Extended Data Figure 2 |. Structure of invertebrate ALK’s EGF-like domains.
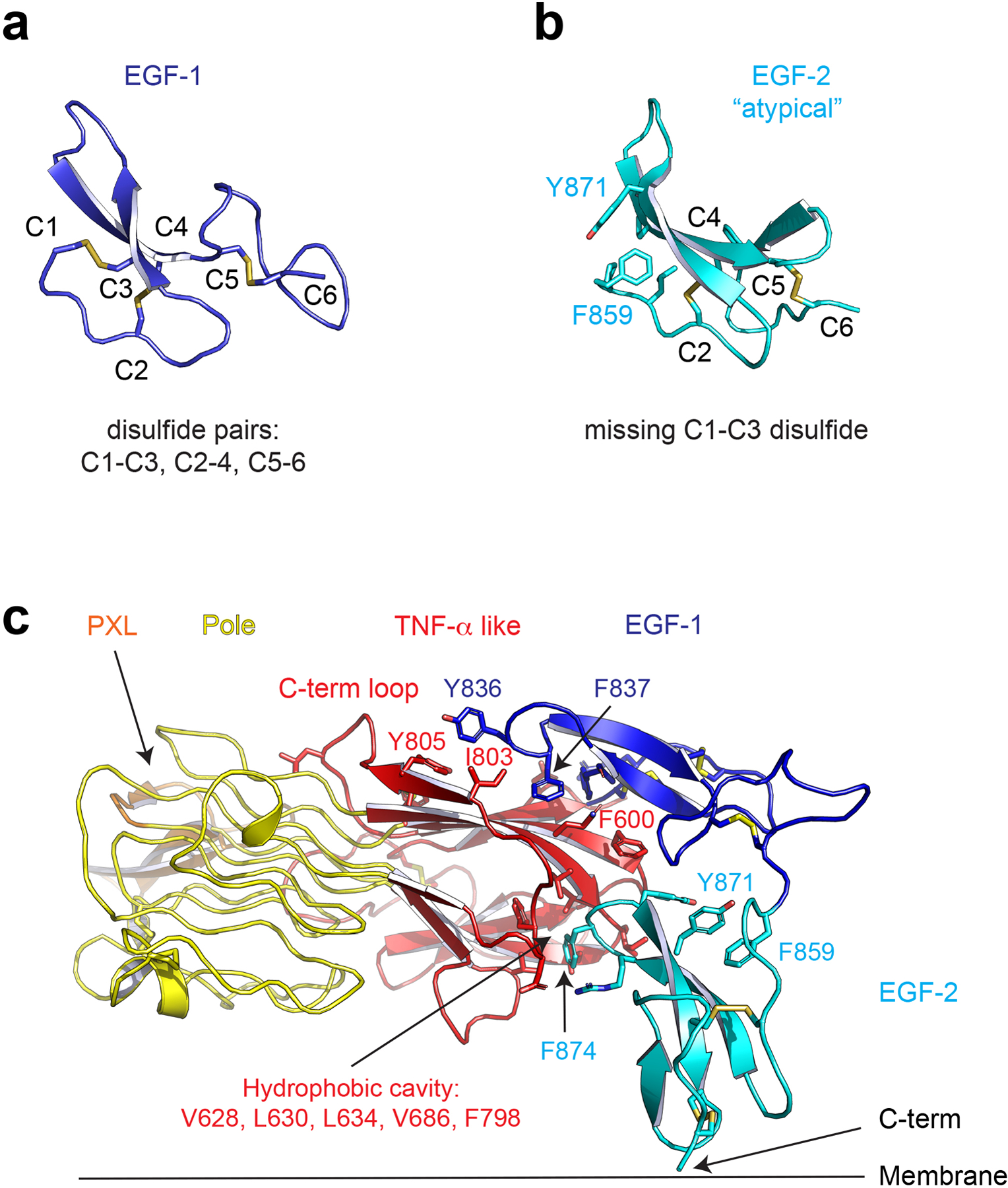
a, The first EGF-like domain has canonical disulfide pairing. b, The second EGF-like domain is atypical in that it lacks the first 1–3 disulfide bond, the stabilizing role of which is replaced by hydrophobic interactions involving Y871 and F859. c, The EGF-like domains pack tightly to the TNF-α like domain with hydrophobic interactions. Y836 and F837 of the first EGF domain bind to the proximal C-terminal loop. F874 of the second EGF like domain is buried in a hydrophobic cavity.
