Abstract
The ColorPAC Giardia/Cryptosporidium (Becton Dickinson) is a solid-phase qualitative immunochromatographic assay that detects and distinguishes between Giardia lamblia and Cryptosporidium parvum in human stool. Agreement between the Alexon-Trend ProSpecT Giardia Rapid EIA and the ColorPAC assay was 166 of 172 (96.5%). Agreement between the Alexon-Trend ProSpecT Cryptosporidium Rapid EIA and the ColorPAC assay was 169 of 171 (98.8%). No cross-reactions were seen with other parasites or human cells.
With the increasing interest in potential waterborne outbreak situations, fewer well-trained microscopists, and confirmation that both Giardia lamblia and Cryptosporidium parvum can cause severe symptoms in humans, laboratories are reviewing their options with regard to immunoassay kits that can be incorporated into their routine testing protocols (4, 14, 15, 20). Not only must these methods be acceptable in terms of sensitivity and specificity but they must provide clinically relevant, cost-effective, rapid results, particularly in a potential waterborne outbreak situation (1, 2, 7, 13). Antigen detection assays for G. lamblia and C. parvum have proven to be very useful in the diagnosis of these infections (5, 10–12, 16–19). Advantages of these assays include labor, time, and batching efficiencies that may lead to reduced costs. These reagents offer alternative methods to the routine “ova and parasite examination” (O&P) method and provide the added sensitivity required to confirm infections in patients with low parasite numbers.
Most commercially available immunoassays use the enzyme immunoassay (EIA) format that requires multiple reagent additions, washing steps, and incubations. A nonenzymatic rapid immunoassay for Giardia and Cryptosporidium antigens has been developed. This test (Genzyme Diagnostics Contrast Giardia/Cryptosporidium) is marketed commercially (Becton Dickinson ColorPAC Giardia/Cryptosporidium). The assay can be performed in approximately 12 min on formalin-fixed (5 or 10% formalin or sodium acetate-acetic acid-formalin [SAF]) or unfixed stool specimens. In the current environment of managed care and cost containment, this new device may provide diagnostic testing that is more accurate, rapid, and cost-effective than traditional methods.
Human fecal specimens were collected in 10% formalin or SAF and polyvinyl alcohol-mercuric chloride base fixatives by the patients and submitted to the laboratory from various clinics affiliated with the UCLA Medical Center. Different parasites (nine protozoa, including both trophozoites and cysts and 1 helminth; 74 positive specimens) and stools containing human cells (21 positive specimens) were included in the negative specimens; all specimens were patient specimens, not seeded specimens. Specific organisms included Blastocystis hominis (23), Chilomastix mesnili (3), Dientamoeba fragilis (6), Endolimax nana (14), Entamoeba coli (6), Entamoeba hartmanni (3), Entamoeba histolytica/E. dispar (10), Iodamoeba bütschlii (5), Trichomonas hominis (1), and Hymenolepis nana eggs (3). Specific human cells seen in fecal specimens included red blood cells (1), polymorphonuclear leukocytes (19), and macrophages (3); these specimens also contained normal fecal debris, including various yeast cells. All specimens were tested as indicated below; additional testing was performed on discrepant specimens.
The following immunoassay diagnostic kits were used according to the manufacturer's directions: (i) Alexon-Trend ProSpect Giardia Rapid EIA (Alexon-Trend-Seradyn, Ramsey, Minn.), (ii) Alexon-Trend ProSpect Cryptosporidium Rapid EIA, (iii) Alexon-Trend ProSpecT microwell EIA (for Giardia or Cryptosporidium), and (iv) ColorPAC Giardia/Cryptosporidium (Becton Dickinson, Sparks, Md.). The Alexon-Trend-Seradyn rapid EIA methods were selected as being the most comparable to the ColorPAC method in terms of time required for performing the procedure. All immunoassay kits were used with unconcentrated, preserved stool specimens (5 or 10% formalin or SAF). Specimens were tested using the following combinations: (i) Alexon-Trend ProSpect Giardia Rapid EIA and ColorPAC Giardia/Cryptosporidium or (ii) Alexon-Trend ProSpect Cryptosporidium Rapid EIA and ColorPAC Giardia/Cryptosporidium. Discrepant results were tested using the Alexon-Trend ProSpecT microwell EIA tests (for Giardia or Cryptosporidium); this test was selected in order to maintain consistency with a single manufacturer.
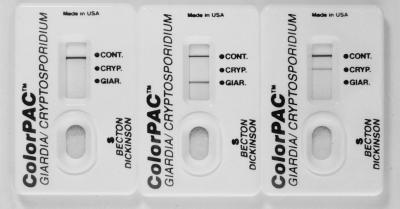
The ColorPAC Giardia/Cryptosporidium assay procedure involves the addition of 2 drops of sample treatment buffer to a tube, the pipetting of 60 μl of uncentrifuged stool specimen into the tube, the addition of 2 drops of a Giardia capture antibody conjugate, and the addition of 2 drops of a colloidal carbon-conjugated detection reagent for Giardia and Cryptosporidium. After the sample is mixed, it is immediately poured into the test device. Assay results are read after 10 min. Positive results are visualized as gray-black lines in the appropriate position in the results window (Fig. 1). The tubes, pipettes, devices, and all reagents are provided with the kit.
FIG. 1.
ColorPac devices demonstrating positive results: left, positive control; middle, positive Giardia; right, positive Cryptosporidium.
By the Merifluor Cryptosporidium/Giardia direct fluorescent-antibody assay (the reference method), the O&P examination (concentration, trichrome stain), and modified acid-fast staining (concentration, modified acid-fast stain), 47 specimens were positive for Giardia, 41 specimens were positive for Cryptosporidium, and 256 were negative for both organisms. Positive Giardia specimens (n = 47) and negative specimens (n = 125) were tested using two different kits. Agreement between the Alexon-Trend ProSpecT Giardia Rapid EIA and the Becton Dickinson ColorPAC was 166 of 172 (96.5%); the six positives by microscopy, the Merifluor direct fluorescent-antibody assay, and the ColorPAC were false negatives in the Alexon-Trend ProSpecT Giardia Rapid EIA (Table 1). There were no cross-reactions with other organisms present in the fecal specimens; specificity was 100%. Positive Cryptosporidium specimens (n = 41) and negative specimens (n = 131) were tested using two different kits. Agreement between the Alexon-Trend ProSpecT Cryptosporidium Rapid EIA and the Becton Dickinson ColorPAC was 169 of 171 (98.8%); the three negatives were false negatives using the Alexon-Trend ProSpecT Cryptosporidium Rapid EIA. The one false-negative C. parvum (ColorPAC) was confirmed by immunofluorescence (Table 1). There were no cross-reactions with other organisms present in the fecal specimens; specificity was 100%.
TABLE 1.
Comparison of rapid test methods for identification of Giardia and Cryptosporidiuma
| Organism | Specimens | Results with:
|
|||
|---|---|---|---|---|---|
| ColorPac rapid test
|
ProSpecT Giardia Rapid EIA
|
||||
| Pos | Neg | Pos | Neg | ||
| Giardia | Pos (n = 47) | 47 | 0 | 41 | 6 |
| Neg (n = 125) | 0 | 125 | 0 | 125 | |
| Cryptosporidium | Pos (n = 41) | 40 | 1 | 38 | 3 |
| Neg (n = 131) | 0 | 131 | 0 | 131 | |
Pos, positive; Neg, negative.
In patients with giardiasis or cryptosporidiosis, the use of routine diagnostic methods such as concentration and trichrome or modified acid-fast staining may be insufficient to demonstrate the presence of these organisms (3, 4, 6, 9, 11, 21). Based on the O&P examination with trichrome stain, as well as the Meridian Giardia/Cryptosporidium Merifluor combination reagent, the sensitivity and specificity, respectively, using the ColorPAC device were as follows: G. lamblia, 100% and 100%; C. parvum, 97.6% and 100%. The Alexon-Trend ProSpecT Rapid EIAs did not perform as well, but results were within previous published expected values regarding sensitivity and specificity. The ability to concurrently detect and distinguish between Giardia and Cryptosporidium antigens in formalin-fixed or unfixed fecal specimens with a 10-min nonenzymatic immunoassay provides the user with another very useful diagnostic kit, the Becton Dickinson ColorPAC. The rapid immunoassays do not take the place of routine O&P examinations, but they are very useful when trying to confirm Giardia and Cryptosporidium infections (8).
REFERENCES
- 1.Addis D G, David J P, Roberts J M, Mast E E. Epidemiology of Giardiasis in Wisconsin: increasing incidence of reported cases and unexplained season trends. Am J Trop Med Hyg. 1992;47:13–19. doi: 10.4269/ajtmh.1992.47.13. [DOI] [PubMed] [Google Scholar]
- 2.Atherton F, Newman C P, Casemore D P. An outbreak of waterborne cryptosporidiosis associated with a public water supply in the UK. Epidemiol Infect. 1995;115:123–131. doi: 10.1017/s0950268800058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappell C L, Okhuysen P C, Sterling C R, Wang C, Jakubowski W, DuPont H L. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999;60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 4.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;3:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doing K M, Hamm J L, Jellison J A, Marquis J A, Kingsbury C. False-positive results obtained with the Alexon ProSpecT Cryptosporidium enzyme immunoassay. J Clin Microbiol. 1999;37:1582–1583. doi: 10.1128/jcm.37.5.1582-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 7.Fayer R, Trout J M, Jenkins M C. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. J Parasitol. 1998;84:1165–1169. [PubMed] [Google Scholar]
- 8.Garcia L S. Practical guide to diagnostic parasitology. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 9.Garcia L S, Bruckner D A. Diagnostic medical parasitology. 3rd ed. Washington, D.C.: ASM Press; 1997. [Google Scholar]
- 10.Garcia L S, Shimizu R Y. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for the detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35:1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia L S, Shum A C, Bruckner D A. Evaluation of a new monoclonal antibody combination reagent for direct fluorescent detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol. 1992;30:3255–3257. doi: 10.1128/jcm.30.12.3255-3257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehl K C, Cicirello H, Havens P L. Comparison of four different methods for the detection of Cryptosporidium species. J Clin Microbiol. 1995;33:416–418. doi: 10.1128/jcm.33.2.416-418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie W R, Hoxie N J, Proctor M E. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 14.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieniazek N J, Bornay-Llinares F J, Slemenda S B, da Silva A J, Moura I N S, Arrowood M J, Ditrich O, Addis D G. New Cryptosporidium genotypes in HIV-infected persons. Emerg Infect Dis. 1999;5:444–449. doi: 10.3201/eid0503.990318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priest J W, Kwon J P, Moss D M, Roberts J M, Arrowood M J, Dworkin M S, Juranek D D, Lammie P J. Detection of enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenblatt J E, Sloan L M. Evaluation of an enzyme-linked immunosorbent assay for detection of Cryptosporidium spp. in stool specimens. J Clin Microbiol. 1993;31:1468–1471. doi: 10.1128/jcm.31.6.1468-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblatt J E, Sloan L M, Schneider S K. Evaluation of an enzyme-linked immunosorbent assay for the detection of Giardia lamblia in stool specimens. Diagn Microbiol Infect Dis. 1993;16:337–341. doi: 10.1016/0732-8893(93)90086-m. [DOI] [PubMed] [Google Scholar]
- 19.Rosoff J D, Sanders C A, Sonnad S S, De Lay P R, Hadley W K, Vincenzi F F, Yajko D M, O'Hanley P D. Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia-specific antigen 65 (GSA 65) J Clin Microbiol. 1989;27:1997–2002. doi: 10.1128/jcm.27.9.1997-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe M S. Giardiasis. Clin Microbiol Rev. 1992;5:93–100. doi: 10.1128/cmr.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman S K, Needham C A. Comparison of conventional stool concentration and preserved-smear methods with Merifluor Cryptosporidium/Giardia direct immunofluorescence assay and ProSpecT Giardia EZ microplate assay for detection of Giardia lamblia. J Clin Microbiol. 1995;33:1942–1943. doi: 10.1128/jcm.33.7.1942-1943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]