Abstract
Objective:
This observational study examined the effects of electroconvulsive therapy (ECT) on suicide and all-cause mortality risk.
Methods:
Participants were Medicare-insured psychiatric inpatients aged 65 years and over. Patients receiving ECT were exact matched to controls (1:3 ratio) on age, gender, principal hospital diagnosis, past year psychiatric hospitalizations, past year suicide attempts, and Elixhauser Comorbidity Index. Cox proportional hazard models were risk-adjusted for race, year of hospitalization, rural-urban continuum code, year of index hospitalization, median income of zip code, and all matched covariates to estimate hazard ratios (HR) with 95% confidence intervals (CI). Analyses were conducted between March 2019 and March 2021.
Results:
A total of 41,620 patients were included in the analysis (65.4% female, mean age 74.7 [SD 7.09]) including 10,460 in the ECT group and 31,160 in the control group. Compared to the control group, patients receiving ECT had lower all-cause mortality for up to 1 year following hospital discharge (adjusted hazard ratio [HR]=0.61, 95% CI:0.56–0.66). For death by suicide, one-year survival analysis showed no group difference. A significant association was observed in the first months following ECT, but this pattern waned over time (1 month: HR=0.44, 95% CI:0.21–0.91; 2 months: HR=0.52, 95% CI:0.29–0.92; 3 months: HR=0.56, 95% CI:0.37–0.92; 6 months: 0.87, 95% CI:0.59–1.28; 12 months: 0.92, 95% CI:0.68–1.25).
Conclusions:
In this observational study, ECT was associated with lower one-year all-cause mortality and short-lived protective effects on suicide risk. These findings support greater consideration of ECT for inpatients with mood disorders at short-term risk of suicide.
INTRODUCTION
Mood disorders, including major depressive disorder and bipolar disorder, are a leading cause of disability worldwide (1). Individuals who suffer from mood disorders are at greater risk for premature mortality compared to the general population. For males, this translates to approximately 10 years of life expectancy lost for individuals with major depressive disorder or bipolar disorder; among females, 7–11 years of life expectancy are lost (2). This increased risk of mortality has been reported across several causes of mortality, including death from suicide, cardiovascular disease, and cancer (3–5). The risk of premature mortality is also considerably higher among individuals with psychosis compared to the general population (6).
With respect to suicide, the period immediately following psychiatric hospitalization is of extreme high risk. In one report of Medicaid patients, suicide rates per 100,000 person-years were 235 for MDD, 216 for bipolar disorder, and 168 for schizophrenia in the 3 months following psychiatric hospitalization (7). Studies in Europe have documented similar findings (8). Inpatients who were not well connected to outpatient healthcare in the 6 months prior to admission are also at increased short-term suicide risk (7), suggesting that at least part of the increased risk is mediated by the poor transition of care that occurs as patients shift from inpatient to outpatient care.
Some prior research suggests that ECT may reduce all-cause mortality. However, narrow target populations, small sample sizes, and concerns over confounder control cast some uncertainty over this work. The most recent study, which reported decreased mortality and suicide risk over an 8-year follow-up for adults with major depression or post-traumatic stress disorder, was from a single Veterans Affairs center and included 92 ECT patients and therefore may not generalize to broader practice (9). A larger study of 783 ECT patients in the UK also found lower mortality among those receiving ECT than among a comparison cohort; however, this study did not account for potentially confounding baseline clinical differences between cohorts (10). In addition, an early cohort study of 135 ECT patients from a single state psychiatric hospital reported a reduced risk of all-cause mortality, but this study also did not control for baseline clinical differences between study cohorts (11). By contrast, an early study from upstate New York in 1984 found higher mortality among depressed inpatients receiving ECT compared to those not receiving ECT. However, this study also did not account for potential confounding patient characteristics other than age (12).
Prior studies on the effects of ECT on suicide are inconsistent. Early studies comparing suicide rates across eras (i.e., when ECT was the primary approach to treating psychiatric disorders in the 1940s v. when ECT was not yet available in the 1930s) suggested an effect on suicide but were subject to methodological limitations such as cohort effects (13, 14). Some later studies have shown a long-term association of ECT with lower rates of suicide but were limited to a single site (9) or areas of the world where ECT is much more common and used on less severely ill patients (15). A recent large, multisite cohort analysis of Veterans Affairs hospitals showed no effect on suicide over a one-year follow-up period (16).
In the present study, we examine the association of ECT with important clinical outcomes: all-cause mortality and suicide in a large, nationally representative cohort of Medicare patients. Given that ECT is particularly effective in older adults (17, 18), we chose to focus our analyses on older patients.
METHODS
Data Source
The data for this study were obtained from the Medicare fee-for-service claims database (including Part D) and the National Death Index (NDI) from 2010–2016, the most recently available data at the time the study was initiated. The Medicare data are derived from claims records, including the Master Beneficiary Summary File, Part D Event Drug files, outpatient and inpatient files, and carrier files.
Sample
Data from all older Medicare beneficiaries (≥65 years) who received any ECT procedures from 2010–2015 (identified by CPT code 90870 or 90871, ICD-9 code 94.27, or ICD-10 codes GZB0ZZZ-GZB4ZZZ) were obtained. We also obtained data from a 25% random sample of all Medicare beneficiaries aged 65 or over who had codes for major depressive disorder during this time period (ICD-9-CM: 296.2x, 296.3x; ICD-10-CM: F32.X and F33.X). To facilitate an appropriate index date, we restricted the analysis to inpatients.
Analytic Sample
Medicare beneficiaries were required to be aged 65 or older, have continuous Medicare coverage (fee-for-service and Part D) for the 12 months prior to index date (see below), and have a psychiatric hospitalization (based on principal hospital diagnosis, see eTable 1) between 2011–2015. Individuals in US territories were not included.
Exact Matching, Index Date
ECT patients were matched in a 1:3 ratio with patients who did not receive ECT. The index date for ECT patients was defined as the discharge date of the first observed hospitalization with ECT treatment. The index date for the control cohort of patients not receiving ECT was the discharge date of a psychiatric hospitalization meeting the matching parameters. Index hospitalization for the control group was chosen such that the number of psychiatric hospitalizations in the pre-index period matched the ECT group. Exact matching was used to create comparable cohorts in regard to measured factors that are most strongly related to risk of suicide and all-cause mortality (7, 19, 20). These included gender, age, number of medically injurious suicide attempts in the prior year (as identified by E-codes E950-E959), number of psychiatric hospitalizations in the prior year, principal diagnosis of the index hospitalization (to the 4th digit of ICD code), and modified Elixhauser Comorbidity Index (20), a measure of medical mortality risk. Additional variables (including race, rural-urban continuum code, US Census region, median income of zip code, and psychotropic medications in the prior year) were included as covariates in the Cox proportional hazard models.
The length-of-stay of the index hospitalization was imbalanced between groups, with ECT patients having longer lengths of inpatient stay. This was likely in part because many ECT centers do not provide outpatient ECT; providers thus will keep patients in the hospital to allow for them to finish a course of ECT, which lengthens their overall hospital stay. A sensitivity analysis was conducted using length-of-stay in the matching algorithm. A 1:1 matching ratio was used in this analysis to allow for matching with more parameters.
Limiting the control cohort to patients hospitalized for psychiatric disorders, but not receiving ECT, created a comparable cohort of patients that, similar to the ECT cohort, required an active inpatient intervention to ameliorate symptoms leading to hospitalization. Use of an active comparator has been shown to minimize bias in observational cohort studies (21).
Exposure
In our primary analysis, we defined exposure to ECT as one or more ECT treatments during the index hospitalization. This is a conservative definition of exposure that is analogous to an intent-to-treat approach in clinical trials, given that some patients stop treatment early due to adverse events. We also conducted a secondary analysis (analogous to a per protocol approach in clinical trials) including only individuals who were likely to receive at least a minimally adequate course of ECT, defined as ≥5 ECT sessions within a 30-day period, as per prior literature (22).
Outcomes
The primary outcomes of interest were all-cause mortality and suicide death, as recorded in the National Death Index (NDI). The NDI is maintained by the National Center for Health Statistics (NCHS) and is regarded as the most authoritative source of death information for the US population, including over 99% of all deaths in the US (23–26). This data provides date and cause of death using International Classification of Diseases (ICD) codes, version 10. The definition of suicide death included mechanisms such as injury/poisoning with undetermined intent (Y10-Y34) classified as probable suicide deaths (see eTable 2).
Analysis
We compared matched ECT and non-ECT groups on measured characteristics prior to index date. Covariate balance was compared using standardized differences for all baseline covariates between ECT and non-ECT patient groups. We used Cox proportional hazards regressions to model the effect of ECT on all-cause mortality and suicide death, for up to one year following index date (hospitalization discharge date). The analyses were adjusted for all matched variables and race/ethnicity, rural-urban continuum codes, year of hospitalization, median income of zip code, and psychotropic medications. We reported the adjusted hazard ratios [HR] and their corresponding 95% confidence intervals [CI] at 30, 60, 90, 180, and 365 days. Our primary analysis included any patient receiving 1 or more ECT sessions and matched controls (i.e., intent-to-treat). A secondary analysis categorized patients as those who received a therapeutic course (≥5 ECT sessions within first 30 days), subtherapeutic course (<5 sessions), and their corresponding matched controls (i.e., per protocol).
As an alternative to exact matching, a sensitivity analysis was performed using inverse probability treatment weighting. In this analysis, propensity scores (the probability of initiating ECT or not) were calculated using a logistic regression model, with covariates of age, gender, race, median income of zip code, US region, psychiatric hospitalizations in the prior year, suicide attempts in the prior year, psychotropic medications in the prior year, year of hospitalization, Elixhauser score, and principal hospital diagnosis (eTable 3). Score distributions were trimmed at the 2.5th and 97.5th percentiles to reduce potential unmeasured confounding bias by excluding patients treated contrary to strong predictions (27). Cox proportional hazard models were used to examine effect of ECT on all-cause mortality and suicide death.
Analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina), R (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 16 (College Station, Texas) between March 2019 and March 2021. All p-values were 2-sided with a p-value < 0.05 indicating statistical significance. The study used de-identified data and was approved with a waiver of informed consent by the Yale School of Medicine Institutional Review Board (#2000024893).
RESULTS
There were 11,654 ECT patients that met inclusion and exclusion criteria (Figure 1). Following matching, 1,194 (10.2%) ECT recipients had no suitable matches and were excluded from analysis; 10,460 ECT patients had ≥1 matched controls, resulting in 31,160 matched controls. Of all patients who received ≥1 ECT sessions, 8,425 (82.9%) received ≥5 sessions within a 30-day period, which we categorized as the group likely to have received a therapeutic course of ECT. To avoid immortal time-bias, we only analyzed the sample of those receiving a therapeutic course of ECT if they completed 5 treatments prior to discharge (n=6,670).
Figure 1.
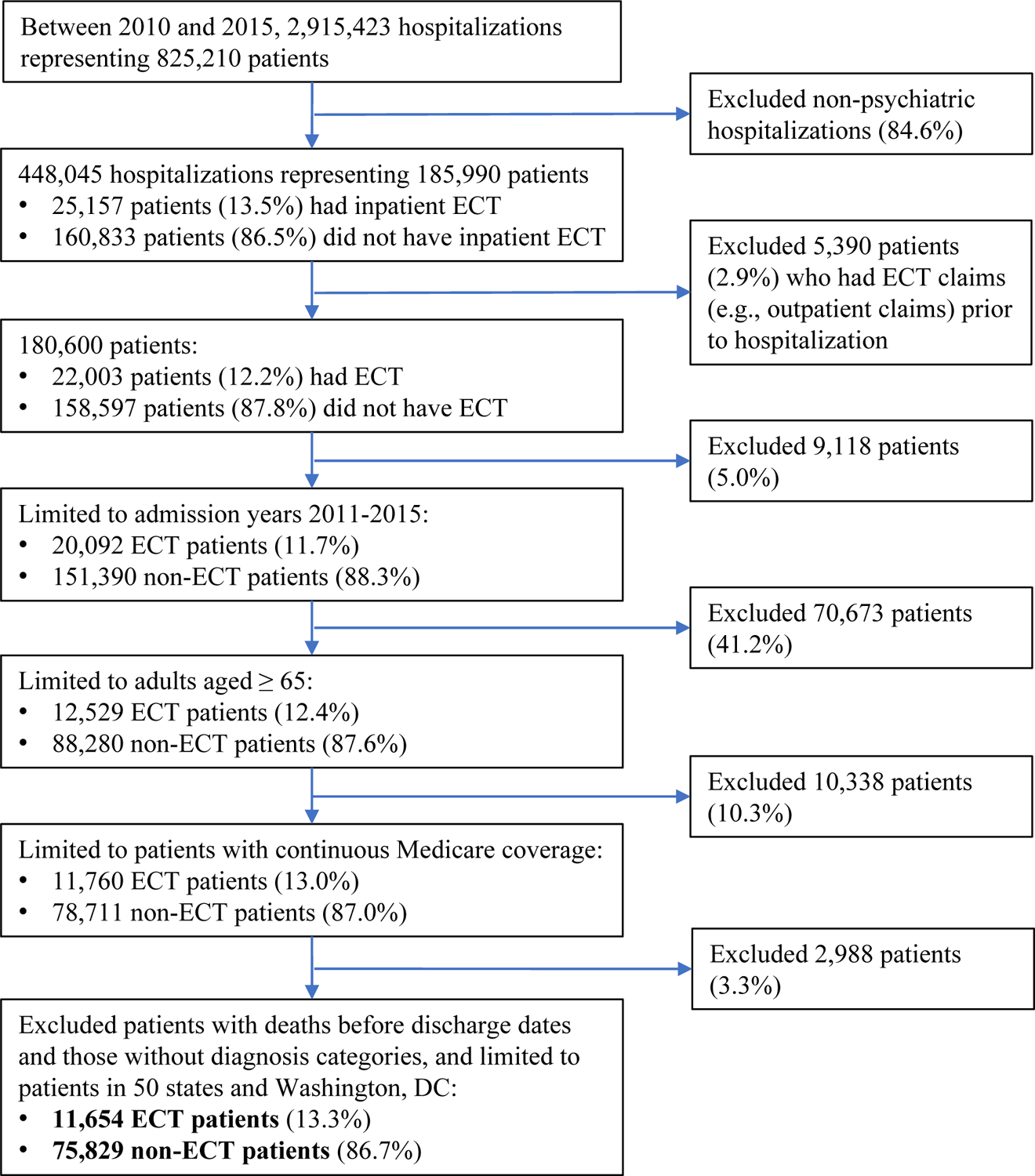
Flowchart of study cohorts
Following the application of eligibility criteria and matching, ECT and control groups were well balanced in all matching characteristics: age, gender, principal hospital diagnosis, suicide attempts in the prior year, and psychiatric hospitalizations in the prior year, and mean Elixhauser Comorbidity Index (Table 1). In addition, the study groups were also similar in year of index hospitalization, urban setting, region, medication history in the prior year (except for antipsychotic and lithium use, which were higher in the ECT group). Consistent with prior research (28), those in the ECT group lived in areas with higher median income and were more likely to be white compared to the control group. Notably, the ECT group had longer hospital admissions compared to the control group.
Table 1.
Baseline clinical and demographic characteristics
| No ECT (N=31,160) | ECT (N=10,460) | Standardized Difference | |||
|---|---|---|---|---|---|
| Age, by category | N | % | n | % | <0.01 |
| 65–74 | 17,196 | 55.2 | 5,760 | 55.1 | |
| 75–84 | 10,600 | 34.0 | 3,564 | 34.1 | |
| 85+ | 3,364 | 10.8 | 1,136 | 10.9 | |
| Female | 20,379 | 65.4 | 6,833 | 65.3 | <0.01 |
| Year of Index Hospitalization | 0.08 | ||||
| 2011 | 6,765 | 21.7 | 2,379 | 22.7 | |
| 2012 | 6,546 | 21.0 | 2,324 | 22.2 | |
| 2013 | 6,278 | 20.2 | 2,100 | 20.1 | |
| 2014 | 5,683 | 18.2 | 1,946 | 18.6 | |
| 2015 | 5,888 (18.9) | 18.9 | 1,711 | 16.4 | |
| Urban setting | 25,149 | 80.7 | 8,946 | 85.5 | 0.13 |
| Modified Elixhauser Score | 0.05 | ||||
| 0 | 8,691 | 27.9 | 2,921 | 27.9 | |
| 1–5 | 6,090 | 19.5 | 2,049 | 19.6 | |
| 6–12 | 8,616 | 27.7 | 2,889 | 27.6 | |
| >12 | 7,763 | 24.9 | 2,601 | 24.9 | |
| Psychiatric Hospitalization in Prior Year | 0.04 | ||||
| 0 | 18,904 | 60.7 | 6,306 | 60.3 | |
| 1 | 7,649 | 24.6 | 2,572 | 24.6 | |
| 2 | 2,783 | 8.9 | 950 | 9.1 | |
| ≥3 | 1,824 | 5.9 | 632 | 6.0 | |
| Suicide Attempt in Prior Year | 0.15 | ||||
| 0 | 29,623 | 95.1 | 9.910 | 94.7 | |
| 1 | 1,351 | 4.3 | 474 | 4.5 | |
| ≥2 | 186 | 0.6 | 76 | 0.7 | |
| Race | 0.29 | ||||
| Non-Hispanic White | 26,840 | 86.1 | 9,597 | 91.8 | |
| Hispanic | 1,346 | 4.3 | 259 | 2.5 | |
| African American | 2,397 | 7.7 | 337 | 3.2 | |
| Other | 506 | 1.6 | 224 | 2.1 | |
| Region | 0.34 | ||||
| Northeast | 7,219 | 23.2 | 2,995 | 28.6 | |
| South | 6,054 | 19.4 | 3,006 | 28.7 | |
| Midwest | 14,553 | 46.7 | 3,363 | 32.2 | |
| West | 3,334 | 10.7 | 1,096 | 10.5 | |
| Principal Hospital Diagnosis | 0.14 | ||||
| Major depressive disorder | 22,007 | 70.6 | 7,372 | 70.5 | |
| Bipolar disorder | 5,802 | 18.6 | 1,957 | 18.7 | |
| Psychotic depression | 278 | 0.9 | 102 | 1.0 | |
| Schizophrenia/schizoaffective disorder | 2,034 | 6.5 | 681 | 6.5 | |
| Other | 1,039 | 3.3 | 348 | 3.3 | |
| Length of stay of index hospitalization in days (SD) | 11.2 | 11.8 | 25.1 | 17.2 | 0.94 |
| Median Income of Zip Code | 0.37 | ||||
| <$40,000 | 7,967 | 25.6 | 1,444 | 13.8 | |
| $40,000 – $60,000 | 12,707 | 40.8 | 4,010 | 38.3 | |
| ≥$60,000 | 9,818 | 31.5 | 4,860 | 46.5 | |
| Psychotropic medication use in prior year | |||||
| Antidepressant | 17,793 | 57.1 | 6,329 | 60.5 | 0.07 |
| Lithium | 838 | 2.7 | 758 | 7.3 | 0.21 |
| Antiepileptic Mood stabilizer | 7,939 | 25.5 | 2,713 | 25.9 | 0.01 |
| Antipsychotic | 10,264 | 32.9 | 4,755 | 45.5 | 0.26 |
All-Cause Mortality
Compared to the control group, the ECT group had significantly lower all-cause mortality at all time points for up to one year (HR=0.61, 95% CI 0.56 to 0.66). A survival curve is shown in Figure 2 for all ECT patients as well as those who met criteria for a therapeutic course. Hazard ratios (adjusting for unmatched covariates) ranged from 0.53 to 0.61 (p<0.01 for all) for all patients who received ≥1 ECT session in the time points up to 1-year following index date (Figure 3). For the sample with only those who met criteria for a therapeutic course, hazard ratios ranged from 0.40 to 0.56 (p<0.01 for all) during this time period. When the survival analysis included those who received ≥5 ECT sessions within 30 days (therapeutic ECT course) and those who received a subtherapeutic course of ECT (<5 treatments within the first 30 days of treatment), those with a subtherapeutic course had a survival trajectory similar to those who did not receive ECT (Figure 2). Results of the model using inverse probability weighting yielded similar results (eTable 4). In a sensitivity analysis that included length-of-stay in matching algorithm (1:1 ratio), ECT was also associated with a lower risk of all-cause mortality (one-year HR=0.56, 95% CI 0.51 to 0.61; eTable 5).
Figure 2.
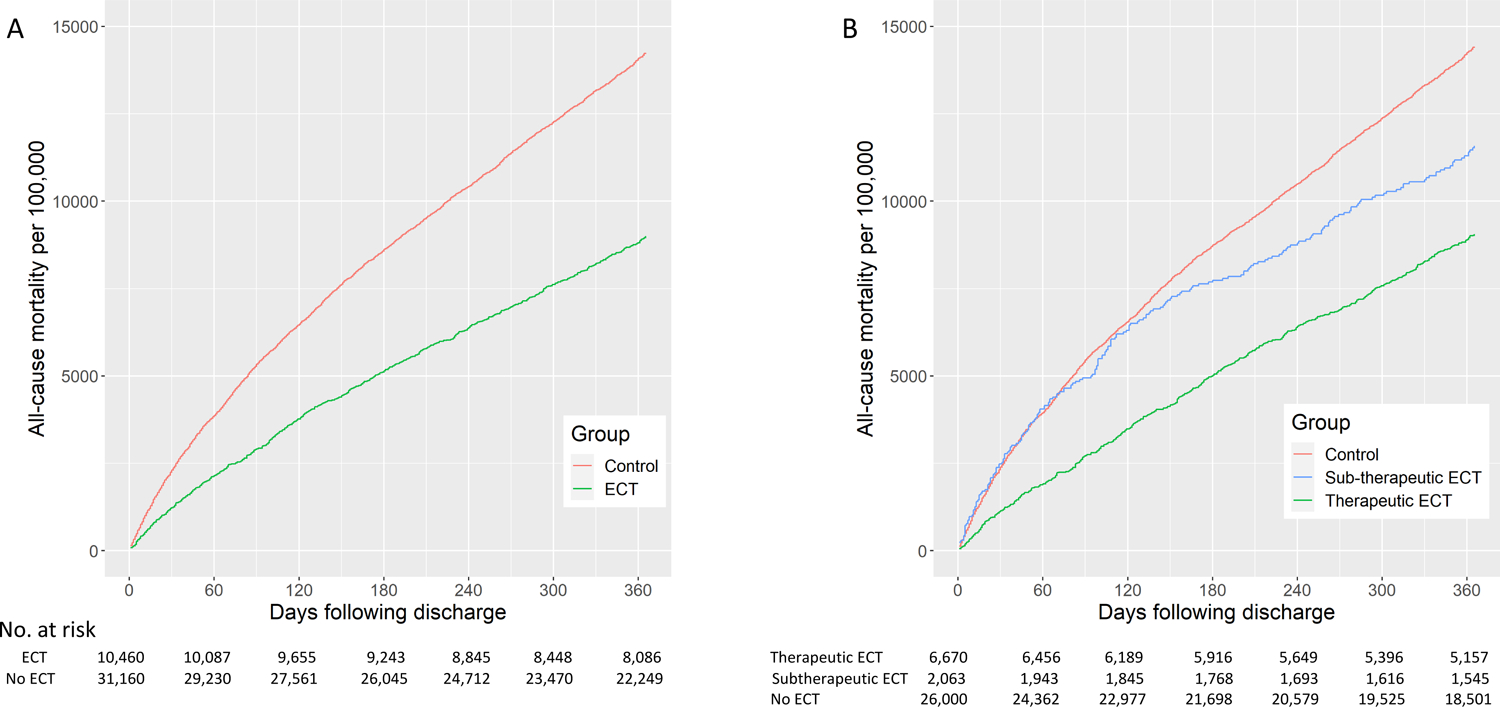
Effect of ECT on all-cause mortality. Analysis in Panel A includes any patient receiving 1 or more ECT and matched controls (Hazard Ratio=0.61, 95% CI 0.55 to 0.66). Analysis in Panel B defines therapeutic ECT as patients receiving 5 or more ECT sessions within 30 days (therapeutic course; Hazard Ratio=0.56, 95% CI 0.52 to 0.61 v. matched controls) and those receiving less than 5 ECT sessions (subtherapeutic; HR=0.80, 95% CI 0.69 to 0.94). There was no difference in trajectory between matched controls for therapeutic and subtherapeutic ECT groups.
Figure 3.
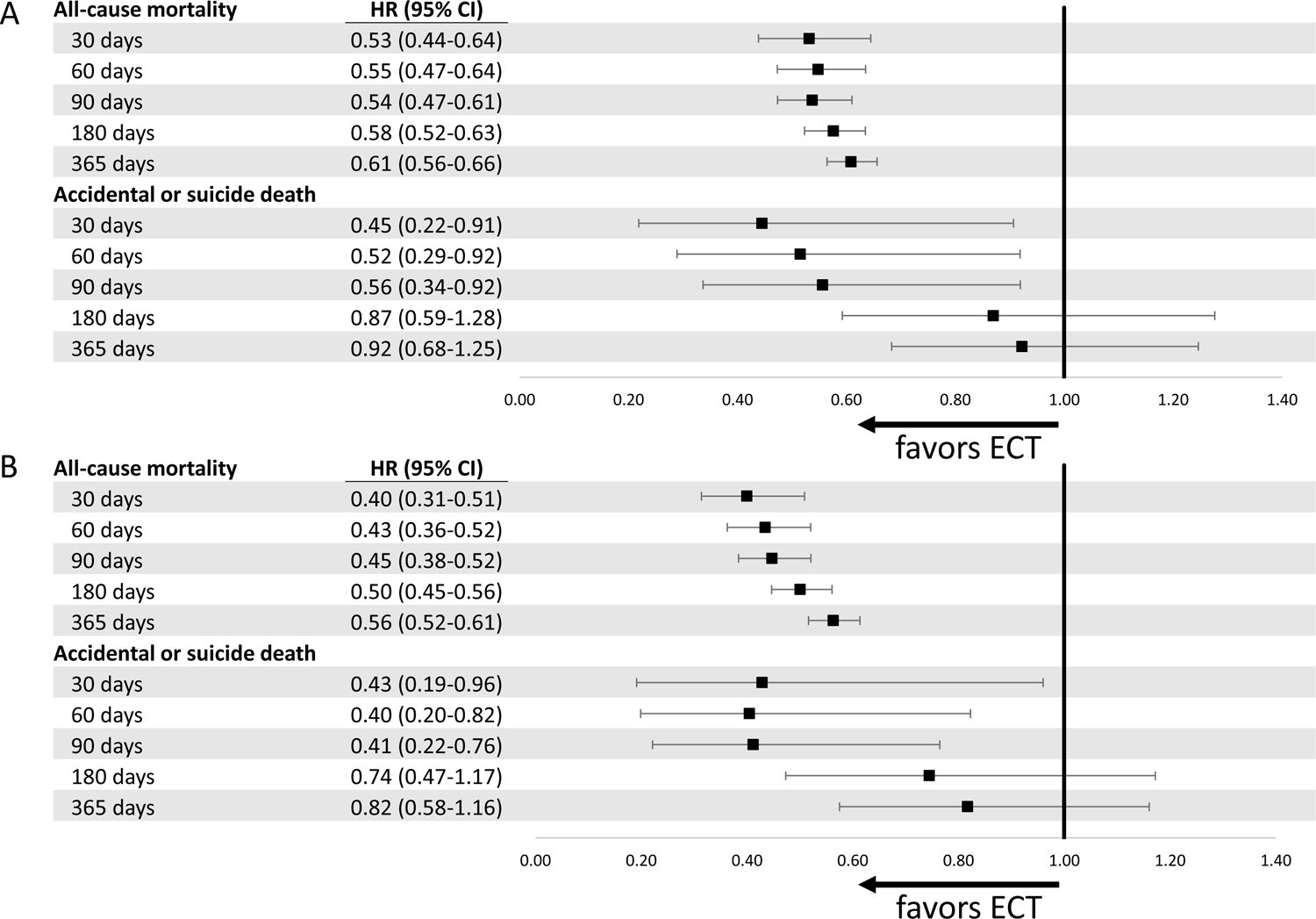
Forest plot showing effects of ECT on all-cause mortality and suicide death among hospitalized geriatric patients. Subjects are exact-matched on age, gender, Elixhauser comorbidity index, suicide attempt in prior year, psychiatric hospitalization in prior year, principal hospital diagnosis; models are adjusted for race, rural-urban setting, year of hospitalization, median income of zip code, and psychotropic medications in the prior year. Panel A includes all patients who receive 1 or more ECT sessions; Panel B includes those receiving a therapeutic course.
In exploring cause of death, ECT patients were less likely to die from several broad mortality categories compared to patients in the control group, including circulatory diseases (1-year HR=0.63, 95% CI 0.58 to 0.69), diabetes-related causes (HR=0.51, 95% CI 0.38 to 0.69), smoking-related diseases (HR=0.45, 95% CI 0.38 to 0.52), and cancer (HR=0.80, 95% CI 0.69 to 0.93).
Suicide Death
With respect to suicide death, over 1-year of follow-up, there was not a significant difference between groups (Figure 4). In the full sample, adjusted hazard ratios suggested a lower risk of suicide in the 90 days following hospital discharge, but this association waned over time (Figure 3A). In the sample including only patients who likely received a therapeutic ECT course, adjusted hazard ratios suggested a similar trajectory, with a waning association over time (Figure 3B). Results from inverse probability weighting model yielded similar results, with statistically significant associations seen at 30, 60, and 90 days (eTable 4). In a sensitivity analysis including length-of-stay in the matching with lower statistical power (1:1 ratio), the hazard ratio estimates were similar but not statistically significant at days 30, 60, and 90 (see eTable 5).
Figure 4.
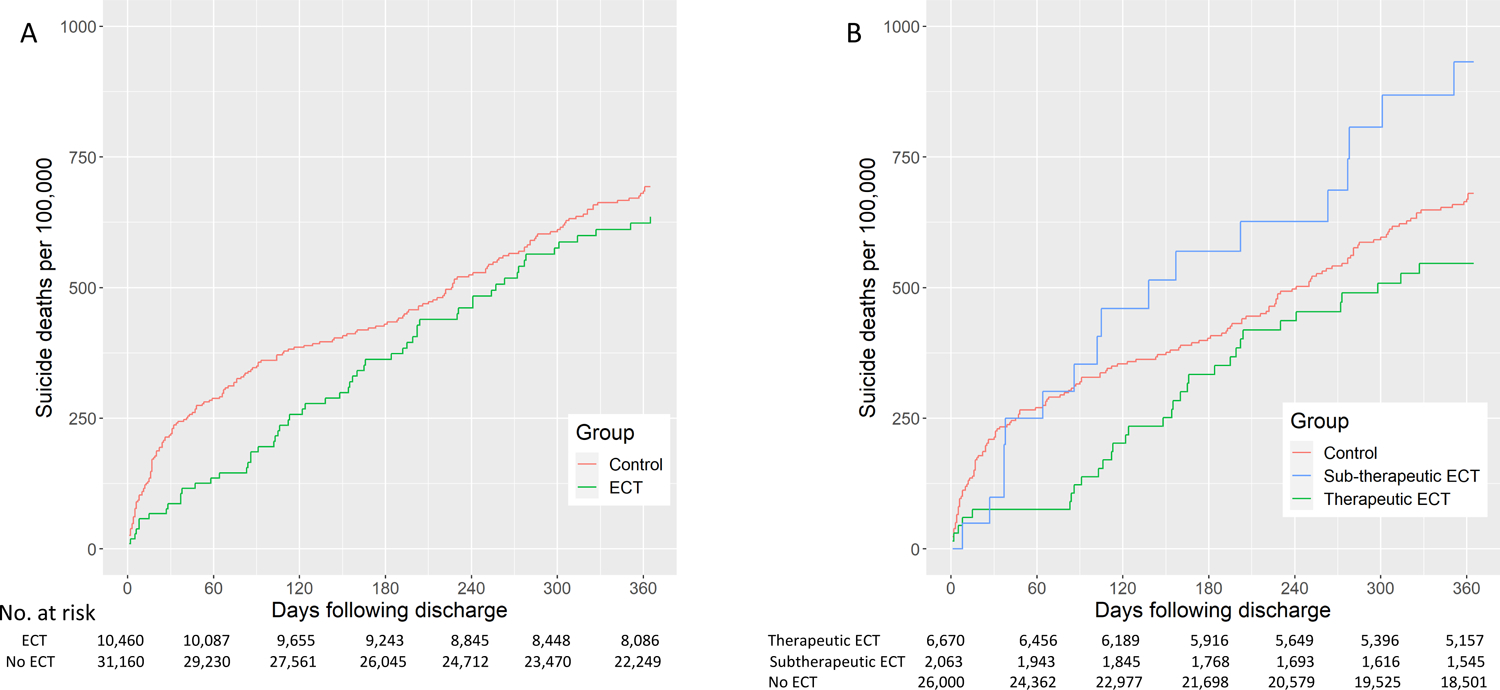
Effect of ECT on suicide death. Analysis in Panel A includes any patient receiving 1 or more ECT and matched controls (1-year Hazard Ratio=0.92, 95% CI 0.68 to 1.25). Analysis in Panel B defines therapeutic ECT as patients receiving 5 or more ECT sessions within 30 days (therapeutic course; Hazard Ratio= 0.82, 95% CI 0.58 to 1.16 v. matched controls) and those receiving less than 5 ECT sessions (subtherapeutic; HR=1.34, 95% CI 0.73 to 2.46). There was no difference in trajectory between matched controls for therapeutic and subtherapeutic ECT groups.
DISCUSSION
In a large sample of Medicare psychiatric inpatients, ECT was associated with a protective though short-lived effect on suicide risk and an enduring protective effect on all-cause mortality risk. As compared to matched patients who did not receive ECT, patients who received ECT had a lower suicide rate during the 3 months following discharge. However, this association was not observed at 6 or 12 months. Patients who received ECT had a lower rate of all-cause mortality for up to 12 months following treatment, consistent with prior literature (9, 11, 29).
ECT and All-cause Mortality
Our finding suggesting that ECT is protective against all-cause mortality generates further evidence consistent with earlier reports (9, 11, 29). Given that this study was not a randomized trial, concern for residual confounding exists. One alternative hypothesis is that patients who are severely medically ill are not selected for ECT because of the medical risk of the procedure. While this may partially contribute to the difference observed, the finding that those receiving a subtherapeutic course of ECT have a similar survival curve compared to those who do not receive ECT in the first 6 months does not support this alternative hypothesis. It should be noted that patients who received ECT were more likely to be from higher income zip codes and slightly more likely to be white compared to patients in the control group; however, even after accounting for these potential confounders including the Elixhauser Comorbidity Index in the adjusted hazard models and inverse probability weighting, a robust association of ECT with lower mortality persisted.
In exploring categories of mortality, ECT patients were less likely to die from a number of causes, including smoking-related diseases, cancer (excluding smoking-related cancer), circulatory disease, diabetes, and substance use deaths. While several mechanisms may be at play, one potential way in which ECT might lead to reduction in mortality risk is that improved functioning following ECT allows patients to better engage in treatment for whatever medical conditions they face or maintain a healthier lifestyle.
ECT and Suicide
Prior studies on the effects of ECT on suicide have yielded inconsistent results (29). Nonetheless, treatment guidelines from the American Psychiatric Association specifically recommend the use of ECT for patients with acute suicidal ideation (30). A recent observational study by Peltzman et al., examined ECT and suicide in the Veterans Health Administration. This study did not find an effect of ECT on suicide in veterans, though it did not look at outcomes prior to the 12-month timepoint (16). Though not specifically explored in this study, it would be expected (31) that the majority of veteran ECT recipients had stopped treatment before 12-month follow-up, when the effects of an acute treatment series have long worn off. The data in the current study suggest that there may be an early association between ECT on reduced suicide risk for up to 3–6 months, but that this association diminishes with time as the antidepressant effects of ECT wear off. This is in line with the traditional understanding of the treatment course of ECT, where a large portion of ECT patients relapses after 6 months without a continuation treatment (32).
Limitations
Several limitations require comment. In evaluating the association between ECT and suicide risk, it is important to bear in mind that it was not possible to control for several suicide risk factors, such as a lifetime history of suicide attempts (33) or a family history of suicide (34), that might have confounded and therefore biased the observed association of ECT with short-term suicide risk. Without an understanding of the mechanisms connecting ECT to suicide risk, the short-term protective associations should be interpreted with caution. In some cases, it is expected that unmeasured confounding with respect to depression severity and suicide risk may bias against ECT. In other cases, however, ECT is more commonly available to those of higher socioeconomic means who may have lower risks of all-cause mortality (35) or suicide (36). Another limitation is that we were unable to ascertain reasons for discontinuing ECT if a full course was not completed. It would be reasonable to assume that discontinuation was often related to emergence of intolerable side effects or lack of efficacy; however, other reasons may have contributed such as administrative barriers during the transition from inpatient to outpatient care. A third limitation of this study is that changes in coding from ICD-9-CM to ICD-10-CM occurred during the study period. However, the minimal differences between groups in the year of index hospitalization (standardized difference of 0.08) ameliorates concern that this is a substantial cause of bias. Furthermore, the year of index hospitalization was included as a covariate in the hazard regression models. Finally, findings might not generalize to populations under age 65.
Conclusion
In this large observational study, ECT was associated with a consistently lower risk of all-cause mortality and a reduced though short-lived association with the risk of suicide death. This report adds to a growing body of research suggesting a positive effect of ECT from a population health perspective (37, 38). Future efforts should focus on ways to ensure broader implementation of this treatment and to improve the maintenance therapy of severely ill patients who receive ECT.
Supplementary Material
Acknowledgments
Funding: This project was funded by the National Institute of Mental Health (R21MH117438) and by a young investigator grant (STW) from the American Foundation for Suicide Prevention. Dr. Wilkinson also acknowledges support from the Agency for Healthcare Research and Quality (K12HS023000).
Footnotes
Conflicts of Interest: Dr. Wilkinson acknowledges receiving contract funding from Janssen, Sage Therapeutics, and Oui Therapeutics for the conduct of clinical trials administered through Yale University. He has received consulting fees from Janssen, Oui Therapeutics, Sage Therapeutics, and Biohaven Pharmaceuticals. None of the other authors have conflicts of interest or disclosures.
REFERENCES
- 1.Depression. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/depression. 30 January 2020. Accessed on March 31, 2021. [Google Scholar]
- 2.Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, Hotopf M, Stewart R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PloS one. 2011;6:e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemogne C, Nabi H, Melchior M, Goldberg M, Limosin F, Consoli SM, Zins M. Mortality associated with depression as compared with other severe mental disorders: a 20-year follow-up study of the GAZEL cohort. Journal of psychiatric research. 2013;47:851–857. [DOI] [PubMed] [Google Scholar]
- 4.Lemogne C, Niedhammer I, Khlat M, Ravaud JF, Guillemin F, Consoli SM, Fossati P, Chau N. Gender differences in the association between depressive mood and mortality: a 12-year follow-up population-based study. Journal of affective disorders. 2012;136:267–275. [DOI] [PubMed] [Google Scholar]
- 5.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychological medicine. 2010;40:1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA psychiatry. 2015;72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfson M, Wall M, Wang S, Crystal S, Liu SM, Gerhard T, Blanco C. Short-term Suicide Risk After Psychiatric Hospital Discharge. JAMA psychiatry. 2016;73:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Archives of general psychiatry. 2011;68:1058–1064. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi N, Moss L, Simon E, Nemeroff CB, Atre-Vaidya N. EFFICACY AND LONG-TERM CLINICAL OUTCOME OF COMORBID POSTTRAUMATIC STRESS DISORDER AND MAJOR DEPRESSIVE DISORDER AFTER ELECTROCONVULSIVE THERAPY. Depression and anxiety. 2016;33:640–647. [DOI] [PubMed] [Google Scholar]
- 10.Munk-Olsen T, Laursen TM, Videbech P, Mortensen PB, Rosenberg R. All-cause mortality among recipients of electroconvulsive therapy: register-based cohort study. The British journal of psychiatry : the journal of mental science. 2007;190:435–439. [DOI] [PubMed] [Google Scholar]
- 11.Avery D, Winokur G. Mortality in depressed patients treated with electroconvulsive therapy and antidepressants. Archives of general psychiatry. 1976;33:1029–1037. [DOI] [PubMed] [Google Scholar]
- 12.Babigian HM, Guttmacher LB. Epidemiologic considerations in electroconvulsive therapy. Archives of general psychiatry. 1984;41:246–253. [DOI] [PubMed] [Google Scholar]
- 13.Huston PE, Locher LM. Involutional psychosis: Course when untreated and when treated with electric shock. Archives of Neurology & Psychiatry. 1948;59:385–394. [PubMed] [Google Scholar]
- 14.Huston PE, Locher LM. Manic-depressive psychosis; course when treated and untreated with electric shock. Arch Neurol Psychiatry. 1948;60:37–48. [PubMed] [Google Scholar]
- 15.Liang CS, Chung CH, Ho PS, Tsai CK, Chien WC. Superior anti-suicidal effects of electroconvulsive therapy in unipolar disorder and bipolar depression. Bipolar disorders. 2018;20:539–546. [DOI] [PubMed] [Google Scholar]
- 16.Peltzman T, Shiner B, Watts BV. Effects of Electroconvulsive Therapy on Short-Term Suicide Mortality in a Risk-Matched Patient Population. The journal of ECT. 2020;36:187–192. [DOI] [PubMed] [Google Scholar]
- 17.Weiner R, Coffey C, Fochtmann L, Greenberg R, Isenberg K, Kellner C, Sackeim H, Moench L. The practice of electroconvulsive therapy. Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. 2001;2.
- 18.Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2005;13:268–281. [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Warner CH, Ivany C, Petukhova MV, Rose S, Bromet EJ, Brown M 3rd, Cai T, Colpe LJ, Cox KL, Fullerton CS, Gilman SE, Gruber MJ, Heeringa SG, Lewandowski-Romps L, Li J, Millikan-Bell AM, Naifeh JA, Nock MK, Rosellini AJ, Sampson NA, Schoenbaum M, Stein MB, Wessely S, Zaslavsky AM, Ursano RJ. Predicting suicides after psychiatric hospitalization in US Army soldiers: the Army Study To Assess Risk and rEsilience in Servicemembers (Army STARRS). JAMA psychiatry. 2015;72:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical care. 2009;47:626–633. [DOI] [PubMed] [Google Scholar]
- 21.Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM: Developing a protocol for observational comparative effectiveness research: a user’s guide, Government Printing Office; 2013. [PubMed] [Google Scholar]
- 22.Rhee TG, Olfson M, Sint K, Wilkinson ST. Characterization of the Quality of Electroconvulsive Therapy Among Older Medicare Beneficiaries. The Journal of clinical psychiatry. [DOI] [PubMed]
- 23.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12:259–261. [DOI] [PubMed] [Google Scholar]
- 24.Patterson BH, Bilgrad R. Use of the National Death Index in cancer studies. J Natl Cancer Inst. 1986;77:877–881. [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. [DOI] [PubMed] [Google Scholar]
- 26.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40:808–813. [DOI] [PubMed] [Google Scholar]
- 27.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010;172:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olfson M, Marcus S, Sackeim HA, Thompson J, Pincus HA. Use of ECT for the inpatient treatment of recurrent major depression. The American journal of psychiatry. 1998;155:22–29. [DOI] [PubMed] [Google Scholar]
- 29.Prudic J, Sackeim HA. Electroconvulsive therapy and suicide risk. The Journal of clinical psychiatry. 1999;60 Suppl 2:104–110; discussion 111–106. [PubMed] [Google Scholar]
- 30.Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, Van Rhoads RS, Reus VI, J Raymond DePaulo M Jr, Fawcett JA. Practice guideline for the treatment of patients with major depressive disorder third edition. The American journal of psychiatry. 2010;167:1.20068118 [Google Scholar]
- 31.Rhee TG, Olfson M, Sint K, Wilkinson ST. Characterization of the Quality of Electroconvulsive Therapy Among Older Medicare Beneficiaries. The Journal of clinical psychiatry. 2020;81. [DOI] [PubMed]
- 32.Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. Jama. 2001;285:1299–1307. [DOI] [PubMed] [Google Scholar]
- 33.Bostwick JM, Pabbati C, Geske JR, McKean AJ. Suicide Attempt as a Risk Factor for Completed Suicide: Even More Lethal Than We Knew. The American journal of psychiatry. 2016;173:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. The Lancet. 2002;360:1126–1130. [DOI] [PubMed] [Google Scholar]
- 35.Bassuk SS, Berkman LF, Amick BC 3rd. Socioeconomic status and mortality among the elderly: findings from four US communities. Am J Epidemiol. 2002;155:520–533. [DOI] [PubMed] [Google Scholar]
- 36.Olfson M, Cosgrove C, Altekruse SF, Wall MM, Blanco C. Deaths Of Despair: Adults At High Risk For Death By Suicide, Poisoning, Or Chronic Liver Disease In The US: Study examines US adults at highest risk for death by suicide, drug poisoning, or chronic liver disease. Health Affairs. 2021;40:505–512. [DOI] [PubMed] [Google Scholar]
- 37.Slade EP, Jahn DR, Regenold WT, Case BG. Association of Electroconvulsive Therapy With Psychiatric Readmissions in US Hospitals. JAMA psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 38.Ross EL, Zivin K, Maixner DF. Cost-effectiveness of Electroconvulsive Therapy vs Pharmacotherapy/Psychotherapy for Treatment-Resistant Depression in the United States. JAMA psychiatry. 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


