Abstract
In June 2021, Thailand was hit by the delta variant of SARS-CoV-2 resulting in the biggest wave of COVID-19. Due to the widespread delta variant, more than 600 healthcare workers had COVID-19 despite completion of two-dose CoronaVac. The Ministry of Public Health recommended that healthcare workers received a third dose of AZD1222 to increase level of protection against SARS-CoV-2. However, immune response after the AZD1222 booster in individuals who completed the two-dose CoronaVac vaccine are limited. In this study, sera from those who received a booster of AZD1222 in June-July 2021 were tested for SARS-CoV-2 spike receptor-binding-domain (RBD) IgG, anti-RBD total immunoglobulins and anti-spike protein 1 (S1) IgA. The neutralizing activities in a subset of serum samples were tested against the wild type and variants of concern (B.1.1.7, B.1.617.2, and B.1.351) using an enzyme-linked immunosorbent assay-based surrogate virus neutralization test. Participants who received the booster of AZD1222 possessed higher levels of spike RBD-specific IgG, total immunoglobulins, and anti-S1 IgA than the two-dose vaccinees (p < 0.001). They also elicited higher neutralizing activity against the wild type and all variants of concern than the recipients of the two-dose vaccines. This study demonstrated a high immunogenicity of the AZD1222 booster in individuals who completed the two-dose inactivated vaccines.
Keywords: COVID-19, Inactivated vaccine, Virus vector, Immunogenicity, Booster dose
1. Introduction
Widespread vaccination against the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is crucial in mitigating the global pandemic of coronavirus disease 2019 (COVID-19). Despite the expedited development of effective vaccines, the ability to acquire sufficient vaccine supplies varies greatly between industrialized and developing countries. Beginning in February 2021, Thailand was only able to procure in large quantities the inactivated COVID-19 vaccine CoronaVac. Healthcare workers 18–59 years of age were among the first to receive two doses of CoronaVac administered 3 to 4 weeks apart. Smaller quantities of the adenovirus-vectored vaccine (AZD1222) arrived the following month, with additional doses domestically produced from licensing with AstraZeneca available several months later. Vaccination with AZD1222 was approved by the Thai FDA for adults ≥ 18 years. Due to the limited supply initially, AZD1222 vaccination administered 10 weeks apart was prioritized for those with underlying comorbidities and adults ≥ 60 years of age.
Two doses of most COVID-19 vaccines were required to elicit an immunological response and protection against symptomatic disease [1], [2], [3], [4], [5]. Nevertheless, the emergence of SARS-CoV-2 variants with accumulated multiple mutations had raised concerns about the efficacy of the two-dose regimen [6]. Data gathered from mass vaccination in some countries have suggested that the effectiveness of mRNA [7] and AZD1222 [6] vaccines was reduced over time and varied among emerging viral variants. Therefore, many countries currently are considering the implementation of a third shot to boost the existing immune response. Ongoing clinical trials to investigate the immunogenicity, safety, and efficacy of a booster dose with the same (homologous) or different (heterologous) vaccine are underway [5], [8], [9].
Since June 2021, reports of hundreds of fully vaccinated healthcare workers who were subsequently infected with the delta variants of SARS-CoV-2 have spurred the implementation of a third COVID-19 vaccine dose in Thailand, either with AZD1222 or BNT162b2 [10]. However, no data were available as to whether heterologous vaccination with an adenovirus vectored vaccine in adults who previously received two doses of an inactivated vaccine was safe and effective. To address this knowledge gap, we characterized the increase in immune response and neutralizing antibody induced by heterologous vaccination with AZD1222 in Thai healthcare workers who were previously fully vaccinated with CoronaVac.
2. Methods
2.1. Study cohort
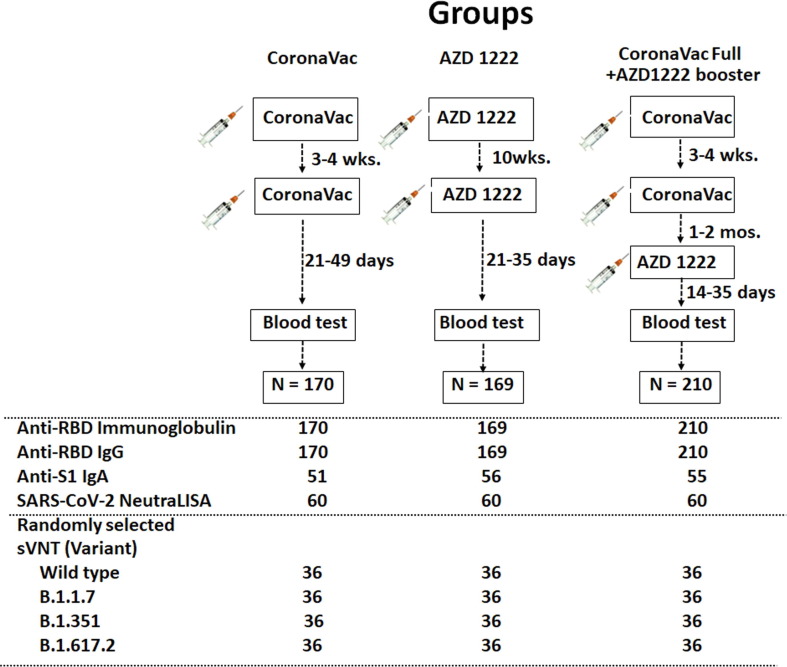
The study flow showing participant enrollment and sample size in this study is shown in Fig. 1 . The first group (n = 170) received two doses of CoronaVac. The second group (n = 169) received two doses of AZD1222. The third group (n = 210), all healthcare workers, received two doses of CoronaVac, followed by AZD1222. An additional comparison group consisted of unvaccinated, laboratory-confirmed COVID-19 patients who have recovered from illness as previously reported [11]. The study protocols were approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University (IRB numbers 192/64 and 546/64). The study is registered with the Thai Clinical Trials Registry (TCTR) with identifiers TCTR20210319003 and TCTR20210910002.
Fig. 1.
Schematic diagram of this study. Three groups of vaccinees and samples done for each test are depicted. Randomly selected samples subjected to testing listed as numbers under each group.
2.2. Vaccination
COVID-19 vaccines were available in Thailand beginning in March 2021. CoronaVac was approved for Thai adults ages 18–59 and was administered 21–28 days apart [12]. Since ADZ1222 was authorized for use in adults ≥ 18 years of age, priority was given to those ≥ 60 due to its limited supply in Thailand [13] and vaccination was done 10 weeks apart. Beginning in June 2021, healthcare workers who were previously fully vaccinated with CoronaVac (those who received two doses) were offered a third vaccination with AZD1222. In this study, the two-dose CoronaVac vaccine recipients received the first dose between March 3 and 31, 2021, and the two-dose AZD1222 vaccine recipients received the first dose between March 26 and April 3, 2021. The participants who had previously received two doses of CoronaVac were immunized with AZD1222 as a third dose between June 11 and July 23, 2021.
2.3. Blood samples
Between April 22 and August 24, 2021, blood samples were collected once between 2 and 7 weeks after the second or third vaccination in all vaccinated participants. For the recovered patients with SARS-CoV-2 infection, samples were collected between 4 and 6 weeks after symptoms appeared or after laboratory confirmation (from testing nasopharyngeal swabs with reverse-transcription polymerase chain reaction).
2.4. Antibody assays
Serum samples were evaluated for total immunoglobulins specific to the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein using Elecsys SARS-CoV-2 S according to the manufacturer’s instruction (Roche Diagnostics, Basel, Switzerland). Values ≥ 0.8 U/mL was considered positive. To quantify only IgG specific to the RBD, we utilized SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Abbott Park, Ill) according to the manufacturer’s instruction. Values ≥ 50 arbitrary U/mL (AU/mL) was considered positive. Multiplying the numerical AU/mL by 0.142 converts it to binding antibody units per milliliter (BAU/mL). A subset of samples was evaluated for anti-S1 IgA using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction (Euroimmun, Lübeck, Germany). The results were derived from the ratio in optical density (OD) obtained from samples and the calibrator. Maximum cut-off ratio (OD/CO) was 9; results greater than 9 was recorded as 9.
A subset of samples was also evaluated for neutralizing activity against the SARS-CoV-2 wild-type and variants of concern B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) using an ELISA-based surrogate virus neutralization test (sVNT). NeutraLISA (Euroimmun, Lübeck, Germany) was used to evaluate the wild-type strain only. Results ≥ 35% inhibition was considered positive. Additionally, cPass SARS-CoV-2 neutralizing antibody detection kit (GenScript, Piscataway, NJ) was used for all strains. The recombinant RBD from B.1.1.7 (containing N501Y), B.1.351 (containing N501Y, E484K, and K417N), and B.1.617.2 (containing L452R and T478K) were also used with this kit. Briefly, the serum samples were diluted 1:10 with buffer and incubated with RBD conjugated to horseradish peroxidase for 30 min. at 37 °C. Next, 100 µL of the sample mixture was added to a capture plate pre-coated with human angiotensin-converting enzyme 2 (ACE2) and incubated for 15 min at 37 °C. After washing, 100 µL of TMB chromogen solution was added and the plate incubated in the dark for 15 min at room temperature. After the addition of 50 µL stop solution, samples were read at 450 nm. The ability of a serum to inhibit binding between RBD and ACE2 was calculated as percentage as follows: 1 – (average OD of sample/average OD of negative control), multiplied by 100. Inhibitions ≥ 30% was considered positive.
2.5. Statistical analysis
Baseline characteristics were reported as mean and standard deviations (SD), or median and interquartile range (IQR). Antibody levels were presented as geometric mean titers (GMT) with 95% confidence interval (CI), or median with interquartile range when appropriate. The difference in baseline characteristics such as sex and age were calculated by Chi-squared test and t-test, respectively. The difference in antibody titers, OD/CO, and percent inhibition between groups was calculated using ANOVA or the Kruskal–Wallis test. Statistical analysis was done using Prism 8.0 (GraphPad, San Diego, CA). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic data of vaccinees
The fully vaccinated AZD1222 group was older (mean age 47.9 years), while the fully vaccinated CoronaVac followed by the AZD1222 booster group was younger (mean age 40 years). There were significantly more women in the fully vaccinated CoronaVac + AZD1222 group (Table 1 ). Time interval between the last dose vaccination and blood collection in the fully vaccinated CoronaVac followed by AZD1222 group was shorter (14–35 days) whereas the longer interval (22–49 days) was found in the fully vaccinated CoronaVac group (supplementary Table 1).
Table 1.
Characteristics of the vaccinees in this study.
| Characteristics | CoronaVac (fully vaccinated) n = 170 |
AZD1222 (fully vaccinated) n = 169 |
CoronaVac (full vac.) + AZD1222 n = 210 |
p-value |
|---|---|---|---|---|
| Mean age, years (SD) | 42.3 (9.6) | 47.9 (15.5) | 40.0 (9.8) | p < 0.05a,p < 0.001b |
| Sex and age group | ||||
| Male no. (%) | 81 (47.6%) | 71 (42.0%) | 59 (28.1%) | |
| 18–29 y | 8 | 4 | 2 | |
| 30–59 y | 73 | 46 | 55 | |
| ≥ 60 y | – | 21 | 1 | |
| ND | – | – | 1 | |
| Female no. (%) | 89 (52.4%) | 98 (58.0%) | 151 (71.9%) | p < 0.001c, p = 0.001d |
| 18–29 y | 10 | 18 | 29 | |
| 30–59 y | 79 | 55 | 118 | |
| ≥ 60 y | – | 25 | 3 | |
| ND | – | – | 1 | |
| Days (median) between dose 1 and 2 | 23.0 | 70.0 | 21.0 | |
| [IQR] | [21.0, 26.0] | [70.0, 70.0] | [21.0, 26.0] | |
| (min–max) | (21.0–28.0) | (68.0–76.0) | (14.0–37.0) | |
| Days (median) between dose 2 and 3 | – | – | 70.0 | |
| [IQR] | [61.0, 79.0] | |||
| (min–max) | (30.0–99.0) | |||
| Days (median) between last dose and blood collection | 29.0 | 30.0 | 28.0 | |
| [IQR] |
[28.0–31.0] | [26.0–31.0] | [20.0–32.0] | |
| (min–max) | (27.0, 49.0) | (19.0, 41.0) | (14.0, 35.0) | |
represents the comparison between fully vaccinated CoronaVac and fully vaccinated CoronaVac + AZD1222 group using t-test. b represents the comparison between fully vaccinated AZD1222 and fully vaccinated CoronaVac + AZD1222 group using t-test. c represents the comparison between fully vaccinated CoronaVac and fully vaccinated CoronaVac + AZD1222 group using Chi-square test. d represents the comparison between fully vaccinated AZD1222 and fully vaccinated CoronaVac + AZD1222 using Chi-square test. ND indicates no data available.
3.2. RBD-specific SARS-CoV-2 antibodies
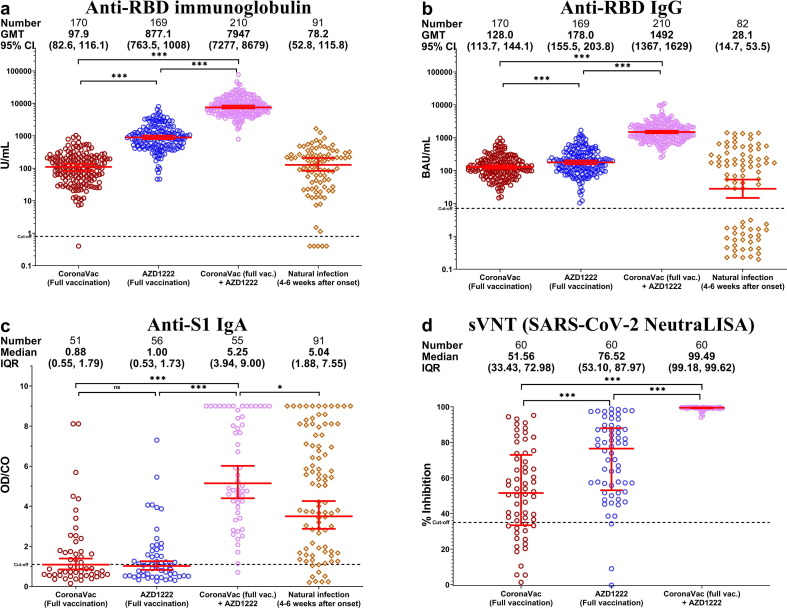
We first evaluated the GMT of total anti-RBD immunoglobulins in vaccinated individuals and recovered COVID-19 patients. The GMT of total anti-RBD immunoglobulins among vaccinees fully vaccinated with CoronaVac was comparable to that of unvaccinated convalescent patients (<100 U/mL) (p = 0.7608) (Fig. 2 a.). The GMT of fully vaccinated CoronaVac + AZD1222 (7,947 U/mL) was nine-fold greater than that of fully vaccinated AZD1222. When we determined only the contribution of anti-RBD IgG, the GMT among convalescent patients (28.1 BAU/mL) was lower than that of fully vaccinated CoronaVac group (128 BAU/mL) (p = 0.0375). In contrast, the fully vaccinated CoronaVac + AZD1222 vaccinees possessed the highest antibody levels (1,492 BAU/mL) compared to other groups (Fig. 2b.). Serum anti-S1 IgA was also highest in this group (Fig. 2c.). The presence of serum anti-S1 IgA was observed only after a third vaccination and in convalescent patients. In addition, the sVNT assay, which was based on antibody-mediated inhibition of ACE2 and S protein interaction, showed that the fully vaccinated CoronaVac + AZD1222 recipients had higher neutralizing activities (demonstrated as percent inhibition) against the original Wuhan (wild-type) strain compared to fully vaccinated CoronaVac and fully vaccinated AZD1222 groups (Fig. 2d.). We analyzed the immunogenicity data in two sets. The first set included all available data. The second set included only participants whose blood samples were at the same interval between last dose vaccination and blood sampling. Differences between all available data and data from participants with similar intervals between vaccination and blood samples among all groups were not significant (supplementary Fig. 1).
Fig. 2.
Immunoassays for SARS-CoV-2 in serum samples from fully vaccinated and unvaccinated convalescent COVID-19 patients. (a) Total anti-RBD immunoglobulins. (b) Anti-RBD IgG. (c) Anti-S1 IgA. (d) Surrogate virus neutralization test (sVNT). Geometric mean titer (GMT) with 95% confidence intervals are shown as red lines within each group. * denotes p < 0.05 and *** denotes p ≤ 0.001.
3.3. Neutralizing antibodies against SARS-CoV-2 variants
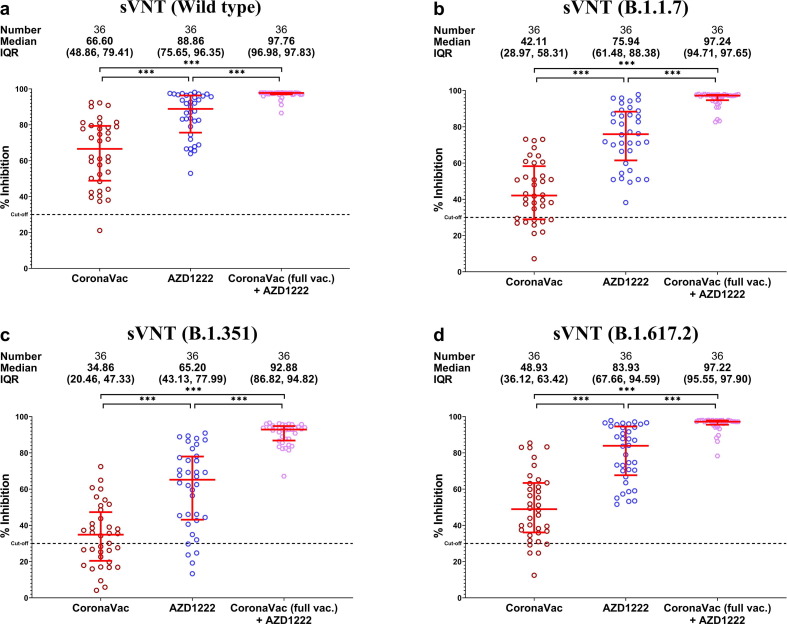
Although serum samples from the fully vaccinated AZD1222 group was able to better inhibit wild-type SARS-CoV-2 and variants than those from the fully vaccinated CoronaVac (p < 0.01), the fully vaccinated CoronaVac + AZD1222 group demonstrated higher neutralizing antibodies compared to two doses of either vaccine (p < 0.0001) (Fig. 3 a-d.).
Fig. 3.
Surrogate virus neutralization test against SARS-CoV-2 wild-type and variants. (a) Wild-type. (b) B.1.1.7. (c) B.1.351. (d) B.1.617.2. Percent inhibition is shown with mean and IQR (red lines). *** denotes p ≤ 0.001.
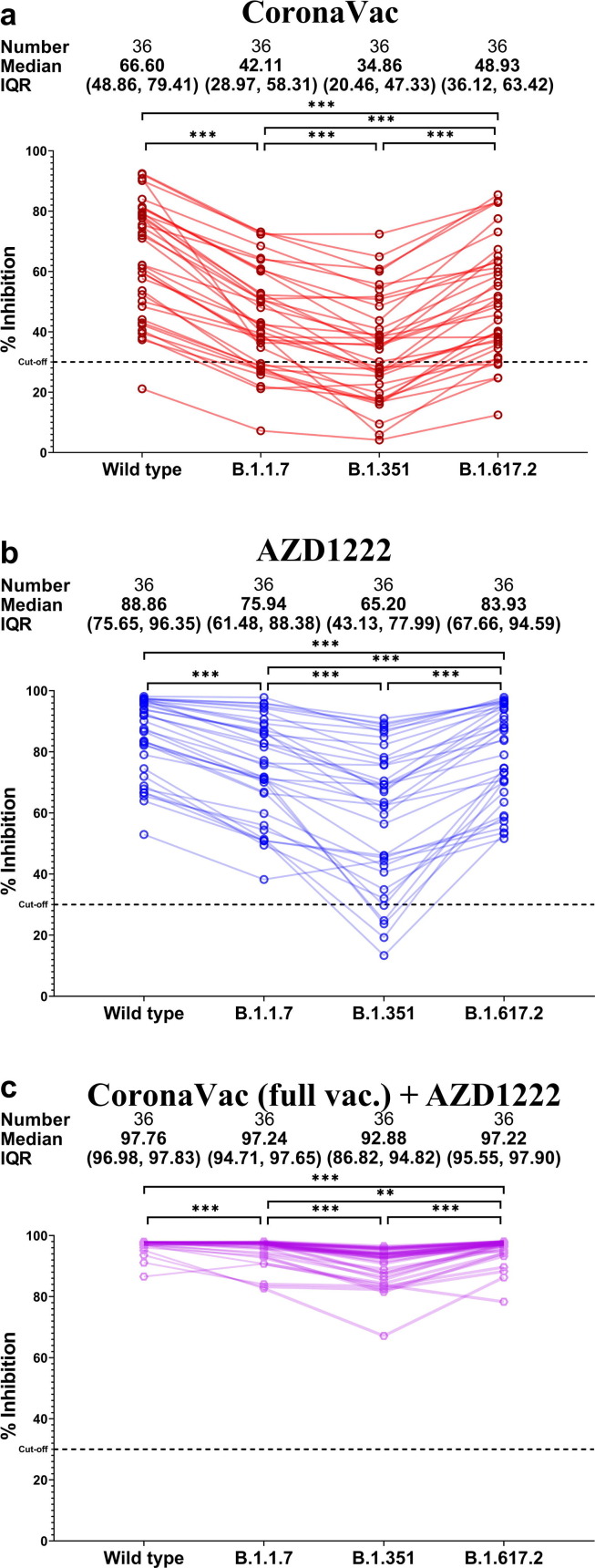
Comparison of neutralizing activities within the same participants against the wild-type and variant strains showed that all vaccinated groups elicited higher neutralizing activities in the following order: wild type > B.1.617.2 > B.1.1.7 > B.1.351 (Fig. 4 a-c.). The fully vaccinated CoronaVac group showed the lowest inhibition against B.1.351 (Fig. 3c.).
Fig. 4.
Comparison between neutralizing activities against variants relative to the wild type in serum samples obtained from (a) two-dose CoronaVac/CoronaVac, (b) two-dose AZD1222/AZD1222, and (c) two-dose CoronaVac + one-dose AZD1222 vaccinees. *** denotes p ≤ 0.001. ** denotes p ≤ 0.01.
4. Discussion
Recent data suggest that the immunity elicited by two-dose vaccination against COVID-19 waned over time resulting in reduced protection against wild type SARS-CoV-2 and variants of concerns [14], calling into question whether a third dose would be advantageous or potentially required for long-term protection. This study showed that participants who received the third dose AZD1222 vaccine after completion of the two-dose CoronaVac vaccines possessed higher levels of spike RBD-specific IgG, total immunoglobulin, and anti-S1 IgA than that of two-dose CoronaVac, two-dose AZD1222 vaccinated and previously-infected individuals. In addition, sera samples from booster dose vaccine recipients elicited higher neutralizing activity against the wild type and all variants of concern than those in the recipients of the two-dose CoronaVac and AZD1222 vaccines.
These results follow similar trends expressed in research on the immunogenicity of a homologous three-dose regimen of the CoronaVac vaccine [8], [15], AZD1222 vaccine [16] or mRNA-1273 vaccine [17] indicating that three-dose vaccination showed a higher immune response than two-dose vaccination. Commonly, it was shown that a third dose evokes a higher immunological response surpassing levels post-second dose of the primary vaccines as a booster effect. In addition, mouse models using the heterologous vaccine regimen have shown that the three-dose vaccine schedule can promote higher humoral and cellular responses than that induced by the same three-dose regimen [18]. The higher antibody responses elicited by the heterologous two-dose vaccine regimen when compared to the two-dose homologous vaccine regimen have also been reported [19], [20]. Furthermore, sVNT experiments presented significantly showed higher post-third dose inhibition levels of neutralizing activities against the wild type and variants of concerns in the third dose vaccine recipients, which is consistent with that of a high immunoglobulin titer.
Aside from heightened immunoglobulin titers and sVNT inhibition percentages, a notable discrepancy of immunoglobulin subtype A was observed. Serum anti-spike1 protein-specific IgA OD/CO ratios significantly increased following an AZD1222 vaccine as a third booster, compared to after two-dose homologous CoronaVac and AZD1222 regimen. The presence of serum spike-specific IgA was observed only after a third vaccination and natural infection. IgA is a potent immunoglobulin [21] found mainly in mucosal regions and is fundamental for inhibiting respiratory viruses from epithelial cell attachment [22]. Despite differing mechanistic characteristics, our results highlighting an absence of serum anti-spike specific IgA after a two-dose homologous CoronaVac and AZD1222 schedule. In contrast, some two-dose regimens can elicit serum IgA responses despite the absence of mucosal IgA responses. Limited data is available on the clinical benefit of serum IgA in protection, however, a recent in vitro experimental study has shown that serum IgA contributed to the neutralization of the SARS-CoV-2 [21], [23], [24].
Our study had a few noteworthy limitations. First, only immunological data was collected and therefore this investigation lacks information regarding the efficacy of a heterologous third AZD1222 vaccination. Second, demographic data collected highlighted a difference in the averaged age among vaccinated groups and a gender disparity with a large majority of female participants in the fully vaccinated CoronaVac followed by AZD1222 group. In general, the antibody responses to vaccine are lower in older adults and the vaccine efficacy declines with age [25]. Our fully AZD1222 vaccinated group were older adults indicating that the immune response in this group might be lower than their younger counterparts. Moreover, most of the participants in the fully vaccinated CoronaVac followed by AZD1222 group were young and pre-menopause female. Typically, the younger female can elicit the antibody response to vaccination better than men [26]. Lastly, a cell-mediated response was not examined. Only a humoral response was investigated, accounting for a portion of an immunological response to vaccination.
In conclusion, a three-dose heterologous regimen, two initial CoronaVac followed with a third AZD1222 vaccine, induced a strong immunological response. Additional research into age-related efficacy, vaccination timing, cell-mediated response, and effectiveness data should be considered for a comprehensive understanding of the benefit of this vaccine schedule. Further investigations into the vaccine-induced protection against SARS-CoV-2 emerging variants and their impact on this vaccination regimen’s effectiveness are to be further investigated.
Author contributions
Conceptualization, R.Y., N.S., N.S., N.W. and Y.P.; data collection, D.S. and T.T.; formal analysis, R.Y., N.S., N.W. and Y.P.; methodology, N.S., S.A., S.K., T.T., P.V., C.A. and L.W.; project administration, Y.P.; writing-original draft, R.Y., H.P., N.W. and Y.P.; writing-review and editing, N.W., N.S. and Y.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Research Council of Thailand (NRCT), Health Systems Research Institute (HSRI), MK Restaurant Group, Center of Excellence in Clinical Virology at Chulalongkorn University, and King Chulalongkorn Memorial Hospital.
Institutional Review Board Statement
The study protocols were approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University.
Informed Consent Statement
This study was carried out following the principles expressed in the Declaration of Helsinki. The written inform consent was obtained from the CoronaVac, AZD1222 and natural infection groups. Patient consent in the CoronaVac Full + AZD1222 group was waived due to the fact that the datasets used in this study were anonymous and prevent the identification of any individual study subject by the research team at any stage of the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Ritthideach Yorsaeng: Conceptualization, Formal analysis. Nungruthai Suntronwong: Conceptualization, Formal analysis, Methodology, Writing – original draft. Harit Phowatthanasathian: Writing – original draft. Suvichada Assawakosri: Methodology. Sitthichai Kanokudom: Methodology. Thanunrat Thongmee: . Preeyaporn Vichaiwattana: Methodology. Chompoonut Auphimai: Methodology. Lakkhana Wongsrisang: Methodology. Donchida Srimuan: . Thaksaporn Thatsanatorn: Methodology. Sirapa Klinfueng: . Natthinee Sudhinaraset: Conceptualization, Writing – review & editing. Nasamon Wanlapakorn: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Yong Poovorawan: Conceptualization, Formal analysis, Project administration, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all the staffs of the Center of Excellence in Clinical Virology and all the participants for helping and supporting in this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.11.083.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. PMID: 33306989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. PMID: 34192428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;(1):32635–32645. doi: 10.1001/jama.2021.8565. PMID: 34037666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Z.P., Yang M., Lai C.L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals (Basel) 2021;14(5):406. doi: 10.3390/ph14050406. PMID: 33923054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. PMID: 33725432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. medRxiv 2021 [preprint]; doi: https://doi.org/10.1101/2021.08.25.21262584. [DOI] [PMC free article] [PubMed]
- 8.Pan, H.; Wu, Q.; Zeng, G.; Yang, J.; Jiang, D.; Deng, X.; Chu, K.; Zheng, W.; Zhu, F.; Yu, H.; et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv 2021[preprint]; https://doi.org/10.1101/2021.07.23.21261026.
- 9.He Q, Mao Q, An C, Zhang J, Gao F, Bian L, et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect 2021;10(1):629-637. http://doi: 10.1080/22221751.2021.1902245. PMID: 33691606. [DOI] [PMC free article] [PubMed]
- 10.Pietsch B. Hundreds of Thais inoculated with Sinovac are infected as cases spike in Southeast Asia. The Washington Post. 2021 https://www.washingtonpost.com/world/2021/07/12/coronavirus-latest-updates [Internet] [cited 2021 September 8] [Google Scholar]
- 11.Chirathaworn C., Sripramote M., Chalongviriyalert P., Jirajariyavej S., Kiatpanabhikul P., Saiyarin J., et al. SARS-CoV-2 RNA shedding in recovered COVID-19 cases and the presence of antibodies against SARS-CoV-2 in recovered COVID-19 cases and close contacts, Thailand, April-June 2020. PLoS ONE. 2020;15(10):e0236905. doi: 10.1371/journal.pone.0236905. PMID: 33119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thailand Food and Drug Administration. Summary of Product Characteristic CoronaVac, 2021[Internet] [cited 2021 September 01] Available from: https://www.fda.moph.go.th/sites/drug/Shared%20Documents/Vaccine/U1DR1C1072640000311C-SPC-EN.pdf.
- 13.Thailand Food and Drug Administration. COVID-19 Vaccine AstraZeneca package leaflet, 2021[Internet] [cited 2021 September 01] Available from: https://www.fda.moph.go.th/Pages/covidvaccine/Package_Leaflet_TH.html.
- 14.Wilder-Smith A., Mulholland K. Effectiveness of an Inactivated SARS-CoV-2 Vaccine. N Engl J Med. 2021;385(10):946–948. doi: 10.1056/NEJMe2111165. PMID: 34469651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Cao Y., Zhou Y., Wu J., Jia Z., Hu Y., et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.09.02.21261735. [DOI] [PubMed] [Google Scholar]
- 16.Flaxman A., Marchevsky N.G., Jenkin D., Aboagye J., Aley P.K., Angus B., et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398(10304):981–990. doi: 10.1016/S0140-6736(21)01699-8. PMID: 34480858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall V.G., Ferreira V.H., Ku T., Ierullo M., Majchrzak-Kita B., Chaparro C., et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021 doi: 10.1056/NEJMc2111462. PMID: 34379917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., He Q., An C., Mao Q., Gao F., Bian L., et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg Microbes Infect. 2021;10(1):1598–1608. doi: 10.1080/22221751.2021.1957401. PMID: 34278956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi: 10.1038/s41591-021-01449-9. PMID: 34262158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanlapakorn N., Suntronwong N., Phowatthanasathian H., Yorsaeng R., Vichaiwattana P., Thongmee T., et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccines in healthy adults. medRxiv. 2021 doi: 10.1101/2021.11.04.21265908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. PMID: 33288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortari E.P., Russo C., Vinci M.R., Terreri S., Salinas A.F., Piccioni L., et al. Highly-specific memory B cells generation after the 2nd dose of BNT162b2 vaccine compensate for the decline of serum antibodies and absence of mucosal IgA. medRxiv. 2021 doi: 10.1101/2021.06.08.21258284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klingler J., Weiss S., Itri V., Liu X., Oguntuyo K.Y., Stevens C., et al. Role of IgM and IgA Antibodies in the Neutralization of SARS-CoV-2. J Infect Dis. 2021;223(6):957–970. doi: 10.1101/2020.08.18.20177303. PMID: 33173891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Viant C., Gaebler C., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. PMID: 34619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T., Iwasaki A. Sex differences in immune responses. Science. 2021;371(6527):347–348. doi: 10.1126/science.abe7199. PMID: 33479140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.