Abstract
Background:
Universal testing and treatment (UTT) for all persons living with HIV has only been assessed under experimental conditions in cluster-randomized trials. The public health effectiveness of UTT policies on the HIV care cascade under real-world conditions is not known.
Methods:
We used a regression discontinuity design (RDD) to assess the real-world effectiveness of Zambia’s adoption of universal HIV treatment on January 1, 2017. We used data from Zambia’s routine electronic health record to analyze antiretroviral therapy (ART)-naïve adults who newly enrolled in HIV care between January 1, 2016 and January 1, 2018 at 117 clinics supported by the Centre for Infectious Disease Research in Zambia (CIDRZ). We estimated the effects of implementing UTT on ART initiation and retention on ART at 12 months (defined as clinic attendance 9 to 15 months after enrollment and at least 6 months on ART), under the assumption that those presenting immediately before and after UTT implementation were balanced on both measured and unmeasured characteristics. We performed an instrumental variable (IV) analysis to estimate the effect of same-day ART initiation under routine conditions on 12-month retention.
Findings:
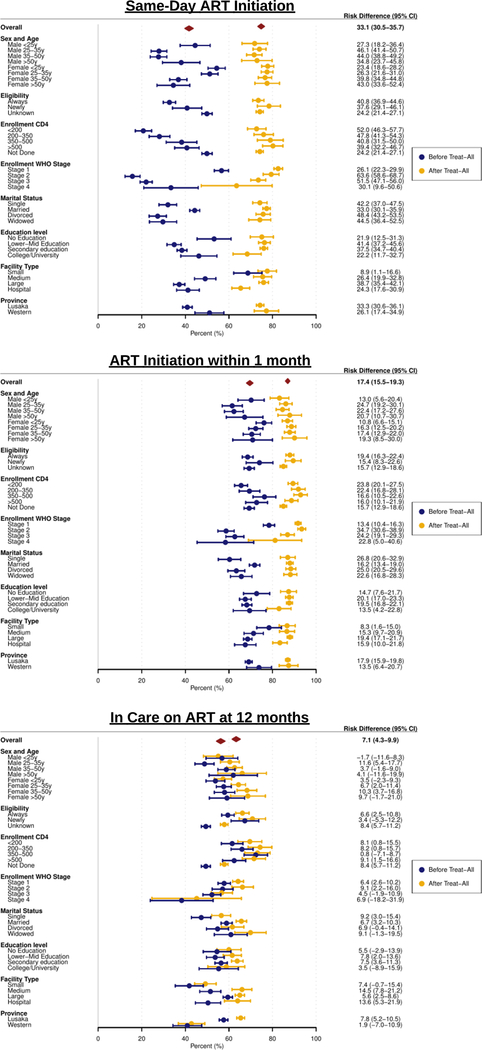
Among 65,673 newly enrolling HIV patients (62.2% female, median age 32 years [IQR 26–39], median CD4 287 cells/μL [IQR 147–466]), implementation of universal treatment increased same-day ART initiation from 41.7% to 74.8% (risk difference [RD] +33.1%, 95% CI 30.5–35.7%), ART initiation by 1 month from 69.6% to 87.0% (RD +17.4%, 95% CI 15.5–19.3%), and 12-month retention on ART from 56.2% to 63.3% (RD +7.1%, 95% CI 4.3–9.9%). ART initiation rates became more uniform across patient subgroups after implementation of universal treatment, but heterogeneity in 12-month retention on ART between them was unchanged. IV analyses indicated that same-day ART initiation in routine settings led to a 15.8% increase (95% CI 12.1–19.5%) in 12-month retention on ART.
Interpretation:
Implementing universal and rapid HIV treatment in Zambia substantially increased same-day and overall ART initiation, and also reduced disparities in treatment initiation among newly enrolling patients. UTT policies also led to modest improvements in retention in care, but disparities in 12-month retention remained largely unchanged. Natural experiments reveal both the anticipated and unanticipated impacts of real-world implementation and indicate the need for new strategies leveraging the short-term impacts of UTT to cultivate long-term treatment success.
Funding:
National Institutes of Health
Keywords: universal test and treat, UTT, treat-all, same-day ART, retention in care, implementing HIV guidelines, regression discontinuity, instrumental variable, natural experiment
INTRODUCTION
Universal testing and treatment (UTT) and rapid ART initiation are currently recommended by the WHO for all persons living with HIV (PLWH), and national governments across the world have adopted this approach as a cornerstone of their HIV control programs. Still, their overall effects on the success of HIV treatment programs are not well understood. Several large population-level UTT trials reported significantly improved outcomes across the HIV care cascade, but these experimental trials often included comprehensive service delivery packages incorporating interventions such as recurrent community-wide testing campaigns, linkage to care support, and enhancements to routine clinical care that are not typically present in real-world program delivery1,2. Existing real-world studies on universal and rapid ART have yielded indeterminate results, largely due to their smaller sample sizes and differences in methodologies3–11. Thus, despite widespread adoption, the impact of both universal and rapid treatment policies as implemented in real-world settings on both short- and long-term patient outcomes is inadequately understood.
Assessing the real-world impact of UTT on care cascade outcomes is essential for optimizing the next phase of the HIV treatment response. Previous research suggests that expanding treatment eligibility from CD4 levels of 350 to 500 increased overall retention12, but quantifying the additional gains from adopting so called “treat-all” is needed to understand the value and impact of these policies, particularly since national adoption may lead both intended and unintended consequences. For example, universal HIV treatment expands the pool of eligible patients, but also removes the need for additional steps to assess treatment eligibility, thereby simplifying and potentially improving access to ART initiation. Yet, concerns also exist that rapid ART initiation in routine settings could adversely affect patient retention if counseling is limited or of poor quality or there is decreased identification of people with advanced HIV13,14.
On January 1, 2017, Zambia implemented new national HIV treatment guidelines to adopt universal and rapid HIV treatment for all PLWH regardless of CD4 count or WHO stage15. We leveraged this policy change in a regression discontinuity design (RDD) to examine the impact of UTT as implemented in real-world settings on ART initiation and retention on ART at one year based on the assumption that patients presenting to care immediately before and after the policy change were comparable, and therefore exchangeable. We also used exposure to these new policies as an instrumental variable (IV) to estimate the effect of same-day ART initiation in routine settings on retention on ART. RD and IV methods assess causal effects based on plausible assumptions that make patients “as if” randomized and thereby offer both highly rigorous (i.e., internally valid) as well as relevant (i.e., externally valid) estimates. We use these “natural experiment” techniques to provide evidence on the impacts and gaps of real-world UTT policies on both immediate and 12-month patient outcomes in Zambia’s national treatment program.
METHODS:
Patient Population and Setting:
We analyzed ART naïve, adults (greater than 18 years old) who newly enrolled in HIV care up to one year before and after Zambia adopted universal treatment for all persons living with HIV (i.e., January 1, 2016 to January 1, 2018). Patients were from 117 clinics operated by the Zambian Ministry of Health with technical support from the Centre for Infectious Disease Research in Zambia (CIDRZ), a Zambian nongovernmental organization that supports HIV care delivery and research across two of the ten provinces in Zambia, and the CDC/PEPFAR initiative.
Prior to January 1, 2017, patients with a CD4 count below 500 cells/μL, WHO Clinical Stage 3 or 4, active tuberculosis (TB), or who were pregnant or breastfeeding women were eligible for treatment with ART. Eligible patients underwent three evaluation and counselling sessions (approximately every 1–2 weeks) prior to initiating ART approximately 4 weeks later; thereafter, visits were approximately every 3 months. Ineligible patients received follow-up every 6 months until they became eligible for ART. After Zambia began to implement universal HIV treatment policies on January 1, 2017, all persons living with HIV were immediately eligible for ART treatment. The new guidelines included a provision to consider same-day ART initiation in appropriate patients; counseling sessions occurred the day of enrollment but follow-up schedules were otherwise unchanged15.
Measurements:
Sociodemographic (e.g., age, sex, clinic site), clinical (e.g., enrollment CD4 count, WHO Stage, TB diagnoses, pregnancy, breastfeeding), facility-level (e.g., size), and visit history (e.g., HIV clinic enrollment date, ART initiation date, follow-up visits) measurements were obtained from the national electronic health record (EHR) and laboratory systems used in routine HIV care in Zambia (SmartCare). At the time of the study, the EHR was populated by providers first completing standardized paper clinical forms during patient encounters and trained CIDRZ data clerks then entering this information into the electronic database on an ongoing basis. Additionally, CIDRZ performed routine data quality audits and updates at least quarterly to ensure relatively high-quality data.
Statistical Analysis
We used an RDD to estimate the effect of implementing universal and rapid ART treatment in Zambia on three main outcomes from the time individuals newly enroll in care: (1) ART initiation on the day of enrollment (i.e., same-day ART initiation); (2) ART initiation within 1 month of enrollment; and (3) retention in care on ART at 12 months (defined as having made a clinic visit between 9- and 15-months post-enrollment and having been on ART for at least 6 months at that time). RDDs enable causal inferences when a treatment is partially or fully assigned by an arbitrary threshold value16,17. In this analysis, we used the date implementation of new guidelines began (i.e., January 1, 2017) as the threshold value, and compared outcomes in patients enrolling in care immediately before and after that date to estimate the effect of this policy change. The key underlying assumption is that individuals’ enrollment behaviors did not immediately change right at the time of guideline implementation, even though they may over longer periods of time. Under these conditions, patients enrolling immediately before and after guideline implementation would be similar on both observed and unobserved characteristics (i.e., exchangeable) and exposure to the new treatment guidelines is “as if random”16,17. Thus, comparing patients within a small window around guideline implementation (i.e., “locally”) can yield causal effect size estimates. To avoid bias from patients “crossing-over” and having outcomes influenced by exposure to practices both before and after guideline change, we excluded patients who enrolled 30 days or less before new guideline implementation in our analyses. Thus, all ART outcomes were determined under exposure to a single guideline period and we assume that the effect of new guidelines on retention is only mediated by individuals’ experience during first 30 days after enrollment. To allow for a transition period as the new guidelines were being fully implemented across all clinics, we also excluded patients who enrolled 90 days or less after the new guideline date. Lastly, for each outcome, we excluded patients who had EHR documentation of officially transferring out before the time at which that outcome was determined. After applying these restrictions, we performed standard assessments for violations of the underlying RD assumptions (appendix p 2).
We estimated the risk difference for each outcome right at the time of new guideline implementation using Poisson regression with robust variances that included an interaction between guideline change and time to allow for slope and intercept changes at the threshold16,17. We used the Imbens-Kalyanaram data-driven bandwidth algorithm to identify the optimal window around the threshold for each analysis18. This algorithm objectively identifies the largest window around the cutoff with an approximately linear relationship between time and outcome, thereby maximizing precision and minimizing bias from nonlinear relationships further away from the cutoff window18. We performed analyses in the overall population and in stratified subgroups based on sociodemographic and clinical characteristics. Our final models were unadjusted based on the assumption that patients were exchangeable on either side of the cutoff. We also assessed heterogeneity in the effects between clinics with at least 100 patients using mixed-effects models that included facility as a random effect (but otherwise were per RD specifications) and estimating the intraclass correlation. We conducted multiple sensitivity analyses to ensure the results remained robust under various specifications, including performing an adjusted analysis to assess for any bias from small differences in baseline characteristics and assessing for any differences at sites that were also included in the PopART trial (appendix pp 3–4).
We also sought to assess the effects of same-day ART initiation in routine settings on 12-month retention on ART using exposure to the new guidelines as an IV. The observational nature of our cohort makes it likely that there are unobserved common causes of same-day ART initiation and retention on ART that would confound the relationship using standard regression methods. IV analyses overcome this unmeasured confounding provided that four assumptions, which are plausible in our setting, are met16,19,20; we empirically tested for violations of these underlying assumptions before conducting the IV analyses (appendix pp 2–3). This method estimates the causal effect of same-day ART initiation on retention on ART at 12-months among those patients who were initiated same-day due to implementing universal treatment (and thus no need for assessing ART eligibility)—commonly referred to as the complier average causal effect (CACE)16,19,20.
We used a two-stage least squares bivariate probit regression for the IV analysis. During the first stage, we modelled the relationship between exposure to the new guidelines and same-day ART initiation, and then used the predicted values for same-day ART initiation in the second stage to estimate its association with retention on ART. UTT guidelines could impact retention via both the rapidity of ART initiation (since ART eligibility assessments are no longer required) as well as new eligibility for ART (among those with a CD4 above 500 cells/μL). Therefore, we adjusted for patient eligibility subgroup during both stages so that results only represent the effect of more rapid ART initiation on retention.
All analyses were conducted with Stata MP 16.1 (StataCorp LLC, College Station, Texas) and R 3.2.4 software (R Foundation for Statistical Computing, Vienna, Austria). This manuscript was prepared according to STROBE guidelines (STROBE Checklist).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS:
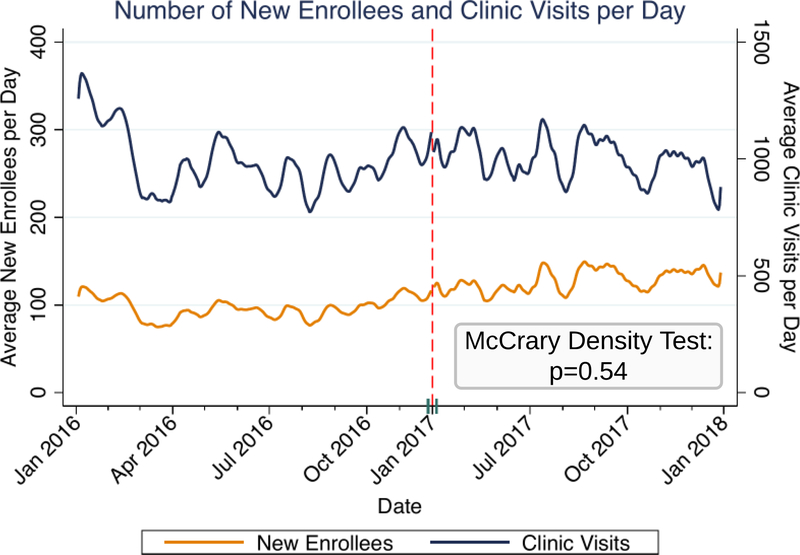
Between January 1, 2016 and January 1, 2018, 79,617 ART naïve patients newly enrolled at one of 117 ART clinics in two provinces in Zambia; after restriction, 65,673 patients were included in subsequent RD analyses (appendix p 5). Most were female (40,858 [62.2%]), the median age was 32 years (IQR 26, 39), and the median enrollment CD4 count was 287 cells/μL [IQR 147, 466]. Only 16,055 patients (51.5%) had a CD4 count performed at enrollment before UTT implementation, whereas 10,014 (29.0%) had it performed after UTT implementation; there were also small differences in WHO stage at enrollment (Table 1, appendix pp 6–7). Baseline patient characteristics were otherwise also similar before and after UTT guideline implementation when specifically assessing for discontinuity in patient characteristics at the time of implementation using RD methods (appendix pp 8–9). The density of new patient enrollments was continuous at the time of guideline implementation (McCrary density test p=0.54) (Figure 1).
Table 1:
Baseline Patient Characteristics, n=65,673
| Pre-Guidelines (n=31145) | Post-Guidelines (n=34528) | |
|---|---|---|
| Sex, n (%) | ||
| Female | 19446 (62.4%) | 21412 (62.0%) |
| Male | 11699 (37.6%) | 13116 (38.0%) |
| Age Category, years, n (%) | ||
| <25 | 6844 (22.0%) | 7604 (22.0%) |
| 25–35 | 12103 (38.9%) | 12950 (37.5%) |
| 35–50 | 10232 (32.9%) | 11731 (34.0%) |
| >50 | 1966 (6.3%) | 2243 (6.5%) |
| Enrollment CD4 performed, n (%) | 16055 (51.5%) | 10014 (29.0%) |
| Enrollment CD4 Category*, cells/μL, n (%) | ||
| <200 | 5697 (35.5%) | 3300 (33.0%) |
| 200–350 | 4118 (25.6%) | 2594 (25.9%) |
| 350–500 | 2903 (18.1%) | 1848 (18.5%) |
| >500 | 3337 (20.8%) | 2272 (22.7%) |
| Enrollment WHO Stage, n (%) | ||
| 1 | 13355 (42.9%) | 17889 (51.8%) |
| 2 | 3658 (11.7%) | 3459 (10.0%) |
| 3 | 5988 (19.2%) | 4179 (12.1%) |
| 4 | 372 (1.2%) | 175 (0.5%) |
| Unknown | 7772 (25.0%) | 8826 (25.6%) |
| TB in past year, n (%) | 1567 (5.0%) | 1865 (5.4%) |
| Pregnant/Breastfeeding at Enrollment, n (%) | 3470 (17.8%) | 3160 (14.8%) |
| Eligibility Category**, n (%) | ||
| Always | 17499 (56.2%) | 13022 (37.7%) |
| Newly | 2571 (8.3%) | 1888 (5.5%) |
| Unknown | 11075 (35.6%) | 19618 (56.8%) |
| Marital Status, n (%) | ||
| Single | 3994 (12.8%) | 4342 (12.6%) |
| Married | 15519 (49.8%) | 16381 (47.4%) |
| Divorced | 4226 (13.6%) | 4295 (12.4%) |
| Widowed | 2145 (6.9%) | 1935 (5.6%) |
| Unknown | 5261 (16.9%) | 7575 (21.9%) |
| Education, n (%) | ||
| None | 1667 (5.4%) | 1679 (4.9%) |
| Primary | 8675 (27.9%) | 8599 (24.9%) |
| Secondary | 14506 (46.6%) | 16056 (46.5%) |
| University | 1256 (4.0%) | 1349 (3.9%) |
| Unknown | 5041 (16.2%) | 6845 (19.8%) |
| Facility Type***, n (%) | ||
| Small Health Center | 1918 (6.2%) | 3810 (11.0%) |
| Medium Health Center | 4136 (13.3%) | 4661 (13.5%) |
| Large Health Center | 21487 (69.0%) | 21316 (61.7%) |
| Hospital | 3604 (11.6%) | 4741 (13.7%) |
| Province | ||
| Lusaka | 28544 (91.6%) | 31284 (90.6%) |
| Western | 2601 (8.4%) | 3244 (9.4%) |
Among those not missing an enrollment CD4 count.
Eligibility subgroups were defined by whether patients would have been eligible for ART according to both the 2014 and 2017 guidelines (“Always Eligible”) or only the 2017 guidelines (“Newly Eligible”) at time of enrollment, regardless of the time period in which they actually enrolled. These were defined using data captured in the EHR; individuals with missing CD4 measurements who did not clearly meet other eligibility criteria were categorized as “Unknown”.
Small Health Center: <500 patients, Medium Health Center: 500–2500 patients, Large Health Center: >2500 patients
Figure 1: Number of New Enrollees and Overall Clinic Visits Per Day.
Lowess curves of the number of patients newly enrolling at HIV clinics per day and overall number of visits (i.e., new and old patients) per day. We used the McCrary density test to formally test for a discontinuity in the density of new enrollees at the time of guideline implementation. Results indicate that he density of new patient enrollments was continuous at that time and support the underlying assumptions of the regression discontinuity analysis.
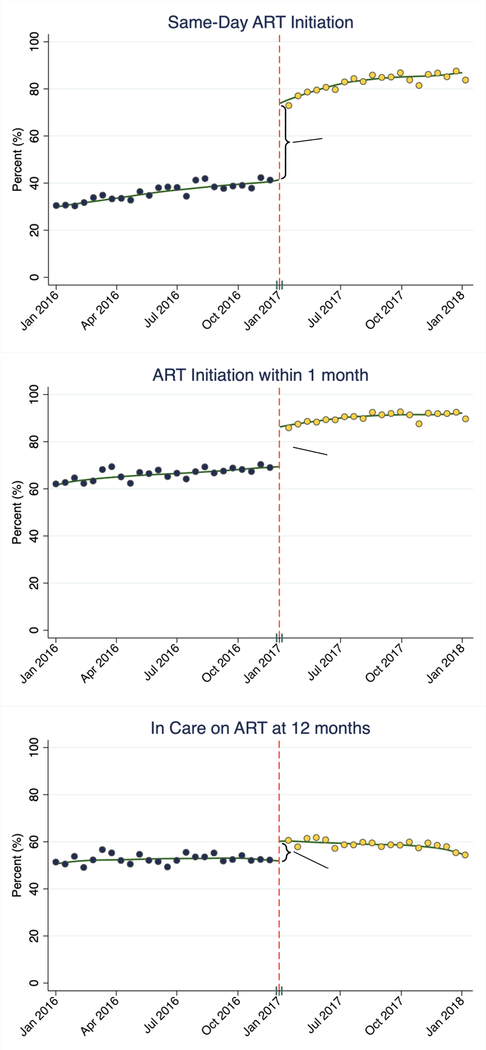
Implementing universal HIV treatment led to overall improvements in both ART initiation and retention in care for all newly enrolling ART naïve patients (Figure 2). UTT policies led to a 33.1% absolute increase in same-day ART initiation (95% CI 30.5–35.7%, p<0.0001), a 17.4% absolute increase in ART initiation within 1 month (95% CI 15.5–19.3%, p<0.0001), and an 8.7% absolute increase in the percentage of patients who were retained in care on ART at 12 months (95% CI 5.8–11.7%, p<0.0001) (Table 2 and Figure 2). All results were robust to various model specifications in sensitivity analyses (appendix pp 10–12). There was no evidence of negative spillover effects and increased rates of missed visits or becoming lost to follow-up (LTFU) in the overall clinic population (i.e., new and old patients) (appendix pp 13).
Figure 2:
Results from Regression Discontinuity Analyses on the Effects of Implementing Universal HIV Treatment on Same-Day ART Initiation, ART Initiation within 1 month, and Retention in care on ART at 12 months.
Table 2:
Results of Regression Discontinuity Analysis on the Effects of Implementing Universal and Rapid HIV Treatment on ART Initiation and Retention in Care
| Pre-Guidelines (95% CI) | Post-Guidelines (95% CI) | Risk Difference (95% CI) | p-value | Bandwidth | |
|---|---|---|---|---|---|
| Same-Day ART Initiation | 41.7 (39.7–43.7) | 74.8 (73.2–76.4) | +33.1 (30.5–35.7) | <0.0001 | 92 |
| ART Initiation within 1 month | 69.6 (68.0–71.1) | 87.0 (85.9–88.0) | +17.4 (15.5–19.3) | <0.0001 | 134 |
| In Care on ART at 12 months | 56.2 (54.2–58.3) | 63.3 (61.4–65.3) | +7.1 (4.3–9.9) | <0.0001 | 90 |
Marginal estimates derived using Poisson regression with robust variances
In stratified analyses, there was substantial heterogeneity in rates of both ART initiation and retention on ART across subgroups prior to UTT. After UTT implementation, there were consistent trends towards improvement across patient subgroups with respect to all three outcomes. These improvements led to more uniform and consistent rates of ART initiation across all subgroups post-UTT, but heterogeneity in retention on ART between subgroups still remained unchanged, with individuals who were younger (both men and women), single, did not have an enrollment CD4 performed, had higher WHO stage, presented to smaller facilities, and from Western province having the lowest retention rates post-UTT (Figure 3).
Figure 3: Forest Plot of Effects of Implementing Universal HIV Treatment Stratified by Patient Subgroups.
Results are from stratified regression discontinuity analyses. Unique Imbens-Kalyanaram data-driven bandwidths were calculated for each analysis.
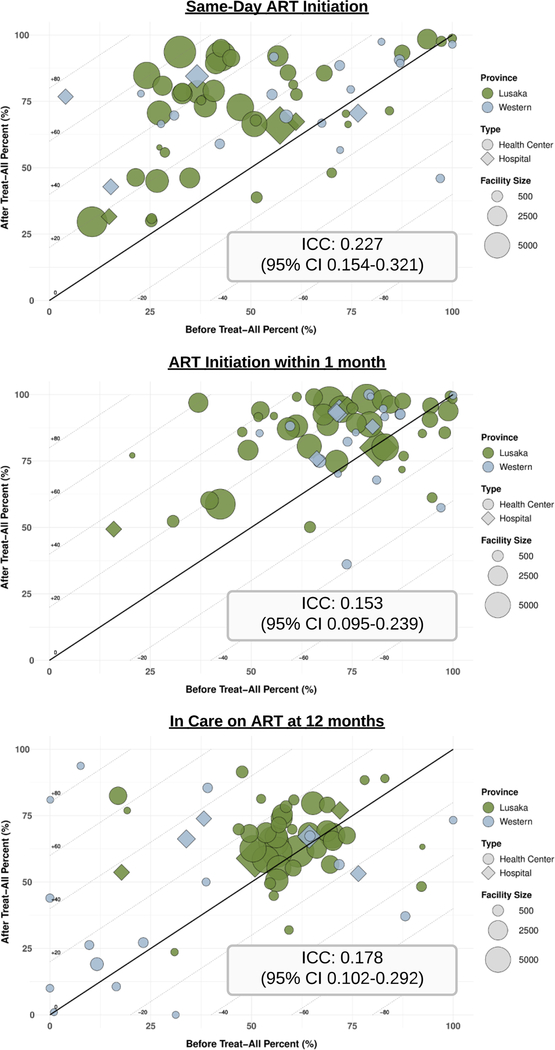
The impact of UTT guidelines also varied significantly across clinics. Mixed-effects models indicated that the clinic of enrollment accounted for 22.7% of the total variability in same-day ART initiation (95% CI 15.4–32.1%), 15.3% of the total variability in ART initiation within 1 month (95% CI 9.5–23.9%), and 17.8% of the total variability in retention on ART at 12 months (95% CI 10.2–29.2%) (Figure 4).
Figure 4: Heterogeneity in the Effects of Implementing Universal HIV Treatment Across Health Facilities.
Each bubble represents a facility and is plotted based on outcomes before vs. after implementation of universal HIV treatment; diagonal lines indicate the risk difference. Intraclass correlations (ICC) were estimated using mixed-effects regression per the RD specifications with facility included as a random effect. Only clinics with at least 100 patients (n=62) were included.
Using the implementation of UTT guidelines as an IV, we estimated the CACE of same-day ART initiation on retention on ART at 12 months was a 15.8% increase (95% CI 12.1–19.5%, p<0.0001). This result indicates that rapidly initiating ART in 6.3 patients (95% CI 5.1–8.3) would prevent one patient from becoming LTFU by one year.
DISCUSSION:
Our study provides rigorous assessment of the impact of implementing UTT in routine settings on both shorter- and longer-term outcomes among PLWH newly linking to care. Implementing universal and rapid HIV treatment substantially and consistently increased the proportion of patients who were initiated on ART the same-day as enrollment, and also led to modest improvements in the proportion of patients who were in care and on ART after one year, overall and across patient subgroups. Our findings are likely explained by two distinct mechanisms. First, implementing UTT expanded ART eligibility to those with a CD4 count above 500 cells/μL. Second, it also removed requirements for assessing ART eligibility and waiting for CD4 results to return, allowing for a more streamlined ART initiation process that benefitted all patients newly linking to care21,22. Ultimately, implementing these policies in real-world settings resulted in improvements in both the rapidity and uptake of ART initiation in the short-term, which in turn also led to small but meaningful increases in the proportion who were in care and on ART at 12 months.
Our findings align with previous studies that examined universal treatment1–9, but extend the existing literature in several ways. First, we estimated the real-world impact of UTT by assessing patients receiving routine care delivered within the public health HIV infrastructure in Zambia. This is in contrast to several UTT trials that demonstrated very high levels of retention and viral suppression, but often also implemented additional co-interventions such as community/home-based testing, enhanced linkage to care, clinic mentorship, or specialized counseling to facilitate fidelity to intervention protocols1,2. This difference in context is likely an important reason why our results qualitatively align, but have differing magnitude of effects. Interestingly, 3 facilities included in our study also participated in the PopART trial: two facilities were in communities that received annual household visits for HIV testing and care referrals (i.e., PopART intervention) and one also had universal ART implemented as part of the trial in 2014 (a third facility served as a control). In sensitivity analyses restricted to these sites, however, the new guideline implementation still had a similar impact on ART initiation and retention in care at these 3 facilities as compared to the remaining facilities, further emphasizing that the impact of such policies may be quite different when implemented as part of a research study compared to when routinely implemented as standard of care. Second, we used an RDD to ensure rigorous, robust, and unbiased results. This study design limits bias from selection or unmeasured confounders between those who did and did not initiate ART before and after UTT, which may have existed in other observational studies6–9. Third, we assessed the impact of UTT on both shorter- (i.e., ART initiation) and longer-term (i.e.,12-month retention on ART) outcomes, which has yet to be assessed rigorously in routine treatment settings. Lastly, this is one of the largest real-world studies of the impact of UTT to date, including approximately 65,000 patients across 117 clinics in two provinces in Zambia.
An important finding from our study is that implementation of universal treatment substantially reduced existing disparities and led to more uniform ART initiation. Prior to UTT, rates of ART initiation across different patient subgroups were heterogeneous, with men and those with more advanced disease (i.e., lower CD4 count and higher WHO stage) initiating ART at lower rates. After UTT, rates of both same-day and one-month ART initiation became substantially more uniform across subgroups, likely reflecting the system-level improvements in treatment access. In contrast, heterogeneity in 12-month outcomes remained largely unchanged after implementing UTT, likely because many other patient-level determinants of retention remained unaddressed. Still, most subgroups did have trends towards some level of improvement in 12-month retention on ART in the pre- versus post-UTT periods (although confidence intervals are wide and definitive conclusions for specific subgroups are difficult to make). Lastly, for both ART initiation and retention on ART, heterogeneity between clinics remained quite high in both the pre- and post-UTT periods. Thus, additional patient- and health-system-level interventions will likely be needed to help sustain these improvements over the long-term and ensure consistent care quality between health facilities.
Our analyses provide important information on the impact of same-day ART initiation as it is implemented in routine care settings on longer-term patient outcomes. For a small but significant proportion of patients, the fewer treatment barriers associated with rapid ART policies may be sufficient to not only influence their time to ART initiation, but also both whether they even initiate ART or remain engaged in care or not21,22. Previous experimental studies reported stable to improved outcomes with rapid ART initiation in controlled settings23–26. Lingering concerns still remain about how these procedures translate to real-world settings, particularly in terms of counseling quality, inappropriate ART initiation in patients with concurrent opportunistic infections or those not yet ready to start, and how this could increase patient attrition13,14. Several existing real-world studies have shown worse retention with same-day ART initiation, but these studies also only assessed outcomes among those who initiated ART7,9–11. These results thus do not incorporate failures to initiate ART and lead to selection bias, since some patient attrition—that would have likely occurred regardless—simply shifts from pre- to post-ART initiation21. A recent study from Botswana reported that same-day ART initiation increased by 50% and viral suppression rates remained stable at 90% after implementing rapid ART policies on top of universal treatment, but this was a substudy from a UTT trial with particularly high levels of retention and viral suppression5. Our use of new treatment guidelines as an instrumental variable and an outcome that assesses the combined changes to both ART initiation and retention in care provides additional rigorous evidence that same-day ART initiation as implemented in routine care settings is feasible, safe, and, ultimately, likely improves patient retention on ART up to 12 months later.
Though we identified overall improvements to patient outcomes, it is also essential to consider the gaps that remain with universal and rapid treatment policies. First, many patients still present with low CD4 counts and would have been ART-eligible even prior to UTT. Innovations in community- or home-based testing campaigns with active linkage to care are needed to facilitate engagement at earlier disease stages1,2,25. Second, there are clear reductions in CD4 staging at enrollment (as they are no longer needed to determine ART eligibility) but this also precludes identifying and providing evidence-based treatment packages to those with advanced disease27. Lastly, though there were high levels of ART initiation, the improvements in retention on ART were overall modest and disparities between patient subgroups and health facilities remained. This is likely reflective of the different barriers and needs beyond easier access to treatment that these diverse groups have and targeted and patient-centered health-system interventions are urgently needed to better address them28–30.
Our study has several limitations. First, our results are, strictly speaking, only applicable right at the time of guideline implementation based on the underlying RD assumptions. Thus, generalizability to time periods further away from the cutoff is uncertain since there could be changes to demographics and subsequent adoption of health-system innovations such as home-based testing or differentiated service delivery. Second, it is difficult to disentangle the effects of universal ART versus rapid ART as both policies were written into guidelines, although emphasis was only on universal ART initially with the push for rapid ART only occurring in 2018. Additionally, several months (but not immediately) after new policies were implemented, CIDRZ, the Ministry of Health, and the CDC began community sensitization campaigns and provided technical assistance for linking newly diagnosed patients and implementing universal treatment. RD analyses, however, only focus right at the time of rollout and we also did not detect changes in the rates of patients enrolling in care that would suggest any immediate impact on the rates of testing and linkage to care prior to versus after the guideline change. Third, we excluded patients enrolling closest to the implementation date to minimize both “cross-over” bias and also to account for a transition period as guidelines were being fully implemented. Still, it is unlikely that secular trends significantly affected our results within the context of this restriction as calendar time was not a significant predictor in our models. Additionally, baseline characteristics remained similar and model diagnostics, sensitivity analyses (including adjusted analyses), and falsification tests did not suggest that the underlying RD assumptions were violated. Fourth, we were unable to assess whether implementing UTT led to population-level changes at earlier stages in the HIV care cascade (e.g., testing). Fifth, there are inherent limitations to routine data sources including missing data. Although some missingness accurately reflects real-world care delivery (e.g., CD4 measurements not always being performed), there is still the possibility of incomplete or inaccurate data entry even with the routine data quality audits by CIDRZ. Nevertheless, our primary analyses only required complete variables and results remained consistent in adjusted sensitivity analyses that used multiple imputation to address missingness. Lastly, we were unable to assess virologic outcomes because viral loads were not consistently collected at scale in Zambia at the time. Still, just under 90% of patients in care and receiving ART are virally suppressed31; thus, being in care on ART at 12 months represents a clinically relevant patient outcome. We were also unable to identify individuals who were considered LTFU but had silently transferred to new facilities, although previous studies have demonstrated that this group still experiences prolonged interim lapses in care and low rates of viral suppression32,33.
In conclusion, we found that implementation of universal and rapid HIV treatment in Zambia improved both the rapidity and incidence of ART initiation, as well as retention on ART at 12 months, although overall retention on ART remained suboptimal. Rates of ART initiation became more uniform across patient subgroups, but differences remained between facilities, as did differences in retention on ART. We found that same-day ART initiation in routine care settings was associated with increases in retention on ART at 12 months. This study provides important new data on the overall impact of implementing UTT in routine care settings in sub-Saharan Africa. UTT policies led to consistent but modest improvements in shorter- and longer-term patient outcomes. Future health-system innovations must now focus on targeted and patient-centered intervention strategies that address gaps in the HIV care cascade that remain even in the setting of universal testing and treatment.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for studies that assessed the effect of universal antiretroviral therapy (ART) treatment for all persons living with HIV in resource-limited settings. We used the following combinations of search terms without any language or date restrictions: “HIV”, “universal test and treat”, “treat-all”, “test and treat”, “test and start”, “universal HIV treatment”, “same-day ART initiation”, and “rapid ART”. We identified relevant clinical trials and cohort studies from this PubMed search. Additional references were identified by manually searching the citation lists of relevant manuscripts. Several clinical trials (individual-level and cluster-level) assessed universal test and treat (UTT) or rapid ART initiation in resource-limited settings, and reported improved ART initiation, retention in care, and viral suppression. However, these trials often contained additional co-interventions (e.g., recurrent community-wide testing campaigns, linkage to care support, enhancements to routine clinical care) to facilitate fidelity to optimal guideline-based care, which does not reflect real-world implementation of UTT policies. Observational studies that reflected real-world settings reported mixed results, and were frequently limited due to methods that may introduce selection bias, unmeasured confounding, and/or had small sample sizes. One study leveraged UTT policies in a rigorous natural experiment, but only examined the impact on ART initiation within one month of enrollment. A study from Botswana examined the impact of adding rapid ART policies to universal treatment and reported stable levels of viral suppression care retention. However, that analysis was still conducted in the context of a cluster-level intervention trial seeking to improve the HIV care cascade and reduce HIV incidence.
Added value of this study
We used a regression discontinuity design to evaluate the effects of implementing UTT in Zambia on same-day ART initiation, ART initiation within one month, and retention on ART at 12 months. The study design allowed us to rigorously examine the impacts of implementing UTT in a real-world setting on shorter- and longer-term patient outcomes, and quantify how these impacts differed across patient subgroups and between clinics. We also leveraged this new treatment policy as an instrumental variable to assess the effect of same-day ART initiation in routine settings on retention on ART at 12 months. These results inform and quantify the public health effectiveness of two key cornerstones of current HIV treatment programs—universal and rapid ART initiation—in one of the largest studies to date assessing the impact of UTT in real-world settings (approximately 65,000 patients in 117 clinics across two provinces in Zambia).
Implications of all the available evidence
All available evidence suggests that UTT policies implemented in real-world settings increase the rapidity and uptake of ART, and improve longer-term patient outcomes such as retention on ART at 12 months. Thus, implementation of these policies likely results in individual- and population-level benefits. UTT policies reduced disparities and led to more uniform ART initiation rates across patient subgroups; however, heterogeneity between clinics and disparities in longer-term patient outcomes (e.g., 12-month retention on ART) remained high. Same-day ART initiation in routine care settings likely improves 12-month patient outcomes. Despite the positive benefits of implementing UTT, improvements are still modest and longer-term outcomes will likely remain suboptimal. Targeted and patient-centered health system interventions are needed to translate these benefits into sustained treatment success for individuals and national HIV treatment programs.
Acknowledgements:
We want to thank all staff at the Centre for Infectious Diseases Research in Zambia (CIDRZ). We also thank the Zambian Ministry of Health, Center for Disease Control, and PEPFAR for developing the health systems and electronic medical record necessary to conduct this research. The manuscript was edited by the Scientific Editing Service of the Institute of Clinical and Translational Sciences at Washington University, which is supported by an NIH Clinical and Translational Science Award (UL1 TR002345).
Financial Support: This work was supported by the National Institutes of Health (KL2 TR002346 to AM and K24 AI134413 to EHG). This project has also been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Declarations of Interests: AM and EHG report funding from the NIH during the conduct of this study. IS, MWM, TS, MEH, and CBM report support from the United States President’s Emergency Plan for AIDS Relief/Centers for Disease Control and Prevention during the conduct of this study. IS, CBM, IEW, CBH, CBM, and EHG report grants from the Bill and Melinda Gates Foundation outside the submitted work. EHG reports educational grants from Viiv Healthcare outside the submitted work. CBH reports consulting for the Bill and Melinda Gates Foundation and support for participating in an expert panel from Merck outside the submitted work. All other authors declare no competing interests.
Ethics Statement: The study was approved by the University of Zambia Biomedical Research Ethics Committee (UNZABREC), institutional review boards at the Washington University School of Medicine, University of North Carolina School of Medicine, and the U.S. Centers for Disease Control and Prevention (CDC) Center for Global Health.
CDC Authorship disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies. The study was reviewed in accordance with the U.S. Centers for Disease Control and Prevention (CDC) human research protection procedures and determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The Government of Zambia allows data sharing when applicable local conditions are satisfied. To request data access, contact the CIDRZ Ethics and Compliance Committee chair/Chief Scientific Officer, Dr. Roma Chilengi (Roma.Chilengi@cidrz.org), or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope.Mwanyungwi@cidrz.org), mentioning the intended use for the data.
REFERENCES
- 1.Perriat D, Balzer L, Hayes R, et al. Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc 2018; 21(1): e25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir D, Lockman S, Ayles H, et al. What do the Universal Test and Treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc 2020; 23(2): e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tymejczyk O, Brazier E, Yiannoutsos CT, et al. Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis. PLoS Med 2019; 16(6): e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S, Spiegelman D, Walsh F, et al. Early access to antiretroviral therapy versus standard of care among HIV-positive participants in Eswatini in the public health sector: the MaxART stepped-wedge randomized controlled trial. J Int AIDS Soc 2020; 23(9): e25610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebelonyane R, Bachanas P, Block L, et al. Rapid antiretroviral therapy initiation in the Botswana Combination Prevention Project: a quasi-experimental before and after study. Lancet HIV 2020; 7(8): e545–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross J, Sinayobye JD, Yotebieng M, et al. Early outcomes after implementation of treat all in Rwanda: an interrupted time series study. J Int AIDS Soc 2019; 22(4): e25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puttkammer N, Parrish C, Desir Y, et al. Toward Universal HIV Treatment in Haiti: Time Trends in ART Retention After Expanded ART Eligibility in a National Cohort From 2011 to 2017. J Acquir Immune Defic Syndr 2020; 84(2): 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makurumidze R, Buyze J, Decroo T, et al. Patient-mix, programmatic characteristics, retention and predictors of attrition among patients starting antiretroviral therapy (ART) before and after the implementation of HIV “Treat All” in Zimbabwe. PLoS One 2020; 15(10): e0240865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerschberger B, Schomaker M, Jobanputra K, et al. HIV programmatic outcomes following implementation of the ‘Treat-All’ policy in a public sector setting in Eswatini: a prospective cohort study. J Int AIDS Soc 2020; 23(3): e25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph Davey D, Kehoe K, Serrao C, et al. Same-day antiretroviral therapy is associated with increased loss to follow-up in South African public health facilities: a prospective cohort study of patients diagnosed with HIV. J Int AIDS Soc 2020; 23(6): e25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: Analysis of routine data. PLoS One 2020; 15(1): e0227572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mody A, Sikazwe I, Czaicki NL, et al. Estimating the real-world effects of expanding antiretroviral treatment eligibility: Evidence from a regression discontinuity analysis in Zambia. PLoS Med 2018; 15(6): e1002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng EH, Havlir DV. The science of rapid start-From the when to the how of antiretroviral initiation. PLoS Med 2017; 14(7): e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen S, Fox MP, Larson BA, et al. Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda. PLoS Med 2016; 13(8): e1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection 2016. Available from: https://www.nac.org.zm/sites/default/files/publications/Zambia%20Consolidated%20Guidelines%202016.pdf. Accessed on 9/27/2019.
- 16.Barnighausen T, Oldenburg C, Tugwell P, et al. Quasi-experimental study designs series-paper 7: assessing the assumptions. J Clin Epidemiol 2017; 89: 53–66. [DOI] [PubMed] [Google Scholar]
- 17.Moscoe E, Bor J, Barnighausen T. Regression discontinuity designs are underutilized in medicine, epidemiology, and public health: a review of current and best practice. J Clin Epidemiol 2015; 68(2): 122–33. [DOI] [PubMed] [Google Scholar]
- 18.Imbens G, Kalyanaraman K. Optimal Bandwidth Choice for the Regression Discontinuity Estimator. The Review of Economic Studies 2011; 79(3): 933–59. [Google Scholar]
- 19.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology 2006; 17(4): 360–72. [DOI] [PubMed] [Google Scholar]
- 20.Swanson SA, Hernan MA. Commentary: how to report instrumental variable analyses (suggestions welcome). Epidemiology 2013; 24(3): 370–4. [DOI] [PubMed] [Google Scholar]
- 21.Fox MP. Are we shifting attrition downstream in the HIV cascade? Lancet HIV 2016; 3(12): e554–e5. [DOI] [PubMed] [Google Scholar]
- 22.Scott NA, Maskew M, Fong RM, et al. Patient Perspectives of Quality of the Same-Day Antiretroviral Therapy Initiation Process in Gauteng Province, South Africa: Qualitative Dominant Mixed-Methods Analysis of the SLATE II Trial. Patient 2021; 14(2): 175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV 2016; 3(11): e539–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maskew M, Brennan AT, Fox MP, et al. A clinical algorithm for same-day HIV treatment initiation in settings with high TB symptom prevalence in South Africa: The SLATE II individually randomized clinical trial. PLoS Med 2020; 17(8): e1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstutz A, Brown JA, Ringera I, et al. Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement After Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: A Two-Year Follow-up of the CASCADE Trial. Clin Infect Dis 2020; 71(10): 2608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med 2017; 14(7): e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasuuna E, Tenforde MW, Muganzi A, Jarvis JN, Manabe YC, Kigozi J. Reduction in Baseline CD4 Count Testing Following Human Immunodeficiency Virus “Treat All” Adoption in Uganda. Clin Infect Dis 2020; 71(9): 2497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mody A, Sikombe K, Beres LK, et al. Profiles of HIV Care Disruptions Among Adult Patients Lost to Follow-up in Zambia: A Latent Class Analysis. J Acquir Immune Defic Syndr 2021; 86(1): 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng EH, Holmes CB, Moshabela M, Sikazwe I, Petersen ML. Personalized public health: An implementation research agenda for the HIV response and beyond. PLoS Med 2019; 16(12): e1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimsrud A, Wilkinson L, Eshun-Wilson I, Holmes C, Sikazwe I, Katz IT. Understanding Engagement in HIV Programmes: How Health Services Can Adapt to Ensure No One Is Left Behind. Curr HIV/AIDS Rep 2020; 17(5): 458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: First Report. Zambia Ministry of Health. Lusaka, Zambia, December 2017. [Google Scholar]
- 32.Sikazwe I, Eshun-Wilson I, Sikombe K, et al. Retention and viral suppression in a cohort of HIV patients on antiretroviral therapy in Zambia: Regionally representative estimates using a multistage-sampling-based approach. PLoS Med 2019; 16(5): e1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikombe K, Mody A, Kadota J, et al. Understanding patient transfers across multiple clinics in Zambia among HIV infected adults. PLoS One 2020; 15(11): e0241477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Government of Zambia allows data sharing when applicable local conditions are satisfied. To request data access, contact the CIDRZ Ethics and Compliance Committee chair/Chief Scientific Officer, Dr. Roma Chilengi (Roma.Chilengi@cidrz.org), or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope.Mwanyungwi@cidrz.org), mentioning the intended use for the data.