Highlights
-
•
The gastroprotective activity of HMP is related to the triple helix structure.
-
•
nSiO2 can increase the gastroprotective activity of HMP.
-
•
Polysaccharides and nSiO2 may interact through hydrogen bonds.
Keywords: Hericium erinaceus mycelium polysaccharide, Degradation, Food additive, Silica nanoparticles, Interaction
Abstract
Gastric mucosal injury is a common gastrointestinal disorder. Hericium erinaceus polysaccharide, the major active ingredient in Hericium erinaceus, can reduce gastric mucosal damage to some extent. In this study, two different products HMP-Vc and HMP-Ce were obtained by Vitamin C and cellulase degradation of Hericium erinaceus mycelium polysaccharide (HMP). The gastroprotective activity of polysaccharides and its interaction products with food additives silica nanoparticles (nSiO2) were studied in GES-1 cells. It was found that gastroprotective activity of HMP was significantly higher than that of degradation products, and the addition of nSiO2 could enhance this activity of HMP. The greatest difference between the degradation products and HMP was the reduction of the triple helix structure, which might be the reason of the gastroprotective activity was less than that of HMP. Moreover, nSiO2 might interact with HMP through hydrogen bonding to enhance its activity.
Introduction
Gastrointestinal health is an important indicator of human health. There is about 10% of the world’s population suffering from the gastric ulcer. Gastric mucosal damage is a type of peptic disease, which is caused by gastric acid and pepsin destroying the gastric mucosa. In addition to the lesions of the stomach itself, gastric mucosal damage can be caused by multiple external factors, including unhealthy lifestyle habits, drug side effects, bacterial infections, and more (Graham, 2014).
Hericium erinaceus is widely used in functional foods as a Chinese herbal medicine due to its rich nutrients. Hericium erinaceus polysaccharide is currently the most used active ingredient of Hericium erinaceus. It has a variety of pharmacological effects, including neuroprotective, anti-inflammatory, antioxidant, and gastric mucosal protection (Kushairi, Phan, Sabaratnam, David & Naidu,2019). Hericium erinaceus polysaccharide is mainly derived from fruiting bodies and mycelium, which reduces the ethanol-induced gastric mucosal damage by reducing the inflammatory response and regulating epidermal differentiation (Jiang, Wang & Zhang, 2016). Hericium erinaceus fruiting body polysaccharide (HFP) significantly improves the gastric mucosa morphology and gastric mucosal immune cells of Muscovy ducks infected with Muscovy Duck Reovirus (Jiang, et al., 2016). However, the gastroprotective activity of Hericium erinaceus mycelium polysaccharide (HMP) has been rarely studied. Our group has found that in animal and vitro models, HMP has stronger gastroprotective activity than HFP.
Structural diversity is the key to polysaccharides having different biological activities (Lee, et al., 2016). Polysaccharide is similar to protein or DNA, the basis of molecular conformation its primary structure is, but the structure of polysaccharide chain is more complex and diverse, usually related to monosaccharide composition and ratio, molecular weight, main chain structure, uronic acid type and ratio, pyran or furan ring configuration and composition, etc (Qu, et al., 2020). Currently, the relationship between the gastroprotective activity and structural properties of Hericium erinaceus polysaccharide is not clear, which limits its application and the development of related products to some extent.
In the development of functional foods, nano-level food additives may significantly impact the color, taste, texture and shelf life of the food. Therefore, nanomaterials are often designed as carriers for color or flavor additives, preservatives or food supplements (He, Deng & Hwang, 2018). Among them, silica nanoparticles (nSiO2) is often added to functional solid beverages as a food anticaking agent to maintain the stability of powdered or granular products (Jin, Kim, Kim, Oh & Choi, 2020). In addition, it can also be used to improve the taste and texture of food and to maintain the color and durability of food (Guo, Martucci, Liu, Yoo, Tako & Mahler, 2018). In functional foods with polysaccharides, nSiO2 is often added to prevent caking.
Due to the special physical and chemical properties of nanomaterials, the changes that may be caused when they are used in functional foods need to be concerned. Different sources and structures of food matrices (proteins, polysaccharides, lipids, etc.) have different digestion rates and degrees in different areas of the gastrointestinal tract, which may affect the interface properties (Ranjan, Dasgupta, Srivastava & Ramalingam, 2016), kinetic changes (Dona, Pages, Gilbert & Kuchel, 2010) .etc. Nanoparticles can also affect the digestion, absorption and biological activity of the food matrix itself. nSiO2 may be combined with food ingredients to increase the oral absorption of polysaccharides and proteins in rats (Lee, et al., 2016). Nanoparticles can adhere to the lipid surface to prolong the digestion of lipids by lipase (Tzoumaki, Moschakis, Scholten & Biliaderis, 2013).
Nanomaterials are widely used as food additives in functional foods, but the study on the interaction between food nutrients and nanoparticles is mainly focused on the effect of protein on the properties of nanoparticles, and there are few studies on the effect of nanoparticles on the properties of the food itself. Moreover, compared with proteins, due to the complex composition and structure of polysaccharides, little progress has been made in the interaction mechanism between polysaccharides and nanoparticles in recent years. nSiO2 is a common food-grade additive. In order to enable it to be effectively promoted and applied, it is necessary to conduct in-depth research on its biological properties. And it needs to be studied intensively on its interaction properties with food matrix to make it further applicable in the future.
This study used HMP as raw material, and two degradation products (HMP-Vc, HMP-Ce) were obtained through Vitamin C acid hydrolysis and cellulose enzymatic hydrolysis. Comparing the effects of HMP, HMP-Vc, HMP-Ce and their interaction products with nSiO2 on the proliferation of GES-1 cells. Ethanol was used to construct cell damage models to explore the preventive effects of different samples on cell damage. SEM, DLS, XRD, FTIR, and Congo red experiments were used to study the morphological characteristics, the changes in hydrodynamic radius, crystal structure, functional groups, and advanced structure differences. The structure–activity relationship was investigated and the polysaccharides-nSiO2 interaction mechanism was preliminarily studied. The novelty of the paper is that it takes into account the actual functional food system. The degradation process that may exist during HMP processing and whether the use of food additives will affect the activity of HMP itself were studied, which is of great significance for the actual production and application of HMP.
Materials and methods
Materials
Hericium erinaceus mycelium polysaccharide was produced from Jiahe Biotechnology Co., Ltd., Shaanxi Province, China. Phosphate buffered solution (PBS, pH 7.4), Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA (0.25%), penicillin–streptomycin solution were purchased from Gibco Life Technologies, USA. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich Chemical Co. St. Louis, MO, USA. Silica nanoparticles (nSiO2, food grade) were purchased from Xinrurong Trading Co., Ltd., Guangzhou, China. Sephadex G-50 Medium was purchased from GE Healthcare, USA. The dialysis membranes (35 00 Da) was cobtained from Shanghai Yuanye Bio-Technology Co., Ltd, China. Ascorbic acid (Vitamin C) was purchased from Boao Jingdian Biotechnology Co., Ltd., Shanghai, China. Cellulase (11 000 U/g) was purchased from Xiasheng Industrial Group Co., Ltd., Ningxia, China. MTT reagents were purchased from Aladdin Reagent Co., Ltd, Shanghai, China. All other chemicals and solvents used in this study were of analytical grade.
Preparation of HMP degradation product
Degradation of HMP by Vitamin C
A series of single-factor experiments were conducted to study the effect of the following factors (Vitamin C concentration, degradation time and degradation temperature) on the degree of HMP degradation. 5 mL of a certain concentration of Vitamin C solution (0.025, 0.05, 0.10, 0.20, 0.25%) was added to 10 mL of HMP aqueous solution (10 mg/mL), and the mixture was stirred at a certain temperature (30, 40, 50, 60, 70 °C) for a certain time (0.5, 1.0, 1.5, 2.0, 2.5 h). After the reaction, supernatant was removed after centrifugation at 8 000 × g at 4 °C for 10 min. The supernatant was dialyzed with a dialysis membrane at 4 °C for 48 h, and then its DPPH radical-scavenging capacity was determined as previously reported (Blois, 1958).
Degradation of HMP by cellulase
A certain concentration of cellulase solution (5, 10, 15%) was added to HMP. The pH was adjusted to 7, and the mixture was stirred at a given temperature (37, 58, 80 °C) for a given time (0.5, 1.25, 2 h). The cellulase were inactivated at 100 °C for 5 min. After the centrifugation (8 000 × g, 10 min, 4 °C), the amount of reducing sugars in the supernatant was quantified by a modified DNS method (Miller, 1959). The enzymatic hydrolysis rate was calculated by reducing sugar content. The formula is as follows.
× 100%
The data was present in the form of an average value, the test was repeated three times, and the enzymatic hydrolysis rate of HMP was the response value. The design-Expert 8.0 software was used to perform quadratic regression fitting analysis on the experimental results.
Purification of polysaccharide degradation products
Distilled water was used as the eluent, the HMP degradation solution (10 mg/mL) obtained in 2.2.1 and 2.2.2 was subjected to Sephadex G-50 chromatography (2.5 × 80 cm) at a flow rate of 1 mL/min. A total of 50 tubes of eluted fractions were collected, 5 mL per tube. Total sugar of each tube was analyzed by the phenol–sulfuric acid method (DuBois, Gilles, Hamilton, Rebers, & Smith, 1956).The fractions were collected, concentrated and dialyzed for 48 h. After vacuum freeze drying for 48 h, the degradation products of HMP were obtained and named HMP-Vc and HMP-Ce.
Preparation of polysaccharides-nSiO2 interaction products
HMP, HMP-Vc, HMP-Ce were ground with a mortar and pestle, respectively. After adding 1.5% nSiO2 (w/w), the mixture was continuously stirred for 30 min to obtain polysaccharides-nSiO2 interaction products and named HMP-nSiO2, HMP-Vc-nSiO2, HMP-Ce-nSiO2.
Study on gastroprotective activity of HMP-nSiO2, HMP-Vc-nSiO2, HMP-Ce-nSiO2
Cell culture
The human gastric epithelial cell line GES-1 used in this study was a generous gift from Tea Research Institute, Guangdong Academy of Agricultural Sciences. GES-1 cells were cultured in DMEM,10% FBS, 100 units/mL penicillin and 100 units/mL streptomycin in a humidified CO2 (5%) incubator (HERAcell 150, Thermo Scientific, USA) at 37 °C.
MTT assay
GES-1 cells in log growth phase were seeded in a 96-well plate at a density of 8 × 103 cells per 100 μL/well and cultured for 24 h (37 °C, 5% CO2). Medium was aspirated and replaced with 100 μL fresh complete medium or 100 μL experimental sample solution and incubated for 24 h. Then, the cells were cultured with 5 mg/mL MTT (20 μL/well) for 4 h. Medium was aspirated and formazan crystals were dissolved with DMSO (150 μL/well). The optical density (O.D.) values at 490 nm were determined and cell viability was evaluated as the ratio of the O.D. values of experimental group relative to those of the control group.
Effect on the proliferation of GES-1 cells
The GES-1 cells were divided into a control group and seven experimental groups (nSiO2, HMP, HMP-Vc, HMP-Ce, HMP-nSiO2, HMP-Vc-nSiO2, HMP-Ce-nSiO2). The MTT assay was performed asdescribed in 2.4.2. The control group was added with serum-free DMEM, each experimental group was added with different concentrations of sample solutions (25, 50, 100, 250 and 500 µg/mL), and the nSiO2 group was added with 0.375, 0.75, 1.5, 3.75 and 7.5 µg/mL nSiO2 solution.
Prevention of GES-1 cell damage induced by ethanol
The GES-1 cells were divided into a control group, a model group and seven experimental groups (nSiO2, HMP, HMP-Vc, HMP-Ce, HMP-nSiO2, HMP-Vc-nSiO2, HMP-Ce-nSiO2). The MTT assay was performed as described in 2.4.2. It should be noted that ethanol induced GES-1 cell injury model was produced before adding MTT reagent to study the preventive effect of each compound. After diluting ethanol to 1 M in serum-free DMEM, add 100 µL/well to each group except the control group, incubate for 2 h (37 °C, 5% CO2), and add MTT to continue the MTT test.
Scanning electron microscopy (SEM)
SEM image was collected from a field-emission scanning electron microscopy (G6 Phenom Pro, Rena Scientific Instruments Co. Ltd. Shanghai, China) after gold coating for 3 min (EM-SCD500, Leica, Germany).
Dynamic light scattering analysis (DLS)
After each sample was prepared into a 1 mg/mL solution with water, the Laser particle size analyzer (ZEN3600, Malvern Instruments Ltd., U.K.) was used to measure the hydrodynamic radius. The measuring temperature was 25 °C and the scattering angle was 173°.
X-ray diffraction (XRD)
The samples were analyzed by X-ray diffraction using an X-ray polycrystalline diffractometer (D8 Advance, Bruker, Germany). The measurement conditions were: Cukα radiation, a scanning step of 0.04°, and a scanning range of 2θ = 4°-50°; the tube flow was 40 mA, and the tube pressure was 40 kV; narrow peak DS was 0.5°, RS was 8 mm.
Fourier transform infrared spectroscopy (FTIR)
The sample was placed on the sample crystal stage for infrared spectrum scanning (FTIR spectrometer, LX100877, Perkin Elmer, Inc., USA), the scanning range was 4 000–400 cm−1, and the wave number accuracy was 0.01 cm−1.
Congo red assay
The triple helical conformation of polysaccharide was investigated using the azo dye Congo red as described (Semedo, Karmali & Fonseca, 2015). Each sample was prepared into a 2 mg/mL solution with distilled water. Three milliliters NaOH solution of different concentrations (0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.4 M),1.5 mL Congo red solution (0.2 mM) and 0.5 mL distilled water were added to 1 mL sample solution. After the mixed solution was reacted for 1 h, a full-wavelength scan was performed (the scan wavelength was 200–800 nm, UV–visible spectrophotometer, UV-1800, Shimadzu, Japan), and the maximum absorption wavelength of the reaction solution in the NaOH solution system with different final concentrations was recorded.
Statistical analysis
All data were obtained in triplicates and presented as mean ± SD. The experimental data used the Duncan multiple comparison method (Duncan) in the IBM SPSS Statistics 22.0 statistical software for one-way analysis of variance (one-way ANOVA). p < 0.05 was regarded as statistically significant. The experimental data chart was drawn using Prism 8.0.
Results and discussion
Determination of degradation conditions of HMP-Vc and HMP-Ce
Low concentration of Vitamin C can be used as a prooxidant to randomly break glycosidic bonds. Cellulase is a commonly used compound enzyme for the enzymatic hydrolysis of polysaccharides, mainly composed of exo-β-glucanase, endo-β-glucanase, β-glucosidase, etc. (Chandel, Chandrasekhar, Silva & Silvério, 2011). Compared with Vitamin C, cellulase selectively breaks the glycosidic bonds of polysaccharides.
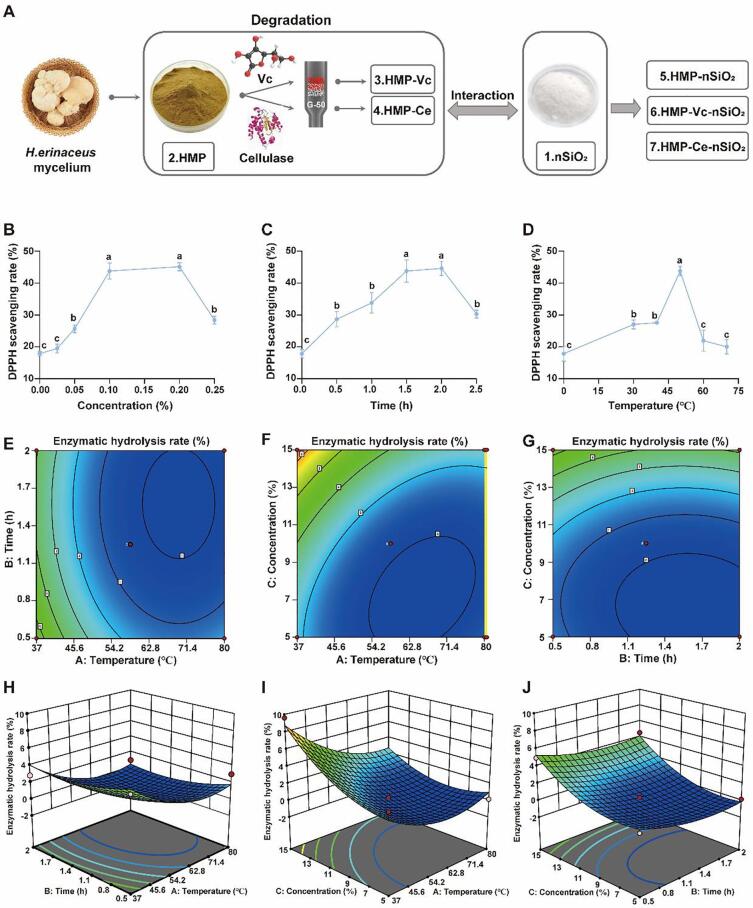
In order to compare the changes of HMP activity before and after degradation and the changes in structure and efficacy caused by the interaction of polysaccharides with different structures and nSiO2, HMP was degraded by Vitamin C or cellulase, and HMP-Vc, HMP-Ce were obtained through gel column chromatography (Fig. 1A).
Fig. 1.
Degradation of HMP. (A) Sample preparation flow diagrams. (B, C, D) Effect of Vitamin C concentration, degradation time and degradation temperature on DPPH scavenging rate. (E-J) Response surface and contour of the interaction of various factors to enzymatic hydrolysis rate.
Degradation of HMP by vitamin C
A single factor experiment was carried out to explore the impact of Vitamin C concentration (0.025, 0.05, 0.10, 0.20, 0.25%), time (0.5, 1.0, 1.5, 2.0, 2.5 h) and temperature (30, 40, 50, 60, 70 °C) on the scavenging rate of DPPH free radicals of HMP degradation products. As shown in Fig. 1B-D, the antioxidant activity of polysaccharide degradation products treated with Vitamin C was enhanced. It may be that the degradation of polysaccharides produces more free hydroxyl groups, increases water solubility and specific surface area, so it has better hydroxyl radical scavenging activity. (Zhang, Wang, Zhao & Qi, 2014), reaching the maximum (45.14 ± 1.25%) when the Vitamin C concentration was 0.2%, but there was no significant difference from 0.1% (p > 0.05). With the increased concentrations of Vitamin C, the DPPH free radical scavenging activity of the degradation products first increased and then decreased. The maximum of Vitamin C concentration was 0.2% (45.14 ± 1.25%), but there was no significant difference from 0.1% (p > 0.05). Similarly, when the degradation time was 2.0 h, the DPPH scavenging rate reached the maximum (44.49 ± 2.25%), but there was no significant difference from 1.5 h (p > 0.05). The antioxidant activity of the degradation products increased with the increase of temperature within a certain range, reached the maximum value (43.78 ± 1.50%) at 50 °C, and then decreased rapidly. This may be owing to the decomposition of Vitamin C due to excessive temperature, Which slows down the generation rate of free radicals, thereby affecting the degradation of polysaccharides. In the upper temperatures, Vitamin C was readily decomposed, causing the rate of free radical generation to slow down, thereby affecting the effect of polysaccharide degradation (Shen, et al.,2019). Based on the above experimental results, as shown in Supplementary Table 1, three-factors-two-levels experiments were conducted by the orthogonal list L4 (23).
The orthogonal experimental scheme is shown in Supplementary Table 2. Among the three factors, the concentration of Vitamin C has the greatest impact on the antioxidant activity of degraded polysaccharides, and the degradation time has the least impact. According to the orthogonal experiment results, the best process for Vitamin C degradation of HMP was obtained. The DPPH scavenging rate of degradation products was 48.5 ± 0.52% (0.2% Vitamin C, 2 h, 50 °C).
Degradation of HMP by cellulase
The response surface methodology (RSM) was employed to optimize the process conditions of cellulase enzyme HMP. According to the Box-Behnken design, the experiment design and results are shown in Supplementary Table 3,4. The software Design-Expert 10 was used for regression analysis. It was noted that the second-degree polynomial regression equations of enzymatic hydrolysis rate (Y) could relatively well describe the relationship between temperature (A), time (B), amount of enzyme (C), and polysaccharide enzymatic hydrolysis rate. So the model could be used to analyze and predict the effect of various enzymatic hydrolysis conditions on the degradation efficiency of HMP. From the analysis of the effects of three factors on the rate of polysaccharide digestion (Y), the response of each factor to the rate of polysaccharide digestion was not a simple linear relationship. F value indicated that the influencing factors on the enzymatic hydrolysis rate are ranked as the amount of enzyme added > temperature > time.
The response surface and contour lines of the interaction of various influencing factors on the enzymatic hydrolysis rate are shown in Fig. 1E-G, which reflects the interaction between temperature (A), time (B), and amount of enzyme (C) and its influence on the enzymatic hydrolysis rate. The steeper the slope of the response surface, the greater the impact of condition changes on the response value is. If the interaction between these two factors is stronger, the contour line will be closer to an ellipse; If the interaction between the two factors is weaker, the contour line is closer to a circle (Sun, Zhang, Zhang & Niu, 2010). Therefore, the interaction between temperature and the amount of added enzyme is the strongest, followed by time and the amount of enzyme added, and the interaction between temperature and time is the weakest. Taking the enzymatic hydrolysis rate as an indicator, the best process for cellulase degradation of HMP were 37 °C, 0.5 h, enzyme added 15%, and the theoretical enzymatic hydrolysis rate was 10.10%. The actual enzymatic hydrolysis was 9.55 ± 0.42%.
Sephadex G-50 chromatography
Gel filtration chromatography is a method for separation of substances with different molecular weights. The components with large molecular weight can’t stay long in the gel column, so they are eluted first, while the components with small molecular weight will be trapped in the gel column, thus having a longer elution time (Wang, Tong., Li, Cao & Su, 2012). Sephadex G-50 was used to separate the degradation products of HMP, and obtain components with similar molecular weights. Supplementary Fig. 1 shows the elution curves of two degraded polysaccharides. The peak time of Vitamin C-degraded polysaccharides was earlier than cellulase-hydrolyzed polysaccharides. The higher molecular weight of Vitamin C-degraded polysaccharides indicated that cellulase degrades HMP to a higher degree. The eluate corresponding to the absorption peak was collected, concentrated and dialyzed at 4 °C for 48 h. Two degradation products were obtained after vacuum freeze drying, and were named as HMP-Vc and HMP-Ce, respectively.
Biological activity research
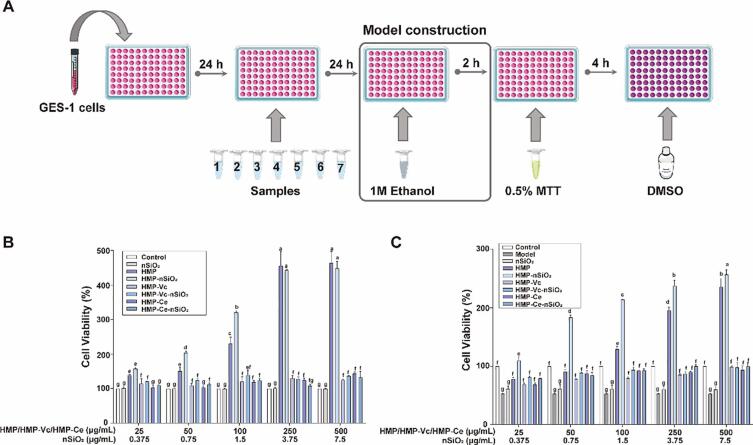
In order to study the changes in the biological activity of polysaccharides before and after degradation and the effect of nanoparticles on the biological activity of polysaccharides. nSiO2 was added to HMP, HMP-Vc, HMP-Ce to prepare interaction products HMP-nSiO2, HMP-Vc-nSiO2, HMP-Ce-nSiO2 (Fig. 1A). The MTT experiment was performed to evaluate the effects of the above 6 samples and nSiO2 alone on the proliferation of GES-1 cells and the preventive effect on cell damage induced by ethanol (Fig. 2B). It should be noted that the time point of ethanol modeling (2 h, 1 M ethanol) was before adding MTT, and the rest of the operations remain unchanged.
Fig. 2.
The effect of different samples on GES-1 cells. (A)Schematic of experimental flow for preventing GES-1 cell damage. (B) Effect on cell viability. (C) Protective activity against cell damage. Different lowercase letters indicate significant differences, p < 0.05.
Cell proliferation
The interaction between apoptosis and proliferation determines the formation of gastric ulcer. As shown in Fig. 2B, in the concentration range (0.375–7.5 μg/mL), nSiO2 had no significant effect on the cells. Previous studies have shown that nSiO2 particles are small and can enter the mitochondria of the cell. However, a trace amount of nSiO2 will not affect the structure of the cell. (Al-Rawi, Diabaté & Weiss, 2011).HMP had a significant activity promoting the proliferation of GES-1 cells at various experimental concentrations (25–500 μg/mL) was concentration-dependent. When the concentration reached 250 μg/mL, the proliferation activity on the cells no longer continued to increase, cells might have reached their maximum limit of uptake (Liu, et al., 2017). When the concentration was up to 500 μg/mL, there was still no cytotoxicity to gastric mucosal epithelial cells, indicating that HMP is safe for functional foods. Compared with HMP-Vc, HMP had a significant proliferation-promoting effect at various concentrations, HMP-Ce had a significant proliferation-promoting activity only when the concentration of HMP-Ce was higher than 100 μg/mL (p < 0.05). This may be associated with the degree of degradation of polysaccharides. From the results of section 3.2, the degradation degree of cellulase to HMP is higher than that of Vitamin C, which may degrade more groups or structures that play a role in gastroprotective activity.
Regarding the interaction product, cell proliferation activity of HMP-nSiO2 was significantly superior to HMP, and there was a significant difference between 50 and 100 μg/mL (p < 0.05), indicating that nSiO2 contributes to enhancing the cell proliferation activity of HMP. Studies have shown that nSiO2 can increase the permeability of the cell membrane to some extent (Melchiorri, Sewerynek, Reiter, Ortiz, Poeggeler & Nisticò, 1997), thereby promoting the entry of polysaccharides into the cell and making it more effective. After adding nSiO2, the proliferation-promoting activities of HMP-Vc-nSiO2 and HMP-Ce-nSiO2 were not significantly different from those of HMP-Vc and HMP-Ce (p > 0.05). Some groups bound to nSiO2 might be reduced after the degradation of polysaccharides, resulting in decreased interaction.
In summary, compared with HMP, HMP-Vc and HMP-Ce have significantly lower proliferation-promoting activities. The interaction between HMP and nSiO2 enhanced the proliferation effect of HMP on GES-1 cells, and the addition of nSiO2 in the degradation products HMP-Vc and HMP-Ce did not significantly affect its properties.
Preventive effect on ethanol-induced cell damage
Ethanol directly damages the epithelial cells in the gastric mucosa, destroying the barrier of the gastric mucosa (Liu, Feng, Zhang, Wei & Zhao, 2019). Therefore, ethanol was chosen to construct a GES-1 cell injury model in vitro. Samples were added to the cells and incubated at 37 °C for 24 h, then 1 M ethanol was added to construct models for 2 h to study the preventive effect of each sample on cell damage.
As shown in Fig. 2C, the cell activity of the nSiO2 group was not significantly different from that of the model group, this revealed that nSiO2 has no protective activity against ethanol-induced damage. The cell viability of injured cells in the HMP group was significantly higher than that of the model group (p < 0.05), indicating that HMP can achieve the effect of protecting the stomach by reducing cell damage. Both HMP-Vc and HMP-Ce can prevent ethanol from damaging GES-1 cells, but compared with HMP, the activities of HMP-Vc and HMP-Ce were significantly reduced. After interacting with nSiO2, the cell damage protection activity of HMP-nSiO2 was better than HMP, and the cell damage protection activity of HMP-nSiO2 was enhanced drastically (p < 0.05). Previous studies have clearly shown that nSiO2 can accumulate on the cell surface (Wang, Luo & Xia, 2018). Therefore, HMP-nSiO2 can make polysaccharides gather more on the cell surface and form a tighter protective layer, which can better prevent ethanol from damaging GES-1 cells. HMP-Vc-nSiO2 and HMP-Ce-nSiO2 also have the effect of preventing ethanol from damaging GES-1 cells, but the results are similar to those in 3.2.1, and there was no significant difference compared with HMP-Vc and HMP-Ce.
The biological activity of polysaccharides is extremely important to living organisms, and their biological activity depends on the chemical structure. When the polysaccharide was degraded, the structure changes, thereby changing the activity. The gastroprotective activity of the two methods of degrading polysaccharides were significantly reduced, which means that different degradation methods may destroy the functional groups of polysaccharides to exert gastroprotective activity. Furthermore, nanoparticles interact with polysaccharides, which may have an impact on the structure of polysaccharides, in turn affects its biological activity (Xu, Wu, Sun, Zhang, Linhardt & Zhang, 2019). In addition, when the structure of polysaccharides changes, their interaction with nanoparticles may also change accordingly. By studying the structural changes before and after the interaction, a certain understanding of the interaction mechanism can be obtained. A series of structural identifications were carried out to explore the relationship between the structure and properties of HMP before and after degradation and the interaction between polysaccharides and nSiO2.
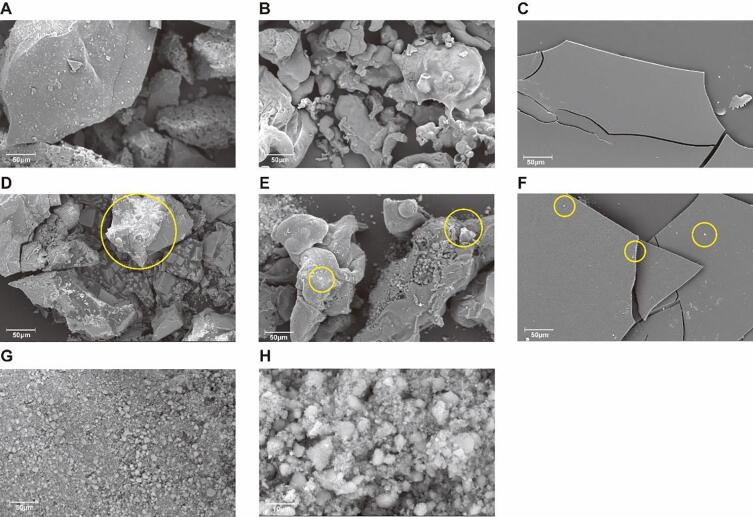
Morphology characterization
The morphology and structure characteristics were characterized by SEM images. As shown in Fig. 3, HMP, HMP-Vc and HMP-Ce presented completely different morphological characteristics. HMP was a rough and porous block structure with obvious large aggregates. HMP-Vc was a relatively uniform and smooth irregular particle conformation, while HMP-Ce was a flat conformation with a smooth surface. Studies have shown that cellulase can cause the depolymerization of polysaccharide molecules, making its appearance smooth (Li, Wang, Liu, Yin & Nie, 2020). It also proved that the degradation degree of HMP by cellulase was higher than that of Vitamin C. nSiO2 had irregular particle morphology and monodisperse particle size. When HMP-Vc and HMP-Ce interact with nSiO2, the surface of the interaction product became apparently rough, with nSiO2 particles attached, and the edges of HMP-Ce-nSiO2 were rough, which might be caused by the interaction of polysaccharides and nSiO2.
Fig. 3.
SEM images of different samples. (A) HMP, (B) HMP-Vc, (C) HMP-Ce, (D) HMP-nSiO2, (E) HMP-Vc-nSiO2, (F) HMP-Ce-nSiO2, (G,H) nSiO2.(A-G)0.630 ×, (H) 4 700 × .
Determination of hydrodynamic radius
The hydrodynamic radius reflects the diffusion of molecules in the solution, the rate of anterior diffusion is inversely proportional to the molecule’s hydrodynamic radius (Shatz,et al., 2016). The hydrodynamic radius of each sample was determined using dynamic light scattering. As shown in Table 1, the hydrodynamic radius of HMP was 688 nm, while HMP-Vc and HMP-Ce were 245 nm and 191 nm, respectively. The hydrodynamic radius after HMP degradation was significantly reduced, indicating that the degradation products are more easily diffused in the solution. When nSiO2 existed alone, its hydrodynamic radius was 1 233 nm, which was due to silica nanoparticles were easy to agglomerate together in the solution (Ma, Lee, Oh, Hwang & Kim, 2010).
Table 1.
Average hydrodynamic radius of the sample.
| Sample | Z-Ave (d.nm) |
|---|---|
| HMP | 688 ± 0.1d |
| HMP-Vc | 245 ± 0.3e |
| HMP-Ce | 191 ± 0.5f |
| HMP-nSiO2 | 1202 ± 0.1b |
| HMP-Vc-nSiO2 | 1510 ± 0.1a |
| HMP-Ce-nSiO2 | 1105 ± 0.6c |
| nSiO2 | 1233 ± 0.3b |
Different letters indicate significant differences, p < 0.05.
When a small amount (1.5%) of nSiO2 interacted with polysaccharides, the hydrodynamic radius of HMP-nSiO2, HMP-Vc-nSiO2 and HMP-Ce-nSiO2 were significantly increased compared with HMP, HMP-Vc and HMP-Ce. Previous studies have found that nSiO2 will adsorb to the substance in the solution medium, which will increase its hydrodynamic radius. (Sun, et al., 2011). It is speculated that HMP can interact with nSiO2 to form a complex. Therefore, a denser polysaccharide protective film was formed on the cell surface, which increases the preventive effect of alcohol-induced cell damage.
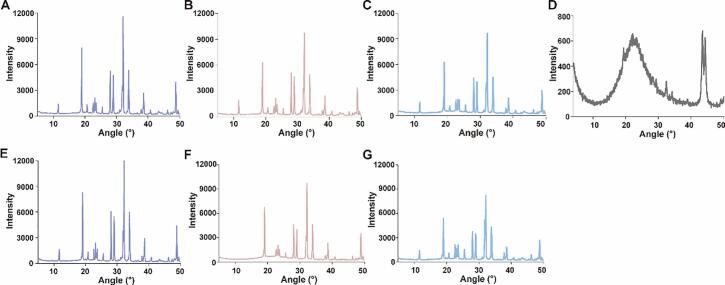
Identification of crystal structures
XRD analysis XRD is a powerful method used to investigate the structure of polysaccharides. It can be seen from Fig. 4 that HMP, HMP-Vc and HMP-Ce had many diffraction peaks above 10°, and these diffraction peaks were all sharp and narrow, indicating that HMP, HMP-Vc and HMP-Ce had ordered crystal conformation. The positions of the diffraction peaks of the three were basically the same, but the intensities of the diffraction peaks of HMP-Vc and HMP-Ce were significantly reduced. In general, X-ray diffraction peak intensity reflects the grain size of the internal crystalline region, and smaller grain sizes lead to lower diffraction peak intensities (Niu, et al., 2018), indicated that the grain size of polysaccharides was reduced after degradation, which was consistent with the results of SEM images.
Fig. 4.
X-Ray Diffraction. (A) HMP, (B) HMP-Vc, (C) HMP-Ce, (D) nSiO2, (E) HMP-nSiO2, (F) HMP-Vc-nSiO2, (G) HMP-Ce-nSiO2.
nSiO2 has broad and blunt diffraction peaks, indicating that nSiO2 is an amorphous semi-crystalline structure (Song, et al., 2019). However, in the XRD diffraction patterns of HMP-nSiO2, HMP-Vc-nSiO2 and HMP-Ce-nSiO2, the peak intensity and position were not significantly different from those of HMP, HMP-Vc and HMP-Ce. It is inferred from this that the interaction of nSiO2 and HMP,HMP-Vc ,HMP-Ce did not significantly change the crystal conformation of polysaccharides.
Identification of functional groups
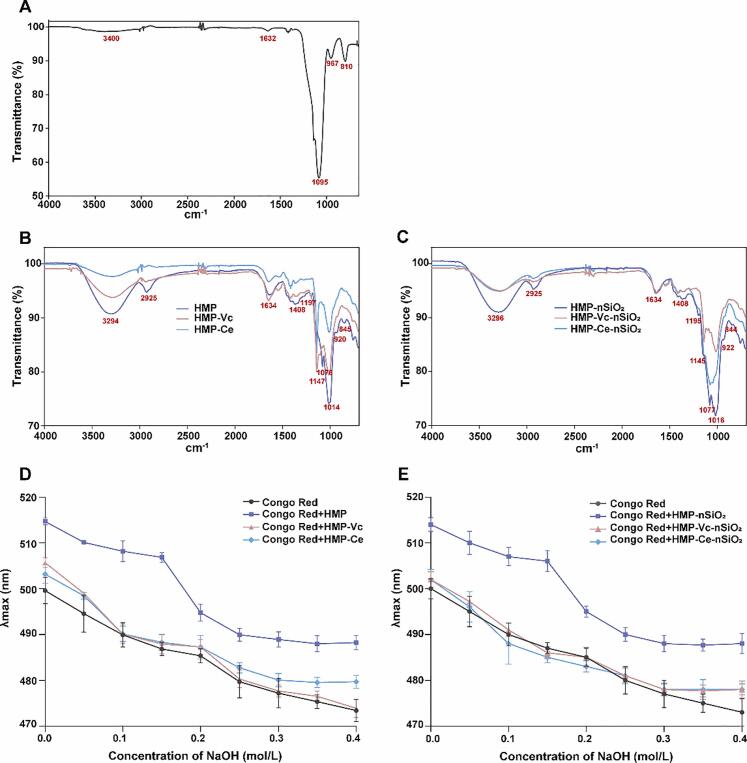
Fourier transmission infrared spectroscopy (FTIR) results is always used to identify functional groups and molecular structure (Mirzaei & Javanbakht, 2019). The scanning result of the FTIR of nSiO2 is shown in Fig. 5A. The bending vibration of Si-O-Si bonds peak of Si-O-Si at 810 cm−1 and the stretching vibration peak of Si-O-Si at 1 095 cm−1, both of them were characteristic absorption peaks of nSiO2. The Si-OH stretching peak at 967 cm−1 indicated the presence of crystal water in nSiO2. At 1 632 cm−1 was proton-containing components σOH (silanol groups and the deformation vibrations of the O—H groups in physically adsorbed molecular water at the silica surface). There was a broad weak absorption peak at 3 400–3 000 cm−1 with a lower response value, which was ν hydrogen-bonded silanols (overlapping the stretching modes in hydrogen-bonded hydroxyl bands produced by OH bonds adsorbed water and Si-OH). It showed that there was a small amount of isolated –OH on the surface of nSiO2, which helped to form hydrogen bonds between the hydroxyl groups of nSiO2 and the hydroxyl groups of polysaccharides (Azizova, Kulik, Palianytsia, Zemlyakov, Tsikalova & Chirva, 2014).
Fig. 5.
FTIR spectrum and Congo red staining (A-C) FTIR spectra of nSiO2, polysaccharides and polysaccharides-nSiO2 interaction products. (D, E) The maximum absorption wavelength of polysaccharides and polysaccharides-nSiO2 interaction products-Congo red complex at different concentrations of NaOH. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig.5B. shows the FTIR of three polysaccharides. From the absorption spectra of HMP, the broadband around 3 600–3 200 cm−1 was characteristic for O—H stretching, and the hydroxyl group had an association reaction between the molecules, showing a non-free state. The absorption peaks at 2 925 cm−1 and 1 410 cm−1 were response for the stretching vibration and bending vibration of C—H, respectively, and they were the characteristic absorption peaks of polysaccharides. The peak near 1650 cm−1 might be attributed to the asymmetric stretching of C O, indicating that the polysaccharide had an amide bond. The strong absorption peak near 1 080 cm−1 was the characteristic peak of polysaccharides composed of the asymmetric stretching vibration of the C—O—O ether bond on the sugar ring. The absorption peaks at 1 014 cm−1 and 1 197 cm−1 might be due to the pyranose ring. The absorption bands near 840 cm−1 and 910 cm−1 were responsible for the formation of α and β glycosidic bonds (Varma & Jayaram Kumar (2018)). Compared with HMP, the absorption peaks of HMP, HMP-Vc and HMP-Ce had no obvious red/blue shift, but the absorption intensity was reduced, or even the absorption front disappeared. This might be due to the degradation of some functional groups. In particular, the absorption peaks at 840 and 910 cm−1 corresponding to the glycosidic bond, HMP-Ce completely disappeared, once again proving that the degree of cellulose degradation was higher than Vitamin C. The results showed that HMP, HMP-Vc and HMP-Ce were all glycoconjugated mixtures containing amino groups. The main functional groups of HMP-Vc and HMP-Ce were similar to HMP, which indicated that the degradation process of polysaccharides did not cause changes types in the main functional groups of polysaccharides.
Advanced structure research
Congo red is an acid dye that can form compounds with polysaccharides in a triple-helix conformation. In a certain range of NaOH concentration, the maximum absorption wavelength of the complex is red-shifted compared with Congo red dye (Chen & Kan, 2018). The triple helix structure of each sample was determined.
Fig.5C. shows the FTIR of the interaction product between polysaccharides and nSiO2. The addition of nSiO2 did not significantly change the position and type of the polysaccharides absorption peak, indicating that the interaction of polysaccharides and nSiO2 would not form new functional groups nor destroy the main functional groups of polysaccharides. Therefore, polysaccharides and nSiO2 are more likely to bond through non-covalent interactions. Due to the presence of –OH in polysaccharides and nSiO2, there is a high possibility that hydrogen bonds will interact as the main force. Studies have shown that polysaccharides and nSiO2 interact mainly through hydrogen bonds and hydrophobic bonds (Li, Zeng, Fu, Wan, Liu & McClements, 2018). Polysaccharide with higher molecule weight are more likely to form hydrogen bonds between polysaccharides, while polysaccharides with low molecule weight tend to form hydrogen bonds with water. Polysaccharide with higher molecule weight is more likely to form hydrogen bonds between polysaccharides, while polysaccharides with low molecule weight tend to form hydrogen bonds with water (Tan, et al., 2020). After being degraded, polysaccharides are more likely to combine with water to form hydrogen bonds and less bind to nSiO2. Therefore, the weak interaction may be the reason that nSiO2 had no obvious effect on the gastroprotective activity of HMP degradation products.
Fig. 5D shows the maximum absorption wavelengths of HMP, HMP-Vc and HMP-Ce and Congo red complexes under different NaOH concentrations. When the concentration of NaOH was 0–0.15 mol /L, the maximum absorption wavelength of each compound had a certain degree of redshift compared with Congo red solution. The redshift of the maximum absorption wavelength of HMP was greater than that of HMP-Vc and HMP-Ce indicated that HMP has a tighter triple-helical structure. Vitamin C oxidative degradation and cellulase enzymatic hydrolysis will destroy the triple-helical structure of HMP to some extent. With the increase of NaOH concentration, the maximum absorption wavelength of HMP, HMP-Vc, HMP-Ce and Congo red complex decreased. This was due to the high concentration of NaOH will destroy the hydrogen bond of the polysaccharide while destroying the polysaccharide. The triple helix structure lead the polysaccharide to a random coiled state in the solution. Studies have shown that polysaccharides will become random helical structures in aqueous solution after being degraded by Vitamin C (Sun, Zhang, Zhang & Niu, 2010).
Fig. 5E shows the maximum absorption wavelengths of HMP-nSiO2, HMP-Vc-nSiO2 and HMP-Ce-nSiO2 and Congo Red composites under different NaOH concentrations. The results showed that the maximum absorption wavelength of the mixed solution of HMP-nSiO2, HMP-Vc-nSiO2 and HMP-Ce-nSiO2 and Congo Red varies with the concentration of NaOH in accordance with HMP, HMP-Vc, and HMP-Ce. It indicated that the existence of nSiO2 will not affect the triple helix structure of HMP and its degradation products.
The above results indicated that the difference between HMP-Vc and HMP-Ce and HMP was mainly the higher-level structure, which is the triple helix structure. It was speculated that the existence of the triple helix structure was an important condition for HMP to have gastroprotective activity. When HMP was degraded, the triple helix structure was greatly reduced, and the effect on the proliferation of GES-1 cells and the preventive effect of cell damage were drastically reduced. It is reported that the triple helix structure of polysaccharides is the spatial configuration with the highest biological activity, polysaccharides with a triple-helical structure usually possess better biological activities (Zheng, Fan, Chen & Liu, 2019).
Conclusion
This manuscript studied the interaction between different structures of polysaccharides and nSiO2, analyzed the changes in its structure and protective activity of the gastric mucosa, and preliminary explores the interaction mechanism of polysaccharide-nSiO2. Using Vitamin C and cellulase enzymatic hydrolysis, two degraded polysaccharides with different structures were obtained. The optimal conditions for Vitamin C degradation of Hericium erinaceus polysaccharide were determined by orthogonal test 0.2% Vitamin C concentration, 50 °C, 2 h. The optimal conditions for cellulase hydrolysis of Hericium erinaceus polysaccharides by response surface analysis were as follows: 15% cellulase, 37 °C, 0.5 h. The results of cell experiments showed that the gastroprotective effect of HMP was significantly higher than that of degradation products, and the addition of nSiO2 could enhance the gastroprotective effect of HMP without significant influence on the degradation products. The structure identification results showed that nSiO2 might interact with polysaccharides through hydrogen bonding, while without affect the crystal configuration of polysaccharides, nor will it change its triple helix structure. After HMP was degraded by Vitamin C and cellulase, its activity of proliferation-promoting and preventing ethanol-damaged cells were significantly weakened, indicating that the gastroprotective activity of HMP is mainly related to its advanced structure. The destruction of advanced structure will significantly reduce its gastroprotective activity. nSiO2 can significantly enhance the activity of HMP, but has no noticeable effect on the activity of HMP-Vc and HMP-Ce. The application of polysaccharides and nSiO2 in functional foods of Hericium erinaceus mycelium in this study provides theoretical support. For future research, it is necessary to further explore the relationship between the gastroprotective activity and structure of the Hericium erinaceus polysaccharide and the interaction mechanism between the polysaccharide and nSiO2.
CRediT authorship contribution statement
Erdong Yuan: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. Shiying Nie: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft. Liangyun Liu: Data curation, Formal analysis, Supervision, Writing – review & editing. Jiaoyan Ren: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge GES-1 cells provided by Tea Research Institute, Guangdong Academy of Agricultural Sciences. This work was supported by the [Guangdong Province Key Field R&D Program Project] under number [2019B020210002].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100172.
Contributor Information
Erdong Yuan, Email: erdyuan@scut.edu.cn.
Jiaoyan Ren, Email: jyren@scut.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al-Rawi M., Diabaté S., Weiss C. Uptake and intracellular localization of submicron and nano-sized SiO2 particles in HeLa cells. Archives of Toxicology. 2011;85(7):813–826. doi: 10.1007/s00204-010-0642-5. [DOI] [PubMed] [Google Scholar]
- Azizova L.R., Kulik T.V., Palianytsia B.B., Zemlyakov A.E., Tsikalova V.N., Chirva V. Investigation of chemical transformations of thiophenylglycoside of muramyl dipeptide on the fumed silica surface using TPD-MS, FTIR spectroscopy and ES IT MS. Nanoscale Research Letters. 2014;9(1):234. doi: 10.1186/1556-276x-9-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1190–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chandel A.K., Chandrasekhar G., Silva M.B., Silvério da Silva S. The realm of cellulases in biorefinery development. Critical Reviews in Biotechnology. 2011;32(3):187–202. doi: 10.3109/07388551.2011.595385. [DOI] [PubMed] [Google Scholar]
- Chen G., Kan J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. International Journal of Biological Macromolecules. 2018;107:166–174. doi: 10.1016/j.ijbiomac.2017.08.160. [DOI] [PubMed] [Google Scholar]
- Dona A.C., Pages G., Gilbert R.G., Kuchel P.W. Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydrate Polymers. 2010;80(3):599–617. doi: 10.1016/j.carbpol.2010.01.002. [DOI] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Graham D.Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World Journal of Gastroenterology. 2014;20(18):5191. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Martucci N.J., Liu Y., Yoo E., Tako E., Mahler G.J. Silicon dioxide nanoparticle exposure affects small intestine function in an in vitro model. Nanotoxicology. 2018;12(5):485–508. doi: 10.1080/17435390.2018.1463407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Deng H., Hwang H. The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis. 2018;27(1):1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Wang Y., Zhang X. Comparative studies on extracts from Hericium erinaceus by different polarity reagents to gain higher antioxidant activities. Experimental and Therapeutic Medicine. 2016;12(1):513–517. doi: 10.3892/etm.2016.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.U., Kim Y.-H., Kim H.-M., Oh J.-M., Kim Y.-R., Choi S.-J. Determination of the fate and biological responses of food additive silica particles in commercial foods. Food Chemistry. 2020;331:127304. doi: 10.1016/j.foodchem.2020.127304. [DOI] [PubMed] [Google Scholar]
- Kushairi N., Phan C.W., Sabaratnam V., David P., Naidu M. Lion's mane mushroom, Hericium erinaceus (Bull.: Fr.) pers. suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. Antioxidants. 2019;8(8):261. doi: 10.3390/antiox8080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-A., Kim M.-K., Song J.H., Jo M.-R., Yu J., Kim K.-M.…Choi S.-J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids & Surfaces B Biointerfaces. 2016;150:384–392. doi: 10.1016/j.colsurfb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Li O.Y., Wang L., Liu X.Y., Yin J.Y., Nie S.P. Interactions between ascorbic acid and water soluble polysaccharide from the seeds of Plantago asiatica L.: Effects on polysaccharide physicochemical properties and stability. Food Hydrocolloids. 2020;99 doi: 10.1016/j.foodhyd.2019.105351. [DOI] [Google Scholar]
- Li R., Zeng Z., Fu G., Wan Y., Liu C., McClements D.J. Formation and characterization of tannic acid/beta-glucan complexes: Influence of pH, ionic strength, and temperature. Food Research International. 2018;120:748–755. doi: 10.1016/j.foodres.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Liu B., Feng X., Zhang J., Wei Y., Zhao X. Preventive effect of anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in kunming mice. Biomolecules. 2019;9(4):137. doi: 10.3390/biom9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liang J., Wu J., Chen H., Zhang Z., Yang H.…Li Y. Transformation of patchouli alcohol to β-patchoulene by gastric juice: β-patchoulene is more effective in preventing ethanol-induced gastric injury. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-05996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Lee N.H., Oh H.J., Hwang J.S., Kim S.J. Preparation and characterization of silica/polyamide-imide nanocomposite thin films. Nanoscale Research Letters. 2010;5(11):1846–1851. doi: 10.1007/s11671-010-9726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri D., Sewerynek E., Reiter R.J., Ortiz G.G., Poeggeler B., Nisticò G. Suppressive effect of melatonin administration on ethanol-induced gastroduodenal injury in rats in vivo. British Journal of Pharmacology. 1997;121(2):264–270. doi: 10.1038/sj.bjp.0701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Analytical Chemistry. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mirzaei S., Javanbakht V. Dye removal from aqueous solution by a novel dual cross-linked biocomposite obtained from mucilage of Plantago Psyllium and eggshell membrane. International Journal of Biological Macromolecules. 2019;134:1187–1204. doi: 10.1016/j.ijbiomac.2019.05.119. [DOI] [PubMed] [Google Scholar]
- Niu F., Kou M., Fan J., Pan W., Feng Z.J., Su Y., Zhou W. Structural characteristics and rheological properties of ovalbumin-gum arabic complex coacervates. Food Chemistry. 2018;260(15):1–6. doi: 10.1016/j.foodchem.2018.03.141. [DOI] [PubMed] [Google Scholar]
- Qu J., Huang P., Zhang L., Qiu Y., Qi H., Leng A., Shang D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. International Journal of Biological Macromolecules. 2020;161:24–34. doi: 10.1016/j.ijbiomac.2020.05.196. [DOI] [PubMed] [Google Scholar]
- Ranjan S., Dasgupta N., Srivastava P., Ramalingam C. A spectroscopic study on interaction between bovine serum albumin and titanium dioxide nanoparticle synthesized from microwave-assisted hybrid chemical approach. Journal of Photochemistry and Photobiology B: Biology. 2016;161:472–481. doi: 10.1016/j.jphotobiol.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Semedo M.C., Karmali A., Fonseca L. A high throughput colorimetric assay of β-1,3-d-glucans by Congo red dye. Journal of Microbiological Methods. 2015;109:140–148. doi: 10.1016/j.mimet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Shatz W., Hass P.E., Mathieu M., Kim H.S., Leach K., Zhou M., Kelley R.F. Contribution of antibody hydrodynamic size to vitreal clearance revealed through rabbit studies using a species-matched fab. Molecular Pharmaceutics. 2016;13(9):2996–3003. doi: 10.1021/acs.molpharmaceut.6b0034. [DOI] [PubMed] [Google Scholar]
- Shen X., Liu Z., Li J., Wu D., Zhu M., Yan L.…Chen S. Development of low molecular weight heparin by H2O2/ascorbic acid with ultrasonic power and its anti-metastasis property. International Journal of Biological Macromolecules. 2019;133:101–109. doi: 10.1016/j.ijbiomac.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Song Q., Jiang L., Yang X., Huang L., Yu Y., Yu Q.…Xie J. Physicochemical and functional properties of a water-soluble polysaccharide extracted from Mung bean (Vigna radiate L.) and its antioxidant activity. International Journal of Biological Macromolecules. 2019;138:874–880. doi: 10.1016/j.ijbiomac.2019.07.167. [DOI] [PubMed] [Google Scholar]
- Sun L., Li Y., Liu X., Jin M., Zhang L., Du Z.…Sun Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicology in Vitro. 2011;25(8):1619–1629. doi: 10.1016/j.tiv.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Sun Z., Zhang L., Zhang B., Niu T. Structural characterisation and antioxidant properties of polysaccharides from the fruiting bodies of Russula virescens. Food Chemistry. 2010;118(3):675–680. doi: 10.1016/j.foodchem.2009.05.036. [DOI] [Google Scholar]
- Tan M., Chang S., Liu J., Li H., Xu P., Wang P.…Zhao Q. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules. 2020;25(17):3840. doi: 10.3390/molecules25173840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoumaki M.V., Moschakis T., Scholten E., Biliaderis C.G. In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food & Function. 2013;4(1):121–129. doi: 10.1039/c2fo30129f. [DOI] [PubMed] [Google Scholar]
- Varma C.A.K., Jayaram Kumar K. Characterization and evaluation of smart releasing polysaccharide from yellow poinciana seed of Jharkhand. International Journal of Biological Macromolecules. 2018;118:2156–2162. doi: 10.1016/j.ijbiomac.2018.07.057. [DOI] [PubMed] [Google Scholar]
- Wang, J., Tong, X., Li, P., Cao, H., & Su, W. (2012). Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. Journal of Ethnopharmacology, 139(3), 788-795. https://doi:10.1016/j. jep.2011.12.019. [DOI] [PubMed]
- Wang Z., Luo H., Xia H. Theaflavins attenuate ethanol-induced oxidative stress and cell apoptosis in gastric mucosa epithelial cells via downregulation of the mitogen-activated protein kinase pathway. Molecular Medicine Reports. 2018;18:3791–3799. doi: 10.3892/mmr.2018.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Wu Y., Sun P., Zhang F., Linhardt R.J., Zhang A. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. International Journal of Biological Macromolecules. 2019;132:970–977. doi: 10.1016/j.ijbiomac.2019.03.213. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang X., Zhao M., Qi H. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydrate Polymers. 2014;112:578–582. doi: 10.1016/j.carbpol.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Fan J., Chen H., Liu E. Trametes orientalis polysaccharide alleviates PM2.5-induced lung injury in mice through its antioxidant and anti-inflammatory activities. Food & Function. 2019;10(12):8005–8015. doi: 10.1039/c9fo01777a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.