Summary
Spatial single cell transcriptomics of primary patient diffuse type gastric cancer reveals distinct cancer and stromal cell differences based on location. Expression of CCL2 by stromal cells in deep tumor regions is highlighted as potentially driving the immunosuppressive microenvironment and enhancing (directly or indirectly) the invasive capacity of tumor cells.
In this issue of Clinical Cancer Research, Jeong and colleagues (1) applied single-cell RNA sequencing (scRNA-seq) technology to profile the cellular heterogeneity of patient tissues from superficial and deep invasive layers of diffuse type gastric cancer (GC). This novel study exploits cutting-edge tools to provide a comprehensive understanding of the complexity underlying the most aggressive form of GC. Results of the analysis highlight landscape features of the invasive front of diffuse type GC and reveal potential therapeutic targets for more precise therapies in the future.
GC is the third most common cause of cancer-related death throughout the world. It is a heterogeneous disease characterized by diverse subtypes and clinical features (2). Currently, there are two major ways of classifying GC, the Lauren Classification system and the WHO classification system with the Lauren Classification system being most commonly used. Based on the Lauren Classification system, which utilizes structural/histological characteristics, GC is categorized into three major subtypes: intestinal, diffuse and mixed type. Prognosis of the disease is associated with subtype. For example, intestinal GC is typically associated with older patients and has better prognosis than the diffuse subtype, which is commonly found in younger patients. However, despite differences in biology and prognosis, treatment strategies are essentially the same regardless of subtype. Typical therapeutic strategies include surgical resection and chemotherapy, with targeted therapies used in a minority of cases, again regardless of GC subtype. The limited spectrum of therapeutic options is due in large part to our limited understanding of this heterogeneous disease.
Compared to intestinal type GC, diffuse type GC features a more aggressive phenotype. Diffuse type GC consists of poorly cohesive/poorly differentiated cells such as signet ring cells that often spread via lymphatic dissemination or local invasion at an early stage of disease. Multiple efforts have profiled intestinal type and diffuse type GC by bulk genomic and transcriptomic analyses. This has led to the identification of common mutations including in CDH1 among diffuse type tumors. In addition, diffuse type GC has been characterized as genomically stable and mesenchymal-like by these global expression studies. However, bulk-level omics studies have not provided an accurate profile of cellular heterogeneity within diffuse type gastric tumors. Recently, scRNA-seq technologies have substantially advanced our knowledge of biological systems (3). These techniques enable an analysis of cellular heterogeneity in a designated tissue, highlight novel cell populations and provide an opportunity to analyze interactions between cell populations.
In this study, Jeong and colleagues (1) obtained fresh diffuse type GC specimens from patients and dissected them into superficial layers within the depth of 5 mm from the tumor surface and deep layers which included the tumor invasion front. These two layers of the tumor as well as matched normal gastric tissues were digested into single cells and subjected to scRNA-seq analysis. This analysis led to the identification of seven major cell types within diffuse type GC, including epithelial cells, fibroblasts, endothelial cells as well as immune cells such as T cells, B cells, plasma cells, and myeloid cells. Interestingly, they found that epithelial cells, plasma cells and B cells were enriched in the superficial layers, while fibroblasts, endothelial cells and myeloid cells tended to accumulate in the deep layers. There was no statistical difference of T cell distribution between the two layers. In addition, along the superficial-to-deep layer transition, there is an increase of inflammatory cellular signatures. For example, the fibroblast, endothelial cell and myeloid cell populations within the deep layers featured expression of inflammatory chemokines and cytokines that are known to be immunosuppressive and tumor-promoting. Moreover, although there was no difference of the number of total T cells between the two layers, exhausted T cells and regulatory T cells (Tregs) were enriched in the deep layers of the tumors. These data suggest that stromal cells of the deep layers contribute to the formation of a more immune evasive and tumor-promoting microenvironment. Consistently, they found that cancer cells up-regulated inflammatory response-related and cancer aggressiveness-related genes and gained an undifferentiated (mesenchymal) gene signature during the transition from superficial-to-deeper locations in GC.
Importantly, this study identified that expression of CCL2 in stromal cells is elevated highly in the deep layers of the tumor (Fig. 1). Further, CCL2 expression correlated significantly with poor survival in GC patients. CCL2, which is also called monocyte chemoattractant protein 1, is a key factor in macrophage chemotaxis (4). It has been shown to recruit immunosuppressive cells such as myeloid-derived suppressor cells and metastasis-promoting monocytes in other cancer types such as breast cancer and colorectal cancer. Targeted therapies against CCL2 or its receptors CCR2 and CCR4, in combination with conventional therapies or immunotherapies are being tested preclinically and clinically and have shown some promise in other types of cancer (5). Results from Jeong et al (1) nominate the testing of the efficacy of blocking CCL2 in diffuse type GC. This study also opens multiple directions for further study to explain mechanisms of diffuse type GC progression. CCL2 is produced by stromal cells (fibroblasts, endothelial cells and myeloid cells) and pseudo-trajectory analysis revealed that stromal cells in the deep layers are derived from fibroblasts and endothelial cells within normal and superficial layers. This strongly implicates the local microenvironment as being a critical stimulus for the production of immunosuppressive cytokines. While this is not surprising, it further validates the intense focus on tumor microenvironmental interactions in controlling the phenotype and behavior of stromal cells in tumors. In the future, the relationship of distinct cell populations from different layers/areas of GC should be investigated by lineage tracing. Moreover, specific cell-ablation studies could yield insight into the functions and importance of individual cell types during the transition from superficial-to-deep layers. In addition, pathway and motif analyses that compare cancer cells or stromal cell populations between superficial and deep layers could reveal signaling pathways that promote the formation of aggressive cancer cells and inflammatory stromal cells. The result of which would certainly reveal additional and maybe new pathways for potential therapeutic intervention in diffuse type GC.
Figure 1. Overview of superficial to deep cell transition in diffuse type gastric cancer.
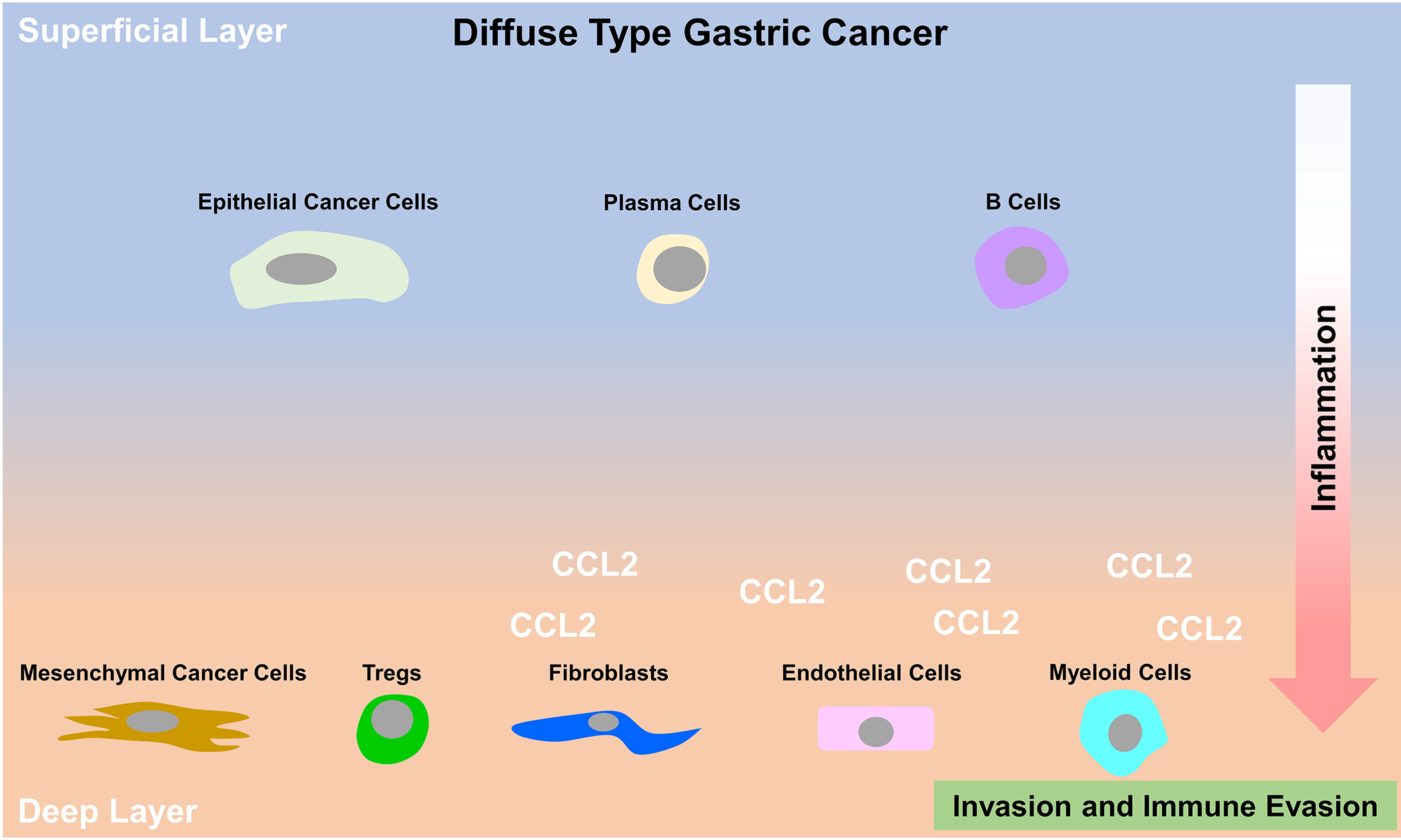
scRNA seq of superficial and deep layer tissues of primary patient diffuse type gastric cancer reveals that epithelial cancer cells, plasma cells and B cells are enriched in the superficial layers, while fibroblasts, endothelial cells, myeloid cells and Tregs tend to localize in the deep layers. In addition, cancer cells in the deep layer acquire a mesenchymal phenotype. The increase of an inflammatory and immunoevasive signature in multiple cell types during the superficial to deep transition is exemplified by the secretion of inflammatory cytokines and chemokines, mainly CCL2, from fibroblasts, endothelial cells and myeloid cells in deeper layers of diffuse type GC.
In conclusion, Jeong and colleagues (1) have highlighted the impact of spatial location on cancer and stromal cell phenotype in primary patient diffuse type gastric tumors. This is the first study that provides a comprehensive cell atlas of different tissue layers in diffuse type GC. Results presented demonstrate that the inflammatory and immunosuppressive tumor microenvironment within the deep tumor invasion front is a significant contributor to the aggressive phenotype of diffuse type GC cells. Finally, the authors provide the beginning of a roadmap that has potential to lead to a better understanding of this dismal disease and provide promising targeted therapies.
Acknowledgements
This work was supported by NIH grants R01 (CA243577) to RAB and K99/R00 CA252009 to HH.
Footnotes
Disclosure of potential conflicts of interest: none.
References
- 1.Jeong HY, Ham IH, Lee SH, Ryu D, Son SY, Han SU, et al. Spatially distinct reprogramming of the tumor microenvironment based on tumor invasion in diffuse-type gastric cancers. Clin Cancer Res. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–64. [DOI] [PubMed] [Google Scholar]
- 3.Stuart T, Satija R. Integrative single-cell analysis. Nat Rev Genet. 2019;20(5):257–72. [DOI] [PubMed] [Google Scholar]
- 4.Gschwandtner M, Derler R, Midwood KS. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front Immunol. 2019;10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):28697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]


