Abstract
With increasing societal development and the concurrent improvement in people's quality of life, meat consumption has gradually changed from a focus on “quantity” to “quality”. Broiler production is increasingly used as a means to improve meat quality by altering various characteristics, especially its genetic factors. However, until now, little has been known about the genetic variants related to meat quality traits in Chinese purebred chicken populations. To better understand these genetic underpinnings, a total of 17 traits related to meat quality and carcass were measured in 325 Chinese Ningdu yellow chickens. We performed DNA sequencing to detect nucleotide mutations, after which we conducted association studies between PHKG1 gene polymorphisms and traits related to meat quality and carcass. Results indicated a large phenotypic variation in meat quality traits. More specifically, the single nucleotide polymorphism (SNP) rs15845448 was significantly associated with drip loss at 24 h (P = 8.04 × 10−6) and 48 h (P = 5.47 × 10−6), pH (P = 2.39 × 10−3), and meat color L* (P = 9.88 × 10−3). Moreover, the SNP rs15845448 reduced 24 h and 48 h drip loss by 3.62 and 5.97%, respectively. However, no significant associations were found between rs15845448 and carcass traits (P > 0.05). Furthermore, a haplotype block containing 2 adjacent SNPs (rs15845448 and rs15845450) was identified. This block displayed 4 distinct haplotypes that had significant association with drip loss at 24 h and 48 h, pH, and meat color L*. Collectively, these results provide new insights into the genetic basis of meat quality in Chinese Ningdu yellow chickens. Moreover, the significance of SNP rs15845448 could be incorporated into the selection programs involving this breed.
Key words: PHKG1 gene, SNP rs15845448, meat quality traits, carcass traits, Ningdu yellow chickens
INTRODUCTION
With societal development and the concurrent improvement in quality of life, meat consumption has begun to be focused not only on quantity, but also on quality. Major meat quality traits of interest include pH, drip loss, meat color, and shear force; collectively, these have become common selection criteria in chicken breeding programs (Allen et al., 1998). However, it is difficult to improve meat quality using traditional breeding methods, since measurements are time-consuming, expensive, and require slaughtering of the animals. This dramatically increases the generation interval and slows overall genetic progress (Magalhaes et al., 2019). Despite this, heritability estimates of various parameters like meat quality traits (0.35–0.81), decline in postmortem pH (0.35–0.49), lightness (0.50–0.75), and drip loss (0.39) indicate that genetic selection is the best tool for improving broiler meat quality (Mir et al., 2017). Therefore, gaining a better understanding of the genetic and molecular background for traits related to meat quality in chickens is very important.
Meat quality traits have important economic value and are considered to be complex quantitative traits controlled by multiple genes (Cahyadi et al., 2014). For example, many previous studies have shown that the fatty acid binding protein (FABP) gene plays an important role in improving overall meat quality (Cho et al., 2011). The CAPN1 gene was significantly associated with tenderness and other meat quality traits, where animals inheriting the AA genotype had smaller shear force values, lower water loss rates, and higher intramuscular fat contents (Shu et al., 2015). The single nucleotide polymorphism (SNP) V315M in PRKAG3 gene was shown to be significantly associated with drip loss and cooking rate of the meat (Yang et al., 2016). However, to the best of our knowledge, no causative gene(s) and/or mutation(s) associated with drip loss, pH, and/or meat color L* in chicken have been reported.
PHKG1 (Phosphorylase Kinase Catalytic Subunit Gamma 1) is a member of the Ser/Thr protein kinase family and encodes a protein with 1 protein kinase domain and 2 calmodulin-binding domains. This protein is the catalytic member of a 16-subunit protein kinase complex which contains equimolar ratios of 4 subunit types (Ma et al., 2014). Diseases associated with PHKG1 include glycogen storage disease (Kishnani et al., 2019). Several studies have also demonstrated that PHKG1 is a strong candidate gene for meat quality traits in pigs (Liu et al., 2019; Liu et al., 2020) and residual feed intake and feed conversion ratio in chickens (Shah et al., 2016; Liu et al., 2018). Furthermore, a previous study demonstrated that the PHKG1 gene is a causative gene for meat quality traits in White Duroc × Erhualian F2 intercross and Sutai pigs, which could lead to a less active Phosphorylase Kinase (PhK) protein (Ma et al., 2014). Zappaterra et al. (2019) validated these results and found that the PHKG1 gene could significantly influence the meat color and water-holding capacity in Italian heavy pigs. However, there have been no studies on the effect of PHKG1 gene polymorphisms on traits related to meat quality and carcass in Chinese Ningdu yellow chickens.
Ningdu yellow chicken is a local Chinese breed with early maturation, excellent meat quality, high nutritional value, and population-wide genetic performance, and has enjoyed a good culinary reputation for long time. Here, we assessed SNPs in the chicken PHKG1 gene and analyzed the associations between the PHKG1 gene polymorphisms and meat quality and carcass traits in Chinese Ningdu yellow chickens. It is hoped this work can provide a theoretical reference for molecule-assisted breeding of desirable chickens.
MATERIALS AND METHODS
Ethics Statement
All procedures involving animals followed the guidelines for the care and use of experimental animals as approved by the State Council of the People's Republic of China. This study was approved by the ethics committee of Nanchang Normal University.
Experimental Population
The Ningdu yellow population used in this study comprised 325 male chickens provided by Jiangxi Nanshi Science and Technology Co., Ltd (Nanchang, China). Chickens were born and raised for 1 d, and then randomly selected from the same batch and transferred to a farm in the city of Nanchang. They were fed with the same diet (Supplementary Table S1) under a standardized feeding and management regimen, and given ad libitum access to water. At the age of 16 wk, a total of 325 individuals were slaughtered at a commercial abattoir in Nanchang.
Phenotype Measurement
Thirty minutes postmortem, the left side of each breast muscle was sampled to measure meat quality traits. Here, we analyzed data regarding several meat quality traits including pH, drip loss at 24 h and 48 h, L* for lightness (ColorM_L), a* for redness (ColorM_a), b* for yellowness (ColorM_b), and shear force. Muscle pH was measured at 30 min postmortem using a Delta 320 pH Meter (Shanghai, China). Each sample was measured 3 times and the average was then used for subsequent analysis. Muscle color measurements were taken 30 min postmortem on the exposed cut surface of the breast muscle with a CM-2600d/2500d Minolta Chroma Meter. Each sample was measured 3 times and the average was used for subsequent analysis. The shear force was measured by the People's Republic of China Agricultural Industry Standard NY/T 1180-2006 ‘Determination of Meat Tenderness-Shearing Force Determination Method’. The final value used for analysis was the average of 3 shear force measurements. Drip loss (hydraulic) was deterred by weighing a meat cuboid (W1) with length × width × thickness (the measurements here were 55 mm × 50 mm × 15 mm). One end of the meat sample was then bound with a thin thread to pull the muscle fiber downward, sealed into an inflatable plastic bag, and hung in the refrigerator at 4°C for 24 h. After 24 h, the meat sample was weighed (W2) and the 24 h drip loss was calculated as follows: (%) = (W1 − W2)/W1 × 100%. The samples was stored again in the refrigerator under the same conditions for 48 h, after which the meat sample was weighed again (W3) and the drip loss was calculated as follows: (%) = (W1 − W3)/W1 × 100%.
Ten carcass traits were measured using ‘The Poultry Production Performance Terms and Measurement Statistics Method (NY/T823-2004)’, including body length, live weight, carcass weight, carcass rate, semi-eviscerated weight, semi-eviscerated rate, eviscerated weight, eviscerated rate, breast muscle weight, and breast muscle rate.
DNA Extraction
Genomic DNA was isolated from blood tissue of each sample using a standard phenol/chloroform method. DNA quality and concentration were determined using a Nanodrop-2000 spectrophotometer (Thermo Fisher Scientific, MA). All DNA samples were qualified and standardized into a final concentration of 50 ng/μL.
Primer Design, Polymerase Chain Reaction Amplification, and Sequencing
Primers (Supplementary Table S2) were designed using Primer 5.0 software based on chicken PHKG1 gene sequences and synthesized by GENERAY Biotech Co., Ltd (Shanghai, China).
Polymerase chain reaction (PCR) was performed in a 30 μL reaction mixture consisting of 1 μL of DNA, 1 μL of forward and reverse primers (10 μM), 15 μL of 2 × Taq PCR MasterMix, and 12 μL ddH2O. All amplifications included an initial denaturing step of 3 min at 95°C, followed by 35 cycles of 20 s at 94°C, 20 s at an optimized annealing temperature and 40 s at 72°C, with a final extension of 5 min at 72°C. The PCR products were sent to the GENERAY Biotech Co., Ltd (Shanghai, China) for sequencing. The ABI 3730xl DNA Sequencer was used for sequencing by the Sanger method. Long fragments were sequenced by bidirectional sequencing and then assembled using DNAStar software.
Extraction of Total RNA and Quantitative Reverse Transcription PCR Analysis
Breast muscle samples were harvested from Chinese Ningdu yellow chickens within 30 min of slaughter. A total of 12 samples (6 CC and 6 AA for SNP rs15845448) were used for quantitative reverse transcription (qRT)–PCR analysis. Total RNA was extracted from breast muscles using TRIzol (Invitrogen) by following the manufacturer's instruction. The residual DNA was eliminated from total RNA using RNase-free DNase I (New England Biolabs, UK). The quality of total RNA was assessed using a Nanodrop-2000 spectrophotometer (Thermo Fisher Scientific) and 1.0% agarose gel electrophoresis. cDNA was synthesized from 1 μg of total RNA using a PrimerScript RT reagent Kit (Takara, Japan) by following the standard manual. qRT-PCR was performed in a 10 μL reaction system containing 1 μL of 2.5-fold diluted cDNA, 5 μL of Power SYBR Green PCR Master Mix (Applied Biosystems), 2 pM of each forward and reverse primer, and 3.6 μL water. PCR was conducted on a Bio-Rad CFX96 instrument (Bio-Rad) under the following cycling conditions: 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 50 s. GAPDH was used as the endogenous control. The quantification of gene expression levels was performed using the comparative Ct (2−ΔΔCt) method. All assays were performed in triplicate and the average values were calculated.
Statistical Analysis
SNPs were identified by comparative analysis of the complete PHKG1 sequence using Seqman software. Genotypic and allelic frequencies for each SNP were calculated using Microsoft Excel (Microsoft Corporation, Redmond, WA). Association analysis between the SNPs and phenotypic traits was performed using the general linear model procedure in Plink v 1.07 (Purcell et al., 2007). Haplotype or linkage disequilibrium (LD) block analysis was performed for the three SNPs. The LD blocks were determined using Haploview version 4.2 software with default settings (Barrett et al., 2005). A haplotype association study (Druet and Farnir, 2011) was performed to identify genomic regions associated with the pH, ColorM_L, and drip loss at both 24 h and 48 h. Haplotypes corresponding to a predetermined number (K = 20) of hidden haplotype states (Sartelet et al., 2012) were conducted with a hidden Markov model via PHASEBOOK (Druet and Georges, 2010).
RESULTS
Summary Statistics for Meat Quality and Carcass Traits
In this study, 325 Ningdu yellow chickens were phenotyped across 7 meat quality traits and 10 carcass traits. Summary statistic results for the 17 traits are shown in Table 1. Results indicated that most of the tested meat quality traits had high coefficients of variation. The ColorM_a and drip loss at 24 h and 48 h had a coefficient of variation of more than 90%. However, carcass traits had relatively lower coefficients of variation, with the highest value of 24.67% for breast muscle weight.
Table 1.
Summary statistics of meat quality traits of breast muscle and carcass traits from Chinese Ningdu yellow chicken population.
| Traits | Mean | S.D.1 | Min. | Max. | C.V2 (%) |
|---|---|---|---|---|---|
| Meat quality | |||||
| pH | 6.26 | 0.24 | 5.57 | 7.05 | 3.78 |
| ColorM_L | 69.22 | 5.51 | 53.33 | 81.06 | 7.96 |
| ColorM_a | 1.05 | 0.95 | -2.00 | 6.96 | 90.41 |
| ColorM_b | 6.11 | 2.72 | 0.22 | 13.09 | 44.56 |
| Drip loss at 24 h (%) | 7.46 | 7.01 | 0.62 | 31.86 | 93.99 |
| Drip loss at 48 h (%) | 13.01 | 11.81 | 1.25 | 46.67 | 90.74 |
| Shear force | 4.51 | 1.14 | 0.94 | 8.91 | 25.33 |
| Carcass traits | |||||
| Live weight | 937.47 | 115.76 | 602.30 | 1234.60 | 12.35 |
| Carcass weight | 784.62 | 113.65 | 454.00 | 1070.40 | 14.48 |
| Carcass rate (%) | 83.74 | 6.52 | 57.62 | 92.39 | 7.78 |
| Semi-eviscerated weight | 691.86 | 99.40 | 398.40 | 971.70 | 14.37 |
| Semi-eviscerated rate (%) | 73.85 | 6.48 | 50.61 | 84.89 | 8.77 |
| Eviscerated weight | 544.26 | 83.18 | 309.20 | 775.00 | 15.28 |
| Eviscerated rate (%) | 58.03 | 5.55 | 39.07 | 66.28 | 9.56 |
| Breast muscle weight | 60.75 | 14.99 | 20.80 | 104.60 | 24.67 |
| Breast muscle rate (%) | 6.47 | 1.42 | 2.64 | 10.20 | 21.88 |
| Body length | 17.82 | 1.00 | 15.00 | 23.10 | 5.60 |
Standard deviation.
Coefficient of variation.
Correlation Analysis for Meat Quality and Carcass Traits
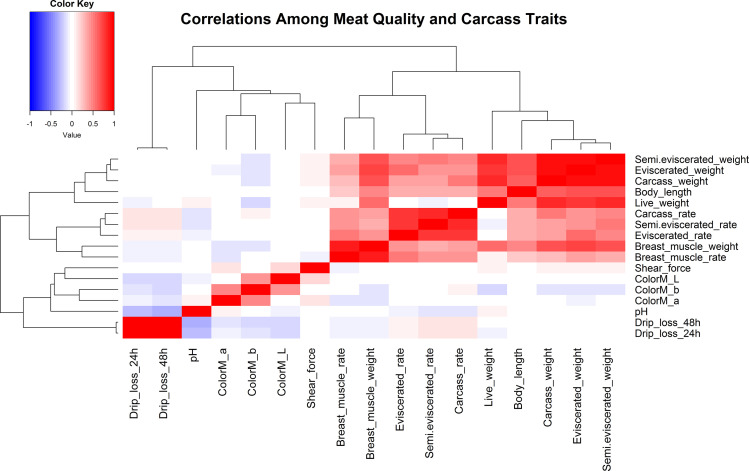
Correlational analyses were conducted among 17 traits for meat and carcass quality (Figure 1, Supplementary Tables S3 and S4). Results indicated that most meat quality traits had low correlations. More specifically, pH was significantly and negatively correlated with drip loss at 24 h and 48 h (r values were −0.27 and −0.30, respectively; P < 0.001). ColorM_b was significantly and positively correlated with both ColorM_L and ColorM_a (r values were 0.41 and 0.46, respectively; P < 0.001).
Figure 1.
Correlation analysis among 17 traits related to meat and carcass quality.
Comparatively, most carcass traits had high correlations. The body length, carcass weight, semi-eviscerated weight, eviscerated weight, and breast muscle weight were all significantly, positively correlated with other carcass traits. For example, eviscerated weight was significantly, positively correlated with all other carcass traits, especially for carcass weight and semi-eviscerated weight (r values were 0.92 and 0.95, respectively; P < 0.001).
Sequencing of Variants in PHKG1 Gene and Association Analysis Between SNPs and 17 Selected Traits
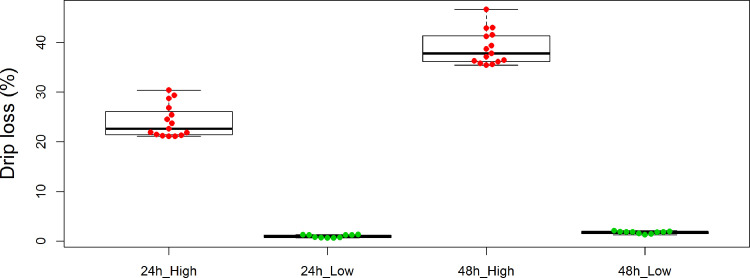
We chose 25 Ningdu yellow chickens for whole-resequencing of the PHKG1 gene, including 15 chickens with high drip loss values and 10 individuals with low drip loss values (Figure 2). We used 7 primers to sequence the whole PHKG1 gene and identified a total of 15 polymorphisms (13 SNPs and 2 indels) in the 25 Ningdu yellow chickens. However, only 1 SNP (rs15845448) of these 15 mutations was concordant with the phenotype values in the aforementioned 25 Ningdu yellow chickens. The SNP rs15845448 was detected in the 3’UTR of PHKG1 gene (position: chr19, 5047116 bp). Two other SNPs (rs15047594 and rs15845450) were also identified using the same primer (Primer 1, FP: GACGGACGCCAGCACGGTCAGACAGAT, RP: ACAGCGAGGGAGGCATTGGCACA).
Figure 2.
The phenotype of high and low drip loss values for 25 Ningdu yellow chickens. The red dot marks 15 chickens with high drip loss values (24 h_High and 48 h_High). The green dot marks 10 individuals with low drip loss values (24 h_Low and 48 h_Low).
We next sought to further analyze the associations between the 3 SNPs (rs15845448, rs15047594, and rs15845450) and measured traits. We sequenced all 325 Ningdu yellow chickens using primer 1 and found that the SNPs rs15845448 and rs15845450 had 3e genotypes. For SNP rs15845448, the heterozygous CA (0.67) genotype had the highest frequency, whereas the homozygous CC (0.12) and AA (0.21) genotype showed relatively low frequencies. The frequencies of AA, AG, and GG in rs15845450 were 0.21, 0.52, and 0.27, respectively. However, the SNP rs15047594 only had 2 genotypes—AA and GA. Finally, the AA genotype frequency (0.90) was higher than that of GA (0.10).
The association analysis between the 3 SNPs and 17 traits assessing meat and carcass quality was then conducted. The results showed that the most significant SNP rs15845448 was significantly associated with drip loss at 24 h (P = 8.04e-6) and 48 h (P = 5.47e-6), pH (P = 2.39e-3), and ColorM_L (P = 9.88e-3). The effects of the SNP rs15845448 on meat quality and carcass traits are illustrated in Table 2. The SNP rs15845448 reduced 48 h and 24 h drip loss by 5.97 and 3.62%, respectively. Comparatively, pH and ColorM_L were improved by 0.09 and 1.45%, respectively. For carcass traits, the SNP rs15845448 was not significantly associated with any of the carcass traits (P > 0.05), but there was a trend for improving the carcass traits. For example, live weight and carcass weight were improved by 12 and 13 g, respectively. Besides the SNP rs15845448, the SNP rs15047594 was only significantly associated with ColorM_a (P = 0.04). The SNP rs15845450 was significantly associated with drip loss at 24 h (P = 1.92e-4) and 48 h (P = 1.63e-4), pH (P = 4.62e-3), and ColorM_L (P = 0.04).
Table 2.
The effects of the SNP rs15845448 on traits related to meat quality and carcass.
| Traits | CC1 (n = 39) | CA1 (n = 217) | AA1 (n = 69) | Effects2 | P value |
|---|---|---|---|---|---|
| Meat quality | |||||
| Drip loss at 24 h (%) | 2.98 ± 0.24 | 7.41 ± 0.56 | 10.22 ± 0.95 | 3.62 | 8.04e-6 |
| Drip loss at 48 h (%) | 5.62 ± 0.89 | 12.94 ± 0.91 | 17.55 ± 1.54 | 5.97 | 5.47e-6 |
| Ph | 6.36 ± 0.02 | 6.26 ± 0.02 | 6.19 ± 0.03 | 0.09 | 2.39e-3 |
| ColorM_L | 70.90 ± 0.88 | 69.30 ± 0.38 | 68.00 ± 0.66 | 1.45 | 9.88e-3 |
| ColorM_a | 0.96 ± 0.25 | 0.99 ± 0.11 | 1.28 ± 0.19 | 0.16 | 0.23 |
| ColorM_b | 6.68 ± 0.44 | 6.03 ± 0.19 | 6.03 ± 0.33 | 0.33 | 0.32 |
| Shear force | 4.24 ± 0.18 | 4.55 ± 0.08 | 4.54 ± 0.14 | 0.15 | 0.29 |
| Carcass traits | |||||
| Live weight | 947.00 ± 19.08 | 940.00 ± 7.97 | 923.00 ± 14.18 | 12.00 | 0.25 |
| Carcass weight | 800.00 ± 18.73 | 785.00 ± 7.83 | 774.00 ± 13.92 | 13.00 | 0.25 |
| Carcass rate (%) | 84.40 ± 1.07 | 83.50 ± 0.45 | 83.90 ± 0.80 | 0.25 | 0.84 |
| Semi-eviscerated weight | 704.00 ± 16.38 | 693.00 ± 6.88 | 681.00 ± 12.27 | 11.50 | 0.24 |
| Semi-eviscerated rate (%) | 74.40 ± 1.07 | 73.70 ± 0.45 | 74.00 ± 0.80 | 0.20 | 0.91 |
| Eviscerated weight | 553.00 ± 13.71 | 546.00 ± 5.76 | 535.00 ± 10.27 | 9.00 | 0.26 |
| Eviscerated rate (%) | 58.40 ± 0.91 | 58.00 ± 0.38 | 58.10 ± 0.68 | 0.15 | 0.88 |
| Breast muscle weight | 62.90 ± 2.47 | 59.90 ± 1.04 | 62.40 ± 1.85 | 0.25 | 0.86 |
| Breast muscle rate (%) | 6.64 ± 0.23 | 6.34 ± 0.10 | 6.78 ± 0.17 | 0.07 | 0.32 |
| Body length | 17.90 ± 0.16 | 17.80 ± 0.09 | 17.70 ± 0.12 | 0.10 | 0.31 |
The phenotypic was calculated by lsmeans package in the R environment. All of the traits are shown as the least square (LS) mean ± standard error (SE) of each genotype.
The effects that allele increases phenotype value in Ningdu yellow chickens.
Haplotype Analyses
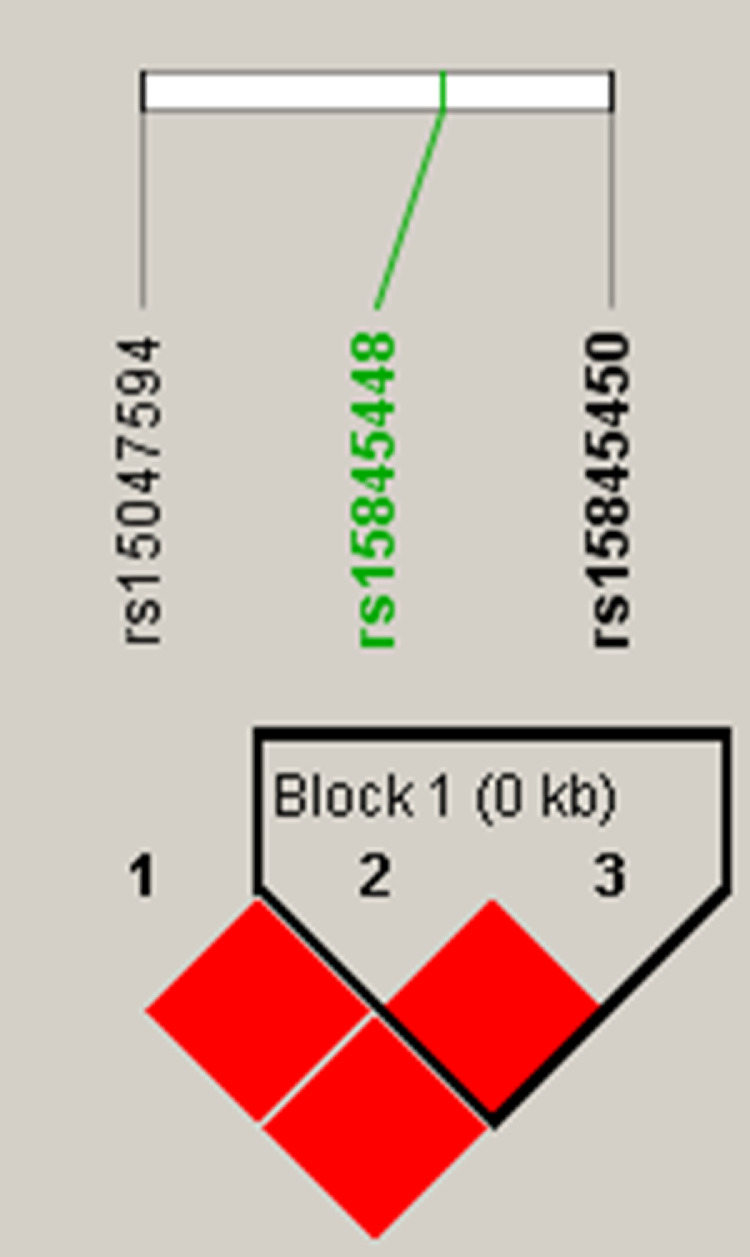
We performed haplotype analysis for the 3 SNPs and the results are shown in Figure 3 and Table 3. We identified one haplotype block in which SNPs were in high LD (r2 > 0.8). Block 1 contained two SNPs including the peak SNP rs15845448. In block 1, four distinct haplotypes (AA, AG, CA, and CG, referred to as Hap1, Hap2, Hap3, and Hap4, respectively) were identified with frequencies of 21, 33, 26, and 20%, respectively. Hap1, Hap2, and Hap4 were each significantly associated with drip loss at 24 h and 48 h (P < 0.05). Hap1 and Hap4 were each significantly associated with pH and ColorM_L (P < 0.05), while Hap3 displayed opposite effects on these traits (P > 0.05; Table 3).
Figure 3.
Haplotype structure of the three SNPs (rs15845448, rs15047594, and rs15845450) associated with drip loss at 24 h and 48 h, pH and ColorM_L. Solid lines mark the identified block.
Table 3.
Haplotype effects on pH, ColorM_L, and drip loss at 24 h and 48 h.
| pH |
ColorM_L |
Drip loss at 24 h (%) |
Drip loss at 48 h (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Haplotype1 | Frequencies | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value |
| Hap1:AA | 0.21 | 6.19 ± 0.02 | ⁎⁎ | 68.00 ± 0.47 | ⁎⁎ | 10.22 ± 0.68 | ⁎⁎⁎ | 17.55 ± 1.10 | ⁎⁎⁎ |
| Hap2:AG | 0.33 | 6.26 ± 0.02 | Ns | 69.30 ± 0.38 | Ns | 7.41 ± 0.56 | * | 12.94 ± 0.91 | ⁎⁎ |
| Hap3:CA | 0.26 | 6.25 ± 0.02 | Ns | 69.40 ± 0.43 | Ns | 7.41 ± 0.64 | Ns | 12.92 ± 1.03 | Ns |
| Hap4:CG | 0.20 | 6.33 ± 0.02 | ⁎⁎ | 70.20 ± 0.49 | ⁎⁎ | 4.55 ± 0.71 | ⁎⁎⁎ | 8.25 ± 1.15 | ⁎⁎⁎ |
Abbreviation: Ns, not significant.
P < 0.05.
P < 0.01.
P < 0.001.
The haplotypes correspond to haplotype block 1 shown in Figure 2.
Analysis of PHKG1 Gene Expression in Breast Muscle Tissue
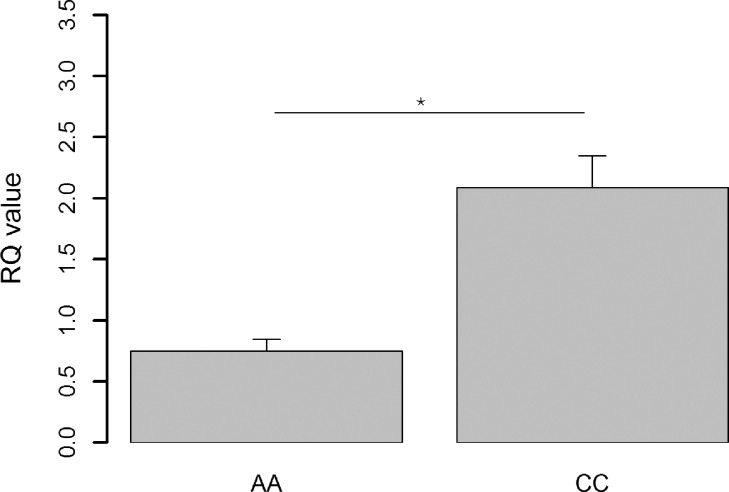
We next explored whether the mutation can lead to variation in the PHKG1 gene expression and related meat quality traits. We determined the mRNA expression level of PHKG1 gene in breast muscle samples of 12 Ningdu yellow chickens, and we found half with very high drip loss values were carriers of the AA genotype of the SNP rs15845448, and the other half with lower drip loss were carriers of the CC genotype. Interestingly, we found the PHKG1 gene expression was significantly different between the 2 phenotypic/genotype groups (P < 0.05; Figure 4).
Figure 4.
The PHKG1 gene expression analysis in breast muscle tissue. Comparison of the expression levels of the PHKG1 gene in breast muscle between two groups (n = 6 per group) with CC and AA genotypes at the SNP rs15845448, which had low and high drip loss values, respectively.
DISCUSSION
The huge phenotypic variation and varied characteristics of the genomic structure of indigenous Chinese chickens allowed us to use them to identify causative mutations for more complex traits. In this study, we used outbreed Ningdu yellow chickens, which show huge phenotypic variation of ColorM_a as well as drip loss at 24 h and 48 h (>90%). These traits are highly varied within the population, which may be related to the isolation of causative gene and mutations controlling these traits. Given this, they should be the focus of future genetic breeding programs.
Drip loss is a growing problem in the meat industry and affects both meat yield and quality (Abdullah et al., 2014), which leads to a huge loss of nutrients, including free amino acids, B vitamins, minerals, and myoglobin (Jauregui et al., 1981). Drip loss is also directly related to the final meat pH. A drop in pH makes the protein molecule polypeptide chains closer and narrows the spaces in the protein's overall spatial structure. Ultimately, this forces water molecules out of the muscle, thereby increasing drip loss (Barbut, 1993; Allen et al., 1998). Furthermore, Xiong et al. (2015) found that pH was significantly and negatively correlated with drip loss at 24 h in Chinese Laiwu pigs. In our present study, results confirmed that pH was significantly and negatively correlated with drip loss. Collectively, this indicated that water drip loss can be effectively reduced by increasing pH, thus reducing the probability of more acidic in the breeding of Ningdu yellow chickens.
Although many QTL and candidate genes have been identified for chicken meat quality traits (Shu et al., 2015; Yang et al., 2016; Pampouille et al., 2018; Yu et al., 2021), no causative genes and/or mutations have been identified for these traits. In the present study, given the large phenotypic variation in drip loss and considering the PHKG1 gene might be significantly associated with drip loss (Ma et al., 2014; Zappaterra et al., 2019), we resequenced the PHKG1 gene and performed an association study of PHKG1 gene polymorphisms with meat quality and carcass traits in Chinese Ningdu yellow chickens. Interestingly, we found the most significant SNP rs15845448 was simultaneously associated with drip loss at 24 h and 48 h, pH, and ColorM_L. Moreover, the SNP rs15845448 (C allele) reduced drip loss at 24 h and 48 h by 3.62 and 5.97%, respectively, whereas improved pH and ColorM_L by 0.09 and 1.45%, respectively. To our knowledge, several previous studies on PHKG1 gene in chickens mainly focused on residual feed intake, growth, and feed conversion ratio (Shah et al., 2016; Xue et al., 2017; Liu et al., 2018). This is the first study reporting that the PHKG1 gene polymorphisms are responsible for chicken meat quality traits.
Phosphorylase kinase (PhK) is comprised of 4 different subunits. Mutations in 4 PhK subunit genes (PHKA1, PHKA2, PHKB and PHKG2) have been implicated in low PhK activity in liver and/or muscle (Wehner et al., 1994; Achouitar et al., 2011; Sibut et al., 2011; Preisler et al., 2012; Bali et al., 2014; ). Furthermore, Ma et al. (2014) confirmed the association between the PHKG1 mutation and PhK deficiency, muscle glycogenosis, and meat quality traits in pigs. In our present study, the PHKG1 gene expression was found to be significantly different between the 2 phenotypic/genotype groups (P < 0.05). Compared with CC group (genotype for PHKG1 rs15845448 SNP), AA group showed lower mRNA levels, consistent with the report by Ma et al. (2014). In addition, Ma et al. (2014) showed that the PHKG1 mutation resulted in a less active protein which ultimately increased accumulation of glycogen and heavy drip loss. We thought that the PHKG1 rs15845448 SNP might affect PhK enzyme activity, and cause a higher drip loss of meat in Chinese Ningdu yellow chickens.
Furthermore, it was observed that the most significant SNP rs15845448 mutation (AA) had negative effects on almost all meat quality traits in the Ningdu yellow population. Although the SNP rs15845448 was not significantly associated with carcass traits (P > 0.05), the AA genotype showed a trend of reducing carcass traits. At the same time, Ningdu yellow chickens carry the mutation AA at relatively high frequencies (0.21). This result may suggest that meat quality has not been the primary breeding goal in the Ningdu yellow chicken population. Ultimately, this study is likely to provide a theoretical reference for molecule-assisted breeding of desirable chickens.
CONCLUSIONS
In conclusion, 325 Chinese Ningdu yellow chickens were phenotyped and a large phenotypic variation was found in traits for ColorM_a as well as drip loss at 24 h and 48 h. We also performed an association study of PHKG1 gene polymorphisms with meat quality and carcass traits. The most significant SNP rs15845448 was simultaneously associated with drip loss at 24 h and 48 h, pH, and ColorM_L. Critically, the SNP rs15845448 (C allele) reduced drop loss at 24 h and 48 h by 3.62 and 5.97%, respectively, and the PHKG1 gene expression was observed to be significantly different between the 2 phenotypic/genotype groups (P < 0.05). Furthermore, a haplotype block with 2 SNPs was identified, and it displayed 4 distinct haplotypes with significant association with drip loss at 24 h and 48 h as well as ColorM_L. Taken together, our findings provide the first insights into the genetic basis of meat quality traits in indigenous Chinese Ningdu yellow chickens and are likely to provide a theoretical reference for molecule-assisted breeding of desirable chickens.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32060740), the Science and Technology Research Project of Education Department of Jiangxi Province (GJJ191135), and the Doctoral Foundation of Nanchang Normal University (NSBSJJ2019009).
Authors’ contributions: Xinwei Xiong conceived and designed the experiments, analyzed the data, wrote and revised the manuscript. Xianxian Liu performed the experiments and revised the manuscript. Xuenong Zhu and Yuwen Tan performed the experiments. Zhangfeng Wang analyzed the data. Jiguo Xu and Xutang Tu collected the samples. Yousheng Rao conceived and designed the experiments. Jinhong Duan and Wenliang Zhao performed the experiments. Min Zhou conceived and designed the experiments and revised the manuscript.
DISCLOSURES
The authors declare that they have no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101556.
Appendix. Supplementary materials
Supplementary Table S1. Ingredient composition of diet for all chickens.
REFERENCES
- Abdullah B.M., Mason A., Cullen J.D., Al-Shamma’a A.I. Assessing water-holding capacity (WHC) of meat using microwave spectroscopy. Sensing Technol. Curr. Status Fut. Trends. 2014;7:117–140. [Google Scholar]
- Achouitar S., Goldstein J.L., Mohamed M., Austin S., Boyette K., Blanpain F.M., Rehder C.W., Kishnani P.S., Wortmann S.B., den Heijer M., Lefeber D.J., Wevers R.A., Bali D.S., Morava E. Common mutation in the PHKA2 gene with variable phenotype in patients with liver phosphorylase b kinase deficiency. Mol. Genet. Metab. 2011;104:691–694. doi: 10.1016/j.ymgme.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Allen C.D., Fletcher D.L., Northcutt J.K., Russell S.M. The relationship of broiler breast color to meat quality and shelf-life. Poult. Sci. 1998;77:361–366. doi: 10.1093/ps/77.2.361. [DOI] [PubMed] [Google Scholar]
- Bali D.S., Goldstein J.L., Fredrickson K., Rehder C., Boney A., Austin S., Weinstein D.A., Lutz R., Boneh A., Kishnani P.S. Variability of disease spectrum in children with liver phosphorylase kinase deficiency caused by mutations in the PHKG2 gene. Mol. Genet. Metab. 2014;111:309–313. doi: 10.1016/j.ymgme.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbut S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in turkey meat. Food Res. Int. 1993;26:39–43. [Google Scholar]
- Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Cahyadi M., Jo C., Lee J.-H. Quantitative trait loci and candidate genes for the economic traits in meat-type chicken. Worlds Poult. Sci. J. 2014;70:329–342. [Google Scholar]
- Cho K.H., Kim M.J., Jeon G.J., Chung H.Y. Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol. Biol. Rep. 2011;38:2161–2166. doi: 10.1007/s11033-010-0344-3. [DOI] [PubMed] [Google Scholar]
- Druet T., Farnir F.P. Modeling of identity-by-descent processes along a chromosome between haplotypes and their genotyped ancestors. Genetics. 2011;188:409–419. doi: 10.1534/genetics.111.127720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druet T., Georges M. A hidden markov model combining linkage and linkage disequilibrium information for haplotype reconstruction and quantitative trait locus fine mapping. Genetics. 2010;184:789–798. doi: 10.1534/genetics.109.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui C.A., Regenstein J.M., Baker R.C. A simple centrifugal method for measuring expressible moisture, a water binding property of muscle foods. J. Food Sci. 1981;46:1271–1273. [Google Scholar]
- Kishnani P.S., Goldstein J., Austin S.L., Arn P., Bachrach B., Bali D.S., Chung W.K., El-Gharbawy A., Brown L.M., Kahler S., Pendyal S., Ross K.M., Tsilianidis L., Weinstein D.A., Watson M.S., A. W. G. o. Diagnosis, V. I. Management of Glycogen Storage Diseases Type, and Ix Diagnosis and management of glycogen storage diseases type VI and IX: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2019;21:772–789. doi: 10.1038/s41436-018-0364-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu R., Wang J., Zhang Y., Xing S., Zheng M., Cui H., Li Q., Li P., Cui X., Li W., Zhao G., Wen J. Exploring genomic variants related to residual feed intake in local and commercial chickens by whole genomic resequencing. Genes. 2018;9:57. doi: 10.3390/genes9020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu X., Zheng Z., Ma T., Liu Y., Long H., Cheng H., Fang M., Gong J., Li X., Zhao S., Xu X. Genome-wide analysis of expression QTL (eQTL) and allele-specific expression (ASE) in pig muscle identifies candidate genes for meat quality traits. Genet. Sel. Evol. 2020;52:59. doi: 10.1186/s12711-020-00579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu Y., Ma T., Long H., Niu L., Zhang X., Lei Y., Wang L., Chen Y., Wang Q., Zheng Z., Xu X. A splicing mutation in PHKG1 decreased its expression in skeletal muscle and caused PSE meat in Duroc x Luchuan crossbred pigs. Anim. Genet. 2019;50:395–398. doi: 10.1111/age.12807. [DOI] [PubMed] [Google Scholar]
- Ma J., Yang J., Zhou L., Ren J., Liu X., Zhang H., Yang B., Zhang Z., Ma H., Xie X., Xing Y., Guo Y., Huang L. A splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes A.F.B., Schenkel F.S., Garcia D.A., Gordo D.G.M., Tonussi R.L., Espigolan R., Silva R.M.O., Braz C.U., Fernandes G.A., Jr, Baldi F., Carvalheiro R., Boligon A.A., de Oliveira H.N., Chardulo L.A.L., de Albuquerque L.G. Genomic selection for meat quality traits in Nelore cattle. Meat Sci. 2019;148:32–37. doi: 10.1016/j.meatsci.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampouille E., Berri C., Boitard S., Hennequet-Antier C., Beauclercq S.A., Godet E., Praud C., Jego Y., Le Bihan-Duval E. Mapping QTL for white striping in relation to breast muscle yield and meat quality traits in broiler chickens. BMC Genomics. 2018;19:202. doi: 10.1186/s12864-018-4598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler N., Orngreen M.C., Echaniz-Laguna A., Laforet P., Lonsdorfer-Wolf E., Doutreleau S., Geny B., Akman H.O., Dimauro S., Vissing J. Muscle phosphorylase kinase deficiency: a neutral metabolic variant or a disease? Neurology. 2012;78:265–268. doi: 10.1212/WNL.0b013e31824365f9. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartelet A., Druet T., Michaux C., Fasquelle C., Geron S., Tamma N., Zhang Z., Coppieters W., Georges M., Charlier C. A splice site variant in the bovine RNF11 gene compromises growth and regulation of the inflammatory response. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T.M., Patel N.V., Patel A.B., Upadhyay M.R., Mohapatra A., Singh K.M., Deshpande S.D., Joshi C.G. A genome-wide approach to screen for genetic variants in broilers (Gallus gallus) with divergent feed conversion ratio. Mol. Genet. Genomics: MGG. 2016;291:1715–1725. doi: 10.1007/s00438-016-1213-0. [DOI] [PubMed] [Google Scholar]
- Shu J.T., Zhang M., Shan Y.J., Xu W.J., Chen K.W., Li H.F. Analysis of the genetic effects of CAPN1 gene polymorphisms on chicken meat tenderness. Genet. Mol. Res. 2015;14:1393–1403. doi: 10.4238/2015.February.13.18. [DOI] [PubMed] [Google Scholar]
- Sibut V., Hennequet-Antier C., Le Bihan-Duval E., Marthey S., Duclos M.J., Berri C. Identification of differentially expressed genes in chickens differing in muscle glycogen content and meat quality. BMC Genomics. 2011;12:112. doi: 10.1186/1471-2164-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner M., Clemens P.R., Engel A.G., Kilimann M.W. Human muscle glycogenosis due to phosphorylase kinase deficiency associated with a nonsense mutation in the muscle isoform of the alpha subunit. Hum. Mol. Genet. 1994;3:1983–1987. doi: 10.1093/hmg/3.11.1983. [DOI] [PubMed] [Google Scholar]
- Xiong X., Liu X., Zhou L., Yang J., Yang B., Ma H., Xie X., Huang Y., Fang S., Xiao S., Ren J., Chen C., Ma J., Huang L. Genome-wide association analysis reveals genetic loci and candidate genes for meat quality traits in Chinese Laiwu pigs. Mamm. Genome. 2015;26:181–190. doi: 10.1007/s00335-015-9558-y. [DOI] [PubMed] [Google Scholar]
- Xue H., Cao S., Li H., Zhang J., Niu J., Chen L., Zhang F., Zhao D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.) PloS One. 2017;12 doi: 10.1371/journal.pone.0178809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xiong D., Yao L., Zhao C. An SNP in exon 11 of chicken 5′-AMP-activated protein kinase gamma 3 subunit gene was associated with meat water holding capacity. Anim. Biotechnol. 2016;27:13–16. doi: 10.1080/10495398.2015.1069300. [DOI] [PubMed] [Google Scholar]
- Yu S., Wang G., Liao J., Chen X. A functional mutation in the AMPD1 promoter region affects promoter activity and breast meat freshness in chicken. Anim. Genet. 2021;52:121–125. doi: 10.1111/age.13025. [DOI] [PubMed] [Google Scholar]
- Zappaterra M., Sami D., Davoli R. Association between the splice mutation g.8283C>A of the PHKG1 gene and meat quality traits in Large White pigs. Meat Sci. 2019;148:38–40. doi: 10.1016/j.meatsci.2018.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Ingredient composition of diet for all chickens.