Significance
The pineal gland secretes melatonin at night mainly driven by adrenergic sympathetic inputs. Many other neurotransmitters are found in the pineal gland, suggesting potential fine modulation of melatonin release. Serotonin as a precursor of melatonin synthesis is known to be significantly released by pinealocytes and its receptor also is expressed in pinealocytes. By identifying the release mechanism, cell signaling in the pinealocytes, and its effect on norepinephrine-induced melatonin secretion, our study defines serotonin as an autocrine neurotransmitter in the pineal gland and suggests possible modulatory targets for melatonin secretion.
Keywords: serotonin receptor, pineal gland, plasma membrane monoamine transporter, melatonin, N-acetylserotonin
Abstract
The pineal gland secretes melatonin principally at night. Regulated by norepinephrine released from sympathetic nerve terminals, adrenergic receptors on pinealocytes activate aralkylamine N-acetyltransferase that converts 5-hydroxytryptamine (5-HT, serotonin) to N-acetylserotonin, the precursor of melatonin. Previous studies from our group and others reveal significant constitutive secretion of 5-HT from pinealocytes. Here, using mass spectrometry, we demonstrated that the 5-HT is secreted primarily via a decynium-22–sensitive equilibrative plasma membrane monoamine transporter instead of by typical exocytotic quantal secretion. Activation of the endogenous 5-HT receptors on pinealocytes evoked an intracellular Ca2+ rise that was blocked by RS-102221, an antagonist of 5-HT2C receptors. Applied 5-HT did not evoke melatonin secretion by itself, but it did potentiate melatonin secretion evoked by submaximal norepinephrine. In addition, RS-102221 reduced the norepinephrine-induced melatonin secretion in strips of pineal gland, even when no exogenous 5-HT was added, suggesting that the 5-HT that is constitutively released from pinealocytes accumulates enough in the tissue to act as an autocrine feedback signal sensitizing melatonin release.
The principal role of pinealocytes of the pineal gland (PG) is to synthesize and secrete the hormone melatonin, driven by the neurotransmitter norepinephrine (NE) released at night from sympathetic nerve terminals. Acting through α1- and β1-adrenergic receptors on pinealocytes, the adrenergic signal generates Ca2+ and cyclic AMP (cAMP) to activate up to 150-fold (in rat) a key synthetic enzyme aralkylamine N-acetyltransferase (AANAT) (1). This signaling pathway is shown in Fig. 1. AANAT in turn converts serotonin (5-HT) from a constitutive pool to generate N-acetylserotonin (NAS), from which hydroxyindole-O-methyltransferase generates melatonin (1). The secretion of melatonin and NAS can be increased 100-fold at night (2). In addition to 5-HT, many other neurotransmitters are present elsewhere in the PG, including acetylcholine (ACh), glutamate, GABA, and neuropeptides (3). Their roles in modulating the activity of pinealocytes and melatonin synthesis remain to be established. Here we focus on modulatory effects of 5-HT.
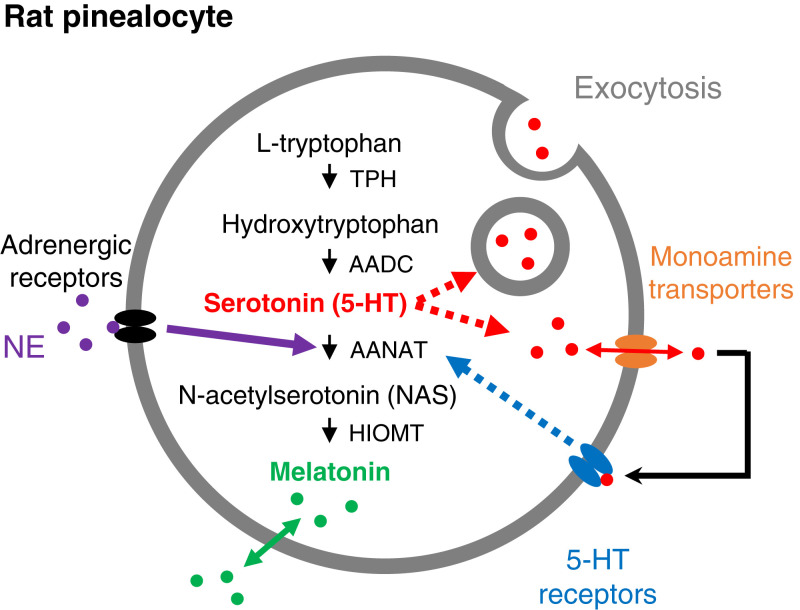
Fig. 1.
Synthesis and release of 5-HT and melatonin in rat pinealocytes. The enzymatic pathway from L-tryptophan to 5-HT and melatonin uses four enzymes. AANAT is regulated by adrenergic signaling. Two possibilities are shown (red dotted arrows) for the mechanism of release of 5-HT from pinealocytes: through vesicular exocytosis or through monoamine transporters running in reverse (orange). Released serotonin activates serotonin receptors (blue) on the plasma membrane of pinealocytes. We are suggesting that the receptors up-regulate AANAT acting as an auto- or paracrine mechanism to promote melatonin synthesis. AADC, aromatic amino acid decarboxylase; HIOMT, hydroxyindole-O-methyltransferase; TPH, tryptophan hydroxylase.
Serotonergic signaling plays diverse roles in the CNS and peripheral tissues. It is critically involved in mood control, the sleep–wake cycle, breathing, locomotion, and more (4, 5) and acts through 14 distinct, mostly G protein–coupled 5-HT receptors. In the mammalian PG, 5-HT content shows a circadian rhythm, higher during daytime and somewhat lower at night while melatonin is being produced (6, 7). Compared to melatonin release, the 5-HT release from the gland shows only modest (±twofold) circadian variation, continuing in appreciable amounts throughout (8–10). The large pineal pool of 5-HT is continually turning over through constitutive synthesis and secretion (11). Exogenous NE increases 5-HT production and secretion in PGs partly by elevating tryptophan hydroxylase activity (10, 12–17).
Secretion of 5-HT from the PG is larger than the elevated nocturnal release of melatonin (9, 10), but the physiological significance of this secretion has not been clear. Released 5-HT is suggested to prime the cAMP-dependent activation of AANAT for melatonin synthesis (17–20). We tested the hypothesis that 5-HT secretion acts as an autocrine signal to promote melatonin synthesis and secretion (Fig. 1) by answering the following questions: What is 1) the secretory mechanism, 2) the type of 5-HT receptor used, and 3) the effect of 5-HT on melatonin secretion? Using optical, electrical, and mass spectrometric (MS) measurements, we found that 5-HT is released from pinealocytes via a nonconventional mechanism and sensitizes NE-induced melatonin synthesis and secretion by activating 5-HT2C receptors present in the PG. We show that 5-HT qualifies as an autocrine transmitter in the PG.
Results
5-HT Secretion Is Promoted by NE.
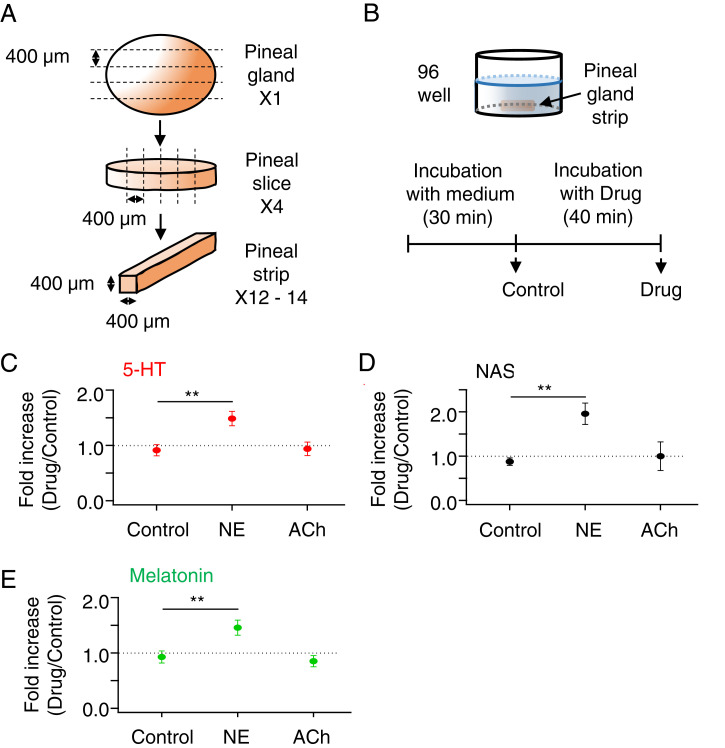
We started with the secretion of 5-HT from pinealocytes and pineal tissue. First, we tested whether 5-HT secretion is controlled by NE using MS and measuring 5-HT, NAS, and melatonin in the same samples. A single PG was cut into several tiny match-stick-like strips (Materials and Methods and Fig. 2A). The reduced tissue size allowed multiple experiments with a single gland. Basal levels of secretion for each strip were measured for the first 30 min in M199 medium before application of NE (Fig. 2B). Then secretion from the same strips was measured with or without 1-μM NE stimulation for 40 min. In unstimulated control strips, the 5-HT secretion rate ran down only slightly with time, with the ratio between the second and first incubations being 0.91 ± 0.10 (n = 8 strips) (Fig. 2C). In contrast, 40-min treatment with NE gave a 5-HT secretory ratio of 1.48 ± 0.13 (n = 8, P = 0.003 compared to the control). Treatment with ACh, which increases intracellular Ca2+ (21), gave a secretory ratio of 0.94 ± 0.12 (n = 8, P = 0.86 compared to the control). Thus, 40 min of NE increased 5-HT secretion significantly by ∼50%, whereas ACh was ineffective. The absolute secreted amount of 5-HT was 3.1 ± 0.5 pmol/h per strip for basal conditions (constitutive) and 4.4 ± 0.59 pmol/h per strip during 40-min NE treatment (n = 8). Typical basal 5-HT secretion from whole glands is 25 ± 1 pmol/h per gland (9). Considering the number (12–14) and size of strips we obtained from the gland, the two measurements were comparable. The M199 medium contains no melatonin, 5-HT, NAS, NE, or ACh.
Fig. 2.
NE promotes an early transient 5-HT release. (A) A single PG from a rat was sliced into three to four slices (400 μm thick), and each slice was further sliced into three to five strips (300 to 400 μm thick). (B) Schematic drawing of a single pineal strip in one well of a 96-well plate. Individual strips were incubated in M199 for 30 min and then in M199 (Control) or M199 plus 1 μM NE or 50 μM ACh for another 40 min. After each incubation, the medium was collected and used to measure 5-HT (C), NAS (D), and melatonin (E) levels by UPLC/MS analysis. Fold increases are given as test period divided by baseline period and compared using a t test. **P < 0.01. Results are presented as mean ± SEM.
Secretion of NAS (Fig. 2D) and melatonin (Fig. 2E) had secretory ratios of 2.23 and 1.63, respectively, in the 40-min NE treatment. Compared to longer stimulation (e.g., 6 h) (see, for example, Fig. 8) (9), the up-regulation of NAS and melatonin was minute but detectable. This early secretion presumably reflects the earliest beginnings of de novo transcription of AANAT by brief adrenergic signaling (22). Again, ACh treatment did not affect NAS or melatonin secretion. These results confirm that short exposure to NE augmented 5-HT release, as reported before with in vivo microdialysis and in experiments using isolated PGs (2, 8, 10, 15). It is also clear that the constitutive background 5-HT secretion was high (12, 15, 16).
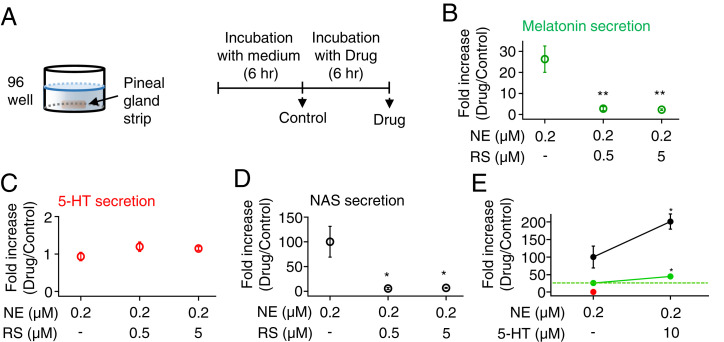
Fig. 8.
5-HT2C receptors sensitize NE-induced melatonin and NAS secretion in pinealocytes. (A) Schematic of a single pineal strip in one well of a 96-well plate. Strips were incubated individually in M199 for 6 h and then in M199 containing different drugs for another 6 h. Next, 0.2 μM NE was used to activate adrenergic receptors, and RS was used to block 5-HT2C receptors. After each 6-h incubation, the solutions were collected and used to measure melatonin (B), 5-HT (C), and NAS (D) levels by UPLC/MS. (E) Normalized levels of secreted melatonin (green), 5-HT (red), and NAS (black) after further application of 5-HT with NE were measured as a positive control. The green dotted line indicates the basal melatonin secretion. When exogenous 5-HT was added to the bath, it was not possible to measure endogenous 5-HT secretion. Fold increases relative to the baseline incubation in M199 were compared using a t test. **P < 0.01 and *P < 0.05.
Exocytosis Is Not Involved in 5-HT Secretion.
Next, we investigated potential mechanisms of 5-HT secretion. In a quantitative biophysical study, we previously showed that melatonin and NAS, both neutral indoleamines, are so lipid-soluble that once synthesized they cannot be retained in a cell for more than a few seconds (23). Secretion would follow synthesis obligatorily and with little delay. On the other hand, 5-HT is a cation and does not cross lipid bilayers easily. In neurons and immune cells, 5-HT is secreted via quantal exocytosis from secretory vesicles (24, 25). Typically, the exocytosis is Ca2+-dependent, and the Ca2+ rise is mediated by voltage-gated Ca2+ channels. A similar mechanism has been suggested in the PG (26). To test for exocytotic 5-HT release from pinealocytes, we used microamperometry. A miniature carbon-fiber probe polarized at +600 mV oxidized 5-HT instantaneously and measured the oxidative current. Single-vesicle fusions would be seen as individual amperometric spikes (25, 27–29). For this experiment, we used either ACh or potassium-rich Ringer’s solution (KCl) as stimuli. Previous studies indicate that ACh and KCl depolarize the cell and elevate [Ca2+]i via Ca2+-permeable nicotinic ACh receptors and voltage-gated Ca2+ channels (21, 30, 31).
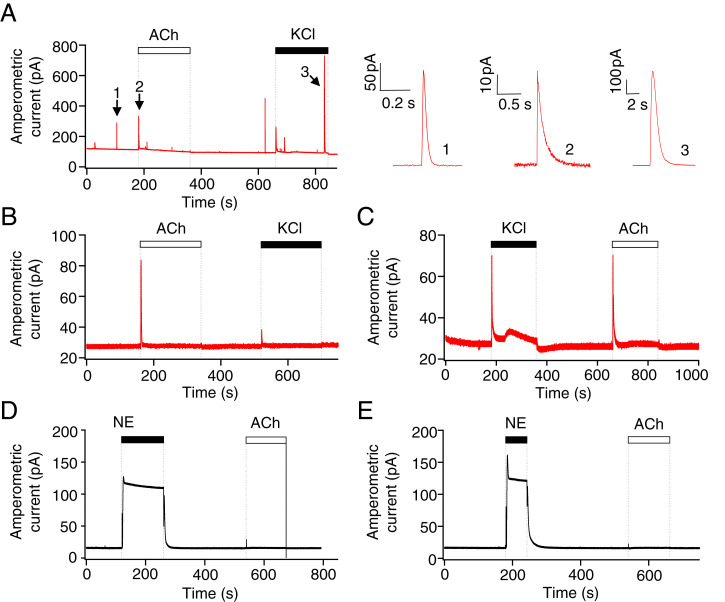
As a positive control, the pinealocytes were loaded with oxidizable dopamine (32, 33). Cells loaded with this artificial reporter showed occasional large quantal events in basal conditions and more frequent exocytotic spikes during depolarization of the membrane using KCl (n = 4). (Fig. 3A), verifying that the electrode can detect quantal release. The magnified traces on the right show that the dopamine spikes have the stereotypical shape of exocytotic quantal secretion (32, 33). If 5-HT were secreted by Ca2+-dependent exocytosis, we should detect similar quantal amperometric currents from the oxidation of released vesicular 5-HT. As a control, amperometric current was recorded with the electrode in the bath solution without pinealocytes (Fig. 3B). Here, the signal was steady except for spikes at the moment of solution change to ACh or KCl. These represent electrochemical reactions of the electrode to the ACh and KCl solutions themselves. Then the same solutions were applied with the probe touching a cluster of pinealocytes (Fig. 3C). Other than the solution-change artifacts, no quantal events were induced during ACh or KCl perfusion in any measurement (n = 7). However, both solutions induced a broader rise of current, larger with KCl than with ACh. Apparently unidentified oxidizable chemicals were released from the cells in a continuous manner so that the current rose and maintained an elevated level during this stimulation. Even if this oxidative current were generated in part by released 5-HT, which it could be, it does not show signs of typical vesicular exocytosis.
Fig. 3.
Serotonin is not released via exocytosis. Representative amperometric current traces measured using carbon-fiber amperometry with a miniature amperometric electrode polarized at +600 mV. (A) Amperometric traces of pinealocytes preloaded with exogenous dopamine. Enlarged single quantal events in the Right are from the numbered spikes from the Left (1: spontaneous event, 2, 3: evoked exocytosis). The three amperometric spikes illustrated correspond to 2-electron oxidation of 1.7 × 107, 2.1 × 107, and 270 × 107 dopamine molecules, respectively. In the remaining panels, pinealocytes were not preloaded with dopamine. The amperometric electrode was placed in the bath solution without pinealocytes (control traces) (B and D) or on a cluster of pinealocytes (C and E). ACh (50 μM) and high potassium chloride (70 mM KCl) were used to elicit membrane depolarization and intracellular Ca2+ increases. Next, 1 μM NE (an oxidizable molecule) was applied to the electrode in the bath (D) or with the same electrode touching pinealocytes (E). No quantal events were observed before or after NE application, only a step up of current as applied NE is being oxidized.
Since NE initially promoted the early 5-HT release seen with MS (Fig. 2C), we performed amperometry during transient application of NE (Fig. 3E) as well. Again, we did not see any quantal events during or after 1 μM NE. The large step increment of the baseline was due to oxidation of the exogenously applied NE solution, as shown when the electrode is positioned in the bath solutions without pinealocytes (Fig. 3D). In sum, we failed to detect constitutive or stimulated exocytotic 5-HT release from the pinealocytes, suggesting that 5-HT is released in some other ways. A more cautious statement would be that if there was any exocytotic release, the quantal units would have to be too small to be resolved.
A Monoamine Transporter Is Involved in Constitutive 5-HT Release from Pinealocytes.
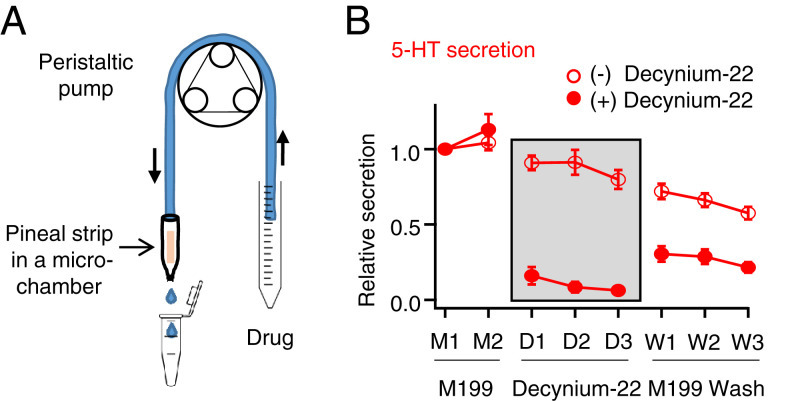
The next candidate mechanism we tested for 5-HT release was equilibrative plasma membrane transporter that transports cationic monoamine neurotransmitters including 5-HT (34). Reversible equilibrative transporters, such as the plasma membrane monoamine transporter (PMAT), normally take up excessive ambient cationic neurotransmitters into cells with energetic coupling only to the negative membrane potential without coupling to Na+ or other ion gradients (34–36). Depending on the membrane potential and the electrochemical gradient of the neurotransmitters, these transporters can reverse direction to release cationic monoamines (37). Release of 5-HT would be favored by depolarization and by intracellular 5-HT concentrations higher than the extracellular concentration. Several of these transporters are blocked by decynium-22. To test this hypothesis, we assessed reduction of 5-HT release by decynium-22. Single pineal strips were perifused continuously with M199 medium (Fig. 4A), and 20-min samples were collected over 160 min. Each sample contained 100 to 200 μL of medium. They were analyzed by ultraperformance liquid chromatography (UPLC)/MS to measure 5-HT (Fig. 4B). For the control group (Fig. 4B, open circles), the pineal strip was perifused with just M199 medium for 160 min to estimate rundown in control conditions. For the drug-treated group (Fig. 4B, closed circles), the strip was perifused with M199 for the first 40 min, and then with 50 μM decynium-22 for the next 60 min. Finally, the strip was again perifused with M199 for the washout. During decynium-22 application, the constitutive 5-HT secretion was profoundly decreased. The secretion with decynium-22 relative to the original baseline was only 0.10 ± 0.05, whereas secretion in control was 0.87 ± 0.06. During washout, secretion recovered partially but not completely, perhaps because of incomplete washout of the blocker during 60 min of slow perifusion. The strips still showed residual red stain from decynium-22 at the end of the experiment. Apparently, at least 89% of the large constitutive release of cationic 5-HT from pinealocytes involves equilibrative transporters sensitive to decynium-22. In contrast, hydrophobic NAS and melatonin diffuse spontaneously out of cells within seconds after synthesis (23).
Fig. 4.
5-HT is released from pineal strips via a decynium-sensitive plasma membrane monoamine transporter. (A) Schematic of a single strip inserted into a microchamber connected to a peristaltic pump for continuous perifusion. (B) Time course of 5-HT secretion during drug exposure from UPLC/MS analysis of 20-min samples of the solution passing through the pineal strip. Open circles are control and closed circles are the secretion in the presence of 50 μM decynium-22, a selective inhibitor of equilibrative cation transporters, applied only during drug periods D1, D2, and D3.
5-HT Does Not Activate Gi/o or Gs in Pinealocytes.
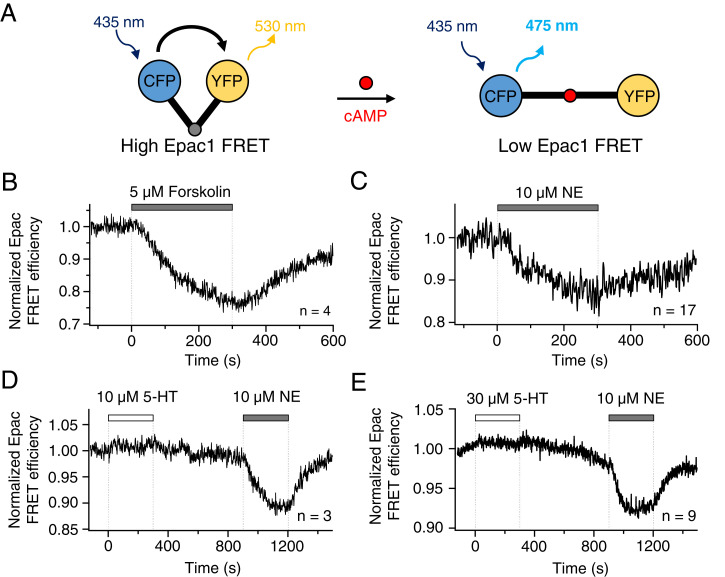
We turn now to functional questions. Could released 5-HT act on 5-HT receptors on the pinealocyte membrane itself? Known 5-HT receptors fall into three functional groups (SI Appendix, Table S1): 1) a cAMP-regulating group coupled to Gi/o or Gs, 2) a ligand-gated ion channel group, and 3) a Ca2+-signaling group, called 5-HT2. We looked for the corresponding signals. The cAMP-regulating group was tested using a cAMP probe derived from the cAMP-binding domain of exchange protein directly activated by cAMP1 (Epac1) (38). The fluorescent reporter, cyan fluorescent protein (CFP)–Epac1-yellow fluorescent protein (YFP), allows FRET measurements to monitor intracellular cAMP levels (39). The probe has high FRET efficiency (FRETeff) without bound cAMP and a decreased efficiency when cAMP is bound (Fig. 5A). Pinealocytes were transiently transfected (Materials and Methods and SI Appendix, Fig. S1) with the probe and identified visually. As a first positive control, 5 μM forskolin, which activates adenylyl cyclase and increases intracellular cAMP, reduced FRETeff by about 25% over several minutes (Fig. 5B). The resting FRETeff was restored after washout of forskolin. As a second positive control, application of 10 μM NE to activate adenylyl cyclase in pinealocytes also decreased FRETeff by about 15% (Fig. 5C). Finally, to test whether cAMP-regulating 5-HT receptors are active in pinealocytes, two different concentrations of 5-HT were applied: 10 μM (Fig. 5D) and 30 μM (Fig. 5E). Neither evoked any FRETeff change, whereas 10 μM NE at the end of each experiment elicited the expected reduction in FRETeff. These results demonstrated that 5-HT secreted from pinealocytes would not affect their intracellular cAMP signaling.
Fig. 5.
5-HT does not change cAMP levels in isolated pinealocytes. The cAMP levels were analyzed by FRET. (A) A schematic diagram of the cAMP biosensor, Epac1, sandwiched between CFP (donor) and YFP (acceptor). Pinealocytes were transfected with the Epac1 probe by magnetofection. Once cAMP binds to Epac1, the conformational change increases the distance between CFP and YFP and thereby decreases FRET efficiency. (B) Forskolin, increasing cAMP, lowers FRET efficiency in pinealocytes. (C) NE activates endogenous β-adrenergic receptors and increases cAMP levels in pinealocytes. Neither 10 μM 5-HT (D) nor 30 μM 5-HT (E) affected the level of cAMP in pinealocytes.
5-HT Does Not Activate a Ligand-Gated 5-HT3 Ion Channel in Pinealocytes.
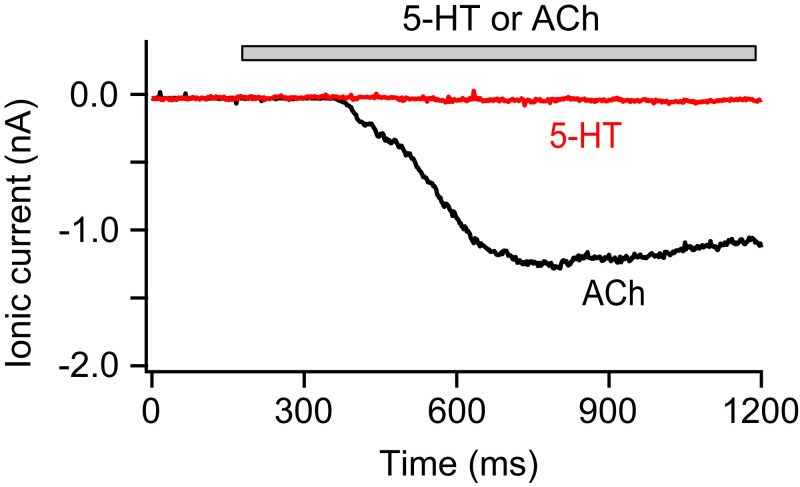
Next, we tested for possible activation of ligand-gated 5-HT3 ion channels (SI Appendix, Table S1). Transmembrane ionic currents were recorded with cultured pinealocytes in whole-cell patch-clamp at −60 mV (Fig. 6). Since depolarizing nicotinic ACh receptors are expressed on pinealocyte plasma membranes (21), we applied ACh as a positive control. In all of the trials (n = 7), 50 μM ACh elicited significant inward currents (1.66 ± 0.36 nA), whereas 30 μM 5-HT, a supramaximal dose for 5-HT3 receptors, did not (n = 6). We concluded that 5-HT3 activity was not present in pinealocyte plasma membranes.
Fig. 6.
5-HT does not activate an ionotropic 5-HT3 receptor in pinealocytes. Representative recordings of 5-HT– and ACh-induced currents from a pinealocyte in whole-cell patch-clamp recording. Six of six cells showed no current with 5-HT and all of them exhibited significant inward currents with ACh. Holding potential was −60 mV. ACh (50 μM) and 5-HT (30 μM) were applied to the cell during the bar using local perfusion.
5-HT Does Activate Gq/11 Signaling in Pinealocytes.
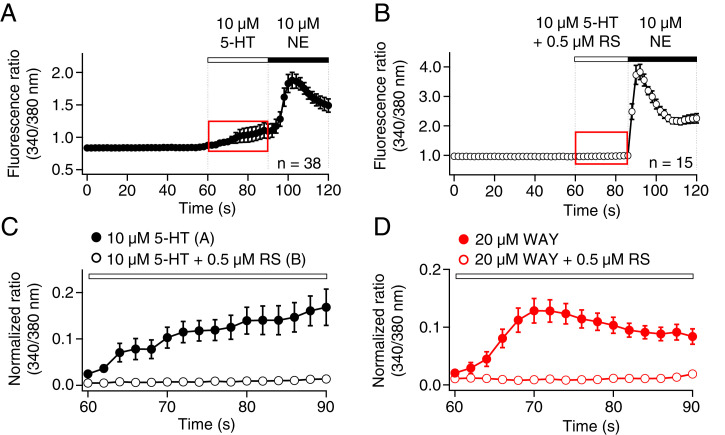
Finally, we tested for activity of 5-HT2 receptors, which couple to Gq/11 and intracellular Ca2+ signaling (SI Appendix, Table S1). Indeed, Ca2+ imaging experiments revealed a modest increase in intracellular free Ca2+ upon perfusion of 10 μM 5-HT (Fig. 7A). As a positive control at the end of each experiment, 10 μM NE further increased intracellular Ca2+, a known response requiring activation of α1 adrenergic receptors (40). Of the three subtypes of 5-HT2 receptors, 5-HT2C are implicated in modulation of melatonin secretion in rat PG (41). When we preincubated cells for 10 min with 0.5 μM RS-102221 (RS), a potent 5-HT2C receptor antagonist (42), 10 μM 5-HT no longer evoked an increase of intracellular Ca2+, whereas 10 μM NE still did (Fig. 7 B and C). RS abolished the 5-HT–induced Ca2+ increase. Additionally, a potent and selective 5-HT2C receptor agonist, WAY-161503 (WAY) (43), evoked Ca2+ signals; its action was also inhibited by RS (Fig. 7D). Thus, 5-HT can evoke Ca2+ responses from 5-HT2C receptors present in the plasma membrane of pinealocytes.
Fig. 7.
5-HT activates 5-HT2C receptors in the membrane of pinealocytes and raises calcium. Intracellular calcium levels were monitored by calcium imaging using Fura-2 AM fluorescence ratios (340/380 nm). (A) Calcium time course during application of 5-HT and NE. (B) Calcium time course in pinealocytes preincubated for 10 min with 0.5 μM RS, a selective 5-HT2C antagonist, and then measured during application of 5-HT with RS and NE. (C) Magnified traces from the red-dashed box (A and B), showing that RS blocked the induction of a modest calcium increase by 5-HT. Closed circles indicate the application of 5-HT (A) and open circles indicate coapplication of 5-HT and RS (B). (D) Like 5-HT, WAY, a potent and selective 5-HT2C agonist, increased intracellular calcium by a pathway blocked by RS.
Tonic 5-HT Up-Regulates NAS and Melatonin Secretion Induced by NE.
Finally, we tested the hypothesis that melatonin synthesis is modulated by locally released 5-HT acting through autocrine 5-HT2C receptors. The receptor blocker RS was used to test whether it affected NE-induced secretion of NAS and melatonin from pinealocytes. Pineal strips were incubated for 6 h in a 96-well plate for an initial drug-free control period with 200 μL M199 medium alone, and then switched for a further 6 h to test medium with NE, with or without RS (Fig. 8A). As the effect of RS on melatonin secretion was not known a priori, we used a low concentration of NE (0.2 μM) to permit detection of either inhibition or potentiation by added 0.5 or 5 μM RS. The control and test media were analyzed, and results were expressed as secretion in test medium relative to drug-free control. Secretion of melatonin was increased 26.3 ± 6.3-fold by 6 h of 0.2 μM NE alone (n = 6), but only 2.7 ± 1.0- and 2.3 ± 0.4-fold when 0.5 or 5 μM RS was included (n = 4 and P = 0.01 compared to NE alone for both RS concentrations) (Fig. 8B). The secretion of 5-HT was not affected by application of NE alone or with RS (Fig. 8C). Like melatonin, NAS secretion also showed a huge increase with 0.2 μM NE alone, 100 ± 31-fold increase. However, with 0.5 or 5 μM RS added to NE, NAS showed only 5.6 ± 2.1- or 6.9 ± 0.9-fold increases (P < 0.03), respectively (Fig. 8D).
The inhibitory effect of RS was weaker and required a higher inhibitor concentration when the pineal strips were activated by strong NE (1 μM). Melatonin secretion was 38.8 ± 4.7-fold increased by 6 h of 1 μM NE alone (n = 8) and 44.9 ± 5.6-fold (n = 9, P = 0.40) and 23.7 ± 3.3-fold (n = 9, P = 0.02) increased with 0.5 and 5 μM RS included, respectively (SI Appendix, Fig. S2A). The secretion of 5-HT was not affected by application of 1 μM NE (SI Appendix, Fig. S2B). RS did not decrease NAS secretion induced by 1 μM NE (182 ± 46-fold increase for NE alone, vs. 264 ± 58-fold increase for NE with 5 μM RS; n = 8 to 9, P = 0.8) (SI Appendix, Fig. S2C).
It is clear that inhibition of 5-HT2C receptors could reduce the NE-induced secretion of NAS and melatonin. The experiments showed that even when the PG is cut into small strips, enough constitutively secreted 5-HT accumulated in the tissue to activate endogenous 5-HT2C receptors, and this activation of receptors increased the responsiveness of pinealocytes to NE stimulation. As further confirmation, we tested the effect of adding exogenous 5-HT (Fig. 8E). Coapplication of 10 μM 5-HT increased NE-induced melatonin secretion by 1.7-fold (n = 6 to 8, P = 0.02). Similarly, NAS secretion was doubled (P = 0.02). This potentiation by 5-HT may better mimic the physiological effects of constitutively secreted 5-HT that is released and retained in glands that have not been sectioned into small pieces. We presume that the effect of 5-HT is exerted on melatonin synthesis (Fig. 1) since melatonin secretion by membrane solubility follows obligatorily after synthesis (23).
Discussion
We studied signaling by 5-HT in the PG, describing 1) continuous spontaneous release of 5-HT via an equilibrative transporter, 2) activation of Gq-coupled 5-HT2C receptors producing a Ca2+ signal, and 3) sensitization of NE-induced melatonin production and the potential for an autocrine action of 5-HT.
Unique Mechanism of Pineal 5-HT Secretion.
We started with previous studies. In the brain, the PG has the highest content of 5-HT (44), the precursor of melatonin. Many-day sampling by in vivo pineal microdialysis shows strong constitutive 5-HT release, day and night, with only modest circadian variations: a transient 100-min doubling prior to nocturnal onset of melatonin release, followed by a nocturnal 50% depression during augmented melatonin synthesis (8, 13). The early nocturnal doubling of 5-HT release can be mimicked in isolated PGs by stimulation of α-adrenergic receptors (Fig. 2) (12) and coincides with adrenergic activation of the 5-HT synthetic enzyme tryptophan hydroxylase 1 (13), a transient rise of intracellular Ca2+, and a small depolarization of the membrane potential (Fig. 7B) (21, 40). A portion of the released 5-HT is taken up by sympathetic nerve terminals and rereleased as 5-hydroxyindoleacetic acid (45). Nerve terminals containing 5-HT in the PG include serotonergic innervation and also the sympathetic endings that can take up 5-HT (45–48). In perifusion studies of acutely dissected and unstimulated PGs, addition of the 5-HT uptake blocker fluoxetine increases the collected 5-HT by 24% and decreases collected 5-hydroyindoleacetic acid (45). Thus, a 5-HT uptake is operative but removes only a minor component of the total secreted 5-HT. This net uptake into neurons is by a fluoxetine-sensitive, Na+-coupled 5-HT uptake mechanism rather than by the equilibrative transporters of pinealocytes. The pinealocytes, isolated PGs, and strips that we and most others have studied were cultured for >48 h to allow degeneration of such nerve terminals, so the 5-HT we measure here would be from pinealocytes and includes the extra ∼20% that would have normally been taken up by nerve endings (9, 12, 20, 45).
The mechanism of 5-HT release has been unclear. NE-induced transient elevations of 5-HT release have been considered as calcium-regulated exocytosis from vesicles (26, 49, 50). However, although high-KCl, ACh, and NE each elevate intracellular Ca2+ (Fig. 7) (21, 30, 31, 40), none of these treatments evoked quantal releases of oxidizable materials like 5-HT from pinealocytes (Fig. 3). As we confirmed for ACh application with MS (Fig. 2C), neither ACh nor KCl elevates basal 5-HT secretion from isolated PGs (26). Furthermore, there is no Ca2+ elevation or adrenergic stimulation of PGs that accompanies the strong constitutive 5-HT secretion during daytime (9, 13). We conclude that very little of the ongoing 5-HT secretion from PGs could be Ca2+-dependent exocytosis.
In the absence of NE, we found that the constitutive efflux of 5-HT from pineal strips was blocked by decynium-22 (Fig. 4B), a blocker of organic cation and monoamine transporters in the OCT1-3 (SLC22A1-3) and PMAT (SLC29A4) gene families (34). Unlike the well-known, high-affinity monoamine transporter families, these are low-affinity, high-capacity equilibrative transporters driven by the electrochemical gradient of the substrate and not coupled to gradients of monovalent Na+, K+, or H+. In the nervous system, equilibrative transporters can take up large amounts of released monoamine neurotransmitters aided by a negative resting potential when extracellular transmitter concentrations are high and intracellular concentrations are low (34). They would operate in parallel with the substrate-specific, high-affinity transporters. Transcripts for OCT3 and PMAT are present in the pineal (51, 52). PMAT has the higher capacity for 5-HT, an uptake Km of 114 μM, and can catalyze reverse transport (efflux) of substrate from cell lines when the electrochemical gradient favors exit (37). For comparison, OCT2 has an uptake Km of 3.6 mM (53). The membrane potential of pinealocytes is −45 to −55 mV, less negative than in neurons (40, 54), but still favoring cation influx. However, we presume that the continual active synthesis of 5-HT in pinealocytes always keeps cytoplasmic 5-HT well above that in the extracellular space, engaging reverse transport constitutively, likely via the equilibrative 5-HT–favoring PMAT transporter.
Pineal 5-HT Receptor Signaling.
The PG is tightly packed with cells, mainly pinealocytes and minor glial and interstitial cells (55–57). Release of 5-HT would elevate the local extracellular concentration as the secreted product builds up in this tight space. Gene-chip studies show that the principal 5-HT receptor subtype in PGs is 5-HT2C (Ki < 0.1 μM 5-HT), whose transcripts are up-regulated 2.2-fold at night (51). These Gq-coupled receptors are sensitive to RS, agonized by WAY, raise intracellular Ca2+ and, likely, may activate protein kinase C. Consistent with this, we demonstrated that 5-HT acted on an RS-sensitive and WAY-stimulated receptor in pinealocytes to raise intracellular Ca2+ and not cAMP, and to sensitize NE-stimulated melatonin secretion from PGs (Figs. 5 D and E, 7 C and D, and 8E). In addition, RS profoundly depressed NE-evoked melatonin secretion even without added 5-HT, confirming the importance of constitutive action of endogenous 5-HT released in the tissue (Fig. 8B). Our work extends a careful in vivo study on freely behaving rats showing that nocturnal but not diurnal melatonin secretion is enhanced by systemic injection of 5-HT2C agonists, and depressed by injection of 5-HT2C antagonists (41, 58).
Effect of 5-HT on Melatonin Synthesis.
When pineal strips were stimulated by a low concentration of NE (0.2 μM), constitutive 5-HT2C receptor stimulation seemed essential for meaningful activation of melatonin secretion (Fig. 8B). But when strips were stimulated by saturating concentrations of NE (1 μM), 5-HT was not needed for maximal melatonin secretion. Thus, we can say that constitutively released 5-HT is increasing the sensitivity of the gland to stimulation by NE. These observations parallel old findings that β-adrenergic receptor induction of AANAT activity in vitro is amplified by 5-HT, that 5-HT does not induce AANAT by itself (20), and that 5-HT2C agonists injected systemically in vivo enhance and 5-HT2C antagonists depress nocturnal melatonin secretion (41, 58). Maximal adrenergic induction of AANAT requires both α1-adrenergic Ca2+ signals and β1-adrenergic cAMP signals (1, 59). Presumably, 5-HT2C receptors potentiate by stimulating the Ca2+ branch, likely acting at the level of AANAT activation. The modulatory effects of 5-HT resemble those of σ-receptors (60) and α1-adrenergic receptors (61) in the PG. Alone they do not activate AANAT, but they potentiate the β-adrenergic signal.
Conclusion
5-HT, synthesized in the PG as a precursor of melatonin synthesis (Fig. 1), is released in a constitutive manner by efflux through equilibrative transporters, possibly PMAT. The released 5-HT acts locally in an autocrine/paracrine fashion on pinealocyte 5-HT2C receptors generating Ca2+ signals and sensitizing stimulation of melatonin synthesis by NE.
Materials and Methods
Animals and Cell Culture.
Male Sprague–Dawley rats (6- to 18-wk-old, ∼150 to 450 g) were housed with a 14:10 light:dark cycle for >2 wk. Animal care and surgery followed protocols approved by the University of Washington Institutional Animal Care and Use Committee. PGs were removed from rats killed by CO2 around midday (Zeitgeber time, ZT: 4 to 6).
Pineal cells were prepared and cultured as described previously (54). Dissociated cells were incubated at 37 °C in a CO2 incubator for 1 to 3 d before use. All single-cell measurements were performed during daytime (ZT: 4 to 12). Pinealocytes were visually identified by small blobs at their cell surface under differential interference contrast optics (54). In contrast, glial cells and fibroblasts have a clear membrane. All single-cell experiments were performed at room temperature in Ringer’s solution.
Pineal Strips.
A single PG was immersed in liquid agar (2%) for slicing. Liquid agar was prepared in Ringer’s by warming to 80 °C and cooling to 45 °C. The immersed PG was suctioned into a cutoff 1-mL syringe, repositioned in the middle of a semiliquid agar block, and allowed to cool. This block was sliced at 400-μm thickness by a Vibratome 1000 Classic (IMEB) (Fig. 2A). Slices containing pineal tissue were transferred into 2% agar solution once again and cooled. The re-embedded agar block was sliced orthogonally at 300- or 400-μm thickness. This two-step procedure yielded 9 to 15 elongated pineal strips from one PG. They were incubated in DMEM for 48 h before use to allow sympathetic terminals to degenerate.
Secretion from Pineal Strips.
For perifusion experiments (Fig. 4), single strips were washed twice with M199 medium (Life Technologies) and inserted into a glass microchamber perfused by a peristaltic pump. The perfusate was collected every 20 min at 37 °C and 5% CO2. Samples (100 to 200 μL) were centrifuged at 800 × g for 3 min to remove any released pinealocytes. The supernatant was centrifuged again at 16,000 × g for 10 min and the clear supernatant was stored at −80 °C until the UPLC/MS analysis.
For short-term (Fig. 2) and long-term (Fig. 8) secretion, each strip was washed with M199 medium, transferred into a 96-well plate with 120 or 200 μL M199, and incubated at 37 °C and 5% CO2. After incubation with M199, solution was collected (Control), and new solution was added for a second incubation (Drug). Samples were centrifuged and stored as in the perifusion experiment. Sampling using PG strips was carried out at ZT: 4 to 12.
UPLC/MS.
Molecules secreted from pineal strips were analyzed with UPLC/MC as described previously (9). Briefly, samples and standard solutions were separated by liquid chromatography and ionized by Xevo TQ-S MS/MS in electrospray ionization (ESI)–positive mode. MRM acquisition monitored fragments that were quantified as peak areas.
Transfection.
To measure cAMP in pinealocytes, we transfected an Epac1-derived plasmid (gift from Martin Lohse, University of Würzburg, Würzburg, Germany). Pinealocytes were hard to transfect by conventional Lipofectamine or by several adenoviruses or baculovirus. Therefore, we used a magnetofection kit for primary cells (OZ Bioscience) following the protocol of the manufacturer: 2 μL of cDNA and 6 μL MTX transfection reagent were allowed to combine with 2 μg of magnetic nanoparticles (CombiMag) for 20 min and transported into cells with 1× MTXBoost by a magnetic field on the top of the supplied magnetic plate for 20 to 30 min. In preliminary experiments, we optimized intracellular transfection and expression of a different fluorescent protein label after standard culture for 1 to 2 d (SI Appendix, Fig. S1). Since the Epac1 probe is also fluorescent, it could be confirmed in each cell we studied.
Amperometry.
A 10-μm amperometric carbon-fiber probe (28) was placed close to or touching the pinealocytes to detect the quantal events from the oxidation of released compounds. An applied electrode potential of +600 mV oxidized 5-HT molecules readily. Amperometric currents were recorded with an EPC-9 amplifier (HEKA Electronic), filtered at 100 Hz and sampled at 500 Hz. The Ringer’s bath solution contained: 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, pH 7.3 adjusted with NaOH. K-rich bath solution contained: 140 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, pH 7.3 adjusted with KOH. A subset of cultured cells was loaded with exogenous dopamine for 35 min at room temperature (32, 33) and measured within 1 h.
FRET.
We measured FRET efficiency by the three-cube method using transfected Epac1 protein tagged with CFP and YFP at N and C termini (38). Transfected pinealocytes were excited at two wavelengths, and fluorescent light was measured at two wavelengths at 1 Hz. Data were corrected for background, analyzed as FRET efficiency, and normalized to the starting baseline of each experiment as described previously (62).
Patch Clamp.
Ionic currents were recorded from single pinealocytes in the whole-cell configuration at room temperature using an EPC-9 patch-clamp amplifier. Data were low-pass filtered at 1 kHz and sampled at 5 kHz (54). The pipette resistance was 2 to 3 MΩ and series resistance was compensated by at least 70%. The bath solution was Ringer’s solution. Pipette solution consisted of: 10 mM NaCl, 140 mM KCl, 1 mM MgCl2, 1 mM EGTA, pH 7.3 adjusted with KOH.
Calcium Imaging.
Pinealocytes were loaded with 2 μM Fura 2-AM with 0.05% pluronic F-127 in Ringer’s in darkness for 45 min at room temperature and then incubated in dye-free Ringer’s for an additional 20 min. Imaging used a Nikon TE2000-U microscope equipped with a Polychrome monochromator (Polychrome IV; TILL Photonics) and a CCD camera (Photometrics). The excitation wavelengths were 340 and 380 nm, and fluorescence emission was collected at 510 nm every 2 s using Metafluor software (Molecular Devices). The ratio of emission of excitation from 340 nm vs. 380 nm (340/380) served to measure free calcium.
Statistical Analysis.
Data are presented as mean ± SEM, n is the number of cells or pineal strips. Student’s t test was used to test the significant difference between two groups. P < 0.05 was regarded as significant.
Acknowledgments
We thank Lea M. Miller for technical assistance; Dr. Joanne Wang for advice on the plasma membrane monoamine transporter; Dr. Jongyun Myeong for help with FRET measurements; and Drs. Jill B. Jensen, Lizbeth de la Cruz, Oscar Vivas, and Joanne Wang for helpful comments on the manuscript. This work was supported by NIH Grant R01-GM083913 (to B.H.) and the Wayne E. Crill Endowed Professorship (B.H.).
Footnotes
The authors declare no competing interest.
Reviewers: J.B., University of Michigan, Ann Arbor; E.M.M., National University of Cuyo Mendoza.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2113852118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Klein D. C., et al., The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog. Horm. Res. 52, 307–357, discussion 357–358 (1997). [PubMed] [Google Scholar]
- 2.Sun X., Liu T., Deng J., Borjigin J., Long-term in vivo pineal microdialysis. J. Pineal Res. 35, 118–124 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Møller M., Baeres F. M., The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 309, 139–150 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Masson J., Emerit M. B., Hamon M., Darmon M., Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 685–713 (2012). [Google Scholar]
- 5.Ray R. S., et al., Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewczuk B., Nowicki M., Prusik M., Przybylska-Gornowicz B., Diurnal rhythms of pinealocyte ultrastructure, pineal serotonin content and plasma melatonin level in the domestic pig. Folia Histochem. Cytobiol. 42, 155–163 (2004). [PubMed] [Google Scholar]
- 7.Snyder S. H., Zweig M., Axelrod J., Fischer J. E., Control of the circadian rhythm in serotonin content of the rat pineal gland. Proc. Natl. Acad. Sci. U.S.A. 53, 301–305 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azekawa T., Sano A., Sei H., Morita Y., Diurnal changes in pineal extracellular indoles of freely moving rats. Neurosci. Lett. 132, 93–96 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Lee B. H., et al., Two indoleamines are secreted from rat pineal gland at night and act on melatonin receptors but are not night hormones. J. Pineal Res. 68, e12622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X., Deng J., Liu T., Borjigin J., Circadian 5-HT production regulated by adrenergic signaling. Proc. Natl. Acad. Sci. U.S.A. 99, 4686–4691 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonneaux V., Ribelayga C., Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 55, 325–395 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Aloyo V. J., Walker R. F., α-Adrenergic control of serotonin release from rat pineal glands. Neuroendocrinology 48, 61–66 (1988). [DOI] [PubMed] [Google Scholar]
- 13.Huang Z., et al., Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J. Pineal Res. 45, 506–514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitaram B. R., Lees G. J., Diurnal rhythm and turnover of tryptophan hydroxylase in the pineal gland of the rat. J. Neurochem. 31, 1021–1026 (1978). [DOI] [PubMed] [Google Scholar]
- 15.Walker R. F., Aloyo V. J., Norepinephrine stimulates serotonin secretion from rat pineal glands, in vitro. Brain Res. 343, 188–189 (1985). [DOI] [PubMed] [Google Scholar]
- 16.Walker R. F., Aloyo V. J., Molecular mechanisms controlling norepinephrine-mediated release of serotonin from rat pineal glands. Adv. Exp. Med. Biol. 221, 223–236 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Wurtman R. J., Shein H. M., Larin F., Mediation by -adrenergic receptors of effect of norepinephrine on pineal synthesis of (14 C)serotonin and (14 C)melatonin. J. Neurochem. 18, 1683–1687 (1971). [DOI] [PubMed] [Google Scholar]
- 18.Miguez J. M., Simonneaux V., Pevet P., The role of the intracellular and extracellular serotonin in the regulation of melatonin production in rat pinealocytes. J. Pineal Res. 23, 63–71 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Olcese J., Münker M., Extracellular serotonin promotes melatonin release from cultured rat pinealocytes: Evidence for an S2-type receptor-mediated autocrine feedback. Brain Res. 643, 150–154 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Sugden D., 5-Hydroxytryptamine amplifies beta-adrenergic stimulation of N-acetyltransferase activity in rat pinealocytes. J. Neurochem. 55, 1655–1658 (1990). [DOI] [PubMed] [Google Scholar]
- 21.Yoon J. Y., Jung S. R., Hille B., Koh D. S., Modulation of nicotinic receptor channels by adrenergic stimulation in rat pinealocytes. Am. J. Physiol. Cell Physiol. 306, C726–C735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schomerus C., Korf H. W., Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. N. Y. Acad. Sci. 1057, 372–383 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Yu H., Dickson E. J., Jung S. R., Koh D. S., Hille B., High membrane permeability for melatonin. J. Gen. Physiol. 147, 63–76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagayasu K., Kitaichi M., Shirakawa H., Nakagawa T., Kaneko S., Sustained exposure to 3,4-methylenedioxymethamphetamine induces the augmentation of exocytotic serotonin release in rat organotypic raphe slice cultures. J. Pharmacol. Sci. 113, 197–201 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Marquis B. J., Liu Z., Braun K. L., Haynes C. L., Investigation of noble metal nanoparticle ζ-potential effects on single-cell exocytosis function in vitro with carbon-fiber microelectrode amperometry. Analyst (Lond.) 136, 3478–3486 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Hayashi M., Haga M., Yatsushiro S., Yamamoto A., Moriyama Y., Vesicular monoamine transporter 1 is responsible for storage of 5-hydroxytryptamine in rat pinealocytes. J. Neurochem. 73, 2538–2545 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Koh D. S., Hille B., Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc. Natl. Acad. Sci. U.S.A. 94, 1506–1511 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh D. S., Hille B., Rapid fabrication of plastic-insulated carbon-fiber electrodes for micro-amperometry. J. Neurosci. Methods 88, 83–91 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Kawagoe K. T., Zimmerman J. B., Wightman R. M., Principles of voltammetry and microelectrode surface states. J. Neurosci. Methods 48, 225–240 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Yu H., et al., GABAergic signaling in the rat pineal gland. J. Pineal Res. 61, 69–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizutani H., et al., Modulation of Ca2+ oscillation and melatonin secretion by BKCa channel activity in rat pinealocytes. Am. J. Physiol. Cell Physiol. 310, C740–C747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K. T., Koh D. S., Hille B., Loading of oxidizable transmitters into secretory vesicles permits carbon-fiber amperometry. J. Neurosci. 20, RC101 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh D. S., Moody M. W., Nguyen T. D., Hille B., Regulation of exocytosis by protein kinases and Ca2+ in pancreatic duct epithelial cells. J. Gen. Physiol. 116, 507–520 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., The plasma membrane monoamine transporter (PMAT): Structure, function, and role in organic cation disposition. Clin. Pharmacol. Ther. 100, 489–499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel K., Zhou M., Wang J., Identification and characterization of a novel monoamine transporter in the human brain. J. Biol. Chem. 279, 50042–50049 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Zhou M., Xia L., Wang J., Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab. Dispos. 35, 1956–1962 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel K., Wang J., Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol. Pharmacol. 68, 1397–1407 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Nikolaev V. O., Bünemann M., Hein L., Hannawacker A., Lohse M. J., Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 279, 37215–37218 (2004). [DOI] [PubMed] [Google Scholar]
- 39.L. R. Landa, Jr, et al., Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J. Biol. Chem. 280, 31294–31302 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zemkova H., Stojilkovic S. S., Klein D. C., Norepinephrine causes a biphasic change in mammalian pinealocye membrane potential: Role of α1B-adrenoreceptors, phospholipase C, and Ca2+. Endocrinology 152, 3842–3851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steardo L., et al., Serotonergic modulation of rat pineal gland activity: In vivo evidence for a 5-hydroxytryptamine(2C) receptor involvement. J. Pharmacol. Exp. Ther. 295, 266–273 (2000). [PubMed] [Google Scholar]
- 42.Bonhaus D. W., et al., RS-102221: A novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36, 621–629 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Di Giovanni G., De Deurwaerdère P., New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol. Ther. 157, 125–162 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Reiter R. J., “Tryptophan metaboliem in the pineal gland” in Progress in Tryptophan and Serotonin Research, Schlossberger H. G., Kochen W., Linzen B., Steinhart H., Eds. (Walter de Gruyter & Co., Berlin, NY, 1984), pp. 251–258. [Google Scholar]
- 45.Míguez J. M., Simonneaux V., Pévet P., Evidence for a regulatory role of melatonin on serotonin release and uptake in the pineal gland. J. Neuroendocrinol. 7, 949–956 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi Y., Kojima M., Matsuura T., Sano Y., Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat. Embryol. (Berl.) 167, 321–333 (1983). [DOI] [PubMed] [Google Scholar]
- 47.Kennaway D. J., Moyer R. W., Serotonin 5-HT2c agonists mimic the effect of light pulses on circadian rhythms. Brain Res. 806, 257–270 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Juillard M. T., Collin J. P., Pools of serotonin in the pineal gland of the mouse: The mammalian pinealocyte as a component of the diffuse neuroendocrine system. Cell Tissue Res. 213, 273–291 (1980). [DOI] [PubMed] [Google Scholar]
- 49.Yamada H., et al., Norepinephrine triggers Ca2+-dependent exocytosis of 5-hydroxytryptamine from rat pinealocytes in culture. J. Neurochem. 81, 533–540 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Chuluyan H. E., Rosenstein R. E., Cardinali D. P., Serotonin release mechanisms in bovine pineal gland: Stimulation by norepinephrine and dopamine. Mol. Cell. Endocrinol. 64, 71–80 (1989). [DOI] [PubMed] [Google Scholar]
- 51.Bailey M. J., et al., Night/day changes in pineal expression of >600 genes: Central role of adrenergic/cAMP signaling. J. Biol. Chem. 284, 7606–7622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang E., et al., Resource: A multi-species multi-timepoint transcriptome database and webpage for the pineal gland and retina. J. Pineal Res. 69, e12673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gründemann D., et al., Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J. Biol. Chem. 273, 30915–30920 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Kim M. H., et al., Glutamate transporter-mediated glutamate secretion in the mammalian pineal gland. J. Neurosci. 28, 10852–10863 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manno F. A. M., Lau C., The pineal gland of the shrew (Blarina brevicauda and Blarina carolinensis): A light and electron microscopic study of pinealocytes. Cell Tissue Res. 374, 595–605 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Khan N. A., et al., Three dimensional culture of pineal cell aggregates: A model of cell-cell co-operation. J. Neuroendocrinol. 7, 353–359 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Ibañez Rodriguez M. P., Noctor S. C., Muñoz E. M., Cellular basis of pineal gland development: Emerging role of microglia as phenotype regulator. PLoS One 11, e0167063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afzal R., Shim W. S., Glucosylsphingosine activates serotonin receptor 2a and 2b: Implication of a novel itch signaling pathway. Biomol. Ther. (Seoul) 25, 497–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugden A. L., Sugden D., Klein D. C., Essential role of calcium influx in the adrenergic regulation of cAMP and cGMP in rat pinealocytes. J. Biol. Chem. 261, 11608–11612 (1986). [PubMed] [Google Scholar]
- 60.Steardo L., et al., (+)-N-allylnormetazocine enhances N-acetyltransferase activity and melatonin synthesis: Preliminary evidence for a functional role of sigma receptors in the rat pineal gland. J. Pharmacol. Exp. Ther. 275, 845–849 (1995). [PubMed] [Google Scholar]
- 61.Klein D. C., Sugden D., Weller J. L., Postsynaptic alpha-adrenergic receptors potentiate the beta-adrenergic stimulation of pineal serotonin N-acetyltransferase. Proc. Natl. Acad. Sci. U.S.A. 80, 599–603 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myeong J., et al., Phosphatidylinositol 4,5-bisphosphate is regenerated by speeding of the PI 4-kinase pathway during long PLC activation. J. Gen. Physiol. 152, e202012627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and SI Appendix.