Abstract
Executive functions (EFs) and intelligence (IQ) are phenotypically correlated. In twin studies, latent variables for EFs and IQ display moderate to high heritability estimates; however, they show variable genetic correlations in twin studies spanning childhood to middle age. We analyzed data from over 11,000 children (9-10-year-olds, including 749 twin pairs) in the Adolescent Brain Cognitive Development (ABCD) Study to examine the phenotypic and genetic relations between EFs and IQ in childhood. We identified two EF factors — Common EF and Updating-Specific — which were both related to IQ (rs = .64-.81). Common EF and IQ were heritable (53-67%), and their genetic correlation (rG = .86) was not significantly different than 1. These results suggest that EFs and IQ are phenotypically but not genetically separable in middle childhood, meaning that this phenotypic separability may be influenced by environmental factors.
Keywords: executive control, working memory, cognitive control, heritability, inhibition, general cognitive ability
Executive functions (EFs) are higher-order cognitive abilities that help regulate thoughts and behavior (Diamond, 2013; Friedman & Miyake, 2017). EFs are related to multiple behavioral outcomes, and are transdiagnostic correlates of psychopathology (Best, Miller, & Naglieri, 2011; Engelhardt, Briley, Mann, Harden, & Tucker-Drob, 2015; Friedman et al., 2020; Harden et al., 2019; Snyder, Friedman, & Hankin, 2019; Snyder, Miyake, & Hankin, 2015; Young et al., 2009). Although moderately to highly correlated, the variance common to multiple EFs (a Common EF factor) and intelligence (IQ) are distinguishable, with respect to their correlations with other measures (Blair, 2006; Brydges, Reid, Fox, & Anderson, 2012; Demetriou et al., 2014; Friedman et al., 2006; Kane & Engle, 2002), and their patterns of genetic and environmental influences (Engelhardt et al., 2015; Friedman et al., 2016; Haworth et al., 2010; Tucker-Drob, Briley, & Harden, 2013; Van Soelen et al., 2011). The genetic correlation between Common EF and IQ varies across studies, particularly across age groups (Engelhardt et al., 2016; Friedman et al., 2008; Gustavson et al., 2018), raising the question of whether this correlation may be higher in younger samples. In this study, we examined the shared genetic influences on EFs and IQ in the Adolescent Brain Cognitive Development (ABCD) Study, a longitudinal study on health and brain development across adolescence that includes over 10,000 singletons and twins (Garavan et al., 2018; Iacono et al., 2018; Jernigan & Brown, 2018; Volkow et al., 2018), to evaluate whether patterns found in older samples hold in this sample of 9-10-year-old children.
Latent variables are unobserved variables that are estimated by statistically extracting the variance shared between measures thought to be influenced by the construct (Kline, 2015). Latent variable models are particularly advantageous when studying EFs because latent variables do not include random error variance and task-specific effects (i.e., “task impurity”). Task impurity may be particularly problematic in EF tasks, because EFs are by definition high-level cognitive processes that control lower-level processes, so any EF task must include lower-level processes (e.g., word reading, categorization, visual tracking) that could also lead to individual differences in performance (Miyake et al., 2000). Because latent variable extract common variance across tasks, when those tasks differ in these lower-level processes, the common variance excludes these task-specific factors, and excludes random measurement error, which would not correlate across tasks. The elimination of random measurement error and task-specific effects often leads to stronger correlations between latent EF variables compared to correlations between individual EF tasks (Miyake et al., 2000, Friedman & Miyake, 2017).
The most commonly examined EFs in latent variable studies are inhibiting prepotent responses, working memory capacity and updating, and shifting between mental sets (Miyake et al., 2000). These EF factors are correlated, but not perfectly so, in adolescence and adulthood (Friedman et al., 2016), and this pattern is established sometime in middle childhood (Brydges et al., 2012; Brydges, Fox, Reid, & Anderson, 2014; Engelhardt et al., 2015). In early childhood, unitary models of EFs fit equally well or better than models with multiple factors (Brydges et al., 2014, 2012; Willoughby, Blair, Wirth, & Greenberg, 2012).
The unity/diversity model (Friedman & Miyake, 2017) decomposes variance in EF tasks into a Common EF factor that influences performance on all tasks, and specific factors (Updating-Specific and Shifting-Specific) that influence performance on updating and shifting tasks, respectively. In several prior studies, Common EF captured all the covariance among inhibition tasks, so there was no Inhibition-Specific factor (see Friedman & Miyake, 2017, for a review). The Common EF factor is thought to reflect individual differences in goal formation and maintenance and the use of those goals to bias ongoing processing (Friedman & Miyake, 2017), enabled in part by a frontal-parietal brain network (Herd et al., 2014). This conceptualization is consistent with classic conceptions of frontal lobe function enabling attentional control and goal-directed behavior (Duncan, Emslie, Williams, Johnson, & Freer, 1996; Miller & Cohen, 2001).
Some researchers have proposed that g (general cognitive ability, which is closely related to IQ) is a reflection of goal-related processes and linked to the multiple-demand network, a set of frontal and parietal brain areas that are recruited across diverse tasks (Duncan, 2010). The similarity of goal-related processes and areas related to g and Common EF is consistent with the observation that these constructs are correlated, but also raises the question of their separability. In adulthood, EFs are correlated with, but distinct from, IQ, and these relationships vary between EF domains (Blair, 2006; Friedman et al., 2006; Kane & Engle, 2002). Friedman et al. (2006) found that when controlling for the correlations among Inhibiting, Updating, and Shifting factors, the Updating factor was strongly related to fluid and crystalized IQ whereas Inhibiting and Shifting factors showed weak relations with IQ. Later bifactor models that separated working memory/updating ability into variance attributable to Common EF and Updating- or Working Memory-specific factors indicated that IQ’s association with working memory abilities results from associations with both Common EF and Updating/Working Memory-Specific factors (Friedman et al., 2008; Gustavson et al., 2018). In childhood and adolescence, the range of correlations between EFs and IQ is broad (rs = .13 to .89) (Brydges et al., 2012; Engelhardt et al., 2016; Friedman et al., 2008; Malanchini, Engelhardt, Grotzinger, Harden, & Tucker-Drob, 2018; Stephens et al., 2018). These different estimates may be related to age or task batteries. Overall, the associations between EFs and IQ seem to vary across development and between EF domains.
Genetically informative studies suggest different patterns of genetic and environmental influences on EFs and IQ (Engelhardt et al., 2015; Friedman et al., 2016; Haworth et al., 2010; Tucker-Drob et al., 2013; Van Soelen et al., 2011). Behavioral genetic studies show individual EF tasks typically display low estimates of genetic and shared environmental influences and high estimates of nonshared environmental influences, which include measurement error (Friedman & Miyake, 2017; Polderman et al., 2006), while latent factors of EFs are highly heritable in middle childhood (additive genetic influences [A] = 94-100%) (Engelhardt et al., 2015, 2016). In contrast, the heritability of IQ is moderate early in life (A = 25-64%) and increases across development (A = 65-72%) (Tucker-Drob et al., 2013; Van Soelen et al., 2011). Importantly, behavioral genetic studies typically find significant estimates of shared environmental influences (C; environmental influences that make siblings correlated) on IQ in childhood (C = 10-40%), but not on EFs (C = 0%) (Bishop et al., 2003; Engelhardt et al., 2015, 2016; Haworth et al., 2010; Tucker-Drob et al., 2013; Van Soelen et al., 2011). Moreover, twin studies typically observe low correlations between the non-shared environmental variance components (E; environmental influences that lead siblings to not correlate) between EFs and IQ (Engelhardt et al., 2016; Friedman et al., 2008; Gustavson et al., 2018), emphasizing environmental separability between these traits. Notably, the A, C, and E variance components identified in the behavioral genetic literature do not represent measured genes or specific environmental factors. However, the different patterns of genetic and environmental influence on EFs and IQ are meaningful in their own right, as they represent possible distinctions between EFs and IQ at the environmental and genetic levels.
Behavioral genetic studies have identified a wide range of genetic correlations between Common EF, Updating-Specific, and IQ (rA = .22 - .92) (Engelhardt et al., 2016; Friedman et al., 2008; Gustavson et al., 2018). These genetic correlations appear stronger in childhood than in adulthood: Engelhardt et al. (2016) reported the genetic correlation between Common EF and a latent factor of IQ was not significantly different than 1.0 in middle childhood, whereas studies of twins in late adolescence (Friedman et al., 2008), and middle age (Gustavson et al., 2018) report genetic correlations that are significantly lower than unity. The Engelhardt et al. study is the only twin study to examine relations of IQ to latent EF factors in children. The ABCD study provides an opportunity to re-examine these relationships in a large and nationally representative sample of children.
In the current study we asked, 1) Do we observe unity and diversity of EFs in this sample with the set of tasks selected for ABCD? 2) What are the phenotypic relationships between EFs and IQ in middle childhood? And 3) What are the genetic and environmental correlations between EFs and IQ in middle childhood? We use the cognitive battery from ABCD to answer these questions, using all data for the phenotypic models, and the twin data for the genetic models.
Method
Sample
Participants were 11,875 children ages 9-10.9 (M = 9.91, SD = .62, 48% female) recruited as part of the ABCD study (http://abcdstudy.org). ABCD comprises sites located across 21 US states, including four twin-pair recruitment sites (Iacono et al., 2018). Phenotypic analyses included the entire baseline sample, while the behavioral genetic analyses included 749 twin pairs (329 monozygotic [MZ], 420 dizygotic [DZ]).
Measures
The ABCD data-collection protocol included NIH toolbox tasks measuring EF, language development, memory, and processing speed (Bleck, Nowinski, Gershon, & Koroshetz, 2013; Gershon et al., 2013; Hodes, Insel, & Landis, 2013). We included flanker, card sort, and list sort1 from the NIH Toolbox, and the Rey auditory verbal learning task (RAVLT), emotional n-back, emotional Stroop, stop signal task (SST), matrix reasoning, and picture vocabulary (see Table 1 for full descriptive statistics of cognitive tasks). Task reliabilities have been established in prior studies and pilot ABCD data (Casey et al., 2018, & Luciana et al., 2018).
Table 1.
Descriptive Statistics for Screened Cognitive Tasks
| Measure | n | M | SD | Min | Max | Range | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Flanker | 11712 | 94 | 9.14 | 51 | 116 | 65 | −1.00 | 1.49 |
| List | 11669 | 96.64 | 12.09 | 36 | 136 | 100 | −0.54 | 0.87 |
| Card | 11713 | 92.52 | 9.51 | 50 | 120 | 70 | −0.82 | 2.04 |
| Picture | 11706 | 102.81 | 12.07 | 76 | 136 | 60 | 0.25 | −0.40 |
| NBack2 | 7938 | .75 | 0.12 | 0.15 | 1 | 0.85 | −0.43 | 0.19 |
| StroopRT | 4776 | 75.99 | 63.42 | −260.9 | 413.36 | 674.26 | 0.25 | 0.76 |
| StroopACC | 4776 | −.03 | 0.05 | −0.3 | 0.19 | 0.49 | −0.77 | 1.23 |
| SST | 8262 | 299.79 | 65.99 | 55.97 | 614.32 | 558.36 | 0.34 | 1.00 |
| PI | 11597 | −.20 | 1.99 | −9 | 7 | 16 | −0.02 | 0.27 |
| RI | 11644 | −1.53 | 1.99 | −7.97 | 4.87 | 12.84 | −0.33 | 1.02 |
| MatrixR | 11620 | 17.90 | 3.84 | 0 | 32 | 32 | −0.40 | 0.80 |
| PicVocab | 11718 | 84.45 | 8.12 | 29 | 119 | 90 | 0.11 | 0.64 |
Note. Descriptive statistics for cognitive tasks after data screening (see supplemental Table 1 for descriptive statistics before screening).
List = list sort; Card = card sort; Picture = picture sequence; NBack = accuracy on 2-back trials; StroopACC = Accuracy Diff Score = difference in accuracy between incongruent and congruent trials (incongruent trials - congruent trials); StroopRT = RT Diff Score = difference in reaction time (RT) between incongruent and congruent trials (incongruent trials - congruent trials); SST = stop signal RT, calculated by the mean “go” trial RT - the mean stop signal delay; RAVLT = Rey auditory verbal learning task; PI = proactive interference; RI = retroactive interference.; Matrix = matrix reasoning; PicVoc = picture vocabulary.
Flanker measures interference control. Participants saw five arrows and indicated which direction the middle arrow was pointing, while ignoring surrounding arrows pointing in the same or opposite direction. Card sort measures cognitive flexibility. Participants matched objects to two shapes at the bottom of the screen based on a particular rule (color or shape). Both accuracy and speed counted toward the final scores for flanker and card sort (Luciana et al., 2018; Zelazo et al., 2013). List sort measures working memory. Participants viewed a series of two to seven pictures in a category (e.g., animals) and said the list back in size order. The final score was the total number of correct responses.
The RAVLT assesses verbal learning and memory. Participants saw a list of words and recalled as many words as possible in any order. That same list and recall period repeated four times. After those first five trials, participants saw a new distractor list (list b, which occurred between lists five and six) and recalled the new words. Finally, participants repeated the original list’s words after being introduced to the new list without seeing the original list again (Luciana et al., 2018). We calculated a proactive interference score comparing accuracy on the new list to the first trial (list b - list one). A retroactive interference score compared accuracy on the trials before and after the distractor list (list six - list five).
Emotional N-Back (Casey et al., 2018) taps both working memory updating and emotional reactivity. Participants indicate whether pictures of places (e.g., buildings), or a diverse set of faces with happy, fearful, or neutral expressions are targets (match the picture two trials back for 2-back condition, or a given stimulus for 0-back condition) by clicking a button to indicate a match. The dependent measure was overall task accuracy in the 2-back condition. Emotional Stroop taps interference control. Individuals viewed a series of positive, negative, and neutral faces with emotionally valenced words overlaid on the image. Blocks contained both congruent (emotional valence of the word and image matched) and incongruent (emotional valence of the word and image did not match) trials. Participants indicated the emotional valence of the word via button press, ignoring the image (Banich et al., 2019; Başgöze, Gönül, Baskak, & Gökçay, 2015, Luciana et al., 2018). We constructed difference scores for reaction time (RT) and accuracy (incongruent trials – congruent trials). ABCD began administering this task at Wave 2, so data were not available for the entire sample.
The SST taps response inhibition. Participants saw a leftward or rightward facing arrow and indicated the direction of the arrow as quickly and as accurately as possible, but made no response when the arrow changed to an upward-facing “stop” arrow (Casey et al., 2018) after a variable delay on 16.67% of trials. The delay was calibrated to result in 50% correct stopping. We subtracted the mean stop-signal delay (the time between the start of the go trial and the stop trial), from the mean “go” trial RT to measure stop-signal RT.
In Matrix Reasoning (perceptual reasoning), participants saw incomplete visual stimuli and selected one of four options to complete the set (Luciana et al., 2018). This matrix reasoning task is a computerized version of the task of the same name from the Wechsler Intelligence Test for Children-V (WISC-V: Wechsler, 2014), which is a well validated measure of fluid or nonverbal reasoning. In Picture Vocabulary, a verbal reasoning task adapted from the Peabody Picture Vocabulary Test (Gershon et al., 2014), participants selected one of four pictures that best represented audi6torily presented words (Luciana et al., 2018).
Statistical Procedures
We accessed data through the ABCD NIH data portal. For flanker, list sort, picture sequence, card sort, matrix reasoning, and picture vocabulary we used the pre-calculated composite scores, and did not apply transformations. For n-back, we removed responses with an accuracy cut-off based on the binomial probability (p < .01) that the participant would have achieved that score by chance (≤ 62.5%), or from non-responders (all non-targets were correctly ignored, and all targets were missed); n excluded = 1,530/9,468). The stop-signal RT is based on an assumed rate of correct stop trials of 50%. We removed scores from participants with stop probabilities outside of 40-60%, indicating that the participant gave up during the task or had overall erratic performance. Based on best practices detailed in Verbruggen et al., (2019), we removed scores from participants whose SST data violated assumptions of the race model (i.e., with an average failed stop RT > average go RT). We also removed observations with overall mean RT scores < 50 milliseconds indicating the participants responded before than stop signals could occur (total SST n excluded = 1,336/9,598). In our models, we used SST RT multiplied by −1 so that so that for all tasks, higher numbers indicate better performance. For RAVLT, the distribution of retroactive interference was skewed with long tails; therefore, we trimmed the ends by replacing scores more than three SDs above the mean with a score three SDs above the mean (n replaced = 196/11,644). For emotional Stroop, we removed participants with accuracy that was not above chance overall (≤ 61.5%) or on incongruent trials (≤ 69.4%) (n excluded 73/4,849). Descriptive statistics before screening are available in supplemental Table 1. We regressed out age and sex for all cognitive tasks and used the standardized residuals in all models.
Models were estimated in Mplus version 8 (Muthén & Muthén, 2017). The chi-square test is sensitive to sample size, so we also assessed fit with root mean square error of approximation (RMSEA) < .06 and confirmatory fit index (CFI) > .95 (Hu & Bentler, 1998). We determined statistical significance of parameter estimates with chi-square difference tests: A significant p value (p < .05) indicates a reduction in model fit, suggesting a significant parameter was dropped. The ABCD cohort includes siblings and twin pairs; we accounted for non-independence using Mplus’ TYPE = COMPLEX, which uses a weighted likelihood function and a sandwich estimator to obtain a scaled chi-square and standard errors corrected for nonindependence.
ABCD twin zygosities were determined by calculating identity by state (IBS), an estimate of how many alleles at any genetic marker are identical, using genetic data. Twins with an IBS estimate between .4-.7 were classified as DZ while pairs with estimates > .9 were classified as MZ.
The classical twin design utilizes data from monozygotic (MZ) and dizygotic (DZ) twins to estimate what proportions of variation in a measure (phenotype) are attributable to additive genetic (A), shared environmental (C; those that lead siblings to correlate, e.g., socioeconomic status; being in the same class or having the same friends), and nonshared environmental (E; those that lead siblings to not correlate, e.g., joining different extracurricular activities or differential parental treatment) influences, as well as genetic (rA) and environmental (rC and rE) correlations across phenotypes. The twin method uses biometrical genetic principles to decompose the variance of a phenotype into A, C, and E components by examining cross-twin, cross-trait and cross-twin, within-trait covariances. Both MZ and DZ twins share familial environments, but MZ twins share 100% of their segregating genes, while DZ twins, like non-twin siblings, share on average 50% of their segregating genes. Therefore, genetic influences (A) are suggested when MZ twin pairs are more similar (show a higher correlation for a trait) compared to DZ twin pairs. Shared environmental (C) influences are suggested when the DZ correlation is greater than half the MZ correlation. Nonshared environmental influences (E), which include measurement error for non-latent variables, are suggested when the MZ correlation is less than 1.
The structural equation twin model formalizes these biometric principles (supplemental Figure 1A). It includes latent factors for A, C, and E, with the path estimates for these three factors predicting the trait of interest as free parameters that are estimated from the data (the variance/covariance matrix for each twin group). To identify the model, the A variance components for twin 1 and twin 2 are set to correlate at 1 for MZ twins and 0.5 for DZ twins (consistent with the proportion of shared segregating genes for MZ and DZ twin pairs respectively), and the estimated paths from these A factors to the trait are constrained to be equal for twin 1 and twin 2 and zygosity groups (i.e., genetic influences account for the same proportion of variance in a trait across the population, though MZ and DZ twins share these genetic influences to different extents). Similar constraints are implemented for the C and E paths, but the C variables are correlated at 1 for both MZ and DZ twins because both types of twin pairs are raised together, and the E variables are not correlated by definition, because they represent environmental influences unique to each twin (Rijsdijk & Sham, 2002 for an overview of twin modeling).
This twin model can be extended to multiple traits by implementing these biometric principles for each trait and allowing the A, C, and E variables to correlate across traits, leading to rA, rC, and rE estimates (supplemental Figure 1B). Bivariate heritability, the correlation between EFs and IQ explained by the genetic correlation, was calculated by multiplying the rA by the square roots of the heritabilities.
Results
Do We Observe Unity and Diversity of EFs in This Sample with the Set of Tasks Selected for ABCD?
We first evaluated whether EFs would show unity and diversity in this sample with this specific task battery. Based on task demands, and informed by the a priori and well replicated unity/diversity model of EFs (Friedman & Miyake, 2017), we hypothesized that Stroop2, flanker, SST, retroactive interference, proactive interference, and card sort would load onto a Common EF factor. Due to their working-memory requirements, we hypothesized that list sort and n-back would load onto both Common EF and an Updating-Specific factors. Consistent with previous behavioral genetic literature, (Friedman & Miyake, 2017; Polderman et al., 2006) we observed low to moderate estimates of A and moderate to high estimates of E for all cognitive tasks except Stroop and RAVLT. As shown in Table 2 and Table 3, the emotional Stroop and RAVLT measures were not substantially correlated with the other cognitive measures. Additionally, these tasks displayed near-zero estimates of both A and C (indicating that siblings’ performance was not at all correlated on these tasks, see Table 3 for task twin correlations), which is highly atypical. Individual cognitive tasks typically show low to moderate inter-correlations and A and C estimates (Friedman & Miyake, 2017; Polderman et al., 2006). The absence of twin correlations means that all of the variance in these tasks is attributable to E, which includes measurement error. Given the lack of shared A and/or C variances with the other cognitive tasks, these tasks could not be used in the latent variable models, so they were excluded from further analyses.
Table 2.
Correlation Matrix of Cognitive Tasks
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Flanker | 1 | |||||||||||
| 2. List | .30* | 1 | ||||||||||
| 3. Card | .44* | .33* | 1 | |||||||||
| 4. Picture | .21* | .35* | .27* | 1 | ||||||||
| 5. NBack | .18* | .23* | .19* | .18* | 1 | |||||||
| 6. StroopRT | −.06* | −.06* | −.05* | .00 | −.06* | 1 | ||||||
| 7. StroopACC | .05* | .08* | .11* | .09* | .05* | −.13* | 1 | |||||
| 8. SST | −.09* | −.09* | −.15* | −.10* | −.12* | −.01 | −.07* | 1 | ||||
| 9. PI | −.01 | −.03* | −.02* | .00 | .00 | .00 | .00 | −.02 | 1 | |||
| 10. RI | .05* | .09* | .06* | .10* | .06* | −.01 | .03* | −.03* | −.05* | 1 | ||
| 11. MatrixR | .28* | .40* | .31* | .30* | .23* | −.04* | .10* | −.08* | −.02 | .06* | 1 | |
| 12. PicVocab | .26* | .42* | .31* | .26* | .24* | −.04* | .06* | −.05* | −.07* | .07* | .41* | 1 |
Note. Correlation matrix of cognitive tasks after data screening.
List = list sort; Card = card sort; Picture = picture sequence; NBack = accuracy on 2 back trials; StroopACC = Accuracy Diff Score = difference in accuracy between incongruent and congruent trials (incongruent trials - congruent trials); StroopRT = RT Diff Score = difference in reaction time (RT) between incongruent and congruent trials (incongruent trials - congruent trials); SST = stop signal RT; PI = proactive interference measured by the Rey auditory verbal learning task (RAVLT); RI = retroactive interference measured by the RAVLT; MatrixR = matrix reasoning; PicVocab = picture vocabulary.
p < .05.
Table 3.
Twin Correlations, Variance Components, and Model Fit Indices for Univariate ACE Models of Cognitive Tasks
| Twin Correlations | Variance Components | Model Fit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | MZ | DZ | A | C | E | χ 2 | df | p | RMSEA | CFI |
| Flanker | .26 | .09 | .23* | .00 | .77* | 11.09 | 6 | .086 | .048 | .786 |
| List | .43 | .25 | .27* | .13 | .60* | 19.55 | 6 | .003 | .078 | .851 |
| Card | .38 | .13 | .35* | .00 | .65* | 2.48 | 6 | .877 | .000 | 1.00 |
| SST | .36 | .21 | .31* | .05 | .64* | 6.57 | 6 | .363 | .017 | .984 |
| N-Back | .31 | .13 | .30* | .00 | .70* | 7.77 | 6 | .256 | .030 | .901 |
| StroopAcc | .07 | .07 | .01 | .06 | .93* | 13.83 | 6 | .032 | .078 | .000 |
| StroopRT | .06 | .09 | .00 | .07 | .93* | 7.78 | 6 | .255 | .037 | .000 |
| MatrixR | .44 | .30 | .24* | .18 | .57* | 3.46 | 6 | .749 | .000 | 1.00 |
| PicVocab | .54 | .37 | .34* | .20* | .46* | 6.73 | 6 | .347 | .018 | .996 |
| RI | .04 | .05 | .00 | .05 | .95* | 2.29 | 6 | .892 | .000 | 1.00 |
| PI | .07 | .07 | .06 | .00 | .94* | 4.41 | 6 | .621 | .000 | 1.00 |
Note.
List = List sort. Card = Card sort. Picture = Picture Sequence. NBack is accuracy on 2-back trials. StroopRT = Stroop reaction time (RT). StroopAcc = Stroop accuracy. SST = stop signal RT. PI = proactive interference measured by the Rey auditory verbal learning task (RAVLT). RI = retroactive interference measured by the RAVLT. MatrixR = Matrix Reasoning. PicVocab = Picture Vocabulary.
Significance was tested using chi-square comparison testing. All cognitive tasks, except RI, PI, StroopAcc, and StroopRT, displayed a modest to moderate contribution from additive genetic factors (A = 23% - 35%) with moderate to large contributions from unique environmental factors (E = 46% - 77%). We observed zero to small contributions from shared environmental factors on individual differences in tasks (C = 0% - 20%). Only picture vocabulary displayed a significant contribution from shared environmental factors (C = 20%).
p < .05.
A phenotypic factor model of just Common EF (supplemental Figure 2A) fit the data adequately, χ2(5) = 123.52, p < .001, RMSEA = .045, CFI = .971, but not as well as a model that also included an orthogonal Updating-Specific factor with loadings for n-back and list sort (see supplemental Figure 2B), χ2(4) = 43.48, p < .001 RMSEA = .029, CFI = .990; Δχ2(1) = 80.04, p < .001. In the model of Common EF and Updating-Specific, the latter factor would not be identified without a constraint because it has only 2 indicators and is uncorrelated with any other factor (Kline, 2015), so we constrained the unstandardized loadings at the values estimated in the full model with the IQ factor.
What Are the Phenotypic Relationships Between EFs and IQ in Middle Childhood?
Informed by both the a priori unity/diversity model of EFs and the significantly improved model fit, we proceeded with a model that included an orthogonal Updating-Specific factor. A model adding a correlated latent factor of IQ to this EF model fit the data well, χ2(10) = 73.81, p < .001, RMSEA = .023, CFI = .993. As shown in Figure 1A, IQ correlated with Common EF at .64 [standard error [se] = .02] and Updating-Specific ability at .81 [se = .04].
Figure 1.
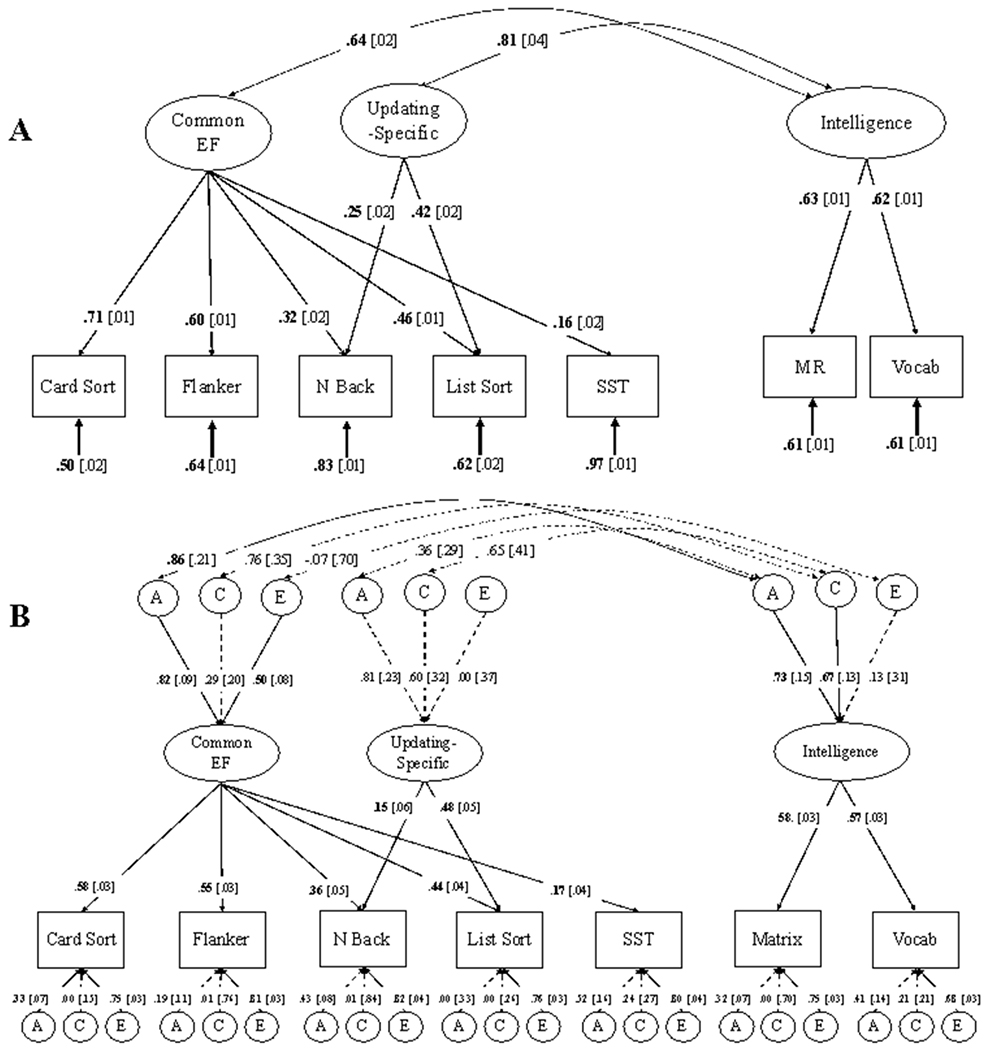
Phenotypic (panel A) and genetic (panel B) model of Common EF, Updating-Specific, and Intelligence (IQ) latent variables. A = additive genetic variance; C = shared environmental variance; E = non-shared environmental variance. Solid lines and boldface type indicate p < .05; dashed lines and regular text indicate p > .05, determined with chi-square difference tests for variance components. MR = matrix reasoning; Vocab = picture vocabulary.
What are the Genetic and Environmental Correlations between EFs and IQ in Middle Childhood?
We examined the shared genetic and environmental contributions to the association of IQ with Common EF and Updating-Specific abilities in a Cholesky decomposition, which we used to calculate genetic and environmental correlations. Supplemental Table 2 contains fit statistics and results of chi-square comparison tests to test the significance of variance components. We estimated correlations between the A, C, and E variance components of EFs and IQ that were greater than 0. Therefore, we did not estimate a correlation between the E variance components for Updating-Specific ability and IQ. This model adequately fit the data χ2(190) = 283.44, p < .001, RMSEA = .036, CFI = .930. Estimates of A, C, and E were similar across the combined model and uncombined models (separate models for Common EF and IQ latent variables). The significance of variance components for Common EF and IQ was tested with 1-df tests in the uncombined models (supplemental Figure 3 and supplemental Table 2).
As shown in Figure 1B, Additive genetic factors explained 67.2% of the variance in Common EF. In the model without IQ this estimate of A was significant, Δχ2(1) = 10.87, p < .001. Shared environmental influences explained a nonsignificant portion of variance (C = 8.4%), Δχ2(1) = .05, p = .823. The remaining variance was attributable to nonshared environmental influences (E = 25%), Δχ2(1) = 11.70, p < .01. For the Updating-Specific factor, additive genetic factors explained 65.6%, shared environmental factors explained 36%, and unique environmental factors explained 0% of the variance. Although chi-square difference tests for dropping each variance component individually in the independent model (supplemental Figure 3B) suggested that the estimates of A, C, and E for Updating-Specific EF were individually non-significant (supplemental Table 2), dropping both A and C components together in a 2-df test resulted in significantly worse fit, Δχ2(2) = 28.88, p < .01. Thus, Updating-Specific variance is attributable to familial influences (A and/or C), but we do not have the power to distinguish between A and C in this case. For IQ, additive genetic (A = 53.3%), Δχ2(1) = 6.68, p = .010, and shared environmental factors (C = 44.9%), Δχ2(1) = 5.62, p = .018, but not nonshared environmental factors (E = 1.7%) explained a substantial portion of the variance.
We observed a significant and strong genetic correlation of .86 [.21] between Common EF and IQ, Δχ2(1) = 15.18, p < .001. We tested if this genetic correlation was significantly different from 1.0 by setting the genetic cross-path from Updating-Specific to IQ and the residual genetic variance for IQ to zero. This correlation did not significantly differ from 1, Δχ2(2) = 1.39, p = .500. Environmental correlations between Common EF and IQ were not significant (rC = .76 [.35], Δχ2(1) = 1.41, p = .234; rE = −.07 [.70], Δχ2(1) = .01, p = .916. The genetic and environmental correlations between Updating-Specific ability and IQ were not significant (rA = .36 [.29], Δχ2(1) = 1.38, p = .240; rC = .65 [.41], Δχ2(1) = 2.23, p = .135). Bivariate heritability is the phenotypic correlation predicted by the genetic association and heritabilities; the bivariate heritability of Common EF and IQ was estimated at .51 [.14], accounting for 79.69% of the phenotypic correlation (.64). The bivariate heritability of Updating-Specific ability and IQ was not significant (.21 [.18]), accounting for 25.92% of the phenotypic correlation (.81).
Discussion
Using data from seven cognitive measures from over 11,000 children enrolled in the ABCD study, we examined three main questions about the phenotypic and genetic relationships between EF and IQ latent variables in middle childhood. First, we examined the factor structure of EFs in this age group and replicated the unity/diversity model of EFs by identifying two EF factors: Common EF and Updating-Specific. Second, we examined the phenotypic relationships between EFs and IQ and found that both Common EF and Updating-Specific correlated with IQ at the phenotypic level. Third, we investigated the strength of the genetic correlations between EFs and IQ and identified significant genetic influences on Common EF and IQ and a strong genetic correlation between Common EF and IQ. These findings are consistent with previous literature demonstrating phenotypic correlations of IQ with both Common EF and an Updating/Working Memory-Specific factor in adults (Friedman et al., 2008; Gustavson et al., 2018), and a strong shared genetic relationship between Common EF and IQ in childhood (Engelhardt et al., 2016).
EFs and IQ are sometime conflated as tapping the same general cognitive function ability (Ackerman, Beier, & Boyle, 2005; Blair, 2006; Heitz et al., 2006). For example, the NIH Toolbox’s fluid cognition composite score includes EF tasks (Zelazo et al., 2013). However, our findings demonstrate that these constructs are phenotypically separate in middle childhood. Although we observed moderate to high phenotypic correlations between latent variables of EFs and IQ, the correlations were still significantly lower than unity, indicating separability. Consistent with past behavioral genetic analyses of latent variable EFs across childhood and adolescence (A = 81-100%; Engelhardt et al., 2015; Friedman et al., 2016), we found a high heritability estimate for Common EF in this sample. However, our Updating-Specific factor did not display significant genetic influences, as twins were strongly correlated on the Updating-Specific factor across both MZ and DZ groups.
Contrasting with these results for Common EF, and consistent with the literature on IQ in middle childhood (Engelhardt et al., 2016; Tucker-Drob et al., 2013), we found significant estimates of shared environmental (C) influences on IQ in this sample. Meta-analyses and longitudinal studies of IQ suggest the importance of the shared environment throughout the lifespan (Briley & Tucker-Drob, 2013 Haworth et al., 2010; Tucker-Drob et al., 2013). These IQ C estimates contrast starkly with C estimates for EFs that are typically close to 0, even in childhood (Engelhardt et al., 2015; Engelhardt et al., 2016; Friedman et al., 2016; Gustavson et al., 2018). Though the genetic correlation between Common EF and IQ was high, the phenotypic correlation that was significantly less than unity. This separability could be driven by the shared environment. These differential patterns of environmental influences indicate that EFs and IQ are etiologically distinct in this age group. We also observed a near-zero correlation between the E variance components of Common EF and IQ, further reinforcing the environmental separability of these traits.
Research on the extent to which EFs and IQ have shared genetic influences is limited, especially in childhood (Engelhardt et al., 2016; Friedman et al., 2016; Gustavson et al., 2018). We identified a strong genetic correlation that was not significantly different than 1 between Common EF and IQ. Our estimated genetic correlation between Common EF and IQ was similar to estimates reported in Engelhardt et al. (2016), which suggested that Common EF and IQ are genetically identical. We did not observe significant shared genetic influences on the relationship between Updating-Specific EF and IQ. This is inconsistent with the findings of Friedman et al. (2008) and Gustavson et al. (2018), who reported moderate shared genetic relations between Working Memory- or Updating-Specific factors and IQ. These inconsistencies could be due to sample age, the use of different working memory/updating measures, or task scoring. The ABCD task battery was not designed to replicate the unity and diversity of EFs framework or tap specific facets of EF, like updating. However, even with this task battery and potentially weak factors, there is still evidence for a relationship of Updating-Specific with IQ at the phenotypic level, which is consitent with prior studies in adults (Friedman et al., 2006; Gustavson et al., 2018).
Interpreting Heritability Estimates
It is important to note that these variance components (A, C, and E) do not represent specific genes or environmental factors but represent the proportion of variance in a given population that can be attributed to additive genetic, shared environmental, or unique environmental influences (Harden, 2021, Turkheimer, 2000). Though not in perfect agreement, heritability estimates from molecular genetic studies have reinforced the twin literature indicating significant genetic influences on cognitive functions (Davies et al., 2018; Savage et al., 2018). Additionally, molecular genetic studies demonstrate the complex polygenicity (influenced by many genes) of cognitive traits by identifying hundreds of relevant genes (Davies et al., 2018; Savage et al., 2018).
Heritability is often misinterpreted through the lens of genetic determinism (Harden, 2021). Importantly, significant heritability estimates for Common EF and IQ do not imply that these traits are immutable or fixed. Heritability is an estimate of the proportion of variation in an outcome, like Common EF or IQ, that is accounted for by genetic differences in a population. Therefore, heritability estimates are specific to the population being studied at a particular time and under specific environmental conditions. For that reason, these estimates can change across age and across different environmental contexts (e.g., different countries) (Friedman et al., 2016; Tucker-Drob & Bates, 2016; Briley & Tucker-Drob, 2013).
Furthermore, significant heritability estimates do not negate the importance of environmental predictors of cognition. For example, factors like socioeconomic status predict many cognitive outcomes, including IQ, EFs, language, and brain structure and function (Farah, 2017; Merz, Wiltshire, & Noble, 2019). Additionally, in the United States, there is support for gene x SES effects on cognitive ability where heritability estimates are lower at higher level of socioeconomic disadvantage (Tucker-Drob & Bates, 2016), indicating that environmental influences may matter more at higher levels of deprivation. Gene x SES effects on cognitive ability further reinforce the hypothesis that changes in the environment can lead to changes in heritability estimates.
In addition to gene x environment interactions, genetic effects can operate indirectly through the environment (Koellinger & Harden, 2018), when genetic differences are associated with particular environmental differences (i.e., gene-environment correlation or rGE). For example, musical parents may pass down genes related to musical skill, but also raise their children in homes with access to musical instruments and encourage musical exposure. In classic twin models, such environmentally mediated genetic influences are included in the A estimate (Tucker-Drob et al., 2013), but they can be disentangled in some designs (adoption studies and using molecular genetic data from families; (Koellinger & Harden, 2018). For example, Kong et al. (2018) found that children were influenced by their parent’s measured genetic variants related to educational attainment that they did not inherit, suggesting that those genetic variants influence educational outcomes indirectly through the family environment, a phenomenon they describe as genetic nurture (Kong et al., 2018). Family studies suggest that genetic nurture and rGE appear to be particularly related to cognitive and educational outcomes instead of anthropometric traits, emphasizing the importance of environmental influences on cognitive outcomes (Kong et al., 2018; Selzam et al., 2019).
Limitations and Future Directions
The ABCD Study is an ongoing, longitudinal, open-access project that currently provides pre-scored summary level data for most cognitive tasks. The scoring algorithms for the NIH Toolbox tasks do not take experimental contrasts in each task into account, and instead provide overall indices of performance (i.e., summing across easier and harder conditions, rather than taking the difference scores). Thus, these scores are unlike most traditional EF task scores, potentially leading to different associations with IQ.
Additionally, we examined the phenotypic and genetic correlations between EFs and IQ at one time point (age 9-10). These correlations may change across age. The longitudinal nature of the ABCD Study presents the possibility of future phenotypic and behavioral genetic work examining the structure of and genetic influences on EFs and IQ across development.
Implications
Our phenotypic findings were consistent with research on adults: IQ and some EFs are correlated, but distinct. It appears that even in childhood, EFs and IQ are separate cognitive processes. This separability of EFs and IQ appears to be driven by environmental influences, as their genetic variance shows considerable overlap. The shared genetic influences underlying the relationship between EFs and IQ may be stronger in childhood than late adolescence or middle adulthood. Longitudinal modeling will help understand how the phenotypic and genetic relationships between EFs and IQ change across development.
Supplementary Material
Research Highlights.
The unity and diversity factor structure of executive functions (EFs) is present in middle childhood.
Common EF and intelligence (IQ) latent variables are phenotypically distinct in middle childhood.
Common EF and IQ display strong genetic overlap in middle childhood.
EFs and IQ display different patterns of environmental influence in middle childhood.
Acknowledgments
There are no conflicts of interest to report. All ABCD procedures were approved by a central institutional review board and comply with the World Medical Association Declaration of Helsinki and APA ethical standards. The University of California San Diego institutional review board has stated that analyses using publicly released ABCD data are not human subjects research and do not require their own protocol approval. This research was supported by NIH grants MH063207, MH016880, and DA046413.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from ABCD release 2.0 (DOI: 10.15154/1503209, Study-specific NDA DOI: 10.15154/1519249). ABCD data is publicly available.
Megan Ross and Jennifer Keith aided in accessing and understanding the ACBD data portal and protocols.
Footnotes
We originally considered including picture sequence task. In this task, participants see a list of photos of various actions and recite the actions back to form a scenario (Luciana et al., 2018). After experiencing the task, we judged it to be tapping long term memory due to the length of the to-be-recalled sequence, so we did not include it in our EF model.
The emotional Stroop task requires participants to ignore task-irrelevant emotional faces from same-aged peers and instead decide if the word printed over the face is negatively or positively valenced. This task examines inhibitory control over distracting emotional information and has been reported as engaging brain regions involved in emotional processing and executive control (Banich et al., 2019). Therefore, we hypothesized that the emotional Stroop should be related to the Common EF factor.
References
- Ackerman PL, Beier ME, & Boyle MO (2005). Working memory and intelligence: The same or different constructs? Psychological Bulletin, 131(1), 30–60. 10.1037/0033-2909.131.1.30 [DOI] [PubMed] [Google Scholar]
- Banich MT, Smolker HR, Snyder HR, Lewis-Peacock JA, Godinez DA, Wager TD, & Hankin BL (2019). Turning down the heat: Neural mechanisms of cognitive control for inhibiting task-irrelevant emotional information during adolescence. Neuropsychologia, 125, 93–108. 10.1016/J.NEUROPSYCHOLOGIA.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başgöze Z, Gönül AS, Baskak B, & Gökçay D (2015). Valence-based word-face Stroop task reveals differential emotional interference in patients with major depression. Psychiatry Research, 229(3), 960–967. 10.1016/j.psychres.2015.05.099 [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, & Naglieri JA (2011). Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learning and Individual Differences, 21(4), 327–336. 10.1016/j.lindif.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop EG, Cherny SS, Corley R, Plomin R, DeFries JC, & Hewitt JK (2003). Development genetic analysis of general cognitive ability from 1 to 12 years in a sample of adoptees, biological siblings, and twins. Intelligence, 31(1), 31–49. 10.1016/S0160-2896(02)00112-5 [DOI] [Google Scholar]
- Blair C (2006). How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral and Brain Sciences, 29(2), 109–125. 10.1017/S0140525X06009034 [DOI] [PubMed] [Google Scholar]
- Bleck TP, Nowinski CJ, Gershon R, & Koroshetz WJ (2013). What is the NIH Toolbox, and what will it mean to neurology? Neurology, 80(10), 874–875. 10.1212/WNL.0b013e3182872ea0 [DOI] [PubMed] [Google Scholar]
- Briley DA, & Tucker-Drob EM (2013). Explaining the increasing heritability of cognitive ability across development: A meta-analysis of longitudinal twin and adoption studies. Psychological Science, 24(9), 1704–1713. 10.1177/0956797613478618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges CR, Fox AM, Reid CL, & Anderson M (2014). The differentiation of executive functions in middle and late childhood: A longitudinal latent-variable analysis. Intelligence, 47, 34–43. 10.1016/j.intell.2014.08.010 [DOI] [Google Scholar]
- Brydges CR, Reid CL, Fox AM, & Anderson M (2012). A unitary executive function predicts intelligence in children. Intelligence, 40(5), 458–469. 10.1016/j.intell.2012.05.006 [DOI] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, … Dale AM (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. 10.1016/J.DCN.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, … Deary IJ (2018). Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nature Communications, 9(1), 1–16. 10.1038/s41467-018-04362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010). The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in cognitive sciences, 14(4), 172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, & Freer C (1996). Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology, 30(3), 257–303. 10.1006/cogp.1996.0008 [DOI] [PubMed] [Google Scholar]
- Demetriou A, Spanoudis G, Shayer M, Van der Ven S, Brydges CR, Kroesbergen E, … Swanson HL (2014). Relations between speed, working memory, and intelligence from preschool to adulthood: Structural equation modeling of 14 studies. Intelligence, 46(1), 107–121. 10.1016/j.intell.2014.05.013 [DOI] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Briley DA, Mann FD, Harden KP, & Tucker-Drob EM (2015). Genes unite executive functions in childhood. Psychological Science, 26(8), 1151–1163. 10.1177/0956797615577209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Mann FD, Briley DA, Church JA, Harden KP, & Tucker-Drob EM (2016). Strong genetic overlap between executive functions and intelligence. Journal of Experimental Psychology: General, 145(9), 1141–1159. 10.1037/xge0000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2017). The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron. Cell Press. 96(1), 56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Friedman NP, Hatoum AS, Gustavson DE, Corley RP, Hewitt JK, & Young SE (2020). Executive functions and impulsivity are genetically distinct and independently predict psychopathology: Results from two adult twin studies. Clinical Psychological Science, 8(3), 519–538. 10.1177/2167702619898814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, & Hewitt JK (2006). Not all executive functions are related to intelligence. Psychological Science, 17(2), 172–179. 10.1111/j.1467-9280.2006.01681.x [DOI] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, & Hewitt JK (2016). Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology, 52(2), 326–340. 10.1037/dev0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137(2), 201–225. 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, … Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, & Nowinski CJ (2013). NIH toolbox for assessment of neurological and behavioral function. Neurology, 80(11 Suppl 3), S2–S6. 10.1212/wnl.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Cook KF, Mungas D, Manly JJ, Slotkin J, Beaumont JL, & Weintraub S (2014). Language measures of the NIH toolbox cognition battery. Journal of the International Neuropsychological Society, 20(6), 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson DE, Panizzon MS, Franz CE, Friedman NP, Reynolds CA, Jacobson KC, … Kremen WS (2018). Genetic and environmental architecture of executive functions in midlife. Neuropsychology, 32(1), 18–30. 10.1037/neu0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP (2021). “Reports of My Death Were Greatly Exaggerated”: Behavior genetics in the postgenomic era. Annual Review of Psychology, 72(1). 10.1146/annurev-psych-052220-103822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Engelhardt LE, Mann FD, Patterson MW, Grotzinger AD, Savicki SL, … Tucker-Drob EM (2019). Genetic associations between executive functions and a general factor of psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 59(6), 749–758. 10.1016/J.JAAC.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, De Geus EJC, Van Beijsterveldt CEM, … Plomin R (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15(11), 1112–1120. 10.1038/mp.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP, Redick TS, Hambrick DZ, Kane MJ, Conway AR, & Engle RW (2006). Working memory, executive function, and general fluid intelligence are not the same. Behavioral and Brain Sciences, 29(2), 135–136. 10.1017/S0140525X06319036 [DOI] [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, & Friedman NP (2014). A neural network model of individual differences in task switching abilities. Neuropsychologia, 62, 375–389. 10.1016/j.neuropsychologia.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Insel TR, & Landis SC (2013). The NIH toolbox: Setting a standard for biomedical research. Neurology, 80(11 Suppl 3), S1. 10.1212/WNL.0b013e3182872e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods, 3(4), 424–453. 10.1037/1082-989X.3.4.424 [DOI] [Google Scholar]
- Iacono WG, Heath AC, Hewitt JK, Neale MC, Banich MT, Luciana MM, … Bjork JM (2018). The utility of twins in developmental cognitive neuroscience research: How twins strengthen the ABCD research design. Developmental Cognitive Neuroscience, 32, 30–42. 10.1016/j.dcn.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, & Brown SA (2018). Introduction. Developmental Cognitive Neuroscience, 32, 1–3. 10.1016/j.dcn.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin and Review, 9(4), 637–671. 10.3758/BF03196323 [DOI] [PubMed] [Google Scholar]
- Kline RB (2015). Principles and practice of structural equation modeling. Guilford publications. [Google Scholar]
- Koellinger PD, & Harden KP (2018). Using nature to understand nurture. Science, 359(6374), 386–387. 10.1126/science.aar6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE, … Stefansson K (2018). The nature of nurture: Effects of parental genotypes. Science, 359(6374), 424–248. 10.1126/science.aan6877 [DOI] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, & Banich MT (2018). Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience, 32, 67–79. 10.1016/J.DCN.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchini M, Engelhardt LE, Grotzinger AD, Harden KP, & Tucker-Drob EM (2018). “Same But Different”: Associations between multiple aspects of self-regulation, cognition, and academic abilities. Journal of Personality and Social Psychology, 117(6), 1164–1188. 10.1037/pspp0000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Wiltshire CA, & Noble KG (2019). Socioeconomic inequality and the developing brain: Spotlight on language and executive function. Child Development Perspectives, 13(1), 15–20. 10.1111/cdep.12305 [DOI] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/COGP.1999.0734 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998-2017). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, De Leeuw CA, … Posthuma D (2018). Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nature Genetics, 50(7), 912–919. 10.1038/s41588-018-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzam S, Ritchie SJ, Pingault JB, Reynolds CA, O’Reilly PF, & Plomin R (2019). Comparing within- and between-family polygenic score prediction. American Journal of Human Genetics, 105(2), 351–363. 10.1016/j.ajhg.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Friedman NP, & Hankin BL (2019). Transdiagnostic mechanisms of psychopathology in youth: Executive functions, dependent stress, and rumination. Cognitive Therapy and Research, 43(5), 834–851. 10.1007/s10608-019-10016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RL, Langworthy B, Short SJ, Goldman BD, Girault JB, Fine JP, … Gilmore JH (2018). Verbal and nonverbal predictors of executive function in early childhood. Journal of Cognition and Development, 19(2), 182–200. 10.1080/15248372.2018.1439493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, & Bates TC (2016). Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 27(2), 138–149. 10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA, & Harden KP (2013). Genetic and environmental influences on cognition across development and context. Current Directions in Psychological Science, 22(5), 349–355. 10.1177/0963721413485087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E (2000). Three laws of behavior genetics and what they mean. Current Directions in Psychological Science, 9(5), 160–164. 10.1111/1467-8721.00084 [DOI] [Google Scholar]
- Van Soelen ILC, Brouwer RM, Van Leeuwen M, Kahn RS, Hulshoff Pol HE, & Boomsma DI (2011). Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Research and Human Genetics, 14(2), 119–128. 10.1375/twin.14.2.119 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Band GPH, Beste C, Bissett PG, Brockett AT, … Boehler CN (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife, 8, e46323. 10.7554/eLife.46323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, … Weiss SRB (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental Cognitive Neuroscience, 32, 4–7. 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2014). WISC-V: Technical and Interpretive Manual. Bloomington, MN: Pearson [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, & Greenberg M (2012). The measurement of executive function at age 5: Psychometric properties and relationship to academic achievement. Psychological Assessment, 24(1), 226–239. 10.1037/a0025361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, & Hewitt JK (2009). Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology, 118(1), 117–130. 10.1037/a0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, & Weintraub S (2013). NIH toolbox cognition battery (CB): Measuring executive function and attention. Monographs of the Society for Research in Child Development, 78(4), 16–33. 10.1111/mono.12032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


