Abstract
The impact of acute liver failure (ALF) etiology on waitlist and post-transplant outcomes, independent of severity of illness is incompletely characterized. All adults (N=1,691) listed for primary liver transplantation (LT) between 2002–2019 with ALF due to acetaminophen (APAP), drug-induced liver injury (DILI), autoimmune hepatitis (AIH) and hepatitis B virus (HBV) were identified in the United Network for Organ Sharing database. ALF etiology was evaluated as an independent predictor of waitlist mortality and spontaneous survivorship (SS; versus outcome of LT), as well as post-LT overall survival, graft survival and in-hospital mortality using multivariable models that accounting for differences in clinical parameters at listing. Accounting for severity of illness at listing, waitlist mortality and SS for DILI, AIH and HBV were each lower than for APAP (adjusted relative risk ratio <1 in all analyses with p<0.001 for both outcomes). ALF etiology was not associated with adjusted overall survival post-LT (p=0.09) or graft survival (p=0.1). Inpatient mortality rate post-LT was high at 9%. While ALF etiology was also not associated with adjusted inpatient mortality (p=0.4), cause of death (COD) was different. For example, the rate of post-LT brain death was 5.3% for APAP, 3.0% for other DILI, 1.1% for AIH and 3.0% for HBV (p=0.02).
Conclusion:
ALF etiology is an independent predictor of waitlist outcome, even after adjusting for severity of illness, but is not associated with post-LT outcomes with the exception of COD. The majority of post-LT deaths for all ALF etiologies studied occurred during the index hospital stay, suggesting a continued need for enhanced prognostic tools to ensure efficient organ utilization, and ALF- and etiology-specific post-LT care to prevent brain death.
Keywords: acute liver failure, waitlist mortality, post-transplant mortality
Introduction
Acute liver failure (ALF) is a hepatology emergency, with modern day mortality approaching 30%1–4. Liver transplantation (LT) is a potentially life-saving intervention, though post-LT mortality for ALF remains higher than for non-ALF indications5. Several underlying ALF etiologies exist, with incidence varying with geography and time6–9. In the United States (US), the most common causes are acetaminophen (APAP) toxicity, hepatitis B virus (HBV), autoimmune hepatitis (AIH), and non-APAP drug induced liver injury (DILI)4,10,11.
ALF etiologies are known to have different epidemiology, potentially targeted treatment options, and prognosis12,13. Studies of ALF patients waitlisted for LT note that patients with APAP are less likely to undergo LT and more likely to experience spontaneous survival (SS), with much more rapid evolution towards these outcomes13. However, other multi-center studies evaluating the impact of ALF etiology on waitlist outcomes have either not adjusted for illness severity or greatly simplified ALF etiology categories13,14.
With regards to post-LT outcomes, the impact of etiology remains poorly characterized and has primarily focused on mid- to long- term survival rather than immediate in-hospital mortality15–18. A 2006 retrospective study of ALF patients in the United Network for Organ Sharing (UNOS) database found that disease etiology did not have a significant association with overall post-LT survival17. In contrast, a large European study found that patients with an identified cause for ALF (either APAP or non-APAP) experienced increased post-LT mortality and graft loss2. These studies primarily included LTs from the 1980s and 1990s, for which both waitlist and post-LT outcome rates are known to be inherently different than those observed today14.
The first objective of this study was to evaluate the impact of ALF etiology on LT waitlist outcome among ALF patients listed for LT in the US, addressing key differences in severity of illness. Second, we evaluated the impact of etiology on short- and long-term post-transplant outcomes in the modern era.
Methods
Study design, data source and study population
This was a retrospective cohort study using the United Network for Organ Sharing (UNOS) database. All adults (≥18 years) waitlisted for LT as Status 1 between 2/27/2002 and 3/31/2019 were included. Subjects were additionally required to have Status 1 priority at the time of waitlist removal. Patients were excluded if they previously received a transplant or if they were listed for and/or received a multiorgan transplant.
Among patients in the starting cohort, the four most common defined etiologies of ALF were identified. These were: APAP, HBV, AIH and non-APAP DILI. ALF etiology was determined from UNOS coding and free-text entry at listing and waitlist removal19. The determination of APAP versus other DILI cases fully relied on free-text entry given the lack of specific UNOS codes. ALF listings with a specific designation of APAP were categorized as such, while those specifying an alternate drug, drug class or those that used the term “drug-induced” without mention of APAP were classified as non-APAP DILI. The combination of diagnosis codes and free-text entries allowed for the determination of HBV and AIH.
The final analytic cohort included only subjects waitlisted for LT with these four ALF etiologies during the study period. A total of 1,713 patients were excluded by this criterion, of whom 1,329 (77.6%) had ALF of unknown or indeterminate etiology (Figure 1). Patients with an unknown etiology included those with a missing etiology (e.g., due to administrative error) or patients for whom the etiology was explicitly stated as ‘unknown’ or those with insufficient granularity in their free text entries to determine an etiology. Patients with an indeterminate etiology were those for whom the etiology was listed as being ‘indeterminate’ or those with multiple different etiologies listed. The population of unknown and indeterminate ALF patients therefore likely included patients with a broad range of ALF etiologies, including a subset of patients with the four etiologies that this study chose to include. Given the heterogeneous nature of this group, interpreting any relevant differences in characteristics or outcomes is inherently problematic, and was the primary reason why these were excluded. For transparency, the clinical characteristics of patients in the unknown/indeterminate group are shown in Supplemental Table 1. We additionally compare the waitlist outcomes and post-LT outcomes of patients in the unknown/indeterminate group to those of the study cohort in Supplemental Table 2 and Supplemental Figure 1, respectively.
Figure 1:
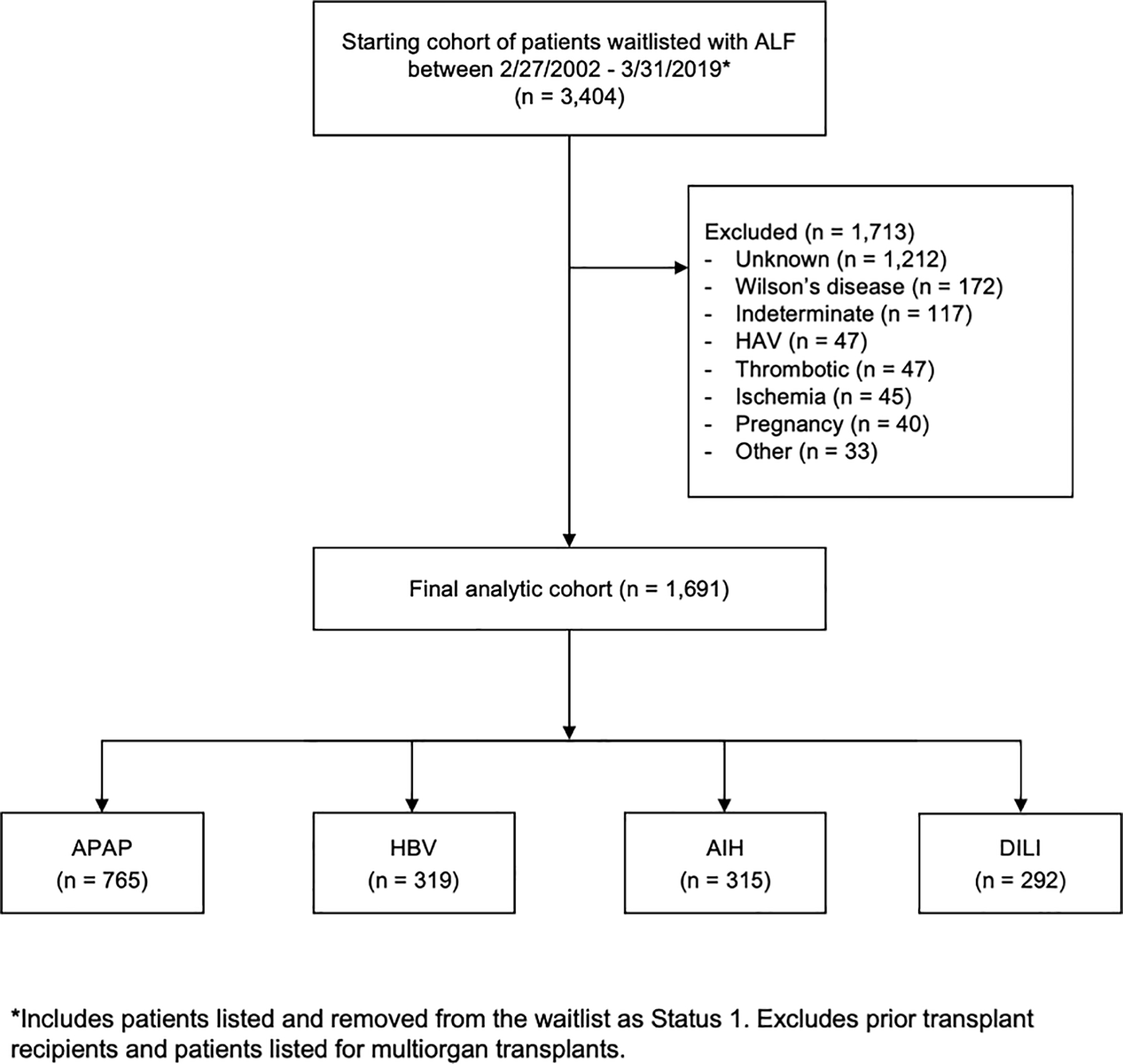
Flowchart detailing construction of study cohort with exclusion criteria
Exposures, covariates and outcomes
The primary exposure of interest was ALF etiology as a 4-tier categorical variable. Additional variables assessed at the time of waitlist entry included: sex, age, race/ethnicity, native Model for End-stage Liver Disease (MELD) score and its components, presence of ascites, presence of grade 3–4 encephalopathy, receipt of mechanical ventilation and receipt of hemodialysis. Among those transplanted, donor quality indicators included donor age, whether the allograft was donated after circulatory determination of death (DCD) and cold ischemic time. Waitlist era was also evaluated (2002–2007, 2008–2013, 2014–2019). Missingness for each variable was estimated and provided.
The primary outcomes of interest included: (i) waitlist outcome (transplanted, removal due to death or clinical deterioration, and removal due to clinical improvement [i.e., spontaneous survival, SS]); (ii) overall post-LT patient and graft survival; and (iii) in-hospital mortality. In-hospital mortality included deaths occurring up to 7 days post-discharge to account for potential transitions to hospice care. Other specific outcomes evaluated included time from hospital admission to waitlisting, in-hospital mortality, need for re-transplantation and cause of death (COD). COD was ascertained via UNOS coding and free-text entry, and subdivided into the following groups: (i) brain death / cerebral edema, (ii) cardiovascular, (iii) infection, (iv) malignancy, (v) other organ failure, (vi) graft-related, (vii) suicide / overdose / trauma, and (viii) other / unknown (see Supplemental Table 3 for further details).
Statistical analyses
Characteristics of waitlisted and transplanted patients were compared by ALF etiology using Chi-squared tests and Kruskal-Wallis tests for categorical and continuous variables, respectively. Univariable linear regression, stratified by ALF etiology, was used to determine the presence of any significant temporal trends using 2002 as the baseline.
Due to the short time-horizon between listing and removal from the waitlist in the setting of ALF, multinomial regression was used to evaluate ALF etiology as a predictor of waitlist outcome (transplanted, died or too sick, SS)19. The multivariable model was additionally adjusted for the following covariates: sex, age, race/ethnicity, presence of ascites, grade 3–4 encephalopathy, receipt of mechanical ventilation, dialysis status and waitlist era. From this multivariable model, adjusted predicted probabilities of each waitlist outcome by ALF etiology were obtained.
Nelson-Aalen cumulative hazard function curves described post-LT mortality trends by ALF etiology. Cox proportional hazards was used to evaluate overall post-transplant patient and graft survival as time-to-event according to ALF etiology, using APAP as the reference group. In these models, failure events were defined as death and death or retransplantation, respectively. Patients were censored at the time of last follow-up. In-hospital mortality was evaluated as a binary event using logistic regression. All post-LT multivariable models were adjusted for the same covariates as those listed for the multinomial model evaluating waitlist outcome above, as well as donor age, DCD allograft and cold ischemic time. All analyses used complete case analysis, as missingness was overall low and suspected to be random.
This study was approved by the Institutional Review Board of the University of Pennsylvania. All statistical analyses were performed using STATA version 14 (College Station, TX).
Results
Overall cohort
The final study cohort included 1,691 Status 1 patients listed for LT between February 27, 2002 and March 31, 2019 with ALF due to either APAP (N=765, 45.2%), HBV (N=319, 18.9%), AIH (N=315, 18.6%) or non-APAP DILI (N=292, 17.3%). Baseline characteristics by etiology are shown in Table 1. Differences in patient demographics were observed between the four groups. HBV patients were more likely to be Asian and male, while APAP patients were more likely to be White and ≤40 years old. Age at waitlisting was heterogeneous with the AIH group having 22.7% of patients who were ≥60 years at waitlisting. At the time of listing, APAP and HBV patients were more likely to be sicker with higher native MELD scores and higher rates of Grade 3–4 encephalopathy and dialysis (all p<0.001; Table 1). There were no significant differences in donor age, use of DCD organs, or cold ischemia time by ALF etiology (p=0.307, 0.254 and 0.142, respectively).
Table 1.
Demographic and clinical characteristics by etiology of patients waitlisted for acute liver failure as Status 1 in the US between 2002–2018
| ALF Etiology |
|||||
|---|---|---|---|---|---|
| APAP | HBV | AIH | DILI | p-value | |
| Variable | N=765 | N=319 | N=315 | N=292 | |
| Female, N (%) | 603(78.8) | 142(44.5) | 256(81.3) | 222(76.0) | <0.001 |
| Race/ethnicity, N (%) | <0.001 | ||||
| White | 614(80.3) | 119(37.3) | 127(40.3) | 152(52.1) | |
| Black | 69(9.0) | 76(23.8) | 114(36.2) | 73(25.0) | |
| Hispanic | 52(6.8) | 27(8.5) | 55(17.5) | 39(13.4) | |
| Asian | 13(1.7) | 93(29.2) | 15(4.8) | 24(8.2) | |
| Other | 17(2.2) | 4(1.3) | 4(1.3) | 4(1.4) | |
| Age (years), N (%) | <0.001 | ||||
| ≤40 | 511(76.7) | 116(38.9) | 112(37.5) | 131(48.3) | |
| 40–59 | 127(19.1) | 139(46.6) | 119(39.8) | 102(37.6) | |
| ≥60 | 28(4.2) | 43(14.4) | 68(22.7) | 38(14.0) | |
| Characteristics at WL | |||||
| MELD score, median (IQR) | 37(31–43) | 39(34–44) | 33(29–37) | 35(31–40) | <0.001 |
| Grade 3–4 encephalopathy, N (%) | 545(71.2) | 210(65.8) | 177(56.2) | 170(58.2) | <0.001 |
| Ascites present, N (%) | 226(29.5) | 179(56.1) | 170(54.0) | 128(43.8) | <0.001 |
| Mechanical ventilation, N (%) | 505(66.0) | 132(41.4) | 91(28.9) | 110(28.9) | <0.001 |
| Dialysis, N (%) | 105(13.8) | 20(6.3) | 14(4.5) | 18(6.2) | <0.001 |
| Characteristics at WL removal* | |||||
| MELD score, median (IQR) | 35(28–41) | 38(32–43) | 33(28–38) | 34(29–42) | <0.001 |
| Grade 3–4 encephalopathy, N (%) | 515(70.0) | 223(70.4) | 185(60.1) | 174(60.8) | <0.001 |
| Ascites present, N (%) | 280(38.0) | 197(62.2) | 199(64.6) | 152(53.5) | <0.001 |
| Dialysis, N (%) | 245(32.1) | 67(21.1) | 33(10.5) | 56(19.2) | <0.001 |
| Donor quality† | |||||
| Donor age (years), N (%) | 37(23–51) | 34(21–50) | 34(22–49) | 36(23–51) | 0.307 |
| DCD Liver, N (%) | 18(2.4) | 3(0.9) | 3(1.0) | 5(1.7) | 0.254 |
| Cold ischemia time (hours), median (IQR) | 6(4.9–7.3) | 6.2(5–8) | 6.2(5–7.7) | 6(4.7–7.6) | 0.142 |
Abbreviations: ALF – acute liver failure; APAP – acetaminophen; DILI – drug induced liver injury; AIH – autoimmune hepatitis; HBV – hepatitis B virus; WL – waitlist; MELD – Model for End-Stage Liver Disease; IQR – interquartile range; DCD – donation after cardiac death
Note:
Missingness was <1% for dialysis at listing/removal and MELD score at removal, 1–3% for ascites and encephalopathy at removal and 5.6% for cold ischemia time.
Includes patients removed from the WL for any reason. Mechanical ventilation at WL removal omitted, given data was only available for patients receiving LT.
Among patients transplanted only (N=1,217)
The overall rate of ALF listings among new first-time waitlist entrants for LT alone has gradually decreased from 2.9% in 2002 to 2.0% in 2018 (p<0.001). An increase in ALF listings with APAP was observed in 2007 with a concurrent decrease in those with other DILI (Figure 2). These isolated trends were significantly different form the baseline rate of ALF listing for APAP and other DILI (p=0.001 and p=0.011; see Supplemental Table 4 for complete regression output). Temporal trends in waitlisting for HBV and AIH did not change over time (p>0.1 for all years versus 2002; data not shown). Trends in waitlistings for ALF with unknown etiology largely mirrored those of ALF listings overall, while those of indeterminate ALF did not markedly change over time (Supplemental Figure 2). The proportion of ALF listings with unknown etiology was not different between 2002 and 2018 (19.6% bs 19.2%, respectively; p=0.926).
Figure 2:
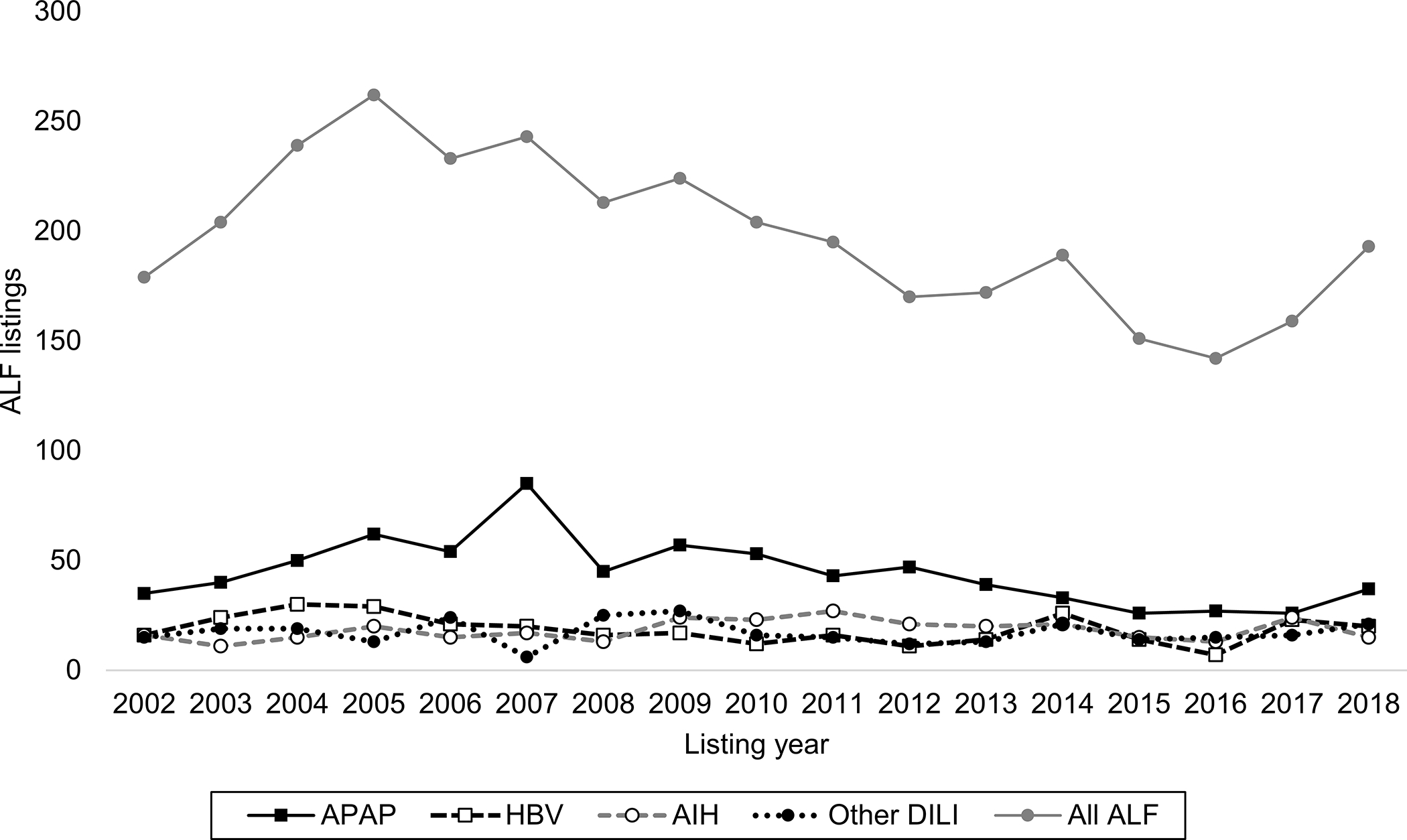
Temporal trends in ALF listings for LT between 2002–2018 overall (N=3,404) and by etiology in the study cohort (N=1,691)
Note:
Increase in APAP and decrease in other DILI waitlistings in 2007 statistically significant (p=0.001 and p=0.011, respectively, vs 2002 as reference). All other years for APAP and other DILI, and all years for HBV and AIH were not statistically different.
Waitlist outcomes
Observed outcome rates for the overall cohort were: 72.7% LT (N=1,217), 17.3% (N=289) waitlist mortality, and 10.0% (N=167) SS. Rates of LT were lower for patients with APAP but were comparable among non-APAP etiologies: 57.9% in APAP vs 86% in HBV, 88.3% in AIH, and 80.0% in DILI (p<0.001). Compared to non-APAP etiologies, APAP patients were more likely to undergo waitlist removal for death/decompensation (22.8% in APAP vs 13.0% in HBV, 10.2% in AIH, and 15.2% in DILI, p<0.001). SS was also more frequent in APAP-induced ALF, for which 19.3% of waitlisted patients were removed for clinical improvement. In contrast, SS occurred rarely in HBV, AIH, and DILI patients (1.0%, 1.6%, and 4.8% respectively, p<0.001).
Time from admission to LT waitlisting varied significantly by etiology. This was longest for patients with AIH, where 20.5% patients had ≥7 days between admission and waitlisting (compared to 1.8% in APAP, 1.8% in HBV, and 14.7% in DILI; p<0.001). In contrast, 94% of APAP patients underwent waitlisting ≤2 days of admission (versus 65.7% in HBV, 45% in AIH, and 62.5% in DILI; p<0.001). For those patients undergoing LT, time from admission to LT was also shortest for APAP (74.1% waiting ≤2 days) and longest for AIH patients (11.3% waiting ≥7 days; p<0.001). Similar trends were observed among those removed due to death or clinical decompensation (data not shown). The number of patients with AIH and HBV experiencing SS was small, precluding a meaningful comparison of time to clinical improvement by etiology.
The results of the multinomial model for each waitlist outcome by etiology are shown in Table 2. Accounting for other patient factors at waitlisting, the relative risk ratio (RRR) for waitlist mortality (versus LT) was lower for HBV, AIH, and DILI (0.43 (95% CI: 0.27–0.67), 0.43 (95% CI: 0.26–0.68), and 0.65 (95% CI: 0.44–0.97) respectively, vs APAP; p<0.001). The RRR for SS was similarly lower for HBV, AIH, and DILI compared to APAP (0.05 (95% CI: 0.01–0.16), 0.04 (95% CI: 0.01–0.10), and 0.15 (95% CI: 0.08–0.28) respectively; p<0.001). Differences in the adjusted predicted probabilities of each outcome according to ALF etiology were greatest APAP, which was associated with the lowest probability of LT (59.7%) and the highest probability of WL mortality (20.9%) and SS (19.4%), despite adjustment for differences in baseline characteristics (Figure 3). The predicted outcomes of HBV and AIH were most comparable. Predicted WL mortality for APAP and other DILI were not different (20.9% vs 17.2%; p=0.196), though differences in LT rate (59.7% vs 78.4%; p<0.001) and SS were observed (19.4% vs 4.4%; p<0.001).
Table 2.
Results of multivariable multinomial model for LT waitlist outcomes (N=1,661)
| WL mortality | Spontaneous survival | |||
|---|---|---|---|---|
| Variable | RRR* (95% CI) | p value | RRR* (95% CI) | p value |
| ALF etiology | <0.001 | <0.001 | ||
| APAP | --- | --- | ||
| HBV | 0.43(0.27–0.67) | 0.05(0.01–0.16) | ||
| AIH | 0.43(0.26–0.68) | 0.04(0.01–0.10) | ||
| DILI | 0.65(0.44–0.97) | 0.15(0.08–0.28) | ||
| Female | 0.99(0.72–1.36) | 0.944 | 0.81(0.52–1.28) | 0.367 |
| Age, per year | 1.01(1.00–1.02) | 0.238 | 0.99(0.97–1.00) | 0.077 |
| Race/ethnicity | 0.990 | 0.267 | ||
| White | --- | --- | ||
| Black | 1.04(0.72–1.52) | 0.55(0.28–1.08) | ||
| Hispanic | 0.91(0.55–1.50) | 1.07(0.57–2.01) | ||
| Asian | 0.96(0.54–1.71) | 0.40(0.11–1.42) | ||
| Other | 1.11(0.41–2.95) | 1.29(0.34–4.91) | ||
| MELD, per point | 1.03(1.01–1.04) | 0.004 | 0.92(0.90–0.94) | <0.001 |
| Clinical Factors at WL | ||||
| Grade 3–4 encephalopathy | 1.69(1.20–2.38) | 0.003 | 0.65(0.44–0.97) | 0.035 |
| Ascites present | 1.00(0.75–1.33) | 0.988 | 0.61(0.40–0.95) | 0.027 |
| Mechanical ventilation | 2.08(1.52–2.86) | <0.001 | 0.35(0.23–0.52) | <0.001 |
| Dialysis | 0.83(0.54–1.30) | 0.417 | 1.45(0.75–2.82) | 0.274 |
| Era | 0.195 | 0.810 | ||
| 2002–2007 | --- | --- | ||
| 2008–2013 | 0.75(0.55–1.03) | 0.87(0.57–1.33) | ||
| 2014–2019 | 0.83(0.58–1.18) | 0.97(0.60–1.57) | ||
Abbreviations: APAP – acetaminophen; DILI – drug induced liver injury; AIH – autoimmune hepatitis; HBV – hepatitis B virus; LT – liver transplantation; WL – waitlist; MELD – Model for End-Stage Liver Disease
Reference outcome is LT
Figure 3:
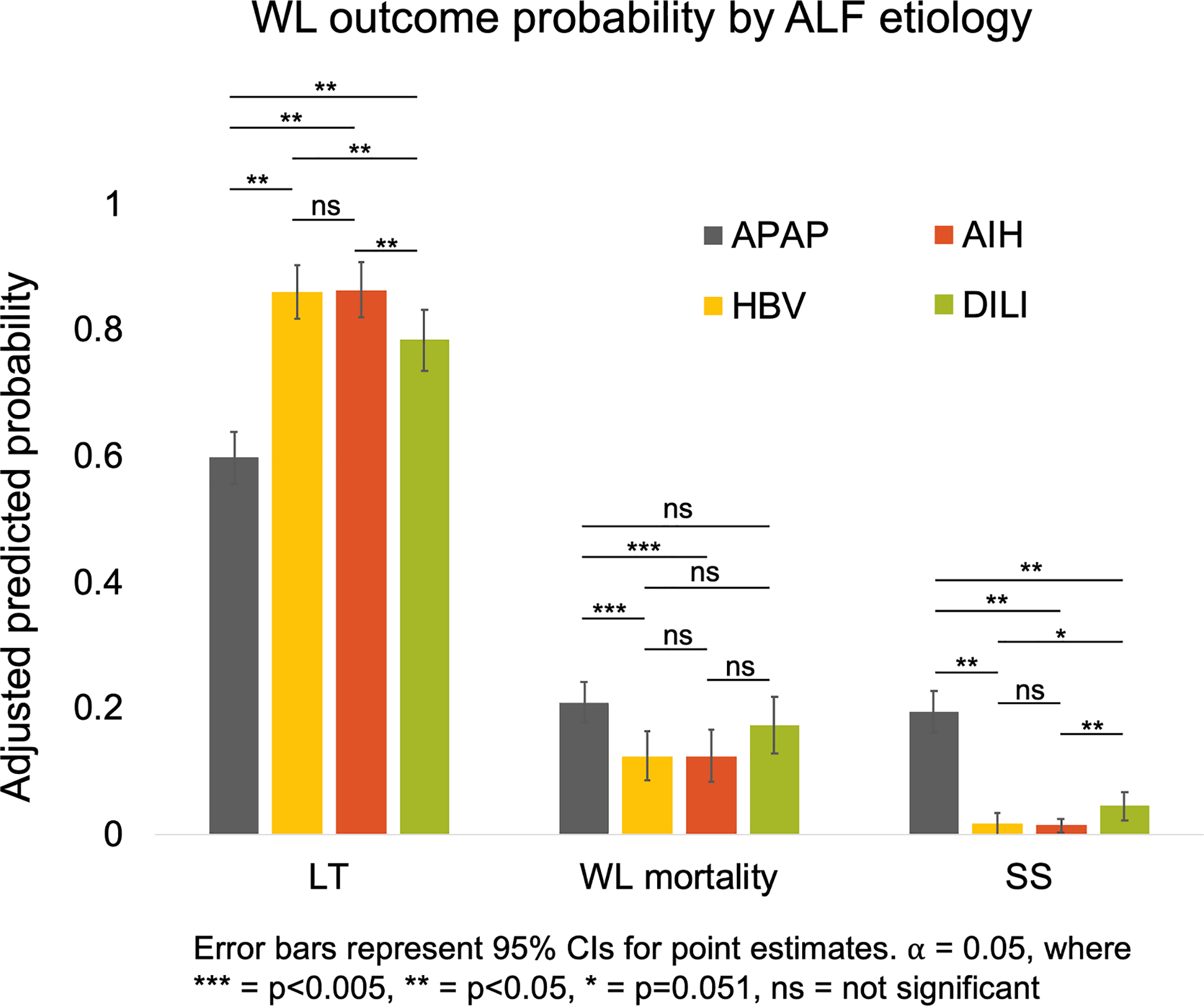
Predicted probabilities of LT, WL mortality and SS by ALF etiology accounting for baseline characteristics at listing
Overall post-LT patient and graft survival
Post-LT outcomes were evaluated for 1,217 patients who underwent LT, with a median follow-up time of 4.7 years (IQR: 1.0– 8.9 years). In the overall cohort, 298 (24.5%) deaths post-LT were observed, with a median time to death of 299 days (IQR: 10–1,805). The majority of post-LT deaths across all etiologies occurred ≤1 month from LT (Figure 4). Rates of early post-LT death were the highest among APAP and HBV patients. Rates of early mortality were lower among patients with other DILI, though this group had higher rates of later post-LT deaths. Retransplantation occurred in 82 patients (6.7% of LT cohort) with a median time to retransplantation of 133 days (IQR: 14–805).
Figure 4:
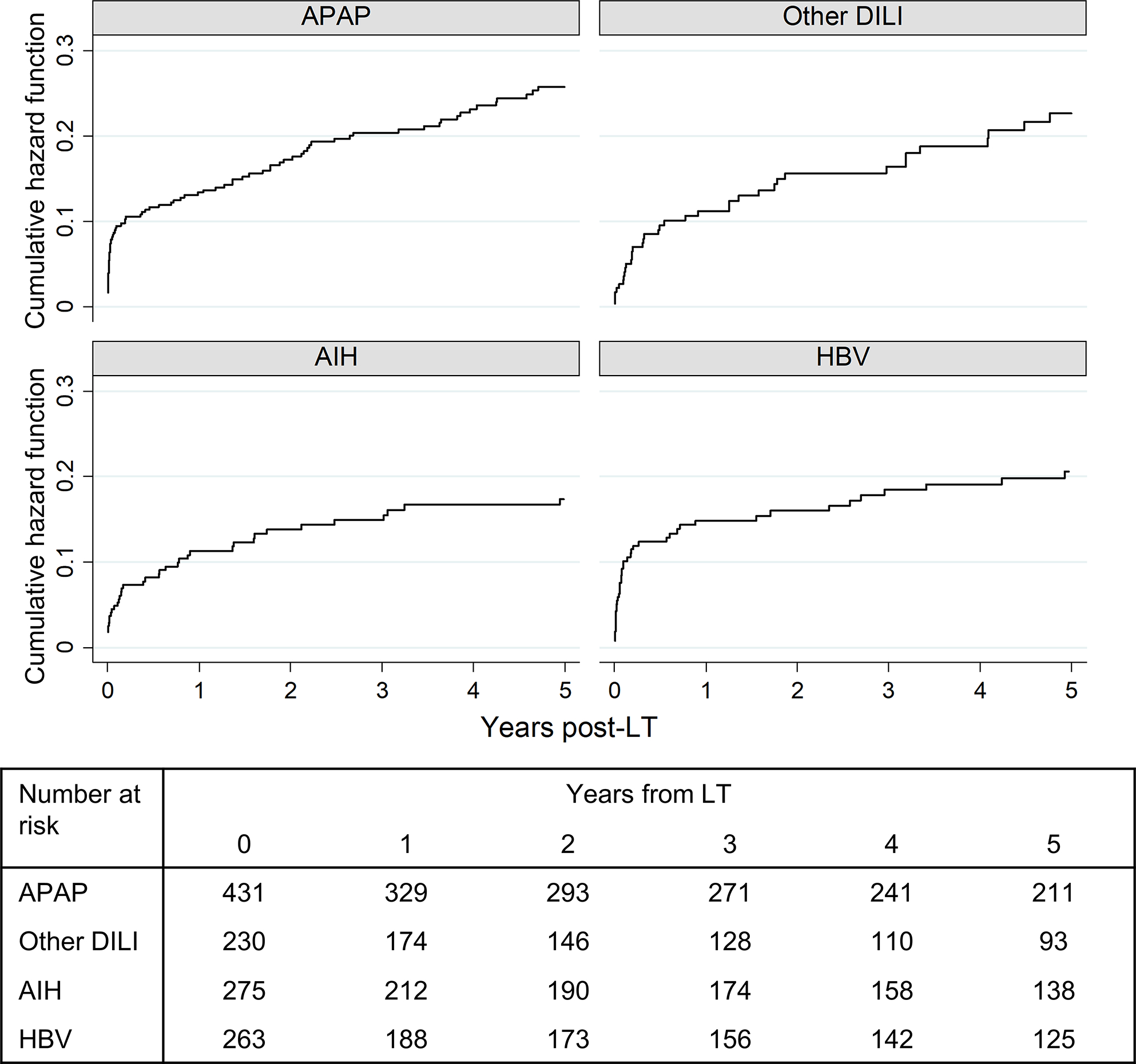
Timing of death from LT by ALF etiology (N=298)
The results of a multivariable model for all-cause post-LT mortality and graft failure are shown in Table 3. For reference, the baseline characteristics of the transplanted ALF patients by etiology are shown in Supplemental Table 5. Post-LT mortality was not significantly associated with ALF etiology, but there was a trend towards lower mortality for AIH compared to APAP (HR 0.59, 95% CI: 0.39–0.89; p=0.087 in overall pairwise comparisons). This was also seen with regards to graft failure (HR 0.66, 95%CI 0.46–0.94; p=0.134 in overall pairwise comparisons). Age ≥60 years was associated with increased post-LT mortality with a HR of 1.81 (95% CI: 1.22–2.70, p<0.001). Black race was an independent predictor of post-LT mortality (HR 1.69, 95% CI: 1.23–2.30, vs White; p<0.001) and of graft failure (HR 1.77, 95% CI: 1.34–2.33; p<0.001). Ascites and mechanical ventilation were also independent risk factors for post-LT mortality (HR 1.38 [95% CI: 1.06–1.79; p=0.016] and HR 1.34 [95% CI: 1.00–1.78; p=0.046], respectively).
Table 3.
Results of multivariable Cox proportional hazards model for all-cause post-LT mortality and graft failure
| Post-LT mortality N=1,129 |
Graft failure N=1,128* |
|||
|---|---|---|---|---|
|
| ||||
| Variable | HR (95% CI) | p value | HR (95% CI) | p value |
| ALF etiology† | 0.087 | 0.134 | ||
| APAP | --- | --- | ||
| HBV | 0.84(0.57–1.24) | 0.89(0.63–1.26) | ||
| AIH | 0.59(0.39–0.89) | 0.66(0.46–0.94) | ||
| DILI | 0.75(0.51–1.11) | 0.89(0.63–1.24) | ||
| Female | 0.82(0.62–1.08) | 0.162 | 0.90(0.70–1.15) | 0.392 |
| Age (years), N (%) | <0.001 | 0.083 | ||
| ≤40 | --- | --- | ||
| 40–59 | 0.96(0.73–1.27) | 0.86(0.68–1.10) | ||
| ≥60 | 1.81(1.22–2.70) | 1.31(0.90–1.90) | ||
| Race/ethnicity | <0.001 | <0.001 | ||
| White | --- | --- | ||
| Black | 1.69(1.23–2.30) | 1.77(1.34–2.33) | ||
| Hispanic | 0.91(0.57–1.44) | 0.97(0.65–1.46) | ||
| Asian | 0.44(0.23–0.83) | 0.58(0.34–0.99) | ||
| Other | 1.11(0.35–3.49) | 1.43(0.59–3.50) | ||
| MELD, per point | 1.00(0.98–1.02) | 0.959 | 1.00(0.98–1.01) | 0.810 |
| Clinical Factors at WL | ||||
| Grade 3–4 encephalopathy | 0.98(0.72–1.32) | 0.884 | 0.93(0.71–1.21) | 0.575 |
| Ascites present | 1.38(1.06–1.79) | 0.016 | 1.39(1.10–1.75) | 0.005 |
| Mechanical ventilation | 1.34(1.00–1.78) | 0.046 | 1.27(0.99–1.64) | 0.060 |
| Dialysis | 0.78(0.56–1.10) | 0.159 | 0.87(0.65–1.18) | 0.378 |
| Donor Age (per 10 years) | 1.16(1.08–1.25) | <0.001 | 1.18(1.10–1.25) | <0.001 |
| Cold Ischemia Time (per hour) | 1.01(0.97–1.04) | 0.731 | 1.01(0.99–1.04) | 0.319 |
| DCD Liver | 1.54(0.76–3.15) | 0.231 | 2.06(1.18–3.62) | 0.012 |
| Era | 0.984 | 0.659 | ||
| 2002–2007 | --- | --- | ||
| 2008–2013 | 1.00(0.75–1.34) | 0.87(0.69–1.11) | ||
| 2014–2019 | 1.04(0.70–1.54) | 0.78(0.56–1.09) | ||
Abbreviations: LT – liver transplant; OR – Odds ratio; ALF – acute liver failure; APAP – acetaminophen; DILI – drug induced liver injury; AIH – autoimmune hepatitis; HBV – hepatitis B virus; MELD – Model for End-Stage Liver Disease; WL – waitlist
Notes:
The number of transplanted ALF patients in the study population included N=436 APAP, N=278 HBV, N=271 AIH and N=232 other DILI. Additional demographic and clinical characteristics of transplanted ALF patients are shown in Supplemental Table 5.
Sample sizes differ due to number of patients experiencing the failure events of death and death/retransplant at t=0 (N=18 and N=19 respectively).
In-hospital mortality
Among the observed 298 post-LT deaths, 36.6% (N=109) occurred during the index LT admission. By comparison, 15.4% (N=46) were ≤1 year of hospital discharge, and 48.0% (N=143) >1 year post-LT. The overall in-hospital mortality rate was 9.0%. In-hospital mortality was noted to decrease over time from 12.2% between 2002–2007, to 8.1% between 2008–2013, and 5.6% between 2014–2019 (p=0.004). In-hospital mortality was not statistically significant by ALF etiology but trended higher among HBV patients: 12.2% versus 9.4% for APAP, 7.6% for AIH and 6.0% for DILI (p=0.084). Median time from LT to death for in-hospital deaths was 6 days (IQR 2–29) and was not different by ALF etiology (p=0.188).
On univariable analyses, the only factors associated with in-hospital mortality were: severe HE (vs none or mild, OR 2.05, 95% CI: 1.25–3.35, p=0.004), mechanical ventilation at LT (OR 2.68, 95% CI: 1.70–4.23, p<0.001), donor age (OR 1.18 per 10 year increase, 95% CI: 1.05–1.32, p=0.006) and waitlist era (OR 0.63 [95% CI: 0.41–0.99] for 2008–2013 and 0.42 [95% CI: 0.24–0.74] for 2014–2019 versus 2002–2007, p=0.005). On multivariable analyses, ALF etiology was again not an independent predictor of in-hospital mortality (p=0.419), while only mechanical ventilation at LT (aOR 2.43, 95% CI: 1.43–4.12; p=0.001) and donor age (aOR 1.19 per 10 year increase, 95% CI 1.05–1.35; p=0.008) remained significantly associated with the outcome; Supplemental Table 6).
Cause of death
Overall COD for patients receiving LT was different by ALF etiology (p=0.007; Figure 5). The most frequent CODs among those occurring during the index transplant admissions were brain death / cerebral edema (29.4%), non-liver organ failure (23.9%), infection (18.4%) and graft-related complications (11.9%).
Figure 5:
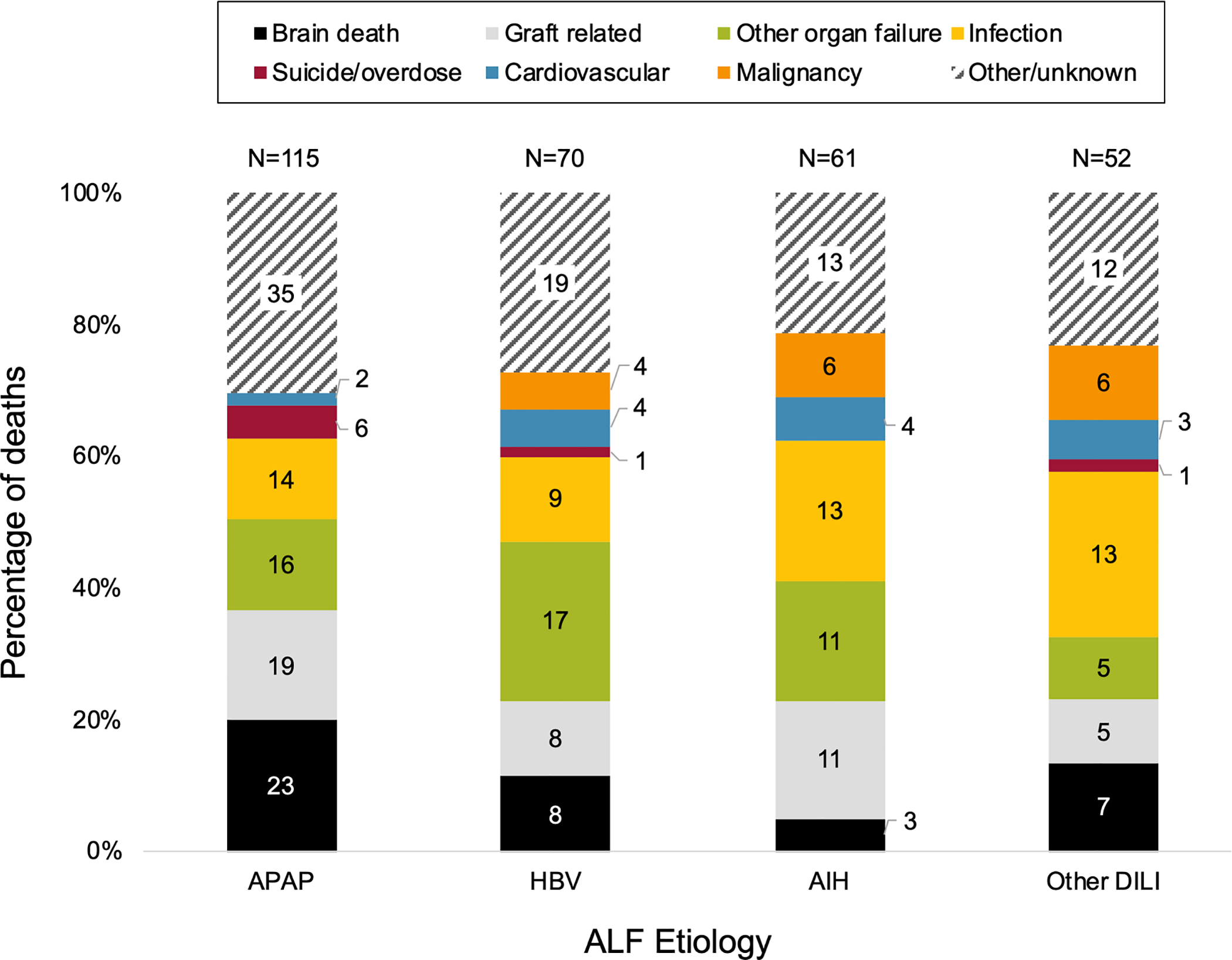
Cause of death post-LT by ALF etiology (N=298)
Note:
Reported COD was significantly different according to ALF etiology with p=0.007.
APAP patients were more likely to die of brain death or suicide/overdose, compared to patients with non-APAP ALF etiologies. The overall rate of post-LT brain death by etiology was 5.3% for APAP, 3.0% for other DILI, 1.1% for AIH and 3.0% for HBV (p=0.02). Stated otherwise, for every 20 LTs performed for APAP during the study period, one post-LT brain death occurred. The proportion of post-LT deaths from suicide was higher among LTs for ALF due to APAP vs non-APAP, though absolute numbers were small (6 of 115 (5.2%) vs 2 of 183 (1.1%); p=0.032). Infection was most common for other DILI, and non-cardiac/non-liver organ failure was most common in HBV cases.
Among the 82 patients who were retransplanted, 22 (26.8%) were performed during the index transplant admission with no difference by etiology (p=0.460). All patients who underwent retransplantation during the index LT admission survived until hospital discharge.
Discussion
ALF etiology is known to have an impact on overall patient survival and waitlist outcomes among those listed for LT1,12,13. However, until now the understanding of the relationship between ALF etiology and waitlist outcomes has not accounted for the marked differences in patients’ baseline severity of disease and has not included a comprehensive range of ALF etiologies. This study found that APAP patients had the shortest time from admission to waitlisting, yet the lowest predicted probability of LT. This is explained by their higher predicted waitlist mortality and SS rates, compared to other ALF etiologies13. In contrast, post-LT patient and graft survival were not significantly impacted by ALF etiology. The majority of post-LT deaths for all ALF etiologies studied occurred during the index hospital stay, raising the question of potentially futile LT. Post-LT deaths due to brain death were most frequent among APAP patients, accounting for 20% of deaths and occurring in 5% of LTs for APAP-induced ALF overall.
The increased rate of SS and waitlist mortality among APAP patients is consistent with prior studies that did not adjust for patient-level factors13. This supports the hypothesis that factors beyond intensive care resource use dictate survival among ALF patients, as these were accounted for in the models. It is possible that unique mechanistic aspects of the liver and associated organ failure in APAP explain these differences in waitlist mortality and SS. Such mechanistic factors could provide enhanced therapeutic strategies that are disease-specific and more tailored to patients’ clinical presentation. For example, the more rapid evolution of the liver injury in APAP and its subsequent consequences, such as cerebral edema, play important roles in the increased risk of waitlist mortality and contributes to the greater burden of in-hospital mortality post-LT. This presents an opportunity to further explore novel therapeutic targets that may be specific to APAP-induced ALF, beyond the use of N-acetyl cysteine, as well as a more aggressive approach to the management of cerebral edema such as with the use of continuous renal replacement therapy20. Similarly, there are few disease-specific evidence-based practices for ALF due to HBV, AIH and other DILI, despite these accounting for over half of ALF cases in the U.S.
We investigated timing from admission to LT by etiology, as ALF etiology alters the trajectory of disease progression and therefore the urgency of clinical interventions13. We observed that timing from admission to LT waitlisting was significantly longer for AIH and non-APAP DILI patients compared to APAP or HBV13. Of waitlisted patients, 20% of AIH patients and 14.7% of non-APAP DILI patients waited ≥7 days from index admission to waitlisting, compared to under 2% for APAP and HBV. Diagnostic uncertainty, perhaps a more indolent symptom development, and potentially attempts by centers to manage patients expectantly prior to considering LT may explain this finding. Among Acute Liver Failure Study Group centers, however, previously published waitlist times were similar across etiologies, suggesting that variability may exist in the decision to pursue LT under different clinical circumstances13.
Patients with ALF who undergo LT are known to have worse immediate survival than patients receiving LT for non-ALF15–18. This study found that nearly 1 in 10 LTs for ALF led to an in-hospital death, demonstrating that prognostication of futile LT is clearly deficient in this setting. This also raises the question as to the efficiency of the organ allocation process for Status 1 patients in its current form2. Our study demonstrates no association between underlying ALF etiology and post-LT patient survival in a modern U.S. national cohort, which is consistent with the prior analysis of the UNOS database from 1988–200317. We hypothesize that, in contrast to waitlist outcomes, survival after LT in ALF is primarily dictated by candidate selection and perioperative events that are more agnostic to the mechanism of liver injury itself. Thus, the transplant community would benefit from both enhanced prognostication of when to avoid LT in such patients, particularly in the setting of cerebral edema, as well as potential therapeutic strategies to decrease early post-LT mortality (e.g., antibiotic management, transfusion parameters, anesthesia considerations). While several scoring systems have been developed to decrease LT futility risk among ALF candidates, such as the Acute Liver Failure-Organ Failure score, these have not been externally validated as of yet and are therefore not frequently used in clinical practice16,17.
A limitation of this study is its focus on patients with known ALF etiologies, which required omission of 1,329 patients with unknown or indeterminate etiology. It is unclear what proportion of these omitted cases were truly of unknown etiology, versus incorrectly reported by centers to UNOS. For example, it has been shown previously that 18% of patients classified as indeterminate ALF etiology actually had unrecognized or uncertain APAP toxicity21. While we did find small but significant differences in waitlist outcomes between patients included in the cohort versus those with unknown/indeterminate ALF, including a higher rate of waitlist mortality and lower rate of SS (Supplemental Table 2), we found no difference in post-LT outcomes (Supplemental Figure 1). These differences may limit the generalizability of our findings, and may be evidence for worse outcomes in the setting of rapidly evolving ALF cases with etiologic uncertainty. Of note, the rate of ALF listings with unknown etiology in 2018 was not markedly different from 2002, unlike what would be perhaps expected if these were all due to data entry quality alone.
Despite being listed as Status 1 priority for fulminant liver failure, HBV and AIH patients in this study had high rates of ascites that potentially reflected chronic liver disease. We also acknowledge the heterogeneity of the non-APAP DILI etiology group, comprised of DILI due to a wide range of medications. Unfortunately, the UNOS database does not permit a reliable analysis of the specific medications implicated. Moreover, the determination of APAP versus DILI cases relies fully on free text entries, as there is no numerical code to differentiate between them. The aberrantly low number of DILI cases and high number of APAP cases in 2007 may be related to inaccuracies in free-text data, and made more significant by the small number per year of ALF listings. Finally, post-LT mortality analysis was limited by the small number of events and potential inaccurate COD reporting. The role of suicide and medication non-adherence are potentially actionable causes, which merit additional studies with patient-level psychosocial data.
This study used updated, national data to demonstrate the impact of underlying ALF etiology in outcomes before and after LT, accounting for differences in baseline severity of illness13,14. While ALF etiology was associated with waitlist outcomes, it was not associated with post-LT mortality, supporting the hypothesis of the differential role of the mechanism of liver injury on these two outcomes. This study added new characterization of the significant burden of in-hospital mortality in transplanted ALF patients, complementing prior studies that have focused on mid- and long-term mortality15,17. Among ALF cases with known etiology, APAP patients stand out as a subgroup in which the high risk of waitlist mortality coupled with high rates of brain death post-LT necessitate novel risk stratification tools to balance timely, life-saving LT and minimize LT futility. Future efforts should also aim to identify perioperative therapeutics that reduce the risk of early adverse post-LT events among patients with ALF.
Supplementary Material
Supplemental Figure 2: Trends in waitlisting for ALF with indeterminate (N=117) and unknown (N=765) etiology over time
Supplemental Figure 1: Unadjusted (A) patient survival and (B) graft survival for study cohort (N=1,217) versus unknown/indeterminate group (N=926)
Acknowledgments
Grants and financial support: Therese Bittermann is supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant K08-DK117013.
Abbreviations:
- APAP
acetaminophen
- ALF
acute liver failure
- AIH
autoimmune hepatitis
- COD
cause of death
- DCD
donor after circulatory determination of death
- DILI
drug induced liver injury
- HBV
hepatitis B virus
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-stage Liver Disease
- RRR
relative risk ratio
- SS
spontaneous survival
- UNOS
United Network for Organ Sharing
- US
United States
- WL
waitlist
Footnotes
Conflicts of interest: The authors of this manuscript firmly declare no conflicts of interest related to this study.
References
- 1.Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. Journal of hepatology. 2013;59(1):74–80. [DOI] [PubMed] [Google Scholar]
- 2.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. Journal of hepatology. 2012;57(2):288–296. [DOI] [PubMed] [Google Scholar]
- 3.Mendizabal M, Tagliafichi V, Rubinstein F, et al. Liver transplantation in adults with acute liver failure: Outcomes from the Argentinean Transplant Registry. Annals of hepatology. 2019;18(2):338–344. [DOI] [PubMed] [Google Scholar]
- 4.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Annals of internal medicine. 2002;137(12):947–954. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RB Jr., Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(4 Pt 2):958–976. [DOI] [PubMed] [Google Scholar]
- 6.Bunchorntavakul C, Reddy KR. Acute Liver Failure. Clinics in liver disease. 2017;21(4):769–792. [DOI] [PubMed] [Google Scholar]
- 7.Thanapirom K, Treeprasertsuk S, Soonthornworasiri N, et al. The incidence, etiologies, outcomes, and predictors of mortality of acute liver failure in Thailand: a population-base study. BMC gastroenterology. 2019;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hey P, Hanrahan TP, Sinclair M, et al. Epidemiology and outcomes of acute liver failure in Australia. World journal of hepatology. 2019;11(7):586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escorsell A, Mas A, de la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(10):1389–1395. [DOI] [PubMed] [Google Scholar]
- 10.Reuben A, Tillman H, Fontana RJ, et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Annals of internal medicine. 2016;164(11):724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14 Suppl 2:S67–79. [DOI] [PubMed] [Google Scholar]
- 12.Stravitz RT, Lee WM. Acute liver failure. Lancet (London, England). 2019;394(10201):869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy KR, Ellerbe C, Schilsky M, et al. Determinants of outcome among patients with acute liver failure listed for liver transplantation in the United States. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(4):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernal W, Cross TJ, Auzinger G, et al. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. Journal of hepatology. 2009;50(2):306–313. [DOI] [PubMed] [Google Scholar]
- 15.Fontana RJ, Ellerbe C, Durkalski VE, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicentre study. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(2):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figorilli F, Putignano A, Roux O, et al. Development of an organ failure score in acute liver failure for transplant selection and identification of patients at high risk of futility. PloS one. 2017;12(12):e0188151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barshes NR, Lee TC, Balkrishnan R, Karpen SJ, Carter BA, Goss JA. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81(2):195–201. [DOI] [PubMed] [Google Scholar]
- 18.Farmer DG, Anselmo DM, Ghobrial RM, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Annals of surgery. 2003;237(5):666–675; discussion 675–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong NZ, Schaubel DE, Reddy KR, Bittermann T. Transplant center experience influences spontaneous survival and waitlist mortality in acute liver failure: An analysis of the UNOS database. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2021;21(3):1092–1099. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology (Baltimore, Md). 2018;67(2):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology (Baltimore, Md). 2011;53(2):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2: Trends in waitlisting for ALF with indeterminate (N=117) and unknown (N=765) etiology over time
Supplemental Figure 1: Unadjusted (A) patient survival and (B) graft survival for study cohort (N=1,217) versus unknown/indeterminate group (N=926)


