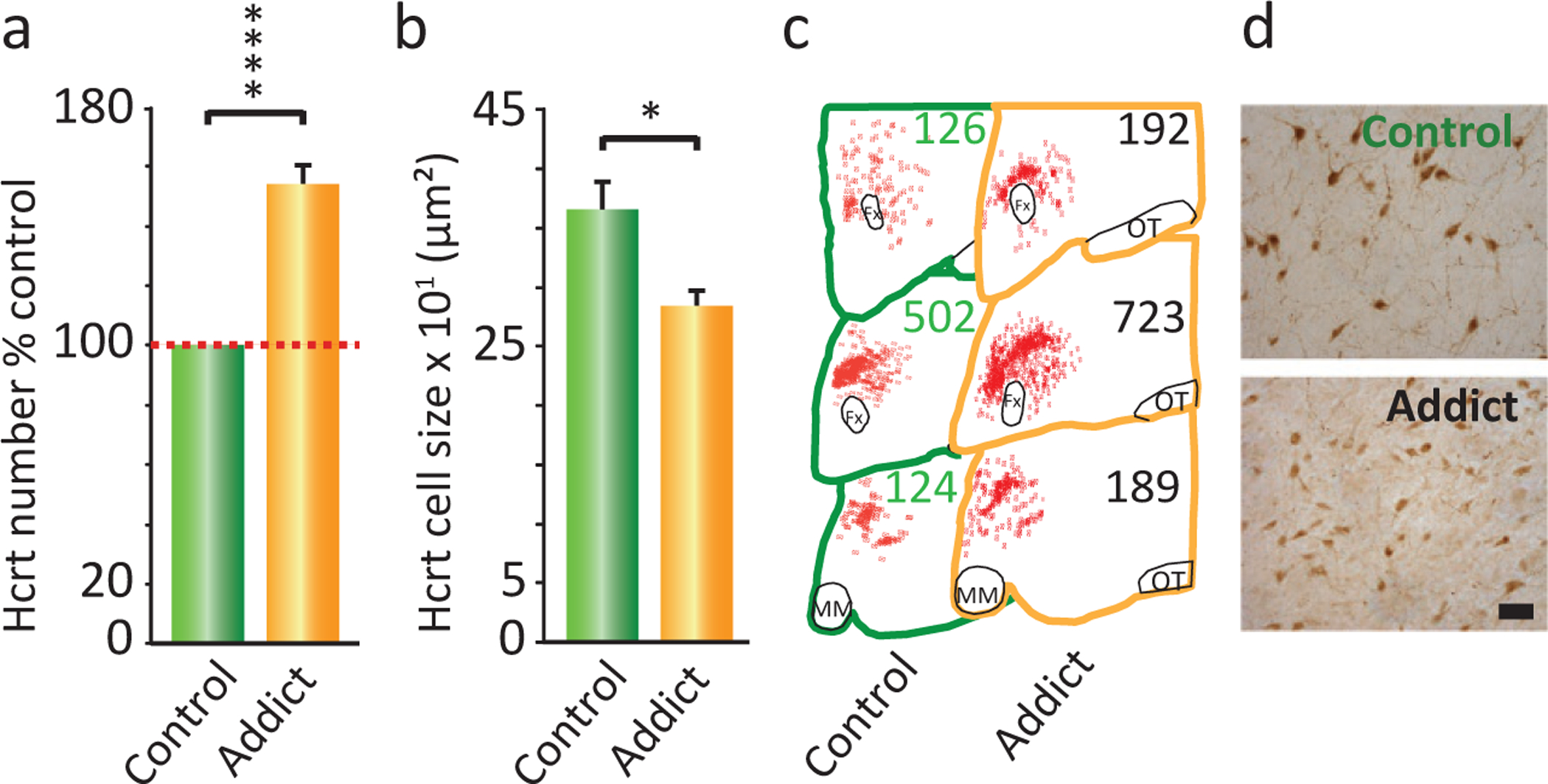
Postmortem brain tissue from heroin addicts shows an increased number of hypocretin-producing neurons. (A) Immunohistochemistry showed that there was a 54% increase in the number of detectable hypocretin neurons in hypothalamic brain tissue from human heroin addicts (n = 5) relative to hypothalamic tissue from human control subjects (n = 7; ****P = 0.0009, t = 8.89, df = 10, t-test). (B) Immunohistochemical staining of postmortem brain tissue showed that hypocretin cells were 22% smaller in cross-sectional area in brain tissue from heroin addicts compared to control subjects [*P < 0.01, t = 2.78, df = 10 (t-test)]. (C) Neurolucida mapping illustrates the distribution and increased number of hypocretin cells in brain tissue from heroin addicts relative to control subjects. Representative counts are given at three anterior–posterior positions: OT, optic tract; MM, mamillary bodies; Fx, fornix. (D) A representative example of immunohistochemical labeling of hypocretin cells in brain tissue from control individuals and heroin addicts is shown. Hcrt neurons are smaller and more numerous in the addicts. Scale bar, 50μm. Redrawn from Thannickal TC, John J, Shan L, Swaab DF, Wu M-F, Ramanathan L, McGregor R, Chew K-T, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM (2018). Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10: p. pii: eaao4953. doi: 10.1126/scitranslmed.aao4953.
Changes in the Hcrt system produced by opioids
Morphine had to be given for at least 2 weeks to produce a significant change in the number of Hcrt cells in mice, whereas cell size reduction was seen as soon as 72 h after subcutaneous implant of a morphine tablet (Thannickal et al., 2018). These changes in Hcrt neuron number and size after morphine were accompanied by an increased expression of preprohypocretin mRNA (Fig. 22.8). The opioid antagonist naltrexone (Narayanan et al., 2004; Skoubis et al., 2005; Shoblock and Maidment, 2006, 2007) given alone on the same dose schedule as morphine did not change the number of Hcrt neurons (data not shown) indicating that the maintenance of baseline number of Hcrt neurons does not require μ-opioid receptor activation. The increased number of Hcrt neurons persisted for at least 4 weeks after discontinuation of 14 days morphine treatment in mice, whereas the decrease in Hcrt cell size lasted for 2 weeks. Our data suggests that the increase may last much longer in human addicts than in mice. One of our addicts had 154% of the number of Hcrt neurons in control brains, even though he had not abused opioids for at least 3 years before his death (Thannickal et al., 2018). Self-administration has been shown to produce longer-lasting behavioral changes compared to passive, involuntary administration (Chen et al., 2006; McNamara et al., 2010; Picetti et al., 2012; Smith and Aston-Jones, 2012; James et al., 2013), suggesting that both species and administration differences may underlie these anatomical changes.
Fig. 22.8.
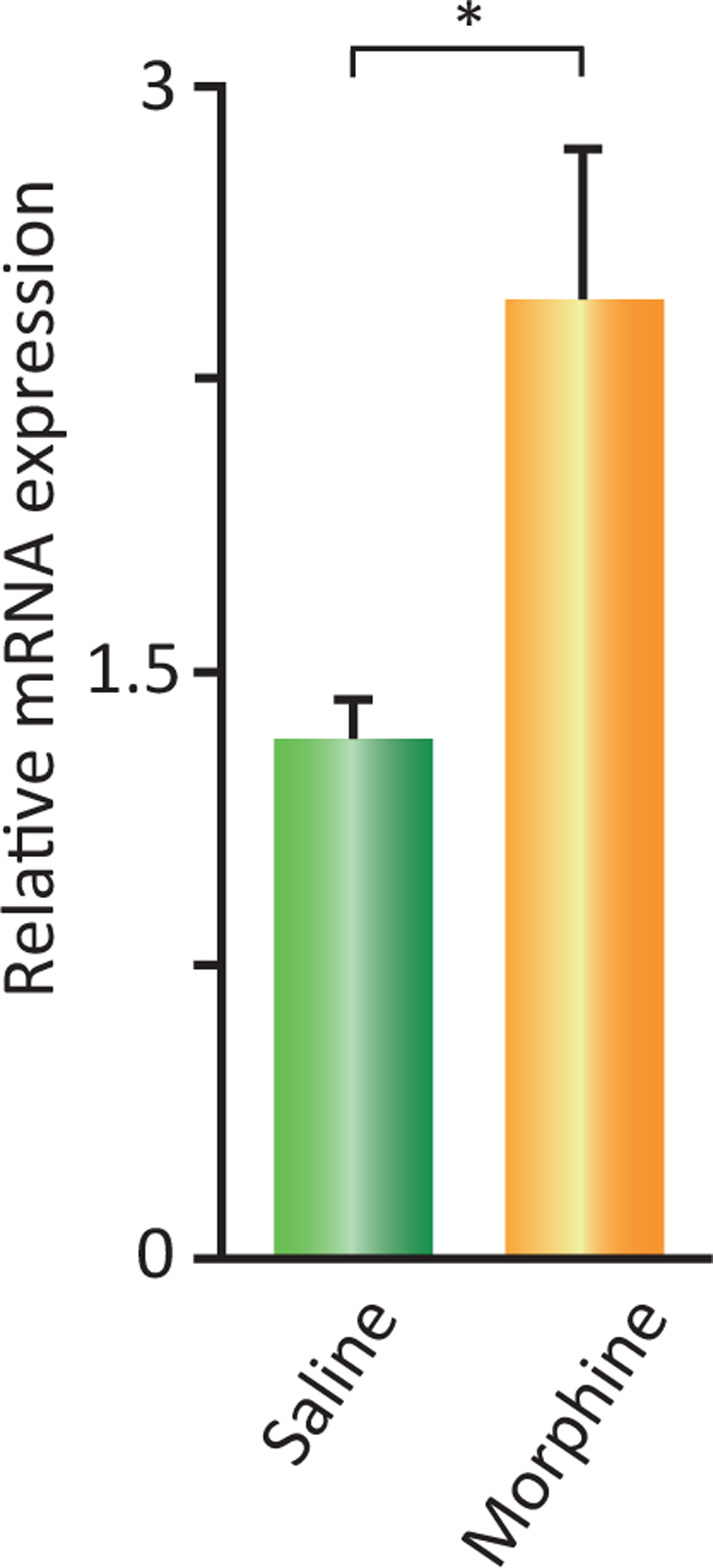
Effect of morphine administration on preprohypocretin mRNA expression in mouse brain. An escalating dose of morphine, starting at 100mg/kg, was given for 14 days to wild-type mice who were compared to saline injected littermates (*P < 0.05, t = 2.99, df = 5, t-test). Redrawn from Thannickal TC, John J, Shan L, Swaab DF, Wu M-F, Ramanathan L, McGregor R, Chew K-T, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM (2018). Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10: eaao4953. doi: 10.1126/scitranslmed.aao4953.
MORPHINE DOES NOT PRODUCE “NEW” HCRT NEURONS
We determined that the increase in the number of detected Hcrt cells was not due to neurogenesis. Both BrdU and doublecortin labeling indicated that no new neurons were produced by morphine (see fig. 4 in (Thannickal et al., 2018)). In a further study, we explored the issue of where the “newly visible” Hcrt neurons are coming from, by giving colchicine to drug naïve mice. Colchicine blocks axonal transport, thereby causing peptide to accumulate in the cell body. We found that this manipulation increased the number of “detectable” Hcrt cells in mice by about 44% (Fig. 22.9A) (McGregor et al., 2017), similar to the amount of increase seen in mice after morphine, i.e., as many as 44% of the neurons capable of producing Hcrt in mice do not produce it at detectable levels under “baseline” conditions. Fig. 22.9B shows that colchicine does not have any effect on the number of melanin-concentrating hormone neurons, a peptide of similar size, whose neurons are intermixed with Hcrt cells. Fig. 22.9C shows a representative hypothalamic section immunostained for Hcrt in a saline (top) and colchicine (bottom)-treated animal.
Fig. 22.9.
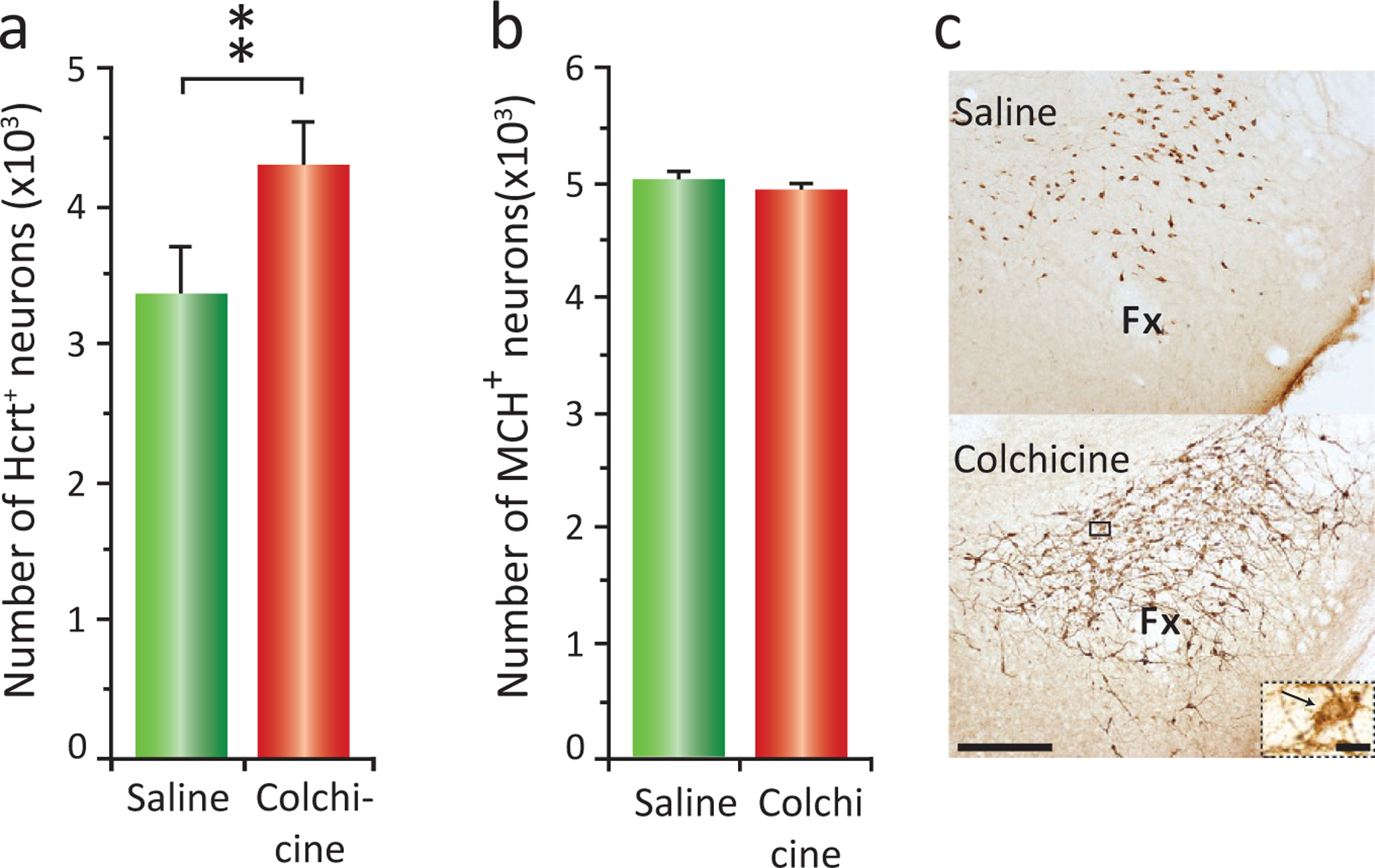
Colchicine given to mice increases the number of Hcrt neurons by 44%. Melanin-concentrating hormone (MCH) neuronal numbers remain unchanged. (A) Total cell counts for saline and colchicine treated subjects showing a 44% increase in the number of detected Hcrt neurons after colchicine (**P < 0.02, t-test). (B) Average number of MCH neurons in animals with ICV saline vs ICV colchicine injections. Number of MCH neurons remains unchanged. (C) Photomicrographs of the same hypothalamic area immunostained for Hcrt of an animal treated with saline (top) or colchicine (bottom). Calibration bar 250 μm. Inset corresponds to a higher (×60) magnification of the selected area (black square) of the animal that received colchicine. Black arrow indicates an Hcrt neuron. Calibration bar 10 μm, Fx, fornix; LH, lateral hypothalamus; MH, medial hypothalamus; PFA, perifornical area. Redrawn from McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM (2011). Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci 31: 15455–15467.
INSOMNIA IS A MAJOR CAUSE OF OPIOID WITHDRAWAL SYMPTOMS, LEADING TO RELAPSE
Increased nocturnal wakefulness is a well-documented effect of opioid withdrawal. Despite progress in treating opioid dependence, sleep disturbance remains an almost universal complaint among withdrawing opioid addicts, persisting for more than 6 weeks and playing a major role in relapse. Longer sleep time is a predictor of increased treatment compliance and better treatment outcome (Gossop and Bradley, 1984; Beswick et al., 2003; Lofwall et al., 2013; Lin et al., 2014). Postaddiction insomnia may be mediated, to some extent, by the increased number of Hcrt-producing neurons, just as the inability to maintain waking in human narcoleptics is linked to decreased Hcrt receptor activation (Peyron et al., 2000; Thannickal et al., 2000a,b; Sharf et al., 2010; Tabaeizadeh et al., 2013; Zarepour et al., 2014; Bali et al., 2015; Bentzley and Aston-Jones, 2015; Guo et al., 2016; Sadeghi et al., 2016; Sadeghzadeh et al., 2016; Alizamini et al., 2017; Farahimanesh et al., 2017; Assar et al., 2019; Azizbeigi and Haghparast, 2019; Azizbeigi et al., 2019; Farzinpour et al., 2019; Pourhamzeh et al., 2019; Shirazy et al., 2020; Zarrabian et al., 2020).
CONCLUSION
The loss of Hcrt neurons causes human narcolepsy. In animal models a clear linkage between the Hcrt system and working for positive reinforcement has been shown. In contrast, Hcrt activity is not strongly altered by working to avoid aversive conditions. A strong circadian modulation of Hcrt function has been shown in both animals and humans. In a human microdialysis study, release was shown to be correlated with pleasurable activities (Blouin et al., 2013). Changes in Hcrt function have been linked to depression (Lu et al., 2017; Thannickal et al., 2018). We found a large increase in the number of Hcrt-producing neurons in human heroin addicts and in mice chronically administered morphine (Thannickal et al., 2018). James et al. reported a nearly identical increase in the number of Hcrt-labeled neurons after chronic cocaine administration in rats (James et al., 2019), suggesting that the increase in Hcrt number may be a correlate of other chemical use disorders. Examining changes in Hcrt anatomy and physiology may shed light on a wide range of behavioral disorders.
Researchers have typically characterized Hcrt neurons as a key part of a waking system. The work reviewed previously suggests that this is an oversimplification. Rather Hcrt activity is linked to particular types of waking behavior. In prior work it has been shown that neurons in the classic brainstem “waking arousal” systems are in fact related to very specific movements that occur in waking rather than relating simply to the waking state (Siegel and McGinty, 1976, 1977; Siegel, 1979; Siegel et al., 1979, 1980, 1983; Siegel and Tomaszewski, 1983). The work on Hcrt neurons suggests that other waking or sleep-related neurons may similarly have positive or negative emotional or behavioral roles. Understanding the behavioral roles of these neuronal groups is critical to understanding the waking state itself.
ACKNOWLEDGMENTS
Supported by RO1 HL148574 and DA034748. Dr. Siegel is the recipient of a Senior Research Career Scientist Award 1IK6BX005245 from the Department of Veterans Affairs.
References
- Alizamini MM, Farzinpour Z, Ezzatpanah S et al. (2017). Role of intra-accumbal orexin receptors in the acquisition of morphine-induced conditioned place preference in the rats. Neurosci Lett 660: 1–5. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R et al. (2003). Distinct recognition of OXl and Ox2; receptors by orexin peptides. J Pharmacol Exp Ther 305: 507. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck J (1951). Hypothalamic control of food intake in rats and cats. Yale J Biol Med 24: 123–140. [PMC free article] [PubMed] [Google Scholar]
- Anderson EM, Wissman AM, Chemplanikal J et al. (2017). BDNF-Trk B controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proc Natl Acad Sci U S A 114: 9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Moorman DE, Becker HC (2018). Contribution of dynorphin and orexin neuropeptide systems to the motivational effects of alcohol. Handb Exp Pharmacol 248: 473–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assar N, Mahmoudi D, Mousavi Z et al. (2019). Role of orexin-1 and −2 receptors within the nucleus accumbens in the acquisition of sensitization to morphine in rats. Behav Brain Res 373: 112090. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC et al. (2010). Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res 1314: 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizbeigi R, Haghparast A (2019). Involvement of orexin-2 receptor in the ventral tegmental area in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Neurosci Lett 696: 121–126. 10.1016/j.neulet.2018.12.029. Epub; %2018 Dec %20. [DOI] [PubMed] [Google Scholar]
- Azizbeigi R, Farzinpour Z, Haghparast A (2019). Role of Orexin-1 receptor within the ventral tegmental area in mediating stress- and morphine priming-induced reinstatement of conditioned place preference in rats. Basic Clin Neurosci 10: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC et al. (2015). Orexin/hypocretin role in reward: Implications for opioid and other addictions. Br J Pharmacol 172: 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali A, Randhawa PK, Jaggi AS (2015). Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci Biobehav Rev 51: 138–150. 10.1016/j.neubiorev.2014.12.018. Epub;%2015 Jan 27. [DOI] [PubMed] [Google Scholar]
- Barateau L, Jaussent I, Lopez R et al. (2016). Smoking, alcohol, drug use, abuse and dependence in narcolepsy and idiopathic hypersomnia: a case-control study. Sleep 39: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard S, Dauvilliers YA (2013). Reward-based behaviors and emotional processing in human with narcolepsy-cataplexy. Front Behav Neurosci 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ (1992). Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci 12: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ (1993). Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem 61: 1766–1773. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G (2015). Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41: 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick T, Best D, Bearn J et al. (2003). The effectiveness of combined naloxone/lofexidine in opiate detoxification: results from a double-blind randomized and placebo-controlled Trial. Am J Addict 12: 295–305. [PubMed] [Google Scholar]
- Bhagwandin A, Gravett N, Hemingway J et al. (2011). Orexinergic neuron numbers in three species of African mole rats with rhythmic and arrhythmic chronotypes. Neuroscience 199: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Thannickal TC, Worley PF et al. (2005). Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology 65: 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, Fried I, Wilson CL et al. (2013). Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Commun 4: 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS et al. (2009). Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29: 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, De Lecea L (2008). Addiction and arousal: the hypocretin connection. Physiol Behav 93: 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Guilleminault C (2011). A review of sodium oxybate and baclofen in the treatment of sleep disorders. Curr Pharm Des 17: 1430–1435. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A et al. (2007). Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol 17: 573–579. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Traskman-Bendz L et al. (2009). Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J Affect Disord 113: 179–182. [DOI] [PubMed] [Google Scholar]
- C.D.C (2017). “search “C.D.C. drug overdose epidemic””, Centers for Disease Control and Prevention. [Google Scholar]
- Cai NS, Quiroz C, Bonaventura J et al. (2019). Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids. J Clin Invest 129: 2730–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Wang JD, Saxon A et al. (2017). Causes of death and expected years of life lost among treated opioid-dependent individuals in the United States and Taiwan. Int J Drug Policy 43: 1–6. 10.1016/j.drugpo.2016.12.003. Epub;%2017 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM et al. (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437–451. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH et al. (1999). Orexin A-like immunoreactivity in the rat brain. Neurosci Lett 260: 161–164. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME et al. (2006). Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31: 2692–2707. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang R, Chen X et al. (2015). Heterodimerization of human orexin receptor 1 and kappa opioid receptor promotes protein kinase A/cAMP-response element binding protein signaling via a Galphas-mediated mechanism. Cell Signal 27: 1426–1438. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J et al. (2001). Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21: RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ (2008). Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 2008/04/14: 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M et al. (2005). Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65: 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Kaye S, Duflou J et al. (2019). Completed suicide among methamphetamine users: a national study. Suicide Life Threat Behav 49: 328–337. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H et al. (1999). Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Montplaisir J, Cochen V et al. (2010). Post-H1N1 narcolepsy-cataplexy. Sleep 33: 1428–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Lopez R, Ohayon M et al. (2013). Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med 11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Siegel JM, Lopez R et al. (2014). Cataplexy: clinical aspects, pathophysiology and management strategy. Nat Rev Neurol 10: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Kilduff T, Peyron C et al. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bello F, Diamanti E, Giannella M et al. (2013). Exploring multitarget interactions to reduce opiate withdrawal syndrome and psychiatric comorbidity. ACS Med Chem Lett 4: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E, Garzon M (2014). Hypocretin1/orexinA-immunoreactive axons form few synaptic contacts on rat ventral tegmental area neurons that project to the medial prefrontal cortex. BMC Neurosci 15: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell LA, Patzke N, Bhagwandin A et al. (2012). Organization and number of orexinergic neurons in the hypothalamus of two species of Cetartiodactyla: a comparison of giraffe (Giraffa camelopardalis) and harbour porpoise (Phocoena phocoena). J Chem Neuroanat 2012/06/08: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell LA, Kruger JL, Pettigrew JD et al. (2013). Cellular location and major terminal networks of the orexinergic system in the brain of two megachiropterans. J Chem Neuroanat 53: 64–71. [DOI] [PubMed] [Google Scholar]
- Dell LA, Karlsson KA, Patzke N et al. (2016a). Organization of the sleep-related neural systems in the brain of the minke whale (Balaenoptera acutorostrata). J Comp Neurol 524: 2018–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell LA, Patzke N, Spocter MA et al. (2016b). Organization of the sleep-related neural systems in the brain of the river hippopotamus (Hippopotamus amphibius): a most unusual cetartiodactyl species. J Comp Neurol 524: 2036–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell LA, Patzke N, Spocter MA et al. (2016c). Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena). J Comp Neurol 524: 1999–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editors (2016). US drug overdose deaths: a global challenge. Lancet 387: 404–6736. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE et al. (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21: 9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahimanesh S, Zarrabian S, Haghparast A (2017). Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides 17: 10. [DOI] [PubMed] [Google Scholar]
- Farzinpour Z, Taslimi Z, Azizbeigi R et al. (2019). Involvement of orexinergic receptors in the nucleus accumbens, in the effect of forced swim stress on the reinstatement of morphine seeking behaviors. Behav Brain Res 356: 279–287. 10.1016/j.bbr.2018.08.021. Epub;%2018 Sep 5. [DOI] [PubMed] [Google Scholar]
- Fronczek R, Overeem S, Lee SY et al. (2007). Hypocretin (orexin) loss in Parkinson’s disease. Brain 130: 1577–1585. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FEJ et al. (1997). Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence [see comments]. Addiction 92: 89–96. [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M et al. (2003). Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23: 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Bradley B (1984). Insomnia among addicts during supervised withdrawal from opiates: a comparison of oral methadone and electrostimulation. Drug Alcohol Depend 13: 191–198. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Cao MT (2011). Narcolepsy: diagnosis and management. In: Kryger MH, Roth T, Dement WC (Eds.), Principles and Practice of Sleep Medicine, Fifth edn. Elsevier Saunders, Missouri, pp. 957–968. [Google Scholar]
- Guo SJ, Cui Y, Huang ZZ et al. (2016). Orexin A-mediated AKT signaling in the dentate gyrus contributes to the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Addic Biol 21: 547–559. [DOI] [PubMed] [Google Scholar]
- Hajek M, Meier-Ewert K, Wirz-Justice A et al. (1989). Bright white light does not improve narcoleptic symptoms. Eur Arch Psychiatry Neurol Sci 238: 203–207. [DOI] [PubMed] [Google Scholar]
- Han F, Lin L, Warby SC et al. (2011). Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 70: 410–417. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G (2006). Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29: 571–577. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF et al. (2007). Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Krause MR, Mainville L et al. (2016). Orexin neurons respond differentially to auditory cues associated with appetitive versus aversive outcomes. J Neurosci 36: 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Berridge KC (2013). An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Doi Y, Juji T et al. (1984). Narcolepsy and HLA: Positive DR2 as a prerequisite for the development of narcolepsy. Folia Psychiatr Neurol Jpn 38: 360. [Google Scholar]
- Horvath TL, Peyron C, Diano S et al. (1999). Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415: 145–159. [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS et al. (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 361: eaau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AS, Chen JY, Cepeda C et al. (2013). Opioid self-administration results in cell-type specific adaptations of striatal medium spiny neurons. Behav Brain Res 256: 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE et al. (2017). A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci 33: 247–281. 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA et al. (2018). Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 10.1016/j.biopsych.2018.07.022. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA et al. (2019). Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 10.1016/j.biopsych.2018.07.022. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N et al. (2002). Release of hypocretin (orexin) during waking and sleep states. J Neurosci 22: 5282–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS (2014). Orexin/hypocretin receptor signalling cascades. Br J Pharmacol 171: 314–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Lee SY, Yuan SS et al. (2016). Comorbidity of narcolepsy and depressive disorders: a nationwide population-based study in Taiwan. Sleep Med 39: 95–100. [DOI] [PubMed] [Google Scholar]
- Li Y & Van Den Pol A 2006, Dynorphin inhibits hypocretin/orexin neurons in hypothalamic brain slice”, Abstract viewer/Itinerary Planner Society for Neuroscience p. 157. 19/V5. [Google Scholar]
- Li Y, van den Pol AN (2008). Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci 28: 2814–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T et al. (2002). Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36: 1169–1181. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Kadotani H et al. (1999). The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor gene. Cell 98: 365–376. [DOI] [PubMed] [Google Scholar]
- Lin SK, Pan CH, Chen CH (2014). A double-blind, placebo-controlled trial of dextromethorphan combined with clonidine in the treatment of heroin withdrawal. J Clin Psychopharmacol 34: 508–512. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Babalonis S, Nuzzo PA et al. (2013). Efficacy of extended-release tramadol for treatment of prescription opioid withdrawal: a two-phase randomized controlled trial. Drug Alcohol Depend 133: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao J, Balesar R et al. (2017). Sexually Dimorphic Changes of Hypocretin (Orexin) in Depression. EBioMedicine 2017/03/31: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ et al. (2008). Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci 28: 3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Ayranci G, Chu-Sin-Chung P et al. (2014). Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology 39: 2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Martin-Fardon R (2018). Drug seeking and relapse: new evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol 9: 720. eCollection;%2018. 10.3389/fneur.2018.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor R, Wu M-F, Barber G et al. (2011). Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J Neurosci 31: 15455–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor R, Shan L, Wu MF et al. (2017). Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A, Lewis DC, O’Brien CP et al. (2000). Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA 284: 1689–1695. [DOI] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW et al. (2010). Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl) 212: 453–464. [DOI] [PubMed] [Google Scholar]
- Meye FJ, van Zessen R, Smidt MP et al. (2012). Morphine withdrawal enhances constitutive opioid receptor activity in the ventral tegmental area. J Neurosci 32: 16120–16128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Reid M, Tafti M et al. (1995). Local administration of dopaminergic drugs into the ventral tegmental area modulates cataplexy in narcoleptic canines. Sleep Res 24: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Lin L, Rogers W et al. (2001). Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet 68: 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005). Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46: 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL et al. (2014). Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA 111: E1648–E1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Lam H, Christian L et al. (2004). Endogenous opioids mediate basal hedonic tone independent of dopamine D-1 or D-2 receptor activation. Neuroscience 124: 241–246. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2013). Cellular basis of memory for addiction. Dialogues Clin Neurosci 15: 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ et al. (2002). Neurobiology of depression. Neuron 34: 13–25. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E (2011). Narcolepsy and cataplexy. Handb Clin Neurol 99: 783–814. [DOI] [PubMed] [Google Scholar]
- Nordstrand SEH, Hansen BH, Rootwelt T et al. (2019). Psychiatric symptoms in patients with post-H1N1 narcolepsy type 1 in Norway. Sleep zsz008. [DOI] [PubMed] [Google Scholar]
- Olateju OI, Bhagwandin A, Ihunwo AO et al. (2017). Changes in the cholinergic, catecholaminergic, orexinergic and serotonergic structures forming part of the sleep systems of adult mice exposed to intrauterine alcohol. Front Neuroanat 11: 110. eCollection;%2017. 10.3389/fnana.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostling PS, Davidson KS, Anyama BO et al. (2018). America’s opioid epidemic: a comprehensive review and look into the rising crisis. Curr Pain Headache Rep 22: 32–0685. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W et al. (2000). A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6: 991–997. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A et al. (2012). Dose escalation and dosepreference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 220: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay S, Bhagwandin A, Bertelsen MF et al. (2017). Regional distribution of cholinergic, catecholaminergic, serotonergic and orexinergic neurons in the brain of two carnivore species: the feliform banded mongoose (Mungos mungo) and the caniform domestic ferret (Mustela putorius furo). J Chem Neuroanat 82: 12–28. [DOI] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazova R et al. (2010a). Reduced amygdala activity during aversive conditioning in human narcolepsy. Ann Neurol 67: 394–398. [DOI] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazova R et al. (2010b). Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann Neurol 67: 190–200. [DOI] [PubMed] [Google Scholar]
- Pourhamzeh M, Mozafari R, Jamali S et al. (2019). Involvement of orexin receptors within the hippocampal dentate gyrus in morphine-induced reinstatement in food-deprived rats. Behav Brain Res 375: 112155. 10.1016/j.bbr.2019.112155. Epub;%2019 Aug 15. [DOI] [PubMed] [Google Scholar]
- Sadeghi B, Ezzatpanah S, Haghparast A (2016). Effects of dorsal hippocampal orexin-2 receptor antagonism on the acquisition, expression, and extinction of morphine-induced place preference in rats. Psychopharmacology (Berl) 233: 2329–2341. [DOI] [PubMed] [Google Scholar]
- Sadeghzadeh F, Namvar P, Naghavi FS et al. (2016). Differential effects of intra-accumbal orexin-1 and −2 receptor antagonists on the expression and extinction of morphine-induced conditioned place preference in rats. Pharmacol Biochem Behav 142: 8–14. 10.1016/j.pbb.2015.12.005. Epub;%2015 Dec 17. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585. [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland SL, Kharazia VN et al. (2002). Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur J Neurosci 26: 749–756. [DOI] [PubMed] [Google Scholar]
- Scammell TE (2006). The frustrating and mostly fruitless search for an autoimmune cause of narcolepsy. Sleep 29: 601–660. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH et al. (1998). Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience 85: 1005–1009. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Ponz A, Poryazova R et al. (2007). Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain 131: 514–522. [DOI] [PubMed] [Google Scholar]
- Schwarzer C (2009). 30 years of dynorphins–new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 2009/05/28: 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR et al. (2010). Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res 1317: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Li Sx, Zhang Xl et al. (2009). Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse 35: 267–272. [DOI] [PubMed] [Google Scholar]
- Shirazy M, RayatSanati K, Jamali S et al. (2020). Role of orexinergic receptors in the dentate gyrus of the hippocampus in the acquisition and expression of morphine-induced conditioned place preference in rats. Behav Brain Res 379: 112349. 10.1016/j.bbr.2019.112349. Epub; %2019 Nov 9. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT (2006). Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology 31: 171–177. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT (2007). Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience 149: 642–649. [DOI] [PubMed] [Google Scholar]
- Siegel JM (1979). Behavioral functions of the reticular formation. Brain Res Rev 1: 69–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM (2004). Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol 55: 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, McGinty DJ (1976). Brainstem neurons without spontaneous unit discharge. Science 193: 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, McGinty DJ (1977). Pontine reticular formation neurons: relationship of discharge to motor activity. Science 196: 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS (1983). Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol 50: 696–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, McGinty DJ (1979). Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res 179: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, Breedlove SM et al. (1980). Brainstem units related to movements of the pinna. Brain Res 202: 183–188. [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Wheeler RL (1983). Behavioral organization of reticular formation: Studies in the unrestrained cat: II. Cells related to facial movements. J Neurophysiol 50: 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Gulyani S et al. (1999). Neuronal degeneration in canine narcoleps. J Neurosci 19: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Moore R, Thannickal T et al. (2001). A brief history of hypocretin/orexin and narcolepsy. Neuropsychopharmacology 25: S14–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Bisaga A, Nunes EV et al. (2012). Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse 38: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Cassidy MP, Sparta A et al. (2011). Effect of DeltaFosB overexpression on opioid and cannabinoid receptor-mediated signaling in the nucleus accumbens. Neuropharmacology 61: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J et al. (2005). Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci 21: 1379–1384. [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ et al. (1999). Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol 128: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G (2012). Orexin-hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci 35: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Kream RM (2007). Endogenous morphine synthetic pathway preceded and gave rise to catecholamine synthesis in evolution (Review). Int J Mol Med 20: 837–841. [PubMed] [Google Scholar]
- Stoeber M, Jullie D, Lobingier BT et al. (2018). A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98: 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaeizadeh M, Motiei-Langroudi R, Mirbaha H et al. (2013). The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naive versus morphine-dependent mice. Behav Brain Res 237: 41–48. 10.1016/j.bbr.2012.09.010.Epub;%2012 Sep 17. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein AN (1962). The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69: 74–90. 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Aldrich M et al. (2000a). Human narcolepsy is linked to reduced number, size and synaptic bouton density in hypocretin-2 labeled neurons. Abstr Soc Neurosci 26: 2061. [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R et al. (2000b). Reduced number of hypocretin neurons in human narcolepsy. Neuron 27: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Siegel JM, Moore RY (2003). Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 13: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM (2007). Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130: 1586–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM (2008). Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson’s disease. Brain 131: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, John J, Shan L et al. (2018). Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10: eaao4953. 10.1126/scitranslmed.aao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB (2003). Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119: 1033–1044. [DOI] [PubMed] [Google Scholar]
- Turner C, Chandrakumar D, Rowe C et al. (2018). Cross-sectional cause of death comparisons for stimulant and opioid mortality in San Francisco, 2005. Drug Alcohol Depend 185: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Volkow ND (2020). Drugs, sleep, and the addicted brain. Neuropsychopharmacology 45: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN (1999). Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 19: 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Woodcock J, Compton WM et al. (2018). Medication development in opioid addiction: meaningful clinical end points. Sci Transl Med 10: eaan2595. [DOI] [PubMed] [Google Scholar]
- Wu MF, Nienhuis R, Maidment N et al. (2011a). Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch Ital Biol 149: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Nienhuis R, Maidment N et al. (2011b). Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J Neurosci 31: 6305–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Tabuchi S, Tsunematsu T et al. (2010). Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci 30: 12642–12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarepour L, Fatahi Z, Sarihi A et al. (2014). Blockade of orexin-1 receptors in the ventral tegmental area could attenuate the lateral hypothalamic stimulation-induced potentiation of rewarding properties of morphine. Neuropeptides 48: 179–185. [DOI] [PubMed] [Google Scholar]
- Zarrabian S, Riahi E, Karimi S et al. (2020). The potential role of the orexin reward system in future treatments for opioid drug abuse. Brain Res 1731: 146028. 10.1016/j.brainres.2018.11.023. Epub;%2018 Nov 23., p. 146028. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wienecke CF, Nachtrab G et al. (2016). A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]


