Significance
Optimal follicular helper T (Tfh) cell differentiation and function are required for effective humoral immunity against infection, while improper Tfh cell responses are associated with autoimmunity and allergy. We demonstrate that Gα13—a Gα protein subunit known to be involved in mediating signals related to cytoskeletal integrity, chemotaxis, and migration—acts as an essential positive regulator in Tfh cell development and function. The deletion of Gα13 in T cells results in dampened germinal center reactions in immunization and viral infection models. Mechanistically, Gα13-RhoA-ROCK2 axis is responsible for the Tfh cell differentiation from naïve precursors, and Rho agonists recuperate hampered Tfh cell function in Gα13-deficient mice. Such mechanistic insight underscores the possibility of targeting Gα13-mediated signaling to maneuver Tfh cell responses.
Keywords: Gα13, Tfh cell, germinal center reaction, ROCK
Abstract
GPCR-Gα protein–mediated signal transduction contributes to spatiotemporal interactions between immune cells to fine-tune and facilitate the process of inflammation and host protection. Beyond this, however, how Gα proteins contribute to the helper T cell subset differentiation and adaptive response have been underappreciated. Here, we found that Gα13 signaling in T cells plays a crucial role in inducing follicular helper T (Tfh) cell differentiation in vivo. T cell–specific Gα13-deficient mice have diminished Tfh cell responses in a cell-intrinsic manner in response to immunization, lymphocytic choriomeningitis virus infection, and allergen challenges. Moreover, Gα13-deficient Tfh cells express reduced levels of Bcl-6 and CXCR5 and are functionally impaired in their ability to adhere to and stimulate B cells. Mechanistically, Gα13-deficient Tfh cells harbor defective Rho-ROCK2 activation, and Rho agonist treatment recuperates Tfh cell differentiation and expression of Bcl-6 and CXCR5 in Tfh cells of T cell–specific Gα13-deficient mice. Conversely, ROCK inhibitor treatment hampers Tfh cell differentiation in wild-type mice. These findings unveil a crucial regulatory role of Gα13-Rho-ROCK axis in optimal Tfh cell differentiation and function, which might be a promising target for pharmacologic intervention in vaccine development as well as antibody-mediated immune disorders.
Gα proteins, activated upon the binding of ligands to their respective G protein–coupled receptors (GPCRs), transduce outside signals to the cell’s interior by binding to either GTP or GDP. Gα proteins are classified into four families—Gαs, Gαi, Gαq, and Gα12/13—with each subfamily consisting of multiple members and many of its members being activated simultaneously by one GPCR to transmit signal. GPCRs are stimulated in response to a variety of ligands, from chemokines, nucleotides, and peptides to hormones and other lipids, and mediate a wide range of cellular function: adhesion, contraction, motility, and proliferation—to name a few. The role of GPCR is fundamental in dynamic biological processes, such as inflammation and immune responses, whereby the careful spatiotemporal orchestration of leukocytes is required. As such, the biological role of Gα proteins in mediating adhesion, chemotaxis, migration, and the motility of immune cells through different tissue types and organ systems has been the interest of many for some time (1). For example, in the context of inflammation, chemokines, sphingosine-1-phosphate (S1P), lysophosphatidic acid, and thrombins signal via Gα12/13-coupled receptors to recruit immune cells to the site of inflammation and promote entry into lymphoid organs, thereby amplifying the inflammatory response (2).
In addition, several groups have provided insight with regard to Gα protein’s role in immune cell survival and retention in lymphoid organs (3–5). For example, the loss of Gα12/13-signaling cells has been shown to promote aberrant B cell survival and skewed retention in secondary lymphoid organs (SLOs), thereby promoting B cell cancer (6–8). Similarly, Gα12/13-coupled receptors have been shown to be critical in T cell polarization, adhesiveness, and retention within the lymph nodes (LNs) (4, 9). Recent efforts have provided detailed descriptions of how motility and chemotactile gradients are fine-tuned by the interplay of various GPCRs (10, 11). In particular, follicular helper T (Tfh) cells seem to be one of the more sensitive subsets to GPCR-mediated signaling out of the helper T cell subsets, presumably because of its requirement to migrate to and from cellular regions within the SLOs to fully mature and accomplish its B cell help function (12). Antigen-specific Tfh cells access the B cell follicle by up-regulating CXCR5 (mediated via Gαi signaling) and by down-regulating CCR7, enabling B cell clonal expansion and subsequent germinal center (GC) response (13). GPR183 (or more commonly known as EBI2) is mediated by Gαi signaling and serves as another important receptor for Tfh cell commitment by guiding progenitor Tfh cells to interact with dendritic cells (DCs) (14). S1PR2, a GPCR mediated by Gα12/13 signaling, regulates LN retention via chemorepulsion and has been shown to be also important in Tfh cell maturation by directing colocalization with B cell within the GCs (9).
Beyond this kinetic insight of Gα protein’s involvement on T cell function, there still exist a significant gap in our understanding of how exactly Gα protein–mediated signaling can regulate the differentiation program of CD4 T cell subsets and the function of effector T cells in vivo. To address the knowledge gap, we employed a T cell–specific knockout (KO) mouse of a particular heterotrimeric Gα protein subunit, Gα13, which in our results was shown to play nonredundant roles with its subfamily member protein. By illustrating how the deficiency of Gα13 signaling can impact the development of a specific helper T cell subset and critically affect its function, our study highlights the importance of Gα13-RhoA–Rho-associated protein kinase 2 (ROCK2) signaling pathway in early Tfh cell differentiation and function in helping B cells.
Results
Contribution of Gα13 Signaling in T Cells to Thymic Development and Distribution to Secondary Lymphoid Organs.
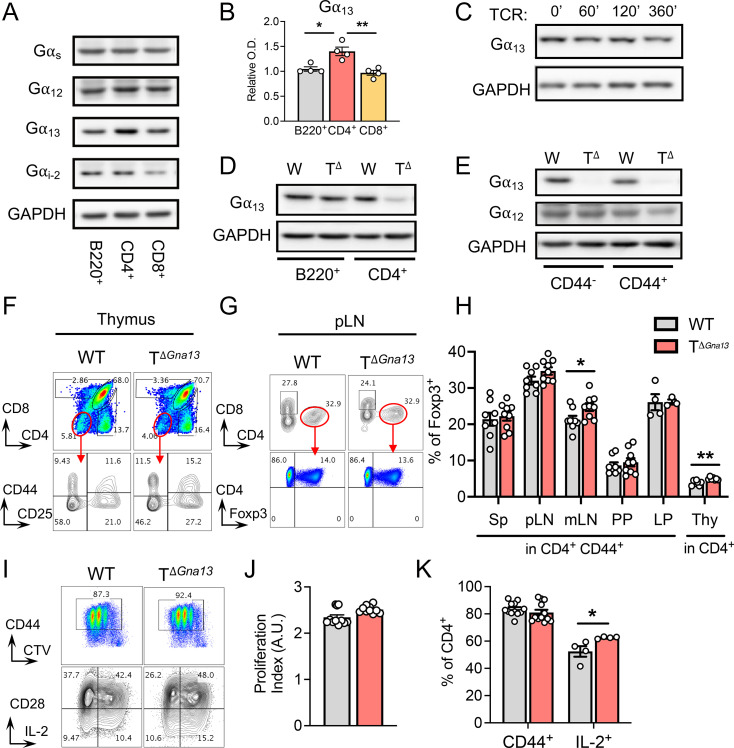
As a first step to investigate the role of Gα13 in T cells, we compared the expression of various members of the G protein α family in flow-sorted B220+ B cells, CD4+ T cells, and CD8+ T cells by Western blot. While the levels of Gαs and Gα12 expression were comparable among the lymphocyte subsets, those of Gα13 were slightly higher in CD4+ T cells compared to other lymphocytes (Fig. 1A and B and SI Appendix, Fig. S1A). T cell receptor (TCR) signaling triggered by anti-CD3/CD28 stimulation induced little changes in the level of Gα13 expression in CD4+ T cells (Fig. 1C). The germline deletion of Gna13 results in embryonic lethality (15). To investigate the role of Gα13 in T cells, we established a T cell conditional Gα13 KO (TΔGna13) mouse system by crossing Gna13f/f strain with CD4Cre mice. We confirmed the efficient deletion of Gα13 in CD4+ T cells but not in B cells (Fig. 1D). While the deletion of Gα13 often leads to the up-regulation of Gα12 as a complementary mechanism (15), the expression of Gα12 was relatively unaltered in Gα13-deficient CD4+ T cells, regardless of the activation status defined by CD44 expression (Fig. 1E).
Fig. 1.
Gα13 signaling in T cells is dispensable for the development and activation of T cells. (A and B) WT lymphocytes were flow sorted and analyzed. (A) Western blot of WT B-, CD4+ and CD8+ T cells to detect Gα subunit proteins, with GAPDH as the loading control and (B) corresponding, relative optical densitometry graphs (n = 4 per group). (C) Western blot of WT, naïve CD4+ T cells that were unstimulated (0’) or stimulated with anti-CD3/CD28 for the indicated times to detect Gα13. (D) Western blot of either WT or TΔGna13 B and CD4+ T cells to detect Gα13 protein. (E) Western blot of either naïve or activated (CD44+) CD4+ T cells to detect Gα12 and Gα13 proteins. (F and G) Representative fluorescence-activated cell sorting (FACS) plots of T cell subset analysis in the thymus (F) and SLO pLN (G), peripheral LNs at steady state. (H) Frequencies of Foxp3+ Tregs in various organs from two independent experiments combined (n = 4 to 9 per group). Sp, spleen; mLN, mesenteric LN; LP; lamina propria; and Thy, thymus. (I–K) Naïve T cells were stimulated with anti-CD3/CD28 for 2 d for proliferation analysis. (I) Representative FACS plots of CD44, CD28, IL-2 expression, and cell division. Calculated proliferation index denoted in arbitrary units (A.U.). (J) and frequencies (K) of CD44+ and IL-2+ CD4+ T cells (n = 4 to 13 per group). All data are representative of two independent experiments, unless stated otherwise. All data are mean ± SEM; *P < 0.05; and **P < 0.01.
Gα13 signaling has been proposed to play a role in early thymocyte proliferation and survival in vitro (16). Additionally, Gα12 and Gα13 double-deficient mice have been shown to harbor increased cellularity in the thymus (4). When we analyzed the subsets of thymocytes between TΔGna13 and littermate control (Gna13f/f) mice, we found that the frequencies and the absolute number of all four double-negative stages were comparable between the two groups (Fig. 1F and SI Appendix, Fig. S1 B–D). Hence, Gα13 signaling in T cells is dispensable for the development of T cells in the thymus. The analyses of SLOs demonstrated that both total cell numbers and frequencies of CD4+ and CD8+ T cells were comparable between the two groups regardless of CD44 expression level (Fig. 1G and SI Appendix, Fig. S1 E and F). In addition, Treg frequencies in the periphery and the thymus were either comparable or slightly higher in the TΔGna13 mice (Fig. 1H). To examine the involvement of Gα13 in TCR-triggered activation, we compared the proliferation and expression of CD44, CD28, and IL-2 after stimulating naïve CD4+ T cells with plate-bound anti-CD3 and anti-CD28 and found similar degrees of activation, proliferation (Fig. 1 I–K), and apoptosis (SI Appendix, Fig. S1 H and I) overall, with an ∼10% increase in IL-2 secretion in the TΔGna13 group. These series of in vitro experiments, along with thymocyte analysis, indicate that the Gα13-mediated signaling in T cells is largely dispensable for the overall development of conventional T cells in the thymus, the distribution of mature T cells into SLOs in vivo, and the TCR-mediated activation and proliferation of CD4+ T cells.
Lack of Gα13 Signaling in T Cells Results in Diminished Tfh Cell Responses In Vivo.
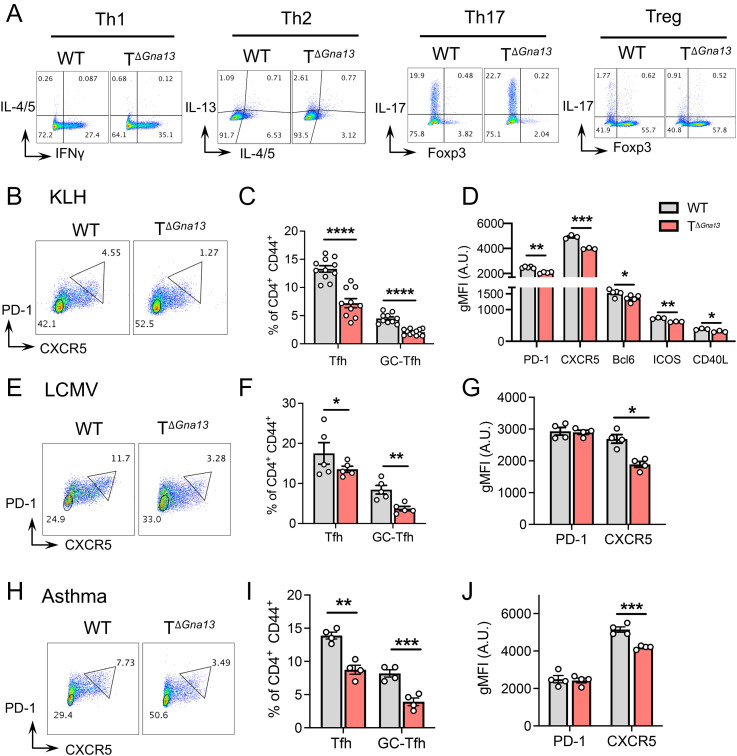
To define the role of Gα13-mediated signaling in peripheral T cell responses, we first investigated the differentiation capabilities of naïve T cells under Th1-, Th2-, Th17-, and Treg-skewing conditions in vitro and found that the differentiated frequencies of Th1, Th2, and Treg cells were comparable, while Th17 cell frequencies were slightly increased in the TΔGna13 group compared to littermate control (Fig. 2A and SI Appendix, Fig. S2A). We then analyzed CD4+ effector Th subsets in the SLOs of TΔGna13 mice at steady state and found that Th1 cell frequencies were slightly elevated in the gut-associated lymphoid tissues of TΔGna13 mice, while the opposite was true in the spleen (SI Appendix, Fig. S2B). The frequencies of Th17 cell and Treg cell were comparable in all examined secondary lymphoid tissues between the two groups overall, except for the slight increase in Treg cell frequencies in the mesenteric LNs (Fig. 1H and SI Appendix, Fig. S2C). Concomitantly, we observed a trend toward diminished Tfh cell frequencies (SI Appendix, Fig. S2 D and E) and absolute numbers (SI Appendix, Fig. S2F) in the Peyer’s patches (PPs) of TΔGna13 mice, albeit no such differences in absolute cell numbers were observed for other Th subsets within the PPs (SI Appendix, Fig. S2F). Additionally, absolute cell counts of the above lymphoid organs were comparable between the two groups, with the exception of the spleen, which was slightly higher in the TΔGna13 group (SI Appendix, Fig. S2G).
Fig. 2.
Gα13 deficiency leads to diminished Tfh cell responses in vivo. (A) Representative fluorescence-activated cell sorting (FACS) plots of naïve T cells differentiated to Th1, Th2, Th17, and Treg conditions in vitro. (B–D) Mice were s.c. immunized with KLH emulsified in CFA at the side flanks, and inguinal LNs were analyzed on day 8. (B) Representative FACS plots of GC-Tfh, gated as CXCR5hi PD-1hi. The image representative of three independent experiments is shown. (C) Total and GC-Tfh frequencies (n = 9 to 10 per group). (D) Geometric mean fluorescence intensities (gMFI) of Tfh markers and molecules (n = 3 to 5 per group). (E–G) Mice were infected with 3 × 105 plaque-forming units (pfu) of LCMV Armstrong, and spleens were analyzed on day 6. (E) Representative FACS plots of GC-Tfh (CXCR5hi PD-1hi) analysis. Frequencies (F) and gMFI (G) of Tfh markers (n = 4 to 5 per group). (H–J) Mice were intranasally challenged with a mixture of OVA and Aspergiluus oryzae (PAO), and mediastinal LNs were analyzed on day 9. (H) Representative FACS plots of GC-Tfh analysis. Frequencies (I) and gMFI (J) of Tfh markers denoted in arbitrary units (A.U.). (n = 4 per group). All data are representative of two independent experiments, unless stated otherwise. All data are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
To evaluate whether Gα13 signaling regulates antigen-specific CD4+ T cell responses in vivo, we subcutaneously (s.c.) immunized TΔGna13 and littermate control mice with KLH in CFA and compared the CD4+ T cell subsets in the draining LNs. We observed that Th1 and Th17 cell frequencies were all comparable between the two groups, with a slight increase in Treg frequencies in the TΔGna13 group (SI Appendix, Fig. S2H). In accordance with these results, ex vivo KLH restimulation of lymphoid cells from the draining LNs showed no difference in IFN-γ and IL-17 production (SI Appendix, Fig. S2I). Total Tfh frequencies were defined to be CXCR5+PD-1+, among which GC-Tfh cells were defined as CXCR5hiPD-1hi (SI Appendix, Fig. S3 A–C). In sharp contrast to the other Th subsets, the frequencies, as well as absolute number, of total and GC-Tfh cells were significantly lower in the TΔGna13 mice than in the littermate control mice (Fig. 2 B and C and SI Appendix, Fig. S3D). In addition, the signature surface markers of Tfh cells, such as CXCR5, PD-1, ICOS, and CD40L, showed significantly lower expression levels in Gα13-deficient Tfh cells than in Gα13-sufficient Tfh cells (Fig. 2D). The expression of Bcl-6, the master transcription factor that commits Tfh cells to the lineage, was also relatively diminished in the former (Fig. 2D). The Ki-67 staining of T cell subsets showed comparable proliferative capacity between the two groups (SI Appendix, Fig. S3E).
To determine further the importance of Gα13 signaling in Tfh cell differentiation in vivo, we employed two additional animal models in which the development of Tfh cells upon antigenic encounter is well-established: lymphocytic choriomeningitis virus (LCMV) infection and allergic asthma (17, 18). In both animal models, we observed a significant reduction in the frequency of Tfh cells among CD4+ T cells in the TΔGna13 mice compared to that of control mice (Fig. 2 E, F, H, and I, respectively, for each model). While frequencies of other helper T cell subsets remained comparable, those of Treg cells detected in the spleens of LCMV model were lower, while Treg cell frequencies in the mediastinal LNs of asthma model were significantly higher in TΔGna13 mice compared to those of control mice (SI Appendix, Fig. S3 F and G). Similar to the KLH model, the expression level of CXCR5 and Bcl-6 was consistently lower in the Gα13-deficient Tfh cells than Gα13-sufficient ones in both models (Fig. 2 G and J and SI Appendix, Fig. S3H). Of note, the frequencies of the fully committed GC-Tfh cells, characterized by CXCR5hi PD-1hi, were all consistently lower in the TΔGna13 group in all three models, indicating that Gα13 signal is integral for triggering Tfh cell commitment (Fig. 2 E, F, H, and I). Altogether, these results indicate that the lack of Gα13-mediated signaling in T cells leads to specifically diminished Tfh cell responses in vivo, regardless of the antigen type or immunization method used.
Gα13 Signaling in T Cell Is Required for Optimal GC Reactions and Humoral Responses.
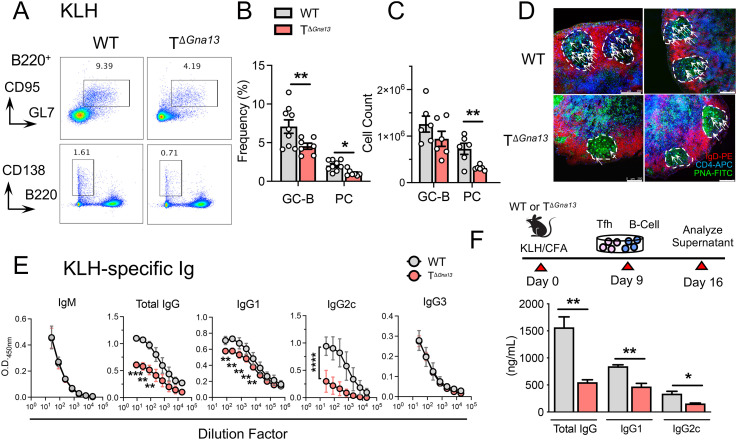
Tfh cells facilitate GC reactions within the SLOs by providing costimulation and cytokines to activated B cells. The diminished Tfh cell responses observed in the TΔGna13 mice prompted us to investigate whether GC reactions are affected in the TΔGna13 mice. While the frequencies of total B220+ B cells were comparable between the two groups at steady state (SI Appendix, Fig. S4A, Left), the frequencies of CD95+GL7+B220+ GC B cells were significantly lower in the PPs of the TΔGna13 mice compared to the littermate control mice (SI Appendix, Fig. S4A, Left). The comparison of absolute cell numbers between the two groups yielded similar trends (SI Appendix, Fig. S4A, Right). Such differences were not observed in other SLOs or were rather reversed in the case of mesenteric LNs (SI Appendix, Fig. S4B). No significant differences were observed in the levels of circulating total IgG at steady state (SI Appendix, Fig. S4C).
Unlike unimmunized mice, we observed a significant reduction in the frequencies of GC B cells and plasma cells (PCs) in the draining LNs of TΔGna13 mice upon s.c. immunization with KLH in CFA, compared to those of littermate control (Fig. 3 A–C). The visualization of the GC showed the relatively poor colocalization of CD4+ T cells within each GC of the draining LNs in the TΔGna13 mice compared to that of control mice, while overall follicle formation seemed to be unaffected (Fig. 3D and SI Appendix, Fig. S4D). Moreover, serum levels of KLH-specific IgG—both IgG1 and IgG2 subclasses—were found to be significantly lower in the former, while those of IgM and IgG3 were comparable between two groups (Fig. 3E). Given that IgM and IgG3 production is largely independent of T cell help, the selective reduction in KLH-specific IgG1 and IgG2 subclasses strongly suggests that diminished Tfh cell responses likely led to reduced KLH-specific IgG in the TΔGna13 mice in this experimental setting.
Fig. 3.
Gα13 signaling in T cells is required for optimal GC reactions and humoral responses. (A–E) Mice were s.c. immunized with KLH in CFA at the side flanks, and inguinal LNs were analyzed on day 8 for cell analysis or day 10 for serum analysis. (A) Representative fluorescence-activated cell sorting plots of GC B and PC analysis from three independent experiments. Frequencies (B) and absolute cell counts (C) of GC B cells and PC (n = 6 to 9 per group). (D) Representative immunofluorescence image of the GC within the draining LNs. White arrows indicate CD4+ T and B cell colocalization within the B cell follicle. White bar on the lower right corner indicates the 250-μm scale. (E) Antigen-specific serum Ig enzyme-linked immunosorbent assay (ELISA) analysis (n = 3 per group). Values are denoted as optical density (O.D.). (F) Tfh cells were flow sorted and cocultured with naïve B cells ex vivo for 7 d prior to Ig ELISA analysis of the culture supernatant. Data are representative of three independent experiments (n = 5 per group). All data are representative of two independent experiments, unless stated otherwise. All data are mean ± SEM; *P < 0.05; and **P < 0.01.
Gα13 deficiency not only led to diminished frequencies of Tfh cells, but also resulted in diminished expression of CXCR5, ICOS, and CD40L on Tfh cells, raising a possibility that Gα13-deficient Tfh cells are functionally impaired. To explore this possibility, we flow-sorted Tfh cells from the KLH-immunized draining LNs of the respective mice and cocultured them with naïve B cells isolated from wild-type (WT) syngeneic mice (Fig. 3F, Top). The amount of IgG in the supernatant was significantly lower in the conditions in which Gα13-deficient Tfh were cocultured with B cells by approximately threefold (Fig. 3F, Bottom). Both IgG1 and IgG2c subclasses were significantly diminished in the coculture supernatant, similar to the in vivo serum analysis (Fig. 3F, Bottom). Trends of reduced humoral responses were also partially observed in LCMV and allergic asthma models, in which GC B cell or PC frequencies, as well as antigen-specific IgG levels, were lower in TΔGna13 mice (SI Appendix, Fig. S4 E and F). Gα13-sufficient, as well as -deficient, Tfh cells showed equivalent survival and proliferation (SI Appendix, Fig. S4G). These results collectively demonstrate that the Gα13-mediated signaling in T cells is integral not only for the differentiation of Tfh cells, but also for its function in facilitating optimal GC reactions and antigen-specific IgG production in vivo.
Gα13 Signaling Promotes Tfh Cell Differentiation in a T Cell–intrinsic Manner.
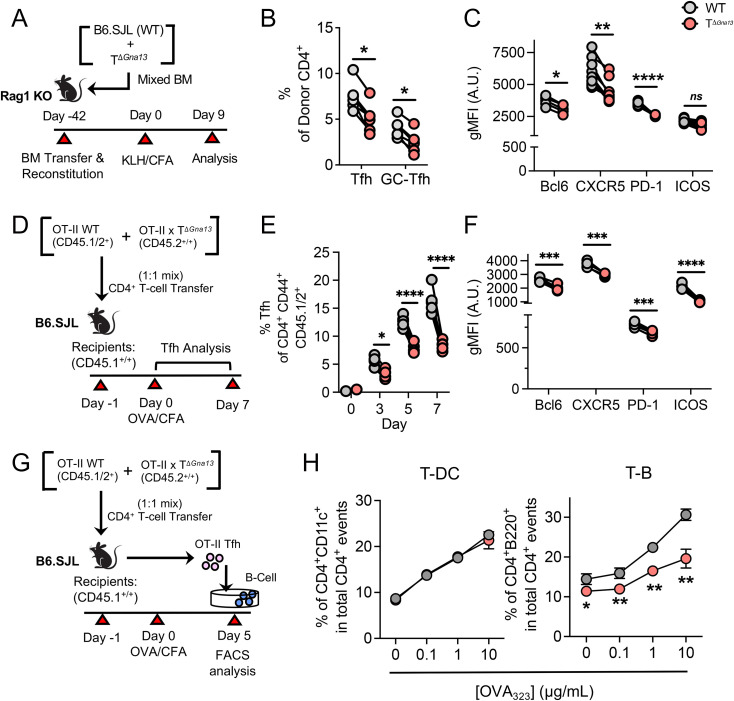
Next, we sought to determine the mechanism by which Gα13 signaling contributes to Tfh cell responses in vivo. To test whether Gα13 triggers Tfh cell differentiation in a cell-intrinsic manner, we employed two different in vivo models. First, we established a mixed bone chimeric mice system, in which congenically marked WT (CD45.1+/+) bone marrow was mixed with TΔGna13 (CD45.2+/+) bone marrow at 1:1 ratio before being adoptively transferred to bone marrow–ablated Rag1−/− mice (Fig. 4A). Following a 6-wk reconstitution period, mice were subjected to KLH immunization and subsequent analysis on day 9, adhering to the gating strategy as shown (SI Appendix, Fig. S5A). The frequencies of Tfh cells, and particularly CXCR5hiPD-1hi GC-Tfh cells, were significantly lower in the TΔGna13 compartment than WT (Fig. 4B). Moreover, the expression levels of Bcl-6, CXCR5, and PD-1 on Gα13-deficient Tfh cells were all significantly diminished compared to those of Gα13-sufficient Tfh cells in the same chimeric mice, although levels of ICOS were comparable (Fig. 4C). Unlike previous models, frequencies of Treg cell were slightly but significantly lower in the TΔGna13 group, while follicular regulatory T cell frequencies were relatively similar between the two donor populations (SI Appendix, Fig. S5 C and D), suggesting that the T cell–intrinsic differences in Tfh responses were not due to differences in Treg cells.
Fig. 4.
Gα13 signaling promotes Tfh cell differentiation in a cell-intrinsic manner. (A–C) Mixed bone marrow (BM) chimera experiments were conducted according to the scheme shown in A. Inguinal LNs were analyzed on day 9. (B) Summary of respective donor Tfh and GC-Tfh frequencies (n = 5 per group). (C) gMFI values of Tfh marker and molecules denoted as arbitrary units (A.U.) (n = 6 per group). (D–F) OT-II cotransfer experiment was conducted according to the scheme shown in D. Inguinal LNs were analyzed on days 0, 3, 5, and 7. (E) Summary of respective donor Tfh and GC-Tfh frequencies. (F) gMFI values of Tfh marker and molecules (n = 5 per group). (G) Experimental scheme of conjugate formation assay. (H) Antigen-specific T-DC and T-B conjugate efficiencies of WT or Gα13-deficient OT-II Tfh cells (n = 3 per group). All data are representative of two independent experiments. All data are mean ± SEM; ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
In the second model, we cotransferred WT (CD45.1+/CD45.2+) and Gα13-deficient (CD45.2+/+) OT-II T cells at 1:1 ratio into congenic mice (CD45.1+/+), followed by immunization with ovalbumin (OVA) in CFA (Fig. 4D and SI Appendix, Fig. S5B). Kinetic analysis revealed that Gα13-deficient OT-II T cells showed consistent impairment in Tfh cell differentiation, starting as early as day 3 postimmunization (Fig. 4E and SI Appendix, Fig. S5E), while the comparable expression of CD44 in both donor groups indicated that the overall activation of OT-II donor cells was comparable (SI Appendix, Fig. S5F). The impairment most affected CXCR5hiPD-1hi GC-Tfh cell population (SI Appendix, Fig. S5G) and led to significantly lower expression levels of Bcl-6, CXCR5, PD-1, and ICOS (Fig. 4F). To compare the efficiency of T-B cell conjugate formation between Gα13-sufficient and -deficient Tfh cells, OT-II Tfh cells were coincubated with either OT-II peptide-loaded DC or B cells (Fig. 4G). While demonstrating comparable T-DC conjugate formation, Gα13-deficient Tfh cells were significantly less efficient than Gα13-sufficient ones in forming conjugates with B cells (Fig. 4H). We observed similar results using in vitro–generated “Tfh-like” cells (SI Appendix, Fig. S5H). Thus, Gα13 signaling plays a crucial, cell-intrinsic role to promote the differentiation of Tfh cells that stably interact with cognate B cells.
Gα13 Signaling Is Required for the Activation of RhoA-ROCK Pathway in Tfh Cell.
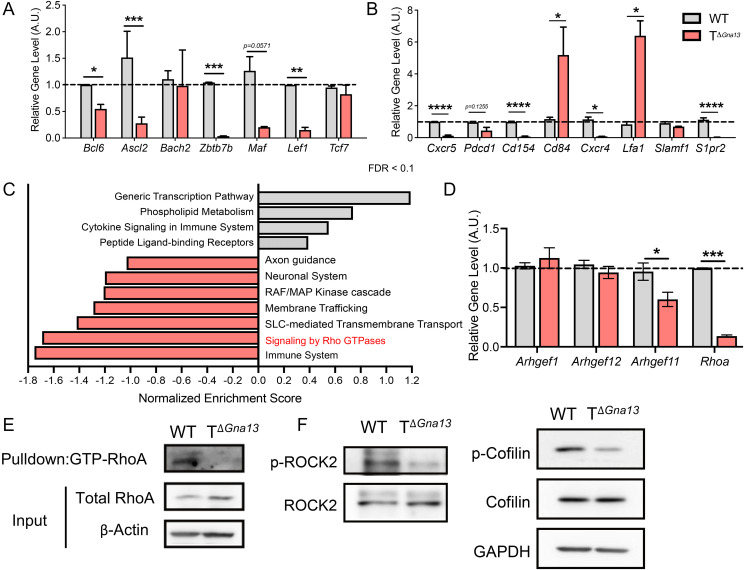
We next compared the relative transcript levels of major transcription factors involved in the Tfh cell differentiation program and found that Bcl6, Ascl2, Zbtb7b (encoding Thpok), and Lef1 expressions, but not Bach2 and Tcf7, were significantly lower, while Prdm1 (encoding Blimp1) expression was higher in Gα13-deficient Tfh cells than those in Gα13-sufficient Tfh cells (Fig. 5A and SI Appendix, Fig. S6A). Levels of messenger RNA–encoding cytokines important for Tfh function, such as Ifng, Il4, and Il21, were comparable or, rather, higher in the former (SI Appendix, Fig. S6B), ruling out the role of Gα13 in regulating these cytokines in Tfh cells. Expression levels of the surface molecules important for Tfh cell function and maturation, including Cd154 (encoding CD40L), Cxcr4, and S1pr2, were significantly down-regulated in Gα13-deficient Tfh cells (Fig. 5B).
Fig. 5.
Gα13 signaling is required for the activation of RhoA-ROCK2 pathway in Tfh cells. (A–G) Tfh from the draining LNs of KLH-immunized mice were flow sorted on day 8 and analyzed. Relative transcript levels of signature transcription factors (A) and surface molecules (B) important in Tfh cell differentiation and function analyzed via qRT-PCR. Values are denoted as arbitrary units (A.U.). (C) DEG from bulk RNA-seq of Tfh cells were subjected to gene ontology analysis. False discovery rate (FDR) cutoff of 0.1 was applied. Solute carrier transporters (SLC). (D) Relative transcript levels of RhoGEFs proteins in Tfh cells. (E) Immunoblotting of active (GTP bound) RhoA. (F) Western blot of total and phosphorylated ROCK2 (Left) and cofilin (Right). All data are mean ± SEM and P values are indicated where appropriate; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
To probe the mechanistic details by which Gα13 signaling regulates Tfh cell differentiation in vivo, we profiled the transcriptome of flow-sorted Tfh cells isolated from KLH-immunized TΔGna13 mice and control mice via RNA sequencing (RNA-seq) analysis. At a setting of greater than twofold expression changes, with a raw P value < 0.05 and false discovery rate < 0.1, 231 genes were up-regulated, and 765 genes were down-regulated in the Gα13-deficient Tfh cells, compared with the Gα13-sufficient Tfh cells (Dataset S1). In particular, we found that among the down-regulated differentially expressed genes (DEGs), genes previously known to be positively associated with the Tfh cell lineage program, such as Apoe, Atf3, Mafb, Scd3, Spp1, and Zeb2, were present (SI Appendix, Table S1) (19–24), indicating that Tfh transcriptome is selectively down-regulated in the absence of Gα13. In fact, out of all the Tfh signature genes found within the DEGs, almost all genes were significantly down-regulated (SI Appendix, Table S1). As expected, nearly half of the top 20 terms of gene ontology enrichment analysis of DEGs using the gProfiler package were related to molecules involved in cell adhesion, chemotaxis, localization, and migration (SI Appendix, Fig. S6C). The gene ontology term analysis of DEGs using a web-based tool indicated that Gα13-deficient Tfh cells had impaired Rho signaling pathways, as demonstrated by the high, negative enrichment scores attributed to genes associated to signaling via Rho GTPase proteins (Fig. 5C). The Gα12/13 signaling of the GPCRs involves the activation of Rho GTPase guanine exchange factors (RhoGEFs), and these link GPCRs to the activation of the small, monomeric GTPase RhoA and other downstream effectors (25). In hematopoietic cells, three RhoGEFs have been identified: PDZ-RhoGEF, leukemia-associated RhoGEF, and p115-RhoGEF (respective gene names: Arhgef1, Argef12, and Arhgef11) (26–28). Among the three RGS-RhoGEFs, the level of Arhgef11 (encoding p115-RhoGEF) was significantly down-regulated in Gα13-deficient Tfh cells (Fig. 5D). The expression of Rhoa transcript and active GTP-bound RhoA levels were also significantly diminished in the Gα13-deficient Tfh cells (Fig. 5E). Since RhoA phosphorylates ROCK2 to regulate cellular proliferation (29) and movement-related factors such as cofilin (30), we examined the phosphorylation of ROCK2 and cofilin and found that the levels of phospho-ROCK2 and phosphocofilin were profoundly diminished in the Gα13-deficient Tfh cells (Fig. 5F and SI Appendix, Fig. S6D). Thus, in the absence of Gα13 signaling, Tfh cells failed to activate the RhoA-ROCK2 signaling pathway. As ROCK2 has been shown to modulate STAT3 signaling (31), we next questioned whether Gα13 deficiency impacts STAT3 phosphorylation in CD4+ T cells. Compared to WT, Gα13-deficient CD4+ T cells displayed a slight but significant reduction in the level of phospho-STAT3 upon IL-6 stimulation (SI Appendix, Fig. S6E), suggesting a role for Gα13 signaling on the optimal STAT3 activation in T cells.
Tfh cells are known to mediate various autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, either via assistance with autoantibody production and/or the formation of tertiary lymphoid structures in nonlymphoid tissues (32, 33). In order to determine whether RhoA signaling is relatively enriched in Tfh cells compared to non-Tfh effector cells in humans, we analyzed available RNA-seq data from SLE patient blood samples (34, 35). Gene set enrichment analysis (GSEA) of Tfh cells compared to non-Tfh cells yielded a positive correlation with genes signatures associated with RhoA and RhoGTPase effectors, suggesting that RhoA activation is positively correlated with human Tfh cell lineage program (SI Appendix, Fig. S6F).
Gα13-RhoA-ROCK Axis Facilitates Tfh Cell Differentiation and Subsequent GC Reactions In Vivo.
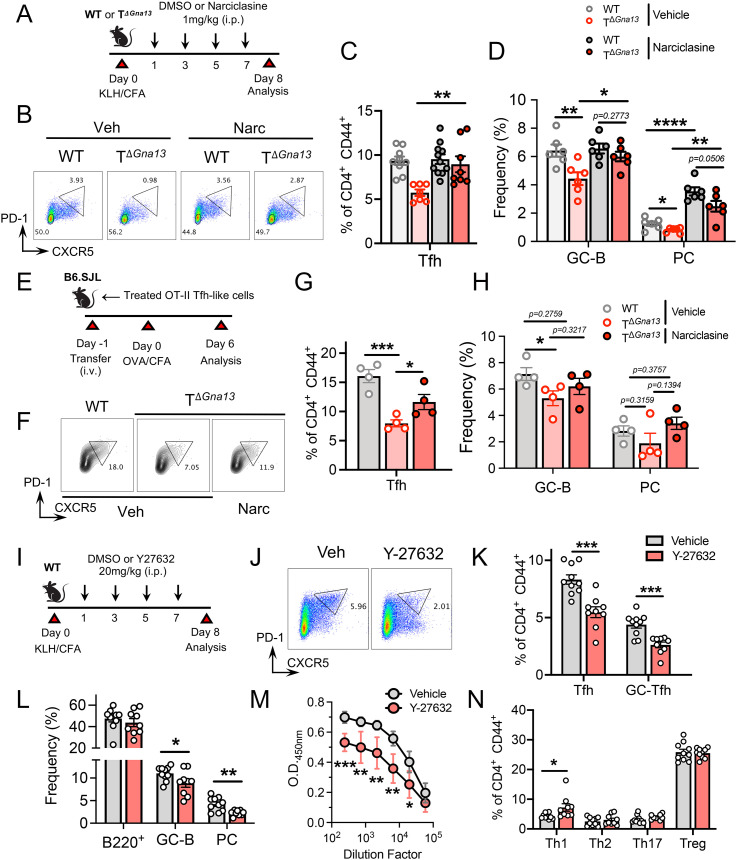
To determine whether the defective RhoA-pROCK2 pathway could account for the observed, diminished Tfh cell responses in TΔGna13 mice in vivo, we examined if treatment with a RhoA agonist, narciclasine, could restore Tfh cell responses in TΔGna13 mice in a KLH immunization model (Fig. 6A). Narciclasine treatment showed little effect on the frequencies of Tfh cells in WT control mice; however, it significantly increased the frequencies of Tfh cells in TΔGna13 mice to the levels comparable to those of control mice (Fig. 6 B and C). Concomitantly, the frequencies of GC B cells and PCs in the TΔGna13 mice were increased by the agonist treatment (Fig. 6D). While the agonist treatment had little effect on the frequencies of GC B cells, it increased that of PCs in control mice, suggesting that, besides regulating Tfh cell responses, the Rho agonist itself might be capable of directly promoting PC differentiation (Fig. 6D). The same observed enhancement in Tfh cell responses was shown in the LCMV infection model; Rho agonist treatment rescued Tfh cell frequencies in the TΔGna13 mice (SI Appendix, Fig. S7 A–C), indicating that Rho activation positively regulates the Tfh cell differentiation program in vivo. The stimulation of naïve CD4+ T cells in the presence of IL-6 and neutralizing antibodies to IFN-γ, IL-4, and TGF-β1 induces IL-21–expressing, Tfh-like cells (20). The increasing concentrations of narciclasine treatment on naïve T cells under Tfh-like–skewing conditions led to higher frequencies of CXCR5-expressing CD4+ T cells in vitro (SI Appendix, Fig. S7D).
Fig. 6.
Modulation of the RhoA-ROCK axis regulates Tfh and GC reactions in vivo. (A–D) WT and TΔGna13 mice were KLH immunized and treated with either DMSO vehicle (Veh) or narciclasine (Narc). Draining LNs were analyzed as indicated in A. (B) Representative fluorescence-activated cell sorting (FACS) plots of GC-Tfh cells. Tfh frequencies (C) combined from two independent experiments and B cell frequencies (D) (n ≥ 6 per group). (E–H) Either DMSO or Narc was treated to WT and Gα13-deficient OT-II cells under Tfh-skewing conditions in vitro and were intravenously (i.v.) transferred to congenically marked WT recipients, which were then OVA immunized as shown in E. (F) Representative FACS plots of Tfh cells. Tfh (G) and B cell (H) frequencies (n = 4 per group). (I–N) KLH-immunized WT mice were subjected to ROCK inhibitor treatment as shown in I. (J) Representative FACS plots of Tfh cells. Tfh, GC-Tfh (K) and B cell (L) frequencies combined from two independent experiments (n = 9 per group). (M) KLH-specific serum total IgG levels. (N) Th subset frequencies in draining LNs. All data are representative of two independent experiments, unless stated otherwise. All data are mean ± SEM and P values are indicated where appropriate; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Since the direct in vivo treatment of narciclasine could introduce indirect effects and skew our interpretation, we first treated agonist on Gα13-deficient, naïve OT-II T cells stimulated under Tfh-like–skewing conditions in vitro and then transferred them into congenically marked WT recipients, followed by immunization with OVA in CFA (Fig. 6E). This also allowed us to precisely determine whether such Rho agonist boosting in CD4+ T cells can truly compensate the defective Tfh cell differentiation in TΔGna13 mice in vivo. Compared to those in the recipients of vehicle-treated, Gα13-deficient OT-II T cells, the frequencies of Tfh cells in the recipients of agonist-treated, Gα13-deficient OT-II T cells were significantly increased, although they were still lower than those in the recipient of WT OT-II T cells (Fig. 6 F and G). While GC B cell frequencies in recipients of vehicle-treated, Gα13-deficient OT-II cells were significantly lower than those that received WT OT-II T cells, no differences were observed in the GC B cell frequencies between the recipients of WT OT-II T cells and agonist-treated OT-II T cells (Fig. 6H). While KLH-specific antibody levels of vehicle-treated TΔ mice were significantly lower than those of vehicle-treated WT mice, narciclasine treatment tended to enhance antibody production in the TΔ group, although not to the level of the WT group (SI Appendix, Fig. S7E). Both the frequency and absolute number of Treg cells did not change as a result of agonist treatment (SI Appendix, Fig. S7F). No differences were observed in endogenous Tfh cells of recipient origin, indicating that recipient Tfh cells did not contribute to the observed differences in B cell responses (SI Appendix, Fig. S7G). These data strongly suggest that the modulation of the Rho pathway can salvage the defective Gα13 signaling in TΔGna13 mice.
Since treatment with a Rho agonist restored the frequencies of Tfh cells and GC B cells in TΔGna13 mice to the levels similar to WT mice in vivo, we hypothesized that the blockade of the RhoA-ROCK2 axis would lead to diminished Tfh cell differentiation and subsequent GC reactions. In order to test this, we treated KLH-immunized C57BL/6 mice with Y27632, a selective ROCK inhibitor, or DMSO as vehicle before analyzing the draining LNs and serum (Fig. 6I). Tfh cell frequencies, as well as the expression levels of CXCR5 on Tfh cells, were significantly diminished in the inhibitor-treated group compared with vehicle-treated group (Fig. 6 J and K and SI Appendix, Fig. S7H). A similar reduction in the frequencies of GC B cells and PC population were also observed (Fig. 6L). Consistently, the levels of KLH-specific IgG were also significantly diminished in the inhibitor-treated group in a IgG2c-dependent manner (Fig. 6M and SI Appendix, Fig. S7I). Frequencies of Th1 cells were slightly elevated in the inhibitor-treated group, which was somewhat in parallel with the results of steady-state analysis conducted previously (Fig. 6N and SI Appendix, Fig. S2 B and E). The frequencies of Th2, Th17, and Treg cells were all unaffected as a result of inhibitor treatment (Fig. 6N and SI Appendix, Fig. S7J). Thus, the blockade of ROCK could suppress the differentiation of Tfh cells and subsequent GC reactions in vivo. Collectively, these results demonstrate that Gα13 signaling in T cells promotes Tfh cell responses via activating the RhoA-ROCK2 cascade, subsequently contributing to the induction of transcription factors and surface molecules required for the Tfh cell lineage program (SI Appendix, Fig. S7K).
Discussion
It has been well documented that GPCRs mediate a wide array of cellular functions in T cells, most notably in cytoskeletal rearrangements and migration. In particular, the modulation of various chemokine receptors on chemotacting T cells has been shown to be mediated by a diverse range of Gα proteins. Among Gα proteins, the Gα12/13 subfamily of proteins have been implicated to negatively regulate T cell trafficking via suppressing expressions of certain integrins. Although the role of Gα proteins in T cell chemotaxis and motility is well documented, how Gα-mediated signaling fine-tunes and modulates T cell subset differentiation and development has not been reported. Here, we demonstrate that the lack of Gα13 signaling in T cells led to defective Tfh cell responses in vivo by using three different animal models: subcutaneous immunization, systemic LCMV infection, and intranasal allergen challenges. Thus, T cell–intrinsic Gα13 signaling is crucially required for the Tfh cell lineage program in vivo, regardless of antigen types. Although differences in Treg frequencies were observed in certain cases at steady state and in KLH and asthma models, similar results were not observed in mixed bone marrow chimeras and cotransfer models, indicating that Treg cell deviations cannot account for the diminished Tfh cell responses in vivo. Many of the Gα13-mediated signals are also shared with Gα12 proteins, and previous works have demonstrated Gα12 activity can partly compensate for signals in the absence of Gα13 (15). As such, we have confirmed that Gα12 expression remains relatively unchanged in our TΔGna13 mouse system. These data indicate that Gα13 protein has a nonredundant role in Tfh cell differentiation in vivo.
Work with other primary and cancer cells have revealed that Gα13 proteins activate Rho GTPases via a direct interaction with RhoGEFs, which in turn activate intracellular mediators such as ROCK2 (36, 37). We have demonstrated that the same molecular pathway seems to be not only pertinent but also critical for naïve T cell commitment to the Tfh cell lineage. In T cell lymphoma, a gain-of-function mutation in RhoA led to increased ICOS expression and a malignant transformation of Tfh cells in vivo (38). Previous work with human T cells has demonstrated that phosphorylated ROCK2 interacts with and binds to phosphorylated STAT3, thereby forming a complex to positively regulate BCL6 (31, 39, 40). Given that the Bcl-6 expression levels of Gα13-deficient Tfh cells, as well as phospho-STAT3 levels, were diminished compared to Gα13-sufficient ones, we propose that Gα13-mediated RhoA-ROCK2-STAT3 signal cascade may account for the diminished Tfh cell responses in TΔGna13 mice in vivo. We have also shown that the failure to transduce optimal Gα13-Rho-ROCK2 pathway in T cells can lead to deterred, antigen-specific humoral responses in vivo, which seems to be somewhat recuperable by the agonistic modulation of the Rho pathway, although not completely. The partial rescue of humoral responses indicates that other intercellular regulations could also be at play besides the aforementioned mechanism. Active Gα12/13 proteins have been shown to bind with and seize cadherins from catenins, which lead to up-regulated, β-catenin–mediated, and TCF/LEF-dependent transcriptional activation in cancer cells (41). TCF/LEF expression and regulation have been shown to be important for Tfh cell polarization (42, 43), and such a pathway is certainly another plausible molecular mechanism. The convergence of multiple transcriptional pathways must produce a synergistic effect and lead to the ultimate cellular consequence to selectively deter commitment to the Tfh cell lineage.
Gα13-mediated signaling appears to be important not only for Tfh cell lineage commitment but also for Tfh cell stimulatory and adhesion function. Our analyses revealed that Gα13 deficiency in T cells prompts multiple defects in Tfh cell function, including the significantly lower expression of receptors important for migration to and within B cell zones, such as CXCR5, S1PR2, and CXCR4, as well as costimulatory molecules important for directly activating B cells, such as ICOS and CD40L, while it seemed to up-regulate the expression of CD84 and LFA1. Histological analysis revealed that Gα13-deficient CD4+ T cells failed to access the GC, presumably because of the reduced expression of CXCR5. Mixed bone marrow chimera studies, as well as OT-II cotransfer studies, indicate that the Gα13 signaling in T cells is indispensable for the surface molecules important for Tfh commitment: CXCR5, CD40L, ICOS, and other cell surface molecules required for helping activated B cells. A similar reduction of CXCR5 expression on Tfh cells was observed in mice treated with ROCK inhibitor in vivo, suggesting that Gα13-Rho-ROCK signaling in T cells facilitates the expression of Tfh cell signature transcription factor and cell surface molecules. In addition to its role in Tfh cell differentiation, Gα13 signaling was found to contribute to the “B cell help” function of Tfh cells, since Gα13-deficient Tfh cells were less efficient not only in forming conjugates with B cells but also in stimulating B cells to produce immunoglobulins.
Our findings raise the possibility that the blockade of Rho-ROCK pathway might be a promising approach to repress Tfh cell differentiation and subsequent GC reactions in antibody-mediated autoimmune diseases, such as SLE and phemphigus vulgaris, in humans. The GSEA of two separate human SLE patient datasets has demonstrated a positive correlation between active Rho gene signatures and DEGs in Tfh cells in comparison to non-Tfh cells. More thorough and comprehensive investigations are needed to determine whether such a relationship is based on cause and effect and whether other autoimmune datasets recapitulate the above correlations; our study presents preliminary grounds to believe that the selective manipulation of the RhoA pathway could salvage Tfh-mediated pathogenicity in humans. Several ROCK inhibitors have been approved for cerebral vasospasm and glaucoma (44, 45), and ROCK2-specific inhibitors are currently under phase II clinical trials for the treatment of multiple indications, including psoriasis, systemic sclerosis, graft-versus-host disease, idiopathic pulmonary fibrosis, and progressive supranuclear palsy (46, 47). It would be important to determine if pan-ROCK inhibitors or ROCK2-specific inhibitors can ameliorate pathogenic Tfh cell responses in humans and more rigorous investigations remain.
While the present study convincingly demonstrates a pivotal role of Gα13 on Tfh cell responses in vivo, remaining questions include what kinds of external stimuli are involved and which of the receptor(s) coupling to Gα13 have the important implications in early Tfh cell differentiation. Many have previously shed some light in an attempt to identify the membrane receptors that couple to Gα13 in lymphocytes, including S1PR2, CXCR4, and LPARs, all of which are involved in cell chemotaxis and adhesion. Although CXCR5 is known to be primarily a Gαi-coupled receptor, there is evidence that it also capable of interacting with Gα13 proteins upon CXCL13 binding (48), indicating that noncanonical signaling possibilities exist, as many of the GPCR signaling is mediated by more than one type of Gα proteins (49). For example, integrins have been suggested to serve as noncanonical, Gα13-coupled receptors, and integrin activation can also lead to cross-talk with GPCRs via a dynamic modulation of Gα13-RhoA activity (50). It is likely that all of the above-mentioned receptors and signals lead to the synergistic amplification of the Gα13-mediated signaling in early antigen exposure and Tfh cell development, rather than one dominant mechanism being at play.
In summary, our work provides a previously unappreciated insight into the mechanism by which Gα proteins positively regulate Tfh cell differentiation and function, thereby fulfilling the need to understand the molecular requirements facilitating humoral responses in vivo. Targeting the Gα13-Rho-ROCK axis might be useful in developing pharmacologic interventions in vaccine development as well as antibody-mediated immune disorders.
Materials and Methods
Mice.
C57BL/6, B6.SJL, and Rag1−/− mice were purchased from Jackson Laboratory (Bar Harbor). Cd4-cre and OT-II Tg mice were kindly provided by Chen Dong, Tsinghua University. Gna12−/− and Gna13fl/fl mice were kindly provided by Melvin I. Simon, California Institute of Technology, Pasadena, CA, and Stefan Offermanns, Max Planck Institute, Bad Nauheim, Germany, respectively. Mice were crossed accordingly and either female or male mice of 6 to 12 wk, sex and age matched, were used for experiments, with Gna13f/f littermate mice as WT controls. All mice were maintained in a specific, pathogen-free facility at the Seoul National University School of Pharmacy Animal Research Facility. For viral infection models, experiments were conducted at Biosafety level-2 research facility (SNUIBC-R190327-1–1 and SNU-190830-2-1). The rest is provided in SI Appendix.
Acknowledgments
We thank Dr. Kyu-Won Kim (Seoul National University) for support in flow cytometry; Dr. Chang-Yuil Kang (Seoul National University) for the provision of materials necessary for viral infection; H.-J. Noh of the National Center for Inter-university Research Facilities for assistance with flow-sorting experiments; and the entire Chung Laboratory members and Dr. Youn Soo Choi (Seoul National University) for discussion and suggestions. This work was supported by the research grants from the following: Global Research Laboratory Program (2017K1A1A2004511 to S.G.K. and Y.C.), Leader Research Program (2020R1A3B207889011 to Y.C.) and the Global Ph.D. Fellowship Program (2017H1A2A1042662 to D.-S.K.) from the National Research Foundation of Korea.
Footnotes
Edited by Jason G. Cyster, University of California, San Francisco, CA, and approved August 19, 2021 (received for review May 6, 2021)
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108376118/-/DCSupplemental.
Data Availability
RNA-seq were performed and analyzed in SI Appendix and Dataset 1.
References
- 1.Lämmermann T., Kastenmüller W., Concepts of GPCR-controlled navigation in the immune system. Immunol. Rev. 289, 205–231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y. M., Kuen D.-S., Chung Y., Kurose H., Kim S. G., Gα12/13 signaling in metabolic diseases. Exp. Mol. Med. 52, 896–910 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieken S., et al., G12/G13 family G proteins regulate marginal zone B cell maturation, migration, and polarization. J. Immunol. 177, 2985–2993 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Herroeder S., et al., Guanine nucleotide-binding proteins of the G12 family shape immune functions by controlling CD4+ T cell adhesiveness and motility. Immunity 30, 708–720 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Muppidi J. R., Lu E., Cyster J. G., The G protein-coupled receptor P2RY8 and follicular dendritic cells promote germinal center confinement of B cells, whereas S1PR3 can contribute to their dissemination. J. Exp. Med. 212, 2213–2222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muppidi J. R., et al., Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature 516, 254–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healy J. A., et al., GNA13 loss in germinal center B cells leads to impaired apoptosis and promotes lymphoma in vivo. Blood 127, 2723–2731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu E., Wolfreys F. D., Muppidi J. R., Xu Y., Cyster J. G., S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature 567, 244–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriyama S., et al., Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J. Exp. Med. 211, 1297–1305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew D., Kremer K. N., Torres R. M., ARHGEF1 deficiency reveals Gα13-associated GPCRs are critical regulators of human lymphocyte function. J. Clin. Invest. 129, 965–968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu E., Cyster J. G., G-protein coupled receptors and ligands that organize humoral immune responses. Immunol. Rev. 289, 158–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty S., T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S., T follicular helper cell biology: A decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Lu E., Yi T., Cyster J. G., EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 533, 110–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu J. L., Müller S., Mancino V., Offermanns S., Simon M. I., Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 99, 9352–9357 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffield V. M., Helms W. S., Jiang Q., Su L., Galpha13 mediates a signal that is essential for proliferation and survival of thymocyte progenitors. J. Exp. Med. 200, 1315–1324 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi Y. S., et al., Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 190, 4014–4026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijayanand P., et al., Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity 36, 175–187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chtanova T., et al., T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Nurieva R. I., et al., Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leavenworth J. W., Verbinnen B., Yin J., Huang H., Cantor H., A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 16, 96–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vella L. A., et al., T follicular helper cells in human efferent lymph retain lymphoid characteristics. J. Clin. Invest. 129, 3185–3200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X., et al., Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper (Tfh) cell differentiation. Cell Rep. 14, 1735–1747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y., et al., Transcriptional factor ATF3 protects against colitis by regulating follicular helper T cells in Peyer’s patches. Proc. Natl. Acad. Sci. U.S.A. 116, 6286–6291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siehler S., Regulation of RhoGEF proteins by G12/13-coupled receptors. Br. J. Pharmacol. 158, 41–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girkontaite I., et al., Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2, 855–862 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Bouafia A., et al., Loss of ARHGEF1 causes a human primary antibody deficiency. J. Clin. Invest. 129, 1047–1060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuner R., Swiercz J. M., Zywietz A., Tappe A., Offermanns S., Characterization of the expression of PDZ-RhoGEF, LARG and G(alpha)12/G(alpha)13 proteins in the murine nervous system. Eur. J. Neurosci. 16, 2333–2341 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Ricker E., Chowdhury L., Yi W., Pernis A. B., The RhoA-ROCK pathway in the regulation of T and B cell responses. F1000Res. 5, F1000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bros M., Haas K., Moll L., Grabbe S., RhoA as a key regulator of innate and adaptive immunity. Cells 8, 733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss J. M., et al., ROCK2 signaling is required to induce a subset of T follicular helper cells through opposing effects on STATs in autoimmune settings. Sci. Signal. 9, ra73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J., et al., Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 39, 770–781 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Gensous N., et al., T follicular helper cells in autoimmune disorders. Front. Immunol. 9, 1637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caielli S., et al., A CD4+ T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat. Med. 25, 75–81 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panwar B., et al., Multi-cell type gene coexpression network analysis reveals coordinated interferon response and cross cell type correlations in systemic lupus erythematosus. Genome Res. 31, 659–676 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng R., et al., Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene 25, 5942–5952 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Fu X.-D., et al., Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS One 3, e2790 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes J. R., et al., RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell 33, 259–273.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W., et al., ROCK2, but not ROCK1 interacts with phosphorylated STAT3 and co-occupies TH17/TFH gene promoters in TH17-activated human T cells. Sci. Rep. 8, 16636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanin-Zhorov A., et al., Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 111, 16814–16819 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meigs T. E., Fields T. A., McKee D. D., Casey P. J., Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for β -catenin release. Proc. Natl. Acad. Sci. U.S.A. 98, 519–524 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi Y. S., et al., LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16, 980–990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L., et al., The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat. Immunol. 16, 991–999 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Feng Y., LoGrasso P. V., Defert O., Li R., Rho kinase (ROCK) inhibitors and their therapeutic potential. J. Med. Chem. 59, 2269–2300 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Tanna A. P., Johnson M., Rho kinase inhibitors as a novel treatment for glaucoma and ocular hypertension. Ophthalmology 125, 1741–1756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulherkar S., Tolias K. F., RhoA-ROCK signaling as a therapeutic target in traumatic brain injury. Cells 9, 245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Humimat G., Marashdeh I., Daradkeh D., Kooner K., Investigational Rho kinase inhibitors for the treatment of glaucoma. J. Exp. Pharmacol. 13, 197–212 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Haibi C. P., et al., Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Mol. Cancer 12, 64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho H., Kehrl J. H., “Regulation of immune function by G protein‐coupled receptors, trimeric G proteins, and RGS proteins” in Progress in Molecular Biology and Translational Science , R. Fisher, Ed. (Elsevier, 2009), pp. 249–298. [DOI] [PubMed] [Google Scholar]
- 50.Gong H., et al., G protein subunit Gα13 binds to integrin aIIbb3 and mediates integrin “outside-in” signaling. Science 327, 340–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-seq were performed and analyzed in SI Appendix and Dataset 1.