Abstract
Difficulty retrieving proper names is common in older adults, coinciding with the accumulation of aggregated proteins in mid-life. We investigated the ability of healthy older adults to retrieve the names of famous faces in relation to positron emission tomography measurements of amyloid-β plaques and tau neurofibrillary tangles. More tau in the left fusiform and parahippocampal gyrus was related to reduced proper name retrieval performance and this effect was potentiated by amyloid-β. These findings provide an explanation for a common complaint of older adults and link proper name retrieval to neural systems involved in face perception, memory, and naming.
Keywords: aging, proper name retrieval, Alzheimer’s disease
Proper names (PNs) are unique lexical items that identify individuals from conceptual structures stored in episodic memory1. While episodic memory decline is affected in healthy aging and Alzheimer’s disease (AD)1,2, cognitively healthy older adults often experience PN retrieval failures despite intact retrieval of common nouns and events1,3. PNs can have both semantic and episodic characteristics, such that they can be associated with a specific event (episodic) or with general knowledge about the individual (semantic)4. PNs engage a large network of neural systems involved in perception, memory, and naming including the fusiform gyrus, occipital gyrus, parahippocampal gyrus, and the temporal pole, among others1,5. Brain regions subserving episodic memory are known to be particularly vulnerable to pathological forms of the microtubule-associated protein tau2,6, one of the two core pathologies of AD. Tau begins depositing as neurofibrillary tangles in the medial temporal lobe during mid-life, at roughly the same time that people begin to experience difficulty with PN retrieval. This pathological tau spread appears to be potentiated by the other core Alzheimer’s pathology, β-amyloid (Aβ), and is associated with decline in episodic memory ability even in the setting of normal cognition2,6,7. That both PN retrieval and episodic memory involve regions in the medial temporal lobe, along with the concurrent appearance of medial temporal tau and PN retrieval failures in mid-life, raises the question of whether tau deposition is associated with this common complaint in older individuals. For this reason, we evaluated relationships between PN retrieval performance, episodic memory, and PET measures of tau and Aβ deposition in a group of healthy older adults.
Methods
Subjects
We studied 85 healthy older adults in the Berkeley Aging Cohort Study. Each subject underwent a neuropsychological exam assessing episodic memory, working memory, and executive function (Table 1), PET scans to measure Aβ using 11C-Pittsburgh Compound-B (PIB)-PET and tau pathology using 18F-Flortaucipir (FTP)-PET, a 1.5T structural MRI scan, and apolipoprotein E (APOE) genotyping. Subjects were deemed cognitively normal based on previous criteria from the Berkeley Aging Cohort Study8. All subjects provided informed consent in accordance with the Institutional Review Boards of UC Berkeley and Lawrence Berkeley National Lab.
Table 1.
Cohort Characteristics
| N = 85 | |
|---|---|
| Age | 79 ± 4.83 |
| Sex (M/F) | 34/51 |
| Education (Yrs.) | 17 ± 2 |
| MMSE | 28.61 ± 1.59 |
| Episodic Memory (z-score) | − .007 ± 0.86 |
| PN Retrieval score | 30.4 ± 6.97 |
| Recognition score | 35.5 ± 4.54 |
| Vegetables score | 13.95 ± 4.28 |
| Animals score | 19.75 ± 5.32 |
| VPA score | 21.41 ± 7.75 |
| LM Score | 43.91 ± 10.40 |
| Testing to PET interval, days | 273 ± 289 |
| PET to MRI interval, days | 18.8 ± 30.8 |
| APOE ε4 carriers | 24 (28%, 1 N/A) |
| Cortical Aβ (PIB-DVR) | 1.16 ± 0.24 |
| Aβ +/− | 40/45 |
Vegetables and animals scores = total free recall after 60 seconds. MMSE = Mini mental state exam, VPA = verbal paired associates, LM = logical memory. VPA & LM are subtests of the Wechsler Memory Scale. We used PET scans closest to the first time they took the Northwestern University Famous Faces task (NUFFACE) to measure PN retrieval and recognition.
We used the Northwestern University Famous Faces task to measure PN retrieval. This test distinguishes the ability to freely retrieve the names of famous faces (“PN retrieval”) versus recognizing and citing semantic facts about the person (“recognition”)9. Participants were shown 20 black-and-white images of generation-appropriate famous faces. If they were able to say the first and last name of the person within five seconds of viewing their picture, they received 2 points for naming and 2 points for recognition. Retrieving only the first name resulted in 1 point for naming and 2 for recognition. If the participant failed to name the person, they could receive 1 or 2 points for citing semantic facts about the person. A perfect score was 40 points for PN retrieval and 40 for recognition.
Neuropsychological Assessment
We examined the relationships between PN retrieval and fluency measures using total recall (after 1 minute) for vegetables and animals and verbal-paired associates. We also examined relationships between PN retrieval and Logical Memory, and a composite episodic memory measure derived from z-transformed scores averaged from the short and long-delay free recall scores on the California Verbal Learning test and Visual Reproduction test10.
1.5 T MRI
For all subjects 1×1×1-mm-resolution T1-weighted magnetization prepared rapid gradient echo images were acquired on a 1.5 T Magnetom Avanto scanner11 and used to coregister FTP-SUVR images. T1 scans were processed with FreeSurfer (v5.3.0) using the Desikan-Killiany atlas12 to derive regions of interest (ROIs) for measures of PET and cortical thickness.
PET data acquisition and analysis
PET data acquisition used standard, previously published methods11. PIB and FTP-PET were performed using a Biograph PET/CT Truepoint 6 scanner on the same day. PIB-PET scans comprised a 90-minute dynamic acquisition with calculation of distribution volume ratios (DVR) and FTP-PET comprised acquisition of data from 80–100 min post injection with a standardized uptake value ratio (SUVR) normalized to the inferior cerebellum gray matter11,13. Global cortical PIB-DVRs were calculated using FreeSurfer-derived gray matter ROIs as previously described14. A global PIB-DVR threshold of 1.065 was used to dichotomize participants as Aβ negative or positive15. FTP-SUVRs were partial volume corrected using a modified Geometric Transfer Matrix approach on FreeSurfer-derived ROIs13,16. Voxelwise PIB-DVR and FTP-SUVR (non-partial volume corrected) images were warped to template space for whole brain analyses.
Statistical analyses
We conducted independent samples t-tests to look for sex differences in PN retrieval and episodic memory and ran Pearson correlations to examine relationships between PN and neuropsycholgical assessments, using jamovi V1.6.
We performed voxelwise PET analyses using FTP-SUVR and PIB-DVR images to measure tau and Aβ. For each tracer, we ran two separate voxelwise linear regression models in SPM12. In one model, PN retrieval scores and age were included as predictors of voxelwise FTP-SUVR; in the other model, the episodic memory composite score and age were used to predict FTP-SUVR. An explicit cortical gray matter mask was used in both models. Results are shown uncorrected at a threshold of P<0.001 at voxel level and family wise error (FWE)-corrected at cluster level (Pcluster< 0.05).
To further examine relationships between tau and PN retrieval, we used a regularized regression approach with an elastic net penalty17,18. This approach allowed us to quantify the associations found in the voxelwise analysis and introduce cortical Aβ and thickness to examine whether tau remained an important component of PN retrieval. We used the “caret” package in R19 which selects the optimal value of alpha (α) and lambda (λ). Due to the size of our dataset, we used a leave one out cross validation strategy. We used the “train” function with method “glmnet” to fit the elastic net model which automatically standardizes predictors for fitting, then reports fitted coefficients using the original scale19. The function then iterated through 25 different alpha values. The smallest root mean squared error was used to select the optimal model. Because the elastic net model selected the interactive effect of fusiform tau by Aβ and not their main effects20, we ran a linear regression model to aid in interpretation. We entered entorhinal tau, fusiform thickness, fusiform tau, Aβ, and their interaction. We ran the same elastic net model with episodic memory performance as the dependent variable.
Results
PN retrieval was significantly correlated with the episodic memory composite (r=0.30, p=0.002) and logical memory (r=0.32, p=0.003) (Fig. 1). We found no significant sex differences in PN retrieval (mean score male = 28.9, mean score female = 31.5, t(84)=1.69, p=0.09, mean difference = 2.59), or episodic memory (mean score M = −0.10, mean score F = 0.038, t(84)=0.51, p=0.61, mean difference = 0.09).
Figure 1.
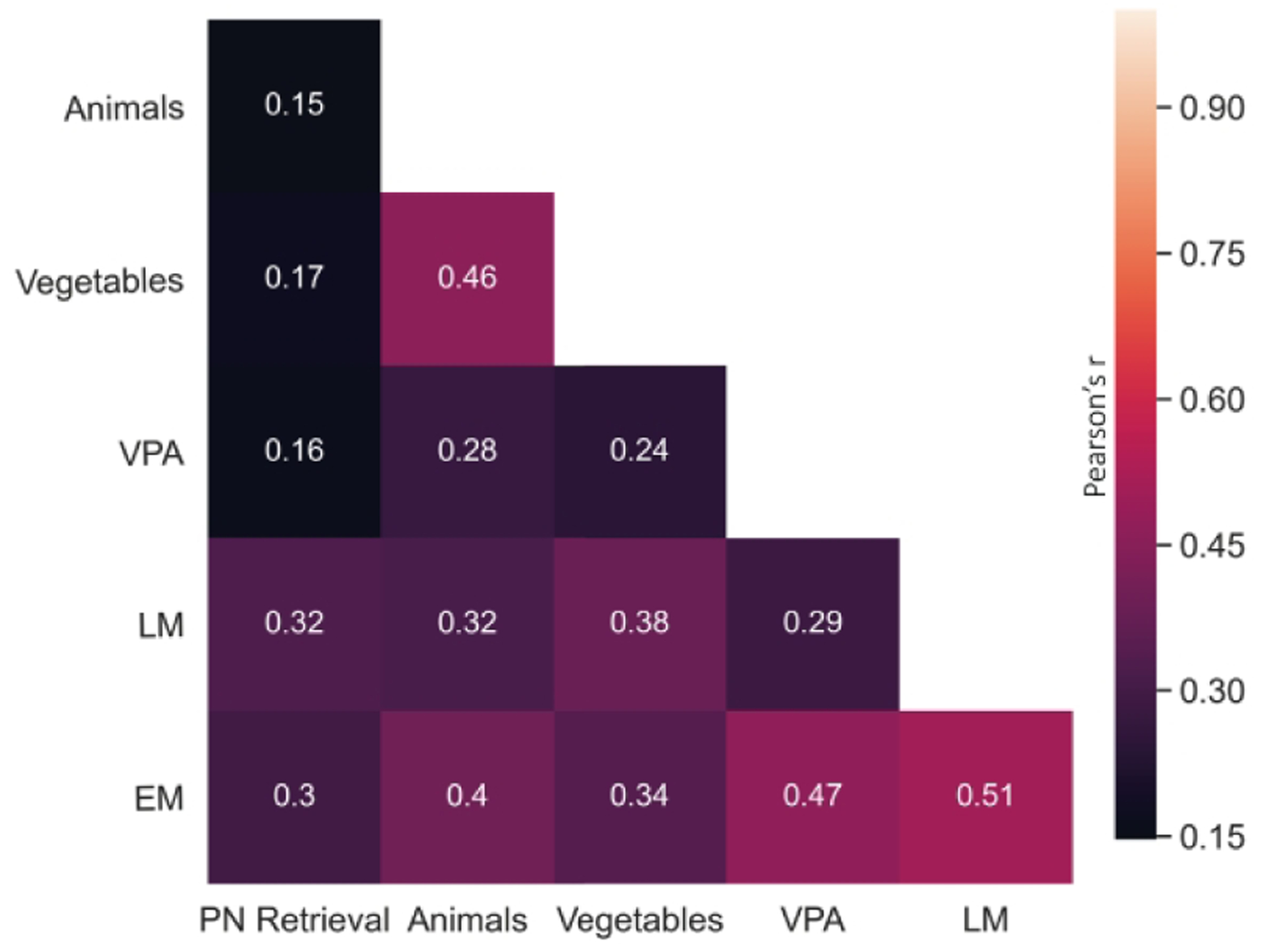
Correlation matrix for associations between proper name (PN) retrieval, episodic memory, and fluency tasks. Pearson correlation coefficients (r) between PN retrieval and vegetable and animal fluency (1-minute free recall), Verbal Paired Associates (VPA) from the Wechsler Memory Scale, and Logical Memory (LM). Episodic memory (EM) comprised long and short delay free recall of the California Verbal Learning Task (CVLT) and Visual Reproduction (VR) memory tests.
In the whole brain voxelwise analysis, worse PN retrieval performance was correlated with more tau in left-lateralized clusters in the fusiform gyrus, parahippocampal gyrus, and other parts of the inferomedial temporal lobe and occipital lobe (Fig. 2A). In contrast, episodic memory performance was associated with tau in more anterior temporal, bilateral medial temporal lobe, and parietal regions (Fig 2B). Overlapping regions included entorhinal cortex, anterior fusiform, and middle temporal gyrus (Fig. 2C). The analyses of voxelwise Aβ with PN retrieval and episodic memory revealed no significant clusters.
Figure 2.
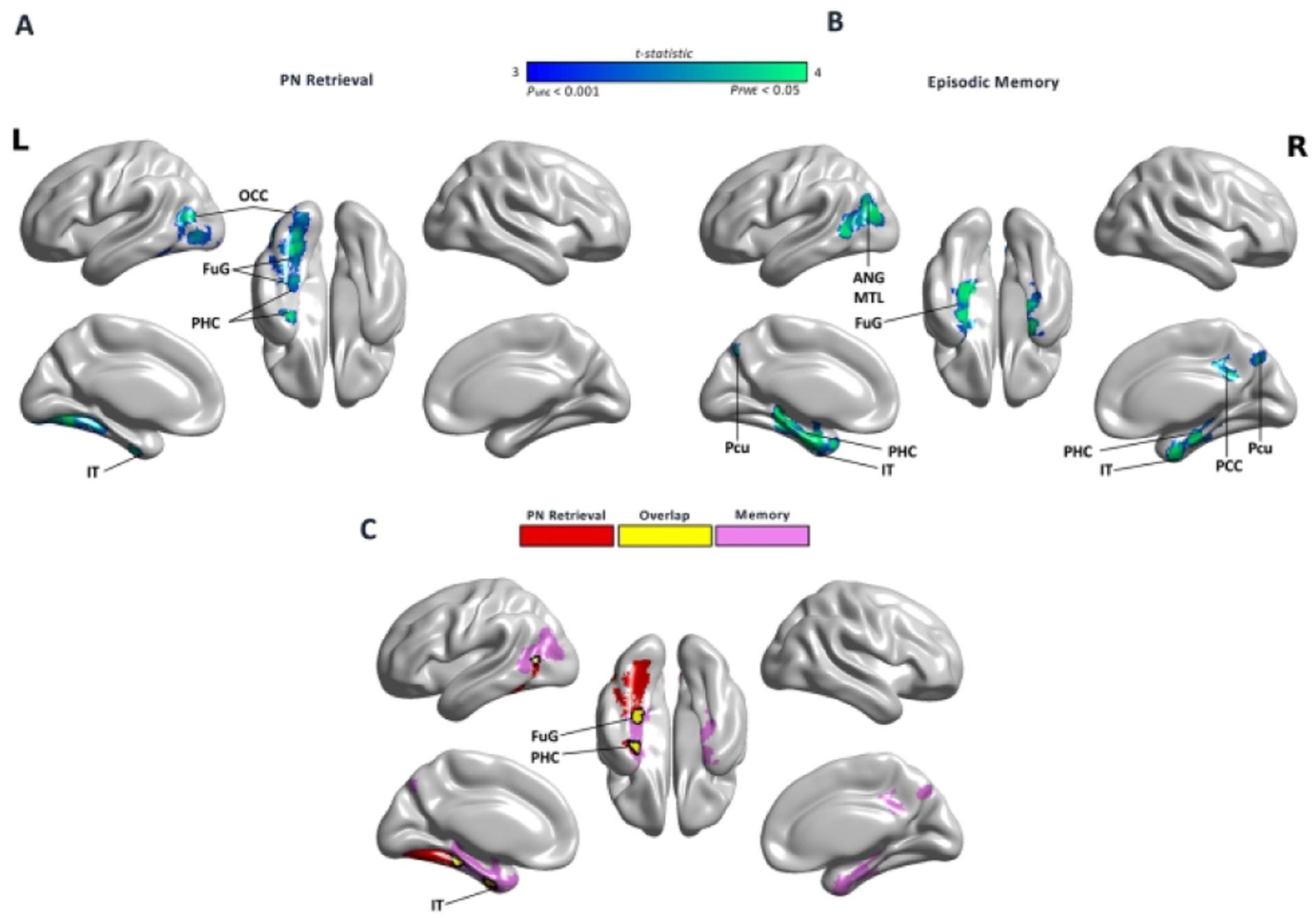
Proper name retrieval and episodic memory associations with tau deposition. Voxelwise regressions in MNI space controlling for age and using an explicit cortical gray matter mask. Results shown at uncorrected (p uncorrected < 0.001) and corrected (using family wise error FWE p<0.05). The color bar illustrates the t-statistic associated with uncorrected and FWE corrected data. (A) Worse proper name retrieval (PN) performance was associated with increased tau (FTP-SUVR) uptake in the fusiform gyrus (FuG), posterior inferior temporal gyrus (IT), parahippocampal gyrus (PHG) including entorhinal cortex (ERC) and parahippocampal cortex (PHC), and middle and inferior occipital gyrus (MOG, IOG). (B) Worse episodic memory was associated with increased tau (FTP-SUVR) uptake in PHC, ERC, FuG, left middle temporal gyrus (MTG), temporal pole (TP), angular gyrus (ANG), posterior cingulate cortex (PCC), and precuneus (Pcu). (C) Overlapping regions comprised ERC, PHC, FuG and MTG.
For the elastic net regression models (Table 2), we included thickness measures because of a known association between tau and cortical thickness2 and added a cortical Aβ measure because Aβ has been shown to exacerbate detrimental effects of tau6,7. The final elastic net model for PN retrieval was consistent with the voxelwise findings and selected fusiform thickness, entorhinal tau, and a fusiform tau × Aβ interaction. The linear regression model showed that increased fusiform thickness was associated with higher PN retrieval scores (p=.05) and more entorhinal tau was associated with lower scores (p<.001). There was no main effect of fusiform tau and Aβ, but the fusiform tau × Aβ interaction indicated a stronger effect of tau on PN retrieval in individuals with high Aβ (p=.03). Confirming this, none of the variables remained significant in the Aβ− group (ps>.05) but did in the Aβ+ group (ps<.05). We believe the flipped sign observed for fusiform tau is due to collinearity17, which is a consequence of using multiregional and multimodel imaging data in the same model. The identical elastic net model with episodic memory as the dependent measure selected entorhinal tau and thickness, parahippocampal tau and thickness, and fusiform thickness, but only entorhinal tau reached significance when put into a multivariate regression model (p=.001).
Table 2.
A. Elastic net variable selections for proper name retrieval and episodic memory by cortical thickness, tau, Aβ, and their interaction. Tau was assessed by FTP uptake (partial volume corrected) and bilateral thicknesses (left and right averaged) were derived by FreeSurfer segmentation from the MRI closest to the tau PET scan. Aβ was measured as a PIB-DVR across cortical regions. FuG = Fusiform gyrus, PHC = Parahippocampal cortex, ERC = entorhinal cortex, RMSE = root mean squared error. The elastic net regression combines the penalties of ridge and lasso regression to regularize coefficient estimates for variable selection, where alpha is the mixing parameter between ridge (α = 0) and lasso (α = 1). It then chooses the variables that are most informative to the overall model and reduces the others to zero. The episodic memory model was run to test the specificity of FuG thickness for PN retrieval. All predictors were standardized for fitting and reported using the original scale. Summary statistics for the final elastic net models are included under Model Summaries.
B. Linear regression models testing chosen variables from elastic net. Because the elastic net model chose the Aβ × FuG tau interaction and not the main effects, we ran linear regression models to aid in interpretation. Only the variables selected by elastic net were included in the linear regression models. The dash [−] indicates that the variable was not included in the linear regression model. Summary statistics for the final linear regression models are included under Model Summaries.
| PN Retrieval | Episodic Memory | ||||||
|---|---|---|---|---|---|---|---|
| A. Elastic Net | B. Linear Regression | A. Elastic Net | B. Linear Regression | ||||
| Predictors | Elastic Net Coeff. | Est. | P | Predictors | Elastic Net Coeff. | Est. | P |
| FuG thickness | 5.93 | 1.42 | 0.05 | FuG thickness | 0.45 | 0.00 | 0.16 |
| ERC tau | −6.60 | −2.83 | 0.00 | ERC tau | −0.95 | −0.36 | 0.001 |
| Aβ × FuG tau | −0.33 | −1.84 | 0.03 | Aβ × FuG tau | 0.00 | – | – |
| Age | 0.00 | – | – | Age | 0.00 | – | – |
| Aβ | 0.00 | 0.54 | 0.53 | Aβ | 0.00 | – | – |
| PHC tau | 0.00 | – | – | PHC tau | −0.20 | −0.01 | 0.92 |
| FuG tau | 0.00 | 1.49 | 0.19 | FuG tau | 0.00 | – | – |
| PHC thickness | 0.00 | – | – | PHC thickness | −0.21 | 0.09 | 0.28 |
| ERC thickness | 0.00 | – | – | ERC thickness | 0.16 | 0.08 | 0.31 |
| Model Summaries | |||||
|---|---|---|---|---|---|
| PN Retrieval | |||||
| A. Elastic Net | B. Linear Regression | ||||
| α | λ | RMSE | R2 | P | R2 |
| 0.55 | 1.21 | 6.56 | 0.18 | < 0.001 | 0.27 |
| Episodic Memory | |||||
| A. Elastic Net | B. Linear Regression | ||||
| α | λ | RMSE | R2 | P | R2 |
| 0.33 | 0.17 | 0.80 | 0.12 | < 0.001 | 0.28 |
Discussion
These findings link the common age-related complaint of PN retrieval to tau deposition in the fusiform and parahippocampal gyrus in cognitively healthy older adults. The data demonstrate that Aβ does not seem to underlie PN retrieval failures but moderates the association with tau, consistent with other studies showing that Aβ does not correlate with cognitive measures but potentiates the effects of tau2,6,7.
The localization of tau in the voxelwise analysis highlights the importance of left-lateralized brain regions for the retrieval of person-specific information21. Our results align with prior literature in that the fusiform is unique to PN retrieval, whereas the parahippocampal gyrus (including the entorhinal and parahippocampal cortices) overlaps with episodic memory2,22. The entorhinal, parahippocampal, and fusiform cortices are affected by early tau pathology, which consequently affects memory networks8. Our results suggest that these networks link identity-specific semantic information, phonological representations, and episodic memory, and become dysfunctional with aging and attendant pathology3,5,22. These results thus align with prior research implicating a network of interacting neural systems that aid in the processing and retrieving of proper names23.
Regardless of underlying pathology, the nature of proper names as ‘arbitrary labels’ makes them a source of vulnerability in memory, requiring a higher level of cognitive demand1,24,25. It is possible that slight disruptions in this network may result in noticeable cognitive changes, leading to PN retrieval failures despite intact episodic retrieval of common nouns and events. It is important to note that our measure of PN retrieval uses retrieval of famous people’s names. Research has shown that difficulty in PN retrieval can vary depending on the type of name, such that individuals sometimes find famous names and proper names of places easier to retrieve than names of acquaintances24. This may be due to the salience and exposure of the name to the person across the lifespan.
Taken together, our findings show that impaired PN retrieval is associated with increased tau deposition in healthy older adults and shares features with episodic memory processes. As previous studies have acknowledged, PN retrieval measures may be able to identify subtle cognitive changes associated with early pathological processes26. In both a voxelwise and penalized regression setting, we have shown that in addition to the effect of entorhinal tau pathology there is a novel effect of fusiform tau and thickness. This demonstrates that PN retrieval is influenced by the spread of tau outside of the entorhinal cortex, which is potentiated by increased Aβ. Our data cannot definitively address the question of whether these cognitive symptoms reflect the early stages of Alzheimer’s disease consequent to Aβ and tau pathology or whether they are a more benign expression of normal aging, reflecting primarily medial temporal lobe tau. However, the very high prevalence of naming complaints among older individuals suggests that the ubiquity of medial temporal tau and its propensity to affect brain regions involved in PN retrieval influences this process.
Acknowledgements:
This research was supported by NIH grants AG034570, AG062542, AG062090, and AG065501. Avid Radiopharmaceuticals enabled the use of the 18F-Flortaucipir tracer but did not provide direct funding and were not involved in data analysis or interpretation.
Footnotes
Potential conflicts of interest: We have no conflicts to disclose.
References
- 1.O’Rourke T & de Diego Balaguer R Names and their meanings: A dual-process account of proper-name encoding and retrieval. Neuroscience and Biobehavioral Reviews (2020) doi: 10.1016/j.neubiorev.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Maass A et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J. Neurosci (2018) doi: 10.1523/JNEUROSCI.2028-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza C The neuropsychology of proper names. Mind Lang. (2009) doi: 10.1111/j.1468-0017.2009.01366.x. [DOI] [Google Scholar]
- 4.Greenberg DL & Verfaellie M Interdependence of episodic and semantic memory: Evidence from neuropsychology. Journal of the International Neuropsychological Society vol. 16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda K, Nakamura T & Beckman B Brain processing of proper names. Aphasiology (2000) doi: 10.1080/02687030050174638. [DOI] [Google Scholar]
- 6.Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica (1991) doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Karran E, Mercken M & Strooper B. De. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nature Reviews Drug Discovery (2011) doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 8.Maass A et al. Alzheimer’s pathology targets distinct memory networks in the ageing brain. Brain (2019) doi: 10.1093/brain/awz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gefen T et al. Naming vs knowing faces in primary progressive aphasia: A tale of 2 hemispheres. Neurology (2013) doi: 10.1212/WNL.0b013e3182a08f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds CR & Powel J Wechsler memory scale-revised. Arch. Clin. Neuropsychol (1988) doi: 10.1093/arclin/3.4.397. [DOI] [Google Scholar]
- 11.Maass A et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage (2017) doi: 10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desikan RS et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage (2006) doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Baker SL, Maass A & Jagust WJ Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Br. (2017) doi: 10.1016/j.dib.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mormino EC et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex (2011) doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villeneuve S et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain (2015) doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousset OG, Ma Y & Evans AC Correction for partial volume effects in PET: Principle and validation. J. Nucl. Med (1998). [PubMed] [Google Scholar]
- 17.Tomaschek F, Hendrix P & Baayen RH Strategies for addressing collinearity in multivariate linguistic data. J. Phon 71, 249–267 (2018). [Google Scholar]
- 18.Zou H & Hastie T Regularization and variable selection via the elastic net. 301–320 (2005). [Google Scholar]
- 19.Max A et al. Package ‘ caret ‘ R topics documented : (2021).
- 20.Hastie T, Martin RT, Hastie W, Tibshirani, • & Wainwright, •. Statistical Learning with Sparsity The Lasso and Generalizations Statistical Learning with Sparsity. [Google Scholar]
- 21.Gainotti G Cognitive models of familiar people recognition and hemispheric asymmetries. Frontiers in Bioscience - Elite (2014) doi: 10.2741/e698. [DOI] [PubMed] [Google Scholar]
- 22.Aminoff EM, Kveraga K & Bar M The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences (2013) doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damasio H, Tranel D, Grabowski T, Adolphs R & Damasio A Neural systems behind word and concept retrieval. Cognition (2004) doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Cohen G & Burke DM Memory for Proper Names: A Review. Memory (1993) doi: 10.1080/09658219308258237. [DOI] [PubMed] [Google Scholar]
- 25.Rentz DM et al. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia (2011) doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller KD et al. Proper names from story recall are associated with beta-amyloid in cognitively unimpaired adults at risk for Alzheimer’s disease. Cortex (2020) doi: 10.1016/j.cortex.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


