Abstract
Chronic social isolation stress (SIS) induces lasting negative effects on the brain, including memory deficits, cognitive impairments, and mood alterations such as depression and anxiety. All these symptoms, at least in part, reflect reduced hippocampal function. In both clinical and preclinical studies, subanesthetic doses of the NMDA receptor antagonist, ketamine (KET), was shown to have rapid and lasting antidepressant effects. Animal studies have shown that biological sex and levels of gonadal hormones alter the behavioral effects of KET, with ovarian hormones increasing sensitivity to the antidepressant-like effects of KET. Since the hippocampus plays a key role in mediating some of the effects of SIS, and considering that KET at low doses has been shown to rescue some of the behavioral deficits of isolation rearing this study aimed to assess the effects of isolation stress on pre- and post-synaptic hippocampal functions in male and female rats reared in SIS, as well as determine whether some of the physiological deficits can be rescued with a single injection of sub-anesthetic doses of KET. To do this, Sprague-Dawley rats were raised from weaning in either social isolation or with same-sex cage mate for 5 to 7 weeks. Male and female rats in either diestrus of proestrus received a single injection of KET (0, 2.5, or 5.0 mg/kg) three hours prior to termination and collection of acute hippocampal slices for ex vivo electrophysiological field potential recordings. Long-term potentiation (LTP) and paired pulse facilitation (PPF) outputs were assessed in a canonical CA3-CA1 dorsal hippocampal circuit. Our data show that SIS inhibits hippocampal LTP without affecting PPF in male rats, an effect that was rescued by KET. In female rats, isolation stress did not alter LTP, but did reduce PPF - especially when females were tested in diestrus-, an effect that was rescued by KET at the highest dose. Our data thus suggest sex differences in the contribution of pre-and postsynaptic hippocampal compartments in response to stress and KET.
Keywords: social isolation, ketamine, long term potentiation, paired pulse facilitation, hippocampus, plasticity, estrous cycle, sex differences
1. Introduction
In humans, early-life social isolation resulting from neglect or abandonment and solitary confinement leads to depression, anxiety and cognitive deficits (Mulder et al 2018, Liang et al 2016, Coid et al 2003, Khan and Leventhal 2020). Like humans, rodents also require continuous interaction with conspecifics to maintain homeostasis and normal brain functions (Hawkley and Cacioppo, 2010; Cacioppo et al., 2011). Therefore, early life SIS can have numerous negative physiological impacts in social species (Mumtaz et al., 2018). For example, early life SIS leads to deficits in recognition and spatial memories (Bianchi et al 2006; Wang et al 2019), depressive- and anxiety-like behaviors (Leussiss & Andersen 2008), and increased aggressive behaviors (Vale & Montgomery 1997).
Early life stress has been clearly shown to alter the hippocampus anatomy and physiology (Teicher, M. H. & Samson, J. A.; 2016; Frodl, T. et al., 2017; Lambert, H. K. et al.2019; De Bellis, et al., 2013). This highly interconnected brain area, which continues to develop during childhood, is extremely sensitive to stress (Khalaf-Nazzal, R. & Francis, F., 2013). It is thus not surprising that early life stress, such as SIS, will alter some of the essential functions of the hippocampus such as cognition and emotion (Teicher, M. H. & Samson, J. 2016; Lambert, H. K. et al.2019; De Bellis, et al., 2013).
One of the important physiological functions of the hippocampus that could be altered by SIS is Long Term Potentiation (LTP), which is a form of synaptic plasticity. LTP studies test the adaptability of a specific brain circuit to changes in inputs. An electrical current stimulates the presynaptic terminal into firing, causing the release of glutamate, which activates AMPA and NMDA receptors on the postsynaptic terminals, leading to the production of a field excitatory post-synaptic potential (fEPSP). With repeated high-frequency stimulation (HFS), the synaptic responses change over time to produce larger fEPSPs. This plasticity in the synapse is termed LTP and reflects the synapses’ ability to adapt to stimuli. LTP thus is an important candidate to the cellular mechanisms of learning and memory (Cooke SF, Bliss TV 2006). SIS has been shown to decrease hippocampal LTP in male mice (Wang et al 2019), and the non-competitive NMDA receptor antagonist ketamine (KET), when administered at lower doses, was able to rescue LTP deficits in a rat model of the studies of depression (Lily R Aleksandrova et al., 2020). Of note, KET, when administered at subanesthetic doses, has rapid and long lasting anti-depressant effects (Berman et al 2000, Duman 2018, Sterpenich et al 2019). Since stress in general reduces LTP (Lily R Aleksandrova et al., 2020; Pereda-Pérez, N. Popović, B.B. Otalora, et al. 2013; P. Kehoe, J.D. Bronzino 1999), while chronic administration of the antidepressant fluoxetine enhances it (K.G. Bath, D.Q. Jing, I. Dincheva, et al.2012), we hypothesize that acute KET administration at subthreshold doses may rescue synaptic plasticity deficits induced by SIS in male and female rats. We decided to include both sexes because our previous work and the work of others clearly show sex differences in KET’s antidepressant-like effects (Carrier and Kabbaj, 2013; Franceschelli et al 2015) and a significant role of female gonadal hormones in mediating these sex differences (Carrier and Kabbaj, 2013). Furthermore, since estrous cycle stages have been shown to have strong influences on hippocampal LTP, with females in proestrus showing greater hippocampal LTP than females in diestrus (Warren et al., 1995), another aim of this study was to examine LTP in female rats at different stages of their estrous cycle. In this work, we will be also assessing Paired-pulse facilitation (PPF) which is a short-term, pre-synaptic form of plasticity. PPF is thought to be caused by an influx of calcium ions (Ca2+ ) into the terminals via the opening of the voltage dependent channels. When there is a short interval between two stimulations, the residual Ca2+ from the first stimulation adds to the Ca2+ influx from the second stimulation, increasing the Ca2+ concentration on the second pulse, thus increasing probability of neurotransmitter release. This method thus allows a relative measure of presynaptic terminals’ short-term plasticity. Hence, performing PPF and LTP measurements in conjunction provides more detailed information about the effect that a treatment has on a synapse.
2. Materials and Methods
2.1. Animals
44 Male and 84 female Sprague Dawley rats aged twenty-two days (P22) were obtained immediately post weaning from Charles River Laboratories (Raleigh, NC). All animals were housed under controlled temperatures and 12-hour light/dark cycles (with lights on at 0900) and were allowed ad libitum access to standard rat chow and water. All procedures were carried out under strict accordance to the NIH Guide for the Care and Use of Laboratory Animals and the protocol was approved by the Florida State University Institutional Animal Care and Use Committee.
2.2. Social Isolation Stress
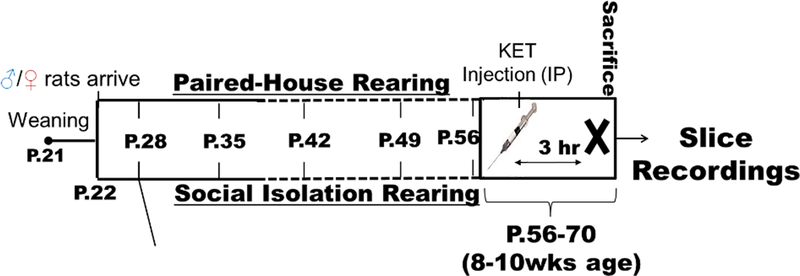
Upon rats’ arrival to the animal facility on P22, they were randomly assigned to be housed either in a same sex pair or in social isolation with opaque barriers between cages to prevent visual contact between animals. Animals were maintained in these conditions for five to seven weeks, only being handled for a weekly body mass measurement and cage/bedding changes (Figure 1). Beginning on the fourth week of housing, female rats were vaginally lavaged once daily during the light cycle to assess estrous cycle stage. Cycle stage was assessed by cell cytology under a light microscope (Goldman et al 2007; Saland and Kabbaj, 2018). After the fifth week of isolation (P56), female animals were selected for hippocampal LTP recording any day they were in either Diestrus 1 or Proestrus. Male and female rats were counterbalanced and were age-matched at time of termination. Rats were 8–10 weeks old when terminated (Figure 1).
Figure 1. Experimental Timeline.
Animals were isolated post-weaning (P22) for a minimum of 5 weeks. Animals aged 56–70 days were selected for experiments. Estrous stage was assessed on P49 until the day of experiment: females were selected for recordings when either diestrus I or proestrus phase was confirmed that morning. The experimental animals received a single acute injection of KET (0, 2.5, or 5.0 mg/kg, i.p.) at lights onset. Three hours later, acute brain slices containing dorsal hippocampus were prepared for electrophysiological field recordings.
2.3. Drug administration
KET hydrochloride (Ketathesia, racemic, Henry Schein Animal Health Inc.) in an injectable solution was diluted to 2.5 and 5.0 mg/mL in sterile saline vehicle (VEH). Animals were weighed and injected intraperitoneally (i.p.) with 1.0ml/kg body mass of either VEH, 2.5 mg/kg KET, or 5.0 mg/kg KET. Immediately following the injection, animals were quietly transported in their home cage from the housing facility to the electrophysiology recording space to rest for exactly 3 hours. Care was taken to maintain a warm and quiet environment for the animals during this time.
2.4. Tissue Preparation
Solutions and acute hippocampal slices were prepared similarly as described in Qiao et al (2014). Exactly 3 hours after i.p. injection, rats were rapidly decapitated. The brain was rapidly dissected and placed into ice-cold carbogenated cutting solution containing: 230 mM sucrose, 2.5 mM KCl, 10 mM MgSO4, 1.25 mM Na2HPO4, 26 mM NaHCO3, 0.5 mM CaCl2, and 10 mM D-glucose. Parasagittal brain slices containing dorsal hippocampus were cut (400μm) using a vibrating blade microtome (Leica VT1200S). Hippocampal slices were then incubated in carbogenated artificial cerebrospinal fluid (ACSF: 124 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 1.1 mM KH2PO4, 1 mM NaHCO3, and 10 mM glucose) at 37°C for 1 hours, then incubation temperature was restored to room temperature.
2.5. Electrophysiological Recordings
Hippocampal slices were individually transferred to the recording chamber. Carbogenated ACSF warmed to physiological temperature (Warner Instruments TC-344B) was continuously perfused onto the stage using a simple gravity siphon, and a vacuum cleared the waste. Tissue was held in place using a slice anchor (Warner Instruments, SHD-26H/2). Slices were visualized using a Nikon Eclipse FN1 at 40x with the image projected to a digital screen using a Nikon DS-Qi1Mc digital camera and NIS-Elements AR 3.2 64-bit software.
Field potentials were evoked using a concentric bipolar stimulated electrode (FHC CBAPC75) placed in the Schaeffer collaterals of CA3, and stimuli were issued by a Master-8 stimulus generator coupled to an AMPI Iso-Flex power box. Field potentials were recorded with a silver electrode in a glass pipette (World Precision Instruments, 4IN Thinwall GL 1.5 OD/1.12ID) made using a Narshige PC-10 pipette puller and backfilled with 4M NaCl. The recording electrode was coupled to an Axon Multiclamp 700B (Molecular Devices) and placed in the CA1 stratum radiatum. Synaptic transmission strength was measured by assessing the initial slope of fEPSPs using stimuli of monophasic 100 μs pulses at 0.033Hz. The responses were tested over a range of stimulus intensities from 0.2 to 0.65 mA. Stimuli was adjusted to produce 50–60% of maximum response. LTP was then induced with two trains of 50 square pulses (100Hz) delivered 30 seconds apart. Slices that did not exhibit an initial increase in slope of the downward deflection of the fEPSP following HFS were considered unhealthy and not included for analysis.
Paired-pulse facilitation and LTP were never measured in the same slice. Synaptic transmission strength was measured in the same manner as previously described, with the stimuli adjusted to 50–60% maximum response before paired pulses were initiated. Two monophasic pulses of 100μs were applied in a single sweep, with the pulses being delivered at intervals of 20ms, 50ms, and 100ms. Sweeps were separated by 30 seconds.
2.6. Statistical Analysis
For statistical purposes, “Experimental condition” was defined as follows: Pair-housed Saline, Isolated Saline, Isolated KET 2.5mg/kg and Isolated KET 5mg/kg. Following HFS, LTP was defined as a significant change of the fEPSP slope from baseline. LTP data are expressed as mean response over the measured time ± SEM, with statistical significance assessed by three-way mixed model ANOVA with post hoc Tukey corrected tests. Due to the large variability of the initial 10 minutes following HFS, these data were not included in the analyses. Paired-pulse facilitation and input/output data were expressed as means ± SEM at each time interval/intensity. Both were analyzed using a three-way mixed model ANOVA test with post hoc Tukey corrected tests. Data were analyzed in R Studio (lme4 package) and GraphPad Prism.
The approach taken to estimate the degrees of freedom is the Kenward-Roger method, implemented in the lme4 R package. This method improves the calculation of the F value in small to moderate samples. In order to improve the computation of the slope differences for LTP, the repeated measures regarding Pre-HFS and post-HFS were averaged, as recommended by Haleko and Højsgaard (2014). Supplementary table 1 shows individual F values of the adopted 3-way ANOVAs along with effect sizes and individual n’s.
3. Results
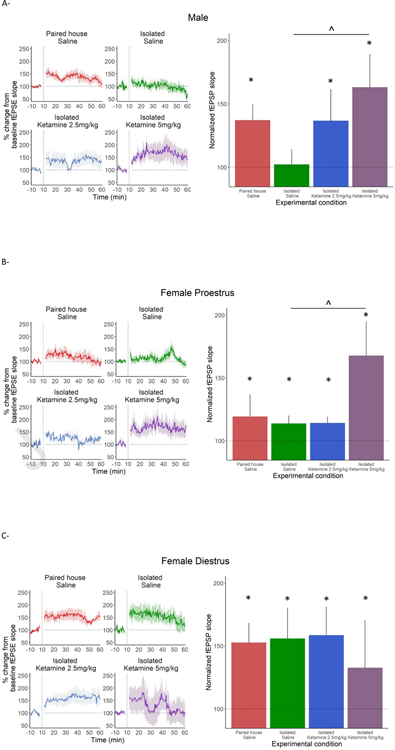
To determine the impact of SIS/KET treatment on long-term synaptic plasticity, we used HFS to induce LTP in CA3-CA1 synapses in acute hippocampal slices. Three-way repeated measures ANOVA revealed a significant effect of Sex (F (1,88.5)= 25.910, p<0.001), Experimental Condition (F (3,114.9) = 33.194, p<0.001) and Time (Pre HFS vs. Post-HFS) (F (1,8837.9) = 1219.844, p<0.001), as well as a three-way interaction between these three factors (F (3,8837.9) = 16.137, p<0.001).
Since there was a significant three-way interaction between Estrous Cycle, Experimental Condition and Time (F (3,5302.0) = 37.3101, p<0.001), we analyzed the effect of KET and housing conditions on LTP separately in males, females during diestrus and females during proestrus.
In males (Fig. 2A), there were significant changes in fEPSP from baseline in all experimental conditions except Isolated Saline (p>0.05). The magnitude of increase from baseline in fEPSP was greater in Isolated KET 5mg/kg than Isolated Saline (p<0.05).
Figure 2. Long-term potentiation recordings.
* indicates difference from baseline. ^ indicates difference between groups.
(A) All male groups except Isolated Saline differ from baseline following HFS. The Isolated KET 5mg/kg has a significantly greater increase from baseline than Isolated Saline (p<0.05). (Paired with saline treatment n=5, Isolated with saline treatment n=5, Isolated with 2.5 mg/kg KET treatment n=5, Isolated with 5.0 mg/kg KET treatment n=5)
(B) All proestrus female groups increased from baseline. The Isolated KET 5mg/kg treatment had a significantly greater increase from baseline than Isolated Saline (p<0.05) (Paired with saline treatment n=6, Isolated with saline treatment n=4, Isolated with 2.5 mg/kg KET treatment n=6, Isolated with 5.0 mg/kg KET treatment n=4)
All diestrus female groups increased from baseline but none differed from each other in responses. (Paired with saline treatment n=5, Isolated with saline treatment n=5, Isolated with 2.5 KET mg/kg treatment n=4, Isolated with 5.0 mg/kg KET treatment n=4).
In proestrus females, there were significant changes from baseline in all experimental conditions, as well as greater increase from baseline in Isolated KET 5mg/kg when compared to Isolated Saline (p<0.05) (Fig. 2B).
In diestrus females, there were significant changes in fEPSP from baseline in all experimental conditions, but no group differences were observed in the increase from baseline (Fig. 2C).
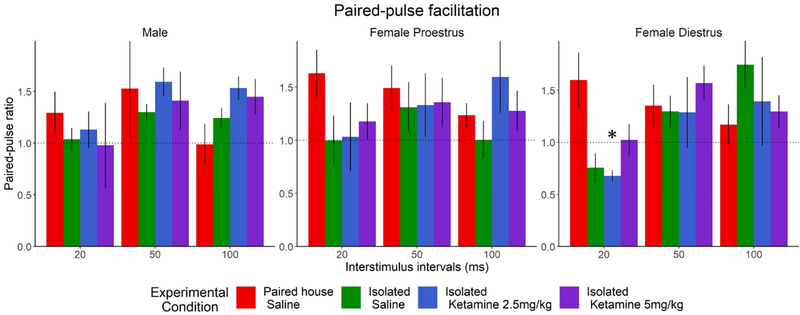
To assess potential presynaptic plasticity alterations by KET and SIS, we measured PPF in the CA3-CA1 synapses of male and female rats in diestrus and proestrus. PPF was assessed in separate slices from those used for LTP measurements. As expected, there was a main effect of stimulus interval (F (2,118) = 8.3019, p<0.05). There was also an interaction between Experimental Condition and Stimulus interval (F(6,118)= 3.7778, p<0.05). To further investigate this, and based on our a priori assumption of Sex and Cycle differences, the data was analyzed with a two-way mixed model ANOVA. In male rats and proestrus females, there were no differences between the 4 groups at any stimulus interval (Fig. 3A, 3B). In diestrus females however (Fig. 3C), a main effect of Stimulus Interval was observed (F (2,38) =4.7280, p<0.05), as well as a two-way interaction between Experimental Condition and Stimulus Interval (F (6,38) =2.5824, p<0.05). Pair-housed diestrus females treated with saline had a greater PPF ratio than Isolated KET 2.5mg/kg at the 20ms interval (p<0.05). A trend was also observed when comparing PPF ratios in Pair-housed vs. Isolated saline at 20ms interval (p=0.07). The observed effects indicate that the effects of SIS at the pre-synaptic site of the CA3-CA1, are only evident during the diestrus phase, when circulating levels of hormones are lower. Our hypothesis is that lower hormones levels in females increase the vulnerability to the effects of SIS at the pre-synaptic site, and that KET (5 mg/kg) abolishes the effects of isolation stress.
Figure 3. Paired Pulse Facilitations.
A main effect of stimulus intensity was present, but there were no differences between sex or treatment in the (A) male (Paired with saline treatment n= 7, Isolated with saline treatment n= 12, Isolated with 2.5 mg/kg treatment n= 11, Isolated with 5.0mg/kg treatment n= 14) or (B) proestrus females (Paired with saline treatment n= 12, Isolated with saline treatment n= 13, Isolated with 2.5 mg/kg treatment n= 9, Isolated with 5.0 mg/kg treatment n= 7). (C) Diestrus females show a trend toward decreased facilitation at the 20ms interval, with the Isolated Saline (p=0.07) and Isolated with 2.5mg/kg KET (p<0.05) groups. This decrease is not present with the 5.0mg/kg treatment (Paired with saline treatment n= 10, Isolated with saline treatment n= 9, Isolated with 2.5 mg/kg treatment n= 12, Isolated with 5.0 mg/kg treatment n= 12).
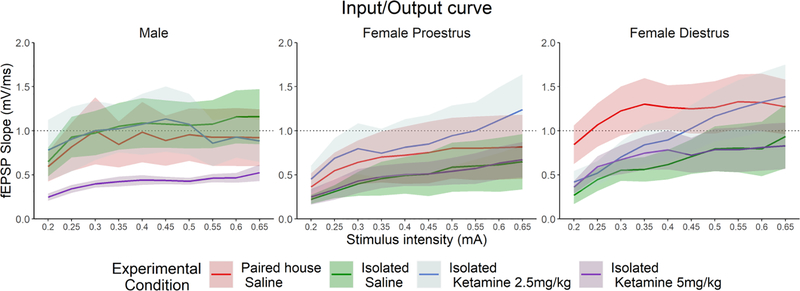
We have also assessed the effects of KET and SIS on Input Output Curves in males and females. As expected, there was a main effect of Stimulus Intensity (F (9,1304.42) =24.9168, p < 2.2e-16). However, there were no differences between experimental conditions within each Sex level (Male, Female Diestrus, Female Proestrus) (Fig. 4).
Figure 4. Input Output Curves.
There was a main effect of stimulus intensity across all groups, but there were no differences by sex or treatment in the (A) male (Paired with saline treatment n= 7, Isolated with saline treatment n= 12, Isolated with 2.5 mg/kg treatment n= 11, Isolated with 5.0 mg/kg treatment n= 14), (B) proestrus females (Paired with saline treatment n= 12, Isolated with saline treatment n= 13, Isolated with 2.5 mg/kg treatment n= 9, Isolated with 5.0 mg/kg treatment n= 7), or (C) diestrus females (Paired with saline treatment n= 10, Isolated with saline treatment n= 9, Isolated with 2.5 mg/kg treatment n= 12, Isolated with 5.0 mg/kg treatment n= 12).
4. Discussion
In this work, we examined the effects of SIS on hippocampal plasticity in both sexes of Sprague-Dawley rats and examined whether low-dose KET can rescue stress-induced deficits. Using slice electrophysiology, we explored the interactions between sex, estrous cycles in females, and KET on hippocampal plasticity following chronic social isolation.
In male rats, SIS led to a complete loss of LTP that was rescued dose-dependently by an acute KET treatment. The increased LTP in isolated male rats by KET is, at least in part, in agreement with a previous report showing that high dose of this drug (10 mg/Kg, i.v.) enhanced LTP in male rats under theta-burst, but not HFS-induced LTP (Widman et al 2018). It is important to note however that Widman et al., used a high dose of KET (10mg/kg), administered intravenously. This is a high dose that is within the anesthetic range of KET and thus its effects cannot be considered related to the antidepressant-like effects of KET, which usually occur below the anesthetic range in rats (Li et al., 2010; Radford et al., 2018; 2020). Radfort et al., reported that KET at this dose (10 mg/kg, i.v.) induced sedation, elevated plasma corticosterone levels and increased short-term fear memory (Radford et al., 2018; 2020), which are not consistent with an antidepressant-like effects in rodents. Another apparent difference between Widman et al. and our study was the fact that we examined the effects of KET in chronically stressed rats, while in their study they used brain tissue from unstressed naïve rats (Wildman et al., 2018). Interestingly, in their study Widman et al. show that KET induced LTP when the slices were potentiated using theta burst stimulation (TBS), but not when using HFS. It is known that TBS activates different intracellular pathways than HFS, with TBS-induced LTP being dependent on BDNF/TrkB signaling (Chen et al 1999, Edelman et al 2015). It is also possible that KET induces BDNF dependent LTP under TBS conditions (although this needs to be demonstrated at antidepressant relevant doses of KET), but BDNF independent form of plasticity under HFS conditions. This latter possibility is however unlikely because other recent studies have shown that KET, at low antidepressant dose, induced BDNF/TrkB dependent LTP under HFS conditions in the hippocampus. For example, in an animal model of inflammation (LPS induced depression rat model), KET administration (10 mg/kg, i.p.) had antidepressant effects that were dependent on increased expressions of p-CREB and BDNF in the hippocampus and prevented the impairment of LTP induction caused by LPS. In this study, LTP induced by HFS was clearly dependent on BDNF signaling (Tang et al, 2020 J of Neuroinflammation). Our results in male rats clearly show that SIS leads to an impairment of LTP in the hippocampus, an effect that was rescued by administration of low doses of KET. It is tempting, when comparing our study to Tang et al. study, to propose that SIS may be causing neuroinflammation that leads to impairment in LTP, which is then rescued by low dose KET. Indeed, previous studies have shown that SIS in male rats and mice have a serious impact on the blood brain barrier integrity and is associated with increased inflammatory markers in the hippocampus (Alshammari et al., 2020, Du Preeze et al., 2020; Calcia et al, 2016). Since PPF, which is a form of short-term plasticity dependent on presynaptic mechanisms (Kamiya and Zucker, 1994; Zucker and Regehr, 2002), was not affected in male rats, we suggest that isolation and the rescuing effects of KET on LTP function are dependent on postsynaptic function of NMDA receptors. The postsynaptic contribution in male rats was also demonstrated by the fact that there were no effects of isolation stress and/or KET in the I/O curve.
Females in Diestrus and Proestrus exhibited LTP that was not affected by the stress history or KET, except for Proestrus female rats which showed increased LTP response at the highest dose of KET. This suggests that KET-induced increased LTP in Proestrus might depend on the presence of high circulating levels of estrogens and progesterone. Previous studies from our group in both rats and mice showed that these hormones are critical in enhancing KET’s antidepressant effects in females (Dossat et al., 2018; Saland et al., 2016, Carrier and Kabbaj, 2013).
Unlike male rats, female isolated rats, especially when tested in diestrus, showed reduced PPF when compared to group-housed control rats. This suggests that SIS alters the short-term presynaptic response in female rats without affecting LTP. The effects of SIS at the pre-synaptic site are only evident during the diestrus phase, when circulating levels of hormones are lower. Our hypothesis is that lower hormone levels in females increase the vulnerability to the effects of SIS at the pre-synaptic site, and that KET (5 mg/kg) abolishes the effects of isolation stress. Also, there were no effects of isolation stress and/or KET in I/O curve in females.
It is important to note that one of the limitations of our study is that we did not include group-housed rats treated with ketamine. However, we believe that ketamine treatment in group housed rats will have no effects on LTP as shown recently in a study by Alexandrova et al. who compared Wistar Kyoto rats (WKR: a model of human depression) to Wistar control rats response to KET (5 mg/kg, i.p.). In saline treated groups, WKR had lower LTP compared to Wistar controls. In WKR, KET increased hippocampal LTP but did not affect LTP in Wistar controls, despite inducing antidepressant like effects in both groups (Aleksandrova et al., 2020). Regardless, it will be important to run these control groups in the future to verify that LTP is not affected by low doses KET in group housed male and female SD rats.
Overall, our data indicate that there is a sexually dimorphic response to SIS. Females present a presynaptic dysfunction that prevents rapid facilitation that is partially ameliorated in proestrus, while males present a post-synaptic deficit following isolation stress that is ameliorated by KET. We thus have shown that the interaction between SIS and low dose KET can alter hippocampal plasticity differently between males and females. Further investigation is needed to determine the mechanisms underlying sex differences in presynaptic and postsynaptic contributions following stress and response to antidepressants.
Supplementary Material
Highlights:
Social isolation stress and low doses of ketamine alter hippocampal plasticity differently between male and female rats.
In male rats, social isolation inhibits hippocampal long-term potentiation, an effect that was rescued by ketamine.
In female rats, social isolation altered the short-term presynaptic response.
Females in Diestrus and Proestrus exhibited long-term potentiation (LTP) that was not affected by the stress history or ketamine, except for Proestrus female rats which showed increased LTP response at the highest ketamine dose.
5. Acknowledgements:
We would like to thank Briauna Norton for assisting with performing the experiments.
8. Funding:
This work was supported by the National Institute of Mental Health (RO1-MH099085 to MK and YZ).
Footnotes
6. Conflict of Interest:
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. Citations
- Aleksandrova Lily R., Wang Yu Tian, and Phillips. Anthony G. “KET and Its Metabolite, (2R,6R)-HNK, Restore Hippocampal LTP and Long-Term Spatial Memory in the Wistar-Kyoto Rat Model of Depression.” Molecular Brain 13, no. 1 (June 16, 2020): 92. 10.1186/s13041-020-00627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari Tahani K., Alghamdi Hajar, Alkhader Lama F., Alqahtani Qamra, Alrasheed Nouf M., Yacoub Hazar, Alnaem Nora, AlNakiyah Maha, and Alshammari Musaad A.. “Analysis of the Molecular and Behavioral Effects of Acute Social Isolation on Rats.” Behavioural Brain Research 377 (January 13, 2020): 112191. 10.1016/j.bbr.2019.112191. [DOI] [PubMed] [Google Scholar]
- Bath Kevin G., Jing Deqiang Q., Dincheva Iva, Neeb Christine C., Pattwell Siobhan S., Chao Moses V., Lee Francis S., and Ninan Ipe. “BDNF Val66Met Impairs Fluoxetine-Induced Enhancement of Adult Hippocampus Plasticity.” Neuropsychopharmacology 37, no. 5 (April 2012): 1297–1304. 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, and Krystal JH. “Antidepressant Effects of KET in Depressed Patients.” Biological Psychiatry 47, no. 4 (February 15, 2000): 351–54. 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Fone KFC, Azmi N, Heidbreder CA, Hagan JJ, and Marsden CA. “Isolation Rearing Induces Recognition Memory Deficits Accompanied by Cytoskeletal Alterations in Rat Hippocampus.” European Journal of Neuroscience 24, no. 10 (2006): 2894–2902. 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo John T., Hawkley Louise C., Norman Greg J., and Berntson Gary G.. “Social Isolation.” Annals of the New York Academy of Sciences 1231, no. 1 (August 2011): 17–22. 10.1111/j.1749-6632.2011.06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia Marilia A., Bonsall David R., Bloomfield Peter S., Selvaraj Sudhakar, Barichello Tatiana, and Howes Oliver D.. “Stress and Neuroinflammation: A Systematic Review of the Effects of Stress on Microglia and the Implications for Mental Illness.” Psychopharmacology 233 (2016): 1637–50. 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier Nicole, and Kabbaj Mohamed. “Sex Differences in the Antidepressant-like Effects of KET.” Neuropharmacology 70 (July 2013): 27–34. 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, and Kossel A. “Relative Contribution of Endogenous Neurotrophins in Hippocampal Long-Term Potentiation.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 19, no. 18 (September 15, 1999): 7983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coid Jeremy, Petruckevitch Ann, Bebbington Paul, Jenkins Rachel, Brugha Traolach, Lewis Glyn, Farrell Michael, and Singleton Nicola. “Psychiatric Morbidity in Prisoners and Solitary Cellular Confinement, II: Special (‘strip’) Cells.” The Journal of Forensic Psychiatry & Psychology 14, no. 2 (September 1, 2003): 320–40. 10.1080/1478994031000095501. [DOI] [Google Scholar]
- Cooke SF, & Bliss TVP (2006). Plasticity in the human central nervous system. Brain, 129(7), 1659–1673. 10.1093/brain/awl082 [DOI] [PubMed] [Google Scholar]
- Bellis De, Michael D, Woolley Donald P., and Hooper Stephen R.. “Neuropsychological Findings in Pediatric Maltreatment: Relationship of PTSD, Dissociative Symptoms, and Abuse/Neglect Indices to Neurocognitive Outcomes.” Child Maltreatment 18, no. 3 (August 1, 2013): 171–83. 10.1177/1077559513497420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat Amanda M., Wright Katherine N., Strong Caroline E., and Kabbaj Mohamed. “Behavioral and Biochemical Sensitivity to Low Doses of KET: Influence of Estrous Cycle in C57BL/6 Mice.” Neuropharmacology 130 (March 1, 2018): 30–41. 10.1016/j.neuropharm.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preez Du, Andrea Thomas Law, Onorato Diletta, Lim Yau M., Eiben Paola, Musaelyan Ksenia, Egeland Martin, et al. “The Type of Stress Matters: Repeated Injection and Permanent Social Isolation Stress in Male Mice Have a Differential Effect on Anxiety- and Depressive-like Behaviours, and Associated Biological Alterations.” Translational Psychiatry 10, no. 1 (September 21, 2020): 1–17. 10.1038/s41398-020-01000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman Ronald S. “KET and Rapid-Acting Antidepressants: A New Era in the Battle against Depression and Suicide.” F1000Research 7 (May 24, 2018): F1000 Faculty Rev-659. 10.12688/f1000research.14344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann Elke, Cepeda-Prado Efrain, Franck Martin, Lichtenecker Petra, Brigadski Tanja, and Leßmann Volkmar. “Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP.” Neuron 86, no. 4 (May 20, 2015): 1041–54. 10.1016/j.neuron.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, and Pitychoutis PM. “Sex Differences in the Rapid and the Sustained Antidepressant-like Effects of KET in Stress-Naïve and ‘Depressed’ Mice Exposed to Chronic Mild Stress.” Neuroscience 290 (April 2, 2015): 49–60. 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Frodl Thomas, Janowitz Deborah, Schmaal Lianne, Tozzi Leonardo, Dobrowolny Henrik, Stein Dan J., Veltman Dick J., et al. “Childhood Adversity Impacts on Brain Subcortical Structures Relevant to Depression.” Journal of Psychiatric Research 86 (March 1, 2017): 58–65. 10.1016/j.jpsychires.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Jerome M., Murr Ashley S., and Cooper Ralph L.. “The Rodent Estrous Cycle: Characterization of Vaginal Cytology and Its Utility in Toxicological Studies.” Birth Defects Research. Part B, Developmental and Reproductive Toxicology 80, no. 2 (April 2007): 84–97. 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Halekoh U, & Højsgaard S (2014). A Kenward-Roger Approximation and Parametric Bootstrap Methods for Tests in Linear Mixed Models - TheRPackagepbkrtest. Journal of Statistical Software, 59(9). doi: 10.18637/jss.v059.i09 [DOI] [Google Scholar]
- Hawkley Louise C., and Cacioppo John T.. “Loneliness Matters: A Theoretical and Empirical Review of Consequences and Mechanisms.” Annals of Behavioral Medicine 40, no. 2 (October 1, 2010): 218–27. 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Haruyuki, and Zucker Robert S.. “Residual Ca 2 + and Short-Term Synaptic Plasticity.” Nature 371, no. 6498 (October 1994): 603–6. 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Kehoe Priscilla, and Bronzino Joseph D.. “Neonatal Stress Alters LTP in Freely Moving Male and Female Adult Rats.” Hippocampus 9, no. 6 (1999): 651–58. . [DOI] [PubMed] [Google Scholar]
- Khalaf-Nazzal R, and Francis F. “Hippocampal Development - Old and New Findings.” Neuroscience 248 (September 17, 2013): 225–42. 10.1016/j.neuroscience.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Khan Israr, and Leventhal Bennett L.. “Developmental Delay.” In StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. http://www.ncbi.nlm.nih.gov/books/NBK562231/. [PubMed] [Google Scholar]
- Lambert Hilary K., Peverill Matthew, Sambrook Kelly A., Rosen Maya L., Sheridan Margaret A., and McLaughlin Katie A.. “Altered Development of Hippocampus-Dependent Associative Learning Following Early-Life Adversity.” Developmental Cognitive Neuroscience 38 (August 1, 2019): 100666. 10.1016/j.dcn.2019.100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis Melanie P., and Andersen Susan L.. “Is Adolescence a Sensitive Period for Depression? Behavioral and Neuroanatomical Findings from a Social Stress Model.” Synapse (New York, N.Y.) 62, no. 1 (January 2008): 22–30. 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, & Duman RS (2010). MTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (New York, N.Y.), 329(5994), 959–964. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Ying, Wang Lei, and Rui Guoqiang. “Depression among Left-behind Children in China.” Journal of Health Psychology 22, no. 14 (December 1, 2017): 1897–1905. 10.1177/1359105316676333. [DOI] [PubMed] [Google Scholar]
- Mulder Tim M., Kuiper Kimberly C., van der Put Claudia E., Stams Geert-Jan J. M., and Assink Mark. “Risk Factors for Child Neglect: A Meta-Analytic Review.” Child Abuse & Neglect 77 (March 2018): 198–210. 10.1016/j.chiabu.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Mumtaz Faiza, Khan Muhammad Imran, Zubair Muhammad, and Dehpour Ahmad Reza. “Neurobiology and Consequences of Social Isolation Stress in Animal Model—A Comprehensive Review.” Biomedicine & Pharmacotherapy 105 (September 1, 2018): 1205–22. 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
- Pereda-Pérez Inmaculada, Popović Natalija, Otalora Beatriz Baño, Popović Miroljub, Madrid Juan Antonio, Rol Maria Angeles, and Venero César. “Long-Term Social Isolation in the Adulthood Results in CA1 Shrinkage and Cognitive Impairment.” Neurobiology of Learning and Memory 106 (November 2013): 31–39. 10.1016/j.nlm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Qiao Haifa, Foote Molly, Graham Kourtney, Wu Yuying, and Zhou Yi. “14–3-3 Proteins Are Required for Hippocampal Long-Term Potentiation and Associative Learning and Memory.” The Journal of Neuroscience 34, no. 14 (April 2, 2014): 4801–8. 10.1523/JNEUROSCI.4393-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KD, Park TY, Jaiswal S, Pan H, Knutsen A, Zhang M, Driscoll M, Osborne-Smith LA, Dardzinski BJ, Choi KH. “Enhanced fear memories and brain glucose metabolism ((18)F-FDG-PET) following sub-anesthetic intravenous ketamine infusion in Sprague-Dawley rats”. Transl. Psychiatry, 8 (1) (2018), p. 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KD, Park TY, Jaiswal S, Pan H, Knutsen A, Zhang M, Driscoll M, Osborne-Smith LA, Dardzinski BJ, Choi KH. “Enhanced fear memories and brain glucose metabolism ((18)F-FDG-PET) following sub-anesthetic intravenous ketamine infusion in Sprague-Dawley rats”. Transl. Psychiatry, 8 (1) (2018), p. 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KD, Spencer Haley F., Zhang Michael, Berman Rina Y., Girase Quinn L., Choi Kwang H“Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats”. Behavioral Brain Research 2020. January 27; 378:112259. doi: 10.1016/j.bbr.2019.112259. Epub 2019 Sep 24. [DOI] [PubMed] [Google Scholar]
- Saland Samantha K., and Kabbaj Mohamed. “Sex Differences in the Pharmacokinetics of Low-Dose KET in Plasma and Brain of Male and Female Rats.” The Journal of Pharmacology and Experimental Therapeutics 367, no. 3 (December 2018): 393–404. 10.1124/jpet.118.251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland Samantha K., Schoepfer Kristin J., and Kabbaj Mohamed. “Hedonic Sensitivity to Low-Dose KET Is Modulated by Gonadal Hormones in a Sex-Dependent Manner.” Scientific Reports 6 (February 18, 2016): 21322. 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich Virginie, Vidal Sonia, Hofmeister Jeremy, Michalopoulos Giorgio, Bancila Victor, Warrot Delphine, Dayer Alexandre, et al. “Increased Reactivity of the Mesolimbic Reward System after KET Injection in Patients with Treatment-Resistant Major Depressive Disorder.” Anesthesiology 130, no. 6 (June 2019): 923–35. 10.1097/ALN.0000000000002667. [DOI] [PubMed] [Google Scholar]
- Tang Xiao-Hui, Zhang Guang-Fen, Xu Ning, Duan Gui-Fang, Jia Min, Liu Ru, Zhou Zhi-Qiang, and Yang Jian-Jun. “Extrasynaptic CaMKIIα Is Involved in the Antidepressant Effects of KET by Downregulating GluN2B Receptors in an LPS-Induced Depression Model.” Journal of Neuroinflammation 17, no. 1 (June 10, 2020): 181. 10.1186/s12974-020-01843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher Martin H., and Samson Jacqueline A.. “Annual Research Review: Enduring Neurobiological Effects of Childhood Abuse and Neglect.” Journal of Child Psychology and Psychiatry 57, no. 3 (2016): 241–66. 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale AL, and Montgomery AMJ. “Social Interaction: Responses to Chlordiazepoxide and the Loss of Isolation-Reared Effects with Paired-Housing.” Psychopharmacology 133, no. 2 (September 1, 1997): 127–32. 10.1007/s002130050382. [DOI] [PubMed] [Google Scholar]
- Wang Bin, Wu Qiong, Lei Hailun Sun, Michael Ntim, Zhang Xuan, Wang Ying, et al. “Long-Term Social Isolation Inhibits Autophagy Activation, Induces Postsynaptic Dysfunctions and Impairs Spatial Memory.” Experimental Neurology 311 (January 1, 2019): 213–24. 10.1016/j.expneurol.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, and Greenough WT. “LTP Varies across the Estrous Cycle: Enhanced Synaptic Plasticity in Proestrus Rats.” Brain Research 703, no. 1–2 (December 12, 1995): 26–30. 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Widman Allie J., Stewart Amy E., Erb Elise M., Gardner Elizabeth, and McMahon Lori L.. “Intravascular KET Increases Theta-Burst but Not High Frequency Tetanus Induced LTP at CA3-CA1 Synapses Within Three Hours and Devoid of an Increase in Spine Density.” Frontiers in Synaptic Neuroscience 10 (May 30, 2018): 8. 10.3389/fnsyn.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker Robert S., and Regehr Wade G.. “Short-Term Synaptic Plasticity.” Annual Review of Physiology 64, no. 1 (2002): 355–405. 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.