Abstract
Objective:
Tuberous Sclerosis Complex (TSC) is highly associated with autism spectrum disorder (ASD). Objectives of the study were to characterize autistic features in young children with TSC.
Methods:
Participants included 138 children followed from ages 3 to 36 months with TSC from the Tuberous Sclerosis Complex Autism Center of Excellence Research Network (TACERN), a multicenter, prospective observational study aimed at understanding the underlying mechanisms of ASD in TSC. Developmental and autism-specific assessments were administered, and a clinical diagnosis of ASD was determined for all participants at 36 months. Further analyses were performed on 117 participants with valid autism assessments based on nonverbal mental age greater than 15 months.
Results:
Prevalence of clinical diagnosis of ASD at 36 months was 25%. Nearly all autistic behaviors on the Autism Diagnostic Observation Schedule-2 (ADOS-2) and Autism Diagnostic Interview-Revised (ADI-R) were more prevalent in children diagnosed with ASD; however, autism-specific behaviors were also observed in children without ASD. Overall quality of social overtures, facial expressions, and abnormal repetitive interests and behaviors were characteristics most likely to distinguish children with ASD from those without an ASD diagnosis. Participants meeting ADOS-2 criteria but not a clinical ASD diagnosis exhibited intermediate developmental and ADOS-2 scores compared to individuals with and without ASD.
Interpretation:
ASD is highly prevalent in TSC, and many additional individuals with TSC exhibit a broad range of sub-threshold autistic behaviors. Our findings reveal a broader autism phenotype that can be identified in young children with TSC, which provides opportunity for early targeted treatments.
Keywords: Tuberous Sclerosis Complex, autism spectrum disorder, TSC-Associated Neuropsychiatric Disorder (TAND)
INTRODUCTION
Tuberous Sclerosis Complex (TSC) is an autosomal dominant genetic disorder that affects multiple organ systems. In addition, nearly 90% of individuals with TSC experience a wide range of neuropsychiatric symptoms, which have been appropriately named TSC-Associated Neuropsychiatric Disorders (TAND) (1–4). TAND is a term developed by the Neuropsychiatry Panel of the 2012 International Consensus Conference for TSC and is comprised of seven levels, including behavioral, psychiatric, intellectual, academic, neuropsychological, and psychosocial (4, 5). At the psychiatric level, neurodevelopmental disorders including autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD) are common (1, 3, 6, 7).
Autism spectrum disorder (ASD) is one of the most common co-occurring TAND diagnoses, and prior studies have estimated that approximately 30–50% of individuals with TSC meet criteria for ASD (6, 8, 9) as compared to a prevalence of 1 in 54 (2%) for the general population (10). Risk correlates for ASD in TSC include presence of developmental delay/intellectual disability, TSC2 mutations, and epilepsy (3, 9, 11–15). A range of social communication difficulties have been reported in individuals with TSC even in the absence of an ASD diagnosis, including poor eye contact and repetitive and ritualistic behaviors (16). In addition, other TAND behaviors not directly included in ASD diagnostic criteria, such as anxiety, self-injury, aggressive outbursts, sleep difficulties, speech and language delay, and temper tantrums, are often seen in individuals with ASD and TSC.
Early emerging characteristics of ASD in infants and toddlers with TSC have been described by Jeste and colleagues (9, 13). In a prospective study of infants with TSC, autistic traits were seen in the first year of life, including alterations in play behavior, social interaction, and eye gaze (9). Additionally, children both above and below diagnostic thresholds for ASD exhibited deficits in imaginative play skills. After the second year of life, abnormal behaviors including hyperactivity, rituals, repetitive behaviors, and temper tantrums were observed. Developmentally, infants who were eventually diagnosed with ASD displayed deficits in nonverbal abilities as early as 6 months of life, with subsequent global delays by 9 months of age (13).
The TSC Autism Center of Excellence Research Network (TACERN) is an NIH-funded, multicenter prospective observational study initiated in 2012 with both short- and long-term goals consisting of: characterizing the ASD phenotype of TSC; identifying clinical, structural, and electrophysiological biomarkers predictive of ASD in children with TSC; and performing a mechanistic analysis of TSC, which is a Mendelian genetic disorder with high prevalence of ASD, to understand molecular pathways involved in ASD and develop precision treatment targets. Children were assessed at multiple time points from age 3 to 36 months, allowing for the examination of the temporal evolution of brain development and behavioral characteristics of children with TSC over the first 3 years of life.
The purpose of the current analysis is to describe the developmental and behavioral profile of ASD in TSC at 36 months, focusing on characteristics of ASD behaviors in this group and determining specific features that may indicate higher suspicion for ASD.
METHODS
Subject recruitment
Participants were enrolled as part of TACERN, a consortium consisting of five hospital programs in the United States established in 2012. TACERN is a multicenter, prospective observational study evaluating early clinical, structural and electrophysiologic biomarkers of ASD in TSC (clinicaltrials.gov, NCT01780441). Institutional Review Board (IRB) approval was obtained at each of the five sites, and informed consent was acquired from all participating families prior to enrollment. A yearly recalibration meeting for developmental testing and autism-specific assessments was held to ensure assessment administration and scoring reliability across all sites.
Participants were included in the study if they met clinical and/or genetic criteria for TSC (17) and were between the ages of 3–12 months at study enrollment. Participants were excluded from the study if gestational age was less than 36 weeks, enrollment in a prior study using oral mammalian target of rapamycin (mTOR) inhibitor (sirolimus, everolimus), subependymal giant cell astrocytoma (SEGA) requiring medical or surgical treatment, or epilepsy surgery prior to enrollment.
Study design
Prospective, longitudinal design incorporated repeated assessments at baseline, 3, 6, 9, 12, 18, 24, and 36 months. In addition, information including demographics, medical history, family history, baseline and interval developmental history, seizure history via seizure diaries (including seizure onset, type, and frequency), therapies, medications, and medical comorbidities, was collected throughout the study. A physical exam was performed at each assessment. Electroencephalogram (EEG) and magnetic resonance imaging (MRI) of the brain was obtained at repeated, scheduled intervals. The current study focuses on the developmental and behavioral assessments and ASD clinical diagnosis at 36 months.
Developmental and behavioral assessments
Developmental and behavioral assessments were administered at 3, 6, 9, 12, 18, 24, and 36 months. Assessments were administered by a licensed psychologist, psychological technician/research assistant, and/or speech-language pathologist. All assessors had attained research reliability on the autism-specific assessments.
The Mullen Scales of Early Learning (MSEL) is a clinician-administered assessment of developmental functioning (18). Due to low developmental functioning in some of the participants and, thus, inability to obtain standard scores in those participants, developmental quotients (DQ) were calculated for all participants (developmental age/chronological age x 100) for the five subdomains (gross motor, fine motor, visual reception, expressive language, and receptive language). A composite developmental quotient was calculated based on an average of all subdomains except gross motor (9, 19). The Vineland Adaptive Behavior Scales, 2nd edition (VABS-II) is a parent-report measure used to evaluate adaptive functioning (20). Domains include communication, socialization, daily living skills, and motor skills. An overall adaptive behavior composite is also obtained. Scores are listed as standard scores. The Preschool Language Scales, 5th edition (PLS-5) is a combination of parent report and clinician assessment evaluating receptive and expressive communication skills (21). Scores are reported as standard scores. The Child Behavior Checklist (CBCL) is a parent-reported measure of behavior in children and consists of 99 items that yield T-scores on clusters of behavior symptoms, broad-spectrum scales, and DSM-oriented disorders (22). T-scores of 64 and higher are considered clinically significant for internalizing/externalizing behaviors and total problem score. A T-score of ≥ 70 is clinically significant for syndrome scales. This version has been used in a multi-site case control study to discriminate children with ASD from children with developmental delay (23).
Autism-specific assessments and determination of ASD
The Autism Observation Scale for Infants (AOSI) is a clinician-administered assessment measuring autism risk behavior in children ages 6–18 months and consists of 18 items meant to evaluate areas of concern for ASD (24); the AOSI was administered at 12 months. A total raw score is obtained with higher scores indicating more autism-risk behaviors. The Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) is a semi-structured observational assessment used to assess behaviors associated with ASD and was administered at both 24 and 36 months (25–27). At 36 months, 117 participants had a valid administration of the ADOS-2 with roughly equal numbers of participants assessed with either module 1 (for nonverbal or minimally verbal children, N=63) or module 2 (for children using phrase speech, N=54). Twenty-one participants were excluded due to having a non-verbal mental age less than 15 months, which has been shown in prior studies to limit the validity of the ADOS-2 (28). Clinical severity scores were used to compare overall ASD severity across modules. Clinical severity scores range from 1–10, with higher scores indicating greater severity of ASD symptoms (25). In addition, clinical severity scores for social affect and repetitive/restrictive behaviors were calculated and used to assess severity within sub-domains (29).
The Autism Diagnostic Interview-Revised (ADI-R) was administered at 24 and 36 months (30). This parent interview evaluates an individual’s social skills, communication, behavior, and development. The ADI-R new algorithm for toddlers and preschool children, ages 12–47 months, was used (31), as this algorithm was created to improve validity in this age group. The new algorithm consists of 13–20 items and separates by level of language; the number of items and cut-offs are different for each algorithm. For the study the clinical cut-off was used, which has a lower threshold and, therefore, has higher sensitivity but lower specificity. Ranges of concern were developed from the cut-offs and consist of little-to-no, mild-to-moderate, and moderate-to-severe level of concern for ASD.
At 36 months, teams at each site consisting of those who administered the ADOS-2 and ADI-R were involved in the diagnostic process. The ASD diagnosis was established by consensus and based on clinical best estimate (CBE) criteria using clinical history, objective assessments, and Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria.
Statistical analysis
ASD clinical diagnosis at 36 months was used as the primary outcome measure for all analyses. Participants were grouped according to ASD or non-ASD clinical diagnosis. Additionally, a third group was created and consisted of those who scored over the cut-off for ASD on the ADOS-2 but did not receive a clinical diagnosis of ASD. Descriptive statistics including means and standard deviations for all measures were reported.
Individual items on the MSEL, VABS-II, PLS-5, CBCL, ADOS-2 scores, and ADI-R algorithm cut-offs were analyzed comparing participants who did and did not meet clinical diagnostic criteria for ASD. Chi-square tests were used to compare frequencies between pairs of categorical variables. Fisher’s exact test was used if the expected cell sizes were too small (less than 5). Results were considered statistically significant if the resulting p-value was < 0.05. Additionally, one-way ANOVAs were calculated when evaluating differences among the three groups (ASD, ADOS+/ASD-, no ASD) for individual items on the ADOS-2, as well as developmental and behavioral assessments listed above.
Univariate logistic regression analyses were performed for all domains and subdomains from the MSEL, VABS-II, PLS-5, as well as individual items on the ADOS-2, AOSI, and ADI-R where the outcome was ASD diagnosis. For these analyses the ordinal categorical variables (with at least three categories) were treated as continuous to avoid multiple intercepts for these simple models. In addition, multivariable logistic regression models were conducted for each of the assessments, and backward elimination was used to identify statistically significant predictors of ASD diagnosis. Lastly, a final multivariable logistic regression model was conducted with the variables selected from each assessment and a backward elimination was conducted to derive the most parsimonious model. In all logistic regression models, variables were removed if their p-value, adjusted for the other variables in the model, was greater than 0.05.
Adjustments for multiple testing using a false discovery rate (FDR) (family-wise error rate = 0.05) were made for each individual table. All statistical analyses were conducted using SAS ® version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics
A total of 169 participants were initially enrolled in the study. Accounting for screen failures and participants who did not complete the study through 36 months of age, 138 participants were eligible for inclusion in the present analysis. Average age at time of enrollment was 5.6 ± 3.2 months (see Table 1 for complete demographics). Overall, 25% of participants were diagnosed clinically with ASD. Approximately 45% of participants were developmentally delayed as defined by an MSEL composite score ≤ 70. Additionally, 38% had speech and language delays as defined by a PLS-5 Total Language Score ≤ 70. Genetic testing results were obtained for 109 participants. Pathogenic variants in TSC1 were found in 14%, in TSC2 75%, and no mutation identified in 11%. One-hundred eight participants (78%) developed seizures during the study with an average age of seizure onset of 5.4 ± 3.8 months (range 0 to 22.5 months). The average number of seizures in the one month preceding 36-month assessments was 9.6 ± 22.3 (range of zero to 96).
Table 1.
Participant Characteristics
| All (N=138) | ASD (N=34) | No ASD (N=104) | p value | |
|---|---|---|---|---|
| Sex | 0.35 | |||
| Male | 71 (51) | 19 (56) | 52 (50) | |
| Female | 67 (49) | 15 (44) | 52 (50) | |
| Ethnicity | 0.42 | |||
| Non-Hispanic | 112 (81) | 26 (76) | 86 (83) | |
| Hispanic | 26 (19) | 8 (24) | 18 (17) | |
| Race | 0.7 | |||
| White | 120 (87) | 29 (85) | 91 (88) | |
| Black/African Amer | 5 (4) | 1 (3) | 4 (4) | |
| Asian | 4 (3) | 2 (6) | 2 (2) | |
| Other | 2 (1) | 0 (0) | 2 (2) | |
| Not Reported | 7 (5) | 2 (6) | 5 (5) | |
| Seizures (all) | 108 (78) | 33 (97) | 75 (72) | 0.002* |
| Focal | 88 (64) | 28 (82) | 60 (58) | 0.009* |
| Generalized | 22 (16) | 8 (24) | 14 (13) | 0.16 |
| Infantile Spasms | 79 (57) | 30 (88) | 49 (47) | <0.0001* |
| Unclassified | 12 (9) | 5 (15) | 7 (7) | 0.15 |
| MSEL Composite DQ | <0.0001* | |||
| ≥70 | 74 (55) | 3 (9) | 71 (70) | |
| <70 | 60 (45) | 29 (91) | 31 (30) | |
| PLS-5 Total Language | <0.0001* | |||
| ≥70 | 79 (62) | 7 (23) | 72 (74) | |
| <70 | 49 (38) | 24 (77) | 25 (26) |
MSEL=Mullen Scales of Early Learning, DQ= Developmental Quotient, PLS-5=Preschool Language Scale, 5th edition. Results are reported as N (%). Four participants had missing data for MSEL Composite DQ (Total= 134, ASD= 32, No ASD= 102), and 10 participants had missing data for PLS-5 Total Language (Total= 128, ASD= 31, No ASD= 97).
Autism summary data
Autism diagnosis was determined clinically and included integration of all information obtained during the clinic visit (i.e. review of clinical history, ADOS-2, ADI-R, developmental and behavioral assessments, and observations throughout the clinic visit). Participants with a nonverbal mental age less than 15 months (n=21) were excluded from analyses comparing ADOS-2 results with clinical ASD diagnosis. Symptom frequency on the ADOS-2 and ADI-R is described in Table 2. As expected, most participants (81%) without ASD scored as being non-spectrum on the ADOS-2. However, 19% of participants without a clinical ASD diagnosis scored in the mild-to-moderate (n = 13) or moderate-to-severe (n = 5) range. Similarly, on the ADI-R 91% of non-ASD participants scored in the little-to-no concern range with 9% of participants who did not have a clinical ASD diagnosis scoring in the ASD range.
Table 2.
Autism Symptom Level by Assessment
| ASD Clinical Diagnosis (N=23) | No ASD Clinical Diagnosis (N=94) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Little-to-no Concern | Mild/Mod Concern | Mod/Severe Concern | Little-to-no Concern | Mild/Mod Concern | Mod/Severe Concern | |
| ADI-R (nonverbal) | 0 (0) | 4 (17) | 4 (17) | 3 (3) | 1 (1) | 0 (0) |
| ADI-R (single words) | 2 (9) | 6 (26) | 3 (13) | 19 (20) | 2 (2) | 3 (3) |
| ADI-R (phrase speech) | 2 (9) | 1 (4) | 1 (4) | 63 (67) | 3 (3) | 0 (0) |
|
| ||||||
| Non-spectrum | Autism Spectrum | Autism | Non-Spectrum | Autism Spectrum | Autism | |
| ADOS-2 | 1 (4) | 5 (22) | 17 (74) | 76 (81) | 13 (14) | 5 (5) |
This table shows the frequency of subjects according to diagnosis and overall level of concern for each algorithm on the ADI-R and ADOS-2. Results are reported as N (%).
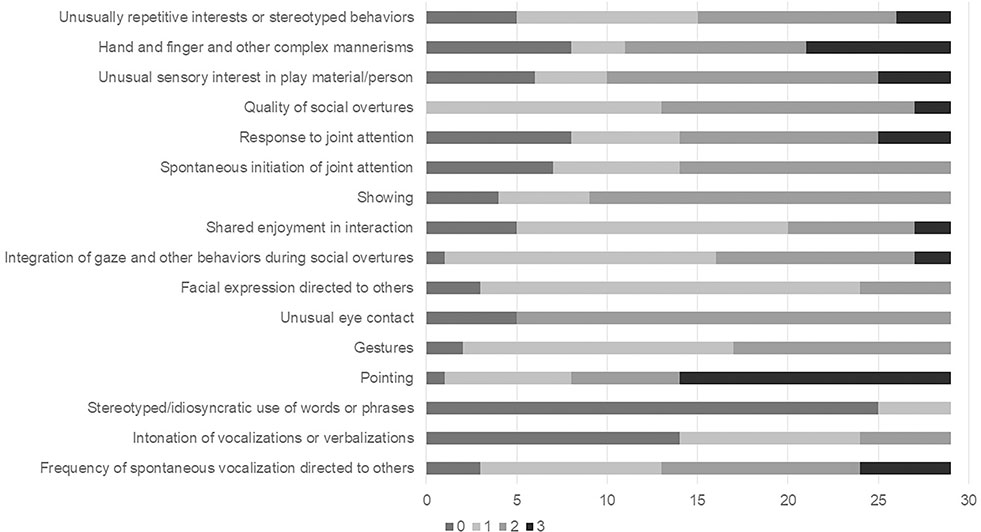
In comparing participants with and without ASD, significant differences were seen across subdomains of functioning in both the ADOS-2 and ADI-R (see Table 3). On the ADOS-2, participants with ASD exhibited significantly higher clinical severity scores for social affect and repetitive and restrictive behaviors, as well as overall, compared to those without ASD. The ADOS-2 Module 1 (Fig 1) shows the range of symptoms in participants with a diagnosis of ASD. On the ADOS-2 high rates of autism symptoms were reported in several items, including pointing, gestures, quality of social overtures, and integration of gaze and other behaviors during social overtures.
Table 3.
ASD Summary Data
| ASD Clinical Diagnosis | No ASD Clinical Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N | Mean | SD | 95% CI | N | Mean | SD | 95% CI | p value | |||
|
|
|||||||||||
| ADOS-2 | |||||||||||
| SA CSS | 23 | 6.87 | 1.89 | 6.05 | 7.69 | 94 | 2.41 | 1.66 | 2.08 | 2.75 | <.0001* |
| RRB CSS | 23 | 6.65 | 2.01 | 5.78 | 7.52 | 94 | 2.54 | 1.97 | 2.14 | 2.95 | <.0001* |
| ADOS-2 CSS | 23 | 7.00 | 1.88 | 6.19 | 7.81 | 93 | 2.03 | 1.53 | 1.72 | 2.35 | <.0001* |
| ADI-R (Phrase Speech) | |||||||||||
| Social Communication (0–22) | 4 | 6.50 | 3.42 | 1.06 | 11.94 | 66 | 1.61 | 1.95 | 1.13 | 2.09 | 0.006* |
| Repet/Restrict Behavior (0–12) | 4 | 4.25 | 2.63 | 0.07 | 8.43 | 66 | 0.85 | 1.42 | 0.50 | 1.20 | 0.003* |
| Reciprocal Peer Interaction (0–6) | 4 | 1.75 | 1.26 | −0.25 | 3.75 | 66 | 0.76 | 1.11 | 0.48 | 1.03 | 0.10 |
| SC+RRB+RPI (0–38) | 4 | 12.50 | 5.07 | 4.44 | 20.56 | 66 | 3.21 | 3.48 | 2.36 | 4.07 | 0.003* |
| ADI-R (Single Words) | |||||||||||
| Social Affect (0–20) | 11 | 7.45 | 4.50 | 4.43 | 10.48 | 24 | 4.08 | 4.19 | 2.31 | 5.85 | 0.018* |
| Repet/Restrict Behavior (0–12) | 11 | 4.64 | 2.16 | 3.19 | 6.09 | 24 | 1.29 | 1.37 | 0.71 | 1.87 | 0.001* |
| Imitation, Gest, Play (0–10) | 11 | 5.45 | 2.34 | 3.88 | 7.03 | 24 | 2.92 | 2.32 | 1.94 | 3.90 | 0.014* |
| SA+RRB (0–32) | 11 | 12.09 | 5.72 | 8.25 | 15.93 | 24 | 5.38 | 4.89 | 3.31 | 7.44 | 0.002* |
| ADI-R (Nonverbal) | |||||||||||
| Social Affect (0–18) | 8 | 11.13 | 2.95 | 8.66 | 13.59 | 4 | 3.75 | 3.50 | −1.82 | 9.32 | 0.04* |
| Repet/Restrict Behavior (0–8) | 8 | 3.63 | 2.13 | 1.84 | 5.41 | 4 | 2.25 | 1.26 | 0.25 | 4.25 | 0.31 |
| Imitation, Gest, Play (0–12) | 8 | 11.50 | 1.07 | 10.61 | 12.39 | 4 | 9.00 | 2.58 | 4.89 | 13.11 | 0.099 |
| SA+RRB (0–26) | 8 | 14.75 | 3.15 | 12.12 | 17.38 | 9 | 8.22 | 5.26 | 4.18 | 12.27 | 0.027* |
RRB= repetitive and restrictive behaviors; CSS= clinical severity score; SC= social communication; RPI= reciprocal peer interaction; SA= social affect. On the ADOS-2, CSS ranges from 1–10. Raw score ranges for the ADI-R are reported in parentheses next to subdomains. ADI-R algorithm for nonverbal and single words does not include “imitation, gestures, and play”. Unadjusted p values are given. P values are based on the Wilcoxon Rank Sum Test.
P values signified by * are statistically significant even after adjusting for multiple testing (FDR).
Figure 1:
Distribution of ADOS-2 Module 1 Items for Children with ASD Diagnosis. This is the frequency of scores for each module item. Note that the following items only had an option of 0,1, or 2: Intonation of vocalizations or verbalizations, Gestures, Showing, Spontaneous initiation of joint attention, and Facial expressions directed to others. Unusual eye contact can only score a 0 or 2. Since only three participants assessed with the Module 2 met criteria for ASD, only Module 1 is graphed.
Relationship between individual items on the ADOS-2, AOSI, ADI-R and ASD diagnosis
Logistic regression was used to determine which items on the ADOS-2 and ADI-R were associated with a diagnosis of ASD. On both assessments most items were significantly associated with an ASD clinical diagnosis. On the ADOS-2 (see Table 4), items including Facial Expression Directed to Others, Quality of Social Overtures, Gestures, Shared Enjoyment in Interaction, Integration of Gaze and Other Behaviors During Social Overtures, and Unusually Repetitive Interests or Stereotyped Behaviors were strongly associated with an ASD diagnosis. On the ADI-R (Table 5), Inappropriate Facial Expressions, Quality of Social Overtures, Offering Comfort, Response to Approaches of Other Children, and Imaginative Play were among those items significantly associated with an ASD diagnosis. The AOSI total score at 12 months was associated with an ASD diagnosis at 36 months (OR 1.12, 95% CI 1.03–1.21, p=0.008). In addition, several individual items on the AOSI were associated with an ASD diagnosis including Coordination of Eye Gaze and Action (OR 4.98, 95% CI 1.61–15.33, p=0.005), Engagement of Attention (OR 4.79, 95% CI 1.57–14.64, p=0.006), Motor Control and Behavior (OR 4.66, 95% CI 2.14–10.17, p<0.001), Sharing Interest (OR 3.52, 95% CI 1.69–7.35, p<0.001), Eye Contact (OR 2.83, 95% CI 1.67–4.79, p<0.001), and Orients to Name (OR 2.32, 95% CI 1.18–4.54, p=0.01).
Table 4.
Odds of individual items on ADOS-2 Module 1 predicting ASD diagnosis at 36 months
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Facial Expressions Directed to Others | 28.57 | 5.73 | 142.42 | <0.0001* |
| Quality of Social Overtures | 27.82 | 3.73 | 207.40 | 0.0012* |
| Unusually Repetitive Interests or Stereotyped Behaviors | 12.65 | 3.51 | 45.57 | 0.0001* |
| Gestures | 12.35 | 2.84 | 53.73 | 0.0008* |
| Shared Enjoyment in Interaction | 11.96 | 3.48 | 41.11 | <.0001* |
| Integration of Gaze/Other Behaviors During Social Overtures | 6.91 | 2.42 | 19.75 | 0.0003* |
| Spontaneous Initiation of Joint Attention | 5.08 | 2.20 | 11.69 | 0.0001* |
| Response to Joint Attention | 4.39 | 1.95 | 9.85 | 0.0003* |
| Unusual Sensory Interest in Play Material/Person | 3.93 | 1.92 | 8.08 | 0.0002* |
| Showing | 3.81 | 1.86 | 7.82 | 0.0003* |
| Pointing | 3.73 | 1.89 | 7.34 | 0.0001* |
| Overall Level of Non-Echoed Spoken Language | 3.70 | 1.84 | 7.45 | 0.0002* |
| Freq of Spontaneous Vocalization Directed to Others | 3.67 | 1.82 | 7.41 | 0.0003* |
| Unusual Eye Contact | 3.04 | 1.61 | 5.75 | 0.0006* |
| Hand/Finger/Other Complex Mannerisms | 2.16 | 1.22 | 3.80 | 0.008* |
| Intonation of Vocalizations/Verbalizations | 2.22 | 0.85 | 5.76 | 0.102 |
| Stereotyped/Idiosyncratic Use of Words/Phrases | 1.50 | 0.37 | 6.04 | 0.57 |
Unadjusted p valued are given. P values are based on the Wilcoxon Rank Sum Test.
P values signified by * are statistically significant even after adjusting for multiple testing (FDR).
Table 5.
Odds of individual items on ADI-R predicting ASD diagnosis at 36 months
| OR | 95% CI | p-value | ||
|---|---|---|---|---|
| Quality of Social Overtures | 12.58 | 4.56 | 34.69 | <.0001* |
| Response to Approaches of Other Children | 11.16 | 3.88 | 32.17 | <.0001* |
| Imaginative Play | 9.01 | 3.63 | 22.35 | <.0001* |
| Offering Comfort | 8.08 | 3.70 | 17.64 | <.0001* |
| Inappropriate Facial Expressions | 7.41 | 2.66 | 20.64 | 0.0001* |
| Conventional/Instrumental Gestures | 6.84 | 3.34 | 14.00 | <.0001* |
| Pointing to Express Interest | 6.76 | 3.31 | 13.80 | <.0001* |
| Hand/Finger Mannerisms | 6.58 | 3.30 | 13.08 | <.0001* |
| Repetitive Use Objects/Interest in Parts of Objects | 6.18 | 2.99 | 12.78 | <.0001* |
| Direct Gaze | 5.77 | 2.42 | 13.77 | <.0001* |
| Range of Facial Expressions Used to Communicate | 5.67 | 2.56 | 12.58 | <.0001* |
| Offering to Share | 5.21 | 2.44 | 11.15 | <.0001* |
| Appropriateness of Social Responses | 4.62 | 2.08 | 10.25 | 0.0002* |
| Showing and Directing Attention | 4.60 | 2.52 | 8.39 | <.0001* |
| Spontaneous Imitation of Actions | 4.38 | 2.30 | 8.33 | <.0001* |
| Interest in Children | 3.89 | 2.01 | 7.55 | <.0001* |
| Seeking to Share Enjoyment with Others | 3.68 | 1.77 | 7.68 | 0.0005* |
| Unusual Sensory Interests | 3.64 | 1.67 | 7.94 | 0.001* |
| Nodding | 3.46 | 1.94 | 6.16 | <.0001* |
| Social Smiling | 3.26 | 1.52 | 7.01 | 0.003* |
| Other Complex Mannerisms/Stereotyped Body Movements | 3.01 | 1.29 | 7.04 | 0.011* |
| Unusual Preoccupations | 2.83 | 1.44 | 5.55 | 0.003* |
| Use of Other's Body to Communicate | 2.29 | 1.13 | 4.64 | 0.023* |
| Compulsions/Rituals | 2.10 | 0.92 | 4.81 | 0.079 |
| Social Verbalization/Chat | 0.63 | 0.19 | 2.06 | 0.44 |
| Stereotyped Utterances/Delayed Echolalia | 0.62 | 0.17 | 2.17 | 0.45 |
Unadjusted p valued are given. P values are based on the Wilcoxon Rank Sum Test.
P values signified by * are statistically significant even after adjusting for multiple testing (FDR).
ASD, ADOS+/ASD−, and no ASD groups
One-way ANOVAs were used to calculate differences among the three groups with the goal of understanding characteristics of participants who exhibited autistic features but did not meet full clinical criteria for ASD. On all developmental assessments significant differences were seen between the ASD and no ASD groups (p<0.0001) (see Supplemental Table 1 for all values). Developmentally, participants with ASD were significantly more delayed across all items on the MSEL (except gross motor), VABS-II, and PLS-5 with a mean MSEL Composite Score of 52.9 (SD 4.17) in the ASD group compared to 86.4 (SD 2.2) in the no ASD group. Similarly, mean Adaptive Behavior Composite on the VABS-II for the ASD group was 69.9 (SD 2.7) compared to 88.4 (SD 1.4) in the no ASD group.
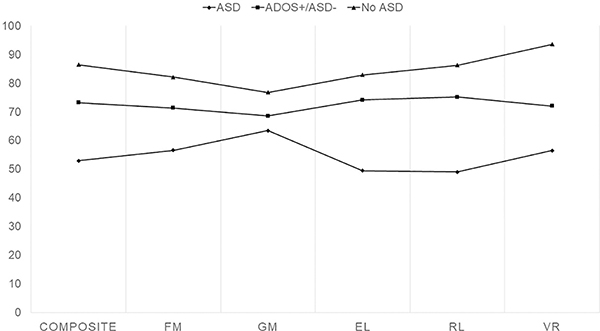
When looking between groups, more differences were seen between individuals with ASD and those with a positive ADOS-2 but who did not meet clinical criteria for ASD (all variables on the MSEL, VABS-II and PLS-5 were significant except MSEL gross motor) compared to this mixed group and those without an ASD diagnosis (Fig 2). Behaviorally, on the CBCL significant differences were seen between the ASD vs no ASD group in all subscales, and there were differences on most subscales between the group with ASD and those with a positive ADOS-2 but no clinical diagnosis, particularly in the areas of anxiety, attention, affective, ADHD, pervasive developmental, emotionally reactive, sleep, somatic, and withdrawn behaviors. However, no behavioral differences were found between the group with no ASD clinical diagnosis and the group with a positive ADOS-2 but no clinical diagnosis.
Figure 2:
Cognitive Domains Across Three Groups. Mullen groups by developmental quotient: Fine motor (FM), gross motor (GM), expressive language (EL), receptive language (RL), visual reception (VR), and composite (FM+EL+RL+VR) by groupings (ASD, met ADOS-2 criteria for ASD but not clinically, and no ASD). All differences had p<0.05 except MSEL gross motor.
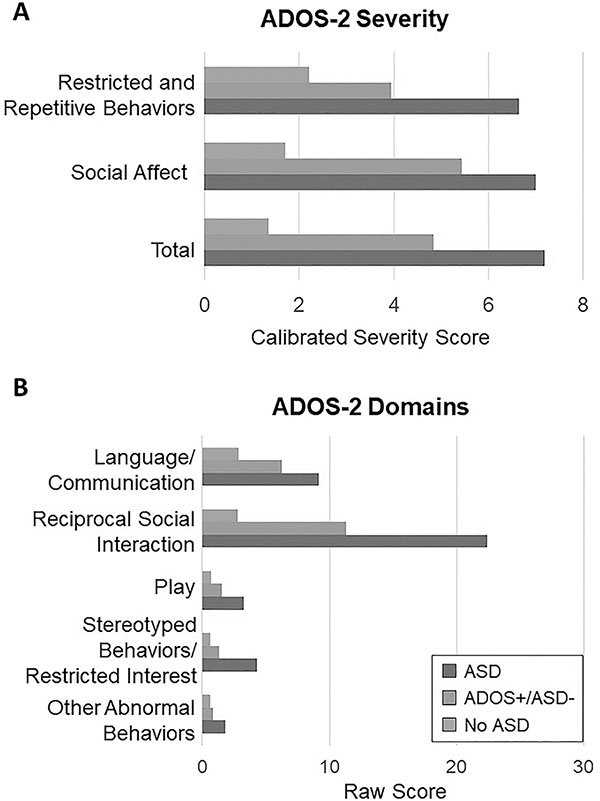
When looking at differences on the ADOS-2, severity scores (total, social affect, and repetitive/restrictive behaviors) were used. Individuals with ADOS+/no ASD diagnosis exhibited social affect severity scores (mean 5.44) closer to the ASD group (mean 7) compared to the no ASD group (mean 1.7). In Figure 3a significant differences were seen across all three groups (p<0.001). See Supplemental Table 1 for all values. To explore this relationship further, individual domains on the ADOS-2 (language/communication, reciprocal social interaction, stereotyped behaviors/restricted interests, other abnormal behaviors) for both modules 1 and 2 were analyzed for the three groups (see Figure 3b). The largest differences were seen in reciprocal social interactions, language/communication, and play (p<0.001). Of note, all the differences in domains were highly significant (p<0.0001) for the ASD vs ADOS+/no ASD and ASD vs No ASD groups, and for the first two domains for ADOS+/no ASD vs No ASD groups.
Figure 3.
A: Group Means Scores on ADOS-2 by Clinical Severity Scores. CSS= Clinical Severity Score. One-way ANOVA was used to calculate differences between each group. All p-values were less than 0.001.
Figure 3B: Group Mean Scores by ADOS-2 Item Domains. One-way ANOVA was used to calculate differences between each group. All p-values were less than 0.001 except for play (0.008), stereotyped/repetitive restrictive behaviors (0.049), and other abnormal behaviors (0.44) in the ADOS+/ASD- versus No ASD group.
DISCUSSION
This multicenter, prospective, longitudinal study has resulted in a better understanding of emerging characteristics of ASD in infants and toddlers with TSC. A relationship between seizures and developmental outcomes in TSC has been previously described by this group (32). The presence of seizures prior to 12 months was associated with worse developmental outcomes and autism-related behaviors at 24 months of age. In addition, age of seizure onset was found to be the most important predictor of later development. Early ASD risk markers in infants with TSC using the AOSI were evaluated at 12 months, and scores were associated with later autism-specific behaviors on the ADOS-2 at 24 months (OR 1.16 [CI 1.06–1.27], p<0.001) (32). Specifically, mean AOSI scores were higher in children who subsequently met ADOS-2 cut-off scores at 24 months compared to those who did not meet ADOS-2 cut-off scores (p<0.001). When accounting for overall development, the AOSI total score was no longer significant. However, development as measured by the Early Learning Composite on the MSEL was a significant predictor for ADOS-2 classification at 24 months indicating that developmental functioning is an important factor in understanding ASD risk behaviors in TSC. The relationship between language, seizures and ASD diagnosis at 36 months was also previously examined (33). Language variables as early as 6 months were noted to be significantly associated with an ASD diagnosis at 36 months. Seizures also negatively correlated with language function.
Results from the current analysis provide a rich description of the profile of ASD in young children with TSC. Prevalence of a clinical diagnosis of ASD was 25%. Prior studies have reported a wide range of prevalence of ASD in TSC (6–69%), with a recent systematic review reporting an average prevalence rate of 30% across 36 studies (9, 13, 34, 35). A unique aspect of our study was the use of gold standard autism measures administered by highly trained researchers who participated in annual calibration meetings, together with careful clinical diagnosis, considering all available data on each individual participant. The result of this clinical best estimate approach (combining clinical history, development, and autism-specific assessments), which aligns more closely with clinical practice, likely led to a lower prevalence of ASD than in many other studies, which either used autism measures alone (without clinician-determined diagnosis (9, 36)) or included clinical diagnosis but without clearly differentiating clinical diagnostic conclusions from scores on autism measures (13, 14, 19, 34, 37). When using only autism measures to calculate prevalence, our results would have been closer to the findings of other studies, with 34% of our participants with valid ADOS-2 administration scoring over the cut-off on the ADOS-2.
Individuals diagnosed with ASD in this group of children with TSC exhibited high rates of abnormal behaviors on the ADOS-2 including reductions in pointing and other gestures, decrease in directed vocalizations, abnormal quality of social overtures, and abnormal integration of gaze and other behaviors during social overtures. Specific ADI-R items associated with ASD diagnosis included quality of social overtures, direct gaze, inappropriate facial expressions, pointing, showing, and offering comfort. Although autism-specific and other developmental measures clearly differentiated children with and without ASD, many children who were not clinically diagnosed with ASD nonetheless exhibited autism-associated behaviors. When examining participants who scored over the cut-off for ASD on the ADOS-2 but did not obtain a clinical ASD diagnosis, this group exhibited intermediate differences in ADOS-2 features as well as developmental and behavioral features. Particularly, this group exhibited clear differences in reciprocal social interactions and social affect on the ADOS-2, which would not be as influenced by lower developmental functioning (compared to language/communication and play, for example). However, in some areas, namely on the VABS-II, CBCL, and expressive language on the MSEL, this mixed group was more like the no ASD group than the ASD group. Due to the high rates of TAND, it is postulated that behaviors such as anxiety and ADHD-type behaviors in combination with cognitive and language delays elevate the overall ADOS-2 score.
Approximately 45% of participants exhibited scores within the delayed range on the MSEL at 36 months. In addition, 38% scored within the language delayed range on the PLS-5. It is likely this percentage will increase as the children age and communication expectations become more sophisticated, so this should not be seen as a static or final representation of language delay-rather a snapshot of those showing early language delay. In addition, overall developmental functioning was higher in the non-ASD group but was delayed in TSC compared to a typically developing population in those children without ASD. It is possible that global developmental delays, as reflected by lower scores on the MSEL, contributed to higher scores on the ADOS-2 or ADI-R. This again highlights the importance of the comprehensive evaluation and multidisciplinary team input for identification of ASD-associated behaviors and establishing the clinical diagnosis of ASD (38). Furthermore, investigation of the individual psychometric properties of the ADOS-2 and ADI-R in making a diagnosis of ASD is underway which will include further analysis of the relationship between global developmental delay and ADOS-2/ADI-R performance in this population.
These findings suggest that children with TSC are at risk for a range of developmental and behavioral challenges. Although autism-specific assessments, such as the ADOS-2 and ADI-R are valuable tools, they should be interpreted carefully in this population as ASD-associated behaviors are present in TSC, but clinical diagnosis is more complex due to broader TAND features. In the majority of cases the ADOS-2/ADI-R hold up very nicely in this population. There are only a relatively few cases in which test scores did not correspond with diagnosis, and there was no single item or items on the ADOS-2, ADI-R, or DSM checklist that clearly differentiated those individuals. In those few cases it is strongly recommended that these individuals be referred for a comprehensive diagnostic evaluation to assess the child’s cognitive, language, and social functioning in the context of assigning an ASD diagnosis. The need for experienced clinical judgement is even more important in lower functioning individuals, particularly those with language difficulties, to be able to discern whether a child’s development and language alone were contributing to a positive ADOS-2 score.
The TAND initiative seeks to identify and treat symptoms and behaviors, not just diagnoses. Leclezio and colleagues developed the TAND checklist to assist clinicians in having a conversation with their patients about TAND, and the TSC Consensus Group recommends that individuals with TSC be screened for TAND once per year (4, 5, 39). Research on the TAND checklist led to developing clusters of naturally occurring symptom groups of TAND behaviors. Delayed language, unusual eye contact, repetitive behaviors, unusual use of language, self-injurious behaviors, difficulties with eating and visuospatial difficulties were associated in an “ASD-like cluster.” Work is currently being done to establish guidelines for each TAND cluster. However, despite progress in understanding TAND, the ability to make a definitive diagnosis of ASD in individuals with TSC poses its own challenges. First there is considerable overlap between the neuropsychiatric features of TSC and ASD (8). Second, the severity of symptoms, particularly for individuals with other comorbid diagnoses such as developmental delay, intellectual disability (ID), speech/language delay, or seizures may impact an individual’s capacity to engage in activities including the requisite tasks of diagnostic batteries, thus making a definitive diagnosis challenging even at 36 months. Third, TSC and ASD are disorders with heterogeneous presentations, which can vary significantly from individual to individual (40–42). The Developmental Synaptopathies Consortium of the Rare Diseases Clinical Research Network (U54-NS092090) is continuing ongoing developmental and ASD-specific assessments of the TACERN cohort, which will help determine if the diagnosis remains stable over time and ideal age for ASD diagnostic evaluation. Despite obstacles, the potential for gain warrants finding solutions in order to reliably differentiate a diagnosis of ASD in children with TSC. The identification of specific behavioral features unique to TSC and ASD (40) can guide treatment decisions (43) and may lead to the development of specific interventions (41, 44).
CONCLUSION
TSC is associated with a broad range of neuropsychiatric manifestations and is among the most common known genetic disorder associated with ASD (45, 46). The prevalence of ASD and TSC offers the potential for increasing our understanding of this disorder, including improvements in screening, diagnosis, and treatment. ASD is highly prevalent in TSC, and many additional TSC patients exhibit a broad range of sub-threshold autistic behaviors. Our findings reveal a broader autism phenotype that can be identified in young children with TSC, which provides opportunity for early targeted treatments.
Supplementary Material
SUMMARY FOR SOCIAL MEDIA IF PUBLISHED.
If you and/or a co-author has a Twitter handle that you would like to be tagged, please enter it here. @DrDarcyKrueger
What is the current knowledge on the topic? Tuberous Sclerosis Complex is highly associated with developmental delay and autism spectrum disorder.
What question did this study address? This study aims to describe the developmental and behavioral profile of autism spectrum disorder in Tuberous Sclerosis Complex at 36 months, focusing on characteristics of autism-specific behaviors in this group and determining specific features that may indicate higher suspicion for autism spectrum disorder.
What does this study add to our knowledge? Many additional individuals with Tuberous Sclerosis Complex exhibit a broad range of sub-threshold autistic behaviors. Our findings reveal a broader autism phenotype that can be identified in young children with Tuberous Sclerosis Complex, which provides opportunity for early targeted treatments.
How might this potentially impact on the practice of neurology? Nearly 90% of individuals with Tuberous Sclerosis Complex experience a wide range of neuropsychiatric symptoms, which are often under-recognized and, thus, under-treated. These findings suggest that children with Tuberous Sclerosis Complex are at risk for a range of developmental and behavioral challenges that should be addressed regularly.
ACKNOWLEDGEMENTS
We are sincerely indebted to the generosity of the families and patients in TSC clinics across the United States who contributed their time and effort to this study. We would also like to thank the Tuberous Sclerosis Complex Alliance for their continued support in TSC research.
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NINDS) and Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) under Award Numbers U01-NS082320, P20-NS0801999, and U54-NS092090. The project was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number UL1-TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- 1.Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14(7):733–45. [DOI] [PubMed] [Google Scholar]
- 2.De Vries P, Humphrey A, McCartney D, Prather P, Bolton P, Hunt A, et al. Consensus clinical guidelines for the assessment of cognitive and behavioural problems in Tuberous Sclerosis. European Child and Adolescent Psychiatry. 2005;14(4):183–90. [DOI] [PubMed] [Google Scholar]
- 3.de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. European child & adolescent psychiatry. 2007;16(1):16–24. [DOI] [PubMed] [Google Scholar]
- 4.de Vries PJ, Whittemore VH, Leclezio L, Byars AW, Dunn D, Ess KC, et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatric neurology. 2015;52(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric neurology. 2013;49(4):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curatolo P, Porfirio MC, Manzi B, Seri S. Autism in tuberous sclerosis. European Journal of Paediatric Neurology. 2004;8(6):327–32. [DOI] [PubMed] [Google Scholar]
- 7.Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy and Behavior. 2007;11(4):506–13. [DOI] [PubMed] [Google Scholar]
- 8.Baker P, Piven J, Sato Y. Autism and tuberous sclerosis complex: prevalence and clinical features. J Autism Dev Disord. 1998;28(4):279–85. [DOI] [PubMed] [Google Scholar]
- 9.Jeste S, Sahin M, Bolton P, Ploubidis G, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol. 2008;23(5):520–5. [DOI] [PubMed] [Google Scholar]
- 10.Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord. 2005;35(1):103–16. [DOI] [PubMed] [Google Scholar]
- 12.Huang CH, Peng SS, Weng WC, Su YN, Lee WT. The relationship of neuroimaging findings and neuropsychiatric comorbidities in children with tuberous sclerosis complex. J Formos Med Assoc. 2015;114(9):849–54. [DOI] [PubMed] [Google Scholar]
- 13.Jeste SS, Wu JY, Senturk D, Varcin K, Ko J, McCarthy B, et al. Early developmental trajectories associated with ASD in infants with tuberous sclerosis complex. Neurology. 2014;83(2):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA. Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011;76(11):981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong V, Khong PL. Tuberous sclerosis complex: correlation of magnetic resonance imaging (MRI) findings with comorbidities. J Child Neurol. 2006;21(2):99–105. [DOI] [PubMed] [Google Scholar]
- 16.de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. TSC-associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric neurology. 2013;49(4):243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullen E Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 19.Jeste SS, Varcin KJ, Hellemann GS, Gulsrud AC, Bhatt R, Kasari C, et al. Symptom profiles of autism spectrum disorder in tuberous sclerosis complex. Neurology. 2016;87(8):766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparrow SS, Balla DA, Cicchetti DV Vineland Adaptive Behavior Scales Second Edition Survey Forms Manual: AGS Publishing; 2005. [Google Scholar]
- 21.Zimmerman I, Steiner V, & Pond R Preschool Language Scale, Fifth Edition (PLS-5): Pearson; 2011. [Google Scholar]
- 22.Achenbach TMRL. An integrated system of multi-informant assessment; Child Behavior Checklist for Ages 1 1/2–5; Language Development Survey; Caregiver-teacher Report Form. Manual for the ASEBA preschool forms & profiles. Burlington: University of Vermont; 2000. [Google Scholar]
- 23.Levy SE, Rescorla LA, Chittams JL, Kral TJ, Moody EJ, Pandey J, et al. ASD Screening with the Child Behavior Checklist/1.5–5 in the Study to Explore Early Development. J Autism Dev Disord. 2019;49(6):2348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord. 2008;38(4):731–8. [DOI] [PubMed] [Google Scholar]
- 25.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules 1–4. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 27.Lord CLR, Gotham K, Guthrie W Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part II): Toddler Module. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 28.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–27. [DOI] [PubMed] [Google Scholar]
- 29.Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44(10):2400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Lord C. Combining information from multiple sources for the diagnosis of autism spectrum disorders for toddlers and young preschoolers from 12 to 47 months of age. Journal of child psychology and psychiatry, and allied disciplines. 2012;53(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capal JK, Bernardino-Cuesta B, Horn PS, Murray D, Byars AW, Bing NM, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy & behavior : E&B. 2017;70(Pt A):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenberger A, Capal JK, Ondracek A, Horn PS, Murray D, Byars AW, et al. Language predictors of autism spectrum disorder in young children with tuberous sclerosis complex. Epilepsy & behavior : E&B. 2020;103(Pt A):106844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moavero R, Benvenuto A, Emberti Gialloreti L, Siracusano M, Kotulska K, Weschke B, et al. Early Clinical Predictors of Autism Spectrum Disorder in Infants with Tuberous Sclerosis Complex: Results from the EPISTOP Study. J Clin Med. 2019;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Specchio N, Pietrafusa N, Trivisano M, Moavero R, De Palma L, Ferretti A, et al. Autism and Epilepsy in Patients With Tuberous Sclerosis Complex. Front Neurol. 2020;11:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benova B, Petrak B, Kyncl M, Jezdik P, Maulisova A, Jahodova A, et al. Early predictors of clinical and mental outcome in tuberous sclerosis complex: A prospective study. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2018;22(4):632–41. [DOI] [PubMed] [Google Scholar]
- 37.Baumer FM, Peters JM, Clancy S, Prohl AK, Prabhu SP, Scherrer B, et al. Corpus Callosum White Matter Diffusivity Reflects Cumulative Neurological Comorbidity in Tuberous Sclerosis Complex. Cerebral cortex (New York, NY : 1991). 2018;28(10):3665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–103. [DOI] [PubMed] [Google Scholar]
- 39.Leclezio L, Jansen A, Whittemore VH, de Vries PJ. Pilot validation of the tuberous sclerosis-associated neuropsychiatric disorders (TAND) checklist. Pediatric neurology. 2015;52(1):16–24. [DOI] [PubMed] [Google Scholar]
- 40.Bruining H, Eijkemans MJ, Kas MJ, Curran SR, Vorstman JA, Bolton PF. Behavioral signatures related to genetic disorders in autism. Mol Autism. 2014;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crino PB. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol. 2013;125(3):317–32. [DOI] [PubMed] [Google Scholar]
- 42.Dawson G Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20(3):775–803. [DOI] [PubMed] [Google Scholar]
- 43.Gipson TT, Poretti A, Thomas EA, Jenkins KT, Desai S, Johnston MV. Autism Phenotypes in Tuberous Sclerosis Complex: Diagnostic and Treatment Considerations. J Child Neurol. 2015;30(14):1871–6. [DOI] [PubMed] [Google Scholar]
- 44.Curatolo P, Napolioni V, Moavero R. Autism spectrum disorders in tuberous sclerosis: Pathogenetic pathways and implications for treatment. J Child Neurol. 2010;25(7):873–80. [DOI] [PubMed] [Google Scholar]
- 45.Davis PE, Peters JM, Krueger DA, Sahin M. Tuberous Sclerosis: A New Frontier in Targeted Treatment of Autism. Neurotherapeutics. 2015;12(3):572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jülich K, Sahin M. Mechanism-based treatment in tuberous sclerosis complex. Pediatric neurology. 2014;50(4):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.