Abstract
Background
Coronavirus disease 2019 (COVID-19) is associated with increased morbidity and mortality in older adults. Although the advent of the first vaccines has significantly reduced these rates, data on older adults in clinical trials are scarce.
Objectives
We quantified and compared the humoral response in individuals with vs. without pre-existing seropositivity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in a cohort of 69 patients living in a nursing home and who had received the recommended two doses of the Comirnaty (Pfizer-BioNTech®) vaccine.
Results
All 69 patients (100%) tested positive for antibodies against SARS-CoV-2 at 2 months post-vaccination. Residents with a pre-vaccination infection had significantly higher titers of anti-spike 1 IgG than those with no prior infection (median [interquartile range]: 55,726 [14463–78852] vs. 1314 [272–1249] arbitrary units, respectively; p < 0.001). The same result was observed for neutralizing antibodies titers (704 [320–1280] vs. 47 [20–40] respectively; p < 0.001). Between the pre-vaccination and post-vaccination periods, for IgG and neutralizing antibodies, we observed a 49 and 8-fold increase respectively. In comparison to the wild-type Receptor Binding Domain (RBD), the binding capacity of these vaccine sera was significantly decreased on the B.1.351 and P.1 variants RBD but not decreased with respect to the B.1.1.7 RBD.
Although all nursing home residents developed a humoral response following Comirnaty vaccine, its intensity appeared to depend on the pre-vaccination serological status.
Conclusion
Our results raise the question of how many doses of vaccine should be administered in older and how long the protection will be effective.
Keywords: SARS-CoV-2, COVID-19, Serological assay, Humoral vaccine response
1. Background
Since early 2020, the world has been facing a pandemic of infections by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is hoped that the advent of the first vaccines will resolve this health crisis [1]. The pandemic of coronavirus disease 2019 (COVID-19) has already claimed more than 4.5 million lives worldwide, with mortality increasing with age [2]. Once the first cases of SARS-CoV-2 infection were identified in nursing homes, the pathogen spread rapidly and resulted in a high incidence and high seroprevalence in this population. Novel messenger RNA vaccine technology has been brought to market in record time and is showing extraordinary results in countries that have been able to vaccinate a high proportion of the population. The results of the phase III trials show that two vaccine doses are highly effective in preventing symptomatic infections in people who have never contracted COVID-19 [3], [4]. However, the need to rapidly implement these pivotal clinical trials means that few adults over the age of 75 could be included. Hence, data on vaccine efficacy and immune response intensity in older adults are scarce. Recent results from groups around the world suggest that one dose of vaccine is sufficient for people who have already been infected with SARS-CoV-2 [5], [6], [7]. Again, little data from these early studies are available for patients over 75 years of age.
2. Objectives
Thus, the objective of the present study was to characterize and quantify the humoral immune response in a prospective cohort of 69 adults aged 75 and over 2 months after full vaccination with the Comirnaty vaccine (Pfizer-BioNTech® BNT162b2). We determined the titer of anti-spike antibodies and neutralizing titers following vaccination and then compared individuals who had previously been infected or not by SARS-CoV-2.
3. Methods
3.1. Study design and cohort
This prospective cohort study was conducted at Amiens University Medical Center (Amiens, France). We sought to monitor the immune response against SARS-CoV-2 for residents in a nursing home that had been severely impacted by SARS-CoV-2 infection during the first wave of the COVID-19 epidemic in France (March-April 2020). Six weeks after the end of the outbreak (June 2020), all residents underwent qualitative serologic testing for antibodies against the SARS-CoV-2 nucleocapsid (Abbott SARS-CoV-2 IgG: Rungis, France) and its spike protein (WANTAI SARS-CoV-2 Ab ELISA: Changping District, Beijing, China). The patients who were seropositive after the first wave were also screened in October and December 2020. Patients were vaccinated between early January and mid-February 2021. Before the first injection of Comirnaty vaccine (Pfizer BNT162b2), 23 patients were seronegative and 46 were seropositive for antibodies against SARS-CoV-2 nucleocapsid or spike proteins (Table 1 ). All 69 patients received a second dose 21 days after the first dose. The blood samples used to assess the humoral immune response were obtained a median of 55 days after the second dose. The mean ages in the seronegative and seropositive groups were 85.1 and 88.2, respectively (Table 1). 8 unvaccinated SARS-CoV-2-seropositive nursing home residents were sampled over the same period and included in the study as a control group. This group consisted of 6 women and 2 men with an average age of 84 years. The present study was approved by the institutional review board at Amiens University Medical Center (PI2020_843_0079) (ClinicalTrials.gov ID: NCT04563650).
Table 1.
Characteristics of fully vaccinated patients with or without pre-existing seropositivity to SARS-CoV-2.
| Seronegative before vaccination n = 23 |
Seropositive before vaccination n = 46 |
|
|---|---|---|
| Female (n (%)) | 16 (70) | 33 (72) |
| Male (n (%)) | 7 (30) | 13 (28) |
| Age, years (mean (SD) and [IQR] |
85.1 (7.5) [77–91] |
88.2 (6.7) [83–92] |
| Time interval between the two vaccine doses (days, median [IQR]) | 21 [21–21] | 21 [21–21] |
| Time interval between the second vaccine dose and the serum sample (days, median [IQR]) |
55 [52–59] | 55 [50–56] |
| Seropositive for anti-spike-1 IgG after full vaccination (n (%)) | 23 (100) | 46 (100) |
IQR: interquartile range
SD: standard deviation
3.2. Serological assays
A chemiluminescent microparticle immunoassay (ABBOTT SARS-CoV-2 IgG IIQuant assay, run on an ALINITY analyzer) was used to quantify IgG antibodies in each patient’s serum sample. The assay detects antibodies against the S1 subunit of the SARS-CoV-2 spike protein. The cut-off for seropositivity was set to 50 arbitrary units per milliliter (AU/ml).
3.3. The neutralization assay
Retroviral particles pseudotyped with the S glycoprotein of SARS-CoV-2 (SARS-CoV-2 pp) were produced as described previously [8] using a plasmid encoding a human codon-optimized sequence of the SARS-CoV-2 spike glycoprotein (accession number: MN908947). Supernatants containing the pseudotyped particles (pp) were harvested at 48, 72, and 96 h after transfection, pooled, and filtered through 0.45-μm pore-sized membranes. Neutralization assays were performed by pre-incubating SARS-CoV-2 pp and serially diluted plasma for 1 h at room temperature prior to contact with 293 T cells (ATCC® CRL-3216TM) transiently transfected with a plasmid encoding the human ACE2 protein (pcDNA3.1-hACE2) 24 h before inoculation. Luciferase activity was measured 72 h postinfection, in line with the manufacturer’s instructions (Promega). Two independent tests were carried out each time in duplicate. The neutralizing antibody titers were defined as the highest dilution of plasma resulting in a 50% decrease of the infectivity. We previously checked the specificity of our neutralization assay using not only plasma samples from patients seropositive for other coronaviruses but also retroviral particles pseudotyped with the vesicular stomatitis virus G glycoprotein.
3.4. CoViDiag+® assay and analysis
Multiplex immunoassays analyses were performed using the commercial SirYus-CoViDiag+® multiplex immunoassay targeting IgG antibodies against eight different antigens of the virus and variants: NP, S1, S2, S1-NTD (N-terminal domain) and S1-RBD wild-type, S1-RBD B.1.1.7 (UK), S1-RBD B.1.351 (SA) and S1-RBD P.1 (BRA). The assays have been performed according to the manufacturer instructions (see Fig. S4 for visual appearance). The results have been automatically delivered using the SirYus-Reader plate reader (Innobiochips SAS, Loos, FRANCE) and associated image analysis software. Raw colorimetric signals were extracted from pictures. The mean of replicates spots were calculated for each printed antigen in each well and then used for further data analysis. A dilution range of First WHO International standard for SARS-CoV-2 immunoglobulin was used to calibrate the assay. Top and bottom asymptotes were calculated when using a 4PL fit. Samples were tested at different dilutions from 1:100 to 1:128000. The sample dilution giving a 50% signal loss was defined for each antigen and then compared to the others.
3.5. Data analysis and statistical analyses
The demographic data for the 69 patients were extracted from the study’s electronic case report forms. Quantitative variables were expressed as the median [interquartile range] or the arithmetic mean ± SD and were compared using Student’s t-test (if normally distributed) or using Kruskal-Wallis/Mann-Whitney U test (if not). Pearson’s correlation coefficient was used to measure the strength of a linear association between two quantitative variables. Statistical analyses were performed using GraphPad Prism software (version 5, San Diego, California). A p-value of less than 0.05 was regarded as statistically significant.
4. Results
4.1. Quantification of the humoral immune response 2 months post-vaccination
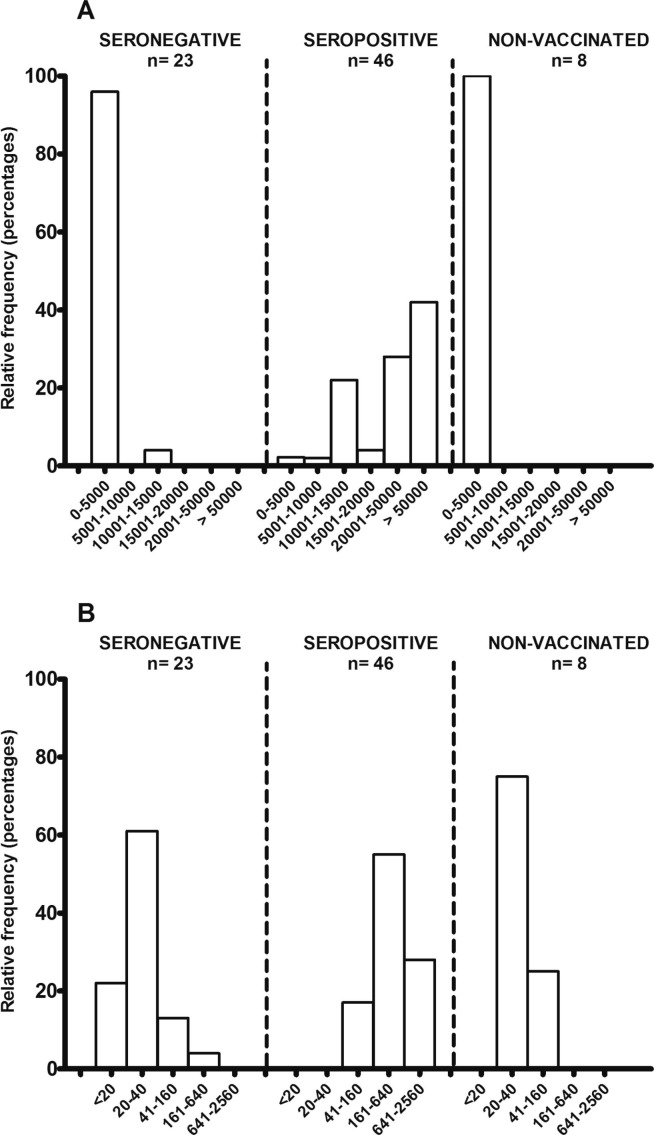
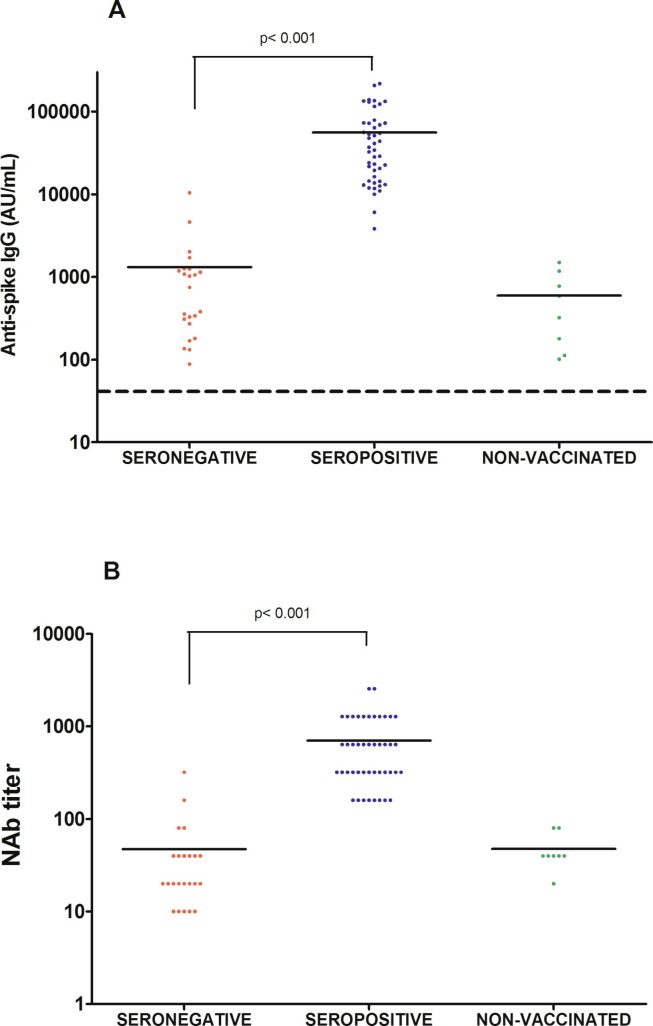
All 69 vaccinated older adults (100%) had developed a detectable humoral immune response 2 months after the second dose (Table 1). However, we observed significant quantitative differences in the distribution of the anti-spike antibody titers (Fig. 1 A) and neutralizing antibody titers (Fig. 1B). By way of a comparison, we also evaluated 8 samples taken at the same time point from SARS-CoV-2-seropositive residents of the same nursing home who did not wish to be vaccinated. Two months after the second dose, for the pre-vaccination seronegative individuals, almost all (96%) of the values were between 50 and 5000 AU, whereas almost all pre-vaccination seropositive individuals achieve values higher than 10,000 AU, and even higher than 50,000 AU for 19 of the 46 individuals (41%). The absolute values measured in each group are summarized in Fig. 2 . The median [IQR] titer was 1314 [272–1249] AU in the seronegative group and 55,726 AU [14463–78852] in the seropositive group (p < 0.001). The same observation was found with regard to the titers of neutralizing antibodies; more than 80% of the values were 40 or less in the seronegative group, whereas more than 80% of the values were greater than or equal to 160 in the seropositive (Fig. 1B). The median [IQR] titers of neutralizing antibodies were 704 [320–1280] vs. 47 [20–40] in the seropositive and seronegative groups, respectively (p < 0.001) (Fig. 2B). Nonvaccinated but seropositive individuals had a median anti-spike IgG titer of 595 AU (p = 0.16, compared to the seronegative vaccinated group) and a median titer neutralizing antibody titer of 47 (p = 0.99) although they had been infected by SARS-CoV-2 nearly 1 year before.
Fig. 1.
Distribution of titers 2 months after vaccination for (A) anti-spike-1 IgG (in AU/mL) and (B) neutralizing antibodies among seronegative, seropositive for SARS-CoV-2 before vaccination and for non-vaccinated home nursing residents.
Fig. 2.
Scatter plot of titers for (A) anti-spike-1 IgG (in AU/mL) and (B) neutralizing antibodies in seropositive and seronegative groups 2 months after vaccination and in the control group. The solid lines correspond to the mean. The dashed line corresponds to the positivity cut-off of 50 AU/mL.
A strong correlation between these two quantitative markers of the humoral response was found for the cohort of 69 patients, with an R2 of 0.71 (p < 0.001) (Supplementary Fig. 1).
Among the 46 patients who were seropositive before their vaccination, we analyzed the anti-Spike and neutralizing antibody titers as a function of age (under 90: 22 patients; 90 and over: 24 patients) and sex (females: 33; males: 13) but did not observe any significant difference between these subgroups (Supplementary Fig. 2A and B, respectively).
4.2. Time course of the pre-vaccine immune response and the response to the vaccine
For the 46 patients who were seropositive for SARS-CoV-2 before vaccination, the anti-spike IgG and neutralizing antibody titers was measured in samples collected in December 2020 (Fig. 3 ). All residents showed a clear rise in titers of IgG and neutralizing antibodies, with a 49 and 8-fold increase respectively. The change over time of these titers (including the June 2020 samples) are shown in Supplementary Fig. 3. As often reported in the literature, serum antibody concentrations tend to decline over time with a clear rebound following vaccination; we observed a 22-fold increase in the anti-spike IgG titer and a 8-fold increase in the neutralizing antibody titer between the June 2020 sample and the specimen sample 2 months after vaccination sample. We did not observe any correlation between the values obtained in June 2020 and those obtained 2 months after vaccination (data not shown).
Fig. 3.
Titers for (A) anti-spike IgG and (B) neutralizing antibodies before and after vaccination in 46 nursing home residents with pre-existing immunity to SARS-CoV-2.
4.3. Binding of vaccine sera to various Receptor-Binding-Domains (RBD)
Using a technique recently developed by Innobiochips CoViDiag+® assay (Lille, France) we evaluated the binding capacity of vaccine serum on wild-type (WT) RBD (Wuhan-Hu1 reference strain) on RBD B.1.1.7 (UK variant), on RBD B.1.351 (South-African (SA) variant) and on RBD P.1 (Brasil (BR) variant). Regardless of the group of patients, the binding on the WT and B.1.1.7 RBD was not different (Fig. 4 ). On the other hand, the binding on B.1.351 RBD and P.1 RBD was significantly lower in seronegative and seropositive before vaccination. Patients with negative pre-vaccination serology had a mean binding titer on wild-type, B.1.1.7, B1.351 and P.1 RBD of 265, 256, 65 and 104 respectively (p = 0.013 between WT and B1.351 and p = 0.047 between WT and P.1). Patients with positive pre-vaccination serology had a mean binding titer on wild-type, B.1.1.7, B.1.351 and P.1 RBD of 26628, 23125, 6213 and 9318 (p < 0.001 between WT and B1.351 and p < 0.001 between WT and P.1).
Fig. 4.
Scatter plot of 2 months post-vaccination samples binding titer on S1-RBD WT, S1-RBD B.1.1.7 (UK), S1-RBD B.1.351 (SA) and S1-RBD P.1 (BRA) among seronegative, seropositive for SARS-CoV-2 before vaccination and for non-vaccinated home nursing residents. The solid lines correspond to the mean.
5. Discussion
In a prospective study of 69 nursing home residents fully vaccinated with Comirnaty vaccine, we observed a significant difference in the humoral immune response between those with documented, pre-existing seropositivity to SARS-CoV-2 and those without. Effective vaccination is critical in this population. Nevertheless, all of the vaccinated residents had a positive serological test afterwards, which demonstrated the vaccine’s effectiveness. We decided to measure the immune response 2 months after the second dose in case the vaccine response was longer in older adults than in healthy younger adults [9]. Reports on transplanted patients with long-term immunosuppression have highlighted the lack of a humoral response to vaccination with the Moderna and Pfizer-BioNTech vaccines [10], [11], [12]. Negativation of serological assays after vaccination is starting to be described for certain categories of patients receiving immunosuppressive drugs. Physicians should be vigilant in this respect and must take into account the serological assay used and its sensitivity. Our group has already identified differences in sensitivity between various serological tests [13], [14]. In our earlier work, we found that the Abbott quantitative anti-spike-1 IgG assay technique was one of the most sensitive on the market when used qualitatively with a positivity threshold set to 50 AU/mL. We obtained a good correlation (R2 = 0.7) between the total antibody titer and the neutralizing antibody titer (Fig. S1). The correlation was not perfect but neutralizing antibodies also bind to the spike 2 protein [15]. Determination of the neutralizing antibody titer is not readily achievable in conventional medical laboratories and requires specific equipment and qualified personnel. The advent of high-performance quantitative IgG assays might help to overcome this problem, provided that they can be harmonized with international standards (e.g. standard 20/136 published by the National Institute for Biological Standards and Control).
Our findings raise the question of whether or not one or more additional doses would be of value in older adults who are seronegative before vaccination, or even whether double-dose vaccines would be useful (as is already the case for the hepatitis B vaccine in liver transplant receipts) [16]. Conversely, and as already demonstrated by many groups, a single dose might suffice for people who have already contracted COVID-19 [5], [6], [7] - even though few data on older patients have been published.
In view of these results, another question arises as to the duration of protection. Titers of anti-spike antibodies and neutralizing titers will necessarily decrease, as observed following a natural infection with SARS-CoV-2 [17]. A very high post-vaccination peak titer might provide longer-term protection, provided that a less susceptible variant does not emerge. It should always be borne in mind that the immune response to a viral infection involves a humoral component and a cellular component. We did not explore the cellular response in the present study. It should also be noted that protective thresholds for anti-spike antibodies or neutralizing antibodies have not yet been defined. It will be very difficult to define a purely analytical threshold, given the difficulty of standardizing techniques. Similarly, when monitoring the immune response after a natural infection or after vaccination, a negative serological test does not necessarily equate to the absence of protection. The memory immune cells obtained at the end of this phase might rapidly mobilize again, which means that the laboratory tests will rapidly become positive [18]. There is a solid body of evidence to show that boosting pre-existing immunity by vaccination with the Wuhan Hu1 spike protein leads to a stronger neutralizing antibody response to not only the vaccine-matched strain but also emerging variants [19].
With regard to neutralization assays, various researchers have shown that post-vaccine sera are less sensitive in detecting emerging variants - particularly those with the 484 mutation in the spike protein [20]. We evaluated the neutralization of pseudotyped particles containing the original (wild-type) Wuhan Hu1 spike protein. The first commercially available mRNA vaccines were designed based on the wild-type sequence, which became available in January 2020. It might be interesting to assess the neutralization against different variants by post-vaccination sera, bearing in mind that the humoral immune response is polyclonal. Thus, a combination of mutations can lower neutralizing capacity of the antibodies. Patients with a very high post-vaccination titer of neutralizing antibodies against the wild-type strain will not have a zero titer against the variants that have emerged and those that are yet to emerge.
The present study’s limitations included a relatively small sample size, the evaluation of a single type of vaccine, and the lack of an assessment of the cellular immune response.
In conclusion, we observed a humoral response in 100% of fully vaccinated nursing home residents. However, the titers varied greatly from one individual to another, and appeared to depend on the pre-vaccination serological status. These data highlight the value of performing a robust serological assay before vaccination, in order to help medical staff decide on the number of vaccine doses required by people who are very vulnerable to SARS-CoV-2 infections.
Funding
The present work was supported with the laboratory's own resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.11.086.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 2.O’Driscoll M., Ribeiro Dos Santos G., Wang L., Cummings D.A.T., Azman A.S., Paireau J., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. C4591001 Clinical Trial Group, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. COVE Study Group, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021;325(14):1467. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochot E., Demey B., Touzé A., Belouzard S., Dubuisson J., Schmit J.-L., et al. Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors J., Bell M.R., Marcy J., Kutzler M., Haddad E.K. The impact of immuno-aging on SARS-CoV-2 vaccine development. Geroscience. 2021;43(1):31–51. doi: 10.1007/s11357-021-00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., et al. Low immunization rates among kidney transplant recipients who received two doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021 doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325(17):1784. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupper A., Rabinowich L., Schwartz D., Schwartz I.F., Ben‐Yehoyada M., Shashar M., et al. Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.v21.810.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brochot E., Demey B., Handala L., François C., Duverlie G., Castelain S. Comparison of different serological assays for SARS-CoV-2 in real life. J Clin Virol. 2020;130:104569. doi: 10.1016/j.jcv.2020.104569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubry A., Demey B., François C., Duverlie G., Castelain S., Helle F., et al. Longitudinal Analysis and Comparison of Six Serological Assays up to Eight Months Post-COVID-19 Diagnosis. J Clin Med. 2021;10(9):1815. doi: 10.3390/jcm10091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 16.Aggeletopoulou I., Davoulou P., Konstantakis C., Thomopoulos K., Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27(6):e1942. doi: 10.1002/rmv.v27.610.1002/rmv.1942. [DOI] [PubMed] [Google Scholar]
- 17.Yamayoshi S., Yasuhara A., Ito M., Akasaka O., Nakamura M., Nakachi I., et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32:100734. doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamatatos L., Czartoski J., Wan Y.-H., Homad L.J., Rubin V., Glantz H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372(6549):1413–1418. doi: 10.1126/science:abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sills J., Saad-Roy C.M., Morris S.E., Metcalf C.J.E., Mina M.J., Baker R.E., et al. Partial immunity and SARS-CoV-2 mutations-Response. Science. 2021;372(6540):354–355. doi: 10.1126/science:abi6719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.