Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The Spike protein that mediates coronavirus entry into host cells is a major target for COVID-19 vaccines and antibody therapeutics. However, multiple variants of SARS-CoV-2 have emerged, which may potentially compromise vaccine effectiveness. Using a pseudovirus-based assay, we evaluated SARS-CoV-2 cell entry mediated by the viral Spike B.1.617 and B.1.1.7 variants. We also compared the neutralization ability of monoclonal antibodies from convalescent sera and neutralizing antibodies (NAbs) elicited by CoronaVac (inactivated vaccine) and ZF2001 (RBD-subunit vaccine) against B.1.617 and B.1.1.7 variants. Our results showed that, compared to D614G and B.1.1.7 variants, B.1.617 shows enhanced viral entry and membrane fusion, as well as more resistant to antibody neutralization. These findings have important implications for understanding viral infectivity and for immunization policy against SARS-CoV-2 variants.
Keywords: Coronavirus, Immune escape, Mutation, Neutralizing antibodies, SARS-CoV-2, Vaccine, Viral entry
Introduction
The novel coronavirus reported in 2019 (2019-nCoV), officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a new type of coronavirus belonging to the genus Betacoronavirus. It is a single-stranded RNA virus with a genome of approximately 29 Kb and with a high pathogenicity and high infectivity. As of July 8, 2021, there were more than 185 million confirmed cases of coronavirus disease 2019 (COVID-19) globally, including more than 4 million confirmed deaths (https://coronavirus.jhu.edu/). As SARS-CoV-2 continues to circulate in the human population, multiple mutations accumulate over time despite its proofreading capacity.1 The Spike glycoprotein mutation D614G became dominant in SARS-CoV-2 during the early pandemic, which displayed increased infectivity and transmission.2
Spike-specific antibodies elicited by natural infection or vaccination contribute the majority of the neutralizing activity in human sera.3 The receptor binding domain (RBD) in the S1 subunit of Spike protein binds to its cellular receptor angiotensin-converting enzyme 2 (ACE2) during viral entry, while the S2 subunit is required for the subsequent fusion of viral and cellular membranes.1 Therefore, RBD is believed to be a major target of neutralizing antibodies (NAbs) and has been a focus of COVID-19 vaccine design.4,5 Our previously studies showed that mutations in SARS-CoV-2 Spike protein could affect viral properties such as infectivity and neutralization resistance.6,7 The newly emerged SARS-CoV-2 variant, B.1.617, first reported from India, which carries two mutations (L452R and E484Q) in its RBD is of particular concern. The AstraZeneca ChAdOx1 nCoV-19 vaccine appeared less effective than the Pfizer-BioNTech (BNT162b2) mRNA vaccine in preventing infection of SARS-CoV-2 B.1.617 variant.8 Although mRNA-based COVID-19 vaccines provide above 90% efficacy against original SARS-CoV-2 strain, breakthrough infections with SARS-CoV-2 variants occur.9,10 However, the efficacy of inactivated and RBD-subunit vaccines against B.1.617 variant is still unknown.
In this study, we used SARS-CoV-2 pseudovirus system to compare the viral entry efficiency in vitro, as well as the neutralization activities of convalescent sera, monoclonal antibodies (mAbs) and COVID-19 vaccine-elicited sera against these newly emerging SARS-CoV-2 variants, including the highly transmissible variants B.1.1.7 and B.1.617.
Materials and methods
Cell culture
HEK 293T (ATCC CRL-3216) and A549 cells (ATCC CCL-185) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco's modified Eagle medium (DMEM; Hyclone, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA), and 1% penicillin-streptomycin at 37 °C in 5% CO2. HEK 293T cells or A549 cells transfected with human ACE2 (293T-ACE2 or A549-ACE2) were cultured under the same conditions with the addition of G418 (0.5 mg/mL) to the medium.
Sera samples
Convalescent sera samples from 20 patients with COVID-19 obtained in February and October 2020 at Yongchuan Hospital of Chongqing Medical University were previously reported.11 All sera were tested positive using magnetic chemiluminescence enzyme immunoassay (MCLIA) kits supplied by BioScience Co. (Tianjin, China).12 Patient sera were incubated at 56 °C for 30 min to inactivate the complement prior to experiments. Twenty CoronaVac vaccinee sera were obtained 14 days following the second dose of vaccine. Eight ZF2001 (RBD-subunit) vaccinee sera were obtained 26–30 days after booster immunization (second dose), and two ZF2001 vaccinee sera were obtained 14 days following the third dose of vaccine. The study was approved by the Ethics Commission of Chongqing Medical University (ref. no. 2020003). Written informed consent was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Plasmids and antibodies
The codon-optimized gene encoding reference strain (GenBank: QHD43416) SARS-CoV-2 Spike protein with C-terminal 19-amino acid deletion was synthesized by Sino Biological Inc (Beijing, China), and cloned into pCMV3 vector. D614G mutation was introduced using site-directed mutagenesis (denoted as pCMV3-S-D614G). SARS-CoV-2 B.1.617 and B.1.1.7 variant Spikes were codon-optimized and synthesized by GenScript Inc (Nanjing, China) and cloned into pCMV3 vector. The HIV-1 NL4-3 ΔEnv Vpr luciferase reporter vector (pNL4-3.Luc.R-E-) constructed by Landau13 was provided by Prof. Cheguo Cai from Wuhan University (Wuhan, China). The expression plasmid for human ACE2 was obtained from GeneCopoeia (Guangzhou, China). Anti-RBD monoclonal antibodies (mAbs) against the SARS-CoV-2 Spike protein were obtained from the blood samples of COVID-19 convalescent patients as described previously.14
Production and titration of SARS-CoV-2 pseudoviruses
SARS-CoV-2 Spike pseudotyped viruses were produced as previously described with some modifications.15,16 In brief, 5 × 106 HEK 293T cells were co-transfected with pNL4-3.Luc.R-E- and recombinant SARS-CoV-2 Spike (D614G) plasmid or its derivatives (B.1.1.7 and B.1.617) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Supernatants containing pseudotyped viruses were harvested 48 h post-transfection, centrifuged, filtered through a 0.45-μm filter, and stored at −80 °C. The titers of pseudoviruses were calculated by determining the number of viral RNA genomes per mL of viral stock solution using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) with primers targeted the LTR.17 Briefly, viral RNAs were extracted using TRIzol (Invitrogen, Rockville, MD, USA), treated with RNase-free DNase (Promega, Madison, WI, USA) and re-purified using mini columns. Then, the RNA was amplified using the TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems, Thermo Fisher). A known quantity of pNL4-3.Luc.R-E- vector was used to generate standard curves. The prepared pseudovirus was adjusted to the same titer (copies/mL) for the following experiments.
SARS-CoV-2 Spike-mediated pseudoviral entry assay
To detect Spike variant-mediated viral entry, 293T-ACE2 and A549-ACE2 cells (1.5 × 104) grown on 96-well plates were infected with 50 μL pseudoviruses (1 × 104 copies). The cells were transferred to fresh DMEM medium 8 h post-infection, and RLU was measured 72 h post-infection using Luciferase Assay Reagent (Promega, Madison, WI, USA) according to the manufacturer's protocol.18
Cell–cell fusion assays
Syncytia formation assays were carried out as previously described with some modifications.19 Briefly, plasmid pAdTrack-TO4-S, encoding SARS-CoV-2 Spike protein and enhanced green fluorescent protein (eGFP), was transfected into HEK 293T cells using Lipofectamine 3000 (Invitrogen). In parallel, another group of HEK 293T cells was transfected with hACE2 expressing plasmids. Two groups of cells were resuspended 24 h post-transfection, mixed at 1:1 ratio, and co-cultured in DMEM medium containing 10% FBS, at 37 °C with 5% CO2, for 24 h, then observed the fusion under the fluorescence microscope. The fusion rate was the ratio of fluorescence fusion areas/total cell areas under the white light measured by Image Pro-Plus (Media Cybernetics, USA).
Western blot
To analyze Spike protein expression in cells, D614G, B.1.1.7, and B.1.617 variant Spike expressing plasmids were transfected into HEK 293T cells. Total protein was extracted from cells using radio immunoprecipitation assay Lysis Buffer (Beyotime, Shanghai, China) containing 1 mM phenylmethylsulfonyl fluoride (Beyotime). Equal amounts of protein samples were electrophoretically separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The immunoblots were probed with the indicated antibodies. Protein bands were visualized using SuperSignal West Pico Chemiluminescent Substrate kits (Bio-Rad, Hercules, CA, USA) and quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Pseudovirus-based neutralization assay
The 293T-ACE2 cells (1.5 × 104 cells/well) were seeded on 96-well plates. For the neutralization assay, equivalent pseudoviruses (1 × 104 copies in 50 μL) were incubated with serial dilutions of sera samples or mAbs for 1 h at 37 °C, then added to the 293T-ACE2 cells (with three replicates for each dilution). Luciferase activity was measured 72 h post infection. The titers of neutralizing antibodies were calculated as 50% inhibitory dose (ID50), the half-maximal inhibitory concentrations (IC50) of monoclonal antibodies (mAbs) against pseudoviruses was calculated using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA).
Statistical analyses
Statistical analyses of the data were performed using GraphPad Prism version 8.0 software. Quantitative data in histograms are shown as means ± SD. Statistical significance was determined using ANOVA for multiple comparisons. Student's t-tests were applied to compare the two groups. Differences with P values < 0.05 were deemed statistically significant.
Results
B.1.617 variant Spike promotes viral entry and membrane fusion
Phylogenetic analysis showed that the newly emerged SARS-CoV-2 B.1.617 variant bearing common signature mutations G142D, L452R, E484Q, D614G and P681R, in its Spike glycoprotein (Fig. 1A). To assess the impact of these mutations on viral entry, synthetic codon-optimized B.1.617 and B.1.1.7 variant Spikes were cloned into mammalian expression vector respectively. Next, we generated pseudotyped SARS-CoV-2 using a lentiviral system, which introduced a Luc (luciferase) reporter gene for quantification of Spike-mediated viral entry. Thereafter, pNL4-3.Luc.R-E- was co-transfected with pS-D614G, pS-B.1.1.7 and pS-B.1.617 to package the Spike pseudotyped single-round Luc virus in HEK 293T cells. The titers of pseudoviruses were determined by RT-qPCR expressed as the number of viral RNA genomes per mL, and then adjusted to the same concentration (1 × 104 copies in 50 μL) for the following experiments.
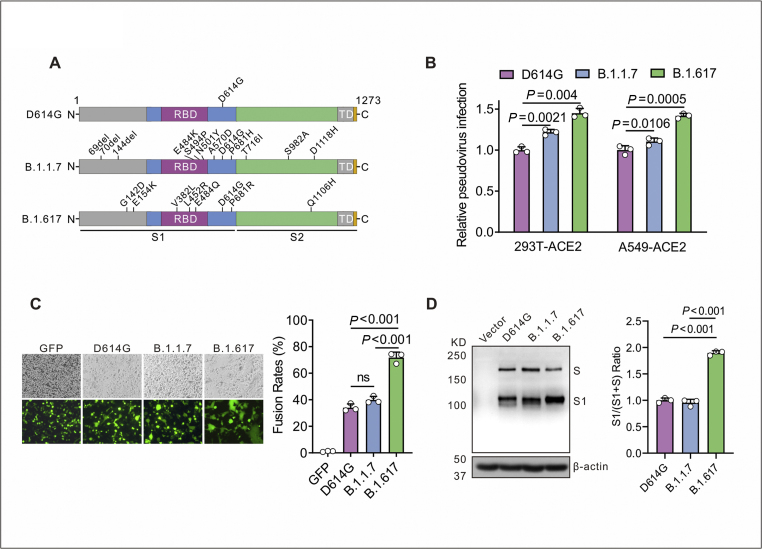
Figure 1.
B.1.617 variant Spike protein of SARS-CoV-2 drives efficient viral entry and cell–cell fusion. (A) The diagram of SARS-CoV-2 Spike protein from D614G, B.1.1.7 and B.1.617 variants. D614G variant pseudovirus (containing the D614G mutation in Spike); B.1.1.7 variant pseudovirus (containing the H69/V70 and Y144 deletions and N501Y, A570D, D614G, P681H, T716I, S982A, and D1118H mutations in Spike); B.1.617 variant pseudovirus (containing the G142D, E154K, V382L, L452R, E484Q, D614G, P681R, and Q1106H mutations in Spike). (B) Infectivity of D614G, B.1.1.7 and B.1.617 variants pseudoviruses was assessed in 293T-ACE2 and A549-ACE2 cells. Cells were inoculated with equivalent doses of each pseudotyped virus, and the supernatants were replaced at 6 h post inoculation with fresh culture. Upon 72 h, cells were lysed with passive lysis buffer and the activity of firefly luciferase was analyzed. (C) Quantitative cell–cell fusion assay. HEK293T cells expressing SARS-CoV-2 Spike variants D614G, B.1.1.7 and B.1.617 were mixed with ACE2-expressing target HEK293T cells (ratio 1: 1), and cell–cell fusion was analyzed by measuring the presence of syncytia by fluorescence microscopy. The fusion rate was the ratio of fluorescence fusion areas/total cell areas under the white light measured by Image Pro-Plus, n = 3. (D) Detection of Spike protein expression of D614G, B.1.1.7 and B.1.617 in HEK 293T cells by Western blot using the anti-RBD (receptor-binding domain) monoclonal antibody. To compare the S1 and S ratio, integrated density of S1/(S + S1) was quantitatively analyzed using ImageJ software, n = 3.
The virus infectivity was determined by a Luc assay as previously described.16 As shown in Figure 1B, to compare the viral entry efficiency meditated by Spike variants, we detected the Luc activity. The B.1.1.7 variant showed a slight increase in viral transduction over the D614G variant was 1.22-fold and 1.17-fold, while the B.1.617 variant over the D614G variant was 1.45-fold and 1.4-fold at 72 h post-infection in 293T-ACE2 and A549-ACE2 cells, respectively. These data suggest that the B.1.617 variant Spike protein significantly promotes viral entry into ACE2-expressing cells.
Next, we investigated Spike protein meditated cell–cell fusion. Coronavirus Spike protein on plasma membrane of effector cells can triggered its fusion of target cells (ACE2-expressing cells). B.1.617 variant Spike protein significantly increased fusion efficacy (2.1-fold or 1.8-fold) compared to D614G or B1.1.7 variant (Fig. 1C). To evaluate the expression and cleavage of SARS-CoV-2 Spike protein in a human cell line, Spike-expressing plasmids (D614G, B.1.1.7 and B.1.617) were transfected into HEK 293T cells. The immunoblot analysis revealed that D614G, B.1.1.7 and B.1.617 Spike proteins showed two major protein bands (unprocessed S and cleaved S1 subunit), when allowed to react with the monoclonal antibody targeting the RBD on the SARS-CoV-2 Spike protein (Fig. 1D). However, the B.1.617-transfected cells showed a stronger S1 signal than D614G-transfected cells, indicating that the B.1.617 variant altered the cleavability of the Spike protein by cellular proteases. Collectedly, our data suggest that Spike protein of B.1.617 variant enhanced viral entry into ACE2-expressing cells and membrane fusion process, which may contribute to SARS-CoV-2 infectivity.
Reduced neutralization by COVID-19 convalescent sera
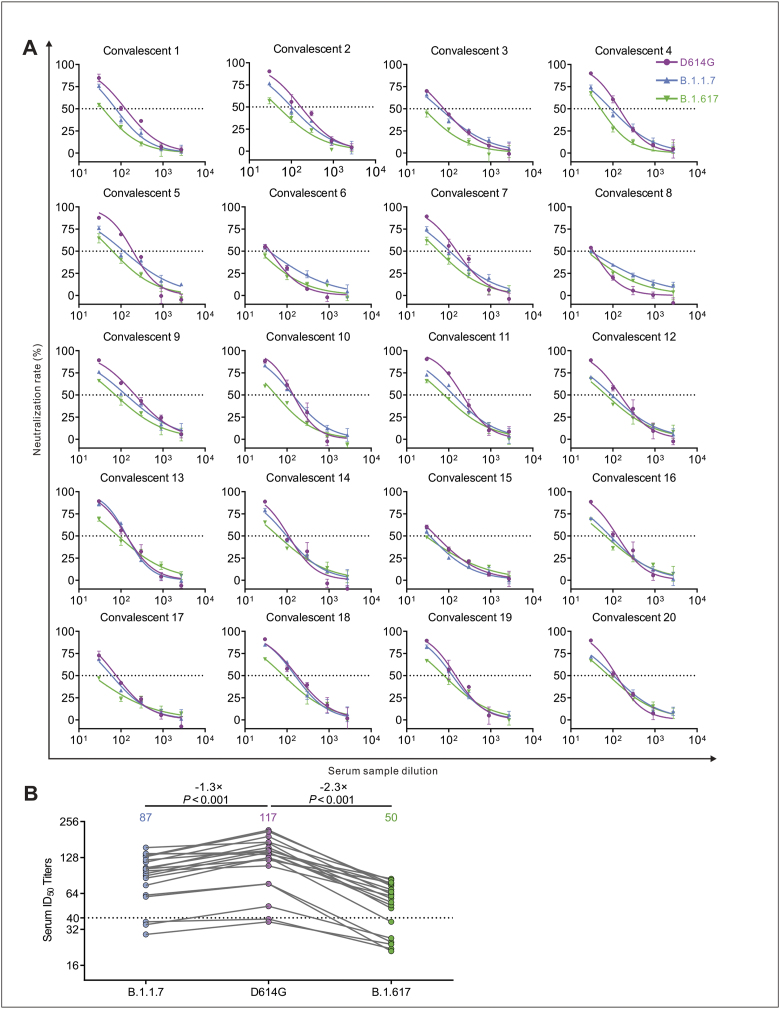
The serum samples of 20 patients with COVID-19 obtained in February and October 2020 in Chongqing were previously reported.11 Using a luciferase-expressing lentiviral pseudotyping system, geometric mean titers (GMTs) of sera were calculated to assess the neutralizing efficacy. The neutralizing activity of 5 samples against B.1.617 variant was reduced by >3-fold compared to D614G (Fig. 2A). Notably, the ID50 titer of 6 samples (30%) was lower than the threshold against B.1.617 (Fig. 2A). Compared with D614G, the ID50 titers of 3 samples (15%) and 6 samples (30%) decreased below the threshold against B.1.1.7 and B.1.617, respectively. The GMTs were 117 for D614G, 87 for B.1.1.7, and 50 for B.1.617 (Fig. 2B). Compared with D614G, the neutralization effects against B.1.617 variant was reduced by 2.3-fold. These data indicate that B.1.1.7 and B.1.617 variants escape from neutralizing antibodies from some COVID-19 convalescent sera. Particularly, B.1.617 variant is less sensitive to the sera neutralization compared to the D614G.
Figure 2.
Neutralization efficiency of convalescent sera against D614G, B.1.1.7 and B.1.617 pseudotyped viruses. (A) Neutralizing activity of convalescent plasma (n = 20) to D614G, B.1.1.7 and B.1.617 variants. Pseudotypes were incubated with different serum dilutions for 60 min at 37 °C, and then were added to the 293T-ACE2 cells. Upon 72 h, cells were lysed with passive lysis buffer and the activity of firefly luciferase was analyzed. The half-maximal neutralizing titer (ID50) was quantitatively analyzed using Graphpad 8.0 and the threshold of ID50 was 1:40. (B) Changes in convalescent sera ID50 against D614G, B.1.1.7 and B.1.617 and the GMT values.
Resistance against monoclonal antibodies targeting the RBD
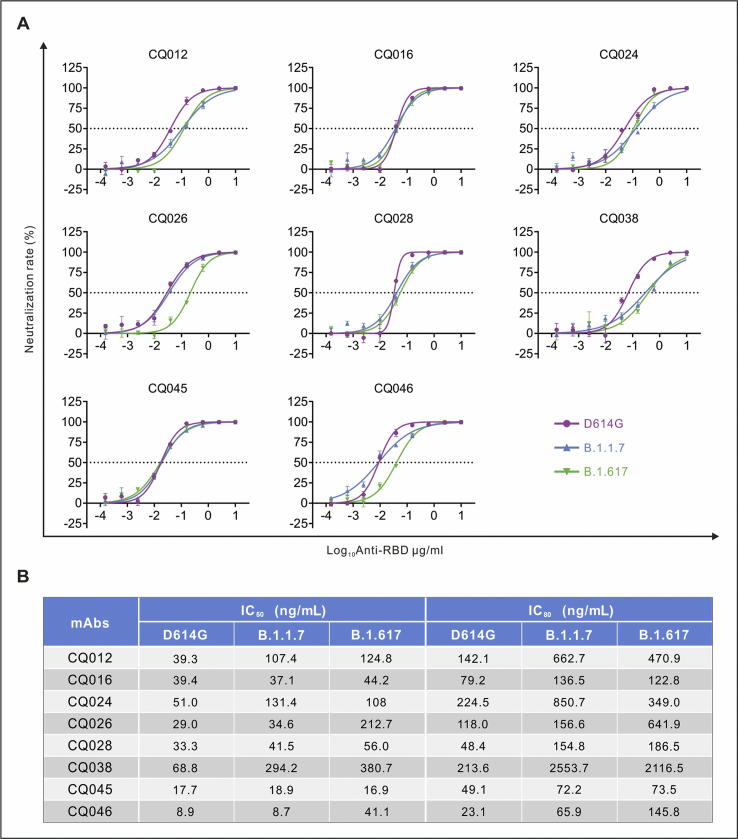
In addition, we assessed the impact of these variants on neutralizing activity of human monoclonal antibodies (mAbs) isolated from COVID-19 convalescent patients. Eight RBD-specific mAbs potently neutralizing SARS-CoV-2 obtained from the blood samples of COVID-19 convalescent patients were selected for this study.14 Among them, three mAbs showed less effective against B.1.1.7, and five against B.1.617 by 3 folds or more (Fig. 3A). Notably, the B.1.1.7 reduced the neutralization sensitivity with three mAbs (CQ012, CQ024 and CQ038) by 2 folds, and B.1.617 reduced the neutralization sensitivity with four mAbs (CQ012, CQ026, CQ038 and CQ046) by 3 folds against D614G pseudovirus. Moreover, IC50 of mAb CQ046 decreased from 8.9 ng/mL (D614G) to 41.1 ng/mL, and IC80 decreased from 23.1 ng/mL (D614G) to 145.8 ng/mL (B.1.617) (Fig. 3B). The B.1.617 variant reduced the neutralization sensitivity with the most potent mAb CQ046 by 4.6 folds, compared with that of D614G, whereas B.1.1.7 did not (Fig. 3B). Together, both B.1.1.7 and B.1.617 reduced neutralization sensitivity to most mAbs tested. Moreover, B.1.617 variant is more resistance to some mAbs targeting the RBD than D614G and B.1.1.7 variants.
Figure 3.
The RBD specific monoclonal antibodies (mAbs) against pseudoviruses. (A) The half-maximal inhibitory concentrations (IC50) representative neutralization curves for tested anti-RBD (receptor-binding domain) monoclonal antibodies (mAbs) against D614G, B.1.1.7 and B.1.617 pseudoviruses. Pseudotypes were incubated with different mAbs dilutions for 60 min at 37 °C, and then were incubated onto 293T-ACE2 cells. Upon 72 h, cells were lysed with passive lysis buffer and the activity of firefly luciferase was analyzed. (B) The IC50 and IC80 were quantitatively analyzed using Graphpad 8.0.
B.1.617 variant reduces sensitivity to vaccine-elicited antibodies
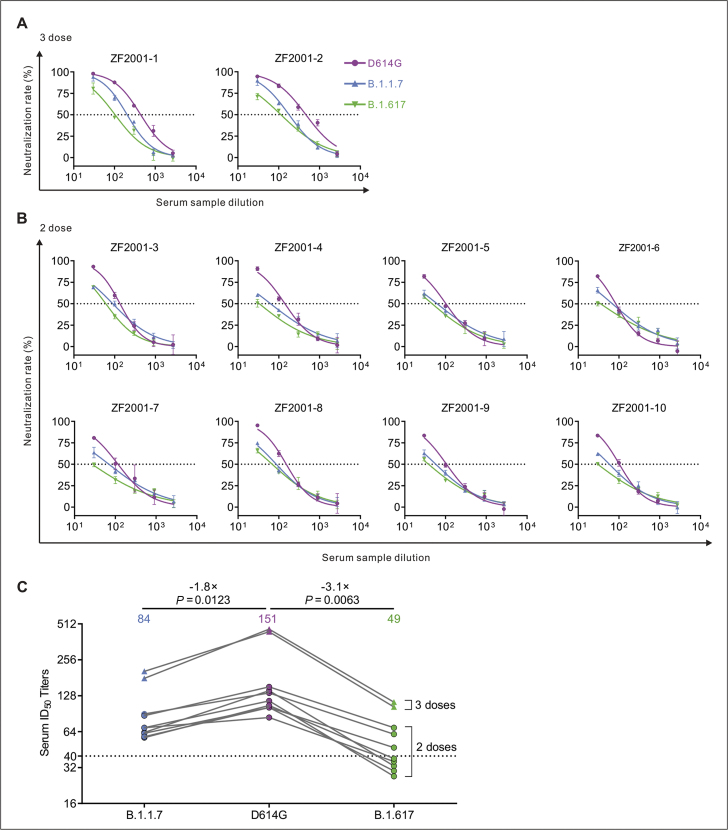
Next, we compared the neutralization potency of COVID-19 vaccine-elicited antibodies against D614G, B.1.1.7 and B.1.617 Spike pseudotyped viruses. We collected serum from twenty individuals who received two doses of CoronaVac (inactivated vaccine) and eight individuals who received two doses of ZF2001 (RBD-subunit vaccine) vaccine, and two individuals who received three doses of ZF2001. The individuals who received three doses of ZF2001 (>14 days out from third dose) had robust neutralization of SARS-CoV-2 Spike D614G, while those who received only two doses had lower but detectable neutralization (Fig. 4A, B). The GMTs of ZF2001-elicited serum against the D614G, B.1.1.7 and B.1.617 were 151, 84 and 49, respectively (Fig. 4C). And the neutralization activities against B.1.617 were reduced by 3.1 folds in ten ZF2001 vaccinee sera compared to D614G (Fig. 4C). Notably, ID50 of five samples against B.1.617 below the threshold were seen in two doses of ZF2001 sera. Together, B.1.617 showed more resistance to the neutralization of vaccinee serum than the D614G.These results indicate that it is of great importance to achieve the third dose of ZF2001 vaccination.
Figure 4.
Detection of neutralizing antibodies against D614G, B.1.1.7 and B.1.617 pseudotyped viruses in ZF2001 vaccinee serum samples. (A–C) Neutralizing activity of ZF2001 (RBD-subunit vaccine) sera to D614G, B.1.1.7 and B.1.617. Two individuals who received three doses (A) and eight individuals who received two doses (B) of ZF2001. Pseudotypes were incubated with different serum dilutions for 60 min at 37 °C, and then were incubated onto 293T-ACE2 cells. Upon 72 h, cells were lysed with passive lysis buffer and the activity of firefly luciferase was analyzed. The half-maximal neutralizing titer (ID50) was quantitatively analyzed using Graphpad 8.0 and the threshold of ID50 was 1:40. (C) The GMT values and changes in ZF2001 vaccinee sera ID50 against D614G, B.1.1.7 and B.1.617.
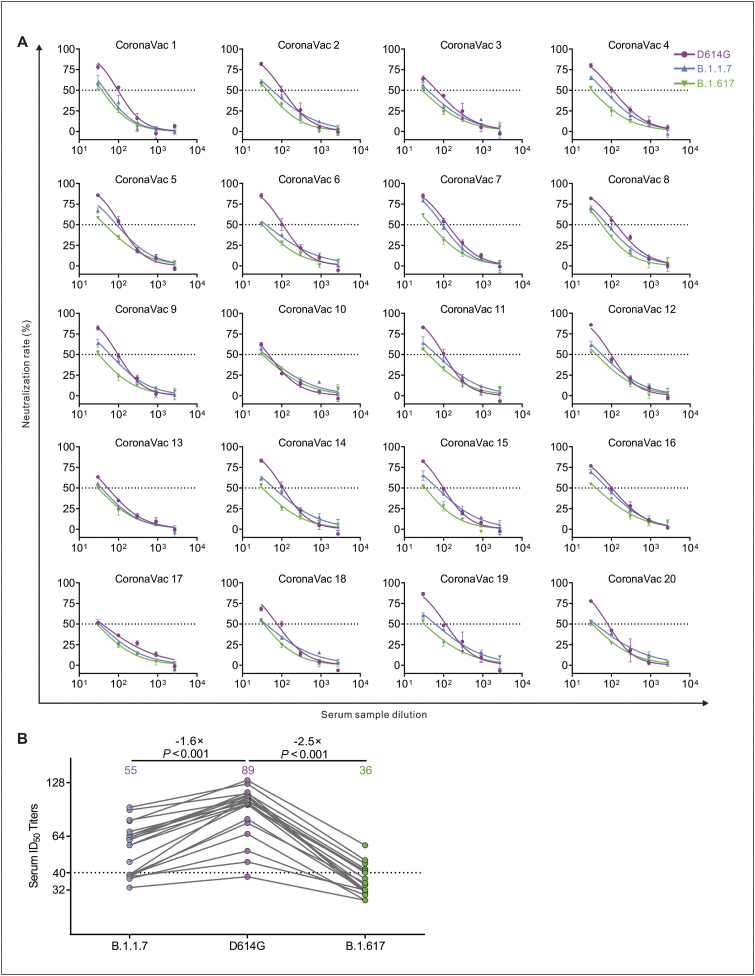
Nineteen CoronaVac-elicited vaccinees had substantial serum neutralizing activity against D614G Spike pseudotyped viruses (Fig. 5A). Compared with activity against the D614G, 35% (7/20) post-vaccination sera were decreased below the threshold against B.1.1.7, and 65% (13/20) were decreased below the threshold against B.1.617 (Fig. 5A). The average neutralization potency of the CoronaVac-elicited serum was reduced 2.5-fold for B.1.617 variant (GMT: 36) compared to D614G (GMT: 89) and reduced 1.6-fold for B.1.1.7 variant (GMT: 55) compared to D614G (GMT: 89) (Fig. 5B).
Figure 5.
Detection of neutralizing antibodies against D614G, B.1.1.7 and B.1.617 pseudotyped viruses in CoronaVac vaccinee serum samples. (A) Neutralizing activity of CoronaVac (inactivated vaccine) sera (n = 20) to D614G, B.1.1.7 and B.1.617. Pseudotypes were incubated with different serum dilutions for 60 min at 37 °C, and then were incubated onto 293T-ACE2 cells. Upon 72 h, cells were lysed with passive lysis buffer and the activity of firefly luciferase was analyzed. The half-maximal neutralizing titer (ID50) was quantitatively analyzed using Graphpad 8.0 and the threshold of ID50 was 1:40. (B) The GMT values and changes in CoronaVac vaccinee sera ID50 against D614G, B.1.1.7 and B.1.617.
Discussion
Due to the highly pathogenic nature of SARS-CoV-2, infectious SARS-CoV-2 must be handled in a biosafety level 3 (BSL-3) facility. Here, using luciferase-expressing lentiviral pseudotype system, we compared viral entry meditated by three SARS-CoV-2 Spike variants: the original D614G variant (identified during the first wave), B.1.1.7 variant (first detected in United Kingdom during the second wave), and B.1.617 variant first reported in India. Our data indicated that B.1.617 variant Spike promotes virus infectivity through enhanced viral entry and membrane fusion, which may play an important role in increased transmissibility of this variant. These findings are highly consistent with previous studies.20 L452R mutation in the RBD was reported to increase SARS-CoV-2 infectivity and fusogenicity.21 P681R, a highly conserved mutation in the B.1.617 lineages, also enhanced the cleavage of SARS-CoV-2 Spike and cell–cell fusion.22 At the time of preparing this manuscript, the B.1.617.2 variant has displaced B.1.1.7 variant as the dominant SARS-CoV-2 strain in UK and other countries.23,24
Another explanation for the increased transmission of B.1.617 variant might be the enhanced ability for the virus to evade immune system. In this study, we compared NAb titers of sera collected from previously SARS-CoV-2 infected individuals, CoronaVac (inactivated vaccine) and ZF2001 (RBD-subunit vaccine) vaccinated persons against three SARS-CoV-2 Spike variants. We found that B.1.617 variant Spike showed more resistant to antibody neutralization. B.1.617 reduced the neutralization of CoronaVac vaccine by 2.5 times, and ZF2001 vaccine by 3.1 times. Consistently, recent studies reported that B.1.617 reduced the neutralization of convalescent plasma by 3.9 times, Oxford-AstraZeneca vaccine by 2.6 times, and Pfizer-BioNTech vaccine by 2.7–3.2 times and mRNA-1273 (Moderna) vaccine by 7 times.25, 26, 27 The B.1.1.7 reduced the neutralization of Pfizer-BioNTech vaccine or mRNA-1273 (Moderna) plasma by 1.6–1.9 times28,29 or even no obvious reduction.30 Therefore, B.1.617 variant was less susceptible to neutralization by sera from convalescent persons and from inactivated virus, RBD-subunit and mRNA vaccinated individuals than was the B.1.1.7 variant.
The RBD of the B.1.617 Spike contains two mutations, L452R and E484Q, which were thought to confer to immune evasion. Several studies have demonstrated that the E484K mutation in the RBD significantly reduced susceptibility to neutralization, as seen in B.1.351 (South Africa) and P.1 (Brazil) variants.31, 32, 33, 34 E484Q mutation occurring in the same position as E484K, was also demonstrated to be associated with immune escape.25,35 Another key mutation in the RBD of B.1.617 is L452R. Recent studies suggested that the L452R mutation of B.1.427/B.1.429 variant Spike also contributes to its escape from NAbs.36,37
The limitation of this study include its small sample size, only focus on pseudovirus-based antibody neutralization in cell culture, and the possibility that mutations may alter neutralization by modulating Spike function rather than its antigenicity. To fully characterize the features of B.1.617 variant, in vivo study with authentic virus and the role of memory T or B cells in protection against this variant will be required. Conclusions about vaccine-mediated protection must be validated by real-world data collected in regions where B.1.617 variant is circulating.
Collectively, this study will be helpful for understanding the increased spread of B.1.617 variant and highlight the need to in depth survey of this variant. Given the evolving nature of the SARS-CoV-2 RNA genome, new variant of concern will continue to arise, which may threaten vaccine efficacy. Therefore, antibody therapeutics and vaccine evaluations against new variants are worthy of further investigation.
Author contributions
Jie Hu, Xiao-yu Wei, Jin Xiang: performed the experiments, performed the statistical analysis, drafted the manuscript.
Pai Peng, Feng-li Xu, Kang Wu: performed the experiments.
Fei-yang Luo, Ai-shun Jin: were responsible for mAb purification. Liang Fang: provided the samples. Bei-zhong Liu, Kai Wang, Ni Tang, Ai-Long Huang: developed the conceptual ideas and designed the study, drafted the manuscript and approved the final version of the manuscript.
Conflict of interests
The authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. U20A20392), the 111 Project (No. D20028); Open Research Fund Program of the Key Laboratory of Molecular Biology for Infectious Diseases, China (No. CQMU202102 and CQMU202105); The Science and Technology Research Program of Chongqing Municipal Education Commission, China (No. KJZD-M202000401); The Natural Science Foundation Project of Chongqing, China (No. cstc2019jscx-dxwtBX0019); The Emergency Project from the Science & Technology Commission of Chongqing, China (No. cstc2020jscx-fyzx0053 and cstc2020jscx-dxwtB0050); Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University, the Emergency Project for Novel Coronavirus Pneumonia from the Chongqing Medical University, China (No. CQMUNCP0302); China Postdoctoral Science Foundation, China (No. 2021M693924); and Chongqing Postdoctoral Science Special Foundation, China (No. 2010010005216630).
Acknowledgements
We would like to thank Professor Cheguo Cai (Wuhan University, Wuhan, China) for providing the pNL4-3.Luc.R-E- plasmid. We also thank all the volunteers who participated in this research.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Bei-zhong Liu, Email: liubeizhong@cqmu.edu.cn.
Kai Wang, Email: wangkai@cqmu.edu.cn.
Ni Tang, Email: nitang@cqmu.edu.cn.
Ai-Long Huang, Email: ahuang@cqmu.edu.cn.
References
- 1.V'kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korber B., Fischer W.M., Gnanakaran S., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamatatos L., Czartoski J., Wan Y.H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbiani D.F., Gaebler C., Muecksch F., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccoli L., Park Y.J., Tortorici M.A., et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J., Peng P., Wang K., et al. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol. 2021;18(4):1061–1063. doi: 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J., He CL, Gao QZ, et al. D614G mutation of SARS-CoV-2 spike protein enhances viral infectivity. BioRxiv. 2020 [Google Scholar]
- 8.Sheikh A., McMenamin J., Taylor B., Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kustin T., Harel N., Finkel U., et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27(8):1379–1384. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng P., Hu J., Deng H.J., et al. Changes in the humoral immunity response in SARS-CoV-2 convalescent patients over 8 months. Cell Mol Immunol. 2021;18(2):490–491. doi: 10.1038/s41423-020-00605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long Q.X., Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 13.Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 14.Han X., Wang Y., Li S., et al. A rapid and efficient screening system for neutralizing antibodies and its application for SARS-CoV-2. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.653189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J., Gao Q., He C., Huang A., Tang N., Wang K. Development of cell-based Pseudovirus entry assay to identify potential viral entry inhibitors and neutralizing antibodies against SARS-CoV-2. Genes Dis. 2020;7(4):551–557. doi: 10.1016/j.gendis.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geraerts M., Willems S., Baekelandt V., Debyser Z., Gijsbers R. Comparison of lentiviral vector titration methods. BMC Biotechnol. 2006;6:34. doi: 10.1186/1472-6750-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang J., Wan Y., Luo C., et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi C., Sun X., Ye J., et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17(6):621–630. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M., Hofmann-Winkler H., Krüger N., et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021;36(3) doi: 10.1016/j.celrep.2021.109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motozono C., Toyoda M., Zahradnik J., et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7):1124–1136. doi: 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito A., Nasser H., Uriu K., et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature. 2022;602(7896):300–306. doi: 10.1038/s41586-021-04266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wall E.C., Wu M., Harvey R., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolze A., Cirulli E.T., Luo S., et al. Rapid displacement of SARS-CoV-2 variant B.1.1.7 by B.1.617.2 and P.1 in the United States. MedRxiv. 2021 [Google Scholar]
- 25.Liu C., Ginn H.M., Dejnirattisai W., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–4236. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Liu Y., Xia H., et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 27.Edara V.V., Pinsky B.A., Suthar M.S., et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385(7):664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caniels T.G., Bontjer I., van der Straten K., et al. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci Adv. 2021;7(36):eabj5365. doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier D.A., De Marco A., Ferreira I.A.T.M., et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136–141. doi: 10.1038/s41586-021-03412-7. [DOI] [PubMed] [Google Scholar]
- 30.Wu K., Werner A.P., Koch M., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaney A.J., Starr T.N., Gilchuk P., et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P., Nair M.S., Liu L., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann M., Arora P., Groß R., et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–2393. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q., Nie J., Wu J., et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184(9):2362–2371. doi: 10.1016/j.cell.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q., Xiong Q., Mei F., et al. Antibody neutralization to SARS-CoV-2 and variants after 1 year in Wuhan, China. Innovation (Camb) 2022;3(1):100181. doi: 10.1016/j.xinn.2021.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCallum M., Bassi J., De Marco A., et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021;373(6555):648–654. doi: 10.1126/science.abi7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng X., Garcia-Knight M.A., Khalid M.M., et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell. 2021;184(13):3426–3437. doi: 10.1016/j.cell.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]