Abstract
Interferon-γ (IFNγ) is a pleiotropic cytokine that has a crucial role in immune response and tumor immunity. Because of its anti-tumor effects, IFNγ has been used in cancer treatment. However, IFNγ also has tumor-promoting functions that are less well understood. Here, we show that IFNγ induces expression of the pro-inflammatory and pro-angiogenic chemokine interleukin-8 (IL-8, CXCL8) in ovarian cancer (OC) cells. The IFNγ-induced IL-8 expression is dependent on JAK1, STAT1, and p65 NFκB, and is associated with an increased occupancy of K314/315 acetylated p65 NFκB and Ser-727 phosphorylated STAT1 at the IL-8 promoter. Neutralization of IL-8 using anti-IL-8 antibody reduces IFNγ-induced migration of OC cells, and their invasion ability in 3D spheroids. Together, these findings identify IL-8 as a novel target induced by IFNγ/JAK1/STAT1/p65 NFκB signaling, and indicate that the IFNγ-induced IL-8 contributes to IFNγ pro-tumorigenic effects in ovarian cancer cells.
Keywords: Interferon-γ, interleukin-8, JAK1, STAT1, p65 NFκB, ovarian cancer, cell invasion
1. Introduction
Interferon-γ (IFNγ) is a pleiotropic cytokine that has a crucial role in immune responses and tumor immunity [1–4]. IFNγ is produced mainly by activated lymphocytes, but its expression is also induced in response to radiation therapy or immune checkpoint blockade used in cancer treatment [5–8]. Because of its anti-tumor effects, IFNγ has been used in cancer treatment, even though with mixed results [9–15]. Indeed, mounting evidence indicates that IFNγ also has important tumor-promoting functions [16–23]. Understanding the tumor-promoting mechanisms and molecular targets of IFNγ is important to minimize its tumor-promoting functions in IFNγ-based therapies, and in cancer treatments associated with IFNγ increase.
Interleukin-8 (IL-8, CXCL8) is a pro-inflammatory and pro-angiogenic chemokine that has a crucial role in cancer progression through its induction of tumor cell proliferation, migration, invasion, immune escape, and metastasis [24–28]. Serum IL-8 levels are increased in patients with solid tumors, reflect the tumor burden, and correlate with poor prognosis [29, 30]. In most cells, the IL-8 expression is regulated by the transcription factor NFκB, particularly by p65 homodimers [31–33]. However, understanding the mechanisms that regulate the IL-8 expression in tumors has been lagging since mice do not have the IL-8/CXCL8 gene [34].
We have recently shown that IFNγ promotes proliferation and migration of ovarian cancer (OC) cells [35, 36]. Considering the strong pro-proliferative and pro-migratory effects of IL-8, in this study we tested the hypothesis that the IFNγ-induced migration of OC cells might be mediated by IL-8. Our results demonstrate that IFNγ induces IL-8 gene expression and chemokine release in OC cells. The IFNγ-induced IL-8 expression is mediated by both JAK1/STAT1 signaling and p65 NFκB. Neutralization of the induced IL-8 decreases migration of IFNγ-stimulated OC cells, and their invasion potential in 3D spheroids. These findings identify IL-8 as a novel target induced by IFNγ/JAK1/STAT1/p65 NFκB signaling and indicate that the IFNγ-induced IL-8 expression contributes to the tumor-promoting functions of IFNγ.
2. Materials and methods
2.1. Cell culture
Human ovarian cancer SKOV3 and OVCAR3 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured (5×105 cells/mL) in 6-well plates in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gemini Bio, West Sacramento, CA, USA) and antibiotics at 37 °C with 5% CO2 as described [35, 36]. For treatment with IFNγ, human recombinant IFNγ (285-IF-100; R&D Systems, Minneapolis, MN) was reconstituted in sterile water. Cell viability was measured by using trypan blue exclusion.
2.2. Transfection with siRNA
Human JAK1 (sc-35719), STAT1 (sc-44123), p65 (sc-29410) and non-silencing control (sc-37007) siRNAs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Prior to transfection, 2×105 SKOV3 cells were seeded into a 6-well plate and incubated in a humidified 5% CO2 atmosphere at 37°C in antibiotic-free RPMI medium supplement with 10% FBS for 24 h to about 80% confluence. For each transfection, 80 pmol of either non-silencing siRNA control or target specific siRNA were used. Cells were transfected 7 h in transfection medium with siRNA transfection reagent according to manufacturer’s instructions (Santa Cruz Biotechnology). After transfection, fresh medium with antibiotics was added, and cells were grown for 24 h before treatment.
2.3. Cell migration transwell assay
The cell migration transwell assay was performed as described by the manufacturer’s protocol (Corning, NY, USA). SKOV3 cells were seeded onto the Corning Biocoat Matrigel Chambers (Corning #354480) at a density of 25,000 cells/0.5 mL in serum-free RPMI medium, with bottom wells containing RPMI medium with 10% FBS. Cells in the top chambers were incubated with recombinant human IFNγ (0 and 50 ng/mL; R&D Systems, 285-IF-100) in the presence of IL-8 neutralizing monoclonal antibody (2 μg/mL; R&D, MAB208) or control IgG (2 μg/mL; Santa Cruz Biotechnology; sc-2025) for 24 h. After incubation, non-migrating cells were scrubbed from the upper surface of the top chambers using cotton swabs. Migrating cells on the bottom membranes were fixed with 100% methanol, stained with crystal violet, washed, and air dried. Migrating cells were counted in five randomly selected fields under a phase-contrast microscope at 10X magnification, and quantified using ImageJ software as described [37].
2.4. 3D spheroid cell invasion assay
3D spheroid cell invasion assay was performed according to the manufacturer’s protocol (Trevigen, MD, USA). Approximately 3000 SKOV3 cells in 50 μL of spheroid formation medium were seeded per well in a 96 well spheroid formation plate. The plate was centrifuged and incubated 72 h to promote spheroid formation. After 72 h, 50 μL of invasion matrix was added per well followed by centrifugation and incubation for 1 h. After incubation, 100 μL of growth medium containing IFNγ and/or anti-IL-8 IgG or control IgG were added into each well, and incubated for up to 25 days. Images were taken at 10X magnification, and spheroid areas were measured using ImageJ software as described [38].
2.5. Quantitative real time RT-PCR
Total RNA was isolated using RNeasy mini-kit (Qiagen, USA). The qScript one-step RT-PCR kit with SYBR Green (Quantabio, Beverly, MA, USA) was used as a super-mix and 20 ng/μL of RNA was used as a template on Bio-Rad MyIQ Single Color Real-Time PCR Detection System (Bio-Rad). The primers used for quantification of human IL-8 and actin mRNA for quantitative (q)RT-PCR were purchased from Qiagen. The mRNA values are expressed as a percentage of control untreated (UT) samples, which were arbitrarily set as 100%.
2.6. ELISA assay
Human IL-8/CXCL8 release was measured by a commercially available ELISA kit (R&D, Minneapolis, MN, USA).
2.7. Western analysis
After SKOV3 cell incubation with IFNγ for indicated times, whole cell extracts were prepared as described [35]. Denatured proteins were separated on 12% denaturing polyacrylamide gels and transferred to nitrocellulose membrane (Hybond C; Amersham, Arlington Heights, IL). Membranes were blocked with a 5% (w/v) nonfat dried milk solution containing 10 mM Tris-Cl, pH 7.5, 140 mM NaCl, 1.5 mM MgCl2, and 0.1% Tween 20 (TBSTM), and incubated with JAK1 (sc-376996; dilution 1:200), STAT1 (Cell Signaling, #9172; dilution 1:1000), Ser-727 pSTAT1 (Cell Signaling, #8826; dilution 1:1000), p65 NFκB (sc-8008; dilution 1:200), K314/315 ac-p65 NFκB (Signalway Antibody, HW136; dilution 1:200), and control actin (Sigma, A5060; dilution 1:2000) antibodies diluted in TBSTM. After washing, the membranes were incubated with HRP-labeled secondary anti-rabbit (Novus NB7185) or anti-mouse (Novus NB7570) antibodies (dilution 1:5000) and the labeled proteins were detected using the ECL detection system (Amersham, Arlington Heights, IL). To confirm equivalent amounts of loaded proteins, the membranes were stripped and re-probed with control anti-actin antibody as described [35].
2.8. Chromatin immunoprecipitation (ChIP)
SKOV3 cells were incubated with IFNγ for 0, 6, 24, and 48 hours, and promoter occupancy by p65 NFκB and STAT1 was analyzed by ChIP as described [39]. Briefly, proteins and DNA were cross-linked by formaldehyde, and cells were washed and sonicated. The lysates were centrifuged (15,000 g, 10 min, 4 °C), and the supernatant extracts were diluted with ChIP dilution buffer and pre-cleared with Protein A/G Agarose (Santa Cruz Biotechnology) for 2 hours at 4 °C. Immunoprecipitations were performed overnight at 4 °C, using 5 μL/mL cell lysate of p65 (Sigma, MAB3026), K314/315 ac-p65 (Signalway Antibody, HW136), STAT1 (Cell Signaling, #9172), and Ser727-pSTAT1 (Cell Signaling, #8826S) antibodies that were pre-incubated (6 h, 4 °C) with Protein A/G Agarose. The immune complexes were collected by centrifugation (150 g, 5 min, 4 °C), washed, and extracted with 1% SDS–0.1 M NaHCO3. After reversing the cross-linking, proteins were digested with proteinase K, and the samples were extracted with phenol/chloroform, followed by precipitation with ethanol. Immunoprecipitated DNA was analyzed by real-time PCR (25 μL reaction mixture) using the iQ SYBR Green Supermix and the Bio-Rad MyIQ Single Color Real-Time PCR Detection System (Bio-Rad). Each immunoprecipitation was performed at least three times using different chromatin samples, and the occupancy was calculated by using the human IGX1A negative control primers (SA Biosciences, Frederick, MD, USA), which detect specific genomic ORF-free DNA sequence that does not contain binding site for any known transcription factors. The results were calculated as a fold difference in p65, K314/315 ac-p65, STAT1, and Ser727-pSTAT1 occupancy at the NFκB and STAT1 binding sites compared to the control negative IGX1A locus that does not bind any transcription factors. The primers used for real time PCR were as follows: IL-8/NFκB (−82 bp from TSS): F, 5-GGGCCATCAGTTGCAAATC-3; R, 5-GCTTGTGTGCTCTGCTGTCTC-3; IL-8/STAT1 (−490 bp from TSS): F, 5-TTTGAAAAGTTGTAGTATGCCCC-3; R, 5-AGAGTGGCAGGTGTTAGAAC-3.
2.9. Statistical analysis
The results represent at least three experiments, and are presented as means ± SE. Data were analyzed by using InStat software package (GraphPAD, San Diego, CA, USA). Statistical significance was evaluated by using one-way ANOVA Tukey-post Hoc-test, and p<0.05 was considered significant.
3. Results
3.1. IFNγ induces IL-8 expression and cytokine release in OC cells
Our recent studies have shown that IFNγ induces proliferation and migration of OC cells [35, 36]; thus, we investigated the possibility that IFNγ might promote expression of the pro-angiogenic chemokine IL-8. As shown in Fig. 1, incubation of ovarian cancer SKOV3 and OVCAR3 cells for 48 hours with IFNγ dose-dependently increased the IL-8 gene expression (Fig. 1A) and cytokine release (Fig. 1B) in both types of OC cells. The IFNγ-induced IL-8 expression in OC cells was also time-dependent; significant IL-8 mRNA (Fig. 1C) and cytokine release (Fig. 1D) levels were induced 24 hours after stimulation with 50 ng/mL IFNγ.
Figure 1. IFNγ induces IL-8 expression in OC cells.
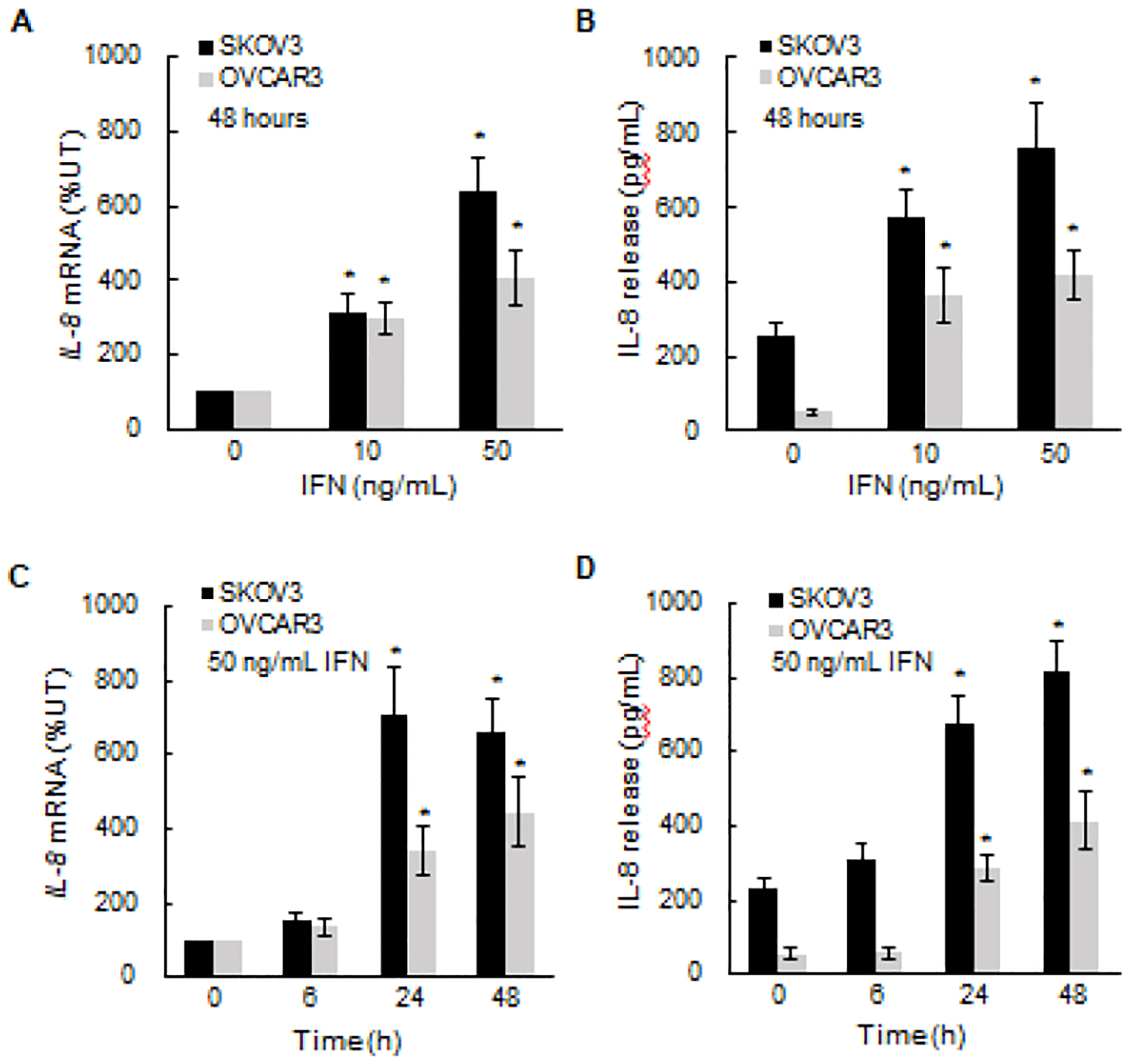
(A) Real time qRT-PCR analysis of IL-8 mRNA levels and (B) ELISA of IL-8 cytokine release in OC cells (5×105 cells/mL) incubated 48 hours with human recombinant IFNγ. (C) qRT-PCR of IL-8 mRNA levels and (D) ELISA of IL-8 cytokine release in OC cells incubated with 50 ng/mL IFNγ for up to 48 hours. The values represent the mean +/−SE (n=4); an asterisk denotes a statistically significant (* p<0.05) change compared to untreated (UT) cells.
3.2. IFNγ-induced IL-8 expression is mediated by JAK1/STAT1 signaling
The primary pathway by which IFNγ induces gene expression is the JAK1-STAT1 canonical pathway [40]; thus, we investigated whether the IFNγ-induced IL-8 expression is regulated by JAK1/STAT1 signaling. The IL-8 expression was first analyzed in SKOV3 cells transfected with control and JAK1 si RNA. As shown in Figs. 2A and B, IFNγ increased the cellular levels of JAK1, which were suppressed by JAK1 si RNA. Importantly, suppression of JAK1 significantly decreased the IFNγ-induced IL-8 expression in SKOV3 cells (Fig. 2C).
Figure 2. IFNγ-induced IL-8 expression is dependent on JAK1.
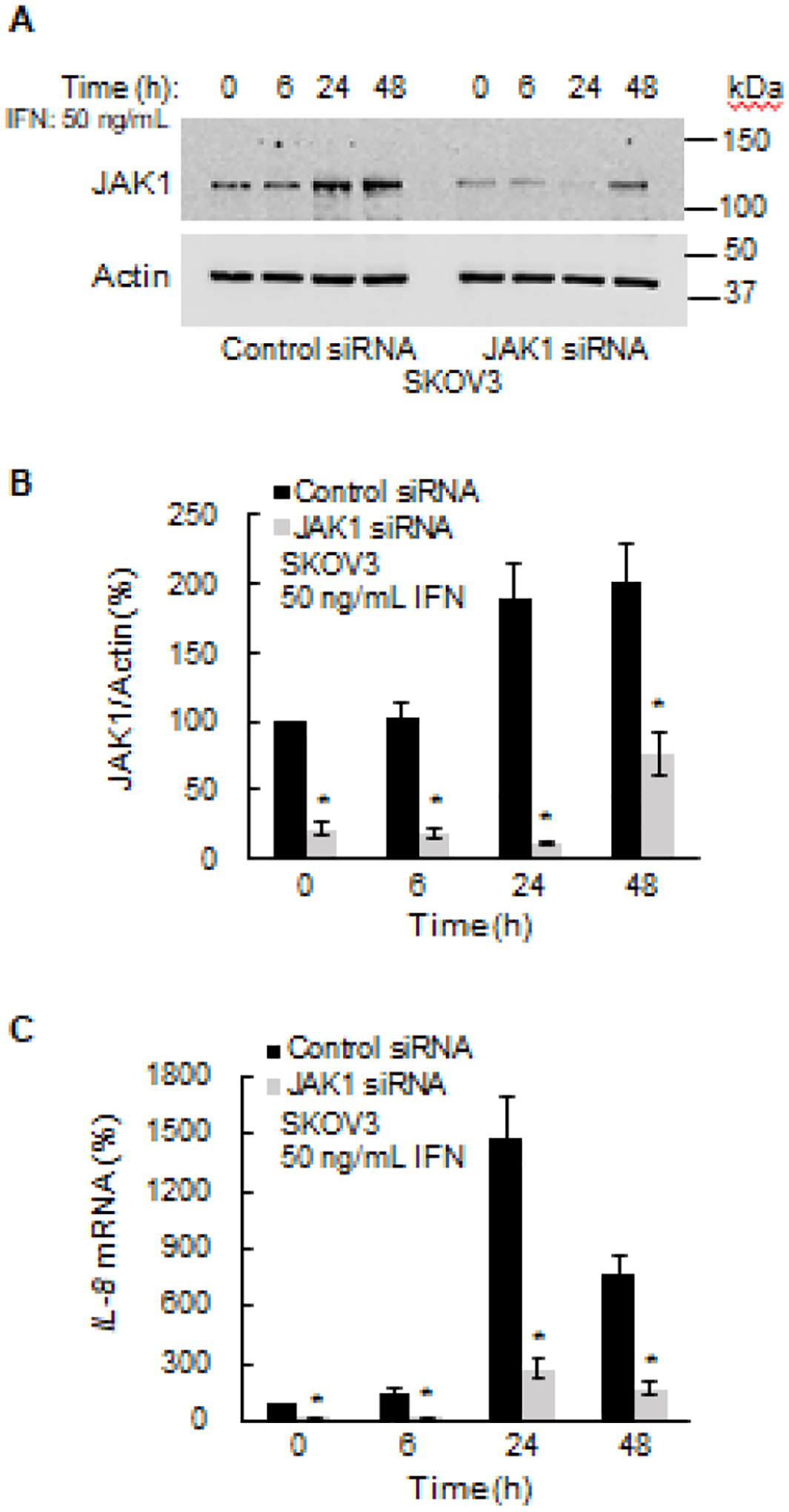
(A) Western blotting of JAK1 and control actin levels analyzed in whole cell extracts (WCE) from SKOV3 cells transfected with JAK1 and control siRNA. (B) Densitometric evaluation of JAK1 protein levels shown in panel A. The JAK1 densities were normalized to actin. (C) Quantitative RT-PCR of IL-8 mRNA in IFNγ-treated SKOV3 cells transfected with JAK1 and control siRNA. The values represent the mean +/−SE (n=3); an asterisk denotes a statistically significant (* p<0.05) change compared to cells transfected with control siRNA.
Since IFNγ signaling induces activation of the transcription factor STAT1 [40], we next analyzed STAT1 involvement in the IFNγ-induced IL-8 expression. As shown in Figs. 3A and B, IFNγ increased the cellular levels of STAT1 in SKOV3 cells, and the IFNγ-induced STAT1 expression was suppressed in cells transfected with STAT1 siRNA. Importantly, suppression of the IFNγ-induced STAT1 resulted in a significantly reduced IL-8 expression (Fig. 3C). Together, these results demonstrate that the IFNγ-induced IL-8 expression is mediated by the JAK1/STAT1 signaling.
Figure 3. IFNγ-induced IL-8 expression is dependent on STAT1.
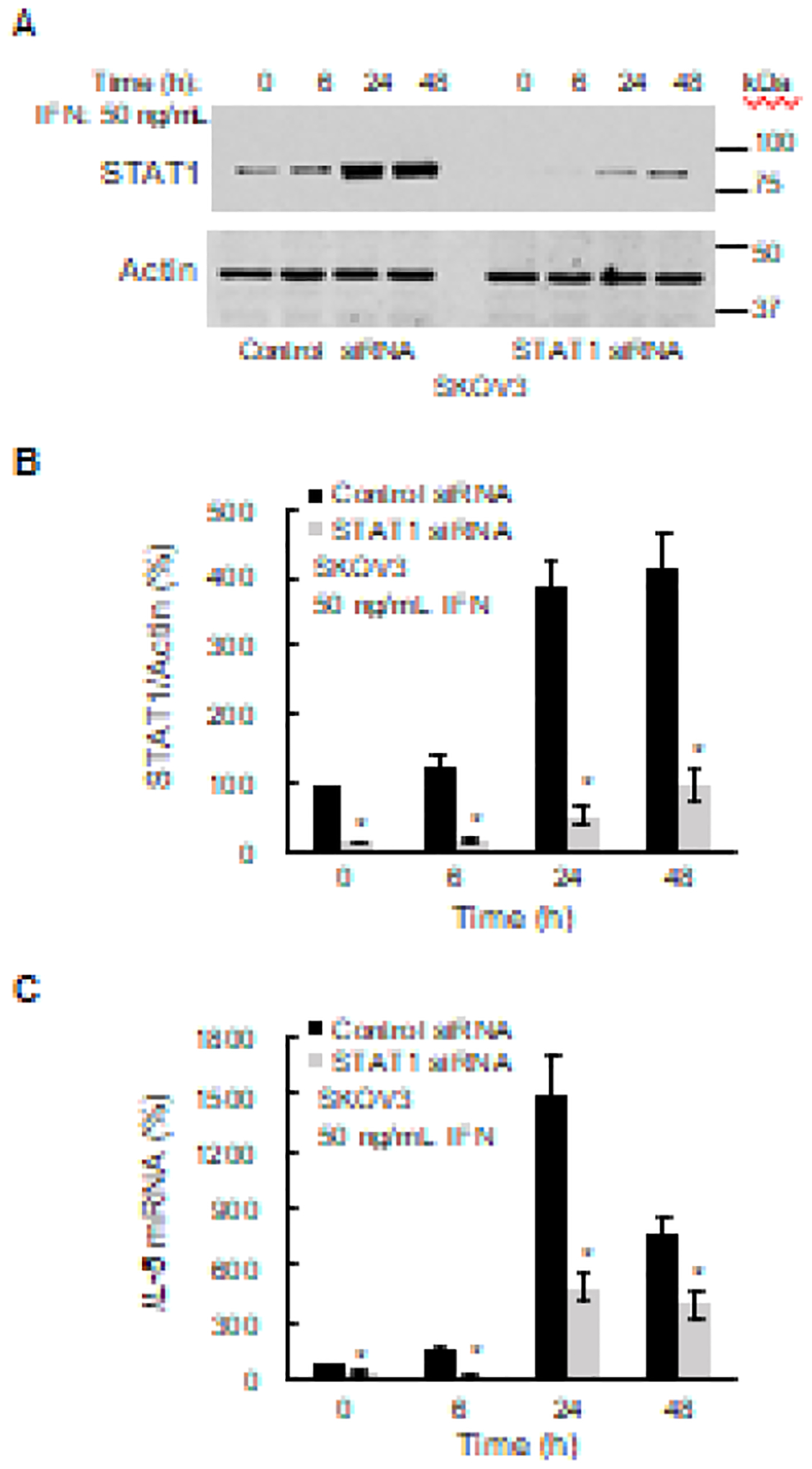
(A) Western blotting of STAT1 and actin levels analyzed in WCE from SKOV3 cells transfected with STAT1 and control siRNA. (B) Densitometric evaluation of STAT1 protein levels shown in panel A. The STAT1 densities were normalized to actin. (C) RT-PCR of IL-8 mRNA in IFNγ-treated SKOV3 cells transfected with STAT1 and control siRNA. The values represent the mean +/−SE (n=3); an asterisk denotes a statistically significant (* p<0.05) change compared to cells transfected with control siRNA.
3.3. IFNγ-induced IL-8 expression is mediated by p65 NFκB signaling
Since in most cells, the IL-8 transcription is regulated by NFκB, particularly by p65 homodimers [31–33], we have also evaluated the IL-8 expression in cells transfected with p65 siRNA. IFNγ increased p65 expression, which was suppressed by p65 siRNA in IFNγ-stimulated SKOV3 cells (Figs. 4A and B). The IFNγ-induced IL-8 mRNA levels were significantly suppressed in cells transfected with p65 specific siRNA (Fig. 4C), indicating that the IFNγ-induced IL-8 expression in OC cells is also regulated by p65 signaling.
Figure 4. IFNγ-induced IL-8 expression is dependent on p65 NFκB.
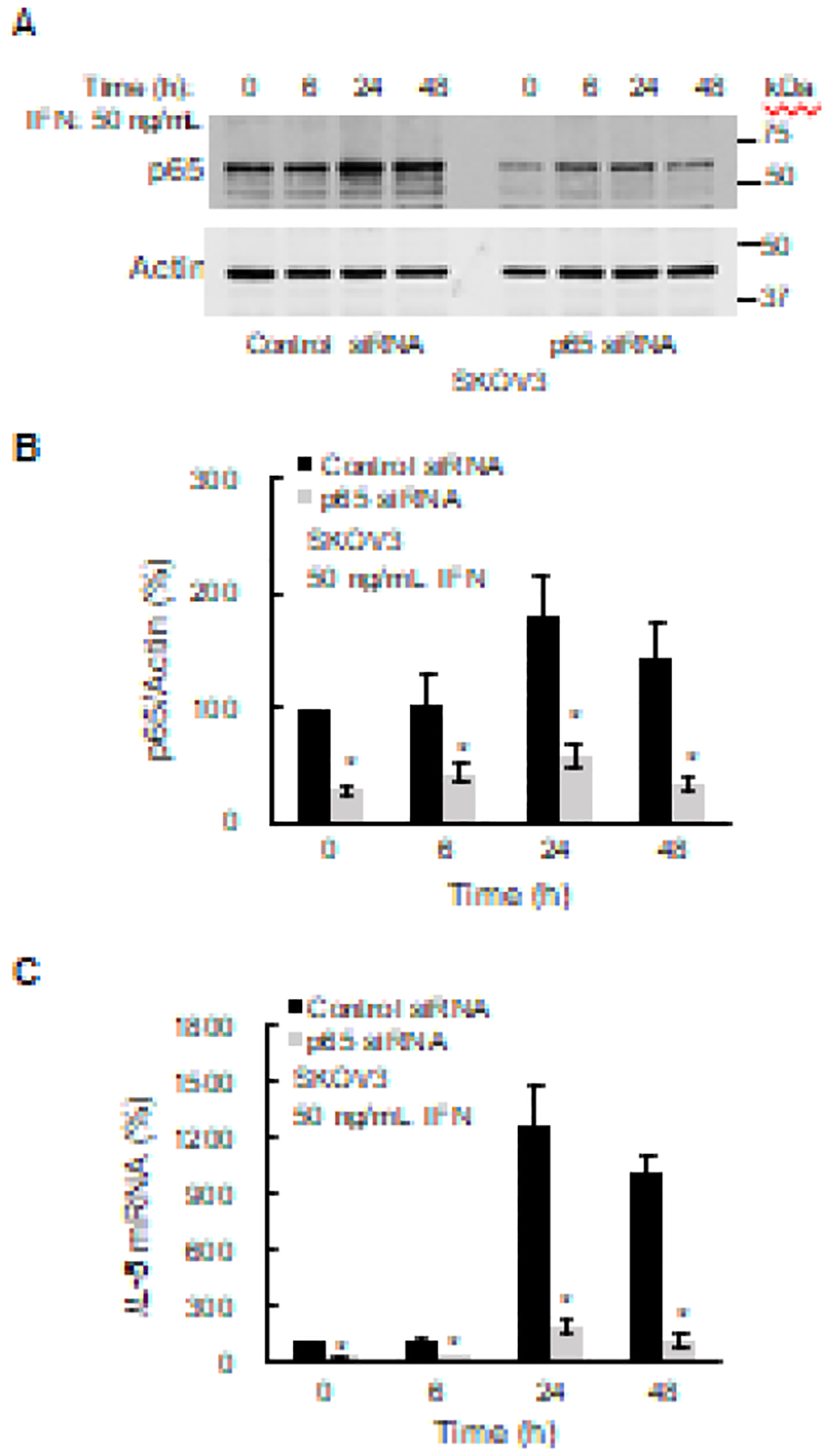
(A) Western blotting of p65 NFκB and actin levels analyzed in WCE from SKOV3 cells transfected with p65 NFκB and control siRNA. (B) Densitometric evaluation of p65 NFκB protein levels shown in panel A. The p65 densities were normalized to actin. (C) RT-PCR of IL-8 mRNA in IFNγ-treated SKOV3 cells transfected with p65 NFκB and control siRNA. The values represent the mean +/−SE (n=3); an asterisk denotes a statistically significant (* p<0.05) change compared to cells transfected with control siRNA.
3.4. IFNγ induces K314/315 ac-p65 and Ser727 p-STAT1 recruitment to IL-8 promoter
The transcriptional activity of p65 NFκB is regulated by its acetylation on Lys 314/315 [41–43]; thus, we have analyzed whether IFNγ increases K314/315 acetylation levels in OC cells. In addition, because Ser-727 phosphorylation of STAT1 is required for its full transcriptional and biological activity [40], we have examined whether IFNγ induces STAT1 Ser-727 phosphorylation. To this end, SKOV3 cells were incubated with IFNγ for 0, 6, 24 and 48 hours, and total cellular levels of p65, K314/315 ac-p65, STAT1, Ser727-pSTAT1, and control actin were analyzed by western blotting. As shown in Fig. 5A, while IFNγ increased the total cellular levels of p65 at 24 and 48 hours, the levels of K314/315 ac-p65 were not increased. However, IFNγ increased the cellular levels of Ser727-pSTAT1, as well as the total levels of STAT1 in SKOV3 cells (Fig. 5A).
Figure 5. IFNγ induces K314/315 ac-p65 and Ser727 p-STAT1 recruitment to IL-8 promoter.
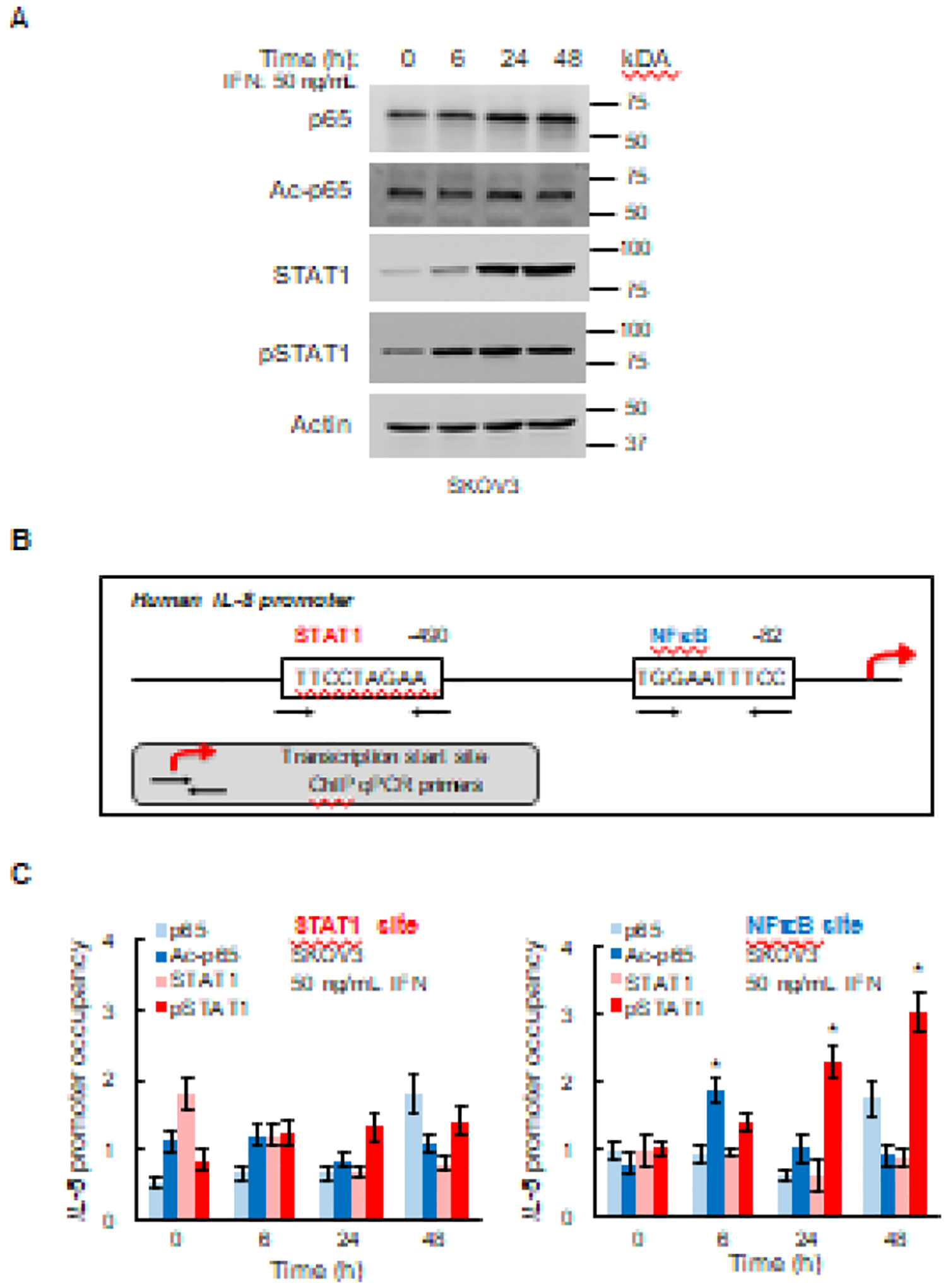
(A) Western analysis of p65, K314/315 ac-p65, STAT1, Ser727 pSTAT1, and control actin in WCE of SKOV3 cells incubated with 50 ng/mL IFNγ. (B) Schematic illustration of STAT1 and NFκB binding sites in human IL-8 promoter. (C) Recruitment of p65, K314/315 ac-p65, STAT1, and Ser727 pSTAT1 to STAT1 and NFκB binding sites in IL-8 promoter. The data are presented as fold-difference in occupancy of the particular protein at the particular locus compared with the negative control human IGX1A (SA Biosciences) locus, which does not contain any transcription factors binding sites. The values represent the mean +/−SE (n=3); an asterisk denotes a statistically significant (* p<0.05) change compared with untreated cells.
IFNγ-activated STAT1 binds to consensus GAS (IFN-γ-activated sequence) motifs (TTCCNNNAA) [40]. We analyzed the human IL-8 promoter and identified a potential GAS binding motif (TTCCTAGAA) located −490 bp from the transcription start site (TSS; Fig. 5B). To determine whether STAT1 binds to this site in OC cells, we measured the recruitment of STAT1 and Ser727-pSTAT1 to IL-8 promoter in IFNγ-treated SKOV3 cells by chromatin immunoprecipitation (ChIP), which measures transcription factor occupancy at endogenous promoter binding sites. In addition, we investigated recruitment of p65 and K314/315 ac-p65 to this potential STAT1 binding site, as well as to the NFκB binding site located −82 bp from TSS.
While we did not detect any substantial recruitment of STAT1, Ser727-pSTAT1, p65, or K314/315 ac-p65 to the STAT1 binding motif in IFNγ-stimulated SKOV3 cells (Fig. 5C, left panel), we found that IFNγ induced K314/315 ac-p65 recruitment to the NFκB binding site at 6 hours, and Ser727 pSTAT1 recruitment to the NFκB site at 24 and 48 hours (Fig. 5C, right panel). The fact that IFNγ induces IL-8 promoter occupancy by K314/315 ac-p65 (Fig. 5C), while it does not increase the cellular levels of K314/315 ac-p65 (Fig. 5A) suggests that IFNγ does not induce p65 acetylation, but rather increases recruitment of K314/315 ac-p65 to the NFκB binding site in IL-8 promoter. Together, these results indicate that the IFNγ-induced IL-8 expression in OC cells is regulated by a direct recruitment of both K314/315 ac-p65 NFκB and Ser727 pSTAT1 to IL-8 promoter.
3.5. Neutralization of IFNγ-induced IL-8 inhibits migration of OC cells
Considering that an increased expression of IL-8 promotes migration and tumor growth in solid cancers [24–26], we hypothesized that the IFNγ-induced IL-8 release in OC cells might contribute to the tumor-promoting functions of IFNγ. To test this hypothesis, we first analyzed migration of OC cells stimulated with IFNγ in the presence of IL-8 neutralizing antibody or control IgG, using the trans-well assay. As shown in Fig. 6, incubation of OC cells with IFNγ significantly increased their migration. Importantly, neutralization of IL-8 by IL-8 neutralizing antibody suppressed the IFNγ-induced OC cell migration (Fig. 6), indicating that the IFNγ-induced migration of OC cells is partly mediated by the IFNγ-induced IL-8.
Figure 6. Neutralization of IFNγ-induced IL-8 inhibits migration of OC cells.
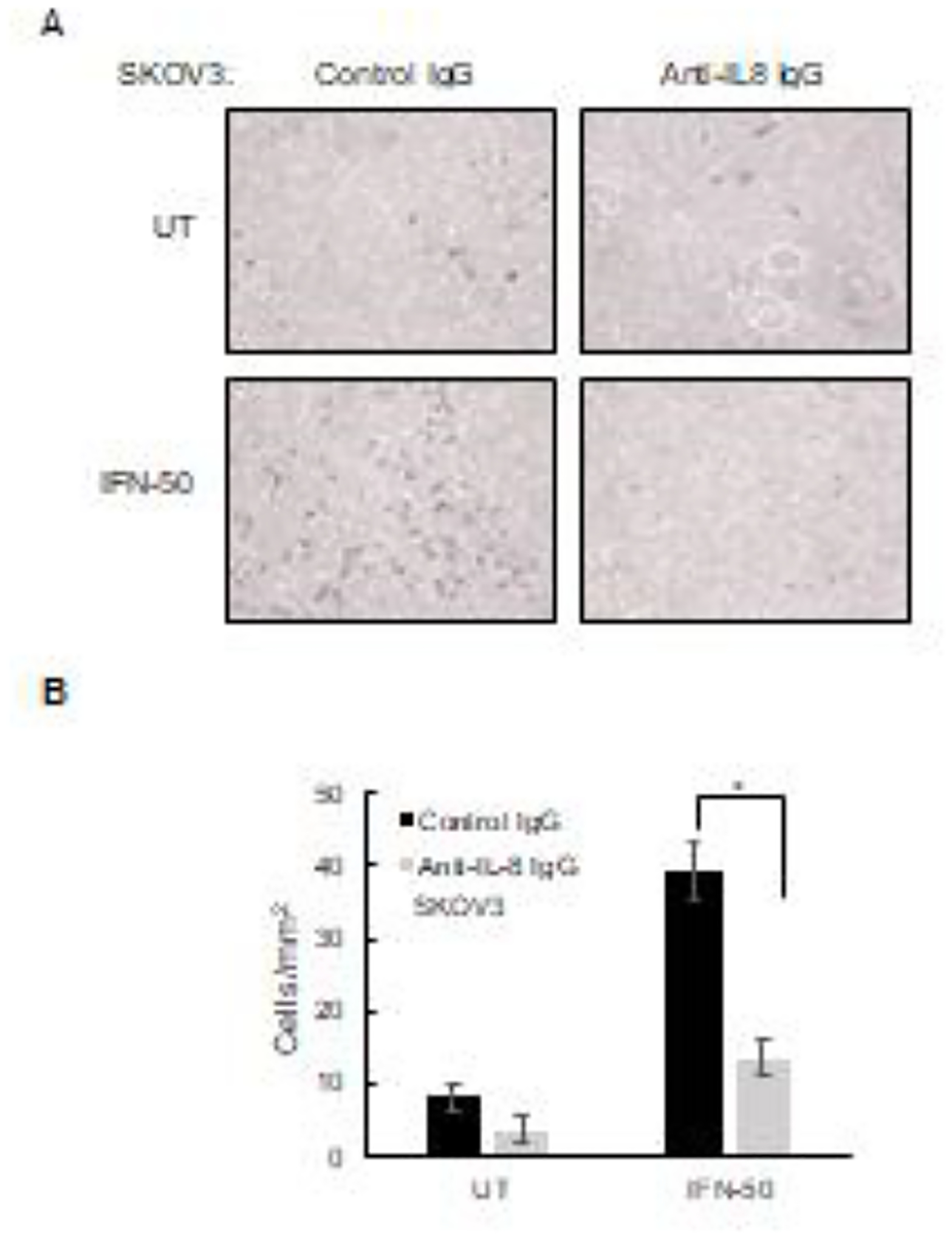
(A) SKOV3 cells were incubated 24 h with IFNγ (0 and 50 ng/mL) in the presence of IL-8 neutralizing monoclonal IgG1 (2 μg/mL) or control IgG1 (2 μg/mL), and cell migration was evaluated by counting the number of migrated cells through the membrane. Magnification: 10X. (B) Images from panel A were quantified using ImageJ software. The results are presented as mean +/−SE, n=4.
3.6. Neutralization of IL-8 suppresses invasion of IFNγ-treated OC cells grown in 3D spheroids
Compared to monolayer culture assays, spheroids 3D culture assays allow cell-to-cell interactions that more closely resemble tumor growth in vivo, while providing environment that allows easy cell manipulations and visualization. To determine whether neutralization of the IFNγ-induced IL-8 inhibits invasion of OC cells grown in 3D spheroids, we analyzed the invasion ability of IFNγ-treated SKOV3 cells grown in 3D spheroids in the presence of anti-IL-8 or control IgG. As shown in Fig. 7, incubation with anti-IL-8 IgG or IFNγ alone did not have a significant effect on the invasion ability of OC cells. However, cells incubated with the combination of IFNγ and anti-IL-8 IgG exhibited a significantly decreased invasion compared to IFNγ or anti-IL-8 IgG alone (Fig. 7). These data demonstrate that neutralization of the induced IL-8 increases effectiveness of IFNγ to inhibit OC cell invasion, indicating that the IFNγ-induced IL-8 contributes to IFNγ pro-tumorigenic effects in OC cells.
Figure 7. Neutralization of IL-8 suppresses invasion of IFNγ-treated OC cells grown in 3D spheroids.
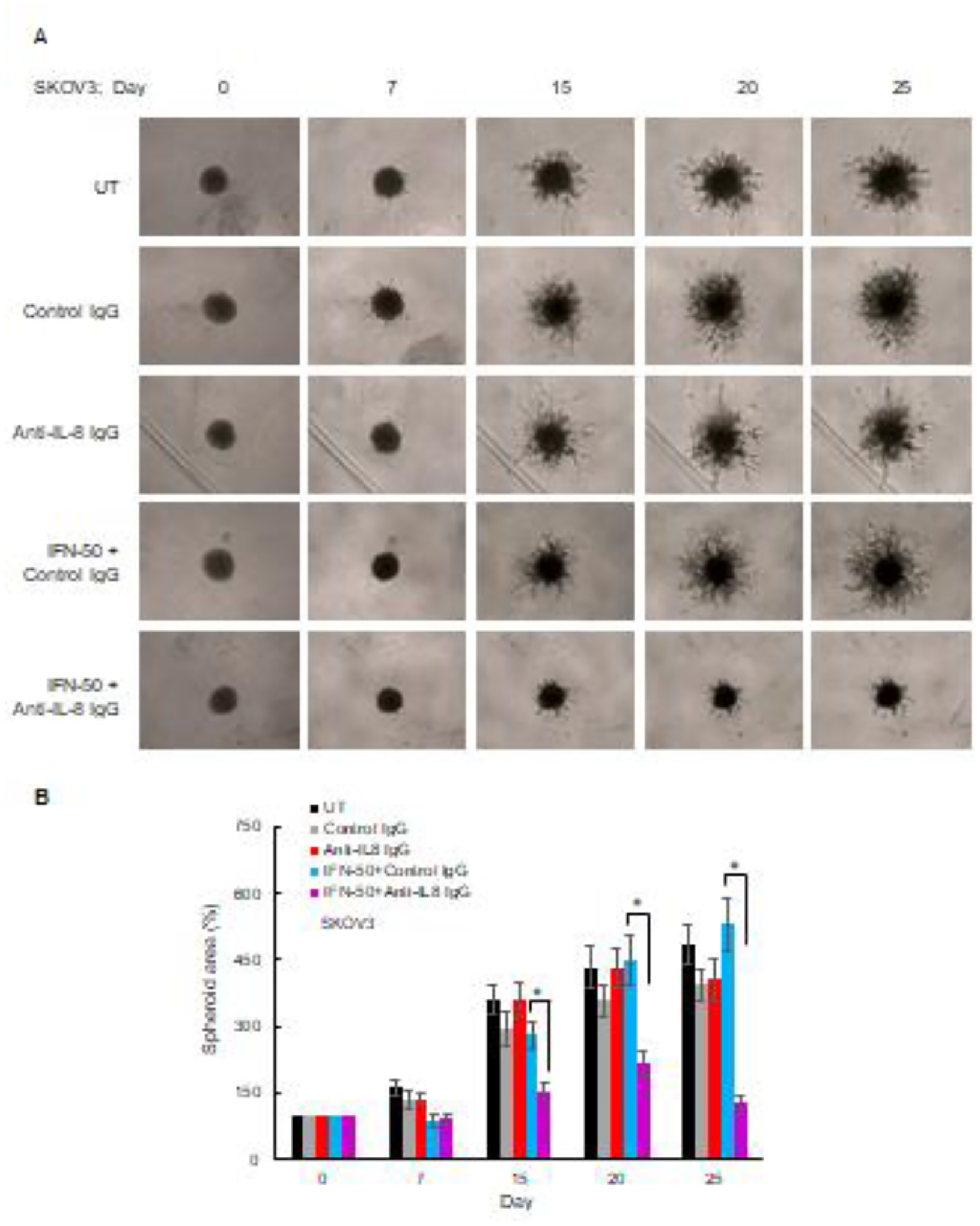
(A) Images of 3D culture spheroids of SKOV3 cells incubated with 2 μg/mL of control IgG1 or anti-IL-8 neutralizing IgG1 with and without IFNγ (50 ng/mL) for up to 25 days. (B) Quantification of images shown in panel A using ImageJ. The data represent the mean +/−SE, n=3.
4. Discussion
Our study identifies IL-8 as a novel target of IFNγ signaling in solid cancer cells, and suggests that the IFNγ-induced IL-8 expression contributes to tumor-promoting effects of IFNγ. The induction of IL-8 expression by IFNγ is consistent with the dependency of IL-8 expression in IFNγ-stimulated cells on JAK1/STAT1 signaling, as the JAK1/STAT1 pathway is the primary mechanism regulating expression of IFNγ-stimulated genes (ISG). However, our results show that the IFNγ-induced IL-8 expression in OC cells is also dependent on p65 NFκB, and suggest a cooperation between STAT1 and p65 NFκB signaling in the transcriptional regulation of ISG in OC cells.
The specific mechanisms how p65 NFκB and STAT1 regulate the IL-8 transcription are currently under investigation; however, our data indicate that IFNγ first induces IL-8 promoter occupancy by K314/315 acetylated p65 NFκB, followed by recruitment of Ser727 pSTAT1, the activated form of STAT1. Several mechanisms are possible; for example, NFκB can induce STAT1 synthesis, promote STAT1 activation, or induce the recruitment of Ser727 pSTAT1 to IL-8 promoter. Indeed, numerous studies have demonstrated a crosstalk between NFκB and STAT1 signaling pathways in the regulation of IFNγ-induced inflammatory genes [44–51].
IFNγ is a type II interferon that can have, depending on cellular and molecular context, both anti-tumor functions and pro-tumorigenic effects [1–4]. The anti-tumor properties of IFNγ include an upregulated expression of MHC proteins on cancer cells, resulting in their increased recognition by T cells. In addition, IFNγ induces cell cycle arrest and apoptosis in cancer cells [1, 2]. Because of these anti-tumor effects, IFNγ has been used in cancer treatment [1–4]. However, most clinical trials using IFNγ in cancer treatment have produced disappointing results [9–15]. Indeed, recent studies have indicated that IFNγ also has important tumor-promoting functions that include its ability to induce expression of immune checkpoints and T cell exhaustion, and increase cancer cell survival and proliferation [16–23]. Nevertheless, because of its anti-tumor potential, IFNγ is currently used in clinical trials for the treatment of ovarian, breast, and other solid cancers (NCT02948426; NCT03112590; NCT02614456). Understanding the tumor-promoting mechanisms and molecular targets of IFNγ is important for development of more effective IFNγ-based combination anti-cancer strategies.
Interleukin-8, originally discovered as the neutrophil chemoattractant and inducer of leukocyte-mediated inflammation, promotes tumor progression through its induction of cancer cell proliferation, migration, invasion, and metastasis [24–28]. IL-8 serum levels are increased in patients with advanced solid tumors, including ovarian cancer, and correlate with higher tumor stage, grade, and tumor burden [29, 30]. Interestingly, recent studies have indicated that increases in IL-8 serum levels may be predictive of resistance to immune checkpoint inhibitors (ICIs) therapy [52–55]. Since ICIs therapy induces IFNγ [6–8], it seems likely that one of the underlying mechanisms consists of the IFNγ-induced IL-8 expression. In this context, it would be important to determine whether IFNγ used in the current clinical trials increases the IL-8 levels in cancer patients. Targeting the IL-8 axis might provide an effective strategy in IFNγ-based cancer treatments and immunotherapies inducing IFNγ expression. The recent clinical trial NCT02536469 in patients with metastatic solid tumors demonstrated that a human monoclonal antibody neutralizing IL-8 (HuMax-IL8) is safe and well-tolerated and suggested the use of anti-IL-8 antibody with other agents [56].
In summary, our data demonstrate that IFNγ induces IL-8 expression in ovarian cancer cells, resulting in their increased migration and invasiveness. The underlying mechanism involves both the canonical JAK1/STAT1 pathway and NFκB signaling. Since both IFNγ and IL-8 neutralizing antibody have been clinically used in cancer patients, neutralizing the IFNγ-induced IL-8 might increase IFNγ effectiveness in cancer treatment.
Highlights.
IFNγ induces IL-8 expression in ovarian cancer cells
IFNγ induced IL-8 expression is mediated by JAK1/STAT1 and NFκB signaling
IL-8 neutralization decreases migration and invasiveness of IFNγ-treated cells
IL-8 is a novel target induced by IFNγ signaling in solid cancer cells
IL-8 neutralization might increase IFNγ effectiveness in cancer treatment
Acknowledgment:
This work was supported by the NIH CA202775 grant (to IV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare no conflict of interest.
CRediT authorship contribution statement
Conceptualization: Sveta Padmanabhan, Bijaya Gaire, Yue Zou, Ivana Vancurova., Formal analysis: Sveta Padmanabhan, Bijaya Gaire, Yue Zou, Ivana Vancurova., Funding acquisition: Ivana Vancurova., Investigation: Sveta Padmanabhan, Bijaya Gaire, Yue Zou, Mohammad M. Uddin, Daniel DeLeon, Ivana Vancurova., Methodology: Sveta Padmanabhan, Bijaya Gaire, Yue Zou, Mohammad M. Uddin, Daniel DeLeon, Ivana Vancurova., Project administration: Ivana Vancurova., Resources: Ivana Vancurova., Supervision: Ivana Vancurova., Writing, review & editing: Sveta Padmanabhan, Bijaya Gaire, Yue Zou, Mohammad M. Uddin, Daniel DeLeon, Ivana Vancurova.,
References
- 1.Alspach E, Lussier DM, Schreiber RD, 2019. IFN-γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity, Cold Spring Harb. Perspect. Biol 11(3). pii: a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi MR, The IFN-γ Paradox in Cancer, J. Interferon Cytokine Res 39 (2019) 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivashkiv LB, IFNγ: signaling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol 18 (2018) 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ, IFN-γ at the Crossroads of Tumor Immune Surveillance or Evasion, Front. Immunol 9 (2018) 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM, Radiation-induced IFNγ production within the tumor microenvironment influences antitumor immunity, J. Immunol 180 (2008) 3132–3139. [DOI] [PubMed] [Google Scholar]
- 6.Curran MA, Montalvo W, Yagita H, Allison JP, PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors, Proc. Natl. Acad. Sci. USA 107 (2010) 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. , CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients, Proc. Natl. Acad. Sci. USA 105 (2008) 14987–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. , PD-1 blockade enhances T-cell migration to tumors by elevating IFNγ inducible chemokines, Cancer Res 72 (2012) 5209–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marth C, Windbichler GH, Hausmaninger H, Petru E, Estermann K, Pelzer A, et al. , Interferon-γ in combination with carboplatin and paclitaxel as a safe and effective first-line treatment option for advanced ovarian cancer: Results of a phase I/II study, Int. J. Gynecol. Cancer 16 (2006) 1522–1528. [DOI] [PubMed] [Google Scholar]
- 10.Alberts DS, Marth C, Alvarez RD, Johnson G, Bidzinski M, Kardatzke DR, et al. , Randomized phase 3 trial of interferon-γ plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: Results from a prospectively designed analysis of progression-free survival, Gynecol. Oncol 109 (2008) 174–181. [DOI] [PubMed] [Google Scholar]
- 11.Meyskens FL, Kopecky KJ, Taylor CW, Noyes RD, Tuthill RJ, Hersh EM, et al. , Randomized trial of adjuvant human IFN-γ versus observation in high-risk cutaneous melanoma: A Southwest Oncology Group study, J. Natl. Cancer Inst 87 (1995) 1710–1713. [DOI] [PubMed] [Google Scholar]
- 12.Schiller JH, Pugh M, Kirkwood JM, Karp D, Larson M, Borden E, Eastern cooperative group trial of interferon-γ in metastatic melanoma: An innovative study design, Clin. Cancer Res 2 (1996) 29–36. [PubMed] [Google Scholar]
- 13.Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. , IFNγ compared with placebo in metastatic renal-cell carcinoma, N. Engl. J. Med 338 (1998) 1265–1271. [DOI] [PubMed] [Google Scholar]
- 14.Von Hoff DD, Fleming TR, Macdonald JS, Goodman PJ, Van Damme J, Brown TD, et al. , Phase II evaluation of recombinant gamma-interferon in patients with advanced pancreatic carcinoma: a Southwest Oncology Group study, J. Biol. Response Mod 9 (1990) 584–587. [PubMed] [Google Scholar]
- 15.Vahdat LT, Cohen D, Zipin D, Lo K, Donovan D, Savage D, et al. , Randomized trial of low-dose IL-2 vs cyclosporine A and IFNγ after high-dose chemotherapy with peripheral blood progenitor support in women with high-risk primary breast cancer, Bone Marrow Transplant 40 (2007) 267–72. [DOI] [PubMed] [Google Scholar]
- 16.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, et al. , IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer, Br. J. Cancer 112 (2015) 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J, IFNγ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression, Oncoimmunology 4 (2015) e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I, Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity, Clin. Cancer Res 22 (2016) 2329–2334. [DOI] [PubMed] [Google Scholar]
- 19.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, et al. , Tumor IFNγ signaling regulates a multigenic resistance program to immune checkpoint blockade, Cell 167 (2016) 1540–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mojic M, Takeda K, Hayakawa Y, The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion, Int. J. Mol. Sci 19 (2017) pii: E89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aqbi HF, Wallace M, Sappal S, Payne KK, Manjili MH, IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression, J. Leukoc. Biol 2018. doi: 10.1002/JLB.5MIR0917-351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu YH, Li ZL, Qiu SF, IFN-γ Induces Gastric Cancer Cell Proliferation and Metastasis Through Upregulation of Integrin β3-Mediated NF-κB Signaling, Transl. Oncol 11 (2018) 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benci JL, Johnson LR, Choa R, Xu Y, Qiu J, Zhou Z, et al. , Opposing Functions of IFNγ Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade, Cell 178 (2019) 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waugh DJ, Wilson C C, The interleukin-8 pathway in cancer, Clin. Cancer Res 14 (2008) 6735–6741. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Allavena P, Sica A, Balkwill F, Cancer-related inflammation, Nature 454 (2008) 436–444. [DOI] [PubMed] [Google Scholar]
- 26.Lazennec G, Richmond A, Chemokines and chemokine receptors: new insights into cancer-related inflammation, Trends Mol. Med 16 (2010) 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David JM, Dominguez C, Hamilton DH, Palena C, The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance, Vaccines (Basel) 4 (2016), pii: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. , The CXCL8-CXCR1/2 pathways in cancer, Cytokine Growth Factor Rev 31 (2016) 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanmamed MF, Carranza-Rua O, Alfaro C, Oñate C, Martín-Algarra S, Perez G, et al. , Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins, Clin. Cancer Res 20 (2014) 5697–707. [DOI] [PubMed] [Google Scholar]
- 30.Sanguinete NM, Oliveira PH, Martins-Filho A, Micheli DC, Tavares-Murta BM, Murta EF, Nomelini RS, Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer, Immunological investigations. 46 (2017) 677–688. [DOI] [PubMed] [Google Scholar]
- 31.Kunsch C, Rosen CA, NFκB subunit-specific regulation of the IL-8 promoter, Mol. Cell. Biol 13 (1993) 6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki CY, Barberi TJ, Ghosh P, Longo DL, Phosphorylation of RelA/p65 on serine 536 defines an IκBα-independent NFκB pathway, J Biol Chem 280 (2005) 34538–34547. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh CC, Ramaswami S, Juvekar A, Vu HY, Galdieri L, Davidson D, Vancurova I, Gene-specific repression of proinflammatory cytokines in stimulated human macrophages by nuclear IκBα, J Immunol 185 (2010) 3685–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomiyama H, Osada N, Yoshie O, The evolution of mammalian chemokine genes, Cytokine Growth Factor Rev 21 (2010) 253–262. [DOI] [PubMed] [Google Scholar]
- 35.Zou Y, Uddin MM, Padmanabhan S, Zhu Y, Bu P, Vancura A, Vancurova I, The proto-oncogene Bcl3 induces immune checkpoint PD-L1 expression, mediating proliferation of ovarian cancer cells, J. Biol. Chem 293 (2018) 15483–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaire B, Uddin MM, Zou Y, Vancurova I, Analysis of IFNγ-Induced Migration of Ovarian Cancer Cells, Methods Mol. Biol 2108 (2020) 101–106. [DOI] [PubMed] [Google Scholar]
- 37.Uddin MM, Gaire B, Deza B, Vancurova I, Interleukin-8-Induced Invasion Assay in Triple-Negative Breast Cancer Cells, Methods Mol. Biol 2108 (2020) 107–115. [DOI] [PubMed] [Google Scholar]
- 38.Uddin MM, Gaire B, Vancurova I, Interleukin-8 Induces Proliferation of Ovarian Cancer Cells in 3D Spheroids, Methods Mol. Biol 2108 (2020) 117–124. [DOI] [PubMed] [Google Scholar]
- 39.Zou Y, Padmanabhan S, Vancurova I, Analysis of PD-L1 Transcriptional Regulation in Ovarian Cancer Cells by Chromatin Immunoprecipitation, Methods Mol. Biol 2108 (2020) 229–239. [DOI] [PubMed] [Google Scholar]
- 40.Stark GR, Darnell JE, The JAK-STAT pathway at twenty, Immunity 36 (2012) 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen LF, Mu Y, Greene WC, Acetylation of RelA at discrete sites regulates distinct nuclear functions of NFκB, EMBO J 21 (2002) 6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buerki C, Rothgiesser KM, Valovka T, Owen HR, Rehrauer H, Fey M, et al. , Functional relevance of novel p300-mediated lysine 314 and 315 acetylation of RelA/p65. Nucleic Acids Res 36 (2008) 1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatla HR, Zou Y, Uddin MM Singha B, Bu P, Vancura A, et al. , Histone Deacetylase (HDAC) Inhibition Induces IκB Kinase (IKK)-dependent Interleukin-8/CXCL8 Expression in Ovarian Cancer Cells, J. Biol. Chem 292 (2017) 5043–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramana CV, Gil MP, Schreiber RD, Stark GR, Stat1-dependent and-independent pathways in IFN-γ-dependent signaling, Trends Immunol 23 (2002) 96–101. [DOI] [PubMed] [Google Scholar]
- 45.Ganster RW, Guo Z, Shao L, Geller DA, Differential effects of TNF-α and IFN-γ on gene transcription mediated by NF-κB-Stat1 interactions, J. Interferon Cytokine Res 25 (2005) 707–719. [DOI] [PubMed] [Google Scholar]
- 46.Krämer OH, Baus D, Knauer SK, Stein S, Jäger E, Stauber RH, Grez M, Pfitzner E, Heinzel T, Acetylation of Stat1 modulates NF-κB activity, Genes Dev 20 (2006) 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu X, Ivashkiv LB, Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases, Immunity 31 (2009) 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chmielewski S, Olejnik A, Sikorski K, Pelisek J, Błaszczyk K, Aoqui C, Nowicka H, Zernecke A, Heemann U, Wesoly J, Baumann M, STAT1-dependent signal integration between IFNγ and TLR4 in vascular cells reflect pro-atherogenic responses in human atherosclerosis, PloS One 9 (2014) e113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platanitis E, Decker T, Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation, Front. Immunol 9 (2018) 2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piaszyk-Borychowska A, Széles L, Csermely A, Chiang HC, Wesoły J, Lee CK, Nagy L, Bluyssen HA, Signal integration of IFN-I and IFN-II with TLR4 involves sequential recruitment of STAT1-Complexes and NFκB to enhance pro-inflammatory transcription, Front. Immunol 10 (2019) 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbosa Lima LE, Muxel SM, Kinker GS, Carvalho-Sousa CE, da Silveira Cruz-Machado S, Markus RP, Fernandes PA, STAT1–NFκB crosstalk triggered by interferon gamma regulates noradrenaline - induced pineal hormonal production. J. Pineal Res 67 (2019) e12599. [DOI] [PubMed] [Google Scholar]
- 52.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, et al. , Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients, Ann. Oncol 28 (2017) 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, et al. , Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors, Nat. Med 26 (2020) 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, Rishipathak D, et al. , High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med 26 (2020) 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakouny Z, Choueiri TK, IL-8 and cancer prognosis on immunotherapy, Nat. Med 26 (2020) 650–651. [DOI] [PubMed] [Google Scholar]
- 56.Bilusic M, Heery CR, Collins J, Donahue RN, Palena C, Madan R, Karzai F, et al. , Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors, J. Immunother. Cancer 7 (2019) 240. [DOI] [PMC free article] [PubMed] [Google Scholar]


