Summary
Objectives:
Benzodiazepines are the standard of care for the management of sustained seizure emergencies, including status epilepticus (SE) and seizure clusters. Seizure clusters are a variably defined seizure emergency wherein a patient has multiple seizures above a baseline rate, with intervening periods of recovery, distinguishing clusters from SE. Although these seizure emergencies are phenotypically distinct, the precise pathophysiological and mechanistic differences between SE and seizure clusters are understudied. Emergency-specific preclinical models may differentiate the behavioral and pathological mechanisms that are acutely associated with seizure emergencies and seizure termination to better manage these events.
Methods:
Herein we characterize a novel model of sustained seizure emergency induced in CF-1 mice through the combined administration of high-dose phenytoin (PHT; 50 mg/kg, i.p.) and pentylenetetrazol (PTZ; 100 mg/kg, s.c.).
Results:
We presently describe a mouse model of sustained seizure emergency that is pathologically, pharmacologically, and behaviorally distinct from SE. Acute administration of PHT 1 h prior to PTZ led to significantly more mice with unremitting continuous seizure activity (CSA; 73.4%) vs vehicle-pretreated mice (13.8%; p < .0001). CSA was sensitive to lorazepam and valproic acid when administered at seizure onset and 30 minutes later. Carbamazepine worsened seizure control and post-CSA survival. Mice in CSA exhibited electroencephalography (EEG) patterns distinct from kainic acid–induced SE and PTZ alone, clearly differentiating CSA from SE and PTZ-induced myoclonic seizures. Neuropathological assessment by Fluoro-Jade C staining of brains collected 24 h post-CSA revealed no neurodegeneration in any mouse that underwent CSA, whereas there was widespread neuronal death in brains from KA-SE mice. Finally, immunohistochemistry revealed acute seizure-induced astrogliosis (glial fibrillary acid protein; GFAP) in hippocampal structures, whereas hippocampal neuronal nuclei (NeuN) protein expression was only reduced in KA-SE mice.
Significance:
We present a novel mouse model on which to further elucidate the mechanistic differences between sustained seizure emergencies (ie, SE and seizure clusters) to improve clinical interventions and define mechanisms of seizure termination.
Keywords: astrocytes, carbamazepine, continuous seizure activity, pentylenetetrazol, phenytoin
1 |. INTRODUCTION
Although most are brief, extensively long seizures require pharmacological intervention. Seizure emergencies include status epilepticus (SE) and seizure clusters. Clinically, SE is defined as a continuous seizure lasting >5 min or two or more sequential seizures without intervening recovery of consciousness.1 SE affects 50 000–150 000 people in the United States each year, with associated adult mortality ranging from 10% to 30%.2,3 Between 4% and 16% of patients with epilepsy will have at least one SE event.4 Conversely, seizure clusters constitute a seizure emergency broadly defined by acute episodes wherein seizure control is interrupted by recurrent seizures lasting <5 min with bouts of seizure freedom.5,6 This seizure cessation and onset cycle uniquely differentiates clusters from SE. However, a lack of consensus in clinical definition may lead to great variability in clinical prevalence.7 In any case, cluster seizures are a medical emergency unique to patients with epilepsy, whereas SE can present in any individual, highlighting a key pathogenic difference.
The pharmacological sensitivity of each seizure emergency depends on a multitude of factors. Seizure clusters remain acutely sensitive to benzodiazepines (BDZs) no matter the delay in which rescue intervention is administered after cluster onset.8–11 Conversely, SE can quickly become refractory to BDZs in as little as 10 min after onset in preclinical models,12–14 leading to increased morbidity and mortality.1,15–17 However, if left untreated, cluster seizures can rapidly progress to SE,18–20 implicating conserved mechanisms in two otherwise distinct indications.
Numerous models of SE reproduce the clinical facets of SE, including behavioral and electrographic features, BDZ resistance, high seizure-induced mortality, and neurodegeneration.21,22 Kainic acid (KA)–induced SE in rodents produces a sustained behavioral and electrographic seizure that can become refractory to delayed administration of BDZs and cause neuronal death throughout the limbic system.14 Rat and mouse models of KA-induced SE have been instrumental in understanding pathogenic mechanisms,23 defining biological underpinnings,14,24 and identifying novel interventions.25 It is important to note that rodent models of spontaneous recurrent seizures, including those that arise post-SE, experience sporadic and infrequent “cluster” seizures.26,27 However, there is as yet no reliable way to evoke cluster seizures in epilepsy models because of the high degree of interindividual variability28 and lack of consensus definition of cluster seizures. It has thus been challenging to consistently and reliably model all types of clinical seizure emergencies to elucidate the pathophysiological differences between SE and seizure clusters.
Acute administration of high doses of sodium channel-blocking antiseizure drugs (ASDs), including phenytoin (PHT), can be proconvulsant and reduce seizure threshold in rodents.29,30 Previous studies have suggested that high-dose PHT pretreatment in the subcutaneous pentylenetetrazol (s.c.PTZ) model of myoclonic seizures in mice results in continuous seizures that were sensitive to midazolam and thus by de facto represented SE.31 Furthermore, others have shown that PHT may potentiate the efficacy of various BDZs in the mouse s.c.PTZ model.32,33 Yet, the phenotypic, neuropathologic, and electrographic characterization of this model has been notably absent. In combination with our serendipitous observations and prior publications demonstrating a proconvulsant effect of high-dose PHT,29–31 we sought to define whether the seizure induced by high-dose PHT administration in the s.c.PTZ model would generate a sustained seizure with a distinguishable behavioral phenotype, electroencephalography (EEG) profile, or neuropathology.21,29 Mice pretreated with high-dose PHT prior to s.c.PTZ exhibit worsened acute seizure severity and duration; we thus hypothesized that this drug combination and resulting aberrant continuous seizure activity (CSA) represented a preclinical seizure that is behaviorally similar to, yet pharmacologically and pathologically distinct from, SE. The present investigation thus sought to establish the phenotypic, electrographic, and neuropathologic features of this mouse model of CSA. The present CSA model is additionally suitable for high-throughput drug screening to potentially identify new treatments for non-SE seizure emergencies. Furthermore, we present a new preclinical platform, which when compared with SE models may help to clarify the mechanistic basis underlying sustained seizures, seizure termination, and neurodegeneration.
2 |. METHODS
2.1 |. Animals
Male CF-1 mice (25–40 g; 5 to 6 weeks old; Envigo) were housed five mice/cage with corncob bedding in a temperature-controlled specific pathogen-free vivarium on a 14:10 light/dark cycle (on: 0600 hours, off: 2000 hours). Animals were given free access to irradiated chow (Picolab 5053) and filtered water, except during periods of behavioral manipulation. Mice were allowed to acclimate to the housing facility for at least 5 days and to the testing room for at least 1 h prior to testing. All studies were conducted during the animals’ light phase. Animals were euthanized by CO2 asphyxiation. This study was not designed to assess the impact of sex as a biological variable, thus only male mice were used. All animal use was approved by the University of Washington Institutional Animal Care and Use Committee, conformed to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines,34 and was conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
2.2 |. Chemical compounds
PTZ (catalog #P6500), PHT (catalog #PHR1139), methylcellulose (VEH; catalog #M0430), valproic acid (VPA; catalog #P4543), carbamazepine (CBZ; catalog #C4024), potassium permanganate (KMnO4; catalog #223468), xylenes (catalog #XX0060), xylazine (X1251–1G), and DPX mounting medium (catalog #06522) were all from Sigma Aldrich (St. Louis, MO, USA). Lorazepam (LZP; NDC 0641–6046-01) was from Westward Pharmaceuticals as a solution, which was further diluted in 40% hydroxypropyl-β--cyclodextrin. Formal Fixx (catalog #9990244), Hoeschst 33342 (catalog #62249), and NaOH (catalog #SS254) were from Thermo Fisher Scientific (Waltham, MA, USA). Fluoro-Jade C (FJ-C; catalog #1FJC) was from Histo-Chem Inc (Jefferson, AR, USA). Kainic acid (KA; catalog #0222) was from Tocris (Bristol, UK). Ketamine (Ketaset) was from Zoetis, Inc (Parsippany, NJ). Anticonvulsant drugs (PHT; CBZ; VPA) administered to mice were suspended in VEH; PTZ was administered after dissolving in saline.
2.3 |. Induction of continuous seizure activity
Each mouse was pre-treated with either 50 mg/kg PHT or VEH administered by the i.p. route (n = 8/pre-treatment group). One hour later, 100 mg/kg PTZ was administered s.c. to each mouse, which was then placed in an individual observation chamber (7.6H × 10.1W × 12.7L cm). At this dose, the seizure associated with s.c.PTZ is characterized by hindlimb extension and differs phenotypically from the minimal clonic seizure endpoint typically evaluated in drug-intervention studies.35 The latency to onset of the PTZ-induced seizure, characterized by first twitch, clonic seizures, and onset of CSA, was recorded for each mouse. Onset of CSA was visually defined as the time when the mouse jumped violently and then exhibited unremitting forelimb and hindlimb clonus. Body weights were recorded 24 h post-CSA and brains were collected for histology.
2.4 |. Assessing the impact of pharmacological intervention on continuous seizure activity
To define whether CSA was phenotypically distinct from acute SE, we employed two ASD intervention paradigms (all i.p.): (1) immediate intervention (administration of ASDs within ≤1 min CSA onset); or (2) delayed intervention (administration of ASDs 30 min post-CSA onset). Once a mouse entered CSA, it was randomized to receive VEH (0.5% methylcellulose), CBZ (20 or 40 mg/kg), LZP (2 or 4 mg/kg), or VPA (150 or 300 mg/kg). Each mouse was then monitored for 1 h and all seizure activity was recorded. There were n = 8 mice/group, which gives 80% power at 95% significance to detect a 1.5 standard deviation difference in seizure severity between each group vs VEH with p < .05. Low and high doses of VPA and CBZ were chosen as those that can block a maximal electroshock (MES) tonic hindlimb extension seizure in 50% of mice (ED50) and produce rotarod impairment in 50% of mice (TD50), respectively.36,37 The dose of LZP was selected based on our ED50 in CF-1 mice in the MES test (1.20 mg/kg [95% confidence interval (CI) 0.801–1.79 mg/kg]; not shown) and was consistent with the efficacy of other BDZs in the MES and rotarod tests.36,37
2.5 |. Kainic acid–induced status epilepticus
Status epilepticus was induced with KA according to a modified method for C57BL/6 mice.38 Male CF-1 mice (n = 10 for FJ-C histology; n = 6 for immunohistochemistry; n = 3 for EEG) were treated with an initial 10 mg/kg (i.p.) dose of KA, followed by an additional 5 mg/kg (i.p.) dose of KA every 20 min until the presentation of multiple Racine stage 4 or stage 5 generalized seizures. Seizures were recorded during the SE induction period and for 1 h after the onset of secondarily generalized behavioral seizures. Brains of mice that survived for 24 h after the onset of SE were collected by flash-freezing on dry ice.
2.6 |. Surgical implantation of EEG electrodes for video-EEG monitoring
A separate cohort of mice (n = 12) was used for video-EEG (vEEG) recordings. Mice were anesthetized with ketamine/xylazine (100/10 mg/kg; i.p.) and placed in a stereotaxic instrument. A midline incision of ~1–2 cm was made exposing the skull. A three-channel stainless steel electrode unit (MS333/1-A; Plastics One, Roanoke, VA) was placed in two holes, which were drilled bilaterally, 4 mm posterior and lateral to bregma to overlay the hippocampus. The third electrode was placed as a ground posterior to lambda to overlay the cerebellum, a region known to be electrically silent. Three stainless steel mounting screws (00–96 X 1/16; InVivo1, Roanoke, VA) were placed, two posterior and lateral to the hippocampal electrodes and one anterior and lateral to bregma.39 The entire assembly was anchored with dental cement (Lang, 1223CLR). Mice were allowed to recover from anesthesia on a heating pad until ambulatory, and given oral Rimadyl (Bio-Serve, MD150–2) as an analgesic (0.5 mg). All implanted mice were individually housed and allowed to recover for 1 week after surgery.
2.7 |. Video-EEG monitoring of electrographic seizures
On the vEEG acquisition day, implanted mice (n = 12) were connected to EEG electrodes and allowed 1 h to acclimate to the room before obtaining a baseline 1 h recording. Implanted mice were randomized into one of three experimental groups: VEH + PTZ (n = 3; ie, s.c.PTZ control group); KA-induced SE (n = 3); or PHT + PTZ induced CSA (n = 6). Recording of behavioral and electrographic seizures continued for >2 h after onset for each experimental condition.
Video-EEG was recorded using a customized data acquisition system with an MP160 and EEC100 (Biopac, Goleta, CA) and three-channel electrodes (InVivo1).28,39 The electrographic seizure analysis was conducted offline following completion of in-life studies using the baseline and active seizure period according to the following methods: the average power of two, 10 min EEG recording bins was first defined from the 1 h baseline period. Then, the average power of two, 10 min active EEG recording bins was defined from the 1 h period surrounding the onset of active VEH + PTZ induced clonic seizures, KA-induced SE, or PHT + PTZ induced CSA. The 10 min recording period for both baseline and active seizures was binned in 30 s epochs by Assyst EEG analysis software (KaosKey; Sydney, Australia). The time of onset of clonic seizures, SE, or CSA was visually noted by a trained investigator and the EEG spectrum was analyzed for each mouse in the 1 h period immediately following the seizure onset.
2.8 |. Fluoro-Jade C assessment of post-insult neurodegeneration
Mice for histology (n = 15 for KA-SE; n = 16 for VEH-rescued CSA; n = 6 for VEH-naive) were euthanized 24 h after seizure onset, and the brains were removed, flash frozen, and maintained at −80°C until processing. Two consecutive 20 μm-thick sections/mouse from the dorsal hippocampus (anteroposterior [AP] from Bregma: −1.34 mm) and ventral hippocampus (AP from Bregma: −2.24 mm) were sectioned on a cryostat (Leica DM1860) and slide-mounted. Slides were fixed in 4% Formal Fixx (10 min) and rinsed in DI water followed by FJ-C and Hoechst staining.40,41 Slides were imaged on an upright fluorescent microscope (Leica DM-4) with a 10x objective (40× final magnification) with constant acquisition settings. Photomicrographs (n = 4 brain sections/mouse) were analyzed by an investigator who was blinded to treatment and visually scored for the presence of FJ-C positive cells in limbic structures (CA1, CA3, and dentate gyrus [DG] of dorsal hippocampus) and hypothalamic nucleus. If two or more of the consecutive sections from each mouse demonstrated FJ-C- positive staining in the designated brain region, the animal was considered to have FJ-C positive labeling.
2.9 |. Immunohistochemistry for astrocytes (GFAP) and neurons (NeuN)
A subset of tissues from mice for histology studies (n = 6 for KA-SE; n = 6 for CSA; n = 6 for VEH) was processed for semi-quantitative immunohistochemistry for neurons (NeuN) and astrocytes (glial fibrillary acidic protein; GFAP). Briefly, NeuN directly conjugated to Alexa Fluor488 (1:300; MAB377X, Millipore) and GFAP directly conjugated to Cy3 fluorophore (1:1000; C9205 Sigma-Aldrich) antibodies were applied under 200 μL coverwells in a 5% goat serum in 1 × phosphate-buffered saline (PBS) solution overnight at 4°C according to previously published methods.42–44 Photomicrographs were acquired as above for FJ-C at 20× magnification and processed by semi-quantitative analysis of percent field area with fluorescently labeled signal, as previously published.42
2.10 |. Statistics
The dose of PTZ necessary to elicit sustained twitch and clonus in >99% of mice (convulsant dose, CD99) was calculated by Probit.45,46 Weights (in grams) and latency to any seizure (in seconds) for acute pharmacological studies were analyzed by one-way analysis of variance (ANOVA). The number of mice displaying CSA in acute pharmacological studies and mortality rates were compared by Fisher’s exact test. Survival after CSA onset was determined with a Kaplan-Meier plot. The number of animals in each treatment group with FJ-C positive cells within each brain region were compared by Fisher’s exact test; semi-quantitative analysis of NeuN and GFAP immunoreactivity within each brain region were compared by one-way ANOVA with Tukey’s post hoc tests. Except for CD99, all statistical analysis was performed in GraphPad Prism version 8.0 or later, with p < .05 considered significant.
3 |. RESULTS
3.1 |. Pentylenetetrazol and phenytoin dose responses
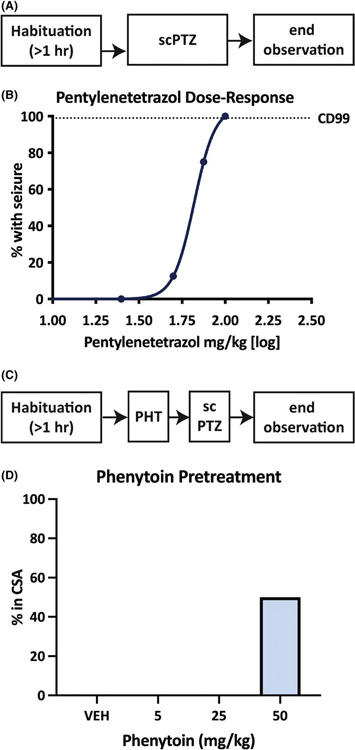
We first sought to establish the dose of s.c.PTZ necessary to reliably induce seizures in >99% of mice (CD99), as well as the dose of PHT necessary to elicit CSA with low mortality when administered in combination of s.c.PTZ. The CD99 of s.c.PTZ was 102 mg/kg [95% CI 83.4–220], thus 100 mg/kg PTZ was used for further studies (Figure 1B). We then quantified the dose-related impact of PHT on the conversion to CSA following administration of 100 mg/kg of PTZ (Figure 1D); pretreatment of mice with high dose PHT (50 mg/kg) induced CSA rather than typical myoclonic seizures, consistent with previous findings.31 Thus subsequent behavioral, pharmacological, and electrographic studies were conducted with this combination; that is, 50 mg/kg, i.p., PHT administered 1 h before 100 mg/kg s.c.PTZ.
FIGURE 1.
Pentylenetetrazol (PTZ) administered by the subcutaneous (s.c.) route normally induces clonic seizures in male CF-1 mice in a dose-related manner. This clonic seizure was shifted to continuous seizure activity (CSA) with pre-administration of high-dose intraperitoneal (i.p.) phenytoin (PHT). (A) The timeline of dose-response studies to determine convulsant doses of PTZ in male CF-1 mice. Mice were allowed to habituate to the testing environment for 1 h prior to administration of escalating doses of s.c.PTZ. (B) The doses of s.c.PTZ were administered until at least two points could be established between the limits of 0% and 100% of mice with seizures to define the convulsant dose of 99% of mice (CD99). The CD99 of s.c.PTZ was determined to be 102 mg/kg. (C) The timeline of the experimental design used to determine the pretreatment dose of i.p. PHT that would result in CSA in male CF-1 mice treated with s.c.PTZ (100 mg/kg). (D) Administration of 50 mg/kg PHT 1 h prior to s.c.PTZ resulted in CSA in at least 50% of tested mice (n = 4), which aligned with our pilot studies to develop this model (not shown) and previously published work.31 Administration of lower doses of PHT 1 h prior to s.c.PTZ administration did not result in CSA onset in any mice
3.2 |. Phenytoin pretreatment precipitates onset of continuous seizure activity
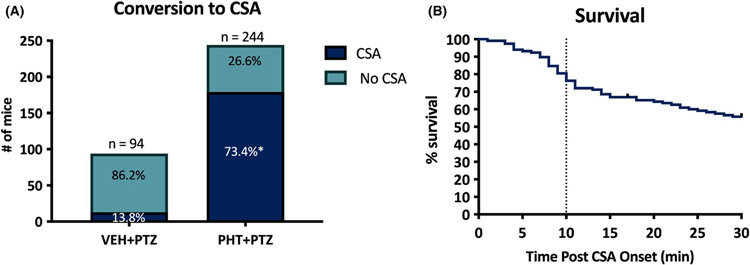
Of the 94 mice pretreated with VEH 1 h prior to 100 mg/kg s.c.PTZ, 13 of 94 (13.8%) developed seizures characteristic of CSA as defined above (Figure 2A). In contrast, 179 of 244 mice (73%) pretreated with high-dose PHT (50 mg/kg) 1 h prior to 100 mg/kg s.c.PTZ developed CSA (Fisher’s exact test, p < .0001; Figure 2A). We then characterized the progression and stability of CSA across time in the delayed intervention studies (Figure 2B). Of the 118 mice enrolled in the delayed intervention studies after PHT + PTZ induced-CSA, 64 of 118 (54.2%) survived while remaining in CSA to the 30 min window to become candidates for pharmacological intervention. Of the mice that did not survive until the 30 min intervention window, the median latency to mortality after CSA onset without pharmacological intervention was 10 min (Figure 2B). Thus there was a notable selection bias for mice that survived the CSA insult to the 30 min intervention. If a mouse survived to the 30 min intervention, it was more likely to survive, which may have also contributed to the discrepancy in improved survival between VEH-pretreated animals in the immediate and delayed intervention studies (Table 1).
FIGURE 2.
The combined administration of phenytoin (PHT; 50 mg/kg, i.p.) 1 h prior to s.c. administration of pentylenetetrazol (PTZ) greatly increased the number of male CF-1 mice that present with continuous seizure activity (CSA). (A) There was a significant increase in the conversion to CSA caused by PHT pretreatment vs VEH treatment alone. *Indicates p < .0001 by Fisher’s exact test. (B) PHT + PTZ treatment groups were divided into immediate (0 min post-seizure onset) and delayed (30 min post-seizure onset) intervention groups for subsequent pharmacology studies. The majority of PHT + PTZ-treated mice that presented with CSA and were candidates for delayed intervention studies survived for at least 30 min after seizure onset (64/118; 54%). Of the PHT + PTZ mice that were enrolled for delayed intervention group, 50% of mice died within 10 min of CSA onset and were thus unable to be rescued with any intervention
TABLE 1.
24-h survival after induction of continuous seizure activity (CSA) and intervention with pharmacological intervention (carbamazepine - CBZ; lorazepam - LZP; valproic acid - VPA; or vehicle - VEH) delivered by the i.p. route (number survived / number tested)
| Vehicle | CBZ 20 mg/kg |
CBZ 40 mg/kg |
LZP 2 mg/kg |
LZP 4 mg/kg |
VPA 150 mg/kg |
VPA 300 mg/kg |
|
|---|---|---|---|---|---|---|---|
| Immediate (0 min) intervention | 5/11 | 6/8 | 3/8 | 7/8 | 8/8 | 6/8 | 9/9 |
| Delayed (30 min) intervention | 12/13 | 7/8 | 3/8 | 8/8 | 9/9 | 6/8 | 8/9 |
3.3 |. Continuous seizure activity is sensitive to administration of both lorazepam and valproic acid
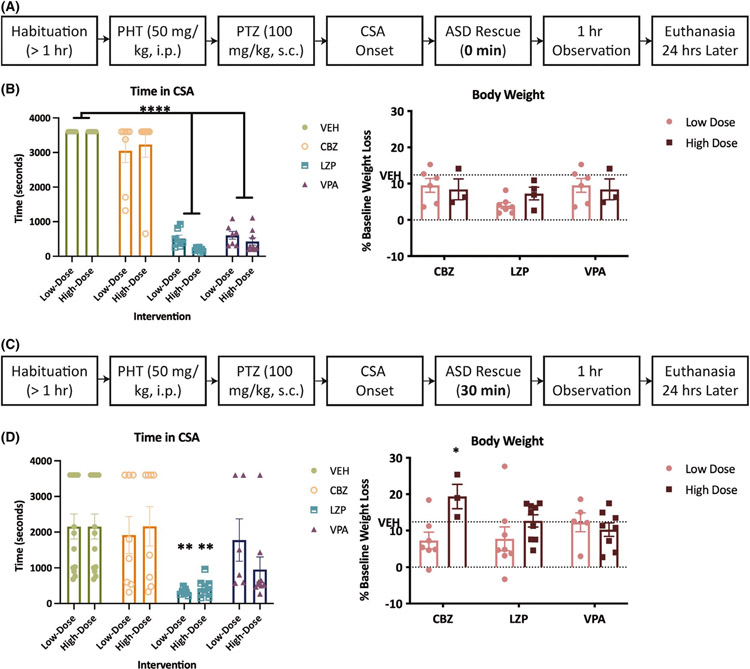
We sought to characterize the sensitivity of CSA to both immediate (0 min) and delayed (30 min) interventions using standard-of-care ASDs: LZP, a frontline rescue BDZ for both seizure clusters and SE15,47; VPA, often utilized as a second-line treatment for BDZ-resistant SE1; and CBZ, which can aggravate seizure clusters in humans19,48,49 and rodent models.26 Pharmacological intervention within 1 min of CSA onset reduced total time in CSA (F(3,61) = 195.7, p < .0001). However, only LZP and VPA significantly reduced the time in CSA (p < .0001; Figure 3B). Conversely, immediate administration of escalating doses of CBZ did not impact CSA duration. Immediate intervention with high doses of both LZP and VPA significantly improved survival (Table 1). Finally, immediate administration of all rescue therapies significantly attenuated CSA-induced 24 h body weight loss vs vehicle-treated CSA mice (F(3,27) = 3.750, p = .0225); however, only surviving mice were weighed at this point and there was significant mortality in VEH-treated mice (Table 1). Therefore, immediate intervention with both LZP and VPA markedly reduced CSA duration, improved survival, and reduced CSA-induced body weight loss, whereas immediate intervention with CBZ did not improve any outcome measures aside from improving 24 h body weight.
FIGURE 3.
Administration of the antiseizure drugs (ASDs) lorazepam (LZP) and valproic acid (VPA) but not carbamazepine (CBZ) reduced the duration of phenytoin (PHT) + pentylenetetrazol (PTZ)–induced continuous seizure activity (CSA) when administered immediately (0 min; A,B) after CSA onset. LZP also reduced CSA duration when administered 30 min after CSA onset (C,D) relative to mice treated with vehicle. (A) The timeline of the immediate intervention paradigm to define the pharmacological efficacy of ASDs in this model of seizure clusters. (B) There was a significant effect of treatment on CSA duration in mice treated with both doses of LZP (2, 4 mg/kg) and VPA (150, 300 mg/kg) vs vehicle (F(3,61) = 195.7, p < .0001). Administration of CBZ immediately after seizure onset did not significantly reduce CSA duration at either a low or high dose. Data are shown as mean percent body weight loss (±SEM) in 24 h. ****Indicates significantly different from VEH-treated mice, p < .0001. (C) The timeline of the delayed intervention paradigm to define the pharmacological efficacy of antiseizure drugs in this model of seizure clusters. (D) There was a significant effect of ASD treatment on CSA duration only in mice treated with low- and high-dose LZP vs vehicle (F(3,66) = 7.246, p = .0003). Administration of VPA and CBZ 30 min after seizure onset did not significantly reduce CSA duration at either a low or high dose. Only LZP effectively reduced seizure duration when administered 30 min after CSA onset; **indicates p < .01. Data are shown as mean percent body weight loss (±SEM) in 24 h. Furthermore, 40 mg/kg CBZ significantly increased the CSA-induced body weight loss relative to VEH-treated mice; *indicates p = .037
Because SE in humans and preclinical models can become refractory to BDZs, we sought to establish whether CSA was also refractory to any intervention administered 30 min post-CSA (Figure 3C). Delayed intervention significantly reduced the total time in CSA (F(3,66) = 7.246, p = .0003). However, only LZP reduced the time in CSA in a dose-related fashion (p < .01; Figure 3D). CBZ and VPA were without significant effect on CSA duration at any dose tested. Delayed intervention with 4 mg/kg LZP also significantly improved survival from CSA (Table 1). There was a main effect of drug dose on 24 h body weight loss (Figure 3D; F(1,34) = 5.20, p = .029); CBZ-treated mice (40 mg/kg) had significantly worse CSA-induced body weight loss vs VEH-treated mice (Figure 3D; p = .0374) Thus PHT + PTZ-induced CSA remained sensitive to LZP up to 30 min post-onset, and administration of this agent alone markedly improved acute CSA-induced behavioral outcomes. Such efficacy of LZP starkly contrasts with traditional SE models that develop BDZ resistance within 30 min of seizure onset.
3.4 |. PHT + PTZ-induced electrographic seizure activity is distinct from KA-and s.c.PTZ-induced electrographic seizure activity
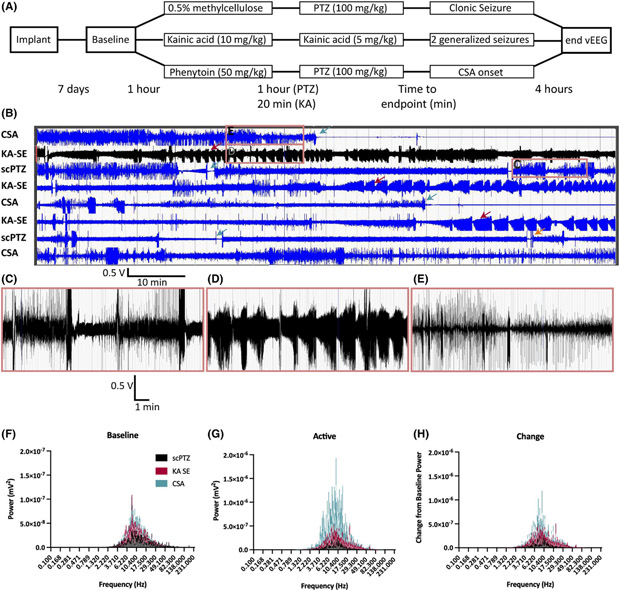
We recorded paired vEEG activity from the PHT + PTZ treated mice to characterize the electrographic seizure profile of a mouse in CSA to more clearly define whether these events were electrographically like those of KA-induced SE or s.c.PTZ-induced myoclonic seizures (Figure 4A). Paired vEEG from mice treated with VEH + PTZ (s.c.PTZ), KA (SE), and PHT + PTZ (CSA) demonstrated qualitatively distinct waveforms in the active seizure period (Figure 4B). Mice treated with VEH + PTZ showed acute electrographic patterns consistent with the behavioral hindlimb extension seizures that are prototypical of high dose s.c.PTZ (Figure 4C). Mice in KA-induced SE showed a rhythmic pattern of electrographic bursting (Figure 4D) consistent with rodent SE.25,38 Mice in CSA experienced continual electrographic activity with consistent dyssynchronous spiking throughout the recording duration, distinct from the patterns of KA-induced SE and s.c.PTZ alone (Figure 4E). All groups showed a 10-fold increase in EEG power during the active seizure vs their respective baseline, but the small group sizes of this qualitative study did not support statistical comparisons (Figure 4F–H). Nonetheless, there were clear behavioral and electrographic distinctions between s.c.PTZ, KA-SE, and CSA.
FIGURE 4.
A novel mouse model of continuous seizure activity was produced through the combined administration of PHT (50 mg/kg, i.p.) 1 h prior to PTZ (s.c., 100 mg/kg) administration. PHT + PTZ produced electrographic patterns visually distinct from those produced during KA-induced status epilepticus (SE). (A) This timeline displays the three distinct experimental flows of the clonic seizure model with VEH + PTZ, the SE model with KA, and the CSA model with PHT + PTZ. (B) 100 min of active EEG with each of the three seizure types. Green arrows indicate time of death during a seizure. Red arrows indicate mice in SE following repeated KA administration. Orange arrow indicates a mouse that replaced a deceased animal and that was pretreated with 0.5% methylcellulose vehicle prior to s.c.PTZ administration but never presented with a clonic spasm during the subsequent observation period. Scale bar for 100 min of recording. (C–E) 10 min of active EEG corresponding to an s.c.PTZ-induced clonic hindlimb extension seizure (C), KA-induced generalized seizures (D), and PHT + PTZ induced CSA (E). (F) Average baseline power at all frequencies for each testing group (n = 2, s.c.PTZ; n = 3, KA SE; n = 3, CSA mice). (G) Average power for the active seizing time measured for a 10 min period following the onset of a clonic seizure induced by s.c.PTZ, a Racine stage 4 or 5 induced by KA, or CSA induced by PHT + PTZ. The y-axis scale shows over a 10-fold increase in power during the active seizing period. (H) The change in EEG power from the baseline recording to active seizing recording periods
3.5 |. Continuous seizure activity does not induce neurodegeneration within 24 h
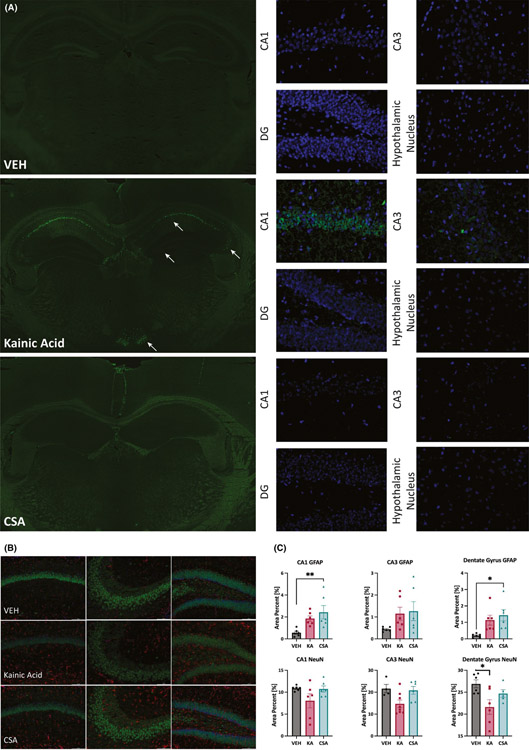
We quantified hippocampal neurodegeneration with FJ-C staining of tissues collected 24 h after visually confirmed behavioral CSA onset (n = 16) vs tissues obtained from visually confirmed KA-induced SE (n = 15 surviving/17 treated mice) and VEH-treated naive mice (n = 6). KA-SE mice demonstrated extensive neurodegeneration in CA1, CA3, and DG of dorsal hippocampus, as well as neurodegeneration in hypothalamic nucleus (Figure 5A). No mouse that entered CSA showed any neurodegeneration, whereas every mouse treated with KA had significant neurodegeneration detectable 24 h after seizure onset (Figure 5A and Table S1). Notably, treatment with ASDs did not affect FJ-C immunoreactivity in any CSA mice (Table S1). FJ-C positive staining was similarly absent in VEH-treated naive mice. This extensive and thorough evaluation conclusively demonstrates that CSA insult was not associated with neurodegeneration 24 h after insult in any brain structure examined across a diversity of treatment paradigms, starkly contrasting this model with KA-SE in CF-1 mice.
FIGURE 5.
(A) Fluoro-Jade C (FJ-C) staining of coronal sections from VEH, kainic acid (KA)–induced SE, and CSA mice collected 24 h after insult demonstrates that CSA did not induce any neurodegeneration in any limbic structure examined, like that which was observed in a vehicle-treated naive mouse (no seizure activity). FJ-C staining of whole brain slices from VEH, KA, and CSA mice only showed evidence of neurodegeneration in the hippocampus and hypothalamic nucleus of a CF-1 mouse that underwent kainic acid–induced SE. Arrows highlight regions with FJ-C positive cells, indicative of dead and dying neurons, which are observed only in KA-treated mice 24 h after SE onset. Higher magnification (40x) photomicrographs reveal that there is widespread and distinct neurodegeneration in CA1, CA3, dentate gyrus, and hypothalamic nucleus of a mouse following KA-induced SE. There were no dead and dying neurons present in the CA1, CA3, dentate gyrus, or hypothalamic nucleus following PHT + PTZ-induced CSA, or in VEH-treated mice. (B) Immunohistochemistry from (left-right) CA1, CA3, and DG for astrocytes (GFAP; red) and neurons (NeuN; green) from a subset of tissues from VEH-treated, KA-SE, and CSA mice collected for histology. DAPI (blue) was used as nuclear counter stain. (C) Semi-quantitative analysis of total field area with GFAP-positive immunolabeling and NeuN-positive immunolabeling revealed a significant main effect of seizure insult in several limbic brain structures. GFAP immunoreactivity was generally increased by seizure activity in area CA1 (F(2,15) = 6.177, p = .0110) and DG (F(2,15) = 5.932, p = .0126), with post hoc Tukey’s tests demonstrating significant differences from VEH for CSA-treated mice in CA1 (p = .0097) and DG (p = .0127). There was no main effect of seizure on GFAP immunoreactivity in area CA3. NeuN expression was also generally affected by seizure history in area CA3 (F(2,15) = 4.546, p = .0286) and DG (F(2,15) = 4.819, p = .0256). Post hoc Tukey’s test demonstrated that only KA-treated mice showed reduced NeuN expression in DG (p = .0203), although the difference in area CA3 did not attain statistical significance (p = .06). There was no main effect of seizure on CA1 NeuN expression
3.6 |. Continuous seizure activity induces reactive gliosis in hippocampus
To better define the pathological differences acutely associated with CSA and KA-induced SE, we evaluated the extent to which protein markers of astrocytes (GFAP) and neurons (NeuN) were altered in the brains of mice relative to VEH-treated animals (Figure 5B and C) 24 h after insult. Sustained seizures generally increased GFAP expression in area CA1 of the dorsal hippocampus (F(2,15) = 6.177, p = .0110), with Tukey’s post hoc test demonstrating that GFAP immunoreactivity was significantly elevated in CSA-treated vs VEH-treated mice (p = .0097), but did not reach significance in KA-SE mice (p = .0761). This effect was similarly observed in DG (F(2,15) = 5.932, p = .0126), with a significant elevation in CSA-treated vs VEH-treated mice (p = .0127), but did not reach significance in KA-SE mice (p = .0597). There was no effect of insult on GFAP immunoreactivity in area CA3. The extent of NeuN immunoreactivity in dorsal hippocampus was also generally affected by insult in area CA3 (F(2,15) = 4.546, p = .0286) and DG (F(2,15) = 4.819, p = .0256), but not CA1. However, Tukey’s post hoc tests revealed only that NeuN labeling was reduced in KA-treated mice within the DG (p = .0203); the difference did not reach statistical significance in area CA3 (p = .06). These findings further illustrate that both CSA and KA are associated with sustained behavioral seizures, but only KA significantly reduced NeuN immunoreactivity in DG.
4 |. DISCUSSION
Status epilepticus is a distinct seizure emergency characterized by prolonged seizures lasting >5 min with no recovery of consciousness. BDZs are the standard-of-care, but untreated SE can quickly become refractory to BDZs, increasing morbidity and mortality risk. We presently demonstrate that mice pretreated with high-dose PHT (50 mg/kg) 1 h prior to s.c.PTZ (100 mg/kg) exhibit a seizure phenotype distinct from the hindlimb extension seizures characteristic of s.c.PTZ.29 These seizures are unequivocally distinct from KA-induced SE at the behavioral, pharmacological, electrographic, and neuropathological levels. CSA is thus a unique seizure emergency model.
The precise mechanisms that contribute to the presently described onset of CSA following PHT + PTZ remain to be elucidated; future mechanistic studies are also needed to interrogate the processes underlying seizure termination. PHT, an ASD that blocks sodium channels, does not prevent minimal clonic seizures induced by s.c.PTZ, even at PHT doses above 100 mg/kg, but PHT effectively prevents maximal hindlimb tonic extension seizures resulting from suprathreshold doses of s.c.PTZ.29 PHT likely blocks seizure spread but does not increase seizure threshold, in contrast to other ASDs.29 The National Institute of Neurological Disorders and Stroke’s Epilepsy Therapy Screening Program has even demonstrated that PHT (>41 mg/kg) can actually decrease seizure threshold in the mouse i.v.PTZ test (https://panache.ninds.nih.gov). Consistent with earlier reports,31 we herein demonstrate that pretreatment with high-dose PHT (50 mg/kg) shifts the phenotype of high-dose s.c.PTZ-induced seizures from hindlimb tonic extension to sustained CSA. PHT + PTZ promotes CSA in over 73% of mice (n = 244 total), a phenomenon that is best described as sustained clonic seizures. Conversely, only 14% of VEH + PTZ-treated mice (n = 94 total) demonstrated CSA; the majority exhibited hindlimb extension seizures typical of suprathreshold doses of s.c.PTZ. The fact that PHT prevents seizure spread and blocks tonic hindlimb extension but reduces seizure threshold at high (ie, proconvulsant) doses likely contributed to the CSA phenomenon when PHT was administered in combination with s.c.PTZ. Seizures in patients and preclinical models will generally self-terminate within 1–2 min and seizure duration is relatively short.50 Until now, this statement generally held true for all events except SE, which likely explains the rationale for a prior study wherein combined administration of PHT + PTZ was used to explore the efficacy of midazolam for SE.31 CSA in mice is distinguished from KA-induced SE based on electrographic, behavioral, and pharmacological features, demonstrating that our model is not only distinct from acute seizures and SE, but that it is also a novel platform on which to better define the mechanisms of seizure initiation and termination.
Seizure emergencies are clinically managed with BDZs.51 The activity of LZP, a first-line therapy for both seizure clusters and SE,9,15,47 administered with a 30-min delay, demonstrates that CSA is not refractory to BDZs.31 Rodent models of SE are refractory to BDZs, consistent with clinical SE.1,52 Furthermore, VPA, a common second-line therapy for SE and seizure clusters,53–55 was as effective as LZP in reducing CSA duration and improving 24 h survival following immediate administration (Figure 3B), but in contrast to LZP, VPA lost efficacy by 30 min (Figure 3D). Efficacy of these two pharmacologically distinct, clinically effective ASDs supports the face validity of this model as a new tool to interrogate the mechanisms underlying the onset and termination of seizure emergencies.
Continuous seizure activity could also be worsened by ASD intervention. Specifically, administration of CBZ 30 min after onset did not reduce CSA duration, increased 24 h body weight loss, and instead worsened overall mortality. These findings align with clinical reports wherein CBZ and other sodium channel blockers can aggravate seizure clusters in patients with epilepsy,19,48,49 as well as in a rat model of temporal lobe epilepsy with documented spontaneous seizure clusters.26 Seizure clusters can also arise in the course of rapid withdrawal of ASDs in patients with drug-refractory epilepsy,56 representing a so-called “rebound” effect, and this clustering phenomenon may be particularly common in patients with a history of lamotrigine and other sodium channel blocking ASD use.56 In fact, Henning et al reported that of the 60 patients with drug-resistant epilepsy who were undergoing ASD withdrawal for EEG monitoring in an epilepsy monitoring unit, 25 (42%) experienced seizure clustering within 24 h of ASD withdrawal.56 Although the patients in that study most commonly used lamotrigine rather than CBZ, it was not specified whether patients who experienced seizure clusters were more likely to have also used lamotrigine. In any case, there is clear preclinical and clinical evidence that sodium channel blocking ASDs can aggravate seizure clusters. Thus use of CBZ in our present study confirmed that CSA was bidirectionally sensitive to ASDs, consistent with seizure clusters. Our current investigation clearly indicates that the observed phenomenon associated with PHT and s.c.PTZ coadministration is not representative of SE, but rather another altogether distinct seizure emergency, that is, non-SE CSA, as evidenced by the findings that LZP (our study), other BDZs,31 and CBZ worsen outcomes.
Systemic administration of KA to rodents is an established method to induce electrographic seizures that arise from limbic structures,14 which progress to rhythmic recurrent electrographic spiking and convulsive SE; these events are detectable by cortical EEG. Although the systemic low-dose KA-induced SE model has been reported previously in inbred C57Bl/6 mice,38 we have established this model in outbred CF-1 mice and find it to reliably evoke acute SE with clear and consistent rhythmic spiking. However, mice that underwent CSA did not demonstrate such rhythmic EEG firing patterns (Figure 4). KA-SE also leads to severe and dispersed neurodegeneration throughout numerous limbic structures, as assessed by FJ-C staining, in all mice 24 h after insult; none of the CSA mice, including all mice rescued with ASDs (Table S1), did not demonstrate consistent evidence of neurodegeneration in any examined brain region 24 h later (Figure 5). Altogether, the electrographic seizure patterns, changes in EEG power between experimental groups, and the clear lack of neurodegeneration 24 h after insult confirm that this CSA model induces a sustained seizure insult that is altogether pathologically distinct from SE.
The CSA model provides a unique platform to define the mechanisms underlying seizure emergencies, as well as their differences in ASD sensitivity. The majority of mice that entered CSA survived for at least 30 min post-insult, allowing for interventions to be administered at an investigator-defined time point in a manner similar to that of preclinical SE models.57 Whether BDZs are the only and best-suited interventions for all seizure emergencies has not yet been rigorously evaluated in a preclinical model. There may be better-tolerated or more effective interventions that are specific to SE vs seizure clusters. Furthermore, this study establishes the CSA model as a platform on which to identify other potential treatments, even with delayed administration, to rescue seizure emergencies. Rescue therapies for seizure emergencies are not often readily available in an outpatient setting; the timing of drug administration may be long after seizure onset.58 In this regard, rodent models with sustained seizures that remain sensitive to protracted interventions could be highly informative to drug discovery and differentiation. Whether this CSA model will ultimately prove useful in the identification of novel interventional agents for the management of seizure emergencies clearly remains to be established.
Supplementary Material
Key Points.
Seizure clusters are a variably defined seizure emergency that are sensitive to benzodiazepines, an event distinct from status epilepticus.
The mechanistic differences between seizure clusters and status epilepticus are not well defined.
We report a mouse seizure emergency model that is phenotypically, pathologically, and pharmacologically distinct from status epilepticus.
This mouse model provides a novel platform on which to further interrogate the mechanisms underlying seizure emergencies.
ACKNOWLEDGEMENTS
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This work was supported by the University of Washington Department of Pharmacy and the NCATS/Institute for Translational Health Sciences (NCATS KL2 TR002317 to MBH). The authors are grateful for the curated list of clinical seizure studies provided by Saifuddin Kharawala and UCB Pharma as AES Abstract 3.331 (December 2019; Baltimore, MD). The authors acknowledge the technical assistance of Zachery Koneval and graphical design assistance of Matthew Haliski.
Funding information
National Center for Advancing Translational Sciences, Grant/Award, Number: 5KL2TR002317
Footnotes
CONFLICTS OF INTERESTS
HSW has served on the Scientific Advisory Board of Otsuka Pharmaceuticals and has served as an advisor to Biogen Pharmaceuticals, Acadia, Neurelis, and Jazz Pharmaceuticals and is a member of the UCB Pharma and SK Life Sciences Speakers Bureau. HSW is scientific co-founder of NeuroAdjuvants, Inc., Salt Lake City, UT. None of the other authors have any conflict of interest to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Trinka E, Shorvon S. A decade of progress in status epilepticus 2007–2017: Proceedings of the 6th London-Innsbruck Colloquium on Status Epilepticus and Acute Seizures. Epilepsia. 2018;59(Suppl 2):67–9. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA. Status epilepticus: epidemiologic considerations. Neurology. 1990;40:9–13. [PubMed] [Google Scholar]
- 3.Neligan A, Noyce AJ, Gosavi TD, Shorvon SD, Kohler S, Walker MC. Change in mortality of generalized convulsive status epilepticus in high-income countries over time: a systematic review and meta-analysis. JAMA Neurol. 2019;76(8):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauser WA, Rich SS, Annegers JF, Anderson VE. Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology. 1990;40:1163–70. [DOI] [PubMed] [Google Scholar]
- 5.Theodore WH, Porter RJ, Albert P, Kelley K, Bromfield E, Devinsky O, et al. The secondarily generalized tonic-clonic seizure: a videotape analysis. Neurology. 1994;44:1403–7. [DOI] [PubMed] [Google Scholar]
- 6.Jenssen S, Gracely EJ, Sperling MR. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47:1499–503. [DOI] [PubMed] [Google Scholar]
- 7.Rose AB, McCabe PH, Gilliam FG, Smith BJ, Boggs JG, Ficker DM, et al. Occurrence of seizure clusters and status epilepticus during inpatient video-EEG monitoring. Neurology. 2003;60:975–8. [DOI] [PubMed] [Google Scholar]
- 8.Wallace SJ. Nasal benzodiazepines for management of acute childhood seizures? Lancet. 1997;349:222. [DOI] [PubMed] [Google Scholar]
- 9.Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunley S, Glynn P, Rust S, Vidaurre J, Albert DVF, Patel AD. A hospital-based study on caregiver preferences on acute seizure rescue medications in pediatric patients with epilepsy: Intranasal midazolam versus rectal diazepam. Epilepsy Behav. 2019;92:53–6. [DOI] [PubMed] [Google Scholar]
- 11.Owusu KA, Dhakar MB, Bautista C, McKimmy D, Cotugno S, Sukumar N, et al. Comparison of intranasal midazolam versus intravenous lorazepam for seizure termination and prevention of seizure clusters in the adult epilepsy monitoring unit. Epilepsy Behav. 2019;98:161–7. [DOI] [PubMed] [Google Scholar]
- 12.Shekh-Ahmad T, Hen N, McDonough JH, Yagen B, Bialer M. Valnoctamide and sec-butyl-propylacetamide (SPD) for acute seizures and status epilepticus. Epilepsia. 2013;54(Suppl 6):99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White HS, Alex AB, Pollock A, Hen N, Shekh-Ahmad T, Wilcox KS, et al. A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage. Epilepsia. 2012;53:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell WG. Status epilepticus and acute repetitive seizures in children, adolescents, and young adults: etiology, outcome, and treatment. Epilepsia. 1996;37(Suppl 1):S74–80. [DOI] [PubMed] [Google Scholar]
- 16.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–10. [DOI] [PubMed] [Google Scholar]
- 17.Kutlu NO, Yakinci C, Dogrul M, Durmaz Y. Intranasal midazolam for prolonged convulsive seizures. Brain Dev. 2000;22:359–61. [DOI] [PubMed] [Google Scholar]
- 18.Cereghino JJ. Identification and treatment of acute repetitive seizures in children and adults. Curr Treat Options Neurol. 2007;9:249–55. [DOI] [PubMed] [Google Scholar]
- 19.Haut SR, Shinnar S, Moshe SL. Seizure clustering: risks and outcomes. Epilepsia. 2005;46(1):146–49. 10.1111/j.0013-9580.2005.29004.x [DOI] [PubMed] [Google Scholar]
- 20.Haut SR, Shinnar S, Moshe SL, O’Dell C, Legatt AD. The association between seizure clustering and convulsive status epilepticus in patients with intractable complex partial seizures. Epilepsia. 1999;40:1832–4. [DOI] [PubMed] [Google Scholar]
- 21.Barker-Haliski M, Harte-Hargrove LC, Ravizza T, Smolders I, Xiao B, Brandt C, et al. A companion to the preclinical common data elements for pharmacologic studies in animal models of seizures and epilepsy. A Report of the TASK3 Pharmacology Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open. 2018;3:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loscher W Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–84. [DOI] [PubMed] [Google Scholar]
- 23.Rogawski MA, Bazil CW. New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels. Curr Neurol Neurosci Rep. 2008;8:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann GM 2nd, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–61. [DOI] [PubMed] [Google Scholar]
- 25.Saporito MS, Gruner JA, DiCamillo A, Hinchliffe R, Barker-Haliski M, White HS. Intravenously administered ganaxolone blocks diazepam-resistant lithium-pilocarpine-induced status epilepticus in rats: comparison with allopregnanolone. J Pharmacol Exp Ther. 2019;368:326–37. [DOI] [PubMed] [Google Scholar]
- 26.Grabenstatter HL, Dudek FE. Effect of carbamazepine on spontaneous recurrent seizures recorded from the dentate gyrus in rats with kainate-induced epilepsy. Epilepsia. 2019;60:636–47. [DOI] [PubMed] [Google Scholar]
- 27.Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno S, Koneval Z, Zierath DK, Knox K, White HS, Barker-Haliski M. Diurnal burden of spontaneous seizures in early epileptogenesis in the post-kainic acid rat model of epilepsy. Epilepsia Open. 2021;6(2):431–6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piredda SG, Woodhead JH, Swinyard EA. Effect of stimulus intensity on the profile of anticonvulsant activity of phenytoin, ethosuximide and valproate. J Pharmacol Exp Ther. 1985;232:741–5. [PubMed] [Google Scholar]
- 30.Loscher W Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur J Pharmacol. 2009;610:1–11. [DOI] [PubMed] [Google Scholar]
- 31.Raines A, Henderson TR, Swinyard EA, Dretchen KL. Comparison of midazolam and diazepam by the intramuscular route for the control of seizures in a mouse model of status epilepticus. Epilepsia. 1990;31:313–7. [DOI] [PubMed] [Google Scholar]
- 32.Czuczwar SJ, Turski L, Kleinrok Z. Diphenylhydantoin potentiates the protective effect of diazepam against pentylenetetrazol but not against bicuculline and isoniazid-induced seizures in mice. Neuropharmacology. 1981;20:675–9. [DOI] [PubMed] [Google Scholar]
- 33.Czuczwar SJ, Turski L, Kleinrok Z. Effects of combined treatment with diphenylhydantoin and different benzodiazepines on pentylenetetrazol-and bicuculline-induced seizures in mice. Neuropharmacology. 1982;21:563–7. [DOI] [PubMed] [Google Scholar]
- 34.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments—the ARRIVE guidelines. Journal of Cerebral Blood Flow & Metabolism. 2011;31(4):991–3. 10.1038/jcbfm.2010.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH, et al. Discovery and preclinical development of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic Drugs, 5th edn. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 36–48. [Google Scholar]
- 36.Bialer M, Twyman RE, White HS. Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav. 2004;5:866–72. [DOI] [PubMed] [Google Scholar]
- 37.Barker-Haliski ML, Johnson K, Billingsley P, Huff J, Handy LJ, Khaleel R, et al. Validation of a preclinical drug screening platform for pharmacoresistant epilepsy. Neurochem Res. 2017;42:1904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umpierre AD, Bennett IV, Nebeker LD, Newell TG, Tian BB, Thomson KE, et al. Repeated low-dose kainate administration in C57BL/6J mice produces temporal lobe epilepsy pathology but infrequent spontaneous seizures. Exp Neurol. 2016;279:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. [DOI] [PubMed] [Google Scholar]
- 41.Wanisch K, Kovac S, Schorge S. Tackling obstacles for gene therapy targeting neurons: disrupting perineural nets with hyaluronidase improves transduction. PLoS One. 2013;8:e53269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, Wilcox KS. Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. J Neuropathol Exp Neurol. 2016;75:366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker-Haliski ML, Heck TD, Dahle EJ, Vanegas F, Pruess TH, Wilcox KS, et al. Acute treatment with minocycline, but not valproic acid, improves long-term behavioral outcomes in the Theiler’s virus model of temporal lobe epilepsy. Epilepsia. 2016;57:1958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker-Haliski ML, Dahle EJ, Heck TD, Pruess TH, Vanegas F, Wilcox KS, et al. Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler’s murine encephalomyelitis virus mouse model. J Pharmacol Exp Ther. 2015;353:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finney DJ. Probit analysis. A statistical treatment of the sigmoid response curve. Cambridge University Press; 1952. [Google Scholar]
- 46.Litchfield JR Jr, Wilcoxon R. A simplified method of evaluating dose-effect experiments. J Pharmacol. 1949;96:99–113. [PubMed] [Google Scholar]
- 47.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–8. [DOI] [PubMed] [Google Scholar]
- 48.Haut SR. Seizure clustering. Epilepsy Behav. 2006;8:50–5. [DOI] [PubMed] [Google Scholar]
- 49.Bauer J, Ghane Y, Flugel D, Wildt L, Stefan H. Etiology, follow-up and therapy of seizure clusters in temporal lobe epilepsy and catamenial epileptic seizures. Schweiz Arch Neurol Psychiatr. 1985;1992(143):117–34. [PubMed] [Google Scholar]
- 50.Loscher W, Kohling R. Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav. 2010;19:105–13. [DOI] [PubMed] [Google Scholar]
- 51.Jafarpour S, Hirsch LJ, Gainza-Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: Definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. [DOI] [PubMed] [Google Scholar]
- 52.Naylor DE, Liu H, Niquet J, Wasterlain CG. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis. 2013;54:225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilad R, Izkovitz N, Dabby R, Rapoport A, Sadeh M, Weller B, et al. Treatment of status epilepticus and acute repetitive seizures with i.v. valproic acid vs phenytoin. Acta Neurol Scand. 2008;118:296–300. [DOI] [PubMed] [Google Scholar]
- 54.Brigo F, Storti M, Del Felice A, Fiaschi A, Bongiovanni LG. IV Valproate in generalized convulsive status epilepticus: a systematic review. Eur J Neurol. 2012;19:1180–91. [DOI] [PubMed] [Google Scholar]
- 55.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 56.Henning O, Baftiu A, Johannessen SI, Landmark CJ. Withdrawal of antiepileptic drugs during presurgical video-EEG monitoring: an observational study for evaluation of current practice at a referral center for epilepsy. Acta Neurol Scand. 2014;129:243–51. [DOI] [PubMed] [Google Scholar]
- 57.Loscher W Strategies for antiepileptogenesis: Antiepileptic drugs versus novel approaches evaluated in post-status epilepticus models of temporal lobe epilepsy. In Noebels JL, Avoli M, Rogawski MA, et al. (Eds) Jasper’s Basic Mechanisms of the Epilepsies: Bethesda (MD): Oxford University Press; 2012. [PubMed] [Google Scholar]
- 58.Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22:207–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.