Summary
Impairment in glucocerebrosidase (GCase) is strongly associated with the development of Parkinson’s disease (PD), yet the regulators responsible for its impairment remain elusive. In this paper, we identify the E3 ligase Thyroid Hormone Receptor Interacting Protein 12 (TRIP12) as a key regulator of GCase. TRIP12 interacts with and ubiquitinates GCase at lysine 293 to control its degradation via ubiquitin proteasomal degradation. Ubiquitinated GCase by TRIP12 leads to its functional impairment through premature degradation, and subsequent accumulation of α-synuclein. TRIP12 overexpression causes mitochondrial dysfunction, which is ameliorated by GCase overexpression. Further, conditional TRIP12 knockout in vitro and knockdown in vivo promotes the expression of GCase, which blocks α-synuclein preformed fibrils (α-syn PFFs)-provoked dopaminergic neurodegeneration. Moreover, TRIP12 accumulates in human PD brain and α-synuclein based mouse models. The identification of TRIP12 as a regulator of GCase provides a new perspective on the molecular mechanisms underlying dysfunctional GCase-driven neurodegeneration in PD.
Keywords: Glucocerebrosidase (GCase); Glucocerebrosidase 1 gene (GBA1); E3 ligase; Thyroid Hormone Receptor Interacting Protein 12 (TRIP12); glucosylceramide (GlcCer); ubiquitin-proteasome system (UPS); α-synuclein; Parkinson’s disease (PD); dementia with Lewy bodies (DLB); Gaucher’s disease (GD); reactive oxygen species (ROS); mitochondria; lysosome, α-synuclein preformed fibrils (α-syn PFFs)
In brief
The E3 ligase TRIP12 interacts with and ubiquitinates GCase associated with the development of PD. The ubiquitinated GCase by TRIP12 leads to its functional impairment, and subsequent accumulation of α-synuclein and mitochondrial dysfunction. TRIP12 depletion promotes the expression of GCase, which blocks dopaminergic neurodegeneration due to α-synuclein preformed fibrils.
Introduction
Homozygous mutations in the glucocerebrosidase 1 gene (GBA1) that encodes the lysosomal enzyme β-glucocerebrosidase (GCase) underlie Gaucher’s disease (GD) (Do et al., 2019). GD is characterized by the accumulation of lipids glucocerebrosides and glucosylsphingosines. Recent studies suggest that patients with Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB) also frequently carry a defective GBA1 gene with heterozygous mutations (Nalls et al., 2013; Sidransky et al., 2009). GCase is synthesized in the endoplasmic reticulum (ER) and transported to the lysosome (Erickson et al., 1985; Zunke et al., 2016). Some of the GBA1 mutants of PD and GD result in misfolded GCases that stay in the ER and are degraded prematurely by the lysosome or the protein quality control system (Bendikov-Bar and Horowitz, 2012; Bendikov-Bar et al., 2011; Maor et al., 2013b). A recent study implicates the E3 ligase Parkin in degrading GCases, specifically the altered ones (Ron et al., 2010).
Growing evidence shows GCase and α-synuclein (α-syn) have a reciprocal relationship that profoundly impacts PD and α-synucleinopathies. Decreased GCase level contributes to α-syn accumulation, and reciprocally, a high level of α-syn inhibits the normal functioning of GCase by inducing aberrant trafficking (Mazzulli et al., 2011). Interestingly, in the substantia nigra (SN) of sporadic PD patients with and without GBA1 mutations, the GCase level and enzymatic activity are significantly reduced (Gegg et al., 2012). The fact that GCase becomes dysfunctional with no mutation in the GBA1 gene suggests that other critical regulators of GCase exist. Therefore, we aimed to identify the key regulators of the wt GCase and study their roles in the pathophysiology of PD and related α-synucleinopathies. To this end, we screened and isolated GCase-interacting proteins using the tandem affinity purification (TAP) technique. Among the interacting proteins, we found TRIP12, a ubiquitin E3 ligase, as a major regulator of wt GCase turnover. We characterized further and uncovered that TRIP12 tightly controls the GCase level via the ubiquitin-proteasome system (UPS), and TRIP12-induced ubiquitination and subsequent degradation of GCase lead to mitochondrial dysfunction. Furthermore, depleting TRIP12 in the human dopaminergic neurons and SN provides neuroprotection against α-syn preformed fibrils (α-syn PFFs)-provoked PD by increasing the GCase activity. Taken together, this study provides new insights into how GCase is dysregulated in sporadic PD and GBA1-linked PD and offers novel molecular therapeutic strategies to restore GCase activity and reverse PD.
Results
GCase interacts with TRIP12
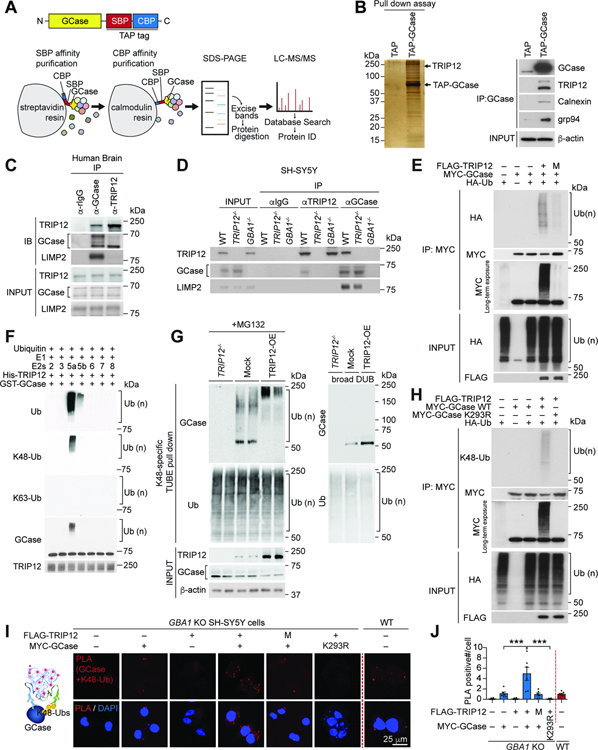
In sporadic PD patients, the GCase level, and subsequently its activity, is reduced in the absence of a GBA1 mutation (Gegg et al., 2012). To identify the critical modulators of GCase, we performed tandem affinity purification (TAP) screening with TAP-tagged GCase (Figure 1A). GCase-interacting proteins were affinity purified, separated on SDS-PAGE, excised, and analyzed by mass spectrometry (Figure 1B). A total of 54 interacting proteins were found in 19 evenly-sized gel slices (Table S1), including the known GCase-binding proteins Calnexin and GRP94 (Hein et al., 2015; Lu et al., 2011). Among the candidates, the E3 ubiquitin-protein ligase TRIP12 (Collado and Serrano, 2010) was seen as one of the most abundant interactors (Table S1). The specific interaction of GCase with TRIP12, Calnexin, and GRP94 was confirmed by immunoblotting the TAP-GCase pulled-down proteins (Figure 1B).
Figure 1. TRIP12 Interacts with GCase and Ubiquitinates Its K293 Residue via K48-specific Ubiquitin Linkage.
(A) Schematic representation of tandem affinity purification of the (TAP)-GCase construct with C-terminal streptavidin-binding peptide (SBP) and calmodulin-binding peptide (CBP) tags.
(B) Silver-stained SDS-PAGE gel (left). Binding between GCase and its interacting proteins calnexin, grp94, and TRIP12 by immunoblot (right).
(C) Co-immunoprecipitation (co-IP) of endogenous TRIP12, GCase, and LIMP2 and their mutual interactions in human brain samples.
(D) TRIP12, GCase, and LIMP2 co-immunoprecipitate from wt SH-SY5Y cells, but not from CRISPR/Cas9 induced TRIP12-knockout (TRIP12−/−) or GBA1-knockout SH-SY5Y cells (GBA1−/−).
(E) FLAG-TRIP12 wt but not its catalytic domain mutant (M; FLAG-TRIP12 C1959A) ubiquitinates MYC-GCase in in vivo ubiquitination assay.
(F) In vitro ubiquitination assay with GST-GCase, E1, E2s Ubch2 (2), UbcH3 (3), UbcH5a (5a), UbcH5b (5b), UbcH6 (6), UbcH7 (7), UbcH8 (8), and His-TRIP12.
(G) Representative western blot shows K48 ubiquitin-linked endogenous GCase enriched by K48-specific TUBE pulldown in SH-SY5Y cells with Mock, TRIP12 overexpression (TRIP12-OE), and TRIP12−/− in the presence of MG132 (left panel). K48-specific ubiquitin-enriched fractions incubated with a DUB (right panel).
(H) In vivo ubiquitination assay shows TRIP12 polyubiquitinates MYC-GCase wt through K48-specific ubiquitination, but not K293R mutant GCase.
(I and J) PLA assay for protein interactions between TRIP12 wt or TRIP12 catalytic domain mutant (M) and GCase WT or GCase K293R mutant in GBA1−/− transfected with the indicated constructs. (I) The PLA-positive signals in each cell were measured and (J) shown in a bar graph (n=6, each group). Data are presented as mean ± SEM (***P < 0.001).
See also Figures S1–S3 and Tables S1 and S2.
Human TRIP12 is 1,992 amino acids long containing an IDR (intrinsically disordered region) and ARM (armadillo/β-catenin-like repeats) domains at the N-terminus and an intermediate WWE and HECT domains at the C-terminus (Figure S1A) (Brunet et al., 2020; Chen et al., 2010). TRIP12 is differentially expressed across the brain regions with high levels in the cortex and hippocampus and low levels in the striatum, midbrain, cerebellum, and brainstem (Figure S1B and S1C). In substantia nigra pars compacta (SNpc), neurons and microglia express TRIP12 but not the astrocytes (Figure S1D). Of note, TRIP12 and GCase are co-expressed in the primary cortical neurons and human neuroblastoma cell line SH-SY5Y (Figure S1E).
We performed co-immunoprecipitation (co-IP) studies in human cortical tissue extracts, and our analysis verified the binding of TRIP12 with GCase (Figure 1C). As expected, no co-IP was seen in TRIP12 or GBA1-knockout generated by the CRISPR/Cas9 system in SH-SY5Y cells (Figure 1D). Consistent with previous findings (Rothaug et al., 2014; Zunke et al., 2016), LIMP2, a protein that translocates GCase to the lysosome, co-immunoprecipitates with GCase (Figure 1C and 1D). Notably, TRIP12 co-IP was seen only with premature forms of GCase (Figure 1C and 1D). The direct interaction between TRIP12 and GCase was further confirmed in vitro using GST-GCase pull-down assay (Figure S1F). To identify which domain of TRIP12 binds with GCase, mapping studies were carried out with deletion constructs of FLAG-tagged TRIP12. SH-SY5Y cells were transiently co-transfected with FLAG-tagged TRIP12, wt or a deletion mutant, and MYC-tagged GCase. Co-IP experiments showed that GCase interacts only with the N-terminal fragment (F1) and not with the middle (F2 and F3) or C-terminal fragments (F4) of TRIP12 (Figure S1G). Likewise, the GCase domain required for TRIP12 binding was identified using MYC-tagged N and C-terminal fragments of GCase, which showed only the C-terminus of GCase interacts with TRIP12 (Figure S1H).
Since only premature GCase, initially synthesized from the ER, interacts with TRIP12 in co-IP assays, it is likely that the GCase:TRIP12-interacting pool is in the ER. We tested this possibility and found that the fluorescence signals from TRIP12 and GCase considerably overlapped in the ER (Figure S1I). Importantly, in situ proximity ligation assay (PLA) showed strong fluorescence signals from both TRIP12 and GCase in the ER of SH-SY5Y (Figure S1J and S1K), whereas those signals were absent in the ER of TRIP12 or GBA1-knockout SH-SY5Y cells (Figure S1J). We further analyzed the intracellular localization of endogenous GCase and TRIP12 by fractionating subcellular organelles of SH-SY5Y (Figure S1L). Only mature GCase (post ER forms) significantly overlapped with LAMP1 and LIMP2 (Figure S1L). Obviously, the distribution of premature GCase (ER forms) and TRIP12 was predominantly stacked with the ER marker calreticulin. These results support the notion that the GCase:TRIP12-interacting pool is in the ER. The intracellular localization of exogenously expressed TAP and MYC-tagged GCases and FLAG-tagged TRIP12 used in this study were validated by subcellular fractionation in GBA1 or TRIP12-knockout SH-SY5Y cells. Most of the immunoreactivity of TAP-tagged or MYC-tagged GCase completely overlapped with LIMP2, and immunoreactivity of FLAG-tagged TRIP12 coincided with calreticulin (Figure S1M–S1O). Similar results were also observed in immunostaining analysis. The tagged mature GCase co-localized with LIMP2 or LAMP1 (Figure S1P and S1Q). Furthermore, the GCase activity assay demonstrates that MYC-GCase is functionally active in the lysosomes (Figure S1R). The quality and integrity of the GCase antibody used in this study have been validated (Figure S1S). These results indicate that TAP-tagged and MYC-tagged GCase are functionally active and predominantly localized in the lysosomes under normal conditions.
TRIP12 ubiquitinates GCase
The HECT domain of TRIP12 E3 ligase ubiquitinates substrates (Park et al., 2009). To determine if GCase is its substrate, we performed in vivo ubiquitination assay and found that TRIP12 significantly ubiquitinated GCase, whereas a TRIP12 catalytic domain mutant (M; TRIP12 C1959A) failed, as observed by anti-HA immunoreactivity (Figure 1E). GCase completely lacked ubiquitination when TRIP12 is knocked out (Figure S2A). To identify the specific ubiquitin chain generated by TRIP12, we investigated K48 or K63-specific ubiquitin linkages on GCase (Tomita et al., 2015). Western blot analysis with K48 and K63-specific ubiquitin antibodies showed that TRIP12 ubiquitinates GCase via the K48-linkage and not K63-linkage (Figure S2B).
We then performed an in vitro ubiquitination assay with ubiquitin and purified recombinant proteins, His-TRIP12, commercially available GST-GCase, E1, and E2 enzymes (UBCH-2, 3, 5a, 5b, 6, 7, and 8). In this assay, TRIP12 polyubiquitinated GCase in the presence of UBCH-5a, indicating that UBCH-5a is the E2 conjugating enzyme for TRIP12 (Figure 1F). This in vitro assay also confirmed ubiquitination via the K48-linkage (Figure 1F). TRIP12-mediated ubiquitination of endogenous GCase was assessed using a tandem ubiquitin-binding entity (TUBE) pulldown assay (Hjerpe et al., 2009). In the presence of proteasome inhibitor MG132, we detected endogenously ubiquitinated GCase (Ub-GCase) in the K48-specific ubiquitin enriched fraction (Figure 1G, left panel). Overexpressing TRIP12 significantly increased K48-specific ubiquitination of GCase, but when TRIP12 is depleted, ubiquitination failed (Figure 1G, left panel). Further, incubating with deubiquitinating enzymes (DUB) resulted in the complete removal of ubiquitin from Ub-GCase (Figure 1G, right panel). No Ub-GCase immunoreactivity was observed in the K63-enriched fraction (Figure S2C). We further examined K48-specific Ub-GCase in primary cortical neurons using in situ PLA (Figure S2D). Abundant PLA signals (red dots) were detected in primary cortical neurons when broad ubiquitin, K48-specific ubiquitin, and GCase antibodies were used, but no PLA signal was seen with GCase and K63-specific ubiquitin antibodies (Figure S2D). Importantly, the PLA signals were abundant in the ER, confirming that K48-specific ubiquitination of GCase by TRIP12 occurs in the ER (Figure S2D).
To determine the exact TRIP12-ubiquitination site of GCase, we performed in vitro ubiquitination assay using TRIP12, GCase, E1, UBCH-5a, and ubiquitin. The end products were analyzed by mass spectrometry, which provided 70% sequence coverage of GCase. The mass spectrometry study indicated five lysine (K) residues of GCase K155, K157, K198, K293, and K408 were ubiquitinated (Table S2). To identify the TRIP12-specific ubiquitin attachment sites, the five K residues were altered to arginine (R) via site-directed mutagenesis and inspected using in situ PLA assay. GCase wt, K155R, K157R, K198R, or K408R overexpression in GBA1-knockout SH-SY5Y with TRIP12 co-expression produced strong PLA signals when GCase and K48-ubiquitin antibodies were used. In contrast, GCase K293R overexpression showed significantly reduced PLA signal (Figure S2E). In vivo ubiquitination assay also showed that TRIP12 failed to generate K48-specific ubiquitination of GCase K293R (Figure 1H). Moreover, in situ PLA assays in GBA1-knockout SH-SY5Y cells showed that the PLA signals with both GCase and K48-specific ubiquitin antibodies were significantly diminished when GCase K293R was overexpressed instead of wt GCase (Figure 1I and 1J). Together, these results demonstrate that TRIP12 specifically ubiquitinates the K293 residue of GCase via the K48-ubiquitin linkage.
TRIP12 regulates GCase protein level, leading to altered α-synuclein expression
Next, we investigated whether TRIP12 reduces the level GCase facilitating proteasomal degradation. Cycloheximide chase experiments demonstrate that the levels of GCase wt, K155R, K157R, K198R, or K408R were significantly lowered by TRIP12 overexpression, whereas GCase K293R mutant resisted TRIP12 (Figure S3A and S3B). The ubiquitin-mediated proteasomal degradation of GCase by TRIP12 was further explored using Tet off-inducible FLAG-TRIP12 SH-SY5Y in the presence and absence of proteasomal inhibitor MG132 and enriching for K48-specific Ub-GCase using TUBE pulldown assay. In the presence of MG132, induced expression of TRIP12 (by withdrawing doxycycline, DOX) significantly increased K48-specific Ub-MYC-GCase immunoreactivity in the cells overexpressing MYC-GCase wt (Figure S3C), but not MYC-GCase K293R (Figure S3D). Notably, DUB treatment completely reduced the K48-specific Ub-MYC-GCase immunoreactivity (Figure S3C). More importantly, in the absence of MG132, TRIP12 overexpression reduced the level of MYC-GCase wt with increase of its ubiquitination, but not MYC-GCase K293R, as determined by the correlation fitting curve suggesting the ubiquitin-mediated proteasomal degradation of GCase by TRIP12 (Figure S3E and S3F). To more precisely measure the degradation rate of MYC-GCase wt by TRIP12, we determined if DOX affects the cell division rate. DOX addition had no effect on Tet off-inducible FLAG-TRIP12 SH-SY5Y cell division rate until 48 h (Figure S3G). However, both MYC-GCases wt and K293R showed approximately 25% reduction 48 h after transfection regardless of DOX in SH-SY5Y cells (Figure S3H and S3I). Therefore, the levels of MYC-GCases shown in Figure S3E were quantitated considering the signal decay (Figure S3F). Similarly, transient TRIP12 wt overexpression, but not TRIP12 catalytic-domain mutant (M), decreased GCase level and activity (Figure S3J and S3K). At the same time, reverse effects were seen when TRIP12 was depleted (Figure S3L and S3M). Previous subcellular fractionations revealed that the exogenously expressed FLAG-TRIP12 is stacked in the ER of SH-SY5Y cells, where the endogenous GCase is low (Figure S1O), indicating that accumulation of TRIP12 in the ER accelerates the degradation of GCase.
Since α-syn levels inversely correlate with GCase activity (Du et al., 2015; Mazzulli et al., 2011), we tested whether TRIP12 modulates α-syn via GCase. We found a close correlation among the levels of TRIP12, GCase, and α-syn when TRIP12 was transiently overexpressed in GCase-stable expressing SH-SY5Y cells (Figure S3N; TRIP12 and GCase: R2=0.965, GCase and α-syn: R2=0.983, TRIP12 and α-syn: R2=0.953). Overexpression of TRIP12 failed to modulate the levels of α-syn in GBA1-knockout SH-SY5Y, indicating that TRIP12 requires GCase to modulate α-syn levels (Figure S3O and S3P). Together, these findings suggest that TRIP12 regulates the steady-state level of GCase via ubiquitination and proteasomal degradation, consequently affecting α-syn protein level.
TRIP12 accumulates in sporadic PD patients and PD mouse models
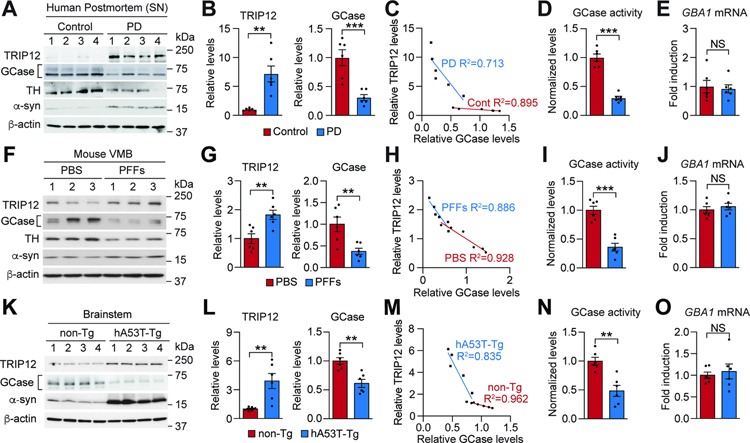
We studied sporadic PD patients and PD mouse models to understand the pathological relevance of TRIP12-mediated GCase degradation. In line with previous reports (Gegg et al., 2012; Murphy et al., 2014), a significant decrease in GCase level was observed in the SN of PD patient postmortem samples compared to age-matched controls (Figure 2A, 2B and Table S3). The levels of TRIP12 and GCase inversely correlated with each other (Figure 2C), and the SN of the PD patient samples had significantly reduced GCase activity (Figure 2D). The alteration of GCase activity can be attributed to its reduced protein level, as there was no change in the GBA1 mRNA level (Figure 2E). Importantly, endogenous TRIP12 immunoreactivity was significantly increased in the dopamine neurons in the SNpc of PD patient brains (Figure S4A–S4D).
Figure 2. TRIP12 Accumulates in Sporadic PD Patients and in PD Mouse Models.
(A) Western blot analysis of TRIP12, GCase, TH, and α-syn protein levels in the SNpc of human PD postmortem brain.
(B) Data in A shown as bar graphs (n=6, each group).
(C) Correlation between TRIP12 and GCase expression (n=6, each group).
(D) GCase activity was measured by inactivating GBA2 protein with CBE treatment in PD postmortem brain and represented in a bar graph (n=6, each group).
(E) GBA1 mRNA levels (n=6, each group).
(F) Western blot analysis of TRIP12, GCase, TH, and α-syn from the VMB of α-syn PFFs-injected mice at 6-months post-injection.
(G) Data in F shown as bar graphs (n=6, each group).
(H) Correlation between TRIP12 and GCase expression (n=6, each group).
(I and J) GCase activity and GBA1 mRNA levels (n=6, each group).
(K) Western blot analysis of TRIP12, GCase, and α-syn from the brainstem region of 9 to 10-month-old human A53T α-syn transgenic mice (hA53T-Tg) or age-matched nontransgenic mice (non-Tg).
(L) Data in K shown as bar graphs (n=6, each group).
(M) Correlation between TRIP12 and GCase expression (n=6, each group).
(N and O) GCase activity and GBA1 mRNA levels. Data are presented as mean ± SEM (NS; not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
The pathological importance of TRIP12-mediated GCase degradation was further investigated using an α-syn preformed fibrils (α-syn PFFs)-induced sporadic PD mouse model (Kim et al., 2018a; Luk et al., 2012; Mao et al., 2016) and a transgenic (Tg) α-synucleinopathy mouse model expressing human A53T α-syn (hA53T α-syn Tg) (Lee et al., 2002b). The level of TRIP12 was high in the ventral midbrain (VMB) of α-syn PFFs-injected mice as assessed by western blot analysis (Figure 2F and 2G), while the GCase level (Figure 2F and 2G) and activity (Figure 2I) decreased. We observed an inverse correlation between the levels of TRIP12 and GCase (Figure 2H) and no difference in the GBA1 mRNA level (Figure 2J). Similarly, the affected brainstem of the hA53T α-syn Tg mice had a high TRIP12 level (Figure 2K and 2L), low GCase level and activity (Figure 2K, 2L, and 2N), an inverse relationship between TRIP12 and GCase (Figure 2M), and unchanged GBA1 mRNA level (Figure 2O). The α-syn level was high in PD patients, α-syn PFFs-induced mice, and hA53T α-syn Tg mice (Figure 2A, 2F, and 2K). Collectively, our results indicate that TRIP12 accumulates in PD patients and α-synuclein-based mouse models, suggesting that it affects the level of GCase and α-syn, contributing to PD pathogenesis.
ER stress induces TRIP12 expression via transcription factor ATF6
ER stress, mitochondrial dysfunction, DNA damage, and oxidative stress have been associated with PD pathophysiology (Puspita et al., 2017). One or more of these events may cause TRIP12 upregulation. To test this possibility, we induced these adverse events in SH-SY5Y cells using specific chemical agents (Legros et al., 2002; Oslowski and Urano, 2011; Sies, 2017; Van Houten and Sancar, 1987). Among the agents used, only ER stress-inducing agents tunicamycin (TM) and thapsigargin (TG) upregulated TRIP12 mRNA expression (Figure S4E). These two agents also increased transcription from TRIP12-luciferase reporter construct, indicating that TRIP12 gene promoter responds to ER stress (Figure S4F). ER stress also increased fluorescence signals from control ER stress-associated reporter genes (Figure S4G). To identify the transcription factor that upregulates TRIP12 expression in response to ER stress, we performed transcription factor array profiling studies. Nuclear extracts were prepared from TG-treated SH-SY5Y cells with biotin-labeled oligos with or without a competing TRIP12 promoter DNA fragment. Profiling studies revealed Activating Transcription Factor 6 (ATF6) as the major transcription factor responsible for TRIP12 upregulation (Figure S4H). Chromatin immunoprecipitation (ChIP) assay also confirmed ATF6 binds to TRIP12 gene promoter (Figure S4I). These results provide first clues that ER stress could cause TRIP12 gene upregulation and accumulation by acting via transcription factor ATF6, contributing to PD pathophysiology.
TRIP12 accumulation affects mitochondria function and α-syn aggregate formation by acting via GCase
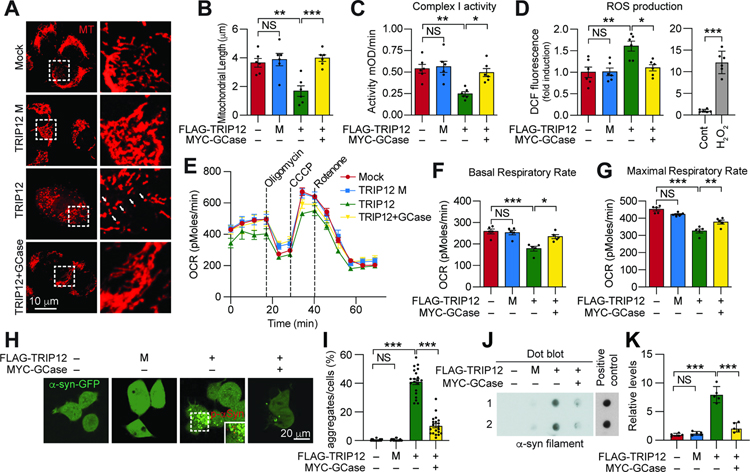
GCase functional impairment is associated with mitochondrial dysfunction (Gegg and Schapira, 2016). Therefore, we monitored the effect of TRIP12-mediated GCase inhibition on mitochondria function. As determined by Mitotracker, the mitochondrial length was reduced by 46% in TRIP12-overexpressing SH-SY5Y, which was restored by GCase overexpression (Figure 3A and 3B). In addition, decreased complex 1 enzyme activity and increased ROS levels were observed in TRIP12-overexpressing SH-SY5Y cells, which were rescued with GCase wt or K293R overexpression (Figure 3C and 3D and S5A and S5B). This effect was consistently observed when the level of MitoSox was measured in the TRIP12-overexpressing SH-SY5Y cells (Figure S5C). Notably, there was a significant increase in lysosomal pH and reduction in Lysotracker-positive structure in the SH-SY5Y overexpressing TRIP12 but not TRIP12 catalytic domain mutant (M), which was rescuable with GCase overexpression (Figure S5D–S5F). Oxygen consumption rate (OCR) was measured as another indicator of mitochondrial function (Stevens et al., 2015). While overexpression of TRIP12 led to a 32% decrease in basal respiration and a 28% reduction in maximal respiration induced by CCCP in SH-SY5Y, GCase overexpression rescued the decreased respiration rate (Figure 3E–3G). Overexpression of the TRIP12 catalytic domain mutant (M) in SH-SY5Y did not affect mitochondrial function (Figure 3A–3G). Next, we investigated whether the mitochondrial dysfunction due to TRIP12 accumulation requires GCase. To this end, we validated the mitochondrial dysfunction in GBA1-knockout SH-SY5Y cells expressing either only TRIP12 or TRIP12 with GCase wt or K293R. Consistent with a previous study (Li et al., 2019), the GBA1-knockout SH-SY5Y had reduced GCase activity accompanied by reduced mitochondrial length and increased ROS (Figure S5G–S5K). TRIP12 overexpression did not worsen the mitochondrial dysfunction in GBA1-knockout SH-SY5Y (Figure S5L and S5M). Importantly, GCase K293R overexpression significantly rescued the impaired mitochondrial function in GBA1-knockout SH-SY5Y expressing TRIP12. Remarkably, the rescue effect of GCase K293R mutant was more pronounced than its wt overexpression (Figure S5L and S5M).
Figure 3. TRIP12 Induces Mitochondrial Abnormalities and Affects the Formation of α-syn Aggregates by reducing GCase level.
(A) SH-SY5Y cells were transfected with FLAG-TRIP12 wt, TRIP12 catalytic domain mutant (M), or both FLAG-TRIP12 wt and MYC-GCase wt. Cells were stained with Mitotracker (MT; Red) (white arrows).
(B) The length of mitochondria (n=6, each group).
(C) Mitochondrial complex I activity (n=6, each group).
(D) Mitochondrial ROS production (left, n=6, each group). H2O2 was used as a positive control for ROS measurement (right)
(E) Microplate-based respirometry readings for SH-SY5Y cells transfected with indicated constructs, measured by the XF24 Seahorse analyzer.
(F and G) Quantitation of the basal and maximal respiratory rates from the respirometry results (n=5, each group).
(H) Representative confocal images of α-syn aggregate formation in GFP-α-syn-expressing SH-SY5Y cells.
(I) Data in H shown as bar graphs (n=30, each group).
(J) Dot-blot with an α-synuclein fibrils specific antibody. α-syn preformed fibrils (α-syn PFFs) were used as a positive control.
(K) Data in J shown as bar graph (n=5, each group). Data are presented as mean ± SEM (NS; not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
See also Figure S5.
Mitochondrial defects contribute to the formation of α-syn aggregates (Esteves et al., 2011; Lee et al., 2002a). As such, we evaluated whether TRIP12-induced mitochondria defect contributes to the formation of α-syn aggregates. Overexpression of TRIP12 wt but not its catalytic domain mutant (M) in the GFP-α-syn stable SH-SY5Y cells significantly induced the formation of α-syn aggregation, which was rescued by GCase overexpression (Figure 3H and 3I). Similar results were observed on the degree of α-syn aggregation, as determined by dot-blot analysis (Figure 3J and 3K). Together, these results indicate that TRIP12 accumulation results in mitochondrial abnormalities via GCase, contributing to pathologic α-syn aggregates.
TRIP12 knockdown rescues mitochondrial dysfunction and α-synuclein pathology caused by α-synuclein PFFs in primary cortical neurons
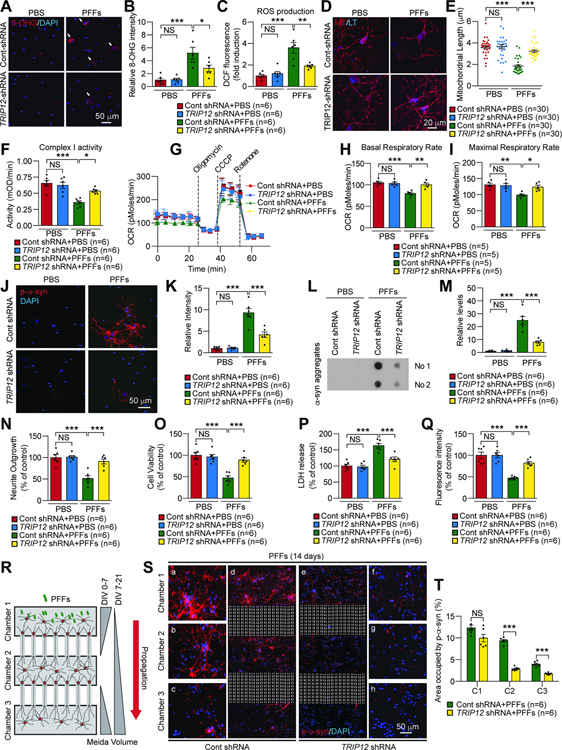
Next, we comprehensively evaluated the effect of TRIP12 knockdown and consequent increase in GCase on α-syn PFFs-induced mitochondrial damage and pathology. Primary cortical neurons were transduced with TRIP12 shRNA or control shRNA lentivirus. Oxidative stress marker 8-hydroxyguanosine (8-OHG) signals (Figure 4A and 4B) and ROS production (Figure 4C) increased after α-syn PFFs treatment, which were prevented by TRIP12 knockdown (Figure 4A–4C). Mitochondrial length (Figure 4D and 4E) and the complex I activity (Figure 4F) decreased in α-syn PFFs-treated primary cortical neurons, which were rescued by TRIP12 knockdown (Figure 4D–4F). α-Syn PFFs treatment also led to a 24% decrease in basal respiration and a 24% reduction in maximal respiration induced by CCCP in primary cortical neurons, which were restored by TRIP12 knockdown (Figure 4G–4I). To investigate the effect of TRIP12 knockdown on phosphoserine 129-α-synuclein (p-α-syn) pathology induced by α-syn PFFs, p-α-syn immunostaining was performed. p-α-syn immunoreactivity significantly increased in α-syn PFFs-treated neurons, which was suppressed by TRIP12 knockdown (Figure 4J and 4K). The level of α-syn aggregates increased by α-syn PFFs was avoided by TRIP12 knockdown, as assessed by dot-blot analysis (Figure 4L and 4M). We measured the α-syn PFFs-induced neuronal toxicity by neurite outgrowth and cell viability, LDH, and AlarmarBlue assays. The α-syn PFFs treated cortical neurons had severe neuronal toxicity, whereas TRIP12 knockdown protected significantly against that toxicity (Figure 4N–4Q). To further examine the effect of TRIP12 knockdown on the transmission of pathologic α-syn, we employed a microfluidic neuronal culture device with three chambers as previously described (Mao et al., 2016; Volpicelli-Daley et al., 2011) (Figure 4R). Lentiviral TRIP12 shRNA was transduced into primary cortical neurons at days in vitro (DIV) 5 and α-syn PFFs treated in chamber 1 at DIV 7. Consistent with a previous observation (Mao et al., 2016), after a 14-day α-syn PFFs treatment, p-α-syn immunoreactivity was observed in chambers 1, 2, and 3 containing cortical neurons with control shRNA indicating successful transmission of pathologic α-syn (Figure 4R–4T). Remarkably, p-α-syn immunoreactivity was gradually and significantly reduced in all chambers containing TRIP12-knocked down cortical neurons (Figure 4R–4T).
Figure 4. TRIP12 Knockdown Rescues the Mitochondria Dysfunction and α-synuclein Pathology caused by α-synuclein PFFs in Primary Cortical Neurons.
(A) Representative images of 8-OHG immunostaining in primary cortical neurons. Lentiviral control shRNA or TRIP12 shRNA was transduced at DIV5, and α-syn PFFs were treated at DIV7 for two weeks.
(B) Quantification of the 8-OHG intensity (n=6, each group).
(C) Quantification of ROS production (n=6, each group).
(D) Representative Mitotracker-positive micrographs.
(E) Alterations in the length of mitochondria (n=30, each group).
(F) Mitochondrial complex I activity (n=6, each group).
(G) Microplate-based respirometry readings of primary cortical neurons.
(H and I) Quantification of the basal and maximal respiratory rates from the respirometry results (n=5, each group).
(J) Representative micrographs of p-a-syn aggregates.
(K) Quantification of data in J (n=6, each group).
(L) Dot-blot analysis with an antibody specific to α-syn fibrils.
(M) Quantification of data in L (n=6, each group).
(N) Quantification of neurite outgrowth with cell membrane stain (n=6, each group)..
(O) Quantification of cell viability with a live-cell indicator (n=6, each group).
(P) Quantification of LDH assay (n=6, each group).
(Q) Quantification of AlamarBlue assay (n=6, each group).
(R) Schematic representation of a microfluidic device for transmission of p-α-syn. Lentiviral control shRNA or TRIP12 shRNA was transduced in chamber 2 primary cortical neuron at DIV5, and α-syn PFFs were treated at DIV7 for two weeks.
(S) Representative confocal images showing p-α-syn immunoreactivity.
(T) Data in S shown as bar graph (n=6, each group). Data are represented as mean ± SEM (NS; not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
See also Figure S5.
TRIP12 knockout rescues α-synuclein PFFs-induced pathology in human dopaminergic neurons
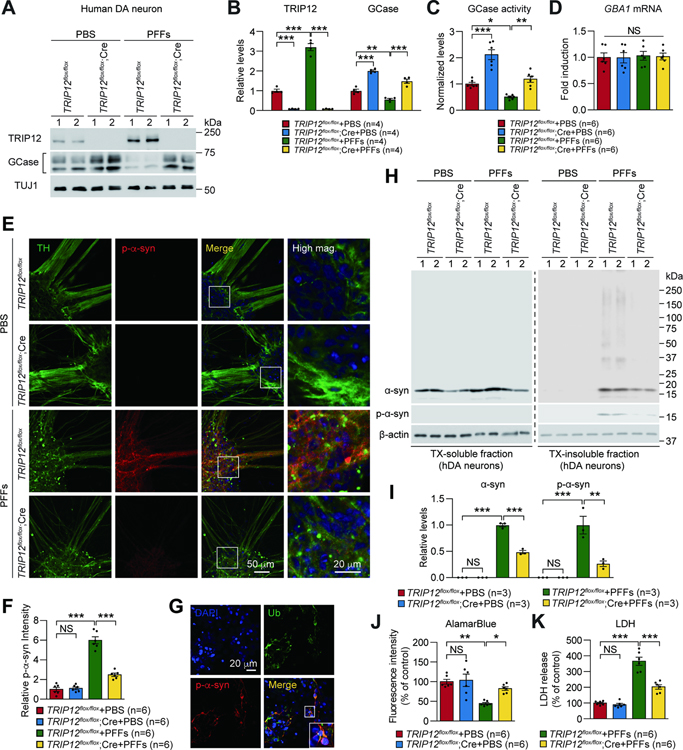
We further evaluated the effect of TRIP12 knockout in α-syn PFF-induced pathology of human dopaminergic (hDA) neurons. Conditional TRIP12 knockout human H9 embryonic stem cells ( TRIP12flx/flx hESCs) were generated using transcription activation-like effector nuclease (TALEN)-mediated gene targeting (Figure S6A and S6B) without off-target effects (Figure S6C). Our analysis confirmed the expression of pluripotent protein markers (Figure S6D and S6E). Following AAV-mediated Cre recombinase transduction into TRIP12flx/flx hESCs-derived DA neurons, depletion of TRIP12 was confirmed by western blot (Figure S6F) and immunofluorescence (Figure S6H). Similar levels of neuronal and DA markers were observed in the knockouts and controls (Figure S6H). Concordant with our results above, depletion of TRIP12 increased the GCase level and activity also in the hDA neurons and reduced the level of α-syn (Figure 5A–5C and Figure S6F and S6G). The GBA1 mRNA level remained unchanged (Figure 5D). Importantly, the immunostaining analysis revealed that the significant increase in the p-α-syn immunoreactivity induced by α-syn PFFs was prevented by TRIP12 depletion (Figure 5E and 5F), and the p-α-syn immunoreactivity co-localized with ubiquitin in hDA neurons (Figure 5G).
Figure 5. TRIP12 Knockout Rescues α-syn PFFs-induced Pathologies in Human Dopaminergic (DA) Neurons.
(A) Western blot analysis of TRIP12 and GCase in conditionally floxed TRIP12 hESCs-derived human dopaminergic (DA) neurons transduced with AAV expressing either control (TRIP12flox/flox) or cre recombinase (TRIP12flox/flox;Cre) and treated with PBS or α-syn PFFs.
(B) Data in A shown as bar graphs (n=4, each group).
(C) GCase activity (n=6, each group).
(D) GBA1 mRNA level (n=6, each group).
(E) Representative confocal images showing p-α-syn aggregates.
(F) Data in E shown as bar graphs (n=6, each group).
(G) The p-α-syn positive signals co-localize with ubiquitin.
(H) Western blot analysis of α-syn, p-α-syn, and β-actin from TX-soluble and TX-insoluble fractions.
(I) Bar graph of the levels of α-syn aggregates and p-α-syn in TX-insoluble fraction (n=3, each group).
(J) Quantification of AlamarBlue assay (n=6, each group).
(K) LDH assay (n=6, each group). Data are presented as mean ± SEM (NS; not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
See also Figure S6.
Next, we determined the amount of pathologic α-syn from Triton X-100 (TX)-soluble (no SDS) and TX-insoluble (with SDS) fractions as previously described (Mao et al., 2016; Yun et al., 2018). TRIP12 depletion significantly reduced the accumulation of TX-insoluble α-syn aggregate species and p-α-syn due to α-syn PFFs (Figure 5H and 5I). To further investigate the effect of TRIP12 depletion on α-syn PFFs-induced dopaminergic neuronal toxicity, AlamarBlue and LDH cytotoxicity assays were performed. In hDA neurons, a 14-day course α-syn PFF treatment was significantly toxic, whereas TRIP12 depletion protected against that toxicity (Figure 5J and 5K).
TRIP12 knockdown rescues α-synuclein PFFs-induced pathology in PD patient-derived hiPSC hDA neurons with GBA1 mutation
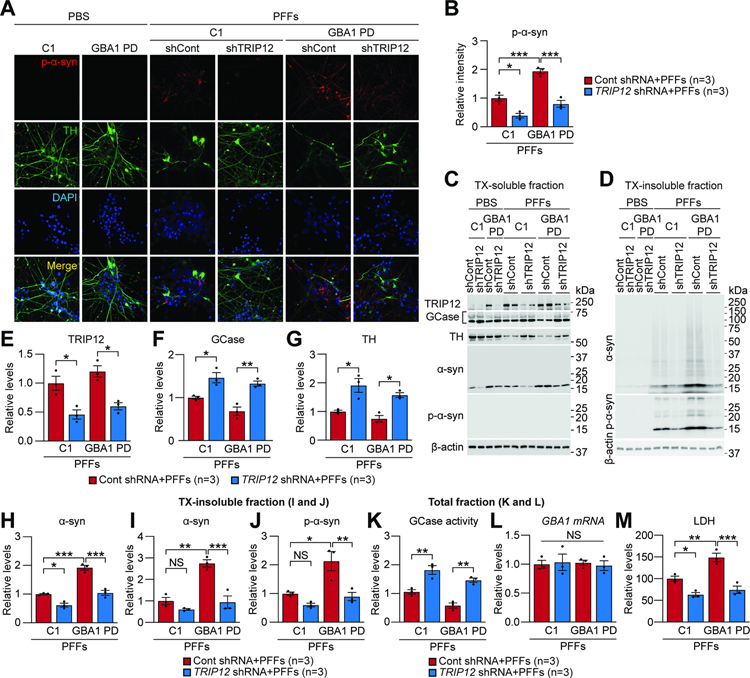
Next, we determined the effect of TRIP12 knockdown in PD patient-derived human induced pluripotent stem cell (hiPSC) DA neurons carrying GCase N370S mutation (GBA1-PD neurons) described in our previous study (Kim et al., 2018b). To this end, control and GBA1-PD neurons were transduced with lentiviral TRIP12 shRNA and control shRNA. When treated with α-syn PFFs, p-α-syn immunoreactivity significantly increased in control and GBA1-PD neurons, and TRIP12 knockdown prevented it in both neurons (Figure 6A and 6B). Importantly, α-syn PFFs treatment in control and GBA1-PD neurons increased TRIP12 levels in the TX-soluble fraction, whereas TRIP12 knockdown precluded TRIP12 accumulation (Figure 6C and 6E). Likewise, the reduction in GCase (Figure 6C and 6F) and TH levels (Figure 6C and 6G) due to α-syn PFFs treatment was significantly restored in both control and GBA1-PD neurons by TRIP12 knockdown (Figure 6C, 6F, and 6G). The increase in α-syn level due to α-syn PFFs treatment was absent in both control and GBA1-PD neurons with TRIP12 knockdown (Figure 6C and 6H). When p-α-syn and α-syn aggregate species were evaluated in TX-insoluble fraction, α-syn PFFs treatment in control and GBA1-PD neurons showed accumulation of abnormal α-syn aggregate species (Figure 6D and 6I) and p-α-syn immunoreactivity in the TX-insoluble fraction (Figure 6D and 6J), which was averted by TRIP12 knockdown (Figure 6D, 6I, and 6J). Importantly, the reduction in GCase activity due to α-syn PFFs was significantly restored in both control and GBA1-PD neurons by TRIP12 knockdown in the total fraction (Figure 6K). Dopamine neuronal toxicity assay revealed that α-syn PFFs treatment for 10 days induced neuronal toxicity in both control and GBA1-PD neurons, which was significantly protected by TRIP12 knockdown as assessed by LDH assay (Figure 6M). GBA1 mRNA level did not change in all experimental groups (Figure 6L). It is noteworthy that all the above effects were significantly greater in GBA1-PD vs control neurons (Figure 6). Taken together, the increase in GCase and activity due to TRIP12 knockdown significantly rescues α-syn PFFs-induced neurodegeneration in both control and GBA1-PD hiPSC DA neurons.
Figure 6. TRIP12 Knockdown Rescues α-syn PFFs-induced Neurodegeneration in Both Control hiPSC- and GCase N370S PD iPSC-derived DA Neurons.
(A-M) Control iPSC (C1) and GBA1-PD hDA neurons (GBA1-PD), respectively, were transduced with the lentivirus expressing either control shRNA or TRIP12 shRNA at three days before α-syn PFFs treatment.
(A) Fluorescent analysis was conducted in 5 μg/ml α-syn PFFs-treated hDA neurons for ten days using anti-p-α-syn and TH antibodies.
(B) In α-syn PFFs-treated hDA neurons, p-α-syn level was quantitated and shown as bar graphs (n=3, each group).
(C-J) hDA neurons were sequentially fractionated in TX-soluble followed by TX-insoluble buffer.
(C and D) Western blot analysis of TX-soluble and TX-insoluble fractions using the indicated antibodies.
(E-H) Quantitation of the levels of TRIP12, GCase, TH, and α-syn in TX-soluble fraction and shown as bar graphs (n=3, each group).
(I and J) Quantitation of α-syn and pS129-α-syn in TX-insoluble fraction (n=3, each group).
(K) GCase activity in total fractions (n=3, each group).
(L) GBA1 mRNA level in total fractions (n=3, each group).
(M) α-syn PFFs-induced cytotoxicity was evaluated using LDH assay in culture media (n=3, each group). All quantitated data shown in bar graphs were conducted in both α-syn PFFs-treated control hiPSC and GBA1-PD hiPSC DA neurons. Data are represented as mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
TRIP12 knockdown protects against α-synuclein PFFs-provoked neurodegeneration in vivo
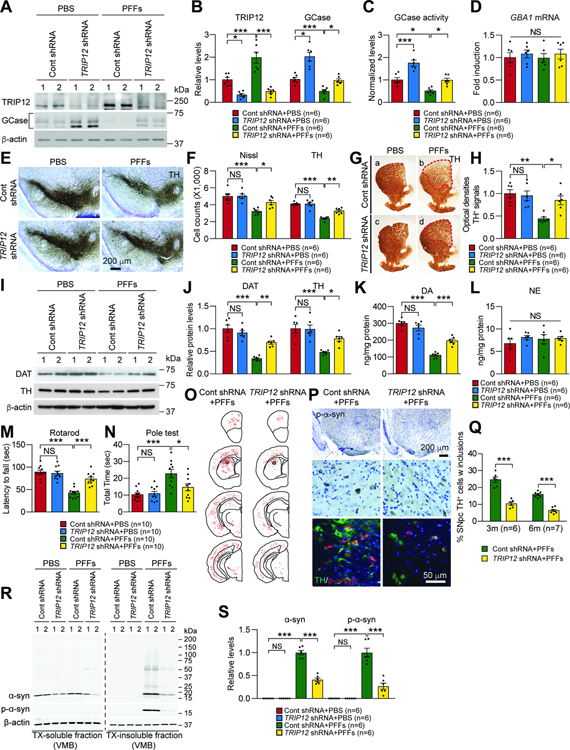
Finally, we studied the effect of TRIP12 knockdown on α-syn PFFs-induced PD pathogenesis in vivo. We generated AAV5 carrying TRIP12 shRNAs with a GFP tag and injected into the striatum (STR) of wt mice stereotaxically and unilaterally (Figure S7A). Injecting TRIP12 shRNA into the STR of wt mice efficiently transduced dopamine neurons in the entire SN and STR (Figure S7B) and resulted in 67% decrease of the mouse TRIP12 protein in the SN (Figure 7A and 7B and Figure S7C–S7F). GCase level (Figure 7A and 7B) and activity (Figure 7C) increased by approximately 2-fold in the SN. After a 6-month course of α-syn PFFs injection, we observed a 2-fold upregulation of TRIP12 protein in the SN, in addition to downregulated GCase, reduced activity, and accumulated glucosylceramide (GluCer), a substrate of GCase (Figure 7A–7C and Figure S7C–S7J). TRIP12 knockdown prevented the reduction in GCase level and activity and the buildup of GluCer in the SN of α-syn PFFs-injected mice (Figure 7A–7C and Figure S7C–S7J). To determine whether the effect is specific for GCase or whether other hydrolases are affected, lysosomes isolated from the VMB of α-syn PFFs-injected mice with or without TRIP12 knockdown were used to analyze hexosaminidase A/B/C (HEX), β-glucuronidase (GUSB), and lysosomal acid phosphatase (LAP) activity. No significant change of the hydrolases was observed except GCase (Figure S7K). Notably, TRIP12 knockdown significantly decreased α-syn protein level in the lysosome compared with control (Figure S7L and S7M). In all experimental groups, GBA1 mRNA level in the SN was unaltered (Figure 7D). Next, we sought to determine whether the reduction in GCase is simply due to toxicity. To this end, we used the VMB at 3 months after α-syn PFFs injection because no nigral toxicity is observed at this time point. Accompanying the upregulation of TRIP12 was the downregulation of GCase in the VMB suggesting that the reduction of GCase was not due to toxicity (Figure S7N and S7O).
Figure 7. TRIP12 Knockdown Protects Against α-synuclein PFFs-induced Neurodegeneration in vivo.
(A-S) PBS or α-syn PFFs containing AAV expressing either Control shRNA or TRIP12 shRNA were stereotaxically injected into the striatum of mice. After six months, motor behavioral deficits, levels of TRIP12 and GCase, GCase activity, DA loss, and neuropathology were evaluated.
(A) Western blot analysis of TRIP12 and GCase.
(B) Levels of TRIP12 and GCase (n=6, each group).
(C) GCase activity (n=6, each group).
(D) GBA1 mRNA level (n=6, each group).
(E and F) The number of TH and Nissl-positive neurons in the SNpc (n=6, each group).
(G and H) Striatal TH-immunopositive fiber density (n=6, each group).
(I and J) DAT and TH levels in mouse striatum (n=4, each group).
(K and L) Striatal dopamine (DA) and noradrenaline (NA) concentrations using HPLC (n=6, each group).
(M and N) Behavioral assessments of mice (Rotarod test and Pole test) (n=10, each group).
(O) Distribution of p-α-syn accumulation in mouse CNS.
(P) Representative images showing α-syn PFFs-induced LB-like inclusion in the SNpc region.
(Q) The percentage of TH-positive cells with inclusion (3 months, n=6; 6 months, n=7; each group).
(R) Representative immunoblots showing the protein levels of α-syn, p-α-syn, and β-actin from TX-soluble and TX-insoluble fractions in VMB of mice.
(S) Bar graph of α-syn aggregates and p-α-syn in TX-insoluble fraction (n=6, each group). Data are presented as mean ± SEM (NS; not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
See also Figure S7.
To examine the pathological relevance of TRIP12-related GCase degradation in α-syn PFFs-induced neurodegeneration, we counted the number of TH-positive neurons by TH-immunoreactivity and Nissl staining. As described previously (Kim et al., 2018a; Luk et al., 2012; Yun et al., 2018), intrastriatal injection of α-syn PFFs led to a 42% reduction in TH-positive and Nissl-stained neurons (Figure 7E and 7F). The TRIP12 knockdown significantly prevented the loss of DA neurons (Figure 7E and 7F), TH-positive fiber degeneration and reduction in STR dopamine (DA) level (Figure 7G, 7H, 7K, and 7L). Additionally, TRIP12 knockdown prevented the α-syn-induced decrease in dopamine transporter (DAT) and TH protein levels, as determined by western blot analysis in VMB (Figure 7I and 7J). In addition, TRIP12 knockdown significantly ameliorated the α-syn PFFs-induced motor deficits in rotarod and pole tests, which are characteristic pathological features that develop after 6-month intrastriatal injection of α-syn PFFs (Figure 7M and 7N). Lastly, TRIP12 knockdown also significantly alleviated LB/LN-like pathology in various brain regions, including SNpc TH-positive neurons at 3 and 6 months of age, as assessed by the immunohistochemical analysis (Figure 7O, 7P, and 7Q). To determine the amount of pathologic α-syn, the VMB of mice was fractionated and analyzed by western blot. The TX-insoluble fraction indicated buildup of pathologic α-syn aggregates and p-α-syn in the α-syn PFF-injected mice, and TRIP12 knockdown prevented the morbid accumulation (Figure 7R and 7S). Taken together, these results indicate that TRIP12 plays a crucial role in the disease progression of the sporadic PD mouse model, and rescuing GCase levels by TRIP12 knockdown prevent against α-syn PFFs-induced neurodegeneration.
Discussion
GCase is conspicuously reduced in the cortex and SN when postmortem brains of sporadic PD patients were examined (Gegg et al., 2012; Murphy et al., 2014), but no apparent alteration of the GBA1 mRNA was seen (Gegg et al., 2012). Indeed, our results from human PD postmortem samples showed no net decrease in GBA1 mRNA level in the SN (Figure 2E), suggesting that the reduction in GCase activity is likely at the protein level. In this study, our investigations of the phenomenon uncover that TRIP12 is a major contributor to the reduction in GCase in sporadic PD, and pathologically overactivated TRIP12 reduces GCase via ubiquitination and subsequent degradation.
Previous studies have reported E3 ubiquitin ligases that might interact with mutant GCases. E3 ligase Itch ubiquitinates mutant GCases in skin fibroblasts of GD patients, HeLa, and HEK293T cells (Maor et al., 2013a). Parkin also interacts with mutant GCase and polyubiquitinates via K48-linkage but does not bind wt GCase (Ron et al., 2010). GCase wt has not been shown to interact with any E3 ligase before. We used wt GCase in our TAP analysis and did not detect Parkin or Itch. Instead, we identified TRIP12 as an E3 ligase that ubiquitinates wt GCase. In our studies, TRIP12 ubiquitinates GCase via the K48 linkage. Mass spectrometry, PLA, and further analysis pinpoint the specific ubiquitination site on GCase is K293 (Table S2). When this lysine is changed to arginine (K293R), ubiquitination of GCase by TRIP12 was significantly reduced in SH-SY5Y cells compared to the wt GCase (Figure S2E and S2F). Thus, our results demonstrate that E3 ligase TRIP12 interacts with GCase, ubiquitinates, and facilitates its proteasomal degradation, which may have profound implications in PD pathologies, as GCase impairment has been identified as a major cause in PD.
TRIP12 was initially identified as a thyroid hormone receptor-interacting protein in a yeast two-hybrid screen (Lee et al., 1995). It is a member of the HECT E3 ubiquitin ligase family. TRIP12 has been implicated in various cellular processes such as cell differentiation, cell cycle progression, chromatin remodeling, and DNA damage repair (Brunet et al., 2020). Importantly, mutations in TRIP12 have been associated with intellectual disability and, more specifically, autism spectrum disorder (ASD), revealing its role in human neuronal diseases (Bramswig et al., 2017; Brunet et al., 2020; Louie et al., 2020; O’Roak et al., 2014; Zhang et al., 2017). A catalytically inactive mutation of the HECT domain in TRIP12 with no ubiquitin ligase activity results in embryonic lethality (Kajiro et al., 2011). Earlier studies in human cancer cell lines found that TRIP12 was predominantly localized in the nucleoplasm (Chen et al., 2010; Gudjonsson et al., 2012). However, we observed a different pattern in the localization of TRIP12 in SH-SY5Y dopaminergic-like neurons. Immunocytochemistry and PLA revealed that TRIP12 is abundant in the ER and the nucleoplasm, and co-localizes with GCase to ubiquitinate it within the ER (Figure S1I–S1K). Furthermore, our density gradient-based subcellular fractionation study also substantiates that the major TRIP12 pool is in the ER-enriched fraction (Figure S1L–S1O).
We further examined the cause of TRIP12 gene upregulation in PD and found that inducing ER-stress with chemical agents TM and TG abnormally increases TRIP12 mRNA expression. Microarray profiling suggests that ATF6 is the likely transcription factor that acts on TRIP12 gene promoter (Figure S4E–S4I). It is interesting to note that, ATF6 has been shown as an ER-associated unfolded protein response (UPR) that activates ER protein synthesis (Ye et al., 2000). ATF6 activity in ER physiology has also been implicated in dopaminergic neuronal survival in a PD mouse model (Egawa et al., 2011). Since TRIP12 accumulation is mainly in the ER where ATF6 acts, it is possible that ATF6 and TRIP12 are a part of the ER-associated degradation (ERAD) pathway that operates under ER stress. These observations await further explorations for confirmation.
Previous reports link reduced GCase activity to mitochondrial dysfunction (Cleeter et al., 2013; Gegg and Schapira, 2016) and demonstrate that autophagic and proteasomal machinery do not completely clear out fragmented mitochondria (Osellame et al., 2013). Consistent with these findings, we find that lack of GCase activity in GBA1-knockout SH-SY5Y cells induces mitochondrial abnormalities, elevates ROS production, and decreases complex I activity (Figure S5I–S5M). Interestingly, we also observe similar effects on mitochondria when TRIP12 is overexpressed in SH-SY5Y cells (Figure 3A–3G). It is possible that a loss of GCase function leads to impaired lysosomal activity, thereby promoting a considerable buildup of damaged mitochondria over time. As a result, the damaged mitochondrial respiratory chain cannot support the membrane potential, eventually decreasing to a level where the ATPase reverses (Li et al., 2019; Plotegher et al., 2020). It is also possible that TRIP12-mediated GCase impairment may hamper the clearance of α-syn in the lysosomes and, in turn, accelerate the formation of α-syn aggregations/inclusions with oxidative stress (Figure 3H–3K). Although we show evidence that TRIP12-GCase interplay in the ER induces mitochondrial toxicity and dysfunction, we acknowledge some limitations in our approach. The experiments were conducted using cell lines overexpressing or knocked down TRIP12. It is also possible other targets of E3 ligase TRIP12 might contribute. Further studies are needed to clarify the precise role of TRIP12-GCase interaction in mitochondrial abnormalities.
We have tested the relationship between TRIP12 and GCase and its impact on PD rigorously using a number of models, including murine models of α-syn PFFs-induced sporadic PD and hA53T α-syn Tg, in vitro studies using hESC or patient-derived hiPSC-derived DA neurons, and in vivo α-synuclein PFFs-provoked neurodegeneration. In all the systems, we observed consistent inverse correlation between the levels of TRIP12 and GCase, while TRIP12 overexpression depleted GCase, TRIP12 knockdown robustly elevated GCase level and activity. The fact that aberrant regulation of TRIP12 is the underlying cause of GCase deficiency-mediated PD is further strengthened by the observation that TRIP12 depletion by knockout or knockdown safeguards against α-syn PFFs-induced neuronal toxicity and reverses p-α-syn pathologies in hDA neurons. Moreover, TRIP12 depletion also shows strong neuroprotection against dopaminergic neurodegeneration in α-syn PFFs-induced PD mice, emphasizing TRIP12 and GCase both have therapeutic potential for PD.
In summary, the identification of TRIP12 as an E3 ubiquitin ligase for GCase provides a foundation to understand the molecular mechanisms underlying neurodegeneration driven by functional impairment of GCase in sporadic PD. Thus, strategies that can rescue GCase activity via blocking the E3 ligase activity of TRIP12 can lay the groundwork for developing efficient neuroprotective therapies for α-synucleinopathies in PD and DLB. The GCase K293R mutant possesses therapeutic potential since it is unaffected by TRIP12 hyperactivation (Figure 1H–1J and S3C–S3F). GCase K293R mutant can maintain normal GCase activity, translocate to the lysosome, and exhibit a low turnover rate in the pulse-chase assay providing long-lasting and sustainable effects of GCase. Therefore, enzyme-replacement therapy with GCase K293R recombinant protein might be considered for treating PD.
STAR METHODS
• RESOURCE AVAILABILITY
LEAD CONTACT
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Han Seok Ko (hko3@jhmi.edu)
MATERIALS AVAILABILITY
All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
DATA AND CODE AVAILABILITY
All raw data for this study is deposited on Mendeley and can be found at https://data.mendeley.com/drafts/g97f9nhbyr
• EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Cell line
Human Cell Line SH-SY5Y cells were purchased from the American Type Culture Collection (ATCC; cat#: CRL-2266, https://www.atcc.org/Products/All/CRL-2266.aspx). Human H9 ES cells (WA09, NIHhESC-10–0062) were obtained from WiCell. Human ES cells were maintained on feeder cells (cell cycle arrested mouse embryonic fibroblasts) at a density of 2–2.5×104 cells/cm2 in a 37°C, 5% CO2 incubator. The cells were fed with human ES cell complete media [DMEM:F12, 4 ng/ml basic FGF, 20% knockout serum replacement (KSR), 0.1 mM non-essential amino acids, 2 mM glutamine, and 0.1 mM β-Mercaptoethanol]. ES cells were then differentiated onto Matrigel® matrix coated (Corning) dishes (50 μl/cm2) and maintained with essential 8 media (Thermo Fisher). Media was changed daily, and cells were dissociated with accutase and re-plated at a density of 5×106 cells/well on the MEF feeder. After 24 hours of passaging, cells were treated with 10 μM Rho kinase–specific (ROCK) inhibitor (Y-27632, Sigma Aldrich).
Mouse primary neurons
Mouse cortical primary neurons were prepared as previously described (Seo et al., 2018). Briefly, primary cortical neurons were isolated from embryonic day 15 CD-1 mice. The isolated neurons were then plated onto poly-D-lysine coated dishes or coverslips and maintained in a 7% CO2 incubator at 37°C. The neuronal cells were maintained with neuronal culture medium consisting of Neurobasal media with B27 supplement and L-glutamine. After 5 days, 30 μM 5-fluoro-2’-deocyuridine was added to the cultures to inhibit the growth of glial cells.
Animal
All experimental procedures were according to the guidelines of Laboratory Animal Manual of the National Institute of Health Guide to the Care and Use of Animals and were approved by the Johns Hopkins Medical Institute Animal Care and Use Committee. Animals were housed in a 12 h dark/light cycle with free access to water and food. Randomized mixed-gender cohorts were used for all animal experiments. All mice were acclimatized for 3 days in the procedure room before any experiments were started. We have taken great effort to reduce animal suffering from pain and discomfort. For the α-synuclein pre-formed fibril (α-syn PFFs) induced animal model, C57BL/6J mice were obtained from the Jackson Laboratories (ME, USA). Mice were mated with C57BL/6J mice for the present study. Mouse recombinant α-synuclein were purified and in vitro fibrils were assembled following a previously described method (Luk et al., 2012). 6 months post-injection, immunohistochemistry and biochemical studies were performed.
Human brain tissues
For the human post-mortem brain tissues in this research, the written informed consent approved by the Johns Hopkins Institutional Review Boards (Approval No. NA00032761) was provided to the patients. Human post-mortem brain tissues were obtained from brain donation programs of the Morris K. Udall Parkinson’s Disease Research Center of Excellence at Johns Hopkins Medical Institutions (JHMI) and the Alzheimer’s Disease Research Center (http://www.alzresearch.org) in compliance with local Institutional Review Board and HIPAA (Health Insurance Portability and Accountability Act) regulations.
• METHOD DETAILS
Plasmid constructions
A full-length TRIP12 was cloned into pcDNA3.1-V5 (Invitrogen) or p3×FLAG-CMV9 vector (Sigma), and a full-length GBA1 was cloned into pcDNA3.1-Myc/His vector (Invitrogen) for in vitro studies such as co-immunoprecipitation and mapping. For the mapping study, fragments of TRIP12 (F1; a.a.1-a.a.500, F2; a.a.501-a.a.1000, F3; a.a.1001-a.a.1500, F4; a.a.1501–1992), as well as the N-terminus (a.a.1-a.a.250) and C-terminus (a.a.251-a.a.536) of GCase were generated. The Tet-off inducible pYR human TRIP12 vector was kindly provided by Dr. Jong-Bok Yoon of Yonsei University of South Korea.
Production of TRIP12 Antibodies
The rabbit polyclonal TRIP12-antibody was generated by Covance with a peptide antigen of human TRIP12 (NCBI reference sequence: NP_004229.1). This peptide contains amino acids 1425–1440 [CKTSPRNAKKHDELWHD] and was generated from the C-terminal region. The antisera were collected from immunized rabbit and purified by affinity chromatography. The mouse monoclonal TRIP12 antibodies were generated by Abmart. The peptides containing amino acids 22–33 [CGAQPQDDSIGGR] or 45–56 [CQPEDPDRANTSE] were used for TRIP12 antibodies producing hybridoma. The mouse monoclonal antibodies were then purified by affinity chromatography.
GCase tandem affinity purification
A full length of the GCase fusion protein was generated by inserting a streptavidin-binding peptide (STP) and a calmodulin-binding peptide (CBP) into GCase-C-terminus expressing vector. The STP-and CBP-fused GCase vector were transfected into SH-SY5Y cells. After 48 hours, GCase was purified from the vector-transfected SH-SY5Y cells by the InterPlay mammalian tandem affinity purification (TAP) system following the manufacturer instruction. The TAP eluate was separated using 8–16% Tris-Gly SDS-PAGE and each SDS-PAGE gel line containing TAP or TAP-GCase interacting proteins was cut into 19 pieces for an increase of protein identification efficiency. The gel pieces were then washed as previously described (Kang et al., 2009), and the GCase interacting proteins were identified by the Nano-LC-ESI-(CID/EDT)-MS/MS analysis via high-capacity ion trap (HCT; Bruker Daltonics, Bremen, Germany). The MS/MS spectra were interpreted, and the peak lists were generated using DataAnlysis 4.0 (Bruker Daltonics, Bremen, Germany). The searches were performed using MASCOT v2.3.02 (Matrix Science) against the UniProtKB/Swiss-Prot database (version 57.14) for protein identification.
Co-immunoprecipitation (Co-IP)
For the co-IP of SH-SY5Y cells, the cells were transfected with 2 μg of V5-TRIP12 and MYC-GCase plasmids. 48 hours later, SH-SY5Y cells were washed twice with cold-PBS and resuspended in immunoprecipitation buffer (IP buffer) containing 1% Triton X-100 and protease inhibitor cocktail in PBS. The supernatants were isolated by centrifugation at 12,000×g at 4°C for 30 mins and then mixed with 50 ml of Protein G Dynabeads that had been pre-incubated with anti-V5 or anti-MYC antibodies. The mixtures were incubated at 4°C overnight in the mixing rotator and then the Protein G Dynabeads were spun down and washed three times with 500 mM NaCl containing IP buffer, followed by three washes with cold-PBS. The isolated proteins were separated by SDS-PAGE and then transferred to a nitrocellulose membrane.
For the co-IP of endogenous proteins from mouse or adult human brain, the samples were homogenized with a tissue lysis buffer consisting of 10 mM Tris-HCl, pH 7.4, 5 mM EDATA, 150 mM NaCl, 10 mM Na-β-glycerophosphate, 0.5% Nonidet P-40, Phosphatase Inhibitor Cocktail I and II, and Complete Protease Inhibitor Mixture. The homogenates were then centrifuged at 12,000×g at 4°C for 30 mins and mixed with Protein G Dynabeads that were pre-incubated with anti-IgG, GCase, or TRIP12. After rotating at 4°C overnight, the Protein G Dynabeads mixtures were pelleted and washed three times with IP-buffer. The isolated proteins were then resolved using SDS-PAGE and transferred to a nitrocellulose membrane. For mapping the binding between TRIP12 and GCase, SH-SY5Y cells were transfected for 48 hours with a full-length GBA1 plasmid and fragmented TRIP12 plasmids, and full-length TRIP12 plasmid and the N-terminus or C-terminus plasmid of GCase. SH-SY5Y cells were then harvested in IP-buffer. The supernatants were then mixed with 50 ml of Protein G Dynabeads that were pre-incubated with anti-FLAG or anti-MYC antibodies. After incubation overnight, proteins were isolated from Protein G Dynabead mixtures and resolved using 8–16% SDS-PAGE.
Generation and validation of GBA1 and TRIP12 knockout SH-SY5Y cell lines
The guide RNA sequences that target exon 3 of human GBA1 was cloned into the LentiCRISPR vector (pXPR_001, plasmid#: 49535, addgene). The single guide RNA sequence 5’-TACACGCAGTGGGCGACGGA-3’ was used for producing the CRISPR lentivirus. SH-SY5Y cells were transduced with GBA1-CRISPR lentivirus to generate the GBA1 knockout. The transduced SH-SY5Y cells were then selected with 1 μg/ml of puromycin (Life Technologies) for 3 days and the single colonies were then isolated using cloning discs (Sigma, Z374431). After 3 weeks, the GBA1 and GCase expression of each colony were validated by real-time RT-PCR and western blotting. For the generation of TRIP12 knockout SH-SY5Y cells, the TRIP12 CRISPR/CAS9 knockout and TRIP12 homology-directed repair (HDR) plasmids were purchased from SantaCruz and transfected into SH-SY5Y cells following manufacturer instructions. After the selection of single colonies, the TRIP12 knockout was validated by real-time RT-PCR and western blotting.
Purification of recombinant TRIP12 protein
A full-length human TRIP12 DNA (accession number D28476) was sub-cloned into a modified baculovirus expression vector, pFasfBac-PS2H (Invitrogen), containing multi-tags flanked by PreScission, StreptacII, and octa-histidine tag at the C-terminus. A baculovirus expression vector system (Invitrogen) was employed for recombinant protein expression in insect cells. General methods for protein purification were described in previous literature (Park et al., 2009). Briefly, High-Five cells were grown to a density of 2.5×106 cells/ml in SFX-Insect media (HyClone) with 100 units/ml of penicillin (100 units/ml), and 100 μg/ml of streptomycin before viral infection. 65 hours post-infection, the cells were harvested using a low-speed centrifugation at 2500 rpm, then were lysed in 30 mL of ice-cold lysis buffer containing 40 mM Hepes pH 7.5, 150 mM NaCl, 3 mM β-mercaptoethanol, 0.5% (v/v) Triton X-100, 10% (v/v) Glycerol, DNase, 5 mM MgCl2, 1 mM PMSF, and protease inhibitor cocktail (Roche, EDTA-free). Cell disruption continued with sonication at amplitude 10% for 10 mins and subsequent dounce homogenization. The cell lysate was cleared twice by centrifuging at 50,000×g for 30 mins at 4°C. The supernatant was incubated for 30 mins by rotating at 4°C for batch binding to TALON Metal Affinity Resin (Clontech) which was prepared by equilibrating in buffer containing 40 mM Hepes pH 7.5, 150 mM NaCl, 3 mM β-mercaptoethanol, 0.5% (v/v) Triton X-100, and 10% (v/v) Glycerol. Additional purification was achieved by applying protein-bound beads onto a gravity column by washing with both 10 column volumes of equilibration and wash buffer (equilibration buffer containing 20 mM Imidazole). The protein was eluted with buffer containing 300 mM imidazole and then was analyzed by SDS-PAGE and MS spectrometry.
GST-pull down assay
For in vitro protein-protein interaction assays, 0.5 μg of GST or GST-GBA were incubated for 1 hour at 4°C with 20 μl of glutathione-sepharose beads. After washing, GST or GST-GBA conjugated beads were re-suspended in 100 ml of binding buffer (20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS) with the protease inhibitor cocktail (Roche). The beads were then incubated for 2 hours at 4°C with the His-TRIP12 protein. After an extensive washing step, retained proteins were boiled in SDS protein loading buffer for elution. The samples were then immunoblotted using anti-GST and anti-TRIP12 antibodies for detection of protein-protein interaction.
In vivo ubiquitination assay
SH-SY5Y cells were transfected with 4 mg of FLAG-tagged WT TRIP12 or C1959A TRIP12, MYC-tagged GCase and 4 mg of pRK5-HA-ubiquitin plasmids for 48 hours. After washing with cold-PBS, total cell lysates were prepared and pellets were solubilized with 200 μl of RIPA buffer consisting of 0.05% SDS and protease inhibitor cocktail. The washed samples were used as input for immunoprecipitation. The samples were immunoprecipitated and the precipitates were used for immunoblotting with anti-HA, anti-MYC, or anti-FLAG antibodies.
In vitro ubiquitination assay
5 ng of GCase-GST was mixed with 10 ng of E1 (10 ng), 100 ng of E2 (His-UbcH5a, 100 ng), and 5mg of E3 ligase TRIP12 in 10 μl of reaction buffer consisting of 40 mM Tris-HCl pH 7.6, 2 mM ATP, 5 mM MgCl2, and 2 mM DTT. After 1 hour of reaction at 37°C, the samples were subsequently resolved on 8–16% Tris-Gly SDS-PAGE gels for western blot analysis. To identify the ubiquitination sites of GCase by TRIP12, the in vitro ubiquitination assay was performed with the same reaction conditions and the end product was sent to Taplin Biological Mass Spectrometry Facility (Harvard Medical School). From the mass spectrometric analysis, 5 specific ubiquitination sites were identified on GCase, shown in Supplemental Table 2.
TUBE pulldown assay
For detection of ubiquitinated endogenous GCase, MG132 1 μM was treated in each cell lines (SH-SY5Y with Mock, TRIP12 knockout SH-SY5Y, and TRIP12 overexpressing SH-SY5Y) 24h before TUBE pulldown. The cells in a 100-mm dish (5×106 cells/dish) were washed 3 times in PBS and lysed in the lysis buffer (150 mM NaCl, 50 mM Tris pH 7.5, 10% glycerol, 1% NP-40, 1 mM EDTA with 6M Urea) with protease and phosphatase inhibitors and 100 mM n-ethylmaleimide (NEM). To enrich for K48- and K63-linked ubiquitinated proteins, lysates were clarified by centrifugation at 14000×g for 10 min and incubated with K48 or K63 linkage specific agarose-TUBE (Lifesensor; UM-0414–4800, UM-0413–6300) at 4°C for overnight. Beads were collected and washed 3 times in lysis buffer without Urea and eluted with elution buffer. The elutes were split into two equal volumes. The first part is added with 2 μL of 10 μM broad spectrum DUB and the other half was added with 2 μL of buffer followed by incubation at 30°C for 1 hours. The reaction is stopped by the addition of SDS-Sample buffer. For detection of ubiquitinated GCase according to increasing amount of TRIP12 expression, Tet off-inducible FLAG-TRIP12 SH-SY5Y cells were transfected with MYC-GCase WT or MYC-GCase K293R in presence or absence of MG132. FLAG-TRIP12 was expressed by withdrawal of doxycycline (DOX). In indicated time point (0, 12h, 24h, 48h), TUBE pulldown assay was conducted as above mentioned.
Site-directed mutagenesis
A full-length GBA1 was cloned into the pcDNA3.1-Myc/His vector (Invitrogen) and GCase mutants K155R, K157R, K198R, K293R, and K408R were created using the QuikChange Lightning site-directed mutagenesis (SDM) kit (Agilent Technologies). The sequences were confirmed by automated DNA sequencing.
In situ proximity ligation assay
SH-SY5Y cells or primary cultured neurons were seeded onto 12 mm poly-D-lysine-coated coverslip in a 12-well plate (5×104 cells/well). The cells were then stained with ER-tracker for visualizing ER structure and fixed with 4% PFA for 10 mins. The fixed cells were then used for the in situ proximity ligation assay following the manufacturer instructions. Briefly, cells were blocked with a provided blocking buffer and incubated with primary antibodies at 4°C for 12 hours. The Minus- or Plus- probe conjugated secondary antibodies were then added and incubated at 37°C for 1 hour. After incubation, the ligation mix was added to each coverslip and incubated at 37°C for another 30 mins. The signals were then amplified by the addition of amplification-polymerase containing reaction solution. The coverslips were mounted with DAPI-containing mounting medium and the images were captured by LSM710 confocal microscopy. The positive signals were measured using ImageJ software.
Fractionation of subcellular organelles from SH-SY5Y cells
The iodixanol-based iso-osmotic density gradient-based subcellular organelle fractionation procedure was developed according to the instructions of the kit manufacturer (OptiPrep, Sigma #D1556), and optimized for 5 ml volume scale. Briefly, homogenized cell lysates were centrifuged for 10 min at 3,000×g, and the pellet was discarded. The supernatant was then centrifuged for 1 h at 100,000×g to remove cytosolic contamination. The resulting second pellet was applied to the top of an OptiPrep discontinuous iodixanol gradient formed by the stepwise addition of solutions with increasing percentages of iodixanol (diluted in PBS). The 5 ml volume gradient was formed by adding 0.385 ml of 2.5%, 0.77 ml of 5%, 0.77 ml of 7.5%, 0.77 ml of 10%, 0.192 ml of 12.5%, 0.77 ml of 15%, 0.192 ml of 17.5%, 0.192 ml of 20%, and 0.192 ml of 30% iodixanol solutions to the bottom of a 5 ml Beckman centrifuge tube as described previously (Seo et al., 2018). To separate effectively ER and Lysosome fraction as described previously (Kawaguchi et al., 2016). The tube was centrifugated for 3 h at 178,000×g using Beckman Optima centrifuge with a SW55i rotor. The 15 fractions were collected from the top of the column, depending on the experimental condition. Proteins in the fraction sets were resolved by SDS-PAGE, followed by western blot analyses with antibodies against the proteins of interest, including organelle markers (anti-calreticulin, 1:1000, 2891, Cell signaling; anti-LAMP1, 1:1000, 3243, Cell signaling; anti-LIMP2, 1:1000, 703037, Thermo; anti-GBA1, 1:1000, G4171, Sigma-Aldrich; anti-TRIP12, 1:1000, this paper; anti-FLAG, 1:1000, F1804, Sigma-Aldrich)
GCase overexpressing SH-SY5Y cells generation.
To prepare the GCase overexpressing SH-SY5Y cells, mock SH-SY5Y cells were transfected with pcDNA3.1-myc-WT-GBA1 plasmids using Lipofectamine Plus according to the manufacturer instructions. 48 hours post-transfection, transfected cells were selected using a selection medium containing 700 μg/ml of geneticin (G418: Invitrogen). The individual colonies were then isolated using cloning discs (Sigma). GCase overexpression was confirmed by western blot and GCase activity assay.
Glucocerebrosidase activity assay
The activity of GCase was measured as previously described (Mazzulli et al., 2011). Briefly, the mouse ventral midbrain tissues were homogenized in buffer consisting of 10 mM HEPES pH 7.4, 0.1 M EDTA, and 0.25 M sucrose. After centrifuging at 6,800×g, 4°C for 5 min, the supernatant was collected and centrifuged at 17,000×g for 10 mins. The lysosome-enriched pellet was then collected in 50 μl of GCase activity assay buffer (0.25% Taurocholic acid, 1 mM EDTA, 0.25% Triton X-100, in citrate/phosphate buffer, pH 5.4). For measuring the GCase activity, 50 μl of 1 mM 4-Methylumbelliferyl b-glucopyranoside (4-MU) and/or 10 mM conduritol B epoxide with 1% BSA was added. After 40 mins at 37°C, the reaction was terminated by addition of 50 μl of 1M glycine, pH 12.5. The fluorescence was analyzed using a Perkin Elmer plate reader (ex = 355 nm, em = 460 nm, 0.1 s). The GCase activity was calculated by subtracting the activity in the presence of GCase inhibitor conduritol B epoxide. We confirmed that about 95–97% of GCase activity was reduced by conduritol B epoxide treatment.
Real-time RT-PCR
Total RNA was isolated from human substantia nigra post-mortem brain and the mouse ventral midbrain using RNeasy® Plus Micro Kit (Qiagen). The first-strand cDNA was then synthesized with SuperScript® IV First-Strand Synthesis System (Invitrogen). The real-time PCR was performed with SYBR Green reagent by a ViiA™ 7 real-time PCR system. The 2−ΔΔCT method (Livak and Schmittgen, 2001) was used for calculating the values. All ΔCT values were normalized to GAPDH. The primer sequences used for real-time PCR were included in the key resources table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-TRIP12 | This paper | N/A |
| Rabbit polyclonal anti-TRIP12 | This paper | N/A |
| Mouse monoclonal anti-alpha-synuclein phosphor (Ser129) | BioLegend | Cat#; 825701; RRID: AB_2564891 |
| Rabbit monoclonal anti-alpha-synuclein phosphor (Ser129) | abcam | Cat#; ab168381; RRID: AB_2728613 |
| Mouse monoclonal anti-alpha-synuclein | BD Biosciences | Cat#; 610787; RRID: AB_398108 |
| Rabbit polyclonal anti-alpha-synuclein | Santa Cruz Biotechnology | Cat#; sc-7011; RRID: AB_2192953 |
| Rabbit monoclonal anti-alpha-synuclein filament | abcam | Cat#; ab209538; RRID: AB_2714215 |
| Rabbit monoclonal anti-APG5L/ATG5 | abcam | Cat#; ab108327; RRID: AB_2650499 |
| Mouse monoclonal anti-beta-actin-peroxidase | Sigma-Aldrich | Cat#; A3854; RRID: AB_262011 |
| Mouse monoclonal anti-GBA1 | abcam | Cat#; ab55080; RRID: AB_2109076 |
| Rabbit polyclonal anti-GBA1 | abcam | Cat#; ab92997; RRID: AB_10712688 |
| Rabbit polyclonal anti-GBA1 | Sigma-Aldrich | Cat#; G4171; RRID: AB_1078958 |
| Rabbit polyclonal anti-GlcCer | Cedarlane | Cat#; RAS0011 |
| Rabbit polyclonal anti-GFAP | Dako | Cat#; Z0334; RRID: AB_10013382 |
| Rabbit polyclonal anti-Iba-1 | Wako | Cat#; 019–19741; RRID: AB_839504 |
| Rabbit polyclonal anti-MAP2 | Millipore | Cat#; AB5622; RRID: AB_91939 |
| Rabbit polyclonal anti-Nurr1 | ThermoFisher Scientific | Cat#; PA5–13416; RRID: AB_2153896 |
| Goat polyclonal anti-PITX3 | Santa Cruz Biotechnology | Cat#; sc-19307; RRID: AB_2165313 |
| Mouse monoclonal anti-HNF-3 (FOXA2) | Santa Cruz Biotechnology | Cat#; sc-374375; RRID: AB_10989476 |
| Mouse monoclonal anti-Tyrosine hydroxylase | Sigma-Aldrich | Cat#; T2928; RRID: AB_477569 |
| Rabbit polyclonal anti-Tyrosine hydroxylase | Novus | Cat#; NB300–109; RRID: AB_10077691 |
| Mouse monoclonal anti-Tyrosine hydroxylase | Millipore | Cat#; AMAB91112; RRID: AB_2665805 |
| Mouse monoclonal anti-Tubulin β 3 (Tuj1) | BioLegend | Cat#; MMS-453P |
| Mouse monoclonal anti-Ubiquitin | ThermoFisher Scientific | Cat#; 13–1600; RRID: AB_86560 |
| Rabbit polyclonal anti-Ubiquitin | Dako | Cat#; Z0458; RRID: AB_2315524 |
| Rabbit monoclonal anti-Ubiquitin, Lys48-specific | Millipore | Cat#; 05–1307; RRID: AB_1587578 |
| Rabbit monoclonal anti-Ubiquitin, Lys63-specific | Millipore | Cat#; 05–1308; RRID: AB_1587580 |
| Rat monoclonal anti-Dopamine transporter | Millipore | Cat#; MAB369; RRID: AB_2190413 |
| Rabbit polyclonal anti-LC3B | Cell signaling Technology | Cat#; 2775; RRID: AB_915950 |
| Rabbit polyclonal anti-SQSTM1/p62 | abcam | Cat#; ab91526; RRID: AB_2050336 |
| Rabbit monoclonal anti-Calnexin | Cell signaling Technology | Cat#; 2679; RRID: AB_2228381 |
| Mouse monoclonal anti-grp78 | Santa Cruz Biotechnology | Cat#; sc-376768; RRID: AB_2819145 |
| Mouse monoclonal anti-TRAP1/hsp75 | BD Biosciences | Cat#; 612344; RRID: AB_399710 |
| Rabbit polyclonal anti-PINK1 | Cell signaling Technology | Cat#; 6946; RRID: AB_11179069 |
| Mouse monoclonal anti-c-myc | Sigma-Aldrich | Cat#; 11667203001; RRID: AB_390911 |
| Mouse monoclonal anti-V5 | Sigma-Aldrich | Cat#; V8012; RRID: AB_261888 |
| Mouse monoclonal anti-FLAG | Sigma-Aldrich | Cat#; F1804; RRID: AB_262044 |
| Rabbit monoclonal anti-LIMP2 | ThermoFisher Scientific | Cat#; 703037; RRID: AB_2734813 |
| Rabbit polyclonal anti-calreticulin | Cell signaling Technology | Cat#; 2891; RRID: AB_2275208 |
| Rabbit monoclonal anti-LAMP1 | Cell signaling Technology | Cat#; 3243; RRID: AB_2134478 |
| Mouse monoclonal anti-c-myc-Peroxidase | Sigma-Aldrich | Cat#; 11814150001; RRID: 390910 |
| Mouse monoclonal anti-V5-Peroxidase | ThermoFisher Scientific | Cat#; R961–25; RRID: AB_2556565 |
| Mouse monoclonal anti-FLAG-Peroxidase | Sigma-Aldrich | Cat#; A8592; RRID: AB_439702 |
| Mouse monoclonal anti-HA-Peroxidase | Sigma-Aldrich | Cat#; 11667475001; RRID: AB_514509 |
| Donkey polyclonal anti-mouse Alexa fluor 488 | Jackson ImmunoResearch | Cat#; 715–545–151; RRID: AB_2341099 |
| Donkey polyclonal anti-rabbit Alexa fluor 488 | Jackson ImmunoResearch | Cat#; 711–545–152; RRID: AB_2313584 |
| Donkey polyclonal anti-mouse CY3 | Jackson ImmunoResearch | Cat#; 715–165–151; RRID: AB_2315777 |
| Donkey polyclonal anti-rabbit CY3 | Jackson ImmunoResearch | Cat#; 711–165-152; RRID: AB_2307443 |
| Mouse IgG isotype control | ThermoFisher Scientific | Cat#; 10400C; RRID: AB_2532980 |
| Rabbit normal IgG control | Cell Signaling Technology | Cat#; 2729; RRID: AB_1031062 |
|
| ||
| Bacterial and Virus Strains | ||
|
| ||
| AAV5-GFP-U6-shRNA | Vector Biolabs | Cat#; 7042 |
| AAV5-GFP-U6-TRIP12-shRNA | Vector Biolabs | N/A |
| GIPZ-non-silencing lentiviral shRNA control | Dharmacon | Cat#; RHS4346 |
| GIPZ-lentiviral TRIP12-shRNA | Dharmacon | Cat#; RMM4532-EG14897 |
|
| ||
| Biological Samples | ||
|
| ||
| Human SN brain tissues | Johns Hopkins Medical Institutions Brain resource center | Table S3 |
|
| ||
| Chemicals, Peptides, and Recombinant Proteins | ||
|
| ||
| Carbonyl cyanide 3-chlorophenylhydrazone | Sigma-Aldrich | Cat#; C2759 |
| Cycloheximide | Sigma-Aldrich | Cat#; C4859 |
| 4-Methylumbelliferyl β-D-glucopyranoside | Sigma-Aldrich | Cat#; M3633 |
| Conduritol-β-epoxide | Sigma-Aldrich | Cat#; C5424 |
| AlarmarBlue Cell Viability Reagent | ThermoFisher Scientific | Cat#; DAL1100 |
| LysoTracker Green DND-26 | ThermoFishfer Scientific | Cat#; L7526 |
| ER-Tracker Green (Bodipy FL Glibenclamide) | ThermoFisher Scientific | Cat#; E34251 |
| MItoTracker Orange DMTMRos | ThermoFisher Scientific | Cat#; M7510 |
| MItoSox Red mitochondrial superoxide indicator | ThermoFisher Scientific | Cat#; M36008 |
| Intracellular pH Calibration buffer kit | ThermoFisher Scientific | Cat#; P35379 |
| CM-H2DCFDA (general oxidative stress indicator) | ThermoFisher Scientific | Cat#; C6827 |
| Recombinant human GBA protein | R&D systems | Cat#; 7410-GH-010 |
| GST-tagged human GBA protein | abnova | Cat#; H00002629-P01 |
|
| ||
| Critical Commercial Assays | ||
|
| ||
| Duolink in situ Oragne Starter Kit Mouse/Rabbit | Sigma-Aldrich | Cat#; DUO92102 |
| Pierce LDH cytotoxicity assay kit | ThermoFisher Scientific | Cat#; 88953 |
| Neurite Outgrowth Staining Kit | ThermoFisher Scientific | Cat#; A15001 |
| In situ cell death detection kit, Fluorescein | Sigma-Aldrich | Cat#; 11684795910 |
| QuikChange Lightning site-directed mutagenesis kit | Agilent Technologies | Cat#; 210518 |
| RNeasy Plus micro kit | Qiagen | Cat#; 74034 |
| SuperScript IV first-strand synthesis system | ThermoFisher Scientific | Cat#; 18091050 |
| Complex I enzyme activity microplate assay kit | abcam | Cat#; ab109721 |
| K48 Linkage Specific Ubitest – Agarose TUBE Elution Kit | LifeSensors | Cat#; UM-0414–4800 |
| K63 Linkage Specific Ubitest – Agarose TUBE Elution Kit | LifeSensors | Cat#; UM-0413–6300 |
|
| ||
| Experimental Models: Cell Lines | ||
|
| ||
| Human: SH-SY5Y cell line | ATCC | Cat#; CRL-2266; RRID: CVCL_0019 |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| Mouse: C57BL/6J | Jackson Laboratory | Cat#; 000664; RRID: IMSR_JAX:000664 |
| Mouse: B6.Cg-Tg(Prnp-SNCA*A53T) 23Mkle/J | Jackson Laboratory | Cat#; 006823; RRID: IMSR_JAX:006823 |
|
| ||
| Oligonucleotides | ||
|
| ||
| Primer: Human GAPDH Forward: AGG GTG GTG GAC CTC AT | Integrated DNA Technologies | Cat#; Hs.PT.58.589510.g |
| Primer: Human GAPDH Reverse: TGA GTG TGG CAG GGA CT | Integrated DNA Technologies | Cat#; Hs.PT.58.589510.g |
| Primer: Human GBA1 Forward: CAG CCT CAC AGG ATT GCT TCT | This paper | N/A |
| Primer: Human GBA1 Reverse: GAC ACA CAC CAC CGA GCT GTA | This paper | N/A |
| Primer: Mouse GAPDH Forward: AGG TCA TCC CAG AGC TGA ACG | This paper | N/A |
| Primer: Mouse GAPDH Reverse: CAC CCT GTT GCT GTA GCC CTA T | This paper | N/A |
| Primer: Mouse GBA1 Forward: ATC GAG GGA TGC AGT ACA GTC A | This paper | N/A |
| Primer: Mouse GBA1 Reverse: GGC CCT CCT TCA GGA TTC AG | This paper | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| pcDNA3.1-myc-human GBA1 | This paper | N/A |
| pcDNA3.1-myc-human GBA1 K155R | This paper | N/A |
| pcDNA3.1-myc-human GBA1 K157R | This paper | N/A |
| pcDNA3.1-myc-human GBA1 K198R | This paper | N/A |
| pcDNA3.1-myc-human GBA1 K293R | This paper | N/A |
| pcDNA3.1-myc-human GBA1 K408R | This paper | N/A |
| pcDNA3.1-myc-human GBA1 N-ter (a.a.1 -a.a.250) | This paper | N/A |
| pcDNA3.1-myc-human GBA1 C-ter (a.a.251-a.a.536) | This paper | N/A |
| pcDNA3.1-V5-human TRIP12 | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 C1959A | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 F1 (a.a.1-a.a.500) | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 F2 | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 F3 (a.a.1001-a.a.1500) | This paper | N/A |
| p3XFLAG-CMV-human TRIP12 F4 (a.a.1501-a.a.1992) | This paper | N/A |
|
Software and Algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Prism 6 | GraphPad software | https://www.graphpad.com/scientific-software/prism/ |
| ZEN lite | Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Endnote 20 | Clarivate™ | https://www.endnote.com/product-details |
Luciferase assay
Dual-reporter promoter clone was generated by GeneCopoeia with pEZX-PG4 plasmid vector (GeneCopoeia, MPRM13584-PG04). SH-SY5Y cells were plated at a density 5×106 cells/dish onto 10 cm dishes. The cells were then transfected with pEZX-PG04-TRIP12 promoter Gaussia luciferase/secreted alkaline phosphatase. After 48 hours, SH-SY5Y cells were re-plated at a density 1×104 cells/well in 96-well plate and then incubated with ER-stress inducing reagent such as 1 mM of thapsigargin or 1 μg/ml of tunicamycin for 24 or 48 hours. The TRIP12 promoter luciferase activity was measured with Dual Luminescence assay kit according to the manufacturer instructions (GeneCopoeia, SPDA-D010).
Promoter binding transcription factor profiling assay
A promoter binding transcription factor profiling plate array was performed according to the manufacturer’s instructions. Briefly, SH-SY5Y cells were plated at a density 5×106 cells/dish onto 10 cm dishes and then incubated with ER-stress inducing reagent such as 1 μM of thapsigargin or 1 μg/ml of tunicamycin for 48 hours. The nuclear extract was prepared and mixed with biotin-labeled 16 ER stress-related transcription factor probes with or without the TRIP12 promoter. The bound probes were detected using streptavidin-HRP conjugate after isolation from the transcription factor-DNA complex.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed according to the manufacturer’s instructions (Pierce, Magnetic ChIP kit). Briefly, SH-SY5Y cells were plated at a density 2×106 cells/dish onto 10 cm dishes and then incubated with ER-stress inducing reagent such as 1 mM of thapsigargin or 1 μg/ml of tunicamycin for 48 hours. The SH-SY5Y cells fixed with 1% formaldehyde for 10 min at 37°C and then the chromatin was digested with MNase, yielding DNA fragments of 150–1000 bps. The digested chromatin and pre-cleared ChIP grade protein A/G magnetic beads mixture samples were incubated with 10 mg of anti-ATF6 antibody, anti-RNA polymerase II antibody as a positive control, or anti-IgG as a negative control with rotation at 4°C for 6 hours. After reverse crosslink, the precipitated DNA was purified and subjected to PCR analysis with the primers to identify the ATF6 binding sequences. The primer information was provided in the KEY RESOURCE TABLE.
Lysotracker and Mitotracker staining
SH-SY5Y cells were transiently transfected with TRIP12 kinase domain mutant (TRIP12 C1959A), WT TRIP12, or both TRIP12 and GCase using Lipofectamine Plus (Invitrogen). 24 hours post-transfection, cells were plated at a density of 1×104 cells/18 mm onto glass coverslips and incubated in 5% CO2 at 37°C for 24 hours. For staining of lysosomes and mitochondria, MitoTracker Orange CMTMRos (Life technologies) and LysoTracker Green DND-26 were used according to manufacturer instructions. The mitochondrial aspect ratio, mitochondrial length, and the Lysotracker positive puncta number/cell were measured using ImageJ.
Reactive oxygen species production
SH-SY5Y cells were plated onto poly-D-lysine coated 6-well plates at a density of 5×105 cells/well. After 24 hours of incubation, the cells were then transfected with TRIP12 kinase domain mutant, TRIP12, or both WT TRIP12 and GCase. The ROS levels were measured using 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate acetyl ester (SM-H2DCFDA, Life technologies) 48 hours post-transfection. For mitochondrial ROS production, SH-SY5Y cells were plated onto poly-D-lysine coated 18 mm glass coverslip at a density of 1×104 cells/coverslip. The cells were then stained with MitoSox Red mitochondrial superoxide indicator following the manufacturer instructions. The MitoSox Red positive images were captured via a Zeiss confocal microscope (LSM710).
Measuring lysosomal pH
SH-SY5Y cells were transiently transfected with TRIP12 kinase domain mutant, WT TRIP12, or both WT TRIP12 and GCase. After 24 hours, cells were plated at a density of 1×104 cells/18 mm glass coverslip and incubated in 5% CO2 incubator at 37°C for 24 hours. SH-SY5Y cells were then labeled with 2 mM of LysoSensor Yellow/Blue DND-160 at 37°C for 30 mins. The fluorescence densities were measured with various pH solutions (Invitrogen). For the generation of pH standard curve, SH-SY5Y cells were stained with 2 mM LysoSensor Yellow/Blue DND-160 for 30 mins at 37°C and treated with 10 mM monesin and 10 mM nigericin in various pH calibration buffer (pH 4.5–7.5, life technologies) as described previously (Bankers-Fulbright et al., 2004). The fluorescence images were captured using an LSM 710 Zeiss confocal microscope.
Complex I activity assay
SH-SY5Y cells were plated at a density of 1×106 cells/dish onto poly-D-lysine coated 6 cm dishes. The cells were then transfected with TRIP12 kinase domain mutant, WT TRIP12, or both WT TRIP12 and GCase. Mitochondrial complex I enzyme activity was measured by complex I enzyme activity assay kit (Abcam) after 48 hours of transfection. Briefly, the proteins were prepared from transfected SH-SY5Y cells by adding 10% detergent containing PBS with 30 mins of incubation on ice. After centrifuging at 12,000×g for 20 mins, the supernatant was collected and adjusted the protein concentration to 5.5 mg/ml. The samples were then loaded on the microplate and incubated for 3 hours at RT. The plate was washed twice with buffer and incubated with 200 μl of assay solution. The values were measured at OD450 with 1-min interval for 30 mins.
8-OHG immunostaining.
Poly-D-lysine-coated 18 mm glass coverslips were employed to 12-well plate the mouse primary cortical neurons at a density of 5×104 cells/coverslip. To fix the neurons, 4% PFA was used and blocked for 1 hour in PBS solution containing 5% normal donkey serum (Cat#: 017–000-121; Jackson ImmunoResearch), 2% bovine serum albumin (Cat#: A7030; Sigma-Aldrich) and 0.1% Triton X-100 (Cat#: T8787; Sigma-Aldrich) at RT. Cells were incubated with anti-8-OHG (Cat#: ab62623; 1:1,000; abcam) overnight at 4°C. After washing the samples with 0.1% Triton X-100 in PBS, the coverslips were incubated with a mixture of Cy3-conjugated (Donkey anti-mouse CY3 secondary antibodies for 1 hour at RT. The coverslips were mounted with DAPI containing mounting solution. The fluorescence images were taken through a Zeiss confocal microscope (LSM 710, Zeiss Confocal).
Cell viability analysis.
The cytotoxicities of human dopaminergic neurons and primary cultured neurons were measured using AlamarBlue cell viability reagent and LDH cytotoxicity assay kit following the manufacturer instructions. For the neurite outgrowth with cell viability was also measured by the neurite outgrowth staining kit (Molecular Probes™), following the manufacturer instructions.
MTS assay
Cell proliferation was measured using the Cell-Titer 96® AQueous One Solution Cell Proliferation Assay kit (MTS, Promega #G3582). Tet off-inducible FLAG-TRIP12 SH-SY5Y cells (1×104 cells/well) were seeded into 96-well plates in triplicate in presence or absence of doxycycline (DOX). MTS solution (20 μl) was added to each well at 0, 12, 24, 48 h and, after two hours incubation, the cell proliferation rate was measured at 490nm.
Determination of oxygen consumption rate
The SH-SY5Y cells were transiently transfected with the TRIP12 kinase domain mutant, WT TRIP12, and both WT TRIP12 and GCase. After 24 hours of incubation, the cells were seeded at a density of 1×105 cells/well onto a Seahorse 24-well culture plate. After 24 hours the cells were then washed with warmed PBS and subsequently incubated with pre-warmed Seahorse assay medium at 37°C for 1 hour. After 45 min of incubation in a CO2-free incubator, the oxygen consumption rate (OCR) was measured using the XF24 Extracellular Flux Analyser (Seahorse Bioscience) with a 1-min mix, 1-min wait, and 2 min measurement at 37°C. Oligomycin, carbonyl cyanide m-chlorophenyllhydrazone (CCCP), and rotenone were sequentially injected into each well to assess basal respiration, the coupling of respiratory, and mitochondrial respiratory capacity. The measured OCRs were normalized by the protein concentration in each well.
Dot-blot assay
A nitrocellulose membrane, pre-wetted with tris-buffered saline, was loaded in the Bio-Dot microfiltration apparatus (Bio-Rad). 20 μg of the primary neuron or tissue lysates were then loaded onto the membrane under mild vacuum. After three washes with Tris-buffered saline, samples were blocked with tris-buffered saline containing 0.1% Tween-20 and 5% non-fat dry milk. Samples were then incubated with α-synuclein filament antibody (abcam, 1:5,000 dilution) at 4°C overnight. The membrane was incubated with HRP-conjugated rabbit secondary antibodies (GE Healthcare) for 1 hour at RT and the signals were visualized using chemiluminescence reagents (Thermo Scientific).
Design and expression of TRIP12 TALEN
Two pairs of TALENs and a donor vector for targeting the human TRIP12 gene (exon 3), were designed by Transposagen, Inc. To reduce a potential off-target effect, we modified the TALENs using a previously published protocol (Yan et al., 2013). A complete list sequences of target sites within the TRIP12 gene is provided in Figure S5. The designed TALENs were cleaved by KpnI and BamHI and ligated into the HBB-TALEN1-R/HBB-TALEN1-L vector. To perform negative selection by gancyclovir, a donor vector containing a puromycin deltaTK cassette was cleaved by SpeI and MfeI and ligated into the donor vector for targeting the human TRIP12 gene. Human embryonic stem cells were harvested as single cells using Stem Pro accutase. A total of 8×105 cells were used for nucleofection using the Lonza neurofection kit. 1ug of each TALEN was nucleofected and the cells were plated in one well of the 24-well plate with a MEF feeder layer along with a ROCK inhibitor. Once ES cell colonies were confluent, the cells were re-plated at a lower density on Matrigel® matrix-coated (Corning) dishes with ROCK inhibitor (Sigma Aldrich). 48 hours after plating, cells were selected with puromycin (300 ng/ml). 10–12 days later, individual colonies were picked for screening. Half of the colonies were used for PCR screening and half were used for expansion of the colonies.
Differentiation of human pluripotent stem cells into DA neurons
The human pluripotent stem cells were differentiated into DA neurons following a DA neuron induction protocol (Kriks et al., 2011). Briefly, the ES cell colonies and N370S GBA1-PD iPSCs were dissociated into a single-cell suspension by 10 mM of ROCK inhibitor Y-27632 containing accutase. The cells were then collected and seeded on Matrigel-coated 6-well plate at a density of 1.5×106 cells/well. Differentiation steps were based on exposure to 100 nM of LDN193189 (Stemgent) on days 0–11; 10 μM of SB431542 (Tocris) on days 0–5; 100 ng/ml of recombinant mouse sonic hedgehog (SHH, C25II; R&D), 2 μM of purmorphamine (STEMGENT) and 100 ng/ml of recombinant human/mouse FGF-8b (R&D) on days 1–7; and 3 mM of CHIR99021 (CHIR; Stemgent) on days 3–13. The cells were maintained on Matrigel with 10% KSR, MEM NEAA, 2 mM L-glutamine and 10 mM β-mercaptoethanol containing DMEM/F12 media for 11 days. KSR-containing DMEM/F12 medium was gradually shifted to N2 medium starting on day 5 of differentiation. On day 11, cells were switched to medium containing the following supplements: 20 ng/ml of brain-derived neurotrophic factor (BDNF; R&D), 0.2 mM of ascorbic acid (Sigma), 20 ng/ml of glial cell line-derived neurotrophic factor (GDNF; R&D), CHIR (until day 13), 1 ng/ml of transforming growth factor type β3 (TGFβ3; R&D), 0.5 mM of dibutyryl cAMP (dbcAMP; Sigma), and 10 mM of DAPT in Neurobasal/B27/L-glutamine containing medium (NB/B27; Invitrogen) for 9 days. On day 21, cells were dissociated using accutase and re-plated on poly-D-lysine and laminin-coated dishes with differentiation medium containing BDNF, ascorbic acid, GDNF, dbcAMP, and DAPT in NB/B27 medium.
Characterization of human DA neurons
When neuronal differentiation was initialized, ES cells and N370S GBA1-PD iPSCs were seeded at a high density of 1.5×106 cells/well. For the characterization of human DA neurons, the differentiated cells were then stained with the following specific markers: tyrosine hydroxylase (TH, dopaminergic neuronal marker, 1:2000), Tuj1 (differentiated neuronal marker, 1:2,000), Microtubule-associated protein 2 (MAP2, neuronal marker, 1:2,000), Nuclear receptor-related 1 protein (Nurr1, DA neuronal marker, 1:500), forkhead box protein A2 (FOXA2, midbrain neuronal marker, 1:500), PITX3 (motor neuron marker, 1:500) at DIV 60.
Production of lentivirus
For the preparation of TRIP12 shRNA lentivirus, GIPZ-non-silencing lentiviral shRNA control and GIPZ-lentiviral TRIP12-shRNA vectors were purchased from Dharmacon. The lentiviral vectors were transiently transfected into HEK293FT cells along with purchased viral packaging with lipofectamine LTX with plus transfection reagents (Invitrogen). 48 hours post-transfection, the infectious lentiviruses were collected from the supernatant, filtered, and concentrated via ultracentrifugation at 25,000×g for 3 hours. The concentrated viruses were re-suspended in 1% BSA in PBS, and then stored at −80°C until use.
α-synuclein PFFs generation
The human or mouse recombinant α-synuclein monomer was purified by following the instructions (Volpicelli-Daley et al., 2014). After purification steps including anion exchange and size exclusion chromatography, the α-synuclein fibrils were assembled by agitation using an Eppendorf orbital mixer at 1,000 rpm at 37°C for 7 days.
Preparation of tissue samples for immunoblot
The post-mortem tissues of human substantia nigra and the mouse brain samples were homogenized in tissue lysis buffer containing 150 mM NaCl, 5 mM EDTA, 10 mM Tris-HCl pH 7.4, Nonidet P-40, 10 mM Na-β-glycerophosphate, complete protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktail I and II (Sigma-Aldrich) as previously described (Ko et al., 2010; Ko et al., 2005). The lysates were then utilized to dilute in 2×Laemmli buffer (Bio-Rad). 20 μg of proteins were resolved on 8–16% gradient SDS-PAGE gels and transferred to nitrocellulose membranes. The nitrocellulose membrane was blocked with 5% non-fat dry milk in 0.1% Tween-20 containing Tris-buffered saline for 1 hour at RT. The membrane was then incubated with primary antibodies as follows: anti-GCase (1:1,000, G4171, Sigma), anti-TRIP12 (1:1,000, this paper), anti-α-synuclein (1:2,000, 610787, BD) antibodies at 4°C overnight. After three washes, the membranes were incubated with HRP-conjugated rabbit or mouse secondary antibodies (GE Healthcare) for 1 hour at RT. The signals were visualized with chemiluminescence reagents (Thermo Scientific). The membranes were then re-probed with HRP-conjugated b-actin antibody (Sigma).
Stereological assessment and immunohistochemistry
The C57BL6 mice were anesthetized with 60 mg/kg of pentobarbital solution at 8–10 weeks of age. The anesthetized mice were then stereotaxically injected into one hemisphere of the striatum with a condonation of +0.2 mm relative to Bregma, +2.6 mm beneath the dura, +2.0 mm from the midline. Mice were injected with 2 μl of PBS or α-syn PFFs (5 μg/2 μl) containing AAV5-shRNA TRIP12 virus (1010 genome copies: GC) or AAV5-scramble shRNA (1010 GC). The mice were perfused with PBS followed by 5% PFA after 6 months of injection. The brain samples were then collected and postfixed with 4% PFA for 12 hours and cryoprotected with 30% sucrose for 48 hours. The brains were cut to 40 μm coronal sections throughout the brain including the SNpc region. Every 4th section was used for stereological counting of TH positive cells in the SNpc. The tissues were incubated with anti-pS129-α-synuclein and anti-TH antibodies. After incubation with secondary antibodies and streptavidin-conjugated HRP, the positive signals were visualized by DAB kit. The tissues were then counterstained with thionin for Nissl structure. The total number of Nissl-positive and TH-positive neurons were measured by the Optical Fractionator probe of Stereo Investigator software. For pathological α-synuclein mapping, inclusions and neurites of phosphor-Ser129-α-synuclein positive signals were observed with 20×magnification at approximately +1.3, +0.26, −1.75, −3.0 mm relative to the Bregma. For immunohistochemistry analysis of human brain, all human brain samples were obtained with proper agreement given before collection of tissue and from the Department of Pathology, Johns Hopkins University School of Medicine (see Table S3). The paraffin-embedded human post-mortem tissue blocks was performed on 10-μm thick sections. The paraffin was removed from tissue samples by consecutive washes with xylene. The 100% ethanol was used to rehydrate xylene-free brain sections. In DAB staining for TRIP12 or p-S129 α-syn, incubations with primary antibodies diluted in PBST were overnight at 4°C (anti-p-S129 α-syn, 1:500, 825701, BioLegend; anti-TRIP12, 1:500, this paper), followed by incubation with biotin-conjugated anti-rabbit or mouse antibody and ABC reagents. Sections were developed using SigmaFast DAB Peroxidase Substrate (D4418, Sigma-Aldrich). In Immunofluorescence double labelling for TH and TRIP12, incubations with primary antibodies diluted in PBST were overnight at 4°C (anti-TH, 1:500, AMAB91112, Sigma; anti-TRIP12, 1:500, this paper). Detection was carried out with appropriate Alexa fluorescent secondaries for 1h at room temperature (Alexa Fluor 488 and 647, 1:1000, Invitrogen).
HPLC analysis
To measure biogenic amine concentrations, the high-performance liquid chromatography with electrochemical detection (HPLC-ECD) was analyzed. Briefly, animals were sacrificed by decapitation and the striatum region was isolated. The striatal tissues were then weighed and homogenized in 0.2 ml of cold-homogenization buffer consisting of 0.01 mM perchloric acid, 0.01% EDTA and 60 ng of 3,4-dihydroxybenzylamine (DHBA) as an internal standard. The homogenized samples were sonicated and centrifuged at 15,000×g for 30 min. After filtering through a 0.2 mm filter, 20 μl of supernatant was analyzed in the HPLC column (4.6 mm × 150 mm C-18 reverse-phase column, MC Medical, Tokyo, Japan) using a dual-channel coulchem III electrochemical detector (Model 5300, ESA, Inc Chelmsford, MA, USA). The measured values were normalized using the protein concentration of tissue homogenates and reported as ng/mg protein.
Behavioral analysis.
For the rotarod test, the animals were trained to acclimate for three days with four 5 min trails. The animals were then placed on the rotarod with specific conditions that increase the speed from 4 rpm to 40 rpm in 300 secs. The latency to fall off was monitored and recorded. For the pole test, the animals were trained three test trials/day for 2 days before the actual test. The pole is a 75 cm metal rod with a diameter of 9mm. The animals were then placed with the head facing up towards the top of the pole. The total taken time to reach the bottom of the pole was measured. 120 secs were the maximum cut-off time to stop the test. All behavioral tests were performed blind.
• QUANTIFICATION AND STATISTICAL ANALYSIS
Data were presented as mean ± SEM from at least 3 independent experiments. To assess statistical significance, Student’s t-tests or ANOVA tests followed by Bonferroni post hoc analysis were performed using GraphPad Prism 9 software. Assessments with p <0.05 were considered significant.
Supplementary Material
Highlights.
GCase is a substrate of E3 ligase TRIP12
TRIP12 accumulates in α-synuclein-based mouse models and sporadic PD brains
TRIP12 ubiquitination of GCase at K293 contributes to mitochondrial dysfunction
TRIP12 depletion protects α-synuclein PFFs-induced pathology both in vitro and in vivo
ACKNOWLEDGMENTS
This work was supported by grants from the NIH/NINDS NS38377 Morris K. Udall Parkinson’s Disease Research Center, NIH/NINDS NS082205, NS098006, NS107404, the JPB Foundation, the Maryland Stem Cell Research Foundation 2012-MSCRFE-0059 and the National Research Foundation of Korea grants funded by the Ministry of Science and ICT (NRF-2016R1A5A2007009). The authors acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation and the Diana Helis Henry Medical Research Foundation through their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Program M-2014, H-1, H-2013. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Disease. The authors acknowledge J.P. Hostetler for assisting in manuscript completion and revision.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bankers-Fulbright JL, Kephart GM, Bartemes KR, Kita H, and O’Grady SM (2004). Platelet-activating factor stimulates cytoplasmic alkalinization and granule acidification in human eosinophils. J Cell Sci 117, 5749–5757. 10.1242/jcs.01498. [DOI] [PubMed] [Google Scholar]
- Bendikov-Bar I, and Horowitz M (2012). Gaucher disease paradigm: from ERAD to comorbidity. Hum Mutat 33, 1398–1407. 10.1002/humu.22124. [DOI] [PubMed] [Google Scholar]
- Bendikov-Bar I, Ron I, Filocamo M, and Horowitz M (2011). Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis 46, 4–10. 10.1016/j.bcmd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Bramswig NC, Ludecke HJ, Pettersson M, Albrecht B, Bernier RA, Cremer K, Eichler EE, Falkenstein D, Gerdts J, Jansen S, et al. (2017). Identification of new TRIP12 variants and detailed clinical evaluation of individuals with non-syndromic intellectual disability with or without autism. Hum Genet 136, 179–192. 10.1007/s00439-016-1743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet M, Vargas C, Larrieu D, Torrisani J, and Dufresne M (2020). E3 Ubiquitin Ligase TRIP12: Regulation, Structure, and Physiopathological Functions. Int J Mol Sci 21. 10.3390/ijms21228515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Shan J, Zhu WG, Qin J, and Gu W (2010). Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature 464, 624–627. 10.1038/nature08820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, and Schapira AH (2013). Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int 62, 1–7. 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, and Serrano M (2010). The TRIP from ULF to ARF. Cancer Cell 17, 317–318. 10.1016/j.ccr.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Do J, McKinney C, Sharma P, and Sidransky E (2019). Glucocerebrosidase and its relevance to Parkinson disease. Mol Neurodegener 14, 36. 10.1186/s13024-019-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du TT, Wang L, Duan CL, Lu LL, Zhang JL, Gao G, Qiu XB, Wang XM, and Yang H (2015). GBA deficiency promotes SNCA/alpha-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy 11, 1803–1820. 10.1080/15548627.2015.1086055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N, Yamamoto K, Inoue H, Hikawa R, Nishi K, Mori K, and Takahashi R (2011). The endoplasmic reticulum stress sensor, ATF6alpha, protects against neurotoxin-induced dopaminergic neuronal death. J Biol Chem 286, 7947–7957. 10.1074/jbc.M110.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AH, Ginns EI, and Barranger JA (1985). Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem 260, 14319–14324. [PubMed] [Google Scholar]
- Esteves AR, Arduino DM, Silva DF, Oliveira CR, and Cardoso SM (2011). Mitochondrial Dysfunction: The Road to Alpha-Synuclein Oligomerization in PD. Parkinsons Dis 2011, 693761. 10.4061/2011/693761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, and Schapira AH (2012). Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol 72, 455–463. 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, and Schapira AH (2016). Mitochondrial dysfunction associated with glucocerebrosidase deficiency. Neurobiol Dis 90, 43–50. 10.1016/j.nbd.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Altmeyer M, Savic V, Toledo L, Dinant C, Grofte M, Bartkova J, Poulsen M, Oka Y, Bekker-Jensen S, et al. (2012). TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709. 10.1016/j.cell.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Hein MY, Hubner NC, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F, et al. (2015). A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723. 10.1016/j.cell.2015.09.053. [DOI] [PubMed] [Google Scholar]
- Hjerpe R, Aillet F, Lopitz-Otsoa F, Lang V, England P, and Rodriguez MS (2009). Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep 10, 1250–1258. 10.1038/embor.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiro M, Tsuchiya M, Kawabe Y, Furumai R, Iwasaki N, Hayashi Y, Katano M, Nakajima Y, Goto N, Watanabe T, et al. (2011). The E3 ubiquitin ligase activity of Trip12 is essential for mouse embryogenesis. PLoS One 6, e25871. 10.1371/journal.pone.0025871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SU, Fuchs K, Sieghart W, Pollak A, Csaszar E, and Lubec G (2009). Gel-based mass spectrometric analysis of a strongly hydrophobic GABAA-receptor subunit containing four transmembrane domains. Nat Protoc 4, 1093–1102. 10.1038/nprot.2009.92. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Okamoto T, Morita M, and Imanaka T (2016). Translocation of the ABC transporter ABCD4 from the endoplasmic reticulum to lysosomes requires the escort protein LMBD1. Sci Rep 6, 30183. 10.1038/srep30183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, Park MJ, Lee M, Choi S, Kwon SH, et al. (2018a). Graphene quantum dots prevent alpha-synucleinopathy in Parkinson’s disease. Nat Nanotechnol 13, 812–818. 10.1038/s41565-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yun SP, Lee S, Umanah GE, Bandaru VVR, Yin X, Rhee P, Karuppagounder SS, Kwon SH, Lee H, et al. (2018b). GBA1 deficiency negatively affects physiological alpha-synuclein tetramers and related multimers. Proc Natl Acad Sci U S A 115, 798–803. 10.1073/pnas.1700465115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HS, Lee Y, Shin JH, Karuppagounder SS, Gadad BS, Koleske AJ, Pletnikova O, Troncoso JC, Dawson VL, and Dawson TM (2010). Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci U S A 107, 16691–16696. 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HS, von Coelln R, Sriram SR, Kim SW, Chung KK, Pletnikova O, Troncoso J, Johnson B, Saffary R, Goh EL, et al. (2005). Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci 25, 7968–7978. 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551. 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Choi C, Lee YH, and Lee SJ (2002a). Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem 277, 5411–5417. 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- Lee JW, Ryan F, Swaffield JC, Johnston SA, and Moore DD (1995). Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature 374, 91–94. 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, and Price DL (2002b). Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 99, 8968–8973. 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Lombes A, Frachon P, and Rojo M (2002). Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell 13, 4343–4354. 10.1091/mbc.e02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ham A, Ma TC, Kuo SH, Kanter E, Kim D, Ko HS, Quan Y, Sardi SP, Li A, et al. (2019). Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy 15, 113–130. 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louie RJ, Friez MJ, Skinner C, Baraitser M, Clark RD, Schwartz CE, and Stevenson RE (2020). Clark-Baraitser syndrome is associated with a nonsense alteration in the autosomal gene TRIP12. Am J Med Genet A 182, 595–596. 10.1002/ajmg.a.61443. [DOI] [PubMed] [Google Scholar]
- Lu J, Yang C, Chen M, Ye DY, Lonser RR, Brady RO, and Zhuang Z (2011). Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc Natl Acad Sci U S A 108, 21200–21205. 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, and Lee VM (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al. (2016). Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353. 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor G, Filocamo M, and Horowitz M (2013a). ITCH regulates degradation of mutant glucocerebrosidase: implications to Gaucher disease. Hum Mol Genet 22, 1316–1327. 10.1093/hmg/dds535. [DOI] [PubMed] [Google Scholar]
- Maor G, Rencus-Lazar S, Filocamo M, Steller H, Segal D, and Horowitz M (2013b). Unfolded protein response in Gaucher disease: from human to Drosophila. Orphanet J Rare Dis 8, 140. 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, and Krainc D (2011). Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52. 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, and Halliday GM (2014). Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain 137, 834–848. 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, Morris CM, Theuns J, Crosiers D, Cras P, et al. (2013). A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70, 727–735. 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Stessman HA, Boyle EA, Witherspoon KT, Martin B, Lee C, Vives L, Baker C, Hiatt JB, Nickerson DA, et al. (2014). Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat Commun 5, 5595. 10.1038/ncomms6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AHV, and Duchen MR (2013). Mitochondria and quality control defects in a mouse model of Gaucher disease--links to Parkinson’s disease. Cell Metab 17, 941–953. 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski CM, and Urano F (2011). Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490, 71–92. 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Yoon SK, and Yoon JB (2009). The HECT domain of TRIP12 ubiquitinates substrates of the ubiquitin fusion degradation pathway. J Biol Chem 284, 1540–1549. 10.1074/jbc.M807554200. [DOI] [PubMed] [Google Scholar]
- Plotegher N, Perocheau D, Ferrazza R, Massaro G, Bhosale G, Zambon F, Rahim AA, Guella G, Waddington SN, Szabadkai G, and Duchen MR (2020). Impaired cellular bioenergetics caused by GBA1 depletion sensitizes neurons to calcium overload. Cell Death Differ 27, 1588–1603. 10.1038/s41418-019-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puspita L, Chung SY, and Shim JW (2017). Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10, 53. 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron I, Rapaport D, and Horowitz M (2010). Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum Mol Genet 19, 3771–3781. 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- Rothaug M, Zunke F, Mazzulli JR, Schweizer M, Altmeppen H, Lullmann-Rauch R, Kallemeijn WW, Gaspar P, Aerts JM, Glatzel M, et al. (2014). LIMP-2 expression is critical for beta-glucocerebrosidase activity and alpha-synuclein clearance. Proc Natl Acad Sci U S A 111, 15573–15578. 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BA, Cho T, Lee DZ, Lee JJ, Lee B, Kim SW, Shin HS, and Kang MG (2018). LARGE, an intellectual disability-associated protein, regulates AMPA-type glutamate receptor trafficking and memory. Proc Natl Acad Sci U S A 115, 7111–7116. 10.1073/pnas.1805060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, et al. (2009). Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361, 1651–1661. 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H (2017). Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol 11, 613–619. 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA, Lee Y, Kang HC, Lee BD, Lee YI, Bower A, Jiang H, Kang SU, Andrabi SA, Dawson VL, et al. (2015). Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci U S A 112, 11696–11701. 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Hamazaki J, Hirayama S, McBurney MW, Yashiroda H, and Murata S (2015). Sirt1-deficiency causes defective protein quality control. Sci Rep 5, 12613. 10.1038/srep12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B, and Sancar A (1987). Repair of N-methyl-N’-nitro-N-nitrosoguanidine-induced DNA damage by ABC excinuclease. J Bacteriol 169, 540–545. 10.1128/jb.169.2.540-545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, and Lee VM (2014). Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc 9, 2135–2146. 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, and Lee VM (2011). Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71. 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Smith C, and Cheng L (2013). Expanded activity of dimer nucleases by combining ZFN and TALEN for genome editing. Sci Rep 3, 2376. 10.1038/srep02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, and Goldstein JL (2000). ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6, 1355–1364. 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, et al. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24, 931–938. 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gambin T, Yuan B, Szafranski P, Rosenfeld JA, Balwi MA, Alswaid A, Al-Gazali L, Shamsi AMA, Komara M, et al. (2017). Haploinsufficiency of the E3 ubiquitin-protein ligase gene TRIP12 causes intellectual disability with or without autism spectrum disorders, speech delay, and dysmorphic features. Hum Genet 136, 377–386. 10.1007/s00439-017-1763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunke F, Andresen L, Wesseler S, Groth J, Arnold P, Rothaug M, Mazzulli JR, Krainc D, Blanz J, Saftig P, and Schwake M (2016). Characterization of the complex formed by beta-glucocerebrosidase and the lysosomal integral membrane protein type-2. Proc Natl Acad Sci U S A 113, 3791–3796. 10.1073/pnas.1514005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data for this study is deposited on Mendeley and can be found at https://data.mendeley.com/drafts/g97f9nhbyr