Abstract
Arbor Acre (AA) broilers were used as the research object to investigate whether glucose oxidase (GOD) has preventive and relieving effects on necrotic enteritis. The experiment was designed as a factorial arrangement of 2 dietary treatments × 2 infection states. Chickens were fed a basal diet or a diet with 150 U/kg GOD, and were challenged with Clostridium perfringens (Cp) or sterile culture medium. In our study, Cp challenge led to intestinal injury, as evidenced by reducing the average daily gain and the average daily feed intake of AA broilers of 14 to 21 d (P < 0.05), increasing the intestinal jejunal lesion score (P < 0.05), reducing the jejunal villi height and villi height/crypt depth (P < 0.05), upregulating the mRNA expression levels jejunal IFN-γ (P < 0.05). The dietary GOD had no significant effects on the growth performance of each growth period, but significantly decreased the ileal pH, increased the height of villi and the ratio of villi height to crypt depth (P < 0.05) and the expression levels of Occludin and Zonula occludens-1 (ZO-1) at d 21. Moreover, dietary GOD and the Cp challenge significantly altered the composition of 21-d ileal microbiota. The Cp challenge decreased the relative abundance of genus Lactobacillus (P = 0.057), and increased the relative abundance of genus Romboutsia (P < 0.05) and genus Veillonella (P = 0.088). The dietary GOD tended to increase the relative abundance of genus Helicobacter (P = 0.066) and decrease the relative abundance of genus Streptococcus (P = 0.071). This study has shown that the supplementation of GOD could promote the integrity of intestinal barrier and the balance of ileal microbiota, but the effects of GOD on NE broilers and its application in actual production need to be further confirmed.
Key words: broiler, necrotic enteritis, clostridium perfringens, glucose oxidase, intestinal health
INTRODUCTION
Necrotic enteritis (NE) is a poultry intestinal disease caused by Clostridium perfringens (Cp), and could cause intestinal and liver lesions (Zhang et al., 2017; Zhang et al., 2019), damage of intestinal villi, intestinal submucosal hemorrhage, intestinal mucosal edema or cell necrosis, destruction of tight junctions and increased intestinal permeability (Van Immerseel et al., 2016). In addition, the excessive proliferation of Cp in the intestine would competitively inhibit the growth of some beneficial bacteria, resulting in the imbalance of intestinal microbiota and accelerate the development of the disease (Feng et al., 2010; Stanley et al., 2012). Therefore, NE could cause weight loss and the decreasing of feed conversion ratio (FCR), increase mortality, and bring a large number of losses to production (Kaldhusdal et al., 2016).
In recent years, the prohibition of medicated feed additives has caused a high incidence of NE, which has brought huge economic losses to the poultry industry (Van Immerseel et al., 2009). There is an urgent need to find effective ways to control the disease. The main nutrition regulation strategies to control NE are as follows: direct feeding of probiotics (Hernandez-Patlan et al., 2019; Whelan et al., 2019), prebiotics (Xue et al., 2017b), organic acids (Stringfellow et al., 2009), enzyme preparations (Xue et al., 2017a; Yin et al., 2017), and adjustment of dietary nutritional composition. Enzyme preparation has great potential for the development of substitute because of its safety, no pollution, no residue and so on. Glucose oxidase (GOD) is a kind of feed enzyme, which has gained much attention due to its beneficial functions on improving the intestinal health of animals (Wu et al., 2019a). It can specifically oxidize β-D-glucose to gluconic acid and hydrogen peroxide when consuming a large amount of oxygen (Bankar et al., 2009). The anaerobic and acidic environment formed by GOD catalytic reaction in the intestine was conductive to the proliferation of anaerobic acid-resistant bacteria. Gluconic acid acts as an acidifier in the intestinal tract (Rafacz-Livingston et al., 2005), which can decrease intestinal pH and improve the activity of digestive enzymes. In addition, gluconic acid also has a potential prebiotic effect, which can reach the hindgut to produce volatile fatty acids by fermentation of specific bacteria, mainly butyric acid (Tsukahara et al., 2002). Butyric acid has been widely proved to have the effects of anti-inflammation, providing energy for intestinal epithelial cells and maintaining the morphology and structure of intestinal mucosa (Peng et al., 2009). The hydrogen peroxide produced also has a certain bactericidal and antibacterial effect.
NE has been reported to be characterized by overgrowth of Cp in the gut, accompanied by impairment of the intestinal barrier and inflammation. Based on the characteristics of GOD catalyzed reaction, it is speculated that GOD plays a certain role in prevention and relief of NE by producing acid and sterilization. So far, the effects of GOD have not been evaluated in broilers challenged by Cp. Therefore, we explored the effects of dietary GOD on the growth performance and intestinal health in Arbor Acre (AA) broilers with Cp infection.
MATERIALS AND METHODS
Animal Feeding and Management
The study was conducted at Zhuozhou Experimental Base of China Agricultural University. Based on a 2 × 2 factorial arrangement with 2 dietary GOD levels (0 or 150 U/kg of diet) and pathogen exposure (with or without Cp challenge), a total of 280 one-day-old AA broilers were randomly assigned to four groups: the control group fed with basal diet (CON), group fed with GOD diet (GOD), Cp challenge group fed with basal diet (Cp), Cp challenge group fed with GOD diet (Cp + GOD). Each group included 7 replications, 10 chickens per replication. The trial lasted 35 d, divided into 3 stages, 1 to 14 d, 14 to 21 d, and 21 to 35 d, and challenged from d 14 to d 20. All chickens were reared in the 3-layer wire cages and were evenly distributed on the top 2 layers. The chickens had free access to feed and water and were maintained on a 23 h constant-lighting program. The unmedicated corn-soybean meal diets were prepared according to the nutritional requirements recommended by American NRC (1994) and Chinese chicken feeding standard (NY/T-33-2004). The GOD was provided by Jinan Bestzyme Biological Engineering Co., LTD. Table 1 presents the composition and nutrient levels of a basal diet. All experimental procedures were approved by the Animal Care and Use Committee of China Agricultural University.
Table 1.
Composition and nutrient levels of basal diet (%, air-dry basis).
| Items | 1–21 d | 21–35 d | Items | 1–21 d | 21-35 d |
|---|---|---|---|---|---|
| Ingredients | Nutrient levels3 | ||||
| Corn | 58.52 | 56.72 | ME (Mcal/kg) | 2.93 | 3.10 |
| Soybean meal | 36.24 | 34.67 | CP | 22.02 | 20.00 |
| Soy oil | 1.54 | 5.32 | Lys | 1.22 | 1.07 |
| Calcium hydrogen phosphate | 1.85 | 1.46 | Met | 0.50 | 0.44 |
| Limestone | 0.88 | 0.95 | Met + Cys | 0.84 | 0.78 |
| Sodium chloride | 0.35 | 0.33 | Ca | 1.00 | 0.90 |
| Mineral premix1 | 0.20 | 0.20 | AP | 0.45 | 0.40 |
| 50% Choline chloride | 0.20 | 0.16 | |||
| DL-Met | 0.17 | 0.14 | |||
| Antioxidant | 0.03 | 0.03 | |||
| Vitamin premix2 | 0.02 | 0.02 | |||
| Total | 100.00 | 100.00 |
Provide per kilogram of diet:copper, 2 mg;iron, 132 mg;zinc, 126 mg;manganese, 129 mg;iodine, 1.8 mg;selenium, 0.6 mg.
Provide per kilogram of diet:vitamin A, 13500 IU;vitamin D3, 3600 IU;vitamin E, 36 IU;vitamin K3, 4.5 mg;vitamin B1, 3.6 mg;vitamin B2, 11.25 mg;vitamin B6, 6 mg;vitamin B12, 0.039 mg;niacin, 39 mg;D-pantothenic acid, 16.5 mg;folic acid, 2.1 mg;biotin, 0.24 mg.
Calculated values.
Clostridium Perfringens Challenge
The Cp challenge was performed on the basis of the study of Liu et al., (2010), with some modifications. From d 14 to d 20, AA broilers in the infected groups were orally challenged with 1 mL of Cp type A (CVCC52) culture broth at 1 × 108 colony-forming units (CFU)/bird per day. Broilers in the uninfected groups received the same amount of sterile culture medium at the corresponding times. The Cp strain was obtained from China Veterinary Culture Collection Center. The strains of Cp were cultured in liquid thioglycolate medium (FTG, Beijing Luqiao Technology Co., Ltd., China) at 37°C for overnight anaerobic culture. The viable bacteria were counted with Tryptose-sulfite-cycloserine (TSC, Beijing Luqiao Technology Co., Ltd.).
Growth Performance and Sample Collection
On d 14, 21, and 35, chickens were weighed by replication, and the feed consumption was recorded by replication. Mortality was recorded as it occurred. The body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and FCR were calculated for the periods during d 1 to 14, 14 to 21, 21 to 35, and 1 to 35. On d 21, one chick of average BW was randomly selected from each cage. The chickens were killed by jugular exsanguination. The midregions of the jejunum (approximately 1 cm) were collected in RNA-free centrifuge tube, rapidly frozen in liquid nitrogen and stored at −80°C for mRNA analysis. Duodenal, jejunal, and ileal content were collected, rapidly frozen with liquid nitrogen and stored at −80°C for the determination of pH and ileal microbiota.
Intestinal pH and Lesion Score
The intestinal contents were weighed and deionized water was added at the dilution ration of 1:10, mixed with a small oscillator (250 bpm) for 5 min, and the pH values of duodenal, jejunal, and ileal contents were determined by pH meter (Mettler Toledo).
The jejunal injury was observed and scored according to the severity of intestinal injury. The scoring criteria for intestinal injury was based on the Dahiya method (Dahiya et al., 2005): 0, no obvious injury; 0.5, severe congestion in the serous surface and mesentery of the small intestine; 1, thinning brittleness and red petechia in the intestinal wall; 2, gas in the intestinal lumen, there is needle-like necrosis or ulceration in the intestinal wall; 3, the intestinal cavity is filled with gas and the intestinal wall appears patchy necrosis or ulcer. 4, there was a large amount of gas in the intestinal cavity, resulting in diffuse necrosis.
Intestinal Morphology
On d 21, the midregions of the jejunum (approximately 1 cm) were collected in 4% paraformaldehyde solution and then embedded in paraffin. Transverse 5-μm sections were stained with hematoxylin and eosin, and histomorphological parameters were examined using an Olympus optical microscope and ProgRes Capture Pro Software (version 2.7, Jenoptik, Jena, Germany). The villus height and crypt depth of the slice samples were read, and the ratio of villus height to crypt depth was calculated. Ten complete and vertical villi were selected for each slice sample. The villus height is the height from the top of the villus to the crypt opening, and the crypt depth is the distance from the crypt opening to the base of the crypt.
Real-Time Quantitative PCR
Total RNA was extracted from intestinal tissues using Trizol reagent (TaKaRa Bio, Japan) according to the manufacturer's protocol. The concentration and purity of RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). In brief, 1 μg of total RNA from each sample was reverse transcribed into cDNA using a PrimeScript RT reagent kit with cDNA eraser (RR036A; TaKaRa Bio). All the measurements were carried out in triplicate. Table 2 lists the quantitative real-time PCR primers used in our study. The relative mRNA expression levels of each target gene were calculated based on the expression of the house-keeping gene GAPDH using the 2−△△Ct method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences of qPCR.
| Gene name | Primer sequence (5′ to 3′) | Accession number |
|---|---|---|
| Occludin | F:ACGGCAGCACCTACCTCAA | NM_20512.81 |
| R:GGGCGAAGAAGCAGATGAG | ||
| Claudin-1 | F:CATACTCCTGGGTCTGGTTGGT | AY750897.1 |
| R:GACAGCCATCCGCATCTTCT | ||
| ZO-1 | F:CTTCAGGTGTTTCTCTTCCTCCTC | XM_413773 |
| R:CTGTGGTTTCATGGCTGGATC | ||
| TLR-4 | F:GGATCTTTCAAGGTGCCACA | AY064697 |
| R:CAAGTGTCCGATGGGTAGGT | ||
| IL-1β | F: ACTGGGCATCAAGGGCTA | NM_204524.1 |
| R: GGTAGAAGATGAAGCGGGTC | ||
| TNF-α | F:GAGCGTTGACTTGGCTGTC | XM_204267 |
| R:AAGCAACAACCAGCTATGCAC | ||
| IFN-γ | F: AGCTGACGGTGGACCTATTATT | NM_205149.1 |
| R: GGCTTTGCGCTGGATTC | ||
| IL-4 | F:GCTCTCAGTGCCGCTGATG | NM-0010079.1 |
| R:GAAACCTCTCCCTGGATGTCAT | ||
| Mucin-2 | F:TTCATGATGCCTGCTCTTGTG | XM_421035 |
| R:CCTGAGCCTTGGTACATTCTTGT | ||
| GADPH | F:TGCTGCCCAGAACATCATCC | NM_204305.1 |
| R: ACGGCAGGTCAGGTCAACAA |
F, forward; R, reverse; Primers were synthesized by Biotech (Shanghai) Co, Ltd.
Bacterial DNA Extraction and Sequencing of 16S rRNA
Ileal content DNA was extracted using PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) according to the manufacturer's protocol. The concentration and purity of total DNA were detected by NanoDrop 2000 (Thermo Scientific, MA) and 1.5% agarose gel electrophoresis. To construct 16S rDNA sequencing libraries, the V3–V4 region of the 16S rDNA gene was amplified from the DNA samples by PCR using primer set of 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′- GGACTACHVGGGTWTCTAAT-3′). The PCR products were purified, quantified, and homogenized to form a sequencing library. After being confirmed qualified, machine sequencing was used on the Novaseq PE250 sequencing platform. Sequence dereplication and denoising were done to generate amplicon sequence variants (ASVs), and Qiime2-2019.7 (Bolyen, 2019, Nature Biotechnology) was used to generate species abundance tables at different classification levels. The Alpha diversity index and Beta diversity of samples were analyzed.
Statistical Analysis
The data were analyzed using the General Linear Model procedure in SPSS version 23.0 (SPSS Inc., Chicago, IL), and subjected to two-way ANOVA in a 2 × 2 factorial arrangement to analyze the main effects of Cp challenge and GOD supplementation, and their interaction. One-way ANOVA and Duncan's multiple comparisons were used when a significant interaction was observed. We used analysis of variance of Permutational analysis of covariance (PERMANOVA) from R's package vegan to compare the effects of dietary GOD on microbial community structures. Results are presented as the means with standard error of the mean. A P value < 0.05 was taken as statistical significance.
RESULTS
Growth Performance and Intestinal pH
Before challenge (from 1 to 14 d), it was observed that the supplementation of GOD had no significant differences in the ADG, ADFI, and FCR (P > 0.05), data were not shown. In Table 3, from 14 to 21 d, Cp challenge significantly decreased the ADG and ADFI of broilers (P < 0.05), but the FCR was not affected. The dietary GOD had no significant effects on the growth performance in each period (P > 0.05), the data were not shown. The effects of the supplement of GOD on duodenal, jejunal and ileal pH of AA broilers are shown in Table 4. We found that dietary supplements of GOD could decrease duodenal (P = 0.094) and ileal (P < 0.05) pH.
Table 3.
The growth performance of AA broilers on d 21.
| Items | Group |
SEM | Main effect |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GOD | Cp | Cp + GOD | Infection |
GOD |
Infection | GOD | Infection × GOD | ||||
| - | + | - | + | |||||||||
| 14-21d | ||||||||||||
| ADG,g/d | 35.98 | 35.87 | 32.56 | 31.42 | 0.827 | 35.92a | 31.99b | 34.27 | 33.64 | 0.018 | 0.690 | 0.744 |
| ADFI,g/d | 56.79 | 57.48 | 53.77 | 52.20 | 0.863 | 57.14a | 52.99b | 55.28 | 54.84 | 0.016 | 0.788 | 0.488 |
| FCR | 1.58 | 1.60 | 1.65 | 1.66 | 0.027 | 1.59 | 1.65 | 1.62 | 1.63 | 0.168 | 0.943 | 0.741 |
| 21-35d | ||||||||||||
| ADG,g/d | 61.60 | 63.31 | 63.52 | 66.03 | 1.521 | 62.46 | 64.77 | 62.56 | 64.67 | 0.471 | 0.511 | 0.900 |
| ADFI,g/d | 100.32 | 103.81 | 100.89 | 105.77 | 1.308 | 102.06 | 103.33 | 100.60 | 104.79 | 0.633 | 0.123 | 0.793 |
| FCR | 1.63 | 1.64 | 1.59 | 1.60 | 0.028 | 1.63 | 1.60 | 1.61 | 1.62 | 0.404 | 0.995 | 0.877 |
| 1-35d | ||||||||||||
| ADG,g/d | 38.66 | 39.52 | 38.76 | 39.52 | 0.635 | 39.09 | 39.14 | 38.70 | 39.52 | 0.972 | 0.550 | 0.970 |
| ADFI,g/d | 61.37 | 62.97 | 60.68 | 62.31 | 0.595 | 62.17 | 61.50 | 61.02 | 62.64 | 0.584 | 0.194 | 0.989 |
| FCR | 1.59 | 1.59 | 1.57 | 1.58 | 0.017 | 1.59 | 1.57 | 1.58 | 1.59 | 0.544 | 0.897 | 0.904 |
Abbreviations: ADG, Average daily gain; ADFI, Average daily feed intake; FCR, Feed conversion ratio; Cp, Clostridium perfringens; GOD, glucose oxidase; CON, The control group; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same row without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Table 4.
The intestinal pH of AA broilers on d 21.
| pH |
|||||
|---|---|---|---|---|---|
| Items | Duodenum | Jejunum | Ileum | ||
| CON | 5.42 | 5.47 | 6.27 | ||
| GOD | 5.25 | 5.17 | 5.80 | ||
| Cp | 5.33 | 5.27 | 6.21 | ||
| Cp + GOD | 5.33 | 5.24 | 6.08 | ||
| SEM | 0.027 | 0.059 | 0.059 | ||
| Main effect | Infection | - | 5.34 | 5.32 | 6.04 |
| + | 5.33 | 5.25 | 6.14 | ||
| GOD | - | 5.38 | 5.37 | 6.24a | |
| + | 5.29 | 5.20 | 5.94b | ||
| P value | Infection | 0.818 | 0.566 | 0.309 | |
| GOD | 0.094 | 0.169 | 0.007 | ||
| Infection × GOD | 0.122 | 0.271 | 0.110 | ||
Abbreviations: Cp, Clostridium perfringens; GOD, glucose oxidase; CON, The control group; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same column without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Lesion Score
As shown in Table 5, Cp infection significantly increased the lesion score of jejunum (P < 0.05). Intestinal mucosal congestion appeared in most broilers in Cp infection groups. However, the dietary GOD had no significant effects on the intestinal score of AA broilers challenged by Cp (P < 0.05).
Table 5.
The jejunual lesion score of AA broilers on d 21.
| Items | Lesion score | |||
|---|---|---|---|---|
| CON | 0.21 | |||
| GOD | 0.57 | |||
| Cp | 1.07 | |||
| Cp + GOD | 1.00 | |||
| SEM | 0.104 | |||
| Main effect | Infection | - | 0.39b | |
| + | 1.04a | |||
| GOD | - | 0.64 | ||
| + | 0.78 | |||
| P value | Infection | 0.001 | ||
| GOD | 0.409 | |||
| Infection × GOD | 0.220 | |||
Abbreviations: Cp, Clostridium perfringens; GOD, glucose oxidase; CON, The control group; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same column without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Jejunal Morphology
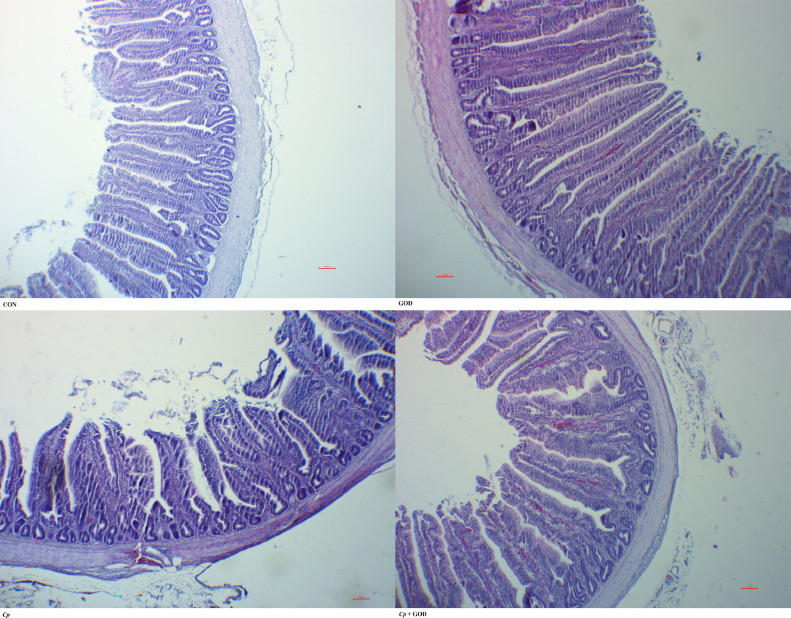
As shown in Figure 1 and Table 6, the Cp infection significantly decreased jejunal villus height and villus height/crypt depth (P < 0.05), but had no significant effects on crypt depth. The dietary supplement of GOD significantly increased the villus height and villus height/crypt depth (P < 0.05).
Figure 1.
The jejunal morphology structure of AA broilers on 21 d. Cp, Clostridium perfringens; GOD, glucose oxidase; CON, The control group; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD. From left to right, top to bottom: CON group; GOD group; Cp group; Cp + GOD group. Bar = 100 μm.
Table 6.
The jejunal histomorphological parameters of AA broilers on d21.
| Items | VH, μm | CD, μm | V/C | ||
|---|---|---|---|---|---|
| CON | 765.39 | 208.40 | 3.67 | ||
| GOD | 832.82 | 208.95 | 3.99 | ||
| Cp | 647.20 | 201.87 | 3.24 | ||
| Cp + GOD | 762.64 | 207.06 | 3.69 | ||
| SEM | 21.276 | 4.280 | 0.084 | ||
| Main effect | Infection | - | 799.11a | 208.67 | 3.83a |
| + | 704.92b | 204.47 | 3.47b | ||
| GOD | - | 706.30b | 205.13 | 3.46b | |
| + | 797.73a | 208.01 | 3.84a | ||
| P value | Infection | 0.015 | 0.645 | 0.015 | |
| GOD | 0.018 | 0.753 | 0.011 | ||
| Infection × GOD | 0.511 | 0.799 | 0.642 | ||
Abbreviations: CD: Crypt depth; Cp, Clostridium perfringens; CON, The control group; GOD, glucose oxidase; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD; VH, Villi height; V/C, Villi height / Crypt depth.
Means in the same column without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
The mRNA Expression Levels of Tight Junction and Inflammatory Cytokines in Jejunum
In Table 7, Cp infection significantly elevated the jejunal IFN-γ mRNA expression (P < 0.05) and numerically decreased the jejunal IL-4 mRNA expression (P = 0.118) of AA broilers on day 21. The dietary GOD significantly elevated the mRNA expression levels of jejunal Occludin and ZO-1, and tended to elevate the mRNA expression levels of Claudin-1 (P = 0.065) and decrease the Mucin-2 mRNA expression (P = 0.085).
Table 7.
The mRNA expressions of tight junctions and inflammatory cytokine in the jejunum of AA broilers on d 21.
| Items | Group |
SEM | Main effect |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GOD | Cp | Cp + GOD | Infection |
GOD |
Infection | GOD | Infection × GOD | ||||
| - | + | - | + | |||||||||
| Occludin | 1.00 | 1.30 | 1.18 | 1.39 | 0.058 | 1.14 | 1.28 | 1.09b | 1.35a | 0.212 | 0.026 | 0.642 |
| Claudin-1 | 1.00 | 1.45 | 1.20 | 1.46 | 0.094 | 1.22 | 1.33 | 1.10 | 1.46 | 0.564 | 0.065 | 0.617 |
| ZO-1 | 1.00 | 1.47 | 1.08 | 1.60 | 0.079 | 1.24 | 1.34 | 1.04b | 1.53a | 0.445 | 0.001 | 0.868 |
| IL-1β | 1.00 | 1.65 | 1.19 | 1.13 | 0.127 | 1.32 | 1.16 | 1.09 | 1.39 | 0.519 | 0.246 | 0.174 |
| IL-4 | 1.00 | 0.96 | 0.80 | 0.91 | 0.039 | 0.98 | 0.86 | 0.90 | 0.94 | 0.118 | 0.624 | 0.326 |
| Mucin-2 | 1.00 | 0.74 | 0.92 | 0.86 | 0.046 | 0.87 | 0.89 | 0.96 | 0.80 | 0.800 | 0.085 | 0.266 |
| IFN-γ | 1.00 | 1.45 | 1.66 | 1.74 | 0.115 | 1.23b | 1.70a | 1.33 | 1.59 | 0.037 | 0.230 | 0.392 |
| TNF-α | 1.00 | 1.15 | 1.06 | 1.16 | 0.041 | 1.07 | 1.11 | 1.03 | 1.15 | 0.693 | 0.144 | 0.719 |
Abbreviations: Cp, Clostridium perfringens; CON, The control group; GOD, glucose oxidase; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same row without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Ileal Microbiota
Twenty-eight ileal chyme samples were processed at the age of 21 d. A total of 4,349,196 data was obtained after 16S rRNA sequencing, and 279,143 valid sequences were obtained after filtering, denoising, and removing chimeras. The rarefaction curves of each group quickly reach the plateau to prove that the sequencing depth is sufficient and can be analyzed later.
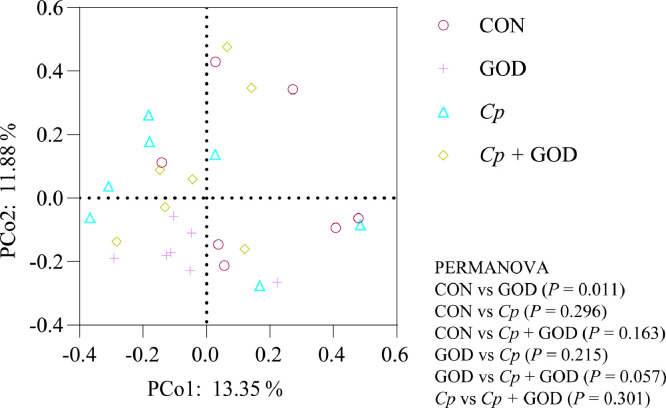
Shannon indexes were used to evaluate the ileal microbial alpha-diversity of 21-day-old AA broilers among the four treatments. The results showed that the supplementation of GOD showed a tendency to increase the Shannon index (P = 0.090) (Table 8). Subsequently, the ileal bacterial compositions with the four treatments were compared based on Bray-Curtis distance. As shown in Figure 2, PERMANOVA was used to examine whether the matrix of major PCoA axes was dependent on the dietary GOD and the Cp infection. According to the results of PCoA, the ileal microbials were significantly isolated among the 4 groups (P < 0.05). Through the pairwise comparison of the four treatments, it was found that there was a significant isolation of intestinal microbiota between the GOD group and the CON group, and there was a trend of isolation between the GOD group and the Cp + GOD group (P = 0.057).
Table 8.
The Shannon index of AA broilers on d 21.
| Items | Shannon index | ||
|---|---|---|---|
| CON | 3.95 | ||
| GOD | 4.65 | ||
| Cp | 4.31 | ||
| Cp + GOD | 4.77 | ||
| SEM | 0.172 | ||
| Main effect | Infection | - | 4.30 |
| + | 4.54 | ||
| GOD | - | 4.13 | |
| + | 4.71 | ||
| P value | Infection | 0.494 | |
| GOD | 0.090 | ||
| Infection × GOD | 0.723 | ||
Abbreviations: Cp, Clostridium perfringens; CON, The control group; GOD, glucose oxidase; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
a–bMeans in the same column without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Figure 2.
Beta diversity of ileal microbiota. Cp, Clostridium perfringens; GOD, glucose oxidase; CON, The control group; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
To determine which bacterial taxa contributed most to the separation of the microbial communities, we compared the ileal compositions of the four treatments at phyla and genus levels. At the phylum level Table 9, Firmicutes, Epsilonbacteraeota, Proteobacteria, Uncultured_bacteria, Bacteroidetes were the most abundant among the four groups. Cp challenge significantly decreased the relative abundance of Cyanobacteria (P < 0.05). The dietary GOD significantly decreased the relative abundance of Firmicutes, and tended to increase the relative abundance of Epsilonbacteraeota (P = 0.063) and Tenericutes (P = 0.096). At the genus level, the top 10 bacteria were Lactobacillus, Helicobacter, Enterococcus, Streptococcus, Escherichia Shigella, Clostridium sensu stricto 1, Veillonella, Uncultured_Bacteria, Gallibacterium, Romboutsia in the 21-day-old AA broilers ileum. As shown in the Table 10, the Cp challenge decreased the relative abundance of Lactobacillus (P = 0.057), and increased the relative abundance of Romboutsia (P < 0.05) and Veillonella (P = 0.088). The dietary GOD tended to increase the relative abundance of Helicobacter (P = 0.066) and decrease the relative abundance of Streptococcus (P = 0.071).
Table 9.
The relative abundance of phyla level in ileal microbiota of AA broilers on d 21 (%).
| Items | Group |
SEM | Main effect |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GOD | Cp | Cp + GOD | Infection |
GOD |
Infection | GOD | Infection × GOD | ||||
| - | + | - | + | |||||||||
| Firmicutes | 82.97 | 62.62 | 68.50 | 61.17 | 3.547 | 72.80 | 64.84 | 75.74a | 61.90b | 0.243 | 0.048 | 0.337 |
| Epsilonbacteraeota | 14.21 | 33.13 | 26.97 | 31.04 | 3.117 | 23.67 | 29.01 | 20.59 | 32.08 | 0.375 | 0.063 | 0.220 |
| Proteobacteria | 2.78 | 3.81 | 4.27 | 3.76 | 1.012 | 3.30 | 4.02 | 3.52 | 3.78 | 0.739 | 0.904 | 0.721 |
| Uncultured_bacteria | 0.03 | 0.07 | 0.02 | 1.90 | 0.312 | 0.05 | 0.96 | 0.03 | 0.98 | 0.128 | 0.108 | 0.121 |
| Bacteroidetes | 0.01 | 0.04 | 0.11 | 0.87 | 0.198 | 0.03 | 0.49 | 0.06 | 0.46 | 0.249 | 0.326 | 0.366 |
| Tenericutes | 0.00 | 0.28 | 0.00 | 0.64 | 0.134 | 0.14 | 0.32 | 0.00 | 0.46 | 0.499 | 0.096 | 0.503 |
| Cyanobacteria | 0.00 | 0.04 | 0.12 | 0.37 | 0.055 | 0.02 | 0.25 | 0.06 | 0.21 | 0.034 | 0.160 | 0.309 |
Abbreviations: Cp, Clostridium perfringens; CON, The control group; GOD, glucose oxidase; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same row without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
Table 10.
The relative abundance of genus level in ileal microbiota of AA broilers on 21 d.
| Items | Group |
SEM | Main effect |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GOD | Cp | Cp + GOD | Infection |
GOD |
Infection | GOD | Infection × GOD | ||||
| - | + | - | + | |||||||||
| Lactobacillus | 64.41 | 60.49 | 43.67 | 39.24 | 5.369 | 62.45 | 41.46 | 54.04 | 49.86 | 0.057 | 0.695 | 0.981 |
| Helicobacter | 14.21 | 33.08 | 26.87 | 30.61 | 3.099 | 23.64 | 28.74 | 20.54 | 31.84 | 0.394 | 0.066 | 0.210 |
| Enterococcus | 9.92 | 0.31 | 11.32 | 14.17 | 2.498 | 5.12 | 12.75 | 10.62 | 7.24 | 0.129 | 0.493 | 0.212 |
| Streptococcus | 8.16 | 1.47 | 7.69 | 1.87 | 1.674 | 4.82 | 4.78 | 7.93 | 1.67 | 0.991 | 0.071 | 0.897 |
| Escherichia Shigella | 2.77 | 3.74 | 2.86 | 3.44 | 1.002 | 3.26 | 3.15 | 2.81 | 3.59 | 0.960 | 0.719 | 0.927 |
| Clostridium sensu stricto 1 | 0.00 | 0.00 | 3.25 | 1.20 | 0.691 | 0.00 | 2.22 | 1.62 | 0.60 | 0.115 | 0.459 | 0.459 |
| Veillonella | 0.00 | 0.00 | 1.23 | 2.30 | 0.504 | 0.00 | 1.77 | 0.62 | 1.15 | 0.088 | 0.593 | 0.593 |
| Uncultured_Bacteria | 0.03 | 0.07 | 0.02 | 1.90 | 0.312 | 0.05 | 0.96 | 0.03 | 0.99 | 0.128 | 0.108 | 0.121 |
| Gallibacterium | 0.00 | 0.00 | 1.38 | 0.03 | 0.246 | 0.00 | 0.71 | 0.69 | 0.02 | 0.142 | 0.157 | 0.157 |
| Romboutsia | 0.00 | 0.00 | 0.78 | 0.57 | 0.148 | 0.00b | 0.68a | 0.39 | 0.29 | 0.024 | 0.720 | 0.720 |
Abbreviations: Cp, Clostridium perfringens; CON, The control group; GOD, glucose oxidase; GOD, The GOD group; Cp, The Cp challenge group; Cp + GOD, The Cp challenge group fed with GOD.
Means in the same row without the same superscript differ significantly (P < 0.05). SEM means standard error of the mean. “+” means challenge or add. (n = 7).
DISCUSSION
In the present study, we induced experimental NE in AA broilers challenge with Cp. The challenge induced macroscopic pathological changes in the intestine, such as distinct hyperemia, hemorrhage, and focal necrosis or ulceration. In addition, Cp challenge resulted in decreased ADG, increased jejunal inflammation, increased jejunal permeability and pathogenic bacteria, which were consistent with the characteristics of subclinical NE established in previous studies (Kaldhusdal et al., 2016; Emami et al., 2019; Huang et al., 2019).
The effects of dietary GOD on growth performance, immune function and intestinal barrier function of poultry were extensively investigated in recent years (Wang et al., 2018; Wu et al., 2019b). Dietary GOD could improve the intestinal environment by utilizing oxygen, producing gluconic acid, and H2O2 (Witt et al., 2000). Considering the characteristics of GOD catalyzed reaction, it is speculated that GOD plays a certain role in prevention and relief of NE in poultry. In our study, dietary GOD had no significant effects on the growth performance of AA broilers during the whole period regardless of Cp challenge.
Acidic environment is conducive to improving the activity of intestinal digestive enzymes, promoting digestion, and absorption, increasing the community of beneficial bacteria so that could promote the growth performance and maintain the intestinal environmental homeostasis (Yadav and Jha, 2019). Gluconic acid produced by catalytic reaction of GOD could decrease intestinal pH, about 70% of which will be fermented by specific bacteria to produce short-chain fatty acids after achieving the hindgut, including acetic acid, butyric acid and so on (Tsukahara et al., 2002). These weak acids could also play a role in decreasing intestinal pH. In our study, the supplementation of GOD significantly decreased ileal pH, and had a tendency to decrease duodenal pH.
The morphology and structure of the intestine determines its ability to absorb nutrients. Intestinal villi are the main tissues for nutrient absorption. The increase in the height of intestinal villi indicates an increase in the number of mature cells and the surface area of intestinal nutrition absorption. At the same time, the ability to absorb nutrients is enhanced. The crypt depth reflects the maturation rate of the cell, and the shallower crypt indicates that the maturation rate of the cell is increased and the secretory function is enhanced. The ratio of villus height to crypt depth reflects the morphological structure and functional state of the intestine, and a larger ratio indicates that the intestinal absorption function is better. Meanwhile, the decrease of villus height, the increase of crypt depth and the decrease of villus height/ crypt depth indicate the need for more cell proliferation to maintain the integrity of the intestinal barrier (Awad et al., 2011; Uni et al., 1998). The excessive proliferation of Cp in the intestine could cause intestinal impair, increase intestinal permeability and impair mucosal barrier function (Zhang et al., 2019). In our study, Cp challenge significantly decreased jejunal villus height and villus height/crypt depth, which was consistent with the results of intestinal lesion score. These results indicated that the integrity of intestinal barrier was impaired by Cp challenge, which might be one of the related reasons for the decreasing growth performance. The dietary GOD significantly increased the villus height and the ratio of villus height to crypt depth, indicating that GOD could improve the morphology and structure of the intestine, which is consistent with the results of previous study (Zhao et al., 2009). This improvement effects of GOD may be due to the probiotic effects of gluconic acid produced by catalytic reaction (Femia et al., 2002), and finally production of butyric acid. Butyric acid could provide energy for intestinal epithelial cells to improve intestinal morphology, or increase the abundance of intestinal beneficial microbiota through oxygen consumption and acid production, including some butyric acid-producing bacteria such as F.prausnitzii (Wu et al., 2019b) to improve intestinal morphology. In this study, GOD played a certain role in improving intestinal injury, reducing ileal pH, but not significantly improving growth performance. After that, it may be necessary to adjust the rearing conditions, the amount of enzyme activity and other factors in order to explore the effects of GOD on NE.
The selective permeability of intestinal epithelium is realized through the tight junction structure (Suzuki, 2013). The destruction of intestinal tight junction will increase the infiltration of macromolecules and harmful molecules in the intestinal lumen, thus causing increased intestinal mucosal immune response and causing inflammation and triggering the occurrence of intestinal and even systemic diseases (Suzuki, 2013). The intercellular tight junction structure is an important structural basis of physical barrier. Occludin is one of the components of intestinal tight junction protein and previous studies have shown that Occludin protein plays an essential role in tight junction of intestinal epithelium (Suzuki, 2013), whose loss will lead to the increase of paracellular permeability to macromolecules (Al-Sadi et al., 2011). Claudin-1 is necessary in barrier formation and pericellular selectivity (Furuse et al., 2002). Zonula occludens-1 (ZO-1) and the other ZO proteins provide intracelluar scaffold for intestinal tight junction proteins, which are necessary to regulate and maintain intestinal tight junction structure, and play a role in tight junction assembly and regulation (Fanning et al., 2002). The Mucin-2 mucin forms the skeleton of the intestinal mucus and covers and protects the intestinal tract from self-digestion and numerous microorganisms. The secretion, composition, and gene expression of mucus would be affected by intestinal microbiota and host inflammatory mediators (Johansson et al., 2008; Wei et al., 2012). In the present study, the supplementation of GOD significantly increased the mRNA expression levels of Occludin and ZO-1, and had a tendency to increase the mRNA expression levels of Claudin-1, indicating that GOD was beneficial to improvement if intestinal tight junction. Related studies (Zhang et al., 2017; Fasina and Lillehoj, 2019) have shown that Cp challenge can damage the integrity of intestinal barrier and induce cytokine response to upregulate the expression of proinflammatory cytokines such as TNF-α and IFN-γ. IFN-γ and IL-4 are characteristic cytokines of Th1 and Th2 cell subsets, respectively. The IFN-γ mRNA expression levels significantly increased in the Cp challenge groups, and the IL-4 had a numerical increase, which means the Cp challenge induced the immuno-homeostasis imbalance and the Th cells developments into proinflammatory response. Although the supplementation of GOD had no significant effect on cytokines, it improved intestinal barrier function mainly by promoting the integrity of intestinal morphology and the maintenance of tight junction structure. However, the Mucin-2 showed a decreased tendency, which may be related to the change of microbiota.
The GOD plays its prebiotic role mainly by regulating intestinal microbiota. In our study, dietary GOD had a tendency to increase the microbial diversity of ileum. Comparing the differences in the composition and structure of ileal microbiota, it was found that there was significant isolation of intestinal microbiota between GOD group and CON group, and there was a trend of separation between Cp + GOD group and GOD group. In our study, the Cp challenge significantly decreased the relative abundance of phylum Cyanobacteria, which is a group of bacteria known for the secretion of H2S (Chi et al., 2020). A related study showed that H2S could reduce disulfide bonds in the mucus layer of the gut epithelium, disrupt its barrier function and potentially play a role in inflammatory bowel disease (Ijssennagger et al., 2016). The Cp challenge decreased the relative abundance of genus Lactobacillus, and increased the relative abundance of Romboutsia and Veillonella. Lactobacillus is a beneficial commensal for humans and animals, which could inhibit pathogenic bacteria and is highly related to host feed efficiency (Kobierecka et al., 2017; Yan et al., 2017). Romboutsia is a short-chain fatty acid-producing bacterium, positively correlated with indicators of body weight and serum lipids. Previous study also showed that Romboutsia was positive related to FCR of laying hens (Gan et al., 2020). Veillonella was a potential pathobionts, which was associated with autoimmune hepatitis and oral diseases and already proved by previous studies (Luo et al., 2020; Wei et al., 2020). So, the Cp challenge led to the decrease of beneficial bacteria and the increase of potential pathogenic bacteria, which may also lead to a possible reduction in feed utilization efficiency. The abundance of the phylum Epsilonbacteraeota and Tenericutes increased in the dietary GOD groups, and a similar effect was found when Bacillus sp. was added to the chick diet (Yang et al., 2020). The dietary GOD decreased the abundance of the phylum Firmicutes and the genus Streptococcus. Streptococcus, which belongs to the phylum Firmicutes, represents common inhabitants of the intestinal tract and mucous of animals and humans with a potential for opportunistic infections. Turkey poults, broiler chickens, and ducklings were the most-commonly affected species with streptococcus (Crispo et al., 2018). Helicobacter pylori is the most common pathogen in the Helicobacter genus, which could cause peptic ulcer disease (Mladenova-Hristova et al., 2017). Helicobacter pylori is a microaerophile bacteria. The increase of Helicobacter in the GOD supplementation groups may be related to the consumption of oxygen by the catalytic reaction of GOD, and provide an explanation for the decrease of Mucin-2. This suggested that GOD did play a role in changing the structure of intestinal microbiota, but deoxygenation may lead to an increase in anaerobic pathogens. Further exploration is needed to maximize the prebiotic role of GOD.
CONCLUSIONS
In conclusion, Clostridium perfringens infection caused the injury of intestinal morphology and intestinal barrier and immuno-homeostasis imbalance, resulting in the decline in growth performance. The supplementation of GOD improved intestinal morphology and barrier function and optimized ileal microbiota by reducing intestinal pH and increased the expressions of tight junction protein, but had no significant effect on the growth performance of chickens regardless of Cp challenge. Although dietary GOD could improve intestinal health, the effects of GOD on NE broilers and its application in actual production need to be further confirmed.
ACKNOWLEDGMENTS
This study was supported by National Key R&D Program of China (2017YFE0129900).
Animal welfare statement: All the experimental procedures and sample collection methods in this study were approved by the Animal Care and Use Committee of China Agricultural University.
DISCLOSURES
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
REFERENCES
- Al-Sadi R., Khatib K., Guo S.H., Ye D.M., Youssef M., Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol.-Gastroint. Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Bohm J. Evaluation of the chicory inulin efficacy on ameliorating the intestinal morphology and modulating the intestinal electrophysiological properties in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2011;95:65–72. doi: 10.1111/j.1439-0396.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- Bankar S.B., Bule M.V., Singhal R.S., Ananthanarayan L. Glucose oxidase–an overview. Biotechnol. Adv. 2009;27:489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Bolyen E., J. R. Rideout, M. R. Dillon, N. A. Bokulich, C. C. Abnet, G. A. Al-Ghalith, H. Alexander, E. J. Alm, M. Arumugam, F. Asnicar, Y. Bai, J. E. Bisanz, K. Bittinger, A. Brejnrod, C. J. Brislawn, C. T. Brown, B. J. Callahan, A. M. Caraballo-Rodríguez, J. Chase, E. K. Cope, R. Da Silva, C. Diener, P. C. Dorrestein, G. M. Douglas, D. M. Durall, C. Duvallet, C. F. Edwardson, M. Ernst, M. Estaki, J. Fouquier, J. M. Gauglitz, S. M. Gibbons, D. L. Gibson, A. Gonzalez, K. Gorlick, J. Guo, B. Hillmann, S. Holmes, H. Holste, C. Huttenhower, G. A. Huttley, S. Janssen, A. K. Jarmusch, L. Jiang, B. D. Kaehler, K. B. Kang, C. R. Keefe, P. Keim, S. T. Kelley, D. Knights, I. Koester, T. Kosciolek, J. Kreps, M. G. I. Langille, J. Lee, R. Ley, Y. X. Liu, E. Loftfield, C. Lozupone, M. Maher, C. Marotz, B. D. Martin, D. Mcdonald, L. J. Mciver, A. V. Melnik, J. L. Metcalf, S. C. Morgan, J. T. Morton, A. T. Naimey, J. A. Navas-Molina, L. F. Nothias, S. B. Orchanian, T. Pearson, S. L. Peoples, D. Petras, M. L. Preuss, E. Pruesse, L. B. Rasmussen, A. Rivers, M. S. Robeson, 2nd, P. Rosenthal, N. Segata, M. Shaffer, A. Shiffer, R. Sinha, S. J. Song, J. R. Spear, A. D. Swafford, L. R. Thompson, P. J. Torres, P. Trinh, A. Tripathi, P. J. Turnbaugh, S. Ul-Hasan, J. J. J. Van Der Hooft, F. Vargas, Y. Vázquez-Baeza, E. Vogtmann, M. Von Hippel, W. Walters, Y. Wan, M. Wang, J. Warren, K. C. Weber, C. H. D. Williamson, A. D. Willis, Z. Z. Xu, J. R. Zaneveld, Y. Zhang, Q. Zhu, R. Knight and J. G. Caporaso. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature biotechnology. 37:852–857.
- Chi L.D., Khan I., Lin Z.B., Zhang J.W., Lee M.Y.S., Leong W., Hsiao W.L.W., Zheng Y. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine. 2020;67:12. doi: 10.1016/j.phymed.2019.153157. [DOI] [PubMed] [Google Scholar]
- Crispo M., Shivaprasad H.L., Cooper G.L., Bickford A.A., Stoute S.T. Streptococcosis in commercial and noncommercial avian species in california: 95 Cases (2000-2017) Avian Dis. 2018;62:152–162. doi: 10.1637/11765-103117-Reg.1. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Hoehler D., Wilkie D.C., Van Kessel A.G., Drew M.D. Dietary glycine concentration affects intestinal Clostridium perfringens and lactobacilli populations in broiler chickens. Poult. Sci. 2005;84:1875–1885. doi: 10.1093/ps/84.12.1875. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A.S., Ma T.Y., Anderson J.M. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femia A.P., Luceri C., Dolara P., Giannini A., Biggeri A., Salvadori M., Clune Y., Collins K.J., Paglierani M., Caderni G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–1960. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- Feng Y.N., Gong J., Yu H., Jin Y.P., Zhu J., Han Y.M. Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Vet. Microbiol. 2010;140:116–121. doi: 10.1016/j.vetmic.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Furuse M., Hata M., Furuse K., Yoshida Y., Haratake A., Sugitani Y., Noda T., Kubo A., Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Zhao Y., Mahmood T., Guo Y. Effects of dietary vitamins supplementation level on the production performance and intestinal microbiota of aged laying hens. Poult. Sci. 2020;99:3594–3605. doi: 10.1016/j.psj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., Lopez-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M.A., Tellez-Isaias G., Latorre J.D. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions, and ileal microbiota in broiler chickens using a laboratory challenge model. Front. Vet. Sci. 2019;6:11. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Peng X.Y., Gao B., Wei Q.L., Xiang R., Yuan M.G., Xu Z.H. The effect of Clostridium butyricum on gut microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front. Microbiol. 2019;10:14. doi: 10.3389/fmicb.2019.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijssennagger N., van der Meer R., van Mil S.W.C. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol. Med. 2016;22:190–199. doi: 10.1016/j.molmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Johansson M.E.V., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldhusdal M., Benestad S.L., Lovland A. Epidemiologic aspects of necrotic enteritis in broiler chickens - disease occurrence and production performance. Avian Pathol. 2016;45:271–274. doi: 10.1080/03079457.2016.1163521. [DOI] [PubMed] [Google Scholar]
- Kobierecka P.A., Wyszyńska A.K., Aleksandrzak-Piekarczyk T., Kuczkowski M., Tuzimek A., Piotrowska W., Górecki A., Adamska I., Wieliczko A., Bardowski J., Jagusztyn-Krynicka E.K. In vitro characteristics of Lactobacillus spp. strains isolated from the chicken digestive tract and their role in the inhibition of Campylobacter colonization. Microbiol. Open. 2017;6:e512–e527. doi: 10.1002/mbo3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo Y.M., Wang Z., Yuan J.M. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010;39:17–24. doi: 10.1080/03079450903447404. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Y.X., Sun M.L., Shi P.L., Liu P., Chen Y.Y., Peng X. [Research progress in the relationship between Veillonella and oral diseases] West China J. Stomatol. 2020;38:576–582. doi: 10.7518/hxkq.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenova-Hristova I., Grekova O., Patel A. Zoonotic potential of Helicobacter spp. J. Microbiol. Immunol. Infect. 2017;50:265–269. doi: 10.1016/j.jmii.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Peng L.Y., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafacz-Livingston K.A., Parsons C.M., Jungk R.A. The effects of various organic acids on phytate phosphorus utilization in chicks. Poult. Sci. 2005;84:1356–1362. doi: 10.1093/ps/84.9.1356. [DOI] [PubMed] [Google Scholar]
- Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Stringfellow K., McReynolds J., Lee J., Byrd J., Nisbet D., Farnell M. Effect of bismuth citrate, lactose, and organic acid on necrotic enteritis in broilers. Poult. Sci. 2009;88:2280–2284. doi: 10.3382/ps.2008-00456. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T., Koyama H., Okada M., Ushida K. Stimulation of butyrate production by gluconic acid in batch culture of pig cecal digesta and identification of butyrate-producing bacteria. J. Nutr. 2002;132:2229–2234. doi: 10.1093/jn/132.8.2229. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ganot S., Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Lyhs U., Pedersen K., Prescott J.F. Recent breakthroughs have unveiled the many knowledge gaps in Clostridium perfringens-associated necrotic enteritis in chickens: the first International Conference on Necrotic Enteritis in Poultry. Avian Pathol. 2016;45:269–270. doi: 10.1080/03079457.2016.1166857. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Wang Y.B., Xu H., Mei X.Q., Gong L., Wang B.K., Li W.F., Jiang S.Q. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poult. Sci. 2018;97:3540–3549. doi: 10.3382/ps/pey216. [DOI] [PubMed] [Google Scholar]
- Wei Y., Li Y., Yan L., Sun C., Miao Q., Wang Q., Xiao X., Lian M., Li B., Chen Y., Zhang J., Li Y., Huang B., Li Y., Cao Q., Fan Z., Chen X., Fang J.Y., Gershwin M.E., Tang R., Ma X. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569–577. doi: 10.1136/gutjnl-2018-317836. [DOI] [PubMed] [Google Scholar]
- Wei X.C., Yang Z., Rey F.E., Ridaura V.K., Davidson N.O., Gordon J.I., Semenkovich C.F. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe. 2012;11:140–152. doi: 10.1016/j.chom.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttila T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S., Wohlfahrt G., Schomburg D., Hecht H.J., Kalisz H.M. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-D-glucose. Biochem. J. 2000;347:553–559. doi: 10.1042/0264-6021:3470553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Li T., Niu H., Zhu Y., Liu Y., Duan Y., Sun Q., Yang X. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult. Sci. 2019;98:828–841. doi: 10.3382/ps/pey393. [DOI] [PubMed] [Google Scholar]
- Wu S.R., Li T.H., Niu H.F., Zhu Y.F., Liu Y.L., Duan Y.L., Sun Q.Z., Yang X.J. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult. Sci. 2019;98:828–841. doi: 10.3382/ps/pey393. [DOI] [PubMed] [Google Scholar]
- Xue G.D., Wu S.B., Choct M., Pastor A., Steiner T., Swick R.A. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017;96:3581–3585. doi: 10.3382/ps/pex164. [DOI] [PubMed] [Google Scholar]
- Xue G.D., Wu S.B., Choct M., Swick R.A. Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Anim. Nutr. 2017;3:399–405. doi: 10.1016/j.aninu.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Sun C., Yuan J., Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017;7:45308. doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Huang K., Wang J., Wu D., Liu Z., Yu P., Wei Z., Chen F. Combined use of Bacillus subtilis yb-114,246 and Bacillus licheniformis yb-214,245 improves body growth performance of Chinese Huainan Partridge Shank Chickens by enhancing intestinal digestive profiles. Probio. Antimicrob. Proteins. 2020;13:327–342. doi: 10.1007/s12602-020-09691-2. [DOI] [PubMed] [Google Scholar]
- Yin D.F., Du E.C., Yuan J.M., Gao J.X., Wang Y.L., Aggrey S.E., Guo Y.M. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017;7:11. doi: 10.1038/s41598-017-07420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.B., Gan L.P., Shahid M.S., Lv Z.P., Fan H., Liu D., Guo Y.M. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J. Anim. Sci. Biotechnol. 2019;10:14. doi: 10.1186/s40104-019-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.B., Lv Z.P., Li H.X., Guo S.S., Liu D., Guo Y.M. Dietary L-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Br. J. Nutr. 2017;118:321–332. doi: 10.1017/S0007114517002094. [DOI] [PubMed] [Google Scholar]
- Zhao G.X., Song H.B., Ma K.W., Zhang Z.H., Ji C., Ma Q.G. Effects of glucose oxidase on intestinal tract pH and cecum microorganism in broiler chickens. J. Hebei Agric. Univ. 2009;32:83–87. [Google Scholar]