Figure 3: ANCs maintain in vitro binding and in vivo therapeutic efficacy of immune checkpoint blockade (ICB) mAbs.
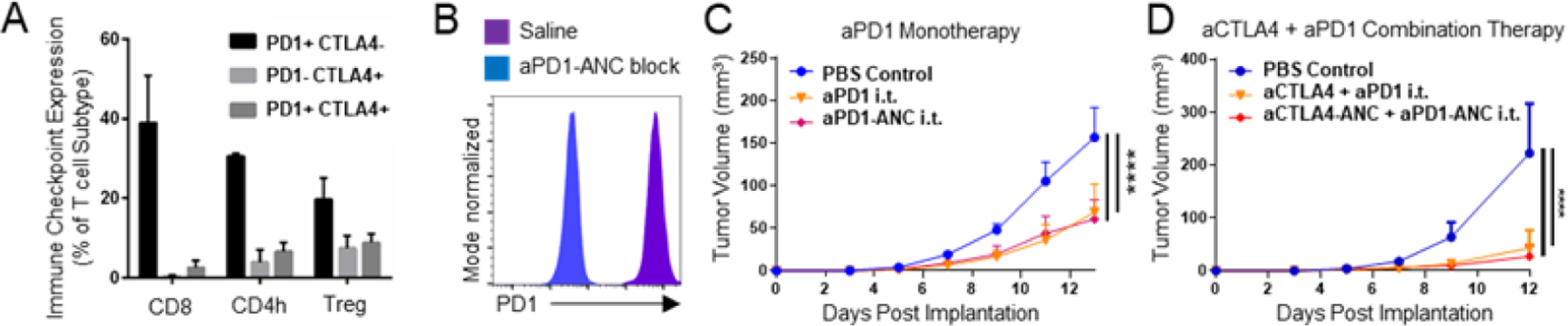
A) Expression of immune checkpoint molecules PD1 and CTLA4 by CD8 (CD8+), CD4h (CD4+FoxP3−) and Treg (CD4+FoxP3+) T lymphocytes (CD3+CD45+). B) Flow cytometry histograms of fluorescent labeling of PD1-expressing EL4 murine lymphoma cells with aPD1 with or without 15 min pre-incubation with aPD1-ANCs. Tumor control by aPD1 (150 ug) as monotherapy (C) or in combination with aCTLA4 (150 ug of each) (D) administered i.t. on days 5, 7, and 9 post-B16F10 implant is equivalent between ICB in its free or ANC form. Statistical analyses were done using two-way ANOVA with Tukey’s test (C-D). ****p < 0.0001.
