Abstract
BACKGROUND AND AIMS:
Chronically administered parenteral nutrition (PN) in patients with intestinal failure carries the risk for developing PN-associated cholestasis (PNAC). We have demonstrated that farnesoid X receptor (FXR) and liver X receptor (LXR), proinflammatory interleukin-1 beta (IL-1β), and infused phytosterols are important in murine PNAC pathogenesis. In this study we examined the role of nuclear receptor liver receptor homolog 1 (LRH-1) and phytosterols in PNAC.
APPROACH AND RESULTS:
In a C57BL/6 PNAC mouse model (dextran sulfate sodium [DSS] pretreatment followed by 14 days of PN; DSS-PN), hepatic nuclear receptor subfamily 5, group A, member 2/LRH-1 mRNA, LRH-1 protein expression, and binding of LRH-1 at the Abcg5/8 and Cyp7a1 promoter was reduced. Interleukin-1 receptor–deficient mice (Il-1r−/−/DSS-PN) were protected from PNAC and had significantly increased hepatic mRNA and protein expression of LRH-1. NF-κB activation and binding to the LRH-1 promoter were increased in DSS-PN PNAC mice and normalized in Il-1r−/−/DSS-PN mice. Knockdown of NF-κB in IL-1β–exposed HepG2 cells increased expression of LRH-1 and ABCG5. Treatment of HepG2 cells and primary mouse hepatocytes with an LRH-1 inverse agonist, ML179, significantly reduced mRNA expression of FXR targets ATP binding cassette sub-family C member 2/multidrug resistance associated protein 2 (ABCC2/MRP2), nuclear receptor subfamily 0, groupB, member 2/small heterodimer partner (NR0B2/SHP), and ATP binding cassette subfamily B member 11/bile salt export pump (ABCB11/BSEP). Co-incubation with phytosterols further reduced expression of these genes. Similar results were obtained by suppressing the LRH-1 targets ABCG5/8 by treatment with small interfering RNA, IL-1β, or LXR antagonist GSK2033. Liquid chromatography–mass spectrometry and chromatin immunoprecipitation experiments in HepG2 cells showed that ATP binding cassette subfamily G member 5/8 (ABCG5/8) suppression by GSK2033 increased the accumulation of phytosterols and reduced binding of FXR to the SHP promoter. Finally, treatment with LRH-1 agonist, dilauroyl phosphatidylcholine (DLPC) protected DSS-PN mice from PNAC.
CONCLUSIONS:
This study suggests that NF-κB regulation of LRH-1 and downstream genes may affect phytosterol-mediated antagonism of FXR signaling in the pathogenesis of PNAC. LRH-1 could be a potential therapeutic target for PNAC. (Hepatology 2021;0:1–17).
Parenteral nutrition (PN) is life-saving for many infants with perturbations of normal intestinal anatomy or function. However, prolonged use of PN in patients with intestinal failure (IF) is associated with an increased risk of developing cholestatic and eventually end-stage liver disease, referred to as parenteral nutrition–associated liver disease or IF-associated liver disease (IFALD).(1–4) Histologic evidence of cholestasis (PNAC) may be evident within 2 weeks of starting PN in infants with IF and may progress to cirrhosis within 6–12 months.(5) The pathogenesis of PNAC has been elusive until the past decade, with mounting evidence implicating synergy between activation of hepatic innate immune cells by intestinally derived bacterial products and the effects of plant-based intravenous lipid emulsions in animal models(6,7) as well as in affected infants, children, and adults.(8,9) In a murine model of PNAC in which intestinal injury and hyperpermeability induced by dextran sulfate sodium (DSS) is followed by total PN for 1–4 weeks, we have reported that bacterial lipopolysaccharide (LPS) absorbed from injured intestine work synergistically with plant sterols (phytosterols) within the IV lipid emulsion to initiate hepatic macrophage activation.(10)Subsequent generation of IL-1β interferes with hepatocyte gene expression of bile and sterol canalicular transporters and nuclear receptors,(10–12) including bile salt export pump (BSEP/ATP-binding cassette, subfamily B member 11 [Abcb11]), multiple resistance protein 2 (MRP2/ATP-binding cassette subfamily C member 2 [Abcc2]), sterolin (ATP-binding cassette subfamily G member 5/8 [ABCG5/8]), farnesoid X receptor (FXR; Nr1h4), and liver X receptor (LXR; Nr1h3).(10,12) Furthermore, we have shown in the PNAC mouse model that IL-1β from activated hepatic macrophages initiates hepatocyte NF-κB signaling with subsequent transcriptional suppression of LXR and FXR target genes, the sterol and bile acid transporters (sterolin and BSEP), resulting in hepatic accumulation of phytosterols (which themselves interfere with FXR signaling) and bile acids, thus mediating cholestatic injury.(12) A number of nuclear hormone receptors including FXR, LXR, and liver receptor homolog 1 (LRH-1) play important roles in bile homeostasis and the development of cholestasis.(13)
LRH-1 is a nuclear receptor that regulates diverse biological functions, including hepatic glucose, cholesterol, and bile acid metabolism,(14–17) partly through its interaction with FXR signaling.(16,18) In the liver and intestine, LRH-1 and LXR regulate secretion of sterols through their target genes ABCG5/8.(19) In this study, we explored the potential role of LRH-1 and its downstream regulation of ABCG5/8 in the pathogenesis of PNAC.
Animals and Methods
PNAC MOUSE MODEL
All animals were treated humanely according to approved protocols by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. C57BL/6 wild-type (WT) or IL-1 receptor knockout (Il-1r−/−) adult male mice (8 weeks old, 22–23 g body weight) ( Jackson Laboratories, Bar Harbor, ME) were subjected to the PNAC model procedures as described in the Supporting Experimental Methods.(10,12)
CULTURED HEPATOCYTE EXPERIMENTS
Mouse primary hepatocytes were isolated from fresh liver tissue as previously described(20) with some modifications (Supporting Experimental Methods). Primary hepatocytes (7.5 × 105/cells/well in Williams E Media (Gibco, Lafayette, CO) were treated with 10 ng/mL recombinant murine IL-1β (BD Biosciences, San Jose, CA) or vehicle for 4 hours followed by 5 μM GW4064 (Tocris, Minneapolis, MN), an FXR agonist, or 10 μM stigmasterol acetate (stig) and 10 μM sitosterol acetate (sito) (Steraloids, Newport, RI) overnight in Williams E media with transfection agent (Dharmafect; Dharmacon, Lafayette, CO), as previously described.(7) Transfection agent was used in all stig and sito incubations to promote phytosterol uptake.(7) HepG2 and Huh7 cells (2.0 × 105 cells/well in DMEM/fetal bovine serum; Gibco) were similarly treated with IL-1β, GW4064, and/or stig or sito, as previously described.(7) In trans-well coculture experiments, the human monocyte cell line THP-1 cells (plated on the inserts) were cocultured with HepG2 cells (plated on bottom of lower chamber) in 12-well plates (Thermo Fisher Scientific, Waltham, MA). THP-1 cells were exposed overnight to 10 μM stig+sito followed by incubation with 100 ng/mL LPS (Salmonella typhimurium; Sigma-Aldrich, St. Louis, MO) for 4 hours. mRNA was isolated and transcribed into complementary DNA (cDNA) from both cell types and analyzed by quantitative real-time PCR.
RNA INTERFERENCE TRANSFECTION AND INCUBATIONS
HepG2 cells were transfected with 100 nM small interfering RNA (siRNA) ABCG8 (cat: L-008397–00-0005), 100 nM siRNA NF-κB (cat: L-003520–00), 100 nM siRNA nuclear receptor subfamily 5, group A, member 2 (NR5A2; cat: L-003430–00; Dharmacon), or a nontargeting siRNA (cat: D-001810–10-05; Dharmacon) in the presence of Dharmafect transfection reagent (Dharmacon) for 24 hours, according to the manufacturer’s instructions. The following day, media was changed and siRNA-transfected HepG2 cells were treated overnight with or without 10 μM stig+sito and with or without 5 μM GW4064. In other experiments, HepG2, Huh7, or primary mouse hepatocytes were incubated for 4 hours with either the LRH-1 inverse agonist 5 μM ML179 (Tocris; Bioscience, Minneapolis, MN) or the LXR antagonist 5 μM GSK2033 (Tocris; Bioscience), followed by overnight incubation with or without 10 μM stig+sito and with or without 5 μM GW4064.
RNA ISOLATION AND QUANTITATIVE GENE-EXPRESSION ANALYSIS
Mouse and human Liver or cellular RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RNA samples were used for cDNA preparation using a kit (Cat: 1708891; BioRad, Hercules, CA) followed by quantitative real-time PCR using TaqMan probes (Thermo Fisher Scientific) (Supporting Table S1), as previously described.(12)
ANTIBODIES AND IMMUNOBLOT ANALYSIS
Total cell lysates and nuclear fractions were isolated using M-PER and NE-PER Extraction Reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions. Proteins were quantified by BCA Assay (Thermo Fisher Scientific) and separated on 4%−20% SDS polyacrylamide gels (BioRad) and transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA). Membranes were then incubated for 1 hour in blocking buffer (Tris-buffered saline, 0.1% Tween), 5% nonfat dry milk, and incubated overnight at 4°C with indicated primary antibodies (Supporting Table S2) followed by 1-hour incubation with indicated secondary antibodies. Blots were developed using enhanced chemiluminescence (Santa Cruz Biotechnologies, Santa Cruz, CA) according to the manufacturer’s protocol.
CHROMATIN IMMUNOPRECIPITATION
Chromatin immunoprecipitation (ChIP) assay was performed on liver samples that were immediately flash-frozen in liquid nitrogen before processing or on freshly obtained cell lysates from HepG2 cells. ChIP was done using specific antibodies for FXR, LRH-1, and NF-κB p65, and amplification of promoter sequences from the Nr5a2, Abcg5/8, and nuclear receptor subfamily 0, group B, member 2 (Nr0b2) genes, using specific primer sets (Supporting Table S3) and subsequent PCR. ChIP assays were performed according to the manufacturer’s instructions using the EZ ChIP/Magna ChIP G Kit from EMD/Millipore (Billerica, MA).
LC/MS QUANTIFICATION OF PHYTOSTEROLS
Ultrahigh-performance liquid chromatography–mass spectrometry (LC/MS) quantification of phytosterol concentrations was performed on HepG2 cell pellets by the University of Colorado Denver Mass-spectrometry core, as described in the Supporting Experimental Methods.(21–23)
STATISTICAL ANALYSIS
Gene expression from quantitative real-time PCR assays was determined in triplicate for each mouse, and the average from each mouse was used to generate the mean ± SEM for each treatment group. The number of animals in each group was three to seven and provided in Table 1. For cell culture experiments, gene expression was determined in triplicate and averaged, and results from three representative experiments are shown. All samples were coded and were analyzed blind to the treatment group. ANOVA and Tukey’s correction for multiple comparisons were used to determine statistical significance. The level of significance between two groups was determined by Student unpaired t test. P value < 0.05 was considered statistically significant. PRISM Graph Pad software (La Jolla, CA) was used for statistical analyses. Animals were randomly assigned to treatment groups. All samples were coded and analyzed blind to the specific treatment of the animals or cell lines.
TABLE 1.
Liver Biochemistries for Experimental Mouse Groups
| AST (U/L) |
ALT (U/L) |
Total Serum Bile Acids (µM) |
Total Serum Bilirubin (mg/dL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Mean ± SEM | P Value vs. DSS-PN | n | Mean ± SEM | P Value vs. DSS-PN | n | Mean ± SEM | P Value vs. DSS-PN | n | Mean ± SEM | P Value vs. DSS-PN | n |
| Chow | 129.9 ± 13.3* | <0.0001 | 23 | 34.1±2.6* | <0.0001 | 23 | 3.30 ± 0.29* | <0.0001 | 25 | 0.063 ± 0.005* | <0.0001 | 11 |
| DSS-chow | 144.3 ± 20.7* | <0.0001 | 25 | 34.5 ± 4.6* | <0.0001 | 23 | 3.10 ± 0.20* | <0.0001 | 25 | 0.085 ± 0.004 | ns | 9 |
| PN | 125.5 ± 26.8* | 0.0048 | 6 | 45.0 ± 12.0 | 0.0185 | 6 | 3.10 ± 0.30* | <0.0001 | 6 | — | — | — |
| DSS-PN | 290.3 ± 25.8 | — | 27 | 66.8 ± 5.8 | — | 25 | 7.28 ± 0.38 | — | 27 | 0.163 ± 0.01 | — | 10 |
| IL-1−/−/chow | 145.0 ± 35.1* | 0.0181 | 6 | 54.0 ± 8.5 | ns | 6 | 4.20 ± 0.42* | <0.0001 | 5 | 0.054 ± 0.00* | <0.0001 | 3 |
| IL-1−/−/DSS-PN | 108.3 ± 9.4* | <0.0001 | 10 | 39.0 ± 4.2 | 0.0092 | 10 | 4.00 ± 0.60* | <0.0001 | 11 | — | — | — |
Note: Values are presented as mean ± SEM.
Significantly different from DSS-PN.
Abbreviation: ns, not significant.
Results
REDUCED EXPRESSION OF LRH-1 AND ITS TARGET GENE ABCG5/8 DURING PNAC
Treatment of adult C57BL/6 mice with 2.0% DSS in drinking water for 4 days followed by PN containing soy lipid emulsion for 14 days (DSS-PN mice) induced cholestasis and hepatocyte injury, as shown by significantly elevated serum aspartate aminotransferase (AST), total bilirubin, and total serum bile acids in DSS-PN mice relative to chow, DSS-chow and PN-only mice, as previously reported (Table 1).(7,12) Serum alanine aminotransferase (ALT) was elevated in DSS-PN mice compared with chow, DSS-chow and PN-only mice. To investigate the role of LRH-1 in PNAC, we first examined mRNA and protein expression of Nr5a2/LRH-1 and ABCG8 protein expression in DSS-PN mice compared with chow-fed mice. We have previously reported reduced mRNA expression of Abcg5/8 in DSS-PN mice.(12) DSS-PN treatment resulted in a significant reduction in both hepatic mRNA for Nr5a2 and LRH1 protein (Fig. 1A–C) as well as ABCG8 protein expression (Fig. 1B,C). To determine whether these findings were present in human infants with PNAC, we analyzed mRNA expression in flash-frozen liver biopsy tissue from 7 infants with PNAC and intestinal failure compared with liver from 4 age-matched control infants without inflammatory or cholestatic liver diseases (Table 2). Similar to our observations in the PNAC mouse model, we found significantly lower hepatic mRNA expression for NR5A2/LRH-1 in infants with PNAC and a failure of the expected up-regulation of ABCG5 and ABCG8 (Supporting Fig. S1G) that would be anticipated during infusion of intravenous lipid emulsions.(7) We also analyzed hepatic macrophage infiltration by CD68 immunostaining(24) in liver from healthy children and children with PNAC and found increased accumulation of macrophages in PNAC liver, similar to the observation in PNAC mice (Supporting Fig. S1H)
FIG. 1.
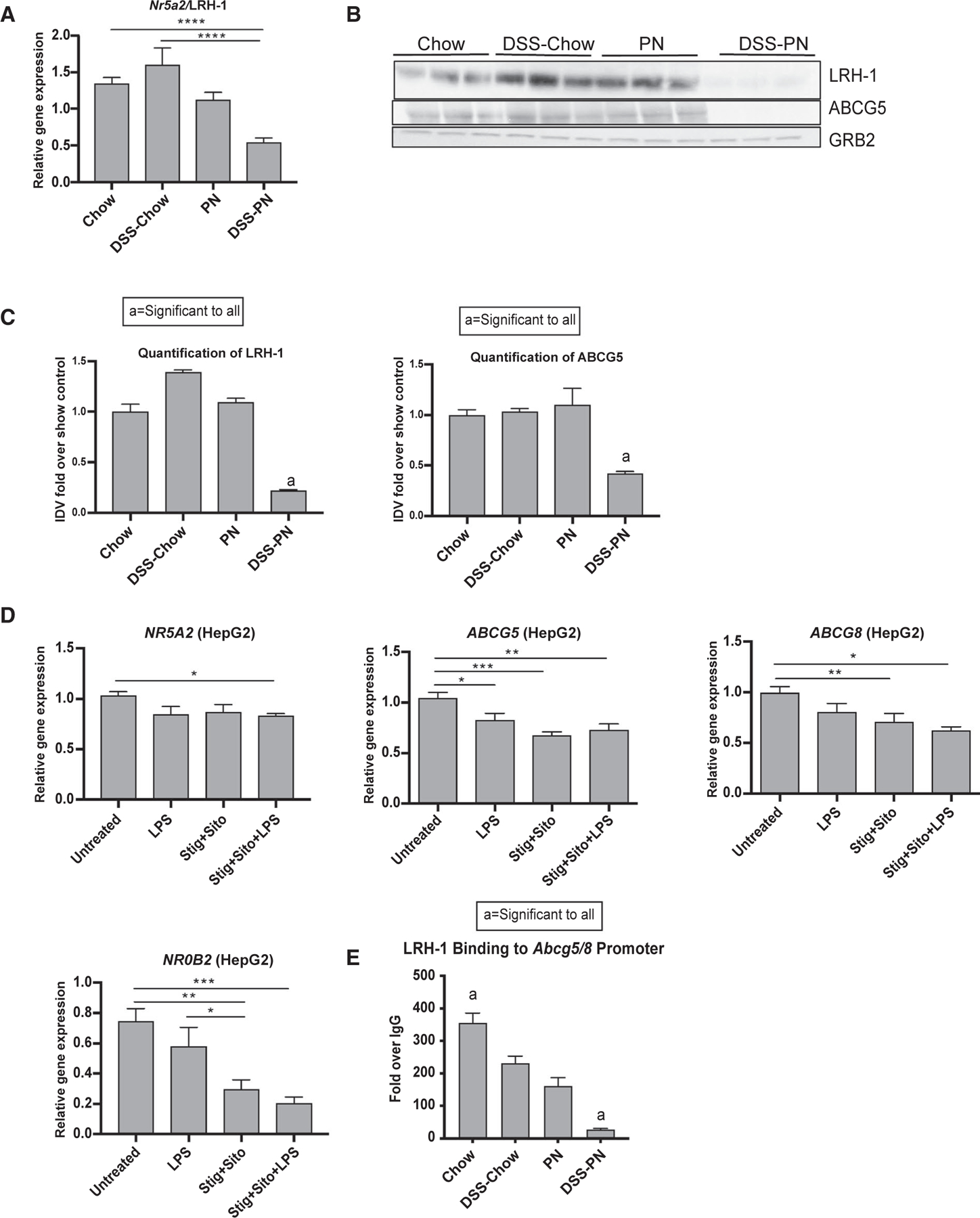
Effect of PNAC, LPS, and phytosterols on LRH-1 and its target gene expression. (A) Gene-expression analysis of hepatic Nr5a2/LRH-1 from Chow, DSS-Chow, PN, and DSS-PN treated mice. mRNA expression was determined after normalization to Hprt1 as an endogenous control gene and expressed relative to results obtained from untreated Chow controls. (B) Western analysis of LRH-1 and ABCG8 protein expression in liver homogenate from Chow, DSS-Chow, PN, and DSS-PN treated mice. (C) Quantification of integrated density values of the LRH-1 and ABCG8 immunoblot in (B). (D) Gene expression of NR5A2/LRH-1, ABCG5, ABCG8, and NR0B2/SHP in HepG2 cells from coculture experiments with human monocyte/macrophage THP-1 cells incubated with and without LPS for 4 hours and with or without stig+sito. Gene expression was determined after normalization to HPRT1 as an endogenous control gene and expressed relative to results obtained from untreated controls. (E) ChIP assay for LRH-1 binding to the promoter of Abcg5/8 in liver homogenate from chow, DSS-chow, PN, and DSS-PN mice. Data are presented as fold change over IgG. Statistical analysis was performed, and adjusted P values obtained using one-way ANOVA with Tukey’s correction for multiple comparisons (A,D) and by Student unpaired t test (C,E). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
TABLE 2.
Liver Biochemistries of Patients
| Patient No. | Disease Control Diagnosis | Age (years) | Sex | Total Bilirubin (mg/dL) | ALT (IU/L) | AST (IU/L) |
|---|---|---|---|---|---|---|
| 1 | Hepatoblastoma | 3.32 | Female | 0.40 | 29 | 27 |
| 2 | Hepatoblastoma | 3.39 | Male | 0.20 | 46 | 63 |
| 3 | Methylmalonic acidemia | 1.65 | Female | 0.60 | 22 | 61 |
| 4 | Ornithine transcarbamylase deficiency | 2.56 | Female | 0.30 | 312 | 331 |
| PNAC Group Diagnosis | Age (years) | Sex | Total Bilirubin (mg/dL) | ALT (IU/L) | AST (IU/L) | |
| 5 | PNAC | 0.10 | Female | 12.10 | 101.00 | 118.00 |
| 6 | PNAC | 0.25 | Female | 8.30 | 226.00 | 213.00 |
| 7 | PNAC | 0.08 | Male | 4.00 | 48.00 | 82.00 |
| 8 | PNAC | 1.02 | Male | 63.2 | 172 | 430.00 |
| 9 | PNAC | 0.18 | Female | 4.80 | 182.00 | 230.00 |
| 10 | PNAC | 0.30 | Female | 5.70 | 36.00 | 70.00 |
| 11 | PNAC | 0.50 | Male | 2.90 | 485.00 | 396.00 |
| Group | Total Bilirubin (mg/dL) (mean ± SEM) | ALT (IU/L) (mean ± SEM) | AST (IU/L) (mean ± SEM) | |||
|
| ||||||
| Disease controls | 0.38 ±0.09 | 102.25 ± 70.10 | 120.50 ± 70.65 | |||
| PNAC | 14.43 ± 8.21 | 178.57 ± 57.65 | 219.86 ± 55.03 | |||
| Liver Biochemistry | Disease Controls vs. PNAC P Value | |||||
|
| ||||||
| Total bilirubin | <0.0001 | |||||
| ALT | ns | |||||
| AST | ns | |||||
Abbreviation: ns, not significant.
We have previously reported that LPS and phytosterols synergistically activate hepatic macrophages to generate IL-1β and TNF-α in the PNAC mouse model and in cultured cells.(7) To examine the effect of macrophage activation on hepatocyte LRH-1 expression, a THP-1 (monocyte cell line)/HepG2 co-culture model was used. Treatment of THP-1 cells in the upper wells with either phytosterols (stig+sito), LPS, or the combination significantly increased THP-1 mRNA expression of IL-1B and TNF and secretion of IL-1β and TNF-α into the medium of the upper and lower wells (Supporting Fig. S1A,B,D,E), which was associated with decreased expression of NR5A2, ABCG5, ABCG8, NR0B2, and ABCC2 in HepG2 cells in the lower wells (Fig. 1D and Supporting Fig. S1C). To further determine whether perturbations of LRH-1 are involved in the suppression of Abcg5/8 in the PNAC model, we performed ChIP assays on liver homogenate and found that LRH-1 binding to the promoter regions of Abcg5/8 and Cyp7a1, which encodes the rate-limiting enzyme for bile acid synthesis, was significantly reduced in DSS-PN mice compared with chow mice, chow-DSS, and PN mice (Fig. 1E and Supporting Fig. S1F). Taken together, these data suggest that in PNAC, suppression of Nr5a2/LRH-1 leads to reduced transcription of its downstream targets Abcg5/8, which is required for canalicular export of the infused PN phytosterols, accounting for, in part, the observed elevation of hepatic phytosterol concentrations in DSS-PN mice and in human patients.(7,12) In addition, reduced occupancy of LRH-1 on the promoter of Cyp7a1 would reduce its transactivation of small heterodimer partner (SHP), and thus reduce SHP suppression of CYP7A1 transcription, which would likely lead to an inappropriate increase in bile acid synthesis and its toxicity in the face of cholestasis (see Discussion).
IL-1β DOWN-REGULATES THE EXPRESSION OF LRH-1 AND ITS TARGETS THROUGH NF-κB DURING PNAC
We have previously shown that IL-1 receptor knockout mice (Il-1r−/− mice) are protected from PNAC, as illustrated by their serum liver biochemistries,(10,12) implicating IL-1 signaling in the induction of PNAC (Table 1). To determine whether IL-1 signaling played a role in the reduced LRH-1 expression during PNAC, hepatic expression of Nr5a2/LRH-1 and Abcg8 was assessed in Il-1r−/− DSS-PN mice; mRNA levels of both Nr5a2 and Abcg8 were significantly higher in Il-1r−/−/DSS-PN compared with WT/DSS-PN mice (Fig. 2A,B), concomitant with the improved liver biochemistries (Table 1). These data suggested a role for IL-1 signaling in suppressing LRH-1 expression and its downstream target genes Abcg5/8 in the PNAC mouse model.
FIG. 2.
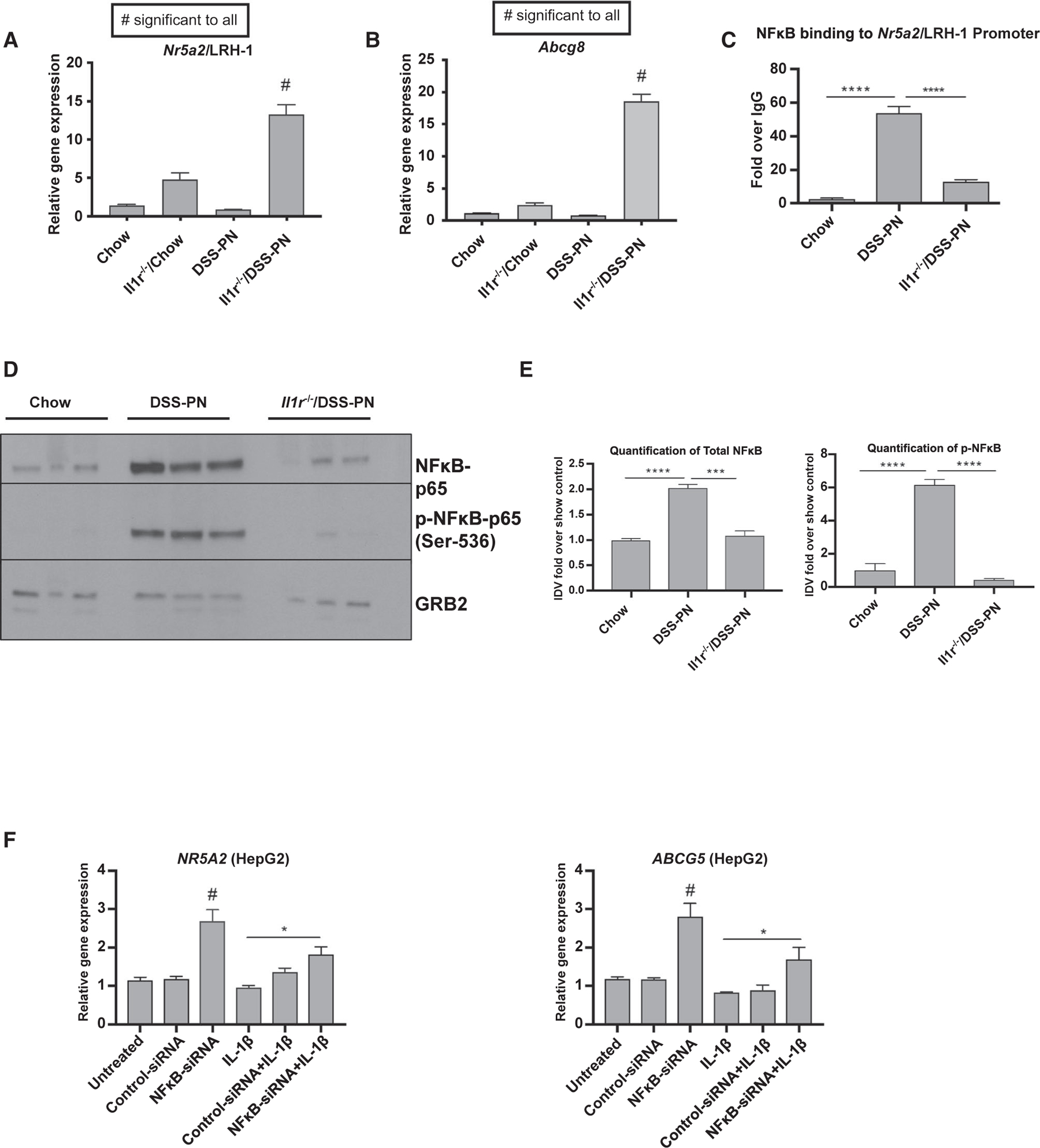
IL-1β-induced NF-κB signaling suppresses LRH-1 and ABCG5 expression. (A) Gene-expression analysis of hepatic Nr5a2/LRH-1 from WT/chow, WT/DSS-PN, Il-1r−/−/chow, and Il-1r −/−/DSS-PN mice. mRNA expression was determined after normalization to Hprt1 as an endogenous control gene and expressed relative to results obtained from chow controls. ****P < 0.0001 versus all other groups. (B) Hepatic expression of Abcg8 in chow, WT/DSS-PN, and Il-1r−/−/DSS-PN mice. #P < 0.0001 for all other conditions. (C) ChIP assay for NF-κB binding to the LRH-1 promoter in liver homogenate from WT/chow, WT/DSS-PN, and Il-1r−/−/DSS-PN mice. Data are presented as fold change over IgG control. Statistical analysis was by one-way ANOVA with Tukey’s correction for multiple comparisons. ****P < 0.0001. (D) Immunoblot of protein expression of total NF-κB-p65 and p-NF-κB-p65 in liver homogenates from chow, WT/DSS-PN, and Il-1r−/−/DSS-PN mice. (E) Quantification of integrated density values of immunoblots of total NF-κB-p65 normalized to GRB2 endogenous control relative to chow control. ***P < 0.001 and ****P < 0.0001. Ratio of p-NF-κB-p65 to total normalized NF-κB. ***P < 0.001. (F) mRNA expression of NR5A2/LRH-1 in HepG2 cells transfected with NF-κB siRNA and nontargeting siRNA control for 48 hours followed by IL-1β treatment overnight. Gene expression was normalized to HPRT1 and expressed relative to results from untreated controls. *P < 0.05 and #P < 0.01 versus all other groups. mRNA expression of ABCG5 in HepG2 cells. Gene expression was normalized to HPRT1 and expressed relative to results from untreated controls. *P < 0.05 and #P < 0.01 versus all other groups. Abbreviation: GRB2, growth factor receptor bound protein 2.
We previously demonstrated that NF-κ B played a role in the IL-1 suppression of FXR and LXR signaling in the PNAC model.(12) Therefore, we next determined whether NF-κB similarly mediated IL-1 suppression of Nr5a2. First, we measured NF-κB binding to the Nr5a2/LRH-1 promoter by ChIP analysis in the liver from DSS-PN-treated WT and Il-1r−/− mice. NF-κB binding to the LRH-1 promoter was markedly increased in WT/DSS-PN mouse liver, concomitant with the decreased LRH-1 expression, which was abrogated in Il-1r −/−/DSS-PN mice (Fig. 2C). We next examined the phosphorylation status of NF-κB-p65, as phosphorylation of NF-κB-p65 on Ser-536 is required for its nuclear translocation and activation.(25) Immunoblotting of liver homogenate showed increased NF-κB-p65 phosphorylation in DSS-PN mice compared with chow mice, which was prevented in Il-1r−/−/DSS-PN mice (Fig. 2D,E). To further explore the role of NF-κB in LRH-1 and ABCG5 expression, siRNA-mediated knockdown of NF-κB expression in HepG2 cells resulted in significantly increased NR5A2/LRH-1 mRNA and protein as well as mRNA of its target ABCG5 (Fig. 2F and Supporting Fig. S2A,B). We previously have shown that IL-1β suppresses expression of ABCG5/8 in cultured hepatocytes.(12) Therefore, we next exposed HepG2 cells to human IL-1β and showed that siRNA knockdown of NF-κB reversed the suppressive effect of IL-1β on both NR5A2/LRH-1 and ABCG5 (Fig. 2F). Finally, siRNA-mediated knockdown of LRH-1 expression in HepG2 cells significantly reduced expression of ABCG5, ABCG8, and NR0B2 (Supporting Fig. S2C–G), confirming the role of LRH1 in regulating these genes. Taken together, in conjunction with our previous report,(12) these findings indicate that hepatic macrophage-derived IL-1 signaling suppresses hepatocyte NR5A2/LRH-1 expression and subsequent ABCG5/8 expression through NF-κB activation.
INTERACTION OF LRH-1 AND PHYTOSTEROLS IN DOWN-REGULATION OF FXR TARGET GENES
Hepatic accumulation of bile acids in cholestasis induces the expression of canalicular bile transport genes and represses the transcription of CYP7A1, the rate-limiting enzyme of bile acid biosynthesis, through LRH-1(26) and FXR/SHP signaling.(27) Because accumulation of phytosterols also interferes with FXR signaling and bile transporter expression,(7) we next examined the interaction among phytosterols, LRH-1, and FXR signaling. HepG2 cells and primary mouse hepatocytes were treated with the LRH-1 inverse agonist, ML179, in the presence or absence of the FXR agonist GW4064 for 4 hours, followed by overnight incubation with stig and sito. The results showed that inhibition of LRH-1 by ML179 in HepG2 cells in the presence or absence of GW4064 markedly suppressed mRNA expression of FXR target genes NR0B2/SHP and ABCC2/MRP2 (Fig. 3A,B and Supporting Fig. S3A,B) as well as the LRH-1 target ABCG8 (Supporting Fig. S3C), and suppressed Abcb11, Nr0b2, and Abcg5 in primary mouse hepatocytes (Fig. 3C,D and Supporting Fig. S3D–F). This suppression of FXR target gene expression by LRH-1 inhibition was additive to that induced by stig+sito incubation in HepG2 cells and primary mouse hepatocytes (Fig. 3A–E). In contrast, Cyp7a1 mRNA and protein expression in primary mouse hepatocytes were not affected by ML179 (Supporting Fig. S3G). In Huh7 cells, in the presence of GW4064-induced up-regulation of ABCB11, treatment with ML179 or stig+sito alone similarly significantly suppressed ABCB11 mRNA expression, with further suppression by the combination of ML179 and stig+sito (Fig. 3E). These data suggest that the role of phytosterols in mediating cholestasis in PNAC may involve LRH-1 pathways.
FIG. 3.
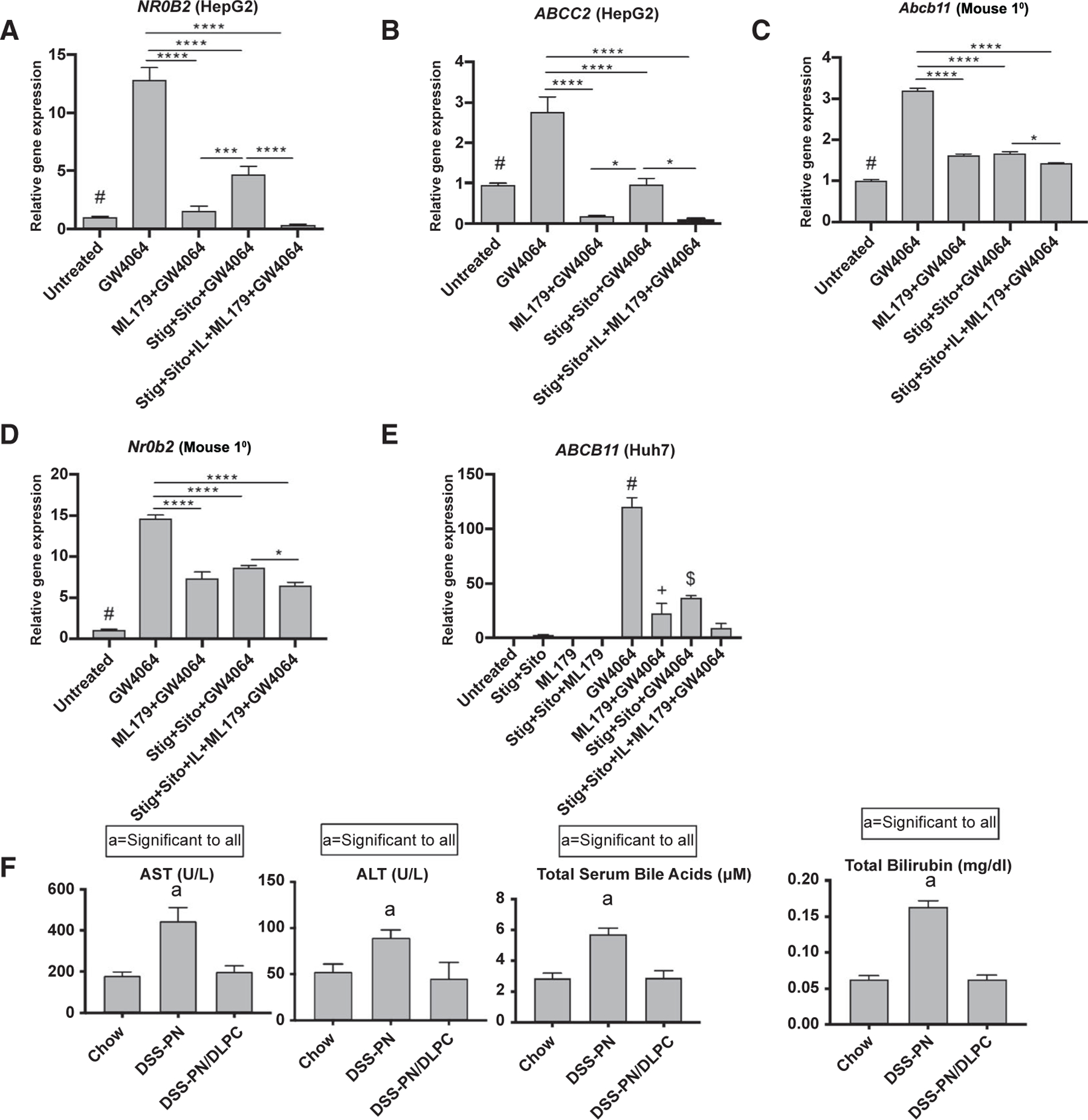
Effect of inhibition of LRH-1 on FXR target genes in cultured cells and of LRH1 agonist treatment in DSS-PN mice. Cultured cells were incubated with LRH-1 inverse agonist ML179 for 4 hours followed by addition of GW4064 +/− stig+sito overnight, and mRNA expression was then analyzed. (A) NR0B2/SHP in HepG2 cells. #P < 0.0001 versus GW4064 and GW4064 + stig+sito. (B) ABCC2/MRP2 in HepG2 cells. #P < 0.05 versus all groups except stig+sito + GW4064. (C) Abcb11/BSEP in primary mouse hepatocytes (Mouse 1°). #P < 0.0001 versus GW4064 group. (D) Nr0b2/SHP in primary mouse hepatocytes. #P < 0.0001 versus all other groups. (E) ABCB11/BSEP in Huh7 cells. #P < 0.0001 versus all other groups. *P < 0.05 versus all groups except stig+sito + GW4064. $P < 0.01 versus all groups except ML179 + GW4064. Gene expression was normalized to HPRT1/Hprt1. (F) Serum AST, ALT, total serum bile acids, and total bilirubin in chow, DSS-PN, and DSS-PN /DLPC (LRH1 agonist)–treated mice. Statistical analysis was performed by one-way ANOVA with Tukey’s correction for multiple comparisons. *P < 0.05 and ****P < 0.0001.
Having demonstrated that the hepatic LRH-1 pathway is down-regulated in PNAC mice and that inhibiting LRH1 signaling down-regulates FXR-induced hepatocyte bile transporters, we next sought to determine whether activation of hepatic LRH-1 signaling would reverse the features of PNAC. In DSS-PN mice, the LRH-1 agonist, dilauroyl phosphatidylcholine (DLPC; 30 mg/kg/body weight), added to the PN solution from day 4 to day 14 prevented the elevation of serum AST, ALT, total serum bile acids, and total bilirubin levels that were observed in DSS-PN mice at 14 days of PN (Fig. 3F). Immunohistochemistry of the liver using the pan macrophage marker, F4/80, showed increased numbers of enlarged macrophages in DSS-PN mice despite no apparent changes on hematoxylin and eosin staining in this early stage of PNAC, as previously described.(10) DLPC treatment of DSS-PN mice reduced hepatic macrophage number and size to that of chow-fed mice (Supporting Fig. S3J). The effect of intravenous DLPC appeared to be limited to the liver inasmuch as DLPC had no significant effects on Fgf15, Abcg5, and Abcg8 mRNA expression in terminal ileum of DSS-PN mice (Supporting Fig. S3I). In summary, inhibition of LRH1 signaling was associated with, and activating LRH-1 signaling in vivo reversed, the liver injury, cholestasis, and macrophage infiltration characteristic of murine PNAC, supporting an important role of LRH-1 signaling in PNAC pathogenesis.
DOWN-REGULATION OF ABCG5/8 INCREASES PHYTOSTEROL RETENTION AND SUPPRESSES FXR TARGET GENES
We have reported down-regulation of hepatic Abcg5 and Abcg8 expression in murine PNAC, which was associated with elevated levels of stigmasterol in liver and serum.(7,9) To further investigate the effect of reduced expression of ABCG5/8 on phytosterol retention and FXR target gene expression, three approaches were used. First, HepG2 cells that were treated with ABCG8 siRNA, and had significantly reduced ABCG8 mRNA levels (Fig. 4A), were subsequently incubated with FXR agonist GW4064, stig+sito, or in combination overnight. The ability of GW4064 to induce transcription of the FXR target gene NR0B2 was significantly reduced in the presence of stig and sito, which was further reduced with ABCG8 siRNA knockdown (Fig. 4B). Second, IL-1β was used to suppress ABCG5/8 expression. We have previously shown that IL-1β, which is up-regulated in PNAC liver macrophages, suppressed ABCG5/8 expression in cultured hepatocytes.(12) Therefore, HepG2 cells and primary mouse hepatocytes were incubated with IL-1β for 4 hours followed by +/− GW4064, +/− stig+sito, or the combination overnight. IL-1β alone or in the presence of stig+sito reduced ABCG5/Abcg5 expression (Supporting Fig. S4A,B). IL-1β or stig+sito alone down-regulated NR0B2/Nr0b2 and Abcb11 in the presence or absence of GW4064 (Fig. 4C–E and Supporting Fig. S4C). Huh7 cells were similarly treated with IL-1β and stig+sito, and ABCB11 expression was shown to be down-regulated by IL-1β and stig+sito, with maximal suppression by the combination (Fig. 4F).
FIG. 4.
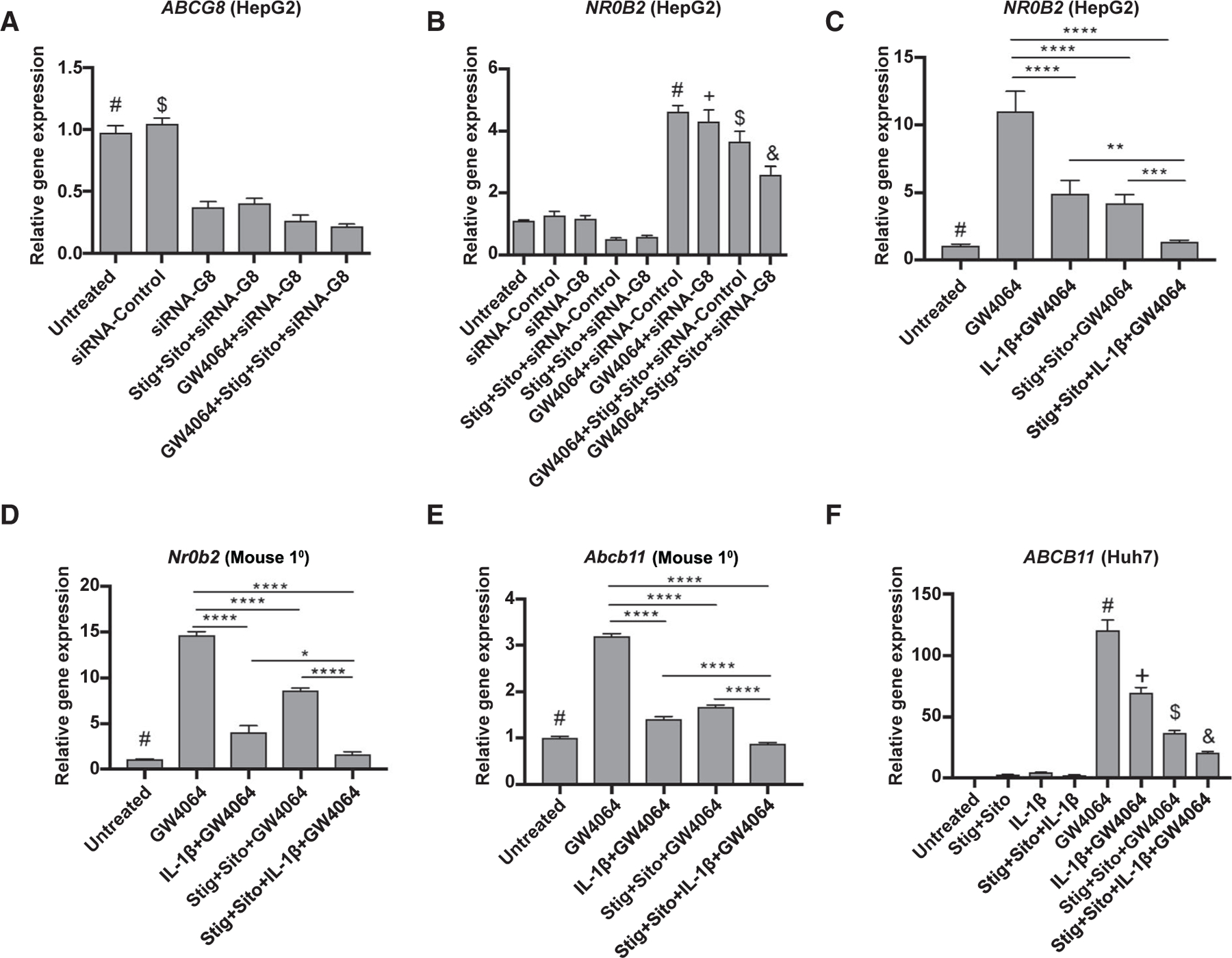
Decreased expression of ABCG5/8 enhances the inhibitory effect of phytosterols on FXR target gene expression. HepG2 cells were transfected with ABCG8 siRNA or nontargeting siRNA for 24 hours followed by addition of +/− GW4064 or +/− stig+sito overnight, after which cells were harvested and mRNA analysis was performed. (A) ABCG8. #P < 0.0001 versus all groups except siRNA control. $P < 0.0001 versus all groups except untreated control. (B) NR0B2/SHP. #P < 0.05 versus all groups except + and $. +P < 0.05 versus all groups except + and #. $P < 0.05 versus all groups except # and +. &P < 0.05 versus all other groups. (C) HepG2 cells were incubated with IL-1β (to reduce ABCG5/8 expression) for 4 hours followed by GW4064 +/− stig+sito overnight, after which cells were harvested and mRNA analysis of NR0B2/SHP was performed. #P < 0.05 versus all groups except GW4064 + stig+sito + IL-1β. (D) Primary mouse hepatocytes (Mouse 1°) were incubated as in (C), and Nr0b2/SHP mRNA was analyzed. #P < 0.01 versus all groups except stig+sito + IL-1β + GW4064. (E) Abcb11 in primary mouse hepatocytes. #P < 0.001 versus all groups except GW4064 + stig+sito + IL-1β. (F) Huh7 cells were incubated as in (C), and ABCB11 mRNA was analyzed. #P < 0.0001 versus all other groups. +P < 0.0001 versus all other groups. $P < 0.05 versus all other groups. &P < 0.05 versus all other groups. Gene expression was determined after normalization to HPRT1 relative to results obtained from untreated controls. For all of these experiments, statistical analysis was performed by one-way ANOVA with Tukey’s correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Third, inasmuch as LXR regulates ABCG5/8 expression,(12) we next used an LXR antagonist, GSK2033,(28) in cultured cells to reduce expression of ABCG5/8 and determine its effects on phytosterol retention and FXR target gene expression. HepG2 and mouse primary hepatocytes were incubated with GSK2033 for 4 hours followed by +/− GW4064, with either the combination of stig+sito, or stig or sito alone. Treatment with GSK2033 reduced the expression of ABCG5 as expected (Supporting Fig. S5A,G), which was associated with suppressed GW4064-induced expression of NR0B2 mRNA and protein (Fig. 5A,B and Supporting Fig. S5C,E,H). Phytosterols (stig or sito alone, or both together) similarly reduced GW4064-induced SHP expression with maximal suppression achieved when combined with GSK2033. In the absence of up-regulation of FXR signalizing by GW4064, similar results were obtained (Supporting Fig. S5B,D,F).
FIG. 5.
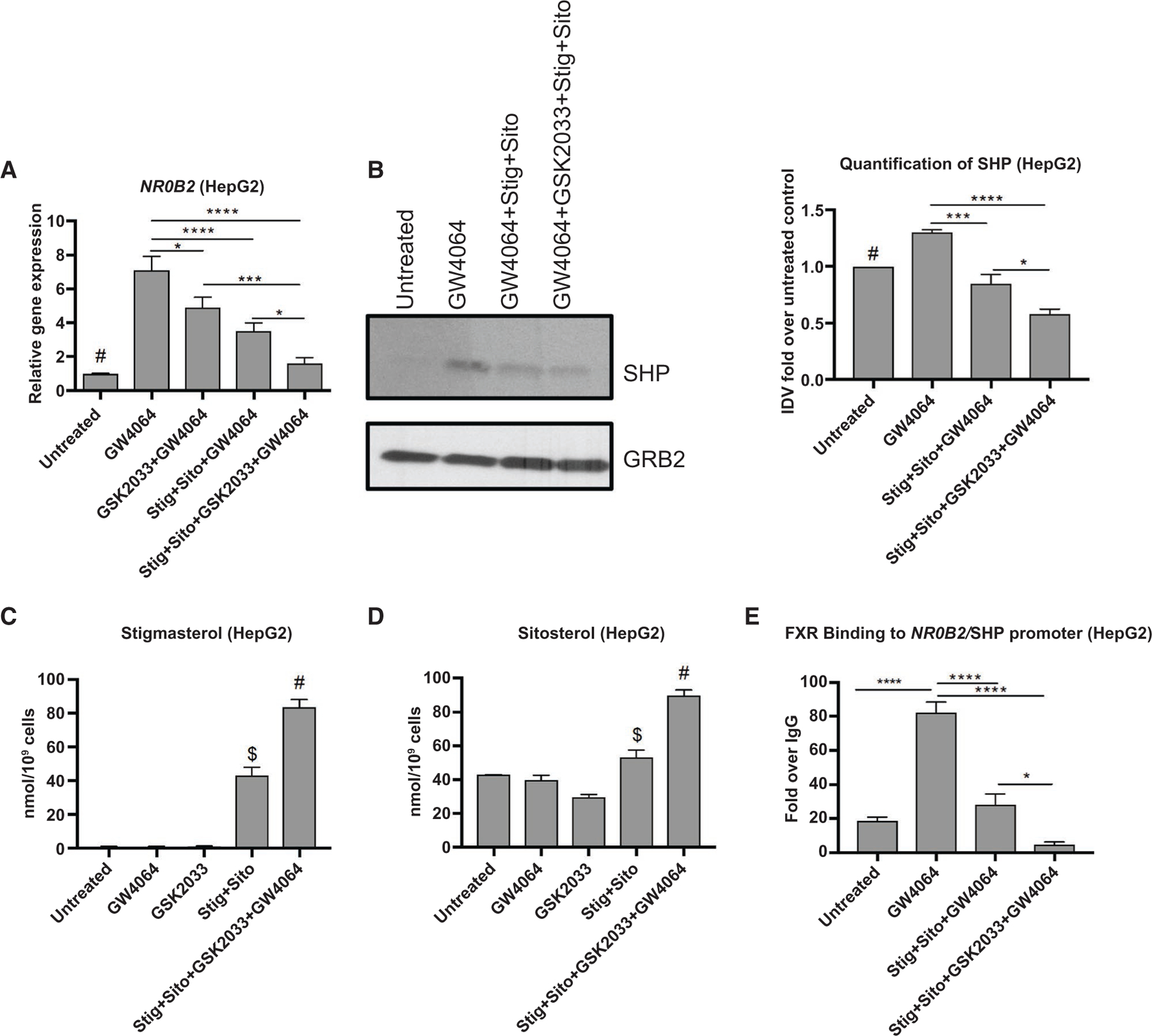
Inhibition of ABCG5/8 in HepG2 cells increases phytosterol accumulation and down-regulation of NR0B2/SHP. HepG2 cells were incubated with LXR antagonist GSK2033 for 4 hours followed by addition of +/− GW4064 or +/− stig+sito overnight; cells were harvested and mRNA analyzed. (A) NR0B2/SHP. #P < 0.05 versus all conditions except stig+sito + GSK2033+GW4064. (B) Immunoblot analysis and quantification of SHP protein in hepatic lysates extracted from HepG2 cells incubated as describe in (A). #P < 0.05 versus all groups except GW4064 + stig+sito. SHP protein was normalized to GRB2 and expressed relative to untreated control. (C) LC/MS quantification of stigmasterol concentrations in HepG2 cells treated as described in (A). #P < 0.0001 versus all other groups. +P < 0.0001 versus all other groups. (D) LC/MS quantification of sitosterol in HepG2 cells treated as described in (A). #P < 0.0001 versus all other groups. *P < 0.05 versus all groups except untreated. (E) ChIP assay demonstrating FXR binding to the promoter of NR0B2/SHP in cell homogenate from HepG2 cells incubated as described in (A). Data are presented as fold change over IgG. For all of these experiments, statistical analysis was performed by one-way ANOVA with Tukey’s correction for multiple comparisons. *P < 0.05, ***P < 0.001, and ****P < 0.0001. Abbreviation: GRB2, growth factor receptor bound protein 2.
To verify that suppression of ABCG5/8 led to enhanced hepatocyte accumulation of sitosterol and stigmasterol, the soy oil phytosterols most implicated in FXR antagonism, HepG2 cells were incubated overnight with stig+sito, GW4064, GSK2033, or the combination of all three, and stigmasterol and sitosterol were measured by LC/MS. Incubation with stig+sito led to a significant increase in hepatocyte concentrations of stig as well as sito, which were further increased by GSK2033 antagonism of LXR (Fig. 5C,D). Finally, to determine whether phytosterol accumulation interfered with FXR signaling by decreasing FXR occupancy on the NR0B2 promoter, HepG2 cells were treated as in Fig. 5A and ChIP assays performed using FXR antibody followed by quantitative real-time PCR. Phytosterol exposure in cells reduced FXR binding to the SHP promoter, which was further suppressed by co-incubation with GSK2033 (Fig. 5E and Supporting Fig. S5I). Taken together, these experiments provide further evidence that the reduced expression of ABCG5/8 in PNAC, which is associated with reduced LRH-1 mRNA and protein, elevates hepatic phytosterol concentrations, which leads to down-regulation of FXR target genes in hepatocytes and cholestasis.
Discussion
In this study, we investigated the role of two phytosterols (stigmasterol and sitosterol), present in commonly used soy lipid–based PN solutions, and IL-1β on the hepatic expression of LRH-1 and its down-stream targets in an established murine model of PNAC and in cultured cell lines and primary mouse hepatocytes. In the PNAC model, intestinal injury and increased intestinal permeability to LPS derived from intestinal microbiota is combined with infusion of soy oil lipid emulsion–containing PN solutions to mimic the pathophysiology present in humans with PNAC and IFALD. We previously validated that this model recapitulated the hepatic inflammatory milieu (increased expression of IL-1b, IL-6, and TNF), hepatic macrophage infiltration, hepatic retention of phytosterols, suppression of FXR and LXR expression, and hepatic bile and sterol gene transporter abnormalities (reduced ABCB11 and ABCC2 expression and suppressed induction of ABCG5/8) reported in children with IFALD.(7,10,12,29–31) In the current study, we found that hepatic LRH-1 mRNA and protein were down-regulated through NF-κB signaling in the PNAC mouse, which was associated with reduced LRH-1 binding to the Abcg5/8 promoter and reduced Abcg5/8 mRNA expression, concomitant with elevated hepatic phytosterol concentrations. These findings were reversed in Il-1r −/− mice and LRH-1 agonist (DLPC)–treated mice, implicating IL-1 signaling in the regulation of LRH-1 during PNAC. Furthermore, the findings suggest that activation of LRH-1 may be an approach to alleviate PNAC liver injury. In vitro studies confirmed that macrophage-derived IL-1β reduced hepatocyte NR5A2/LRH-1 and ABCG5/8 expression, which was mediated by NF-κB.
LRH-1 plays a pivotal role in suppression of hepatocyte bile acid synthesis.(32) When bile acids accumulate in the hepatocyte, as during cholestasis, FXR is activated and binds to the SHP promoter to induce SHP transcription.(32) Elevated levels of SHP protein, in turn, form inhibitory heterodimeric complexes with LRH-1 bound to its response elements on the promoter of CYP7A1 to repress transcription and down-regulate bile acid synthesis.(32) SHP may also interact with LRH-1 bound to the SHP promoter and repress transcription of its own gene.(16) In addition, LRH-1, as well as LXR, activates ABCG5/8 transcription by binding to its response element in the ABCG5/8 intergenic promoter, thereby promoting sterol secretion into the bile canaliculus and reducing phytosterol retention in the hepatocyte.(33–35) We have previously demonstrated that the accumulation of phytosterols plays a pivotal role in the pathogenesis of PNAC,(7,12) leading to the current exploration of the role of LRH-1 in regulating sterol and bile transport in PNAC.
In our previous report we showed that in PNAC, hepatic macrophage–derived IL-1, induces activation of NF-κB in hepatocytes, where it binds to promoter sequences and interferes with expression of FXR-regulated and LXR-regulated bile-transporter and sterol-transporter genes. In this study we expanded on these findings by demonstrating that the IL-1-induced activation of NF-κB in hepatocytes was also associated with down-regulation of Nr5a2/LRH-1 and subsequent downstream suppression of ABCG5/8 transcription. We also show that increased NF-κB phosphorylation and binding to the Nr5a2/LRH-1 promoter and suppressed Nr5a2/LRH-1 transcription were present in livers of mice with PNAC, all of which were reversed in Il-1r−/−/DSS-PN mice. We further show that siRNA knockdown of NF-κB led to increased Nr5a2/LRH-1 mRNA expression in HepG2 cells, and that NF-κB siRNA reversed the IL-1β-mediated suppression of LRH-1 transcription. Thus, the action of both major regulators of ABCG5/8 expression, LXR and LRH-1, are suppressed through NF-κB action in PNAC liver, resulting in failure to up-regulate transcription of ABCG5/8 in response to phytosterol exposure. The resulting insufficient canalicular secretion of sterols by ABCG5/8 promotes hepatocyte accumulation of PN-infused phytosterols, which antagonize FXR regulation of BSEP with resulting retention of bile acids and hepatocyte toxicity.(7,12)
Further supporting the critical role of Abcg5/8 and phytosterol accumulation in this PNAC model are recent studies showing that Abcg5/8−/− mice are more susceptible to cholestasis and have 90-fold-higher tissue concentrations of sitosterol compared with control mice.(35) In humans, mutations in ABCG5/8 cause a rare condition called sitosterolemia, a recessive disorder characterized by sterol accumulation secondary to reduced biliary and intestinal sterol excretion and premature coronary atherosclerosis as well as liver injury.(36–38) In a case report, a patient with sitosterolemia and two mutations in ABCG8 underwent liver transplant for cirrhosis and had significant correction of elevated serum phytosterol levels following transplantation.(36) This case demonstrates both the important role for canalicular rather than intestinal excretion of phytosterols by ABCG5/8, and the hepatic toxicity of retained phytosterols that can lead to progressive liver disease.(36)
Compelling evidence has now established that PN lipid emulsions are involved in the pathogenesis of PNAC.(9,12,39–42) Stigmasterol, a phytosterol present in plant-derived PN lipid emulsions, antagonizes FXR signaling.(7,9,43) FXR is the dominant nuclear hormone receptor playing a key role in bile acid metabolism by regulating both the synthesis and hepatocyte transport of bile acids. This critical role that FXR plays in maintaining hepatocyte bile acid concentrations at a safe level has recently been demonstrated in vivo in children with autosomal recessive biallelic loss-of-function mutations in NR1H4/FXR that result in a severe, progressive cholestatic liver disease in early infancy.(44) We propose that secondary suppression of FXR signaling by phytosterols in PNAC could similarly have major effects on bile acid secretion and promote intrahepatic cholestasis.(45) In this regard, stigmasterol has previously been implicated in the pathogenesis of PNAC(12) through its antagonism of FXR signaling and suppression of Abcb11 transcription.(7,9) Recent experimental and human reports indicate that modification of intravenous lipid emulsions that reduce phytosterol content may reverse PNAC and be used to treat this disorder.(6,7,40,43,46,47) In addition to stigmasterol, in this study we demonstrate that sitosterol, the predominant phytosterol in soy lipid emulsions and the most elevated serum phytosterol in children with IFALD,(41) can similarly suppress FXR target gene expression (Nr0b2/SHP), even in the presence of a potent FXR agonist, and therefore should also be considered a factor in the pathogenesis of PNAC.(9,12)
LRH-1 plays an important role in regulating synthesis of bile acids through its binding to an LRH-1 response element on the promoter of CYP7A1, the rate-limiting enzyme in bile acid synthesis. LRH-1 occupancy of this site transactivates SHP when bound to its own response element on the CYP7A1 promoter, leading to transcriptional repression of CYP7A1 and reduced bile acid synthesis,(16) thus providing a protective mechanism against cholestasis-induced bile acid toxicity to hepatocytes. We have previously shown that there is failure to adequately suppress CYP7A1 mRNA transcription in the PNAC mouse model,(12) although the mechanism was not explored. Here we demonstrate reduced hepatic mRNA and protein levels of LRH-1 in PNAC liver, as well as reduced LRH-1 binding to the CYP7A1 promoter, abrogating its transactivation of SHP, and thus releasing SHP suppression of CYP7A1 transcription. The resultant dysregulated increased synthesis of bile acids concomitant with reduced BSEP expression could elevate hepatocyte bile acid concentrations and exacerbate the cholestatic injury. The beneficial effect of the LRH-1 agonist, DLPC, in the PNAC mouse suggests that LRH-1 signaling plays an important role in the failure to appropriately down-regulate Cyp7a1 in PNAC, and that this may be a potential therapeutic target.
In summary, in the current study we demonstrate an important role for LRH-1 signaling in the pathogenesis of PNAC. We show that inhibition of LRH-1 and its targets ABCG5/8 in hepatocytes contribute to phytosterol accumulation, reduced FXR occupancy on the NR0B2/SHP promoter, and reduced expression of NR0B2, ABCB11, and ABCC2. Furthermore, this study supports the proposed mechanism (Fig. 6) of PNAC, in which pro-inflammatory activation of hepatic macrophages is promoted by the combined effect of intestinal-derived LPS and circulating phytosterols,(7,12) which, in turn, lead to IL-1β–NF-κB–mediated suppression of hepatocyte nuclear receptor signaling, including that of LRH-1, and downstream bile and sterol transporters, with the subsequent retention of phytosterols that further antagonize FXR, down-regulating BSEP and MRP2, culminating in hepatocyte retention of bile acids and cholestatic injury. These results support the role of reducing phytosterol content of PN lipid emulsions in treating PNAC and suggest that methods taken to induce LRH-1 activity may be a therapeutic approach in PNAC.
FIG. 6.
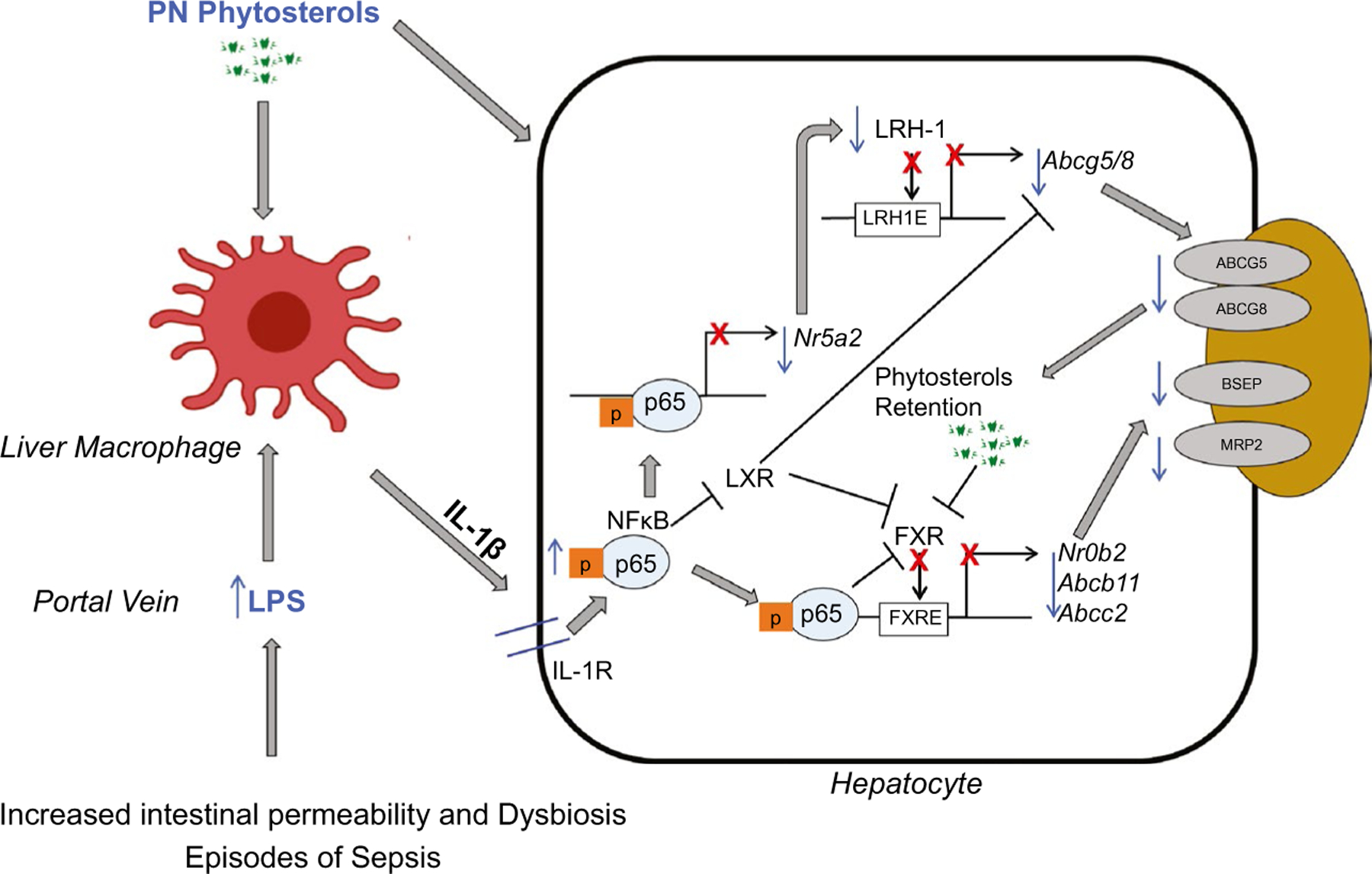
Proposed role of LRH-1 in PNAC pathogenesis. Intestinal injury, dysbiosis, and hyperpermeability caused by intestinal failure promote LPS (and other pathogen-associated molecular patterns) absorption into portal vein, subsequently recruiting and activating liver macrophages to generate IL-1β, which binds to IL-1 receptor on hepatocytes. This triggers activation of NF-κB, which binds to promoter of NR5A2/LRH-1 to inhibit its expression, as well as FXR and LXR target genes. This results in suppression of downstream ABCG5/8 transcription and subsequent hepatocyte accumulation of phytosterols infused in the PN solution, which further antagonize FXR signaling and thus down-regulating canalicular ABCB11/BSEP and ABCC2/MRP2, promoting retention of bile acids and bilirubin and culminating in cholestatic injury. Retained PN phytosterols may also directly activate hepatic macrophages and magnify cytokine production. Abbreviation: FXRE, farnesoid X receptor response element.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (UL1TR002535) and the Gastrointestinal and Liver Innate Immunity Program at the University of Colorado Anschutz Medical Center.
Abbreviations:
- ABCB11
ATP-binding cassette, subfamily B member 11
- ABCC2
ATP-binding cassette subfamily C member 2
- ABCG5/8
ATP-binding cassette subfamily G member 5/8
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BSEP
bile salt export pump
- ChIP
chromatin immunoprecipitation
- DLPC
dilauroyl phosphatidylcholine
- DSS
dextran sulfate sodium
- FXR
farnesoid X receptor
- IF
intestinal failure
- IFALD
IF-associated liver disease
- IL-1β
interleukin-1 beta
- LC/MS
liquid chromatography–mass spectrometry
- LPS
lipopolysaccharide
- LRH-1
liver receptor homolog 1
- LXR
liver X receptor
- MRP2
multidrug resistance-associated protein 2
- NR5A2
nuclear receptor subfamily 5, group A, member 2
- NR0B2
nuclear receptor subfamily 0, group B, member 2
- PN
parenteral nutrition
- PNAC
parenteral nutrition–associated cholestasis
- SHP
small heterodimer partner
- siRNA
small interfering RNA
- sito
sitosterol acetate
- stig
stigmasterol acetate
- WT
wild type
Footnotes
Potential conflict of interest: Dr. Sokol consults for Mirum and Albireo. Dr. D’Alessandro consults for Rubius. He advises Hemanext and Forma. He owns stock in Omix and Altis.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.32071/suppinfo.
REFERENCES
- 1).Orso G, Mandato C, Veropalumbo C, Cecchi N, Garzi A, Vajro P. Pediatric parenteral nutrition-associated liver disease and cholestasis: novel advances in pathomechanisms-based prevention and treatment. Dig Liver Dis 2016;48:215–222. [DOI] [PubMed] [Google Scholar]
- 2).Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology 2006;130:S70–S77. [DOI] [PubMed] [Google Scholar]
- 3).Ganousse-Mazeron S, Lacaille F, Colomb-Jung V, Talbotec C, Ruemmele F, Sauvat F, et al. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clin Nutr 2015;34:428–435. [DOI] [PubMed] [Google Scholar]
- 4).Israelite JC. Pediatric parenteral nutrition-associated liver disease. J Infus Nurs 2017;40:51–54. [DOI] [PubMed] [Google Scholar]
- 5).Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol 2007;27:284–290. [DOI] [PubMed] [Google Scholar]
- 6).Fell GL, Nandivada P, Gura KM, Puder M. Intravenous lipid emulsions in parenteral nutrition. Adv Nutr 2015;6:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).El Kasmi KC, Anderson AL, Devereaux MW, Vue PM, Zhang W, Setchell KD, Karpen SJ, et al. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med 2013;5:206ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Kaufman SS, Gondolesi GE, Fishbein TM. Parenteral nutrition associated liver disease. Semin Neonatol 2003;8:375–381. [DOI] [PubMed] [Google Scholar]
- 9).Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res 2007;62:301–306. [DOI] [PubMed] [Google Scholar]
- 10).El Kasmi KC, Anderson AL, Devereaux MW, Fillon SA, Harris JK, Lovell MA, et al. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 2012;55:1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Lee WS, Sokol RJ. Intestinal microbiota, lipids, and the pathogenesis of intestinal failure-associated liver disease. J Pediatr 2015;167:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).El Kasmi KC, Vue PM, Anderson AL, Devereaux MW, Ghosh S, Balasubramaniyan N, et al. Macrophage-derived IL-1beta/NF-kappaB signaling mediates parenteral nutrition-associated cholestasis. Nat Commun 2018;9:1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Chai J, Feng X, Zhang L, Chen S, Cheng Y, He X, et al. Hepatic expression of detoxification enzymes is decreased in human obstructive cholestasis due to gallstone biliary obstruction. PLoS One 2015;10:e0120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Zambrano E, El-Hennawy M, Ehrenkranz RA, Zelterman D, Reyes-Mugica M. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr Dev Pathol 2004;7:425–432. [DOI] [PubMed] [Google Scholar]
- 15).Oosterveer MH, Mataki C, Yamamoto H, Harach T, Moullan N, van Dijk TH, et al. LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J Clin Invest 2012;122:2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 2000;6:517–526. [DOI] [PubMed] [Google Scholar]
- 17).Stein S, Oosterveer M, Mataki C, Xu P, Lemos V, Havinga R, et al. SUMOylation-dependent LRH-1/PROX1 interaction promotes atherosclerosis by decreasing hepatic reverse cholesterol transport. Cell Metab 2014;20:603–613. [DOI] [PubMed] [Google Scholar]
- 18).Liu DL, Liu WZ, Li QL, Wang HM, Qian D, Treuter E, et al. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol Reprod 2003;69:508–517. [DOI] [PubMed] [Google Scholar]
- 19).Freeman LA, Kennedy A, Wu J, Bark S, Remaley AT, Santamarina-Fojo S, et al. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J Lipid Res 2004;45:1197–1206. [DOI] [PubMed] [Google Scholar]
- 20).Erker L, Azuma H, Lee AY, Guo C, Orloff S, Eaton L, et al. Therapeutic liver reconstitution with murine cells isolated long after death. Gastroenterology 2010;139:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Nemkov T, D’Alessandro A, Hansen KC. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 2015;47:2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom 2017;31:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol Biol 2019;1978:13–26. [DOI] [PubMed] [Google Scholar]
- 24).Shearn CT, Orlicky DJ, Petersen DR. Dysregulation of antioxidant responses in patients diagnosed with concomitant primary sclerosing cholangitis/inflammatory bowel disease. Exp Mol Pathol 2018;104:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem 2005;280:34538–34547. [DOI] [PubMed] [Google Scholar]
- 26).Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 2000;6:507–515. [DOI] [PubMed] [Google Scholar]
- 27).Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009;50:1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Heckmann BL, Zhang X, Saarinen AM, Schoiswohl G, Kershaw EE, Zechner R, et al. Liver X receptor alpha mediates hepatic triglyceride accumulation through upregulation of G0/G1 switch gene 2 expression. JCI Insight 2017;2:e88735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Kurvinen A, Nissinen MJ, Gylling H, Miettinen TA, Lampela H, Koivusalo AI, et al. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J Pediatr Gastroenterol Nutr 2011;53:440–446. [DOI] [PubMed] [Google Scholar]
- 30).Kurvinen A, Nissinen MJ, Andersson S, Korhonen P, Ruuska T, Taimisto M, et al. Parenteral plant sterols and intestinal failure-associated liver disease in neonates. J Pediatr Gastroenterol Nutr 2012;54:803–811. [DOI] [PubMed] [Google Scholar]
- 31).Mutanen A, Lohi J, Heikkila P, Jalanko H, Pakarinen MP. Liver inflammation relates to decreased canalicular bile transporter expression in pediatric onset intestinal failure. Ann Surg 2018;268:332–339. [DOI] [PubMed] [Google Scholar]
- 32).Miao J, Choi SE, Seok SM, Yang L, Zuercher WJ, Xu Y, et al. Ligand-dependent regulation of the activity of the orphan nuclear receptor, small heterodimer partner (SHP), in the repression of bile acid biosynthetic CYP7A1 and CYP8B1 genes. Mol Endocrinol 2011;25:1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Mataki C, Magnier BC, Houten SM, Annicotte J-S, Argmann C, Thomas C, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol 2007;27:8330–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci 2008;13:5950–5958. [DOI] [PubMed] [Google Scholar]
- 35).Wang J, Mitsche MA, Lutjohann D, Cohen JC, Xie XS, Hobbs HH. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res 2015;56:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Miettinen TA, Klett EL, Gylling H, Isoniemi H, Patel SB. Liver transplantation in a patient with sitosterolemia and cirrhosis. Gastroenterology 2006;130:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Sun W, Zhang T, Zhang X, Wang J, Chen Y, Long Y, et al. Compound heterozygous mutations in ABCG5 or ABCG8 causing Chinese familial Sitosterolemia. J Gene Med 2020;22:e3185. [DOI] [PubMed] [Google Scholar]
- 38).Xu L, Wen W, Yang YA, Xie J, Li R, Wu Y, et al. Features of Sitosterolemia in children. Am J Cardiol 2020;125:1312–1316. [DOI] [PubMed] [Google Scholar]
- 39).Btaiche IF, Khalidi N. Parenteral nutrition-associated liver complications in children. Pharmacotherapy 2002;22:188–211. [DOI] [PubMed] [Google Scholar]
- 40).Nandivada P, Carlson SJ, Chang MI, Cowan E, Gura KM, PuderTreatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr 2013;4:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Khalaf RT, Sokol RJ. New insights into intestinal failure-associated liver disease in children. Hepatology 2020;71:1486–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Hukkinen M, Mutanen A, Nissinen M, Merras-Salmio L, Gylling H, Pakarinen MP. Parenteral plant sterols accumulate in the liver reflecting their increased serum levels and portal inflammation in children with intestinal failure. JPEN J Parenter Enteral Nutr 2017;41:1014–1022. [DOI] [PubMed] [Google Scholar]
- 43).Vlaardingerbroek H, Ng K, Stoll B, Benight N, Chacko S, Kluijtmans LeoAJ, et al. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J Lipid Res 2014;55:466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim M-S, Kim KH, et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun 2016;7:10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Cariello M, Piccinin E, Garcia-Irigoyen O, Sabba C, Moschetta A. Nuclear receptor FXR, bile acids and liver dam-age: introducing the progressive familial intrahepatic cholestasis with FXR mutations. Biochim Biophys Acta Mol Basis Dis 2018;1864:1308–1318. [DOI] [PubMed] [Google Scholar]
- 46).Isaac DM, Alzaben AS, Mazurak VC, Yap J, Wizzard PR, Nation PN, et al. Mixed lipid, fish oil, and soybean oil parenteral lipids impact cholestasis, hepatic phytosterol, and lipid composition. J Pediatr Gastroenterol Nutr 2019;68:861–867. [DOI] [PubMed] [Google Scholar]
- 47).Burrin DG, Ng K, Stoll B, Saenz De Pipaon M. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 2014;5:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


