Abstract
Purpose:
Poly (ADP-ribose) polymerase (PARP) inhibitors synergize with topoisomerase inhibitors, and veliparib plus modified (m) FOLFIRI (no 5-FU bolus) had preliminary activity in metastatic pancreatic cancers (mPC). This study evaluated the safety and efficacy of second-line treatment with veliparib and mFOLFIRI vs FOLFIRI (control) for mPC.
Patients and Methods:
This randomized phase II clinical trial led by the SWOG Cancer Research Network enrolled patients between September 1, 2016 and December 13, 2017. The median follow-up was 9 months (IQR 1-27). BRCA1/2 and homologous recombination DNA damage repair (HR-DDR) genetic defects were tested in blood and tumor biopsies. Patients received veliparib 200 mg twice daily, days 1-7 with mFOLFIRI days 3-5, or FOLFIRI in 14 days-cycles.
Results:
After 123 of planned 143 patients were accrued, an interim futility analysis indicated the veliparib arm was unlikely to be superior to control, and the study was halted. Median survival (OS) was 5.4 vs 6.5 months (HR 1.23, p=0.28), and median progression free survival (PFS) was 2.1 vs 2.9 months (HR 1.39, p=0.09) with veliparib vs control. Grade 3/4 toxicities were more common with veliparib (69% vs 58%, p=0.23). For cancers with HR-DDR defects vs wild type, median PFS and OS were 7.3 vs 2.5 months (p=0.05), and 10.1 vs 5.9 months (p=0.17), respectively with FOLFIRI, and 2.0 vs 2.1 months (p=0.62) and 7.4 vs 5.1 months (p=0.10), respectively with veliparib plus mFOLFIRI.
Conclusion:
Veliparib plus mFOLFIRI did not improve survival for mPC. FOLFIRI should be further studied in pancreatic cancers with HR-DDR defects.
Trial Registration:
ClinicalTrials.gov identifier NCT02890355.
Introduction
Metastatic pancreatic ductal adenocarcinoma takes an immense toll on life (1). After progression from first-line therapies, second-line regimens include 5-fluorouracil (5-FU), folinic acid with liposomal irinotecan or irinotecan (FOLFIRI) with median survival of 6 months (2, 3). Pancreatic cancer is characterized by genomic instability and may harbor loss of function mutations in the BRCA-Fanconi anemia (FA) pathway: breast cancer genes 1 and 2 (BRCA1, BRCA2), partner and localizer of BRCA2 (PALB2), and FA genes (4, 5). Additional homologous recombination (HR) DNA damage repair (DDR) gene defects contribute to homologous recombination deficiency (HRD) in approximately 15% of patients (6). The high lethality of pancreatic cancer stems partially from its resistance to cytotoxic agents. Poly (ADP-ribose) polymerase (PARP) enzymes contribute to repair from topoisomerase 1-associated DNA damage (7), and PARP inhibition increases cytotoxicity from camptothecins (8, 9).
PARP inhibitors have modest single agent activity for previously treated or refractory pancreatic cancers associated with germline BRCA1/2/PALB2 loss of function mutations, with overall response rates of 0-22%, progression-free survival averaging 2-4 months, and overall survival up to 10 months (10-13). Nevertheless, olaparib received FDA approval for patients with germline BRCA1/2 mutated cancers as maintenance therapy after response or stable disease from first-line platinum chemotherapy, due to improved progression-free survival (7.4 months with olaparib vs 3.8 months with placebo, p=0.004) (14). Studies of veliparib with 5-FU, folinic acid, and oxaliplatin (FOLFOX) (15), or with gemcitabine plus cisplatin (16,17) demonstrated response rates of 30-77% and survival rates of 9-23 months for patients with germline BRCA1/2/PALB2 mutated cancers. The combination of FOLFIRI with veliparib was tested in a phase I trial among 92 patients with refractory solid tumors (18). Veliparib was dosed from 10 mg to 270 mg BID. To maximize PARP inhibition around the time of FOLFIRI administration and to minimize toxicities, especially myelosuppression, veliparib was dosed intermittently on days 1-5 and days 15-19, with FOLFIRI dosed on days 1-3 and days 15-17 in 28-day cycles. Dose limiting toxicities were grade 4 neutropenia (n=2 at 160 and 270 mg BID), grade 4 febrile neutropenia (100 mg BID) and grade 3 gastritis (270 mg BID). To improve tolerability, the 5-FU bolus was discontinued, and the recommended phase 2 dose was veliparib 200 mg BID with modified (m) FOLFIRI (FOLFIRI without 5FU bolus). Response and stable disease rates among 14 patients with refractory pancreatic cancers without known BRCA1/2 mutations were 14% (n=2, lasting 10 and 25 months) and 43% (n=6), respectively, and the 6-month time-to-progression was 27%. Given the preclinical synergism between veliparib and irinotecan (7-9) and phase I data demonstrating safety and preliminary efficacy of veliparib combined with mFOLFIRI, including for cancers without known BRCA1/2/PALB2 mutations (18), we designed a randomized phase II study to assess whether veliparib improves survival when added to mFOLFIRI compared to FOLFIRI alone for second-line treatment of metastatic pancreatic ductal adenocarcinoma. Secondary objectives were to evaluate overall response rate, progression-free survival, safety, and tolerability, with translational objectives to evaluate if germline or somatic BRCA1/2 mutations and other HR-DDR gene alterations were associated with efficacy outcomes in each arm.
PATIENTS and METHODS
Study Design and Participants
This study was conducted by the SWOG Cancer Research Network and the National Cancer Institute (NCI) National Clinical Trials Network (NCTN), with 28 participating institutions. The applicable regulations and guidelines governing clinical study conduct were followed and the study was performed in compliance with the Declaration of Helsinki. Participating site institutional review board approval and patient written informed consent were obtained prior to enrollment. Pertinent eligibility criteria were age ≥18 years, adequate organ function, Zubrod performance status 0-1, no prior irinotecan or PARP inhibitors, and either one prior therapy for metastatic pancreatic cancer, or progression to metastatic disease within 3 months of gemcitabine/nab-paclitaxel treatment for localized disease. Patients must have been able and willing to undergo pre-treatment biopsy and submit tumor and blood samples for research purposes; archival tumor sample, if available, was required. The trial protocol is available in the Supplementary Appendix 1.
Procedures
Each 2-week cycle of FOLFIRI consisted of irinotecan 180 mg/m2, folinic acid 400 mg/m2, 5-FU bolus 400 mg/m2 and 5-FU infusion 2,400 mg/m2 according to published guidelines (19). mFOLFIRI consisted of irinotecan 180 mg/m2, folinic acid 400 mg/m2, and 5-FU infusion 2,400 mg/m2 with no 5-FU bolus (18). Veliparib (ABT-888, NSC-737664) was provided by the NCI’s Division of Cancer Therapy Evaluation Program (CTEP). To maximize PARP inhibition prior to and during chemotherapy dosing, veliparib was administered orally 200 mg twice daily (BID) on days 1-7, with mFOLFIRI on days 3-5 of every 2-week cycles. If irinotecan or 5-FU infusion were held for toxicity, veliparib was held. Dose reductions for FOLFIRI and mFOLFIRI followed published guidelines (19). Veliparib could be reduced to 150 mg BID, 100 mg BID and 50 mg BID for toxicities.
Adverse events were assessed for severity using NCI Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 (20). Patients were evaluated for tumor response every 8 weeks according to modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (21). Blood and tumor samples from fresh biopsies, and archival samples if available, were collected prior to starting treatment. Blood and tumor DNA was sequenced using BROCA-HR, a targeted capture, massively parallel sequencing test developed at University of Washington (22). BROCA-HR captures > 99% of deleterious BRCA1/2 mutations (including gene rearrangements and copy number variations), and includes other genes in the FA-BRCA HR, mismatch repair, nucleotide excision repair (NER), non-homologous end joining (NHEJ) pathways as well as surveillance and modifier genes of interest (Supplementary Table S1) (23). Homologous recombination DNA damage repair gene defects were categorized in four groups based on degree of HRD conferred by each gene: 1) BRCA1/2; 2) core non-BRCA1/2: PALB2, ATM, RAD51C/D, BRIP1, BARD1; 3) non-core genes which could impact DNA repair: e.g., FANCA-M, CDK12, CHEK2, BLM, SLX4, ERCC1/4; 4) wild type, which includes non-homologous recombination DDR genes: e.g., EZH2, MSH2, MSH6, POLD1, POLE, RIF1, WRN.
Outcomes
The primary outcome was overall survival defined as time from randomization to death of any cause. Secondary outcomes included toxicity; progression-free survival defined as time from randomization to disease progression, symptomatic deterioration, or death by any cause; overall response rate defined as confirmed and unconfirmed complete and partial response, disease control rate defined as confirmed and unconfirmed complete and partial response and confirmed and unconfirmed stable disease; and duration of response defined as time from first documentation of response to progression-free survival failure. Translational secondary outcomes were to evaluate the impact of germline and somatic BRCA1/2 and other homologous recombination DNA damage repair genetic defects on efficacy outcomes in each arm.
Statistical Methods
Patients were enrolled by the treating physicians at the participating hospitals. Patients were randomly assigned in equal proportions to the treatment arms using a dynamic balancing algorithm with stratification by prior systemic treatment for metastatic pancreatic cancer (yes vs no) (24). In this open-label study, patients, investigators, and the study team were not masked to study treatment.
We hypothesized the median survival with FOLFIRI to be 6 months (2). Assuming a one-sided type I error of 10%, approximately 28 months accrual, and 10 months follow-up, 128 eligible patients provided 80% power to detect a 33% increase in median survival to 9 months (hazard ratio [HR]=0.67) with veliparib plus mFOLFIRI. Approximately 110 deaths were expected at the time of the final analysis. An interim futility analysis of progression-free survival was planned when 35% of the expected events (approximately 40 events) in the control arm were observed; evidence suggesting early closure consisted of rejection of the alternative hypothesis (HR=0.67) at one-sided p<0.05. For overall and progression-free survival and duration of response, censoring occurred at the date of last contact. The study did not require selection or stratification based on BRCA1/2 mutation status.
The primary analysis of overall survival was conducted in all eligible and evaluable patients according to the intent-to-treat principle. Probabilities of overall survival, progression-free survival, and duration of response were estimated using the Kaplan-Meier method, with statistical differences in event rates between treatment arms assessed via stratified log-rank test. Overall response rate and disease control rate were compared across treatment arm by Chi-square or Fisher’s exact test in patients with measurable disease. Eligible patients who received at least one dose of any drug were included in the safety analysis. Prespecified analyses between homologous recombination DNA damage repair gene defects and efficacy outcomes were conducted. Associations of HR-DDR gene defects groups: any (core + non-core), and wild-type with overall and progression-free survival were estimated via the Kaplan-Meier method and Cox regression where feasible. Clinical benefit response was defined as overall response plus stable disease lasting for at least 4 months from randomization, and differences in clinical benefit response between HR-DDR gene defects groups were assessed via Fisher’s exact test. All p-values were two-sided with significance level of 5%, apart from the planned interim futility analysis. Analyses were carried out using SAS version 9.4 and R version 3.5.2.
RESULTS
A total of 123 patients were randomized between September 1, 2016 and December 13, 2017 (Fig. 1). Four patients were deemed ineligible due to not receiving prior systemic therapy for metastatic pancreatic cancer or for localized disease with metastatic progression within 3 months from treatment (n=3) or inadequate hematologic function (n=1); two eligible patients refused their assigned FOLFIRI treatment and follow-up and were excluded from efficacy and safety analyses. Baseline characteristics for 117 eligible patients were balanced (Table 1). Among all eligible patients enrolled, 55 (93%) and 50 (86%) in the veliparib and control arm, respectively received prior treatment with gemcitabine plus nab-paclitaxel. No patients were previously treated with FOLFIRINOX. The study was closed to accrual following review of the interim futility analysis by the SWOG Data and Safety Monitoring Committee, given the results suggested that veliparib plus mFOLFIRI was unlikely to be more effective than FOLFIRI (control), based on rejection of the progression-free survival alternative hypothesis. All patients assigned to receive veliparib were advised to discontinue veliparib but were allowed to continue on study with mFOLFIRI alone.
Figure 1:
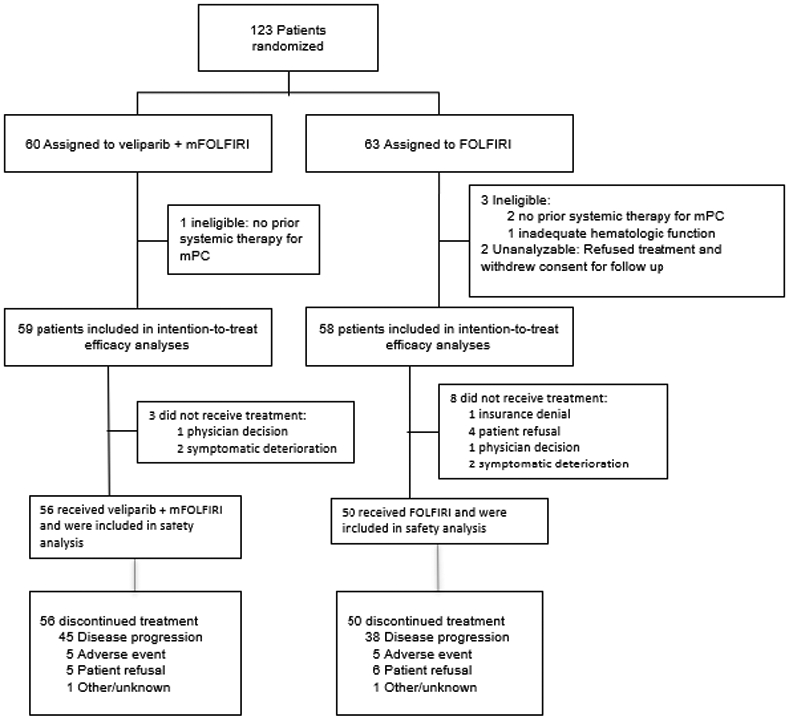
CONSORT Flow Diagram
Random assignment and patient disposition in each treatment arm
Abbreviations: FOLFIRI, folinic acid, 5-fluorouracil (5-FU), irinotecan; mFOLFIRI modified FOLFIRI (no 5-FU bolus)
Table 1:
Patients Characteristics
| Veliparib + mFOLFIRI n=59 |
FOLFIRI n=58 |
|
|---|---|---|
| Age (years) | ||
| median (IQR) | 67 (61 - 72) | 67 (61 - 71) |
| Males | 31 (53%) | 33 (57%) |
| Females | 28 (47%) | 25 (43%) |
| Zubrod (ECOG) PS | ||
| 0 | 14 (24%) | 23 (40%) |
| 1 | 45 (76%) | 35 (60%) |
| Race | ||
| White | 52 (88%) | 47 (81%) |
| Black | 4 (7%) | 5 (9%) |
| Asian | 1 (2%) | 1 (2%) |
| Native American | 1 (2%) | 0 |
| Unknown | 1 (2%) | 5 (9%) |
| Prior treatment for metastatic PCa | ||
| Yes | 53 (90%) | 52 (90%) |
| No | 6 (10%) | 6 (10%) |
| Prior treatments | ||
| Gemcitabine + nab-Paclitaxel | 55 (93%) | 50 (86%) |
| Gemcitabine + capecitabine | 3 (5%) | 1 (2%) |
| Gemcitabine + oxaliplatin + 5-FU | 0 | 1 (2%) |
| Gemcitabine | 0 | 2 (3%) |
| Palbociclib + nab-Paclitaxel | 0 | 2 (3%) |
| FOLFOX | 1 (2%) | 2 (3%) |
| HR-DDR Defects Groupsb | ||
| Group 1: BRCA1/2 | 3 (5%) | 1 (2%) |
| Group 2: core non-BRCA1/2 | 1 (2%) | 4 (7%) |
| Group 3: non-core | 8 (13%) | 5 (9%) |
| Group 4: wild type | 47 (80%) | 46 (82%) |
Patients were eligible if they received one prior line of treatment for metastatic disease or if they were treated with gemcitabine plus nab-paclitaxel for localized pancreatic cancer and developed metastatic disease within 3 months from treatment completion
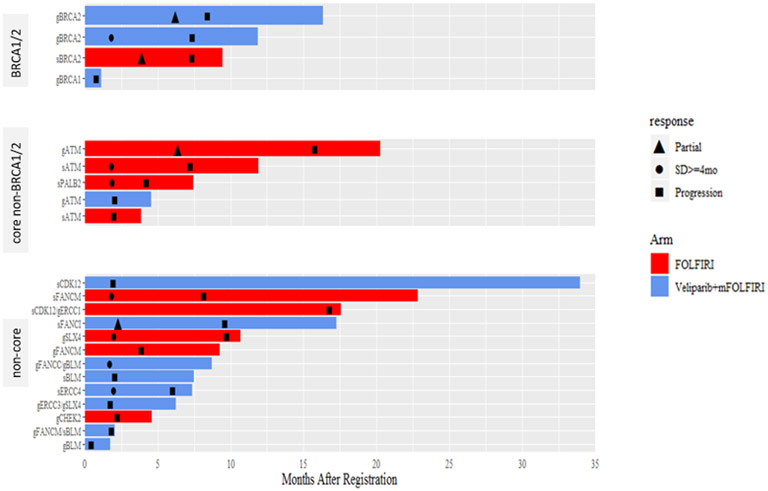
115 patients (59 veliparib arm, 56 control arm) had BROCA-HR analysis: core non-BRCA1/2 genes: ATM (n=4), PALB2 (n=1) non-core HR-DDR genes: BLM (n=2), CDK12, CHEK2, FANCI, FANCM, SLX4. 6 patients had multiple gene defects: CDK12/ERCC1, ERCC4/RIF1, FANCC/BLM, FANCM/BLM, FANCM/MSH6, SLX4/ERCC3
Abbreviations: HR-DDR, homologous recombination DNA damage repair; PC, pancreatic cancer; PS, performance status
Safety and toxicity
The safety analysis included 106 patients, since eleven eligible patients never started treatment. Treatment-related grade 3/4 toxicities observed more often with veliparib vs control were neutropenia, fatigue, nausea, and diarrhea (Table 2). No treatment-related grade 5 toxicities were reported. More patients experienced ≥ grade 3 toxicities in the veliparib arm vs control: 69% vs 58% (p=0.23). Patients had similar treatment exposure: 4 (IQR 3-8) vs 5 (IQR 3-14) cycles (p=0.15) with veliparib vs control (Table 3), and similar proportions had at least one dose reduction, dose held for toxicity, or discontinuation of chemotherapy: 66% vs 56% (p=0.39).
Table 2:
Grades 3 and 4 Treatment-Related Toxicities
| Toxicity | Veliparib + mFOLFIRI (n=56) |
FOLFIRI (n=50) |
p | ||
|---|---|---|---|---|---|
| Grade 3 n (%) |
Grade 4 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
||
| Alkaline phosphatase increased | 2 (4%) | 0 | 3 (6%) | 0 | 0.66 |
| Anemia | 4 (7%) | 0 | 5 (10%) | 0 | 0.73 |
| Hyperbilirubinemia | 2 (4%) | 2 (4%) | 4 (8%) | 1 (2%) | 0.73 |
| Dehydration | 6 (11%) | 0 | 1 (2%) | 0 | 0.12 |
| Diarrhea | 6 (11%) | 0 | 3 (6%) | 0 | 0.50 |
| Fatigue | 10 (18%) | 0 | 3 (6%) | 0 | 0.08 |
| Hyponatremia | 2 (4%) | 0 | 3 (6%) | 0 | 0.66 |
| Lymphopenia | 3 (5%) | 0 | 6 (12%) | 0 | 0.30 |
| Nausea | 7 (12%) | 0 | 2 (4%) | 0 | 0.17 |
| Neutropenia | 10 (18%) | 9 (16%) | 9 (18%) | 2 (4%) | 0.20 |
| Vomiting | 5 (9%) | 0 | 2 (4%) | 0 | 0.44 |
| Leukopenia | 5 (9%) | 1 (2%) | 3 (6%) | 0 | 0.50 |
| Maximum grade any event | 26 (46%) | 13 (23%) | 25 (50%) | 4 (8%) | 0.23 |
Table 3:
Treatment Exposure
| Veliparib + mFOLFIRI |
FOLFIRI | |
|---|---|---|
| Nr. cycles | (n=56) | (n=50) |
| median (IQR)a | 4 (3 - 8) | 5 (3 - 14) |
| ≥ 2 cycles | 51 (91%) | 47 (94%) |
| ≥ 4 cycles | 38 (68%) | 37 (74%) |
| ≥ 6 cycles | 19 (34%) | 23 (46%) |
| ≥ 8 cycles | 15 (27%) | 21 (42%) |
p=0.15
Efficacy
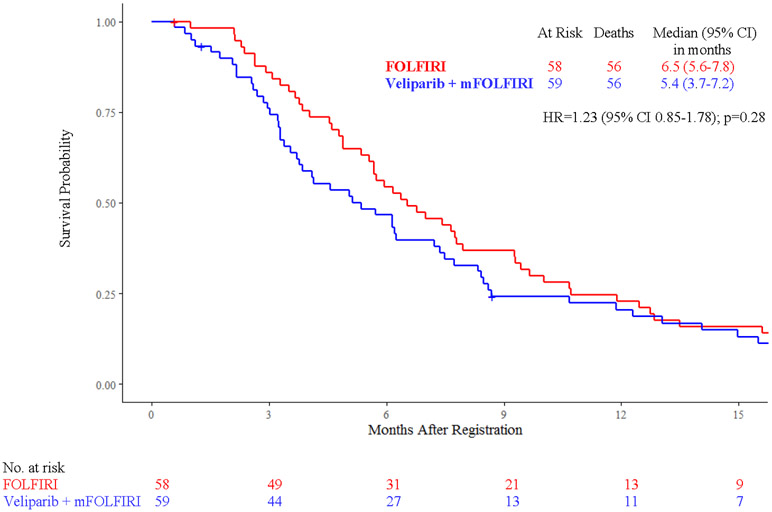
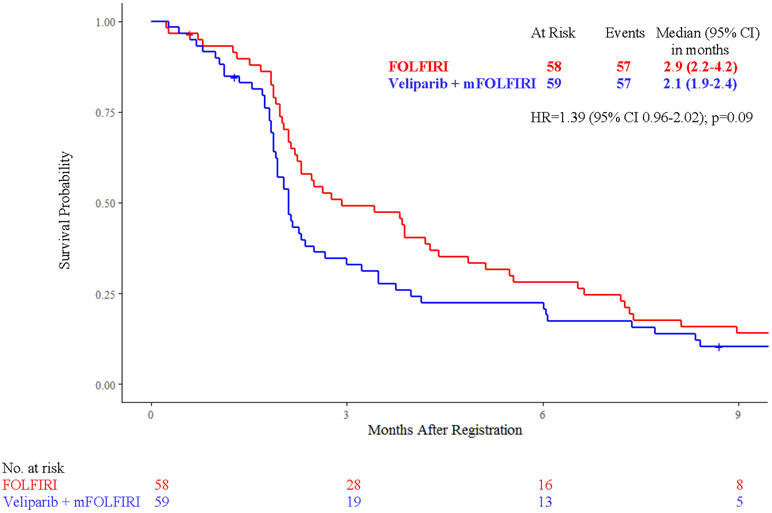
Efficacy analyses were conducted in 117 patients, with median follow up of 9 months (IQR 1-27). Median survival did not vary significantly between treatment arms: 5.4 months [95% confidence interval (CI) 3.7 - 7.2] with veliparib and mFOLFIRI vs 6.5 months (95% CI 5.6 - 7.8) with FOLFIRI (HR 1.23, 95% CI 0.85 - 1.78, p = 0.28) (Fig. 2A). Median progression-free survival was 2.1 months (95% CI 1.9 - 2.4) with veliparib and mFOLFIRI vs 2.9 months (95% CI 2.2 - 4.2) with FOLFIRI (HR 1.39, 95% CI 0.96 - 2.02, p=0.09) (Fig. 2B). Among 106 patients treated, 103 patients (55 in the veliparib arm and 48 in the control arm) had adequate response assessment. Six [11% (95% CI 2.7% - 19.2%)] vs five patients [10% (95% CI 1.8% - 19.1%), p=0.99] had partial response (confirmed or unconfirmed), and 14 [25% (95% CI 13.9% - 37%) vs 21 patients [44% (95% CI 29.7% - 57.8%), p=0.06] had stable disease with veliparib vs control, respectively. Disease control rates were 36% (95% CI 24% - 49%) vs 54% (95% CI 40% - 68%, p=0.07), and median duration of response was 5.1 months (95% CI 2.2 - 5.2) vs 3.4 months (95% CI 2.6 - 3.4, p=0.99) with veliparib vs control, respectively.
Figure 2:
Kaplan-Meier Curves of A) Overall Survival and B) Progression-Free Survival by Treatment Arm
Abbreviations: FOLFIRI, folinic acid, 5-fluorouracil (5-FU), irinotecan; mFOLFIRI modified FOLFIRI (no 5-FU bolus); HR, hazard ratio
Molecular biomarkers and correlations with efficacy
Of 117 patients who underwent BROCA-HR testing, 115 were eligible, and 102 had adequate response assessment. Twenty-two patients (19%) had cancers with HR-DDR gene defects and 10 patients (9%) had cancers with other non-homologous recombination DDR gene defects (Supplementary Table S2)
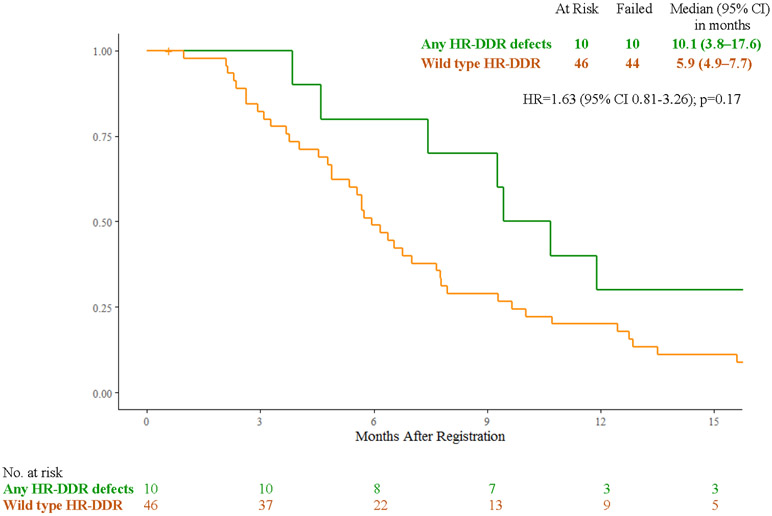
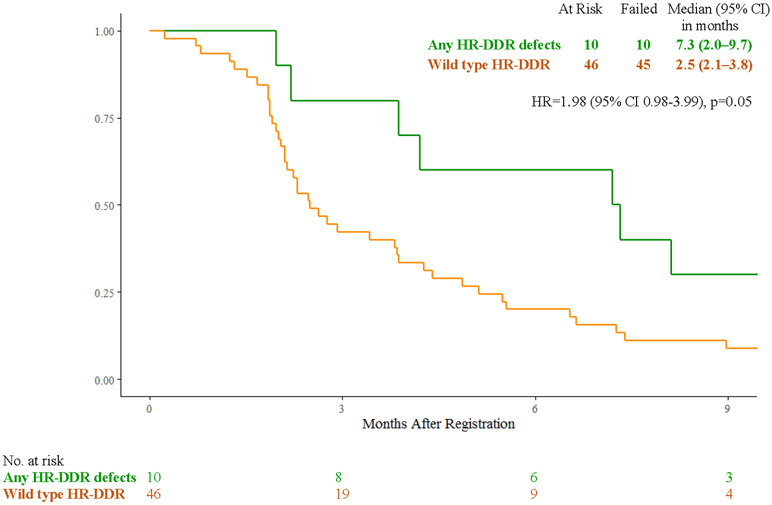
In the FOLFIRI arm, patients with cancers with any HR-DDR gene defects (n=10) vs wild-type (n=46) had median overall survival of 10.1 months (95% CI 3.8 – 17.6) vs 5.9 months (4.9 – 7.7) (HR 1.63, 95% CI 0.81 - 3.26, p=0.17), median progression-free survival of 7.3 months (95% CI 2.0 – 9.7) vs 2.5 months (95% CI 2.1 – 3.8) (HR 1.98, 95% CI 0.98 – 3.99, p=0.05), response rates of 20% (95% CI 0.01% - 44.8%) vs 8% (95% CI 0% - 16.9%, p=0.29), and clinical benefit response rates of 70% (95% CI 41.6% - 98.4%) vs 35% (95% CI 19.8% - 50.5%) (p=0.07) (Fig. 3A, 3C, 3E).
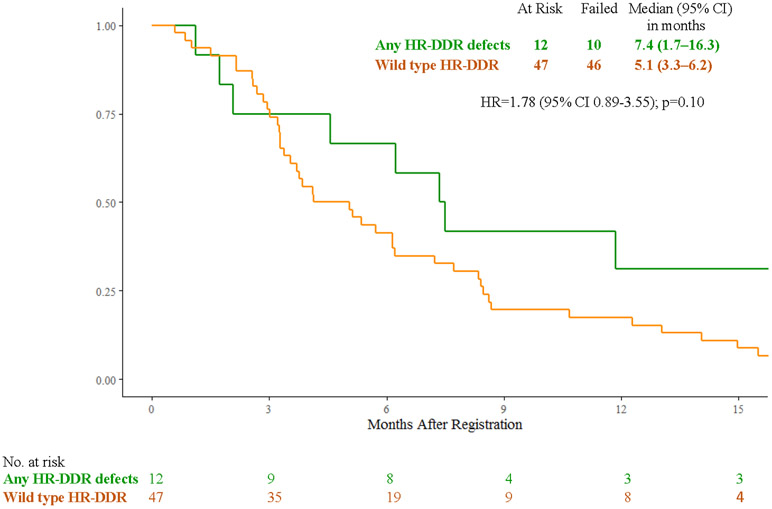
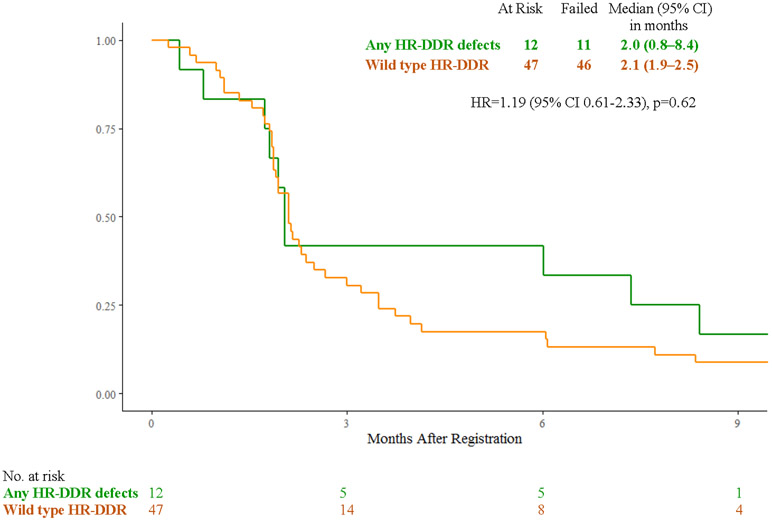
Figure 3:
A) Overall Survival with FOLFIRI for Cancers with HR-DDR Gene Defects vs Wild Type; B) Overall Survival with Veliparib and mFOLFIRI for Cancers with HR-DDR Gene Defects vs Wild Type; C) Progression-Free Survival with FOLFIRI for Cancers with HR-DDR Gene Defects vs Wild Type; D) Progression-Free Survival with Veliparib and mFOLFIRI for Cancers with HR-DDR Gene Defects vs Wild Type; E) Best Response and Survival for Patients with HR-DDR Gene Defects
Abbreviations: HR, hazard ratio; HR-DDR, homologous recombination DNA damage repair; OS, overall survival; PFS, progression-free survival
In the veliparib plus mFOLFIRI arm, patients with cancers with any HR-DDR gene defects (n=12) vs wild-type (n=47) had median overall survival of 7.4 months (95% CI 1.7 – 16.3) vs 5.1 months (95% CI 3.3 – 6.2) (HR 1.78, 95% CI 0.89 – 3.55, p=0.10), median progression-free survival of 2.0 months (95% CI 0.8 - 8.4) vs 2.1 months (95% CI 1.9 – 2.5) (HR 1.19, 95% CI 0.61 – 2.33, p=0.62), response rates of 18% (95% CI 0% - 41%) vs 9% (95% CI 0.60% - 17.6%), (p=0.59), and clinical benefit response rates of 45% (95% CI 16% - 74.9%) vs 25% (95% CI 12.2% - 37.8%), (p=0.27) (Fig. 3B, 3D, 3E). Efficacy outcomes for individual carriers of core and non-core HR-DDR gene defects (n=22) treated with veliparib and mFOLFIRI or FOLFIRI are shown in Fig. 3E and Supplementary Table S2.
DISCUSSION
SWOG S1513 was a randomized phase II study to assess whether the PARP inhibitor veliparib improved survival when added to second line FOLFIRI chemotherapy for metastatic pancreatic cancer patients. The study closed to accrual after the interim analysis (123 patients accrued of 143 patients planned) demonstrated futility with the addition of veliparib. Veliparib did not improve overall survival in combination with mFOLFIRI compared to FOLFIRI alone (5.4 vs 6.5 months, HR=1.23, p=0.28). Notably, the median survival of 6.5 months with FOLFIRI was comparable to the survival of 6.2 months observed with the NAPOLI-1 regimen nal-Iri/5-FU (3).
This was the first study performed through the NCI sponsored National Clinical Trials Network that prospectively tested the presence of germline and somatic DNA damage repair defects in metastatic pancreatic cancer patients and assessed their effects on outcomes with chemotherapy with or without a PARP inhibitor. While the presence of germline or somatic BRCA1/2/PALB2 mutations was not required for study enrollment, 117 patients (95%) provided blood and tumor samples which were analyzed for homologous recombination DNA damage repair gene defects. Twenty-two patients (19%) had germline or somatic defects, including five (4%) patients with BRCA1/2/PALB2 mutations. Small numbers preclude definitive conclusions, but the addition of veliparib did not benefit patients with BRCA1/2 or any HR-DDR defects: median OS 7.4 vs 10.1 months (p=0.65), median PFS 2.0 vs 7.3 months (p=0.15), and response rates 18% vs 20% (p=0.99) with veliparib vs control, respectively.
Toxicities, especially neutropenia, nausea, vomiting, diarrhea, dehydration, and fatigue, were increased with veliparib, although no differences reached statistical significance. While neither the median number of cycles received, nor the incidence of chemotherapy dose modifications varied significantly between arms, numerically fewer chemotherapy cycles were administered in the veliparib arm, which may have led to inferior outcomes. We have not conducted a pharmacokinetic analysis in this study, but previous studies noted no drug-drug interactions between veliparib and irinotecan or veliparib and FOLFIRI, suggesting that veliparib should not have influenced FOLFIRI drug exposure (18, 25).
Based on phase 1 data (18), we dosed veliparib at 200 mg BID on days 1-7 with mFOLFIRI on days 3-5, every 14-days. O’Reilly et al tested first-line treatment with cisplatin/gemcitabine with or without veliparib 80 mg BID on days 1-12 of every 21-days cycle in gBRCA1/2/PALB2 mutations carriers and observed high responses (65-74%) and survival rates (medians 15.5-16.4 months) in both arms, but no benefit from the addition of veliparib (17). Veliparib has been the only PARP inhibitor which could safely combine with chemotherapy, albeit at lower doses than used as monotherapy (400 mg BID), but no evidence of clinical synergism has been observed to date in metastatic pancreatic cancer patients.
The VELIA/GOG-3005 study in ovarian carcinomas showed that veliparib 150 mg BID with carboplatin/paclitaxel, followed by 400 mg BID maintenance significantly improved progression-free survival in patients with BRCA1/2 mutated high grade serous ovarian carcinomas, as well as in cancers with or without HRD compared to chemotherapy alone (PFS 34.7 vs 22 months, 32 vs 20.5 months, and 23.5 vs 17.3 months, respectively) (26). While it is possible that the maintenance (100% dose intensity) rather than the concurrent administration with chemotherapy (37.5% dose intensity) conferred the progression-free survival benefit, to date, VELIA/GOG-3005 is one of the few clinical trials where a PARP inhibitor added to chemotherapy remarkably improved efficacy (p<0.001). Veliparib dosed intermittently at 120 mg BID or 100 mg BID, respectively improved progression-free survival when added to carboplatin/paclitaxel in gBRCA1/2 mutated breast cancers (medians 14.5 vs 12.6 months, p=0.0016) (27), or with cisplatin/etoposide in small cell lung cancers (medians 6.1 vs 5.5 months, p=0.06) (28), but the benefit was modest. Studies to date in pancreatic cancer have not tested continuously dosed veliparib with standard chemotherapy. It is not clear whether the lack of benefit from veliparib in SWOG S1513 was due to the lower than standard dose (50%) and/or intermittent dosing, or, alternatively the lower incidence of HR-DDR defects (approximately 20%) compared to high grade serous ovarian carcinomas (50% HRD) which are inherently more susceptible to PARP inhibition.
It has been postulated that mechanistic differences in catalytic activity and the capacity for PARP1 trapping on DNA account for differential PARP inhibitors’ cytotoxicity or ability to combine with chemotherapy (29), with olaparib and rucaparib having increased PARP1 trapping capacity compared to veliparib. Few patients seem to benefit from PARP inhibitors after progression from first line therapy (10-13). Nevertheless, PARP inhibitors are active as first line maintenance for platinum sensitive pancreatic cancers. Olaparib maintenance after response or stable disease from first line platinum chemotherapy in germline BRCA1/2 mutated pancreatic cancers improved progression-free survival (medians 7.4 vs 3.8 months, p=0.004), and response rates (23% vs 11.5%), but conferred no survival advantage compared to placebo (medians 19 vs 19.2 months, p=0.35) (12, 30). Rucaparib maintenance has also demonstrated encouraging activity, with response rates of 41.7%, median progression free and overall survival of 13.1 and 23.5 months, respectively among patients with germline or somatic BRCA1/2/PALB2 mutated, platinum-sensitive, advanced pancreatic cancers without radiologic or CA19-9 progression within 8 weeks after stopping treatment with platinum chemotherapy (31).
In SWOG S1513, patients with cancers with any germline or somatic HR-DDR gene defects vs wild type treated with FOLFIRI and with veliparib plus mFOLFIRI had numerically longer overall survival (10.1 vs 5.9 months, and 7.4 vs 5.1 months, respectively), and higher clinical benefit responses (70% vs 35%, and 45% vs 25%, respectively), suggesting a prognostic impact from treatment with FOLFIRI. Nevertheless, the small proportion of cancers with BRCA1/2 and other homologous recombination DDR gene defects makes it difficult to determine their predictive value in each treatment arm. Golan and Gallinger have recently described classifiers for an HRD signature and the importance of biallelic inactivation for BRCA1/2 and PALB2 in pancreatic cancers, which may be better predictors of response to DNA damaging agents and/or PARP inhibitors and should be tested in future studies (32).
Given the increased hematologic and gastrointestinal toxicity with chemotherapy and PARP inhibitors, new clinical trials, including SWOG S2001, are testing novel combinations of PARP inhibitors with immune checkpoint blockade, RAS/PI3K/AKT/MEK pathways inhibitors, cell cycle or other DNA damage repair inhibitors, hoping to enhance efficacy without overlapping toxicity (33-37).
In conclusion, SWOG S1513 demonstrated that second-line treatment with veliparib added to mFOLFIRI did not improve survival for patients with metastatic pancreatic cancer and found that HR-DDR genetic defects may be associated with improved prognosis with FOLFIRI chemotherapy. Identifying susceptibility biomarkers beyond BRCA1/2/PALB2 mutations to predict benefit from DNA damaging agents and PARP inhibitors is feasible, and novel treatment approaches for patients with distinct molecular signatures are urgently needed.
Supplementary Material
Translational Relevance.
PARP inhibitors synergize with topoisomerase inhibitors preclinically. SWOG S1513 compared FOLFIRI to veliparib plus mFOLFIRI (no 5-FU bolus) for second line treatment of patients with metastatic pancreatic cancer, and prospectively tested germline and somatic BRCA1/2/PALB2 mutations as well as other homologous recombination (HR) DNA damage repair (DDR) genetic defects to correlate with efficacy outcomes in each treatment arm. Veliparib did not improve survival when added to FOLFIRI. Pancreatic cancers with BRCA1/2/PALB2 mutations have favorable outcomes with platinum chemotherapy, but no prior studies assessed the efficacy of FOLFIRI chemotherapy for cancers with BRCA1/2/PALB2 or other HR-DDR genetic defects. In SWOG S1513 patients with pancreatic cancers harboring HR-DDR gene defects treated with FOLFIRI or with mFOLFIRI plus veliparib had numerically increased survival, progression-free survival (FOLFIRI arm), responses and clinical benefit responses compared to those with wild type status, suggesting a prognostic impact from treatment with FOLFIRI.
ACKNOWLEDGEMENTS:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers CA180888, CA180819, CA180820, CA180821, CA233230, CA189821, CA180818, CA189830, CA189856, CA189858, CA180826, CA190002, CA189957, CA180828, CA189971, CA180835, CA189809, CA46368, CA189960, CA180834, CA239767, CA189860, CA189861, CA189854, CA189872, CA189958, CA180801, and CA189848.
Footnotes
Meeting Presentation: These data were presented in part at the 2019 ASCO meeting, Chicago, IL (J Clin Oncol 37, 2019 (suppl; abstr 4014)
Conflict of Interest Statement:
E.G. Chiorean: consulting/advisory: Astra Zeneca, Bayer, Celgene, Eisai, Ipsen, Legend, Noxxon, Pfizer, Seattle Genetics, Sobi; research grants: NCI/NIH, Boehringer-Ingelheim, Bristol Meyers Squibb, Celgene, Clovis, Corcept, Fibrogen, Halozyme, Incyte, Lilly, MacroGenics, Merck, Halozyme, Rafael, Roche, Stemline.
K.A. Guthrie: no conflicts
P.A. Philip: consulting/advisory: Ipsen, Trisalus, AbbVie, Celgene, Astra Zeneca, Bayer, Merck, Merus; research grants: Celgene, Lilly, Ipsen, Bayer, QED, Incyte, Roche, Rafael, AAA, Novartis, Novocure
E.M. Swisher: no conflicts
F. Jalikis: no conflicts
J.M. Suga: consulting/advisory: Strata Oncology; research funding: NCI NCORP
M.J. Pishvaian: consulting/advisory: Caris Life Sciences, Celgene, Halozyme, Merck, Merrimack, Perthera, Sirtex Medical, AstraZeneca/MedImmune, RenovoR; stock in Perthera; research grants: ARMO BioSciences, Bavarian Nordic, Bayer, Bristol-Myers Squibb, Calithera Biosciences, Celgene, Celldex, Curegenix, Fibrogen, Genentech, Gilead Sciences, GlaxoSmithKline, Halozyme, Karyopharm Therapeutics, MedImmune, Merck, Novartis, Regeneron, Pfizer, Pharmacyclics, Tesaro, Pancreatic Cancer Action Network
J. Berlin: consulting/advisory: Erytech, Gritstone, Celgene, AbbVie, Symphogen, EMD/Serono, Astra Zeneca, Armo, FivePrime, Eisai, Elevar, Bayer, Seattle Genetics, QED, Ipsen, Clovis; DSMB: Astra Zeneca, Novocure, PanCan, NCI; research grants: Symphogen, Abbvie, Immunomedics, Gilead, Boston Biomedical, MacroGenics, Pfizer, Novartis, Roche, Lilly, EMD/Serono, Loxo, PsiOxus, FivePrime, Bayer, Taiho
M.S. Noel: consulting/advisory: Celgene, Ipsen
I. Garrido-Laguna: consulting/advisory: Array Biopharma; research grants: Novartis, Ignyta, Halozyme, Bayer, Bristol Meyers Squibb, Pfizer, NewLink Genetics, MedImmune, Lilly, Incyte, GlaxoSmithKline, OncoMed, ARMO, Glennmark
D. Backlund Cardin: no conflicts
M.R. Radke: no conflicts
M. Duong: no conflicts
S. Bellasea: no conflicts
A.M. Lowy: consulting/advisory: Fount/Kinnate Therapeutics, Bexxion, Pfizer, Merck, Rafael
H.S. Hochster: consulting/advisory: Bayer, Genentech, Amgen, Exelixis
REFERENCES:
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics 2021. CA Cancer J Clin 2021; 71: 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Neuzillet C, Hentic O, Rousseau B, Rebours V, Bengrine-Lefèvre L, Bonnetain F, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol 2012; 18: 4533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang-Gillam A, Hubner RA, Siveke JT, Von Hoff DD, Belanger B, de Jong FA, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 4.Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015; 33: 3124–9. [DOI] [PubMed] [Google Scholar]
- 5.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol 2018; published online July 23. DOI: 10.1200/PO.17.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol 2012; 19: 417–23. [DOI] [PubMed] [Google Scholar]
- 8.Smith LM, Willmore E, Austin CA, Curtin NJ. The novel poly (ADP-Ribose) polymerase inhibitor, AG14361, sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks. Clin Cancer Res 2005; 11: 8449–57. [DOI] [PubMed] [Google Scholar]
- 9.Davidson D, Wang Y, Aloyz R, Panasci L. The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines. Invest New Drugs 2013; 31: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer 2018; 89: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, et al. Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. JCO Precis Oncol 2018; published online May 16. DOI: 10.1200/PO.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn ER, Garrett-Mayer E, Halabi S, Mangat PK, Calfa CJ, Alva AS, et al. Olaparib in patients with pancreatic cancer with BRCA1/2 inactivating mutations: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol 2020; 38 (suppl; abstr 4637) [Google Scholar]
- 14.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 2019; 381: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pishvaian MJ, Wang H, He AR, Hwang JJ, Smaglo BG, Kim SS, et al. A phase I/II study of veliparib (ABT-888) in combination with 5-fluorouracil and oxaliplatin in patients with metastatic pancreatic cancer. Clin Cancer Res 2020; 26: 5092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Reilly EM, Lee JW, Lowery MA, Capanu M, Stadler ZK, Moore MJ, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer 2018; 124: 1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol 2020; 38: 1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berlin J, Ramanathan RK, Strickler JH, Subramaniam DS, Marshall J, Kang YK, et al. A phase 1 dose-escalation study of veliparib with bimonthly FOLFIRI in patients with advanced solid tumors. Br J Cancer 2018; 118: 938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. : FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study J Clin Oncol 2004; 22: 229–37. [DOI] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) version 4.03: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed August 6, 2010.
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 22.Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018; 24: 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nord AS, Lee M, King MC, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genomics 2011; 12: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975; 31: 103–15. [PubMed] [Google Scholar]
- 25.LoRusso PM, Li J, Byrger A, Heilbrun LK, Sausville EA, Boerner SA, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with adanced solid tumors. Clin Cancer Res 2016; 22: 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 2019; 381: 2403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 1269–82. [DOI] [PubMed] [Google Scholar]
- 28.Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL 3rd, Srkalovic G, et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small cell lung cancer. ECOG-ACRIN 2511 study. J Clin Oncol 2019; 37: 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science 2017; 355: 1152–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Final overall survival results from the phase 3 POLO trial: maintenance Olaparib for germline BRCA-mutated metastatic pancreatic cancer. J Clin Oncol 2021;39; (suppl 3; abstr. 378). [Google Scholar]
- 31.Reiss KA, Mick R, O'Hara MH, Teitelbaum U, Karasic TB, Schneider C, et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol. 2021. May 10: JCO2100003. DOI: 10.1200/JCO.21.00003. [DOI] [PubMed] [Google Scholar]
- 32.Golan T, O'Kane GM, Denroche RE, Raitses-Gurevich M, Grant RC, Holter S, et al. Genomic features and classification of Homologous Recombination Deficient pancreatic ductal adenocarcinoma. Gastroenterology. 2021;160: 2119–32. [DOI] [PubMed] [Google Scholar]
- 33.Chung V, Guthrie KA, Pishvaian MJ, Lowy AM, Chiorean EG, Duong MT, et al. Randomized phase II trial of olaparib + pembrolizumab versus olaparib alone as maintenance therapy in metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 (gBRCA1/2+) mutations: SWOG S2001. J Clin Oncol 2021; 39: suppl 3; abstr TPS447. [Google Scholar]
- 34.Stewart RA, Pilié PG, Yap TA. Development of PARP and immune checkpoint inhibitor combinations. Cancer Res 2018; 78: 6717–25. [DOI] [PubMed] [Google Scholar]
- 35.Yap TA, Kristeleit R, Michalarea V, Pettitt SJ, Lim JSJ, Carreira S, et al. Phase I trial of the PARP inhibitor olaparib and AKT inhibitor capivasertib in patients with BRCA1/2- and non-BRCA1/2-mutant cancers. Cancer Discov. 2020. October;10(10):1528–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasanna T, Wu F, Khanna KK, Optimizing poly (ADP-ribose) polymerase inhibition through combined epigenetic and immunotherapy. Cancer Sci 2018; 109: 3383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng-Lin B, O’Reilly EM. Pancreatic ductal adenocarcinoma in the era of precision medicine. Semin Oncol. 2021. February 11: S0093-7754(21)00005-1. DOI: 10.1053/j.seminoncol.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.