Abstract
Cytogenetic studies on fungus-farming ants have shown remarkable karyotype diversity, suggesting different chromosomal rearrangements involved in karyotype evolution in some genera. A notable cytogenetic characteristic in this ant group is the presence of GC-rich heterochromatin in the karyotypes of some ancient and derivative species. It was hypothesized that this GC-rich heterochromatin may have a common origin in fungus-farming ants, and the increase in species studied is important for understanding this question. In addition, many genera within the subtribe Attina have few or no cytogenetically studied species; therefore, the processes that shaped their chromosomal evolution remain obscure. Thus, in this study, we karyotyped, through classical and molecular cytogenetic techniques, the fungus-farming ants Cyphomyrmextransversus Emery, 1894, Sericomyrmexmaravalhas Ješovnik et Schultz, 2017, and Mycetomoelleriusrelictus (Borgmeier, 1934), to provide insights into the chromosomal evolution in these genera and to investigate the presence the GC-rich heterochromatin in these species. Cyphomyrmextransversus (2n = 18, 10m + 2sm + 6a) and S.maravalhas (2n = 48, 28m + 20sm) showed karyotypes distinct from other species from their genera. Mycetomoelleriusrelictus (2n = 20, 20m) presented the same karyotype as the colonies previously studied. Notably, C.transversus presented the lowest chromosomal number for the genus and a distinct karyotype from the other two previously observed for this species, showing the existence of a possible species complex and the need for its taxonomic revision. Chromosomal banding data revealed GC-rich heterochromatin in all three species, which increased the number of genera with this characteristic, supporting the hypothesis of a common origin of GC-rich heterochromatin in Attina. Although a single chromosomal pair carries rDNA genes in all studied species, the positions of these rDNA clusters varied. The rDNA genes were located in the intrachromosomal region in C.transversus and M.relictus, and in the terminal region of S.maravalhas. The combination of our molecular cytogenetic data and observations from previous studies corroborates that a single rDNA site located in the intrachromosomal region is a plesiomorphic condition in Attina. In addition, cytogenetic data obtained suggest centric fission events in Sericomyrmex Mayr, 1865, and the occurrence of inversions as the origin of the location of the ribosomal genes in M.relictus and S.maravalhas. This study provides new insights into the chromosomal evolution of fungus-farming ants.
Keywords: Biodiversity, chromatin, chromosomal rearrangements, Formicidae, karyotype evolution, molecular cytogenetics
Introduction
Fungus-farming ants, included in the subtribe Attina (sensu Ward et al. 2015), have an obligatory symbiotic relationship with fungi (Weber 1966). In this symbiosis, these ants cultivate the fungus for food and, in return, provide the fungus with nutrition, propagate it to new locations, and protect it against parasitic microorganisms (Weber 1966; Little et al. 2005). In this agricultural system, these ants use different types of substrates depending on the genus/species (reviewed by Mehdiabadi and Schultz 2010), and with this, they play important roles in natural ecosystems, such as dispersion and increasing the success of seed germination, soil structuring, and nutrient cycling (Leal and Oliveira 1998; Fernandez-Bou et al. 2019).
Several molecular phylogenetic studies have been conducted in Attina to address the relationships between genera and species (Schultz and Brady 2008; Ješovnik et al. 2017; Sosa-Calvo et al. 2017; Solomon et al. 2019). These phylogenies support the monophyly of the group, with an origin of approximately 50–60 million years ago (Schultz and Brady 2008; Nygaard et al. 2016; Sosa-Calvo et al. 2017). This group includes approximately 280 described taxa distributed in 20 genera (Bolton 2021), which are grouped into two monophyletic sister clades: Paleoattina (Apterostigma Mayr, 1865, Mycocepurus Forel, 1893, and Myrmicocrypta Smith, 1860) and Neoattina (the remaining 17 genera) (Sosa-Calvo et al. 2018; Solomon et al. 2019; Cristiano et al. 2020).
Some Attina genera have been extensively revised (Sosa-Calvo et al. 2017, 2018; Solomon et al. 2019; Cristiano et al. 2020), and in this scenario, cytogenetics is a tool that can help in taxonomic issues, since chromosomal rearrangements can lead to reduced gene flow between populations and reproductive isolation, playing an important role in speciation (Riesemberg 2001; reviewed by Faria and Navarro 2010). In addition to evolutionary, phylogenetic, and chromosomal patterns in different groups, cytogenetic studies on ants, using classical and molecular techniques, are important for the understanding of taxonomically challenging species (Mariano et al. 2012; Santos et al. 2016; Aguiar et al. 2017; Micolino et al. 2019a; Teixeira et al. 2021).
Cytogenetic data are available for 56 taxa of fungus-farming ants with representatives from 12 genera (reviewed by Mariano et al. 2019; Aguiar et al. 2020; Micolino et al. 2020; Barros et al. 2021) and the chromosome number observed for the group ranges from 2n = 8 in Mycocepurusgoeldii (Forel, 1893) and Mycocepurus sp. to 2n = 64 in Mycetophylaxlectus (Forel, 1911) (as Cyphomyrmexlectus) (reviewed by Mariano et al. 2019). A notable cytogenetic characteristic in this ant group is that some Paleoattina and Neoattina species have GC-rich heterochromatin in all chromosomes, with nucleotide composition yet to be determined but may have an origin in the common ancestor needing further investigation (Barros et al. 2018; reviewed by Mariano et al. 2019). Molecular cytogenetic studies using fluorescence in situ hybridization (FISH) for mapping ribosomal genes have already been performed in 17 taxa, including six genera showing a single chromosome pair carrying rDNA genes (reviewed in Teixeira et al. 2021).
According to available cytogenetic data, different chromosomal rearrangements have been proposed to explain karyotype evolution in some Attina genera. The occurrence of centric fissions, according to Minimum Interaction Theory (MIT) (Imai et al. 1994), was suggested to explain the remarkable karyotype variation in Mycetarotes Emery, 1913 (2n = 14 to 54), Apterostigma (2n = 20 to 46), Cyphomyrmex Mayr, 1862 (2n = 20 to 42), and in leaf-cutting ants, in which Amoimyrmexstriatus (Roger, 1863) and Atta spp. present 2n = 22, and most Acromyrmex spp. show 2n = 38 (reviewed by Mariano et al. 2019; Barros et al. 2021). However, chromosomal fusion has been suggested as the origin of the derived karyotype from Acromyrmexameliae De Souza et al. 2007 (2n = 36) (Barros et al. 2021). In Mycetophylax Emery, 1913, both chromosomal fusions and fissions are important for the karyotypic evolution of species (Micolino et al. 2019a). In addition, other mechanisms that do not change the chromosome number were proposed for some species as differential heterochromatin growth in Acromyrmex spp. (Barros et al. 2016), duplications of euchromatic regions by unequal crossing-over or non-homologous translocations in Mycetomoelleriusurichii (Forel, 1893) (as Trachymyrmexfuscus Emery, 1934) (Barros et al. 2013a), paracentric inversion in Acromyrmexechinatior (Forel, 1899) (Barros et al. 2016; Teixeira et al. 2021) and pericentric inversion in Mycetomoelleriusiheringi (Emery, 1888) (Micolino et al. 2020).
There are different possible mechanisms involved in the karyotype evolution of Attina genera, highlighting the need to increase the number of studied species for more robust inferences (Barros et al. 2013b, 2018). The remaining genera of fungus-farming ants have little or no cytogenetically studied species; therefore, the processes that shaped their chromosomal evolution remain obscure. Therefore, using classical and molecular cytogenetic techniques, we determined the karyotypes of three fungus-farming ants – Cyphomyrmextransversus Emery, 1894, Mycetomoelleriusrelictus (Borgmeier, 1934), and Sericomyrmexmaravalhas Ješovnik et Schultz, 2017 – to investigate the presence of GC-rich heterochromatin in these species and understand the patterns of chromosomal evolution in their respective genera as well as in Attina in general.
Material and methods
Colonies of C.transversus, M.relictus, and S.maravalhas were collected in Viçosa, in the Minas Gerais state, Brazil (-20.757041, -42.873516) (Table 1). Sampling permission was given by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (SISBIO accession number 32459). Adult vouchers were identified by Dr. Jacques H. C. Delabie and deposited in the myrmecological collection of the Centro de Pesquisas do Cacau at the Comissão Executiva do Plano da Lavoura Cacaueira (CEPLAC), in Bahia, Brazil.
Table 1.
Species of fungus-farming ants cytogenetically analyzed in the present study collected in Viçosa, Minas Gerais, Brazil. Species, total number of colonies and individuals; diploid chromosome numbers; diploid karyotype formulae, presence of GC-rich heterochromatin, and idiogram showing the location of 18S rDNA genes in the karyotype.
| Species | Col. / Ind. | 2n | Karyotype formulae | GC-rich Het | rDNA 18S location |
|---|---|---|---|---|---|
| Cyphomyrmextransversus | 1 / 6 | 18 | 10m + 2sm + 6a | Yes |
|
| Mycetomoelleriusrelictus | 2 / 7 | 20 | 20m | Yes |

|
| Sericomyrmexmaravalhas | 2 / 14 | 48 | 28m + 20sm | Yes |
|
Mitotic metaphases were obtained from cerebral ganglia of larvae after meconium elimination accordingly to Imai et al. (1988). Chromosome number and morphology of metaphases were analyzed using conventional 4% Giemsa staining. Chromosomes were arranged in order of decreasing size, measured and classified according to the methodology proposed by Levan et al. (1964) that is based on the ratio of the chromosome arm lengths (r = long arm/short arm). The chromosomes were classified as m = metacentric (r = 1–1.7), sm = submetacentric (r = 1.7–3), st = subtelocentric (r = 3–7) and a = acrocentric (r > 7). Chromosomes were organized using Adobe Photoshop CS6 and measured using Image Pro Plus.
The heterochromatin distribution pattern was observed by C-banding technique according to Sumner (1972), with adaptations of Barros et al. (2013b). Metaphases were stained with the fluorochromes chromomycin A3 (CMA3) and 4’6-diamidino-2-phenylindole (DAPI), to the detection of GC and AT-rich regions, respectively based on the technique proposed by Schweizer (1980).
The ribosomal 18S gene clusters were detected by FISH, following the protocol of Pinkel et al. (1986) with the use of the 18S rDNA probes obtained via PCR amplification. The genomic DNA from the ant Camponotusrufipes (Fabricius, 1775) was used for amplification of 18S rDNA using the primers 18SF1 (5’-GTC ATA GCT TTG TCT CAA AGA-3’) and 18SR1.1 (5’-CGC AAA TGA AAC TTT TTT AAT CT-3’) (Pereira 2006). These primers amplify the initial portion of 18S rDNA (for details see Menezes et al. 2021). Gene amplification followed Pereira (2006). 18S rDNA probes were labeled by an indirect method using digoxigenin-11-dUTP (Roche Applied Science, Mannheim, Germany), and the FISH signals were detected with anti-digoxigenin-rhodamine (Roche Applied Science), following the manufacturer’s protocol.
Chromosomes from ten metaphases of each taxon were measured in order to determine the chromosomal morphology. For C-banding, fluorochrome staining, and FISH techniques, at least 30 metaphases of each taxon were analyzed. The metaphases were photographed using an epifluorescent microscope Olympus BX60 attached to an image system QColor Olympus with the filters WB (450–480 nm), WU (330–385 nm), and WG (510–550 nm) for the fluorochromes CMA3, DAPI, and rhodamine, respectively.
Results
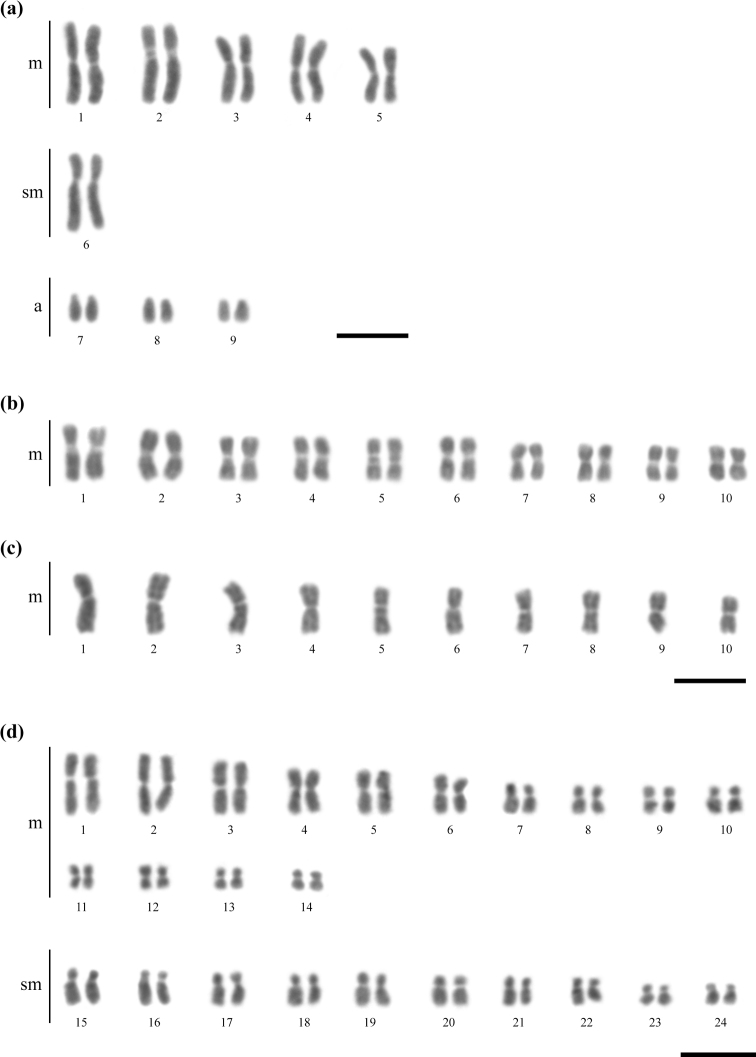
The chromosome numbers and karyotypic formulae observed in the three fungus-farming ant species were as follows: 2n = 18 (10m + 2sm + 6a) in C.transversus (Fig. 1a), 2n = 20 (20m) and n = 10 (10m) in M.relictus (Fig. 1b, c), and 2n = 48 (28m + 20sm) in S.maravalhas (Fig. 1d).
Figure 1.
Karyotypes of fungus-farming ants aCyphomyrmextransversus (2n = 18, 10m + 2sm + 6a) b, cMycetomoelleriusrelictus (2n = 20, 20m and n = 10, 10m), and dSericomyrmexmaravalhas (2n = 48, 28m + 20sm). Scale bars: 5 µm.
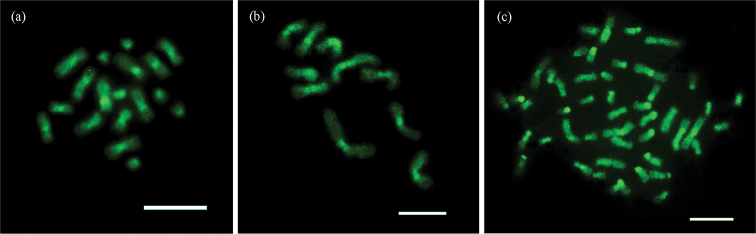
Heterochromatin was observed in the centromeric/pericentromeric regions of all chromosomes besides short arms of acrocentric chromosomes in C.transversus (Fig. 2a). Mycetomoelleriusrelictus presented heterochromatic bands in the centromeric regions of all chromosomes (Fig. 2b). In S.maravalhas, heterochromatin was observed in the centromeric and pericentromeric regions of metacentric chromosomes, and short arms of the 7th, 10th, and 13th metacentric and all submetacentric pairs (Fig. 2c). Most of the heterochromatic regions showed GC-rich patterns in all three species (Fig. 3).
Figure 2.
Heterochromatic patterns after C-banding technique in the karyotypes of the studied fungus-farming ants aCyphomyrmextransversus (2n = 18) bMycetomoelleriusrelictus (2n = 20), and cSericomyrmexmaravalhas (2n = 48). Dark blocks indicate heterochromatin in the centromeric/pericentromeric regions and short arms of the chromosomes. Scale bars: 5 µm.
Figure 3.
GC-rich chromatin patterns using Chromomycin A3 fluorochrome on metaphases of the studied fungus-farming ants aCyphomyrmextransversus (2n = 18) bMycetomoelleriusrelictus (n = 10), and cSericomyrmexmaravalhas (2n = 48). The GC-rich bands in the centromeric/pericentromeric regions and short arms of the chromosomes are colocalized with heterochromatic blocks. Scale bars: 5 µm.
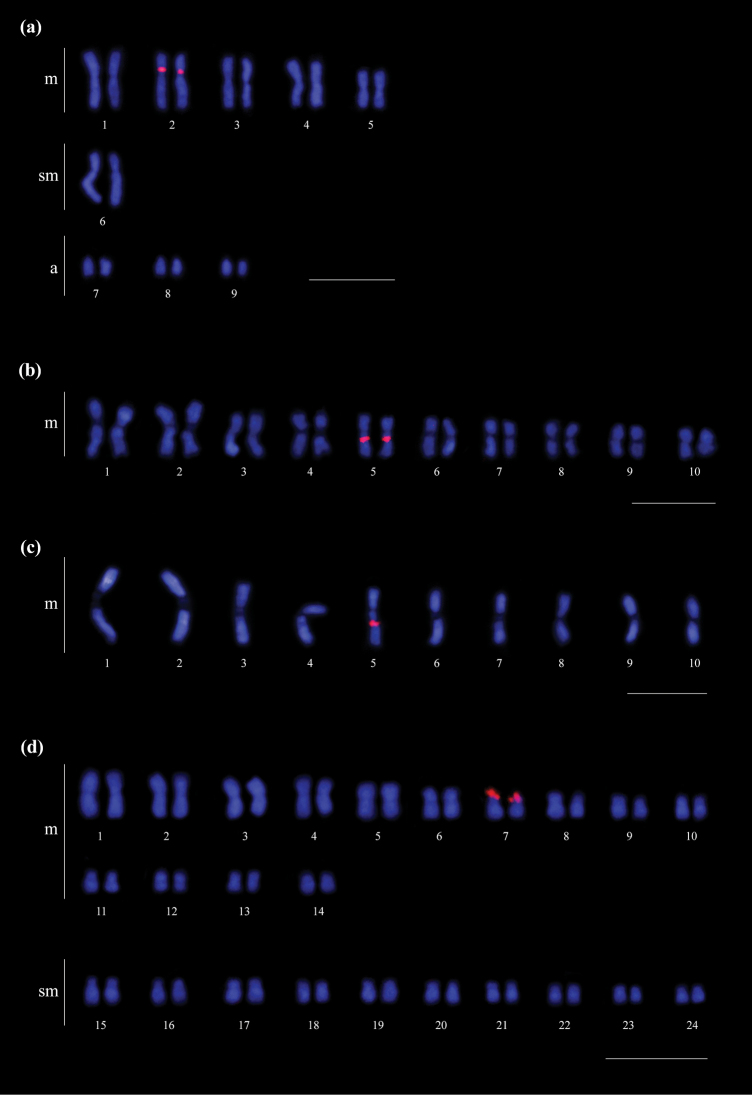
The three species showed a single pair of chromosomes bearing rDNA clusters. The 18S ribosomal gene clusters were mapped in the pericentromeric region of the short arm of the 2nd metacentric pair in C.transversus (Fig. 4a), in the interstitial region of the long arm of the 5th metacentric pair in M.relictus (Fig. 4b, c), and in the terminal region of the short arm of the 7th metacentric pair in S.maravalhas (Fig. 4d).
Figure 4.
18S rDNA clusters (red blocks) location on the karyotypes of the studied fungus-farming ants aCyphomyrmextransversus (2n = 18) b, cMycetomoelleriusrelictus (2n = 20, n = 10), and dSericomyrmexmaravalhas (2n = 48). Scale bars: 5 µm.
Discussion
The association of cytogenetic and molecular data provided insights into the karyotype evolution of the three genera of fungus-farming ants in this study. In Sericomyrmex, the molecular phylogeny proposed by Ješovnik et al. (2017) showed that S.maravalhas, a new species recently described by Ješovnik and Schultz (2017), belongs to the scrobifer clade, which is basal to the other existing clade, the amabilis. Ješovnik and Schultz (2017) highlighted that the distribution data of S.maravalhas are clearly incomplete. This is the first report of this species in the Atlantic rainforest since its known occurrence, to this date, was restricted to Cerrado habitats.
Sericomyrmexmaravalhas (scrobifer clade) has a basal position to Sericomyrmexamabilis Wheeler, 1925 (amabilis clade) (Ješovnik et al. 2017). The former species has 2n = 48, with more submetacentric chromosomes (this study), whereas the latter species has 2n = 50 with only metacentric chromosomes (Murakami et al. 1998). It is possible to suggest an increase in chromosome number from 2n = 48 to 2n = 50. Additionally, the heterochromatic pattern on the short arms of the submetacentric/metacentric chromosomes of S.maravalhas is a strong indicator of centric fission events during the karyotype evolution in Sericomyrmex. The absence of subtelocentric/acrocentric chromosomes in the karyotype of S.maravalhas, which has also been observed in S.amabilis and Sericomyrmex sp. (Murakami et al. 1998; Barros et al. 2013b), can be associated with tandem growth of heterochromatin for telomeric stability after fission, which should have changed the chromosome’s morphology from acrocentric to submetacentric/metacentric. These events of heterochromatin growth may have contributed to differences in chromosomal morphology observed in S.maravalhas in relation to Sericomyrmex sp. and S.amabilis. A similar mechanism has also been suggested to explain interspecific chromosomal variations in leaf-cutting ants Acromyrmex (Barros et al. 2016) and trap-jaw ants Odontomachus (Aguiar et al. 2020).
The molecular phylogeny of Mycetomoellerius, proposed by Solomon et al. (2019), showed two main clades. One clade includes M.urichii with 2n = 18 chromosomes (Barros et al. 2013a), Mycetomoelleriusholmgreni (Wheeler, 1925), and M.iheringi, both of which have 2n = 20 chromosomes (Barros et al. 2018; Cardoso et al. 2018; Micolino et al. 2020; Table 2). Mycetomoelleriusrelictus belongs to the other clade and has 2n = 20 chromosomes (present study; Barros et al. 2013b). Mycetomoellerius sp. (as Trachymyrmex sp.) from the Atlantic rainforest has 2n = 22 (Barros et al. 2013b). Therefore, an ancestor of Mycetomoellerius with the chromosome number between 2n = 18–22 and with a predominance of metacentric chromosomes seems likely.
Table 2.
Summary of available cytogenetic data in the literature and this study for the genera of fungus-farming ants Cyphomyrmex, Sericomyrmex, and Mycetomoellerius. Species, localities, chromosome numbers: diploid (2n)/haploid (n), diploid karyotype formulae, and references. The terminology used for karyotype formulae is in accordance to the published data.
| Species | Localities | 2n/(n) | Karyotype formulae | References |
|---|---|---|---|---|
| Cyphomyrmex | ||||
| C.costatus | Panama | 20 | 20M | Murakami et al. (1998) |
| C.cornutus | French Guiana | 22 | 10M + 12SM | Mariano et al. (2011) |
| C.rimosus | Panama | 32 | 28M + 4A | Murakami et al. (1998) |
| C.transversus | French Guiana | 24/(12) | 14m + 6sm + 4a | Aguiar et al. (2020) |
| C.transversus | SP - Brazil | 42 | 42A | Mariano et al. (2019) |
| C.transversus | MG - Brazil | 18 | 10m + 2sm + 6a | Present study |
| Cyphomyrmex sp. § | MG - Brazil | 32 | 14M + 18A | Mariano et al. (2019) |
| Sericomyrmex | ||||
| S.amabilis | Panama | 50 | 50M | Murakami et al. (1998) |
| S.maravalhas | MG - Brazil | 48 | 28m + 20sm | Present study |
| Sericomyrmex sp. | MG - Brazil | 50/(25) | 44m + 6sm | Barros et al. (2013b) |
| Mycetomoellerius | ||||
| M.urichii* | MG - Brazil | 18 | 16m + 2sm | Barros et al. (2013a) |
| M.holmgreni | MG - Brazil | 20 | 20m | Barros et al. (2018) / Cardoso et al. (2018) |
| M.iheringi | SC - Brazil | 20 | 18M + 2SM | Micolino et al. (2020) |
| M.relictus | MG - Brazil | 20/(10) | 20m | Barros et al. (2013b) / Present study |
| Mycetomoellerius sp.† | MG - Brazil | 22 | 18m + 4sm | Barros et al. (2013b) |
* As Trachymyrmexfuscus in Barros et al. (2013a); † According to new revision by Solomon et al. (2019); § Cyphomyrmex sp. group rimosus. MG: Minas Gerais State; SP: São Paulo State; SC: Santa Catarina State.
The cytogenetic data obtained in this study for C.transversus (2n = 18) showed the lowest chromosome number for this genus. This karyotype is different from the other two previously studied karyotypes in French Guiana (2n = 24) and Brazil (2n = 42) (Mariano et al. 2019; Aguiar et al. 2020; Table 2). The chromosomal morphology also differs among the three karyotypes of C.transversus, with a notable increase in the number of acrocentric pairs in the karyotype from São Paulo-Brazil, which has a higher chromosome number. These data suggest that C.transversus may be a species complex and, therefore, cytogenetic data highlight the need for taxonomic revision of this species. Based on cytogenetic studies available for Cyphomyrmex, Mariano et al. (2019) suggested that centric fissions play a major role in the karyotype evolution within this genus due to an increase in acrocentric chromosome pairs in species with high chromosome numbers. Further molecular phylogenetic studies associated with cytogenetic data will help in the discussion of the karyotype evolution of this genus and the taxonomy of C.transversus.
Regarding heterochromatin constitution, the three species of the present study showed GC-rich heterochromatin, as evidenced by the colocalization of the heterochromatic and CMA3+ bands. These data were first reported in Sericomyrmex and Cyphomyrmex. Other fungus-farming ants showed the same heterochromatic composition such as M.goeldii (Paleoattina) (Barros et al. 2010), M.urichii (Barros et al. 2013a), and M.holmgreni (Barros et. al. 2018), included in Neoattina. This pattern is not common in ants, with few examples in the Dolichoderus genus, which belongs to another subfamily (Santos et al. 2016). Barros et al. (2018) suggested that GC-rich heterochromatin observed in different species of Attina, with representatives in Paleoattina and Neoattina, may have a common origin within the subtribe. The heterochromatic pattern rich in GC observed in this study supports this hypothesis, increasing the number of genera with this characteristic. Further investigation of the chromatin composition of these species should corroborate this hypothesis.
The physical mapping of rDNA genes showed a single chromosome pair bearing these genes for the three species in this study. This pattern is similar to that observed for other fungus-farming ants, which is suggested to be a plesiomorphic characteristic in Formicidae (reviewed by Teixeira et al. 2021), and aculeate Hymenoptera as well (Menezes et al. 2021). Regarding the location of these rDNA genes on the chromosomes in Attina, most species presented these genes in the intrachromosomal region (pericentromeric or interstitial). This characteristic is observed in ancient species such as M.goeldii, Myrmicocrypta sp., Mycetophylax spp., and C.tranversus, in the transition species M.holmgreni and M.relictus, and leaf-cutting ants, most derived from the group, Am.striatus and Atta spp. (reviewed by Teixeira et al. 2021; this study). These data suggest that the intrachromosomal position of rDNA genes seems to be a plesiomorphic character in fungus-farming ants.
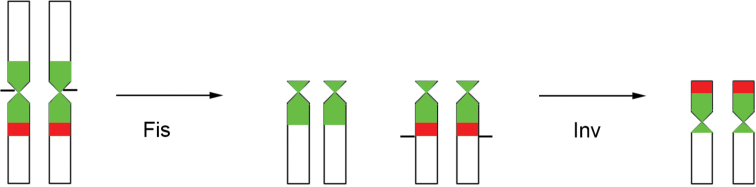
However, in S.maravalhas, the rDNA clusters were mapped in the terminal region of the heterochromatic short arm of the 7th metacentric pair (see Figs 2c, 4c). Considering an ancestor with a low chromosome number and intrachromosomal rDNA clusters, after centric fission events, the occurrence of pericentric inversion would change the pericentromeric rDNA genes to the terminal positions, as observed in S.maravalhas (Fig. 5). In some other fungus-farming ants, rDNA genes are also located in the terminal region, such as Acromyrmex spp. (Barros et al. 2016; Teixeira et al. 2017), Mycetophylaxconformis (Mayr, 1884), and M.morschi (Emery, 1888) (2n = 30) (Micolino et al. 2019a), which are species with derived karyotypes within their respective phylogenetic branches. In the case of M.conformis, the terminal rDNA cluster located on the metacentric chromosome (Micolino et al. 2019a) seems to represent a derived pattern, explained by a single paracentric inversion, considering its ancestor with intrachromosomal rDNA clusters. The rDNA terminal location in Acromyrmex seems to be a derived condition among leaf-cutting ants (Barros et al. 2021).
Figure 5.
Diagram of origin of terminal rDNA clusters in metacentric chromosome from Sericomyrmexmaravalhas, considering its ancestor with intrachromosomal rDNA clusters. Black bars: chromosomal breaks; Fis: centric fission; Inv: pericentric inversion; Green blocks: GC-rich regions; Red blocks: 18S ribosomal clusters.
In addition, a difference in the location of rDNA clusters was observed between M.relictus in this study and M.holmgreni (Barros et al. 2018; Micolino et al. 2019b). The former showed 18S rDNA clusters located in the interstitial region of the 5th metacentric pair while the latter presented these genes in the pericentromeric region of the 4th metacentric pair (Barros et al. 2018; Micolino et al. 2019b). This difference may reflect the phylogenetic position of these species, as they are included in distinct branches of Mycetomoellerius, in which M.holmgreni has a basal position to M.relictus (Solomon et al. 2019). However, the size variation between the 4th and 5th metacentric pairs was very subtle in M.relictus (see Fig. 4b, c). This suggests homeology of the chromosome pair carrying rDNA clusters between M.relictus and M.holmgreni. Therefore, the difference in the location of ribosomal genes between M.relictus and M.homlgreni may be the result of paracentric inversion. In addition, the occurrence of a paracentric inversion involving rDNA genes has already been observed in leaf-cutting ant A.echinatior (Teixeira et al. 2021). Thus, inversions seem to be important rearrangements that generate changes in the position of rDNA genes in the karyotype of fungus-farming ants.
Conclusions
In this study, the distribution of 18S ribosomal genes and GC-rich heterochromatin in Sericomyrmex and Cyphomyrmex, which were reported for the first time, suggest the origin of this heterochromatin in the common ancestor of Attina. The karyotype observed in C.tranversus shows the lowest chromosomal number for the genus, and chromosomal variability among populations of the species highlights the need for taxonomic revision of this species using an integrative approach. Although Sericomyrmex spp. are morphologically complex (Ješovnik and Schultz 2017), karyotype differences were observed in this study, highlighting cytogenetics as an important tool for integrative taxonomy. Cytogenetic data obtained for S.maravalhas suggested centric fission events during chromosomal evolution in Sericomyrmex. Inversions seem to be involved in the origin of location of 18S ribosomal genes in M.relictus and S.maravalhas. Therefore, this study provides new insights into chromosomal evolution in Sericomyrmex, Cyphomyrmex, and Mycetomoellerius. Our data suggest that chromosomal rearrangements have contributed to the species diversification in Attina. We also believe that the increase in the number of species studied using classical and molecular cytogenetic techniques will continue to contribute to discussions about the evolution of fungus-farming ants.
Acknowledgements
We are grateful to Dr. Jacques H. C. Delabie for species identification. GAT thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship granted. This study was supported by Programa de Auxílio ao Pesquisador PAPESQ/UNIFAP.
Citation
Teixeira GA, Barros LAC, de Aguiar HJAC, Lopes DM (2021) Distribution of GC-rich heterochromatin and ribosomal genes in three fungus-farming ants (Myrmicinae, Attini, Attina): insights on chromosomal evolution. CompCytogen 15(4): 413–428. https://doi.org/10.3897/compcytogen.v15.i4.73769
ORCID
Gisele Amaro Teixeira https://orcid.org/0000-0002-7106-5798
Luísa Antônia Campos Barros https://orcid.org/0000-0002-1501-4734
Hilton Jeferson Alves Cardoso de Aguiar https://orcid.org/0000-0001-7738-1460
Denilce Meneses Lopes https://orcid.org/0000-0001-7209-4411
References
- Aguiar HJAC, Barros LAC, Alves DR, Mariano CSF, Delabie JHC, Pompolo SG. (2017) Cytogenetic studies on populations of Camponotusrufipes (Fabricius, 1775) and Camponotusrenggeri Emery, 1894 (Formicidae: Formicinae). PLoS ONE 12(5): e0177702. 10.1371/journal.pone.0177702 [DOI] [PMC free article] [PubMed]
- Aguiar HJAC, Barros LAC, Silveira LI, Petitclerc F, Etienne S, Orivel J. (2020) Cytogenetic data for sixteen ant species from North-eastern Amazonia with phylogenetic insights into three subfamilies. Comparative Cytogenetics 14(1): 43–60. 10.3897/CompCytogen.v14i1.46692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LAC, Aguiar HJAC, Mariano CSF, Delabie JHC, Pompolo SG. (2010) Cytogenetic characterization of the lower-Attine Mycocepurusgoeldii (Formicidae: Myrmicinae: Attini). Sociobiology 56: 57–66. [Google Scholar]
- Barros LAC, Aguiar HJAC, Mariano CSF, Delabie JHC, Pompolo SG. (2013a) Cytogenetic characterization of the ant Trachymyrmexfuscus Emery, 1934 (Formicidae: Myrmicinae: Attini) with the description of a chromosomal polymorphism. Annales de la Société Entomologique de France 49: 367–373. 10.1080/00379271.2013.856201 [DOI] [Google Scholar]
- Barros LAC, Mariano CSF, Pompolo SG. (2013b) Cytogenetic studies of five taxa of the tribe Attini (Formicidae: Myrmicinae). Caryologia: International Journal of Cytology, Cytosystematics and Cytogenetics 66(1): 59–64. 10.1080/00087114.2013.780443 [DOI] [Google Scholar]
- Barros LAC, Aguiar HJAC, Mariano CSF, Andrade-Souza V, Costa MA, Delabie JHC, Pompolo SG. (2016) Cytogenetic data on six leafcutter ants of the genus Acromyrmex Mayr, 1865 (Hymenoptera, Formicidae, Myrmicinae): insights into chromosome evolution and taxonomic implications. Comparative Cytogenetics 10: 229–243. 10.3897/CompCytogen.v10i2.7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LAC, Teixeira GA, Aguiar HJAC, Lopes DM, Pompolo SG. (2018) Cytogenetic studies in Trachymyrmexholmgreni Wheeler, 1925 (Formicidae: Myrmicinae) by conventional and molecular methods. Sociobiology 65(2): 185–190. 10.13102/sociobiology.v65i2.2214 [DOI] [Google Scholar]
- Barros LAC, Aguiar HJAC, Teixeira GA, Souza DJ, Delabie JHC, Mariano CSF. (2021) Cytogenetic studies on the social parasite Acromyrmexameliae (Formicidae: Myrmicinae: Attini) and its hosts reveal chromosome fusion in Acromyrmex. Zoologischer Anzeiger 293: 273–281. 10.1016/j.jcz.2021.06.012 [DOI] [Google Scholar]
- Bolton B. (2021) An online catalog of the ants of the world. http://antcat.org [Accessed 15 January 2021]
- Cardoso DC, Heinze J, Moura MN, Cristiano MP. (2018) Chromosomal variation among populations of a fungus-farming ant: implications for karyotype evolution and potential restriction to gene flow. BMC Evolutionary Biology 18(1): 1–10. 10.1186/s12862-018-1247-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano MP, Cardoso DC, Sandoval‐Gómez VE, Simões‐Gomes FC. (2020) Amoimyrmex Cristiano, Cardoso & Sandoval, gen. nov. (Hymenoptera: Formicidae): a new genus of leaf‐cutting ants revealed by multilocus molecular phylogenetic and morphological analyses. Austral Entomology 59(4): 643–676. 10.1111/aen.12493 [DOI] [Google Scholar]
- Faria R, Navarro A. (2010) Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends in Ecology & Evolution 25(11): 660–669. 10.1016/j.tree.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Fernandez-Bou AS, Dierick D, Swanson AC, Allen MF, Alvarado AGF, Artavia-León A, Carrasquillo-Quintana O, Lachman DA, Oberbauer S, Pinto-Tomás AA, Rodríguez-Reyes Y, Rundel P, Schwendenmann L, Zelikova TJ, Harmon TC. (2019) The role of the ecosystem engineer, the leaf‐cutter ant Attacephalotes, on soil CO2 dynamics in a wet tropical rainforest. Journal of Geophysical Research: Biogeosciences 124(2): 260–273. 10.1029/2018JG004723 [DOI]
- Imai H, Taylor RW, Crosland MW, Crozier RH. (1988) Modes of spontaneous chromossomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Japanese Journal of Genetics 63: 159–185. 10.1266/jjg.63.159 [DOI] [PubMed] [Google Scholar]
- Imai HT, Taylor RW, Crozier RH. (1994) Experimental bases for the minimum interaction theory. Chromosome evolution in ants of the Myrmeciapilosula species complex (Hymenoptera: Formicidae: Myrmeciinae). Japanese Journal of Genetics 69: 137–182. 10.1266/jjg.69.137 [DOI] [Google Scholar]
- Ješovnik A, Schultz TR. (2017) Revision of the fungus-farming ant genus Sericomyrmex Mayr (Hymenoptera, Formicidae, Myrmicinae). Zootaxa 670: 1–109. 10.3897/zookeys.670.11839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ješovnik A, Sosa-Calvo J, Lloyd MW, Branstetter MG, Fernández F, Schultz TR. (2017) Phylogenomic species delimitation and host-symbiont coevolution in the fungus-farming ant genus Sericomyrmex Mayr (Hymenoptera: Formicidae): ultraconserved elements (UCEs) resolve a recente radiation. Systematic Entomology 42: 523–542. 10.1111/syen.12228 [DOI] [Google Scholar]
- Leal IR, Oliveira PS. (1998) Interactions between fungus-growing ants (Attini), fruits, and seeds in cerrado vegetation in southeast Brazil. Biotropica 30: 170–178. 10.1111/j.1744-7429.1998.tb00052.x [DOI] [Google Scholar]
- Levan A, Fredga K, Sandberg A. (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52: 201–220. 10.1111/j.1601-5223.1964.tb01953.x [DOI] [Google Scholar]
- Little AEF, Murakami T, Mueller UG, Currie CR. (2005) Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biology Letters 2(1): 12–16. 10.1098/rsbl.2005.0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano CSF, Santos IS, Groc S, Leroy C, Malé PJ, Ruin-González MX, Cerdan P, Dejean A, Delabie JHC. (2011) The karyotypes of Gigantiopsdestructor (Fabricius) and other ants from French Guiana (Formicidae). Annales de la Société Entomologique de France 47(1–2): 140–146. 10.1080/00379271.2011.10697705 [DOI] [Google Scholar]
- Mariano CSF, Pompolo SG, Silva JG, Delabie JHC. (2012) Contribution of cytogenetics to the debate on the paraphyly of Pachycondyla spp. (Hymenoptera, Formicidae, Ponerinae). Psyche: A Journal of Entomology 2012: 1–9. 10.1155/2012/973897 [DOI] [Google Scholar]
- Mariano CSF, Barros LAC, Velasco YM, Guimarães IN, Pompolo SG, Delabie JHC. (2019) Citogenética de hormigas de la región neotropical. In: Fernández F, Guerrero R, Delsinne T. (Eds) Hormigas de Colombia.Universidad Nacional de Colombia, Bogotá, 131–157. [In Spanish]
- Mehdiabadi NJ, Schultz TR. (2010) Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecological News 13: 37–55. [Google Scholar]
- Menezes RS, Cabral‐de‐Mello DC, Milani D, Bardella VB, Almeida EA. (2021) The relevance of chromosome fissions for major ribosomal DNA dispersion in hymenopteran insects. Journal of Evolutionary Biology 34(9): 1466–1476. 10.1111/jeb.13909 [DOI] [PubMed] [Google Scholar]
- Micolino R, Cristiano MP, Travenzoli NM, Lopes D, Cardoso DC. (2019a) Chromosomal dynamics in space and time: evolutionary history of Mycetophylax ants across past climatic changes in the Brazilian Atlantic coast. Scientific Reports 9(1): 1–13. 10.1038/s41598-019-55135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micolino R, Cristiano MP, Cardoso DC. (2019b) Population-based cytogenetic banding analysis and phylogenetic relationships of the neotropical fungus-farming ant Trachymyrmexholmgreni Wheeler, 1925. Cytogenetic and Genome Research 159(3): 151–161. 10.1159/000503913 [DOI] [PubMed] [Google Scholar]
- Micolino R, Cristiano MP, Cardoso DC. (2020) Karyotype and putative chromosomal inversion suggested by integration of cytogenetic and molecular data of the fungus-farming ant Mycetomoelleriusiheringi Emery, 1888. Comparative Cytogenetics 14(2): 197–210. 10.3897/CompCytogen.v14i2.49846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Fujiwara A, Yoshida MC. (1998) Cytogenetics of ten ant species of the tribe Attini (Hymenoptera, Formicidae) in Barro Colorado Island, Panama. Chromosome Science 2: 135–139. [Google Scholar]
- Nygaard S, Hu H, Li C, Schiøtt M, Chen Z, Yang Z, Xie Q, Ma C, Deng Y, Dikow RB, Rabeling C, Nash DR, Wcislo WT, Brady SG, Schultz TR, Zhang G, Boomsma JJ. (2016) Reciprocal genomic evolution in the ant–fungus agricultural symbiosis. Nature Communications 7(1): 1–9. 10.1038/ncomms12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JOP. (2006) Diversidade genética da abelha sem ferrão Meliponaquinquefasciata baseada no sequenciamento das regiões ITS1 parcial e 18S do DNA ribossômico nuclear. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 141 pp. [In Portuguese] [Google Scholar]
- Pinkel D, Straume T, Gray JW. (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proceedings of the National Academy of Sciences of the United States of America 83: 2934–2938. 10.1073/pnas.83.9.2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesemberg LH. (2001) Chromosomal rearrangements and speciation. Trends in Ecology & Evolution 7(1): 351–358. 10.1016/S0169-5347(01)02187-5 [DOI] [PubMed] [Google Scholar]
- Santos IS, Mariano CSF, Delabie JHC, Costa MA, Carvalho AF, Silva JG. (2016) “Much more than a neck”: karyotype differentiation between Dolichoderusattelaboides (Fabricius, 1775) and Dolichoderusdecollatus F. Smith, 1858 (Hymenoptera: Formicidae) and karyotypic diversity of five other Neotropical species of Dolichoderus Lund, 1831. Myrmecological News 23: 61–69. [Google Scholar]
- Schultz TR, Brady SG. (2008) Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences of the United States of America 105: 5435–5440. 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer D. (1980) Simultaneous fluorescent staining of R bands and specific heterocromatic regions (DA/DAPI-bands) in human chromosomes. Cytogenetics and Cell Genetics 27: 190–193. 10.1159/000131482 [DOI] [PubMed] [Google Scholar]
- Solomon SE, Rabeling C, Sosa‐Calvo J, Lopes CT, Rodrigues A, Vasconcelos HL, Bacci Jr M, Mueller UG, Schultz TR. (2019) The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher‐attine’ ant agriculture. Systematic Entomology 44(4): 939–956. 10.1111/syen.12370 [DOI] [Google Scholar]
- Sosa-Calvo J, Ješovnik A, Vasconcelos HL, Bacci Jr M, Schultz TR. (2017) Rediscovery of the enigmatic fungus-farming ant “Mycetosoritis” asper Mayr (Hymenoptera: Formicidae): implications for taxonomy, phylogeny, and the evolution of agriculture in ants. PLoS ONE 12: e0176498. 10.1371/journal.pone.0176498 [DOI] [PMC free article] [PubMed]
- Sosa-Calvo J, Schultz TR, Ješovnik A, Dahan RA, Rabeling C. (2018) Evolution, systematics, and natural history of a new genus of cryptobiotic fungus-growing ants. Systematic Entomology 43: 549–567. 10.1111/syen.12289 [DOI] [Google Scholar]
- Sumner AT. (1972) A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 83: 304–306. 10.1016/0014-4827(72)90558-7 [DOI] [PubMed] [Google Scholar]
- Teixeira GA, Barros LAC, Aguiar HJAC, Pompolo SG. (2017) Comparative physical mapping of 18S rDNA in the karyotypes of six leafcutter ant species of the genera Atta and Acromyrmex (Formicidae: Myrmicinae). Genetica 145: 351–357. 10.1007/s10709-017-9970-1 [DOI] [PubMed] [Google Scholar]
- Teixeira GA, Aguiar HJAC, Peticlerc F, Jerome O, Lopes DM, Barros LAC. (2021) Evolutionary insights into the genomic organization of major ribosomal DNA in ant chromosomes. Insect Molecular Biology 30(3): 340–354. 10.1111/imb.12699 [DOI] [PubMed] [Google Scholar]
- Ward PS, Brady SG, Fisher BL, Schultz TR. (2015) The evolution of Myrmicinae ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Systematic Entomology 40: 61–81. 10.1111/syen.12090 [DOI] [Google Scholar]
- Weber NA. (1966) Fungus-Growing Ants. Science 153: 587–604. 10.1126/science.153.3736.587 [DOI] [PubMed] [Google Scholar]