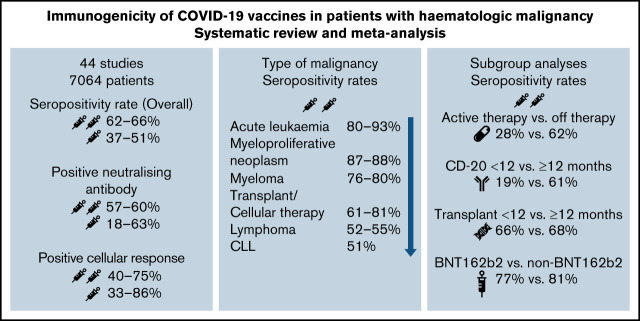
Visual Abstract
Abstract
The objectives of this study were to assess the immunogenicity and safety of COVID-19 vaccines in patients with hematologic malignancies. A systematic review and meta-analysis of clinical studies of immune responses to COVID-19 vaccination stratified by underlying malignancy and published from January 1, 2021, to August 31, 2021, was conducted using MEDLINE, EMBASE, and Cochrane CENTRAL. Primary outcome was the rate of seropositivity after 2 doses of COVID-19 vaccine with rates of seropositivity after 1 dose, rates of positive neutralizing antibodies, cellular responses, and adverse events as secondary outcomes. Rates were pooled from single-arm studies while rates of seropositivity were compared against the rate in healthy controls for comparator studies using a random effects model and expressed as a pooled odds ratios with 95% confidence intervals. Forty-four studies (16 mixed group, 28 disease specific) with 7064 patients were included in the analysis (2331 after first dose, 4733 after second dose). Overall seropositivity rates were 62% to 66% after 2 doses of COVID-19 vaccine and 37% to 51% after 1 dose. The lowest seropositivity rate was 51% in patients with chronic lymphocytic leukemia and was highest in patients with acute leukemia (93%). After 2 doses, neutralizing antibody response rates were 57% to 60%, and cellular response rates were 40% to 75%. Active treatment, ongoing or recent treatment with targeted and CD-20 monoclonal antibody therapies within 12 months were associated with poor immune responses to COVID-19 vaccine. New approaches to prevention are urgently required to reduce COVID-19 infection morbidity and mortality in high-risk patient groups that respond poorly to COVID-19 vaccination.
Introduction
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has resulted in global mortality of more than 3 million deaths. SARS-CoV-2 is likely to remain an endemic viral pathogen, with new variants continuously emerging.1 There is a significant burden of morbidity and mortality from COVID-19 infection in hematology patients. More than 80% of hematology patients require hospitalization, and up to 50% present with severe disease.2-5 Approximately 15% require admission to the intensive care unit, and mortality rates are high at 30% to 40%, depending on underlying disease.2-7 Poor control of infection because of immune compromise lead to emergence of new variants which further complicate management.8
Vaccination is an effective public health measure for reducing the risk of infection and severe complications from COVID-19.9 However, patients with hematologic malignancies were excluded from the pivotal trials that preceded regulatory approvals of the currently recommended COVID-19 vaccines.10-13 Understanding the impact of COVID-19 vaccines on patients with cancer is critical as social distancing restrictions are being eased in countries with relatively high rates of vaccination coverage.14 Assessing immune response after vaccination is the basis of many vaccination studies in patients with hematologic malignancies and is often used as a surrogate marker of vaccine efficacy.15-17 These studies are the basis for vaccination recommendations contained within international guidelines.18,19 Therefore, this study was conducted to systematically review available data on the humoral and cellular immune responses to COVID-19 vaccination in patients with hematologic malignancies to build the evidence base for its utility.
The main objective of this systematic review was to assess the immunogenicity (ie, vaccine-induced immune response) of COVID-19 vaccines in patients with hematologic malignancies, stratified by underlying disease type. The secondary objective was to assess the safety of COVID-19 vaccines in the same patient groups.
Methods
This systematic review was preregistered with PROSPERO (CRD42021276851), and conducted in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.20
Types of studies and participants
Studies eligible for inclusion into the systematic review were those that investigated the vaccine-induced immunity in patients with hematologic malignancies who received at least 1 dose of COVID-19 vaccine. Randomized controlled trials (RCTs), quasi-RCTs, and observational studies were eligible for inclusion. All types of observational studies were included (eg, prospective and retrospective studies and studies with or without a control group). This systematic review evaluated all studies that reported on at least 1 of the following hematology patient groups: all patients with hematologic malignancies; patients with multiple myeloma (MM); patients with chronic lymphocytic leukemia (CLL); patients with lymphoma, Hodgkin lymphoma (HL), or non-Hodgkin lymphoma (NHL); patients with acute myeloid leukemia (AML) or acute lymphocytic leukemia (ALL); patients who are undergoing or have completed hematopoietic stem cell transplantation (HCT) or chimeric antigen receptor (CAR) T-cell therapy; and patients with myeloproliferative neoplasms (MPNs) and chronic myeloid leukemia (CML).
Types of intervention and evaluation
The type of intervention was the use of 1 or 2 doses of COVID-19 vaccine (of any type). Immune response to COVID-19 vaccination included humoral and cellular immune responses. For the purpose of this systematic review, humoral response consists of seropositivity which was defined by the SARS-CoV-2 spike/receptor binding domain–specific immunoglobulin G (IgG) level above the threshold of detection for the assay used in each study. Rate of positive neutralizing antibody (nAb) response was defined by SARS-CoV-2–specific nAb level above the threshold established by the dedicated neutralization assay used in each study. A positive cellular response was defined by the appropriate increase in frequency of SARS-CoV-2–specific CD4+/CD8+ T cells after vaccination according to assays used. Where available, the immune response for each hematologic malignancy patient group was compared with a control group. If there was no comparator group, the immune response for each hematology patient group was described and summarized.
Outcome measures
The primary outcome was rates of seropositivity after 2 doses of COVID-19 vaccine stratified by disease groups. Secondary outcome measures were the rates of seropositivity after 1 dose of vaccine, rates of positive nAb response after 1 or 2 doses of COVID-19 vaccine, rates of positive cellular response after 1 or 2 doses of COVID-19 vaccine, and rates of systemic and/or local adverse events (AEs; whichever rate was higher) after 2 doses of COVID-19 vaccine.
Search strategy
Literature searches were conducted by an experienced research librarian (S.L.) using the Ovid MEDLINE, EMBASE, and Cochrane CENTRAL databases to identify relevant articles published in English from January 1, 2020, to August 31, 2021. A combination of subjects and keyword terms encompassing hematologic cancers, COVID-19, vaccines, and all their associated terms were used for the search. The terms used in our search included: hematologic neoplasms, leukemia, lymphoma, multiple myeloma, myeloproliferative disorders, myelodysplastic-myeloproliferative diseases, stem cell transplantation, bone marrow transplantation, chimeric antigen receptor therapy, COVID-19, SARS-CoV-2, vaccines, vaccination, immunize, immunization, BNT162b2, ChAdOx1, AZD 1222, mRNA-1273, Ad26, Ad5, and NVX-CoV2373. All word variations were searched, and medical subject headings were exploded. The detailed search strategy is summarized in the supplemental data.
Selection of studies
Studies were excluded from the systematic review if they did not measure or report immunogenicity after COVID-19 vaccination, if insufficient details were reported for the hematology patient groups in mixed-group studies, if there were <10 patients including case reports, if data were exclusive to pediatric patients younger than age 18 years, or if they were animal studies. Studies that were not peer reviewed and/or had not been published (eg, preprints, abstracts, and government reports) were excluded. Review articles and other publications without original data such as expert opinions, editorials, and consensus statements were also excluded.
Study eligibility was assessed by 2 independent reviewers (B.W.T., J.S.K.T.), and Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to screen titles, abstracts, and full texts. Irrelevant reports were discarded, and the full texts of the other reports were accessed. Disagreements between the 2 main reviewers with respect to study eligibility were resolved by discussion or via consultation with a third author.
Data extraction
For studies that fulfilled the inclusion criteria, data were extracted manually and independently by authors (J.S.K.T., J.C., B.W.T.) by using a predefined data extraction form. Extracted data elements included study design (enrollment, follow-up period, randomization/allocation, laboratory analysis, predefined outcomes, adjusted analysis, funding source), participant information (inclusion criteria, number of participants, characteristics, disease, treatment), intervention (vaccine type, dose, schedule, comparator group), and outcomes (definitions, timing, AEs).
Assessment of risk of bias
Two review authors (Z.C.F.N., M.A.S.) independently assessed the risk of bias of each cohort study by using the Newcastle-Ottawa Scale.21 Each included study was assessed on the basis of 3 domains: (1) the selection of the study groups, (2) the comparability of the study groups, and (3) the ascertainment of outcome of interest. The rating system proposed by Sharmin et al22 was adopted; a good-quality study scored 3 or 4 stars in the selection domain, 1 or 2 stars in comparability domain, and 2 or 3 stars in the outcome domain. For single-arm studies, a good-quality study scored 3 stars in the selection domain and 2 or 3 stars in the outcome domain.
Measures of treatment effect
For each study, the number of patients who achieved seropositivity and the total number of patients who received vaccination was extracted and expressed as a proportion. For the meta-analysis, rate of response after Ad26 vaccine was analyzed at the same time point and therefore summarized as part of a 2-vaccine dose response. For each hematology disease group, the proportions from each study were pooled using the inverse variance method via the metaprop function in R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and expressed as an overall proportion (response rate) with 95% confidence intervals (CIs).
For each study with a control group, the number of patients with seropositivity in each group and the total number of patients who received vaccination were extracted, expressed as a proportion with 95% CIs, and compared against each other. A meta-analysis was performed if there were 2 or more studies identified with a similar population and sufficient data for the outcomes of interest. The rate of seropositivity as a dichotomous outcome of interest across studies was summarized using a random effects model and expressed as a pooled odds ratios (ORs) with 95% CIs. Comparative analysis was performed using R 4.1.1 software.
Assessment of heterogeneity and missing data
Clinical and methodologic heterogeneity between included studies was assessed by comparing the key patient factors and study factors (vaccine type, type of assay used, threshold for positive response, duration of follow-up). Statistical heterogeneity was assessed by using the I2 statistic and χ2 with a P value ≤ .05 considered significant for the presence of heterogeneity. Reason(s) for missing data were outlined if they were available,. Because of time limitations, further information was not sought from the original or the corresponding author(s).
Subgroup analysis
Preplanned subgroup analysis was performed to evaluate the impact of active treatment (vs no active treatment), anti–CD-20 therapy in the last 12 months, including current CD-20 therapy (vs anti–CD-20 therapy 12 or more months ago), targeted therapy (defined as Bruton tyrosine kinase inhibitor [BTKi], BCL-2 antagonist venetoclax) in the last 12 months (vs no targeted therapy), timing of vaccination in relation to HCT (within 12 months vs longer than 12 months), and response by type of vaccine (BNT162b2 also known as Comirnaty or tozinameran [Pfizer] vs others, including messenger RNA-1273 or mRNA-1273 also known as Spikevax or elasomeran [Moderna], ChAdOx1 also known as Vaxzevria [AstraZeneca], or Ad26 [Janssen]). For subgroup analysis, only studies containing sufficient level of detail for the outcome of interest (eg, therapy <12 months) were included. For analysis by vaccine type, only studies reporting use and immune response of multiple vaccines types were included.
Results
Search results
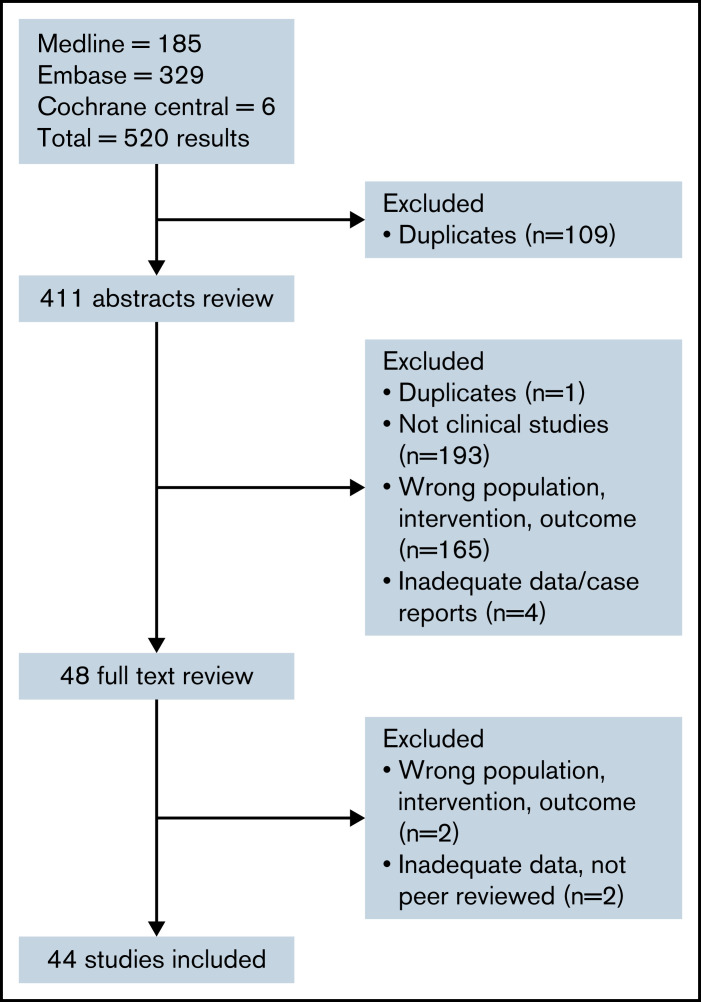
A search of electronic databases yielded 520 results, and after duplicate results were excluded, 411 abstracts were reviewed. Forty-eight studies were identified for full-text review; subsequently, 44 studies fulfilled the inclusion criteria and underwent data extraction (Figure 1). Overall, there were 44 studies of COVID-19 vaccination in patients with hematologic malignancies. Sixteen studies23-38 involved mixed group of patients with various underlying hematologic malignancies, and 28 studies focused on specific malignancies and treatments: 8 myeloma studies,39-46 6 CLL studies,47-52 4 lymphoma studies,53-56 4 studies of HCT57,58 and chimeric antigen receptor (CAR) T-cell therapy,59,60 and 6 MPN studies.61-66 A total of 7064 patients were included in the analysis (2331 after first dose and 4733 after second dose). Characteristics of patients and primary and secondary outcomes in the included studies are summarized in Table 1.
Figure 1.
Flow diagram of studies identified, screened, and included.
Table 1.
Summary of study characteristics and outcomes for patients with hematologic malignancies by overall cohort and specific underlying disease
| Study | Type/location | Study population/comparator | No. of participants analyzed/controls | Median age, y (range or IQR) | M/F | Vaccine type | Analysis | Seropositivity | Rate of positive nAb/cellular response | AEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Mixed-group | ||||||||||
| Addeo et al23 | Multicenter prospective cohort study Europe, North America | Hem Solid tumor | 25 106 | NR for hem | NR for hem | BNT162b2, mRNA-1273, NR for hem | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | Hem: 3-4 weeks after first dose, 18 (72%) of 25; 3-4 weeks after second dose, 17 (77%) of 22. Solid tumor: first dose, 80 (83%) of 96; second dose, 99 (98%) of 101 | NR | NR |
| Agha et al24 | Single-center retrospective cohort study/ United States | Hem | 67 | 71 (IQR, 65-77) | 35/32 | BNT162b2, mRNA-1273, 51%; 42%; unknown, 7% | SARS-CoV-2 spike, IgG Beckman Coulter (≥1.00 = positive) | 21 days after second dose, 36 (54%) of 67 | NR | NR |
| Benda et al25 | Single-center prospective cohort study/ Austria | Hem Solid tumor | 123 34% myeloma, 38% CLL/lymphoma/WM, 28% AML/MDS/MPNs 136 | NR for hem | NR for hem | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | 21 days after first dose, 53 (43%) of 123; 28 days after second dose, 85 (71%) of 119 | NR | NR for hem |
| Cohen et al26 | Single-center retrospective study/Israel | Hem | 54 | 69 (IQR, 61-77) | 32/22 | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | 2-3 weeks after second dose, 34 (63%) of 54 | NR | NR |
| Gavriatopoulou et al27 | Single-center prospective cohort study/ Greece | Hem Control group | 58 48% WM, 38% CLL, 14% NHL 213 | 75 (40-88) | 28/30 | BNT162b2, 76%; ChAdOx1, 24% | nAb GenScript (≥30% = positive; ≥50% = clinically relevant inhibition) | 22 days after first dose, ≥30%, 8 (14%) of 58 patients vs 114 (54%) of 213 controls | 22 days after first dose, ≥50%, 3 (5%) of 58 patients vs 50 (24%) of 213 controls | NR |
| Greenberger et al28 | Multicenter prospective cohort study/ United States | Hem | 1445: 45% CLL, 25% NHL, 5% HL, 15% MM, 4% acute leukemia, 2% CML, 2% MPNs, 2% other | 68 (16-110) | 574/871 | BNT162b2, 55%; mRNA-1273, 45% | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | >14days after second dose, 1088 (75%) of 1445 | NR | NR |
| Herzog Tzarfati et al29 | Single-center prospective cohort study/ Israel | Hem Matched healthy controls | 315 22% MPNs, 17% myeloma, 16% aggressive NHL, 13% indolent NHL, 11% CLL, 7% CML, 5% HL, 5% acute leukemia, 5% MDS 108 | 70 (IQR, 61-77) | 223/200 | BNT162b2 | SARS-CoV-2 spike, IgG DiaSorin (≥12 AU/mL = positive) | 30-60 days after second dose, 235 (75%) of 315 vs 107 (99%) of 108 controls | NR | NR |
| Iacano et al30 | Single-center prospective cohort study/ Italy | Hem ≥80 y; health care worker controls ≥66 y (results NR) | 10 | NR for hem | NR for hem | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (≥50 AU/mL = positive) | 28 days after second dose, 4 (40%) of 10 | NR | NR |
| Jurgens et al31 | Single-center prospective cohort study/ United States | Hem Health care worker controls | 67 31% CLL, 63% NHL, 6% HL 35 | 71 (24-90) | 36/31 | BNT162b2, 46%; mRNA-1273, 54% | SARS-CoV-2 spike, IgG in-house (OD 450 ≥3 = positive) | 21 days after second dose, 41 (61%) of 67 vs 35 (100%) of 35 controls | NR | NR |
| Maneikis et al32 | Multicenter prospective cohort study/ Europe | Hem Health care worker controls | 857 68 | 65 (IQR, 54-72) | 404/453 | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (≥50 AU/mL = positive) | 7-21days after first dose, 481 (56%) of 857; NR for control group | NR | At least 1 AE; dose 1, 56 (9%) of 575; dose 2, 72 (13%) of 575 |
| Malard et al33 | Single-center retrospective cohort study/ France | Hem Healthy controls | 195 27% myeloma, 23% NHL, 3% HL, 13% CLL, 16% AML, 2% ALL, 9% MPNs, 7% other 30 | 69 (22-92) | 117/78 | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (≥50 AU/mL = positive; ≥3100 AU/mL = neutralization >30%; SARS-CoV-2 T-cell response, ELISPOT ≥10 spot-forming cells per 106 PBMCs and ratio 2.5 = positive) | ≥3100 AU/mL threshold*; 28 days after first dose, 3 (2%) of 195; 14 days after second dose, 91 (47%) of 196 vs 26 (87%) of 30 controls | Cellular response 14 days after second dose, 36 (53%) of 68 | At least 1 AE; dose 1, 88 (57%) of 154; dose 2, 55 (34%) of 163 |
| Monin et al34 | Multicenter prospective cohort study/ United Kingdom | Hem Health care worker controls | 56: 68% B-cell malignancies, 9% T-cell malignancies, 18% myeloid or acute leukemia, 5% other 54 | 73 (IQR, 65-80) | NE for hem | BNT162b2 | SARS-CoV-2 spike, IgG (≥70 EC50 = positive); SARS-CoV-2–specific T-cells secreting IFN-γ and/or IL-2 >7 cytokine-secreting cells per 106 PBMCs = positive) | 21 days after first dose, 8 (18%) of 44 vs 32 (94%) of 34 controls; 35 days after first dose, 4 (11%) of 36 vs 18 (86%) of 21 controls; 35 days after first dose (with second dose), 3 (60%) of 5 vs 12 (100%) of 12 controls | Cellular response 21 days after first dose, 9 (50%) of 18 vs 14 (82%) of 17 controls; 35 days after first dose, 6 (33%) of 18 vs 9 (69%) of 13 controls; 35 days after first dose (with second dose), 3 (75%) of 4 vs 3 (100%) of 3 controls | NE for hem patients |
| Ollila et al35 | Single-center retrospective cohort study/ United States | Hem | 160: 36% aggressive and 21% indolent lymphoma, 15% plasma cell leukemia, 12% CLL, 9% other lymphoma, 6% myeloid leukemia | 72 (65-79) | 86/74 | BNT162b2, 60%; mRNA-1273, 31%; Ad26, 7%; ND, 1% | SARS-CoV-2 spike, IgG Abbott (signal/cutoff ratio ≥1.4 = positive) | 56 days after first dose, 63 (39%) of 160 | NR | NR |
| Pimpinelli et al36 | Single-center prospective study/ Italy | Hem Older age (>80 y) control group | 92 46% myeloma, 54% MPN 36 | 73 (47-78)/ 70 (28-80) | Myeloma: 23/19; MPN: 26/24 | BNT162b2 | SARS-CoV-2 spike, IgG DiaSorin (≥15 AU/mL = positive) | 21 days after first dose, 9 (21%) of 42; myeloma, 26 (52%) of 50 MPNs vs 19 (53%) of 36 controls; 14 days after second dose, 33 (79%) of 42; myeloma 44 (88%) of 50 MPNs vs 36 (100%) of 36 controls; ≥80 AU/mL threshold*: 14 days after second dose, 23 (55%) of 42; myeloma 42 (84%) of 50 MPNs vs 34 (97%) of 36 controls | NR | Reported with different patient numbers |
| Re et al37 | Multicenter retrospective cohort study/ France | Hem | 102: 45% lymphoma, 22% myeloma, 13% MDS/AML, 10% CLL, 10% MPNs | 76 (33-93) | 67/35 | BNT162b2, 93%; mRNA-1273, 7% | SARS-CoV-2 spike, IgG; range of commercial kits using their own threshold | 21-28 days after second dose, 64 (62%) of 102 | NR | NR |
| Thakkar et al38 | Single-center prospective and retrospective cohort study/ United States | Hem Solid tumors Healthy controls | 6639% lymphoid malignancies, 27% myeloid malignancies, 33% plasma cell leukemia 134 26 | NR for hem | NR for hem | BNT162b2, 58%; mRNA-1273, 31%; Ad26, 10%; mRNA type unknown, 2% | SARS-CoV-2 spike, IgG Abbott (≥50 AU/mL = positive) | >7 days after second dose, 56 (85%) of 66 vs 131 (98%) of 134 solid tumors; rate NR for controls | NR | NR for hem |
| Myeloma-specific | ||||||||||
| Avivi et al39 | Single-center prospective cohort study/ Israel | Myeloma Healthy volunteers | 171 64 | 70 (38-94) | 96/75 | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | 14-21 days after second dose, 133 (78%) of 171 vs 63 (98%) of 64 controls | NR | At least 1 AE; 90 (53%) of 161 vs 29 (55%) of 53 controls |
| Bird et al40 | Single-center retrospective cohort study/ United Kingdom | Myeloma | 93 | 67 (47-87) | 55/38 | BNT162b2, 52%; ChAdOx1, 48% | SARS-CoV-2 spike, IgG Ortho clinical (≥1 signal/cutoff = positive) | ≥21 days after first dose, 52 (56%) of 93 | NR | NR |
| Ghandili et al41 | Single-center prospective cohort study/ Germany | Myeloma | 82 | 68 (40-85) | 49/33 | BNT162b2, 77%; ChAdOx1, 23% | SARS-CoV-2 spike, IgG DiaSorin (≥34 AU/mL = positive) | 21 days after first dose, 17 (23%) of 74 | NR | NR |
| Ramasamy et al42 | Multicenter web-based prospective cohort study/ United Kingdom | Myeloma | 105-28 patients sampled | 63 | 67/42 | BNT162b2, 42%; ChAdOx1, 58% | SARS-CoV-2 spike, IgG Abbott (COV ≥50 = positive) | >21 days after first dose, 17 (61%) of 28 | NR | NR |
| Stampfer et al43 | Single-center prospective cohort study/ United States | Myeloma Healthy controls Pre-COVID-19 controls | 103 31 34 | 68 (35-88) | 61/42 | BNT162b2, 50%; mRNA-1273, 50% | SARS-CoV-2 spike, IgG in-house (50-250 IU/mL = positive partial response) (>250 IU/mL = clinically relevant response) | 14-21 days after first dose, 20 (21%) of 96; 14-21 days after second dose, 64 (67%) of 96 vs 31 (100%) of 31 controls; >250 IU/mL: 14-21 days after first dose, 2 (2%) of 96; 14-21 days after second dose, 45 (46%) of 96 vs 29 (94%) of 31 controls | NR | NR |
| Terpos et al44 | Single-center prospective cohort study/ Greece | Myeloma Matched controls | 48 102 | 83 (59-92) | 29/19 | BNT162b2 | SARS-CoV-2 nAb GenScript (≥30% = positive; ≥50% = clinically relevant) | ≥30%: 22 days after first dose, 12 (25%) of 48 vs 57 (55%) of 102 controls | ≥50%: 22 days after first dose, 4 (8%) of 48 vs 21 (20%) of 102 controls | NR |
| Terpos et al45 | Single-center prospective cohort study/ Greece | Myeloma Matched controls | 276 77% myeloma, 14% sMM, 9% MGUS 226 | 74 (62-80) | 151/125 | BNT162b2, 78%; ChAdOx1, 22% | SARS-CoV-2 nAb GenScript (≥30% = positive; ≥50% = clinically relevant) | ≥30%; day 22 after first dose, 117 (42%) of 276 vs 145 (64%) of 226 controls; day 50 after first dose, 196 (71%) of 276 vs 204 (90%) of 226 controls | ≥50%; day 22 after first dose, 55 (20%) of 276 vs 73 (32%) of 226 controls; day 50 after first dose, 158 (57%) of 276 vs 183 (81%) of 226 controls | First dose of BNT162b2, 71 (33%) of 215 local reaction; 28 (13%) of 215 systemic reaction; ChAdOx1, 20 (33%) of 61 local reaction. Second dose of BNT162b2, 68 (32%) of 215 local reaction; 45 (21%) of 215 systemic reaction |
| Van Oekelen et al46 | Single-center prospective and retrospective cohort study/ United States | Myeloma Matched health care worker controls | 320 260 sampled 67 | 68 (38-93) | 185/135 | BNT162b2, 69%; mRNA-1273, 27%; unknown, 4% | SARS-CoV-2 spike, IgG in-house (≥5 AU/mL = positive) | 51 days after second dose, 219 (84%) of 260 vs 67 (100%) of 67 controls | NR | NR |
| CLL-specific | ||||||||||
| Benjamini et al47 | Multicenter prospective cohort study/ Israel | CLL patients | 373 | 70 (40-89) | 222/151 | BNT162b2 | SARS-CoV-2 spike, IgG DiaSorin (≥15 AU/mL = positive); Abbott (>50 U/mL = positive); in-house (>1.1 = positive) | 14-21 days after second dose, 160 (43%) of 373 | nAb 14-21 days after second dose, 27 (60%) of 45 | At least 1 AE; 151 (47%) of 331 |
| Del Poeta et al48 | Single-center prospective cohort study/ Italy | CLL patients | 46 | NR | 29/17 | BNT162b2 | SARS-CoV-2 spike, IgG MAGLUMI (≥1.1 = positive) | 14-21 days after second dose, 25 (54%) of 46 | NR | NR |
| Herishanu et al49 | Single-center prospective cohort study/ Israel | CLL patients Age- and sex-matched controls | 167 52 | 71 (63-76) | 112/55 | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | 14-21 days after second dose, 66 (40%) of 167 patients vs 52 (100%) of 52 controls | NR | First dose, 52 (31%) of 167 local reaction; 21 (13%) of 167 systemic reaction. Second dose, 56 (34%) of 167 local reaction; 21 (23%) of 167 systemic reaction |
| Parry et al50 | Single-center prospective cohort study/ United Kingdom | CLL patients Healthy age-matched controls | 299 93 | 69 (IQR, 63-74) | 159/140 | BNT162b2, 52%; ChAxOd1, 48% | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive); dried blood sampling Roche (ratio ≥1.0 = positive) | 43 days after first dose, serum 29 (34%) of 86 vs 89 (94%) of 95 controls; dried blood* 63 (24%) of 267 vs 66 (71%) of 93 controls; 18 days after second dose, serum 9 (75%) of 12 vs 59 (100%) of 59 controls; dried blood* 39 (71%) of 55 vs 36 (97%) of 37 controls | NR | NR |
| Roeker et al51 | Single-center retrospective cohort study United States | CLL patients | 44 | 71 (37-89) | 23/21 | BNT162b2, 57%; mRNA-1273, 43% | SARS-CoV-2 spike, IgG DiaSorin (≥15 AU/mL = positive) | 21 days after second dose, 23 (52%) of 44 | NR | NR |
| Tadmor et al52 | Multicenter prospective observation study/ Israel | CLL patients | 84 | 69 (44-87) | 53/29 | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (≥50 U/mL = positive); SARS-CoV-2 RBD IgG (>1.1 = positive) | 22 days after second dose, 49 (58%) of 84 | NR | NR |
| Lymphoma-specific | ||||||||||
| Ghione et al53 | Single-center prospective cohort study/ United States | B-cell lymphoma Nursing home controls Health care worker controls | 86 47 154 | 70 (35-91) | 45/41 | BNT162b2, 47%; mRNA-1273, 52%; Ad26, 1% | SARS-CoV-2 spike, IgG BioRad (≥1.0 = positive) | 14-56 days after completion of vaccination, 36 (42%) of 86 patients vs 43 (92%) of 47 nursing home controls, 154 (100%) of 154 health care worker controls | NR | NR |
| Gurion et al54 | Multicenter prospective cohort study/ Israel | Lymphoma | 162: 88% NHL, 12% HL | 65 (52-73) | 89/73 | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (≥50 IU/mL = positive) | 28 days after second dose, 83 (51%) of 162 | NR | NR |
| Lim et al55 | Multicenter prospective cohort study interim analysis/ United Kingdom | Lymphoma Healthy controls | 129 recruited, 119 analyzed/ 150: 66% indolent B-cell NHL, 29% aggressive B-cell NHL, 10% HL, 3% other | 69 (IQR, 57-74) | 81/48 | BNT162b2, ChAdOx1 | SARS-CoV-2 spike, IgG Meso Scale Discovery (>0.55 BAU/mL = positive); RBD IgG (>0.73 BAU/mL = positive) | 14 days after first dose, 32 (54%) of 59 patients vs 65 (100%) of 65 controls; 14-28 days after second dose, 61 (71%) of 86 patients vs 85 (100%) of 85 controls | NR | NR |
| Perry et al56 | Single-center prospective cohort study/ Israel | Lymphoma, B-cell NHL Healthy controls | 149 53% indolent NHL, 47% aggressive NHL 65 | 64 (20-92) | 88/61 | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 IU/mL = positive) | 14-21 days after second dose, 73 (49%) of 149 vs 64 (99%) of 65 controls; 38 (48%) of 80 indolent NHL; 34 (49%) of 69 aggressive NHL | NR | At least 1 AE; 60 (51%) of 118; 44 (37%) of 118 local AE; 23 (20%) of 118 systemic AE |
| HCT-specific | ||||||||||
| Easdale et al57 | Single-center retrospective cohort study/ United Kingdom | Allo-HCT >3 months | 55 | 50 (18-73) | 34/21 | BNT162b2, 38%; ChAdOx1, 62% | SARS-CoV-2 spike, IgG Ortho clinical (≥1 signal/cutoff = positive) | 42 days after first dose, 21 (38%) of 55 | NR | NR |
| Redjoul et al58 | Single-center retrospective cohort study/ France | Allo-HCT | 88 | NR | 47/41 | BNT162b2 | SARS-CoV-2 spike, IgG Abbott (>21 AU/mL = positive; >4160 AU/mL = neutralization) | 28 days after second dose, 69 (78%) of 88; >4160 AU/mL 28 days after second dose, 52 (59%) of 88 | NR | NR |
| Ram et al59 | Single-center prospective cohort study/ Israel | Allo-HCT and CAR T cells >3 months | 80: 83% allo-HCT, 17% CAR T cells | 65 (23-83) | 44/37 | BNT162b2 | SARS-CoV-2 spike, IgG Roche (≥0.8 U/mL = positive); SARS-CoV-2–specific cells ELISPOT (IFN, IL-2) (4 spots per well = positive) | 7-14 days after second dose, 47 (75%) of 63 allo-HCT; 5 (36%) of 14 CAR T cells | Cellular response 7-14 days after second dose, 7 (19%) of 37 allo-HCT; 6 (50%) of 12 CAR T cells | At least 1 AE; first dose, 11 (14%) of 80, 3 (4%) of 80 GVHD; second dose, 18 (24%) of 74, 3 (4%) of 74 GVHD |
| Dhakal et al60 | Single-center retrospective cohort study/ United States | Auto-HCT, allo-HCT, CAR T cells | 130: 45 auto-HCT, 71 allo-HCT, 14 CAR T cells | Auto-HCT, 65 (45-75); allo-HCT, 64 (25-77); CAR T cells, NR | NR | BNT162b2, 59%; mRNA-1273, 36%; Ad26, 5% | SARS-CoV-2 spike, IgG EUROIMMUN (≥1.1 signal/cutoff = positive) | 14 days after completion of vaccination, 27 (60%) of 45 auto-HCT; 49 (38%) of 71 allo-HCT; 3 (21%) of 14 CAR T cells | NR | NR |
| MPN- and CML-specific | ||||||||||
| Caocci et al61 | Single-center prospective cohort study/ Italy | MPN | 20: 65% MF, 30% ET, 5% PV | 66 (48-82) | NR | BNT162b2 | SARS-CoV-2 spike, IgG DiaSorin (≥15 AU/mL = positive) | 42 days after second dose, 13 (65%) of 20 | NR | NR |
| Chowdhury et al62 | Single-center retrospective cohort/ United Kingdom | CML and MPN Health care workers age >60 years | 59 232 | 62 (IQR, 52-73) | 27/32 | BNT162b2, 37%; ChAdOx1, 63% | SARS-CoV-2 spike, IgG Abbott (≥50 AU/mL = positive) | ≥2 weeks after first dose, 34 (57%) of 59 vs 224 (97%) of 232 controls | NR | NR |
| Guglielmelli et al63 | Single-center prospective cohort study/ Italy | MPN Healthy controls | 30 43% MF, 33% PV, 23% ET 14 | NR overall | 10/20 | BNT162b2, 83%; mRNA-1273, 17% | SARS-CoV-2 spike/RBD IgG (NS) | 21 to 28 days after first dose, 18 (60%) of 30 vs 14 (100%) of 14 controls | nAb, 21-28 days after first dose, 13 (43%) of 30 vs 14 (100%) of 14 controls | NR |
| Harrington et al64 | Single-center prospective cohort study/ United Kingdom | CML | 16 | 45 (23-74) | 12/4 | BNT162b2 | SARS-CoV-2 spike, IgG in-house (1:25 = positive); SARS-CoV-2 neutralizing in-house (ID50 = positive); SARS-CoV-2 T cells ICA (IFN, IL-2) (threefold increase = positive) | 21 days after first dose, 14 (88%) of 16 | nAb 21 days after first dose, 6 (38%) of 16; cellular response, 14 (80%) of 15 | Local AEs, 8 (50%) of 16; systemic AEs, 9 (56%) of 16 |
| Harrington et al65 | Single-center prospective cohort study/ United Kingdom | MPN | 21 | 58 (36-72) | 7/21 | BNT162b2 | SARS-CoV-2 spike, IgG in-house (1:25 = positive); SARS-CoV-2 neutralizing in-house (ID50 = positive); SARS-CoV-2 T cells ICA (IFN, IL-2) (threefold increase = positive) | 21 days after first dose, 16 (76%) of 21 | nAb 21 days after first dose, 18 (86%) of 21; cellular response (CD4), 15 (75%) of 20 | At least 1 AE; 12 (57%) of 21 local; 10 (48%) of 21 systemic |
| Pimpinelli et al66 | Single-center prospective cohort study/ Italy | MPN | 42: 40% ET, 36% PV, 24% MF | 72 (52-82) | 20/22 | BNT162b2 | SARS-CoV-2 spike, IgG DiaSorin (≥15 AU/mL = positive) | 21 days after first dose, 23 (55%) of 42; 14 days after second dose, 36 (86%) of 42 | NR | NR |
AEs, adverse events; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; BAU, binding antibody unit; CLL, chronic lymphotic leukemia; COV, cut-off value; EC50, median effective concentration; ET, essential thrombocythemia; F, female; GVHD, graft-versus-host disease; hem, hematology; HL, Hodgkin lymphoma; ICA, intracellular cytokine assay; ID50, median infective dose; IFN-γ, interferon-γ; IL-2, interleukin-2; IQR, interquartile range; M, male; MF, myelofibrosis; MGUS, monoclonal gammopathy of unknown significance; MM, multiple myeloma; ND, not determined; NE, not extractable; NHL, non-Hodgkin lymphoma; NR, not reported; OD, optical density; PBMC, peripheral blood mononuclear cell; PV, polycythemia vera; RBD, receptor-binding domain; SMM, smoldering multiple myeloma; WM, Waldenström macroglobulinemia.
Excluded from meta-analysis.
Risk of bias and quality assessment
Overall, 23 studies (52%) were evaluated as being of good or fair quality (good, 11; fair, 12) with low risk of bias, whereas the remaining 21 studies were rated as poor quality (high risk of bias) primarily because of lack of details regarding patient selection, demonstration that outcome of interest was not present at the start of study, comparability of cohorts (design or analysis), and duration of follow-up. Risk of bias and quality assessment of studies are summarized in supplemental Table 1.
Seropositivity rates
There were 44 studies measuring humoral immune responses in patients with hematologic malignancies. The majority of studies evaluated the use of BNT162b2 and used SARS-CoV-2 spike-specific IgG levels above the threshold of detection (seropositivity). Twenty-three studies (52%) reported sufficient data on the spike/receptor binding domain IgG antibody levels (median value or geometric mean titer) after 1 dose (6 studies),27,35,37,41,62,63 2 doses (13 studies),24,26,29,30,38,39,46,48,49,55,59-61 and after both 1 and 2 doses (4 studies).36,43,50,66 Antibody levels achieved were lower compared with those in control cohorts after 1 and 2 doses of COVID-19 vaccine.27,29,30,39,46,49,62,63 In 3 studies,36,43,66 the magnitude of increase in antibody levels between doses was 5-fold to 15-fold, whereas the fourth study by Parry et al50 reported a 132-fold increase between the first and second doses in 12 of 299 patients with CLL.
Several studies attempted to establish potential thresholds for clinical protection with higher antibody levels than the seropositivity threshold used. Malard et al33 correlated SARS-CoV-2 spike IgG levels with nAb levels, and ≥3100 arbitrary units (AU)/mL on the Abbott assay was predictive of an nAb level ≥30%. Using this threshold, the rate of humoral response was 47% in hematology patients compared with 97% in healthy controls after a second dose of the BNT162b2 vaccine.33 Pimpinelli et al36 also evaluated humoral responses using a higher threshold of ≥80 AU/mL (DiaSorin, Saluggia, Italy) and noted response rates of 55% for patients with myeloma and 84% for patients with MPNs compared with 97% for healthy controls. Other studies correlated SARS-CoV-2 IgG levels with plaque reduction neutralization tests or with levels seen in clinical vaccination trials. Stampfer et al43 defined >250 IU/mL (in-house assay) as a clinically relevant response that was attained by 45% of patients with myeloma after 2 doses of a COVID-19 vaccine. For HCT patients, Redjoul et al58 used a threshold of 4160 AU/mL (Abbott Laboratories, Wiesbaden, Germany), and 59% of patients achieved this response after 2 doses.
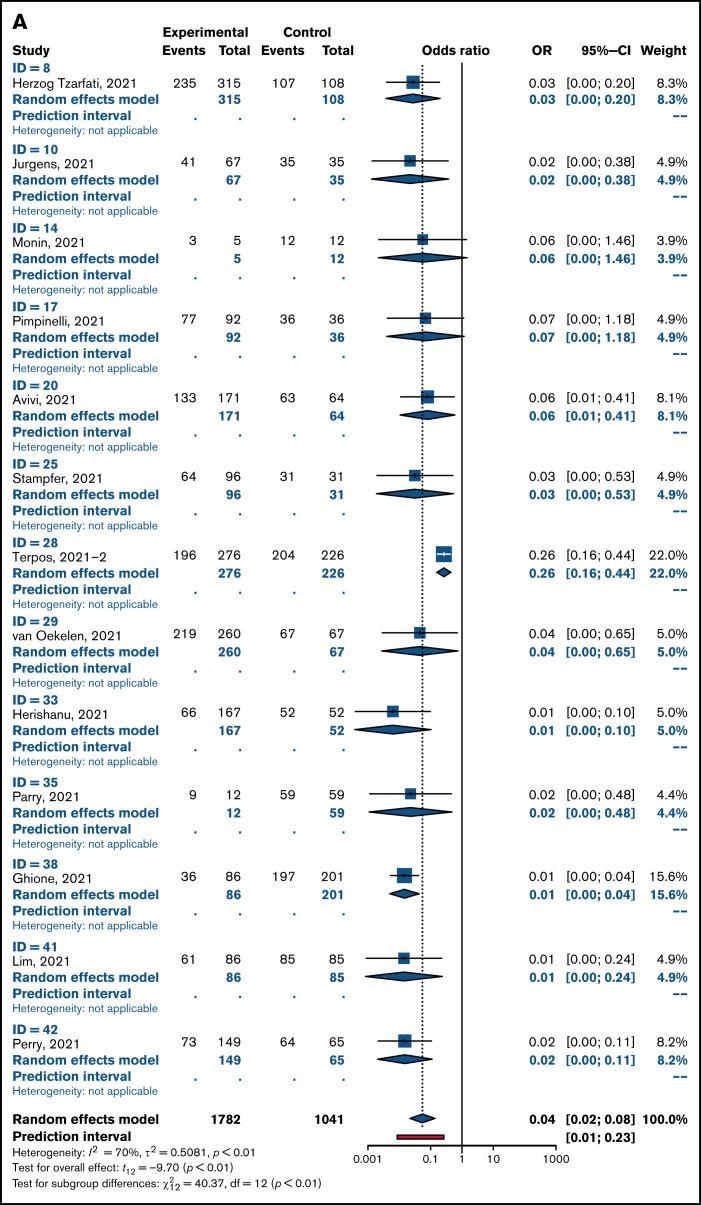
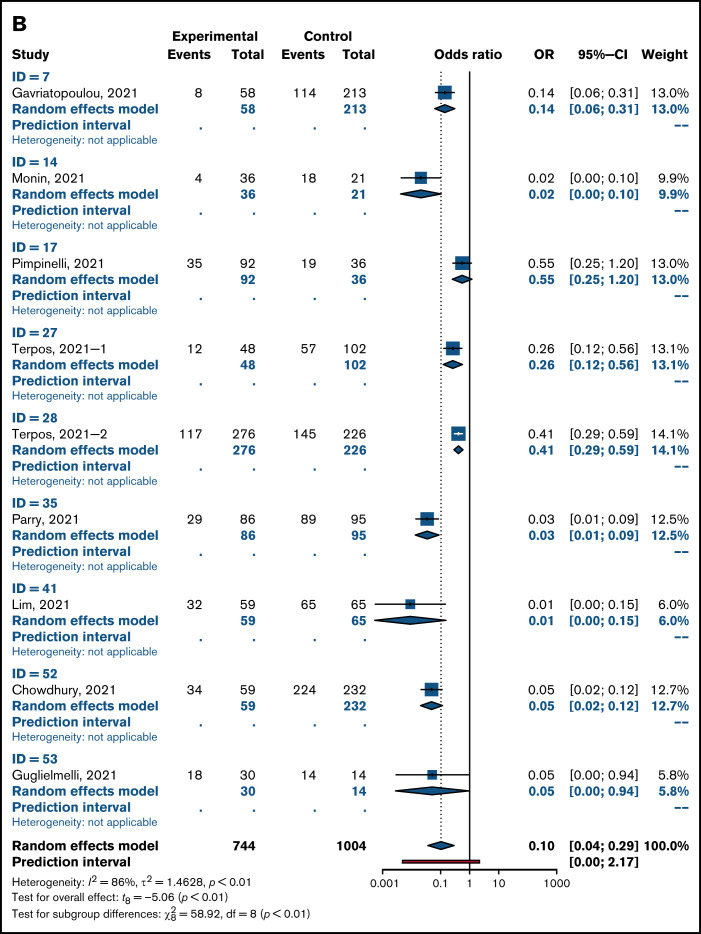
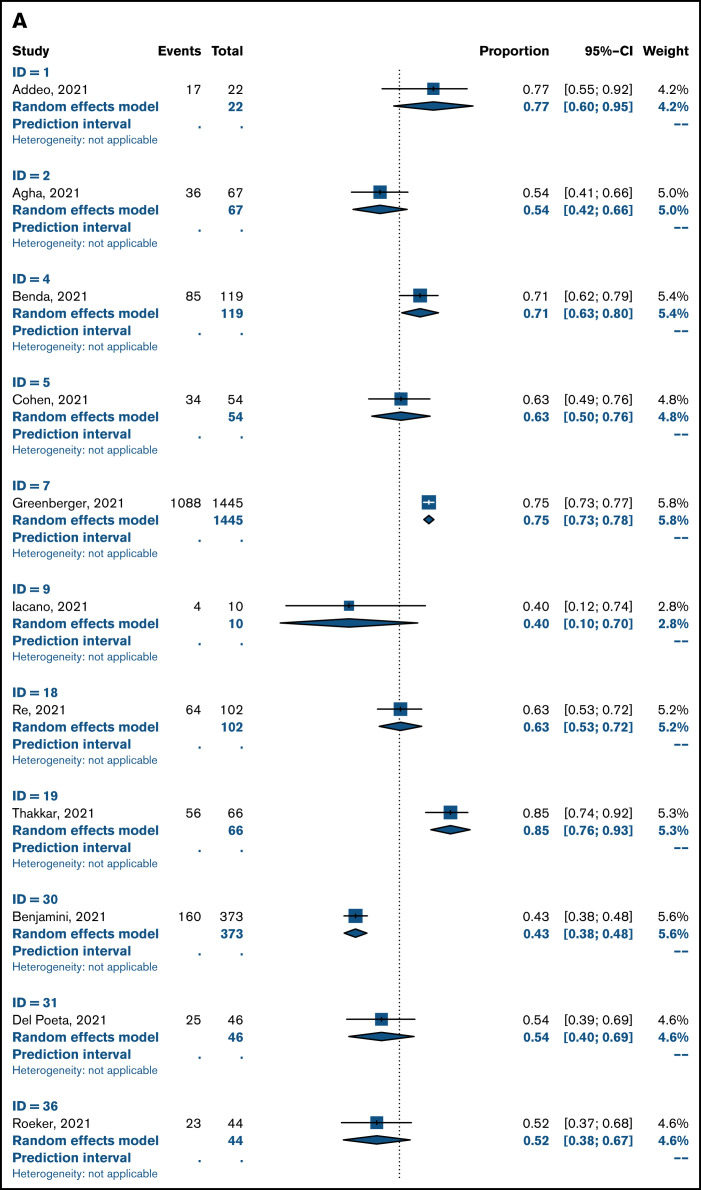
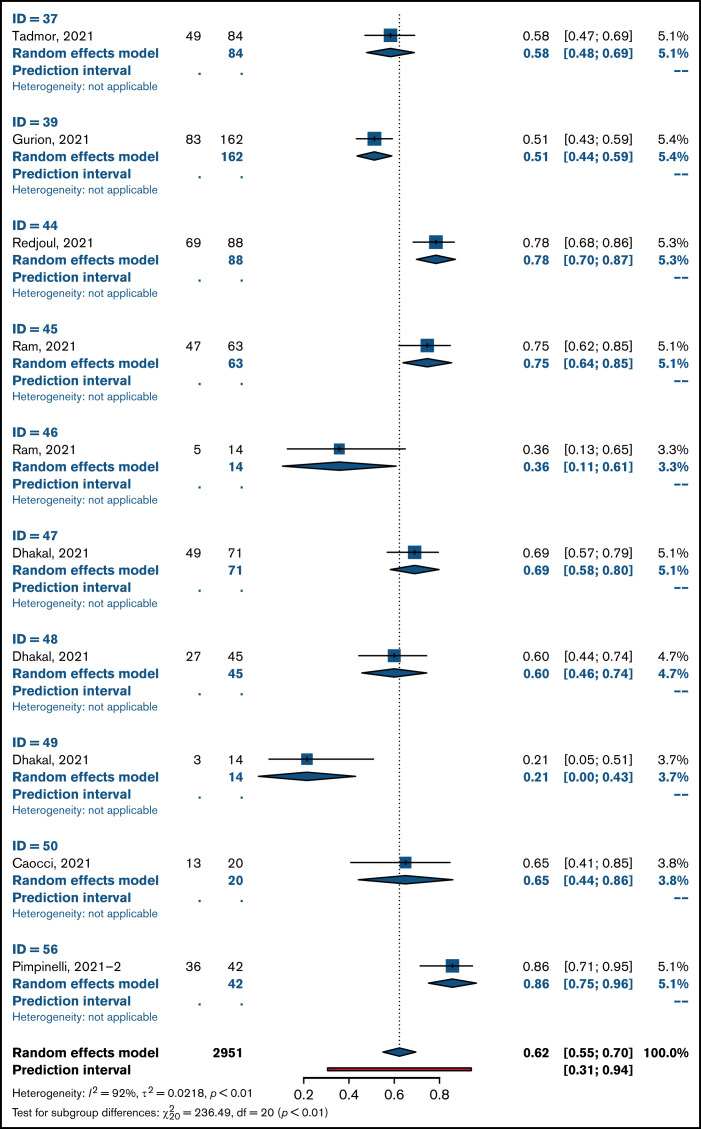
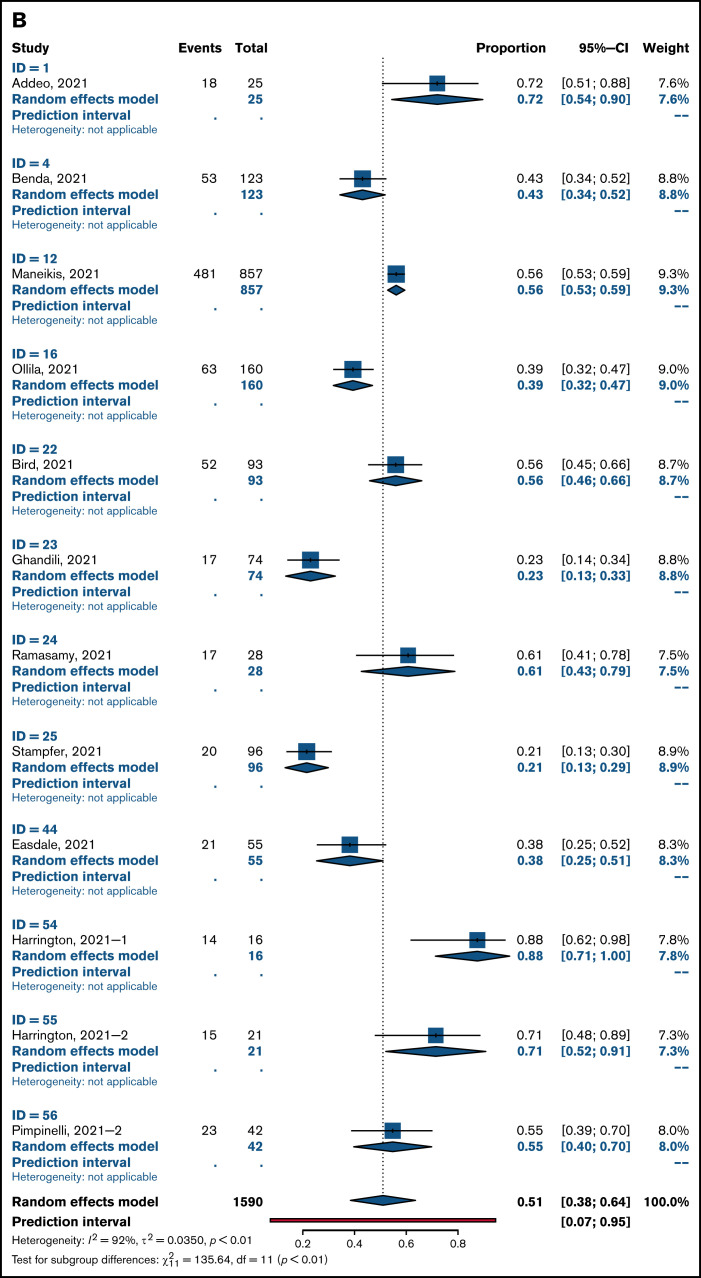
Overall pooled seropositivity rates in patients with hematologic malignancies were 62% in single-arm studies and 66% in comparator studies after 2 doses of COVID-19 vaccination. After a single dose, the pooled seropositivity rates were 51% in single-arm studies and 37% in comparator studies. Compared with healthy or older matched controls, patients with hematologic malignancies were less likely to achieve seropositivity with ORs of 0.04 (95% CI, 0.02-0.08; P < .01) after 2 doses (Figure 2A) and 0.10 (95% CI, 0.04-0.29; P < .01) after a single dose (Figure 2B) of COVID-19 vaccine. Heterogeneity was 70% and 86%, respectively (P < .01). Pooled seropositivity rates by disease type and vaccine doses are summarized in Table 2 and Figure 3. Overall results were graded as having moderate quality due to statistical and clinical heterogeneity and the proportion of studies with a high risk of bias (48%).
Figure 2.
OR for achieving seropositivity in patients with hematologic malignancies vs healthy control group after 2 doses (A) and 1 dose of COVID-19 vaccine (B).
Table 2.
Summary of pooled seropositivity rates for patients with hematologic malignancies by underlying disease type and number of vaccine doses
| No. of patients | Single-arm studies | Malignancy arm of comparator studies | Control arm of comparator studies | Intervention vs control cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled response rate (95% CI) | I2 (%) | P | Pooled response rate (95% CI) | I2 (%) | P | Pooled response rate (95% CI) | I2 (%) | P | OR (95% CI) | P | I2 (%) | P | ||
| Overall: Patients with hematologic malignancies | ||||||||||||||
| After second dose | 4733 | 0.62 (0.55-0.70) | 92 | <.01 | 0.66 (0.57-0.75) | 93 | <.01 | 0.99 (0.97-1.00) | 53 | 0.01 | 0.04 (0.02-0.08) | <.01 | 70 | <.01 |
| After first dose | 2331 | 0.51 (0.38-0.64) | 92 | <.01 | 0.37 (0.23-0.51) | 90 | <.01 | 0.78 (0.62-0.95) | 98 | <.01 | 0.10 (0.04-0.29) | <.01 | 86 | <.01 |
| Myeloma | ||||||||||||||
| After second dose | 1218 | 0.80 (0.64-0.95) | 85 | <.01 | 0.76 (0.70-0.82) | 90 | <.01 | 0.98 (0.95-1.00) | 71 | <.01 | 0.09 (0.03-0.29) | <.01 | 49 | .08 |
| After first dose | 685 | 0.43 (0.18-0.68) | 91 | <.01 | 0.29 (0.09-0.48) | 81 | <.01 | 0.64 (0.42-0.87) | 76 | <.01 | 0.23 (0.05-0.99) | .05 | 49 | .11 |
| CLL | ||||||||||||||
| After second dose | 1446 | 0.51 (0.37-0.65) | 91 | <.01 | 0.51 (0.34-0.68) | 56 | .06 | 1.00 (0.99-1.00) | 0 | .07 | 0.01 (0.01-0.03) | <.01 | 0 | .9 |
| After first dose | 111 | Single study | 0.18 (0.00-1.00) | 89 | <.01 | 0.92 (0.52-1.00) | 0 | .32 | 0.03 (0.00-0.80) | .05 | 0 | .60 | ||
| Lymphoma | ||||||||||||||
| After second dose | 1227 | 0.52 (0.28-0.75) | 97 | <.01 | 0.55 (0.35-0.76) | 84 | <.01 | 0.99 (0.98-1.00) | 0 | .72 | 0.02 (0.01-0.02) | <.01 | 0 | 1.0 |
| After first dose | 69 | No studies available | 0.33 (0.00-1.00) | 93 | <.01 | 0.95 (0.09-1.00) | 71 | .06 | 0.01 (0.0-1.24) | .05 | 0 | .7 | ||
| HSCT and cellular therapies | ||||||||||||||
| After second dose | 401 | 0.61 (0.42-0.80) | 88 | .05 | Single study | Single study | Single study | |||||||
| After first dose | 21 | Single study | No studies available | No studies available | No studies available | |||||||||
| Acute leukemia and MDS | ||||||||||||||
| After second dose | 113 | 0.93 (0.80-1.00) | 29 | .24 | Single study | Single study | Single study | |||||||
| After first dose | 13 | Single study | Single study | Single study | Single study | |||||||||
| MPN and CML | ||||||||||||||
| After second dose | 281 | 0.88 (0.72-1.00) | 70 | .01 | 0.87 (0.81-0.92) | 0 | .83 | 0.99 (0.98-1.00) | 0 | .90 | 0.07 (0.0-1.55) | .06 | 0 | .77 |
| After first dose | 222 | 0.71 (0.30-1.00) | 76 | .01 | 0.54 (0.37-0.71) | 0 | .46 | 0.85 (0.51-1.00) | 90 | <.01 | 0.13 (0.01-1.71) | .09 | 88 | <.01 |
AEs, adverse events; CLL, chronic lymphotic leukemia; CML, chronic myeloid leukemia; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms.
Figure 3.
Pooled rates of seropositivity in single-arm studies involving patients with hematologic malignancies after 2 doses (A) and 1 dose of COVID-19 vaccine (B).
Patients with myeloma.
There were 8 dedicated studies and 9 other studies that reported immune response rates in patients with myeloma (supplemental Table 2). For the 2 studies by Terpos et al,44,45 only nAbs at 2 levels were reported; a threshold of ≥30% was used to define seropositivity and a threshold of ≥50% was used to define rate of positive nAbs. There were 1218 patients with myeloma who received 2 doses of vaccine, and seropositivity rates were 76% to 80%. Seropositivity rates were 29% to 43% after 1 dose of COVID-19 vaccine in 685 patients. The OR for achieving seropositivity was 0.09 (95% CI, 0.03-0.29; P < .01) in patients with myeloma compared with those in the healthy control group after 2 vaccine doses and 0.23 (95% CI, 0.05-0.99; P = .05) after 1 dose (Table 2).
Patients with CLL.
A total of 1557 patients with CLL in 12 studies (6 CLL specific, 6 hematology) were included in this review (supplemental Table 3). Pooled seropositivity rates were 51% in 1446 patients with CLL after 2 doses of COVID-19 vaccine. Rate of seropositivity was 18% after 1 dose of COVID-19 vaccine, and it was 37% in a single-arm study by Ollila et al.35 ORs for seropositivity were 0.01 (95% CI, 0.01-0.03; P < .01) for patients with CLL after 2 doses and 0.03 (95% CI, 0.00-0.80; P = .05) after 1 dose of vaccine compared with that for healthy controls.
Patients with lymphoma.
For 1296 patients with lymphoma across 11 studies (supplemental Table 4), pooled seropositivity rates were 52% to 55% after 2 doses and 33% after 1 dose of COVID-19 vaccine. ORs for achieving seropositivity were 0.01 to 0.02 for patients with lymphoma compared with a healthy cohort (Table 2).
Patients after HCT and CAR T-cell therapy.
A total of 6 studies included in this review reported on immune responses after HCT and CAR T-cell therapy (supplemental Table 5). Two studies involved patients who received an allogeneic HCT (allo-HCT)57,58: One study included patients who received an allo-HCT and those who received CAR T-cell therapy,59 and the other included all patients who received HCTs and those who received CAR T-cell therapy.60 Two studies were larger studies of hematology patients with subsets of patients treated with HCT or CAR T cells.28,29 There were 422 patients of whom 401 received 2 doses of COVID-19 vaccine and the remainder received 1 dose. Pooled seropositivity rate was 61% after 2 doses of COVID-19 vaccine. The seropositivity rate was 74% after allo-HCT and 31% after treatment with CAR T cells. In a single study with a healthy control cohort, seropositivity rate was 81% in patients who received an autologous HCT (auto-HCT) compared with 99% in an age-matched group without haematologic malignancy.29 In the only study of immune response after 1 dose of COVID-19 vaccine, the rate of seropositivity was 38% in patients who received an allo-HCT.57
Patients with acute leukemia or myelodysplastic syndrome (MDS).
There were no dedicated studies of COVID-19 vaccination in patients with acute leukemia. A subset of 126 patients with acute leukemia or MDS were reported in 6 studies (supplemental Table 6), and the seropositivity rate was 93% after 2 doses of COVID-19 vaccine. In a single study, the seropositivity rate was 80% in patients with acute leukemia and 94% in patients with MDS compared with 99% in the control group.29 No patients with acute leukemia mounted an immune response after a single vaccine dose compared with 86% of controls.34
Patients with MPNs or CML.
Of 12 studies (supplemental Table 7) encompassing 503 patients with MPNs or CML (281 after 2 doses, 222 after a single dose), rates of seropositivity were 87% to 88% after 2 doses and 54% to 71% after 1 dose of vaccine. Compared with a healthy patient cohort, the OR was 0.07 (95% CI, 0.0-1.55; P = .06) for patients with MPNs or CML achieving seropositivity with 2 doses of COVID-19 vaccine and 0.13 (95% CI, 0.01-1.71; P = .09) after a single dose.
Rates of nAb and cellular response and AEs
Only 7 studies (16%) reported nAb responses and 5 studies (11%) reported cellular responses. After 2 doses of COVID-19 vaccine, 57% of patients with myeloma achieved a positive nAb response compared with 81% of the control group, whereas 60% of patients with CLL achieved a positive nAb response in a subpopulation of a single-arm study.45,47 After 1 dose of COVID-19 vaccine, the overall pooled rate for a positive nAb response was 18% for all patients with hematologic malignancies (Table 3). The pooled rate of 2 single-arm studies of patients with MPNs was 63%. After COVID-19 vaccination, the rate of achieving a positive cellular response was 40% to 75% for all patients after 2 doses and 33% to 86% after a single dose. In a single study, the cellular response rates after 2 doses of COVID-19 vaccine were 19% for patients who received treatment with allo-HCT and 50% for those who received treatment with CAR T cells.59 In patients with MPNs, this rate was 86% after 1 dose.64,65
Table 3.
Summary of pooled rates of positive nAb, cellular responses, and AEs
| Positive nAb response | Positive cellular response | Atleast 1 AE rate | ||||||
|---|---|---|---|---|---|---|---|---|
| Pooled response rate | 95% CI | Pooled response rate | 95% CI | Pooled response rate | 95% CI | I2 (%) | P | |
| Overall: Patients with hematologic malignancies | ||||||||
| After second dose | Single arm: 0.60 (single study) | Single arm: 0.40 | 0.00-1.00 | 0.36 | 0.24-0.48 | 97 | <.01 | |
| Malignancy arm of comparator studies: 0.57 (single study) | Malignancy arm of comparator studies: 0.75 (single study) | |||||||
| After first dose | Single arm: 0.63 | 0.00-1.00 | Single arm: 0.86 | 0.00-1.00 | 0.39 | 0.18-0.60 | 97 | <0.01 |
| Malignancy arm of comparator studies: 0.18 | 0.00-0.44 | Malignancy arm of comparator studies: 0.33 (single study) | ||||||
In 10 studies (22%), at least 1 systemic or local AE was reported. Overall, the pooled rate of at least 1 AE was 36% after 2 doses and 39% after a single dose (Table 3). Overall, local and systemic AEs were mild (grade 1 to 2) except for a single study in which grade 3 systemic AEs rates were ∼1% to 2%.32,33,39,45,47,49,56,64,65 In patients who received an HCT, a 5% rate of exacerbation of graft-versus-host disease (grade 1 to 2) and grade 3 to 4 neutropenia or thrombocytopenia (self-resolved) was noted.59 Most commonly reported AEs were injection site pain, fatigue, myalgia, headache, and fever.32,33,39,45,47,49,56,59,64,65
Subgroup analysis
Study characteristics and outcomes of studies included in subgroup analyses are summarized in supplemental Tables 8-12. Receiving 2 doses of vaccine during active therapy was associated with seropositivity rates of 28% compared with a rate of 62% when patients were not receiving active therapy with an OR of 0.21 (95% CI, 0.06-0.67; P = .02). Lower seropositivity rates (19% vs 61%) were reported with vaccination during or within 12 months of CD-20 antibody therapy compared with vaccination 12 or more months after completion of therapy. Use of targeted therapy was associated with a pooled seropositivity rate of 35% after 2 doses of COVID-19 vaccine. Seropositivity rates did not differ by timing of vaccination in relation to HCT (66% vs 68%) or vaccine type (BNT162b2 vs others, including mRNA-1273, ChAdOx1, Ad26; [51% vs 64%] [77% vs 81%]) after 1 or 2 doses. Table 4 summarizes the seropositivity rates by each subgroup analyzed.
Table 4.
Summary of subgroup analysis by active treatment, timing of CD20 antibody therapy, HCT, receipt of targeted therapies, and type of COVID-19 vaccine
| Intervention arm | Control arm | Intervention vs control cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled response rate (95% CI) | I2(%) | P | Pooled response rate (95% CI) | I2(%) | P | OR (95% CI) Heterogeneity | P | I2(%) | P | |
| Active therapy vs no active therapy | ||||||||||
| After second dose | 0.28 (0.08-0.48) | 83 | <.01 | 0.62 (0.39-0.86) | 94 | <.01 | 0.21 (0.06-0.67) | .02 | 75 | <.01 |
| After first dose | 0.42 (0.09-0.75) | 49 | .08 | 0.81 (0.66-0.95) | 0 | .62 | 0.19 (0.03-1.19) | .06 | 24 | .27 |
| CD-20 antibody therapy within 12 months vs CD-20 therapy 12 or more months | ||||||||||
| After second dose | 0.19 (0.00-0.50) | 88 | <.01 | 0.61 (0.41-0.82) | 88 | <.01 | 0.08 (0.01-0.59) | .02 | 57 | .04 |
| After first dose | Single study | Single study | Single study | |||||||
| Targeted therapy | ||||||||||
| After second dose | 0.35 (0.19-0.52) | 94 | <.01 | No controls | No analysis | |||||
| After first dose | Single study | No controls | No analysis | |||||||
| HSCT or cellular therapy within 12 months vs after 12 or more months | ||||||||||
| After second dose | 0.66 (0.43-0.88) | 0 | .63 | 0.68 (0.40-0.95) | 45 | .16 | 0.96 (0.11-8.74) | .94 | 36 | .21 |
| After first dose | Single study | Single study | Single study | |||||||
| BNT162b2 vs non-BNT162b2 vaccine type* | ||||||||||
| After second dose | 0.77 (0.40-1.00) | 98 | <.01 | 0.81 (0.56-1.00) | 87 | <.01 | 1.08 (0.20-5.92) | .90 | 64 | .04 |
| After first dose | 0.51 (0.13-0.90) | 86 | <.01 | 0.64 (0.36-0.91) | 73 | .01 | 0.59 (0.22-1.60) | .19 | 7 | .36 |
Non-BNT162b2 includes mRNA-1273, ChAdOx1, and Ad26 vaccines.
Sensitivity analysis
Excluding single-arm studies that were assessed as poor quality or as having a high risk of bias did not alter the pooled seropositivity rates, but in comparator studies, OR was 0.17 (95% CI, 0.04-0.75; P = .03) after 1 dose instead of 0.10 (supplemental Table 13).
Discussion
In this systematic review and meta-analysis of more than 7000 patients with hematologic malignancies, rate of seropositivity was 62% to 66% after 2 doses and 37% to 51% after 1 dose of COVID-19 vaccine. Compared with age-matched and non–age-matched healthy controls (primarily health care workers), odds of achieving seropositivity were significantly lower by 96% after 2 doses of COVID-19 vaccine and 90% after 1 dose. Statistical heterogeneity was substantial at more than 70%, and studies were also clinically heterogenous because of the variety of underlying hematologic malignancies, lack of standardized laboratory platforms by which to measure immune response to vaccination, and variable follow-up periods. Reassuringly, reported rates of at least 1 AE after vaccination were lower at 36% compared with rates of AEs (51% to 88%) reported in clinical trials of the general population.10,13
Among different hematologic malignancies, pooled seropositivity rates after 2 doses were highest at close to 90% in patients with acute leukemia, MDS, or MPNs and the lowest at 51% in patients with CLL. Patients with CLL respond poorly to vaccination, especially to new or novel antigens compared with recall antigens from previous infections or vaccination.67 Poor response is compounded by use of B-cell–depleting and targeted therapies such as CD-20 monoclonal antibodies (mAb) and BTKi’s.67 Immune response to other vaccines, including the seasonal influenza vaccine, is negatively impacted by these therapies, and the poor response persists for at least 6 to 12 months after cessation of therapy.68
A similar negative impact of treatment on COVID-19 vaccine responses was noted in subgroup analysis of published studies. Rates of seropositivity were lower in the setting of active treatment (28%), and the lowest response rates were reported in the setting of current or CD-20 mAb therapy within 12 months (19%), targeted therapies (35%), and after therapy with CAR T cells (31%). Seropositivity rates were 2 to 3 times higher at 62% when patients were vaccinated when they were not receiving active therapy and 61% when vaccinated at 12 or more months after completion of mAb CD-20 therapy.
Unvaccinated patients with CLL have a high burden of morbidity from COVID-19 infection with close to 90% of patients requiring hospital admission, 35% requiring admission to intensive care units, and a mortality rate of 33%.4 Yet patients with CLL have poor humoral responses to vaccination: 18% after 1 dose and 51% after 2 doses. In patients with CLL, immune suppression from underlying disease and ongoing treatments such as anti-CD20 mAb’s and BTKi’s continue to pose a risk for SARS-CoV-2 infection and concurrently limit protective responses from vaccination. Although vaccination remains highly recommended, new strategies are required to further improve immune responses in this high-risk patient population. In other groups of immune compromised patients, the use of an additional dose of COVID-19 vaccine improved serologic response rates by 37%, and the use of heterologous vaccination schedules (mixing vaccine types) appears promising.69-71 International health groups now recommend a third dose of COVID-19 vaccine for immune compromised patients, including those with hematologic malignancies.72,73 Heterologous prime boost or use of high-dose vaccine formulations have been used in randomized trials of seasonal influenza vaccination in hematology and in patients who have received an HCT with mixed success.17,74 In addition, other preventative approaches such as the use of anti–SARS-CoV-2 mAb therapy as pre-exposure (NCT04625725) and post-exposure prophylaxis require further evaluation in vulnerable patient groups who respond poorly to vaccination.75
Serologic responses are classically used as surrogate end points for clinical efficacy in clinical trials of vaccination in patients with hematologic malignancies.15-17 Serologic thresholds for protection (seroprotection) after vaccination have been established for infections such as seasonal influenza.17 In the majority of studies included in this review, however, the outcome of interest was seropositivity as defined by antibody levels above the detection threshold. This is not equivalent to seroprotection because thresholds have not been standardized nor have they been established across the variety of commercial and research platforms used by these studies. Some authors such as Malard et al,33 Stampfer et al,43 and Redjoul et al58 have attempted to define serologic thresholds that correlate with nAb and clinical protection. Unsurprisingly, a lower proportion of patients (by 20% to 35% compared with pooled rates) achieved these higher antibody thresholds.33,43,58 For serologic response measurement to guide clinical management of COVID-19 vaccination, further work is required to achieve harmonization across testing platforms and to derive and validate thresholds that correlate with clinical protection.
Measurement of serologic responses offers only a glimpse of the potential breadth of immune responses to COVID-19 vaccination. Both nAb and cellular responses to vaccination play complementary and vital roles in protection against COVID-19 and remain under-reported.76-78 In 16% of studies, at least 18% of hematology patients achieved a positive nAb response. Positive cellular response rates were at least 15% higher than nAb responses after 1 or 2 doses of vaccine. Although serologic responses have been used as surrogate end points in vaccination studies of immune compromised patients, further large studies are required to identify new immune markers for vaccine response and to determine the efficacy of vaccination.
This review has several limitations. In particular, the findings are of moderate quality because of significant clinical and statistical heterogeneity of included studies and the proportion of studies of poorer quality. In line with other established studies of vaccination in hematology patients, only immune response data were analyzed because clinical efficacy data were limited.
In conclusion, this systematic review and meta-analysis has comprehensively summarized the latest data on response to COVID-19 vaccination in patients with hematologic malignancies. Overall, seropositive rates were reasonable at 66% after 2 doses of vaccine. Higher-risk patient groups were identified, namely patients with CLL and patients receiving active therapy, including targeted and CD-20 mAb therapies. New approaches to treating high-risk patients who are poor responders to vaccination are urgently required.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
B.W.T. is supported by the Australian Government Medical Research Future Fund Investigator Fellowship. M.A.S is supported by a National Health and Medical Research Council Investigator Fellowship.
Authorship
Contribution: B.W.T coordinated the study, performed abstract and full-text screening and data extraction, and wrote the manuscript; J.S.K.T. performed abstract and full-text screening and data extraction; J.C. developed the study protocol and performed data extraction; S.L. developed the search strategy; T.S. conducted the statistical analysis; Z.C.F.N. and M.A.S. assessed the risk of bias in the studies; and all authors reviewed and revised the manuscript.
Conflict-of-interest disclosure: M.A.S. has received research funding and honoraria from Pfizer and Merck Sharp & Dohme and research funding from Gilead. B.W.T has received research funding and honoraria from CSL-Behring, Merck Sharp & Dohme, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Benjamin W. Teh, Department of Infectious Diseases, Peter MacCallum Cancer Centre, 305 Grattan Street, Melbourne, Vic 3000, Australia; e-mail: ben.teh@petermac.org.
References
- 1.Phillips N. The coronavirus is here to stay - here’s what that means. Nature. 2021;590(7846):382-384. [DOI] [PubMed] [Google Scholar]
- 2.Wood WA, Neuberg DS, Thompson JC, et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4(23):5966-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185-e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JA, et al. Risk factors and mortality of COVID-19 in patients with lymphoma: A multicenter study. HemaSphere. 2021;5(3):e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari A, Samur MK, Martinez-Lopez J, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook G, John Ashcroft A, Pratt G, et al. ; United Kingdom Myeloma Forum . Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190(2):e83-e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath PT, Galiza EP, Baxter DN, et al. ; 2019nCoV-302 Study Group . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tau N, Manuel O, Rozen-Zvi B, Shargian L, Yahav D. Precautions after vaccinating immunosuppressed patients with mRNA-based vaccines against SARS-CoV-2: does one size fit all? Clin Microbiol Infect. 2021;27(12):1727-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordonnier C, Labopin M, Chesnel V, et al. ; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation . Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48(10):1392-1401. [DOI] [PubMed] [Google Scholar]
- 16.Cordonnier C, Ljungman P, Juergens C, et al. ; 3003 Study Group . Immunogenicity, safety, and tolerability of 13-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine in recipients of allogeneic hematopoietic stem cell transplant aged ≥2 years: an open-label study. Clin Infect Dis. 2015;61(3):313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teh BW, Leung VKY, Mordant FL, et al. A randomised trial of two 2-dose influenza vaccination strategies for patients following autologous haematopoietic stem cell transplantation [published online ahead of print on 11 November 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa1711. [DOI] [PubMed] [Google Scholar]
- 18.Tomblyn M, Chiller T, Einsele H, et al. ; Centers for Disease Control and Prevention . Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America . 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44-e100. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 22.Sharmin S, Kypri K, Khanam M, Wadolowski M, Bruno R, Mattick RP. Parental supply of alcohol in childhood and risky drinking in adolescence: systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14(3): 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091-1098.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benda M, Mutschlechner B, Ulmer H, et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol. 2021;195(4):523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen D, Hazut Krauthammer S, Cohen YC, et al. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021;48(11):3540-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavriatopoulou M, Terpos E, Kastritis E, et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine published online ahead of print 20 July 2021]. Clin Exp Med. doi: 10.1007/s10238-021-00746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacono D, Cerbone L, Palombi L, et al. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021;12(8):1253-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am J Hematol. 2021;96(11):E410-E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583-e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11(8):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ollila TA, Lu S, Masel R, et al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol. 2021; 7(11):1714-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Re D, Barrière J, Chamorey E, et al. Low rate of seroconversion after mRNA anti-SARS-CoV-2 vaccination in patients with hematological malignancies. Leuk Lymphoma. 2021;62(13):3308-3310. [DOI] [PubMed] [Google Scholar]
- 38.Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081-1090.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195(2):186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389-e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghandili S, Schönlein M, Lütgehetmann M, et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers (Basel). 2021;13(15):3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramasamy K, Sadler R, Jeans S, et al. COVID symptoms, testing, shielding impact on patient-reported outcomes and early vaccine responses in individuals with multiple myeloma [published online ahead of print 2 August 2021]. Br J Haematol. doi: 10.1111/bjh.17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stampfer SD, Goldwater MS, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Oekelen O, Gleason CR, Agte S, et al. ; PVI/Seronet team . Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia [published online ahead of print 29 July 2021]. Haematologica. doi: 10.3324/haematol.2021.279196. [DOI] [Google Scholar]
- 48.Del Poeta G, Bomben R, Polesel J, et al. COVID-19 vaccination: evaluation of risk for protection failure in chronic lymphocytic leukemia patients. Hematol Oncol. 2021;39(5):712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9): 2703-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tadmor T, Benjamini O, Braester A, Rahav G, Rokach L. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2727-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138(9):811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurion R, Rozovski U, Itchaki G, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies [published online ahead of print 29 July 2021]. Haematologica. doi: 10.3324/haematol.2021.279216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542-e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Easdale S, Shea R, Ellis L, et al. Serologic responses following a single dose of SARS-Cov-2 vaccination in allogeneic stem cell transplantation recipients. Transplant Cell Ther; 2021;27(10):880.e1-880.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caocci G, Mulas O, Mantovani D, et al. Ruxolitinib does not impair humoral immune response to COVID-19 vaccination with BNT162b2 mRNA COVID-19 vaccine in patients with myelofibrosis [published online ahead of print 24 July 2021]. Ann Hematol. doi: 10.1007/s00277-021-04613-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowdhury O, Bruguier H, Mallett G, et al. Impaired antibody response to COVID-19 vaccination in patients with chronic myeloid neoplasms. Br J Haematol. 2021;194(6):1010-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guglielmelli P, Mazzoni A, Maggi L, et al. Impaired response to first SARS-CoV-2 dose vaccination in myeloproliferative neoplasm patients receiving ruxolitinib. Am J Hematol. 2021;96(11):E408-E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194(6):999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35(12):3573-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pimpinelli F, Marchesi F, Piaggio G, et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J Hematol Oncol. 2021;14(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv. 2021;5(12):2624-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall VGFV, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Shaw RH, Stuart ASV, et al. ; Com-COV Study Group . Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill JA, Ujjani CS, Greninger AL, Shadman M, Gopal AK. Immunogenicity of a heterologous COVID-19 vaccine after failed vaccination in a lymphoma patient. Cancer Cell. 2021;39(8):1037-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
- 73.United Nations, UN News: Global perspective Human stories. WHO advisory group recommends extra COVID-19 vaccine dose for immunocompromised. https://news.un.org/en/story/2021/10/1102732.
- 74.Halasa NB, Savani BN, Asokan I, et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2016;22(3):528-535. [DOI] [PubMed] [Google Scholar]
- 75.Cohen MS, Nirula A, Mulligan MJ, et al. ; BLAZE-2 Investigators . Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: A randomized clinical trial. JAMA. 2021;326(1):46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dagotto G, Yu J, Barouch DH. Approaches and challenges in SARS-CoV-2 vaccine development. Cell Host Microbe. 2020;28(3):364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flanagan KL, Best E, Crawford NW, et al. Progress and pitfalls in the quest for effective SARS-CoV-2 (COVID-19) vaccines. Front Immunol. 2020;11:579250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.