Abstract
Citrus fruits are well known for their medicinal and therapeutic potential due to the presence of immense bioactive components. With the enormous consumption of citrus juice, citrus processing industries are focused on the production of juice but at the same time, a large amount of waste is produced mainly in the form of peel, seeds, pomace, and wastewater. This waste left after processing leads to environmental pollution and health-related hazards. However, it could be exploited for the recovery of essential oils, pectin, nutraceuticals, macro and micronutrients, ethanol, and biofuel generation. In view of the importance and health benefits of bioactive compounds found in citrus waste, the present review summarizes the recent work done on the citrus fruit waste valorization for recovery of value-added compounds leading to zero wastage. Therefore, instead of calling it waste, these could be a good resource of significant valuable components, in this way encouraging the zero-waste theory.
Keywords: Citrus fruit waste, Valorization, Green extraction techniques, Bioactive compounds, Biofuels
Introduction
Fruits belonging to the genus Citrus and family Rutaceae is one of the largest class of fruits which are extensively grown, processed and consumed all over the globe (Satari and Karimi, 2018). The history with respect to the origin and production of these fruits is as yet inexplicable. However, it is believed that they have origin from lower regions of the Himalayas in Northern India, Northern Myanmar, South China (Yunnan area) and Southeast Asia (Panwar et al., 2021). Among citrus fruits, mandarin (Citrus reticulate L.), sweet orange (Citrus sinensis L.), grapefruit (Citrus paradise L.), lemon (Citrus lemon L.), and lime (Citrus aurantifolium L.) have good commercial value and are extensively grown throughout the world. Citrus fruits are well known owing to their exotic taste, aroma, refreshing fragrance, savor, and adequate vitamin C content (Ledesma-Escobar and de Castro, 2014). Besides, these fruits have great adaptability towards diverse climatic and soil conditions leading to their cultivation in tropical, subtropical, and mild temperature regions.
Citrus fruits are well known for their medicinal and therapeutic potential due to their immense bioactive components. Citrus fruits constitute water (75–90%), sugars (6–9%), and the remaining part consists of pectin, dietary fiber, minerals, and essential oils in a healthy and balanced dietary form. Furthermore, carotenoids and flavonoids are also found in citrus fruits (Santiago et al., 2020).
As per FAO STAT, 2019, the annual citrus fruit production has abundantly grown worldwide in past many years, ascending from around 51.48 MT in 1975 to 158 MT in 2019. China reported 44.10 MT citrus fruit production, accounting for 27.80% of the total world's citrus fruit production in 2019. The other top citrus fruit producing nations are Brazil, India, Mexico, USA, and Spain. India stands at the third position with an annual production of 14.01 MT and 1.07 million hectares of land involved in citrus fruit cultivation in 2019 (FAO STAT, 2019). As per ICAR-Central Citrus Research Institute (2019), citrus crops stand firm on huge foothold in fruit industry of India with 13% contribution to yearly fruit production of the country. India usually exports citrus fruits to neighboring countries and around 4–5% citrus fruit is usually processed annually in food industries, however enormous amounts of lemons and limes used for pickling in unorganized sector goes unaccounted. Most of the citrus fruits such as oranges, mandarins, and grapefruits are commonly eaten in fresh form (75%), approximately 25% of citrus fruits are processed in food industries manufacturing citrus juices/citrus-based drinks, jams, marmalades, and dehydrated citrus-based products (Panwar et al., 2021), leading to generation of huge quantities of waste.
Substantially, the portion of waste material produced from fruits is tremendously high (mango 30–50%, citrus 30–50%, pomegranate 40–50%, banana 20%, guava 10%, grape 20%, pineapple 45–55%) (Banerjee et al., 2018). Citrus fruit waste primarily constitutes pulp, peel, seeds, damaged and rejected fruits. Citrus fruit based industries generate large amounts of citrus waste each year and it represents practically half of the fresh fruit mass. The amount of citrus fruit waste worldwide exceeds approximately 110–120 MT annually (Mahato et al., 2020). Citrus fruit waste has lower pH, a high amount of water, and organic matter which unfits it to be disposed off in landfills as per the European regulations (Santiago et al., 2020). Conversely, massive quantities of citrus fruit waste are normally unloaded on the close by landfill/waterways or it is either scorched leading to ecological pollution, depletion of dissolved oxygen level in polluted water, and health-related hazards (Wadhwa and Bakshi, 2013). Citrus waste is also used as animal feed, yet, an excess of it in animal diet may distress the digestive tract causing a disease named rumen parakeratosis, reflecting this end-use having low profitability (Lotito et al., 2018). In addition, the choices for dealing with this kind of waste incorporate fertilizing the soil through composting, anaerobic co-digestion, and even burning, in spite of the fact that water content makes the last choice not reasonable (Fuertes, 2015).
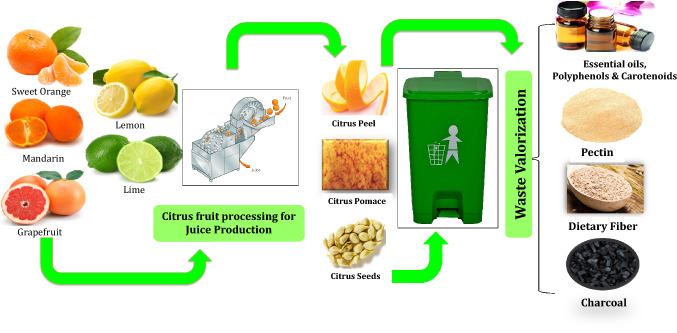
Nonetheless, citrus fruit waste could serve as a potent family of bioactive constituents like phenols, flavonoids, carotenoids, and essential oils. Also in context to the bioeconomy, citrus fruit waste could be used for the generation of biofuels and other commodities (Martin et al., 2010); so the present review as illustrated in Fig. 1 covers the comprehensive summarization and discussion on utilization of citrus fruit waste leading to zero wastage that would help the researchers and policy makers to plan for their valorization for economic growth as well as environmental sustainability.
Fig. 1.
Advanced utilization of citrus fruit waste
Anatomy: components of citrus fruit waste
Citrus processing encompasses varied unit operations leading to the generation of a substantial mass of by-products primarily peel, pulp, seeds, and pomace accounting for 50 to 70%, 60 to 65%, 30 to 35%, and < 10% of the total fruit mass, individually (Panwar et al., 2021). The comprehensive view of citrus fruit comprises of the peel/rind constituting cuticle on the external layer, finely covering the epidermis namely flavedo (exocarp/outermost covering) and albedo (spongy layer of parenchymous cells positioned next to flavedo) (Chavan et al., 2018). Flavedo consists of oil glands filled with essential oils, paraffin waxes, fatty acids, steroids/ triterpenoids, phytochemicals (chlorophyll, carotenoids, flavonoids, and phenolic compounds), and enzymes. Upon ripening the chlorophyll-containing flavedo cell present inside chloroplast gets replaced with carotenoid majorly xanthophyll. This major hormonal-mediated development leads to the change in the color of fruit from green to yellow after ripening. The inner layer of flavedo contains the richness of essential oil embedded in multicellular bodies of spherical/pyriform shapes. Albedo, situated close to the flavedo is the internal layer of the citrus peel that is rich in gelatin, lignin, and dietary filaments (Mamma and Christakopoulos, 2013). The endocarp (pulp/flesh) of citrus fruits is generally formed of sections called segments or carpels, parted through a membrane of thin epidermal tissues consisting of several juice sacs called vesicles and seeds. The core white spongy tissues resembling albedo is known as the axis of the fruit. Collectively the core plus segments are referred to as "rag" of the extracted juice (Chavan et al., 2018).
Characterization of citrus processing waste
Citrus fruits are consumed in high amounts throughout the nations in the natural peeled form or in the form of juice. Citrus fruit processing industry is viewed as a favored financial exercise worldwide leading to the production of a variety of citrus products mainly juice and essential oils. Around 33% of all citrus produce harvested worldwide is utilized for juice production (Lohrasbi et al., 2010). The huge amount of citrus waste left after juice production could be utilized for the extraction of essential oils, pectin, nutraceuticals, macro and micronutrients, ethanol, and biofuel generation. Nonetheless, to foster the effective innovation for the valorization of citrus waste into value-added items, it is needed to comprehend its chemical composition which is boundlessly influenced by the variety of fruit, climatic conditions, and the kind of juice extraction procedure (Chen et al., 2019). Citrus by-products usually contain high moisture (80–90%) and organic matter (Satari and Karimi, 2018). Additionally, the citrus waste contains plentiful amounts of simple carbohydrates (glucose, fructose, sucrose), complex carbohydrates (dietary fiber, cellulose, starch, pectin), protein, natural acids (citrus, malic, oxalic acids), lipids (linolenic, oleic, palmitic, stearic acids), fundamental peel oil (d-limonene), pigments/carotenoids (carotene, Xanthophyll and lutein), water-soluble vitamins (Vitamin C and B-complex vitamins), minerals (calcium and potassium) and polyphenols (flavonoids- hesperidin, naringin, and phenolic acids) (Boukroufa et al., 2015). These also contain enzymes (pectinesterase, phosphatase, peroxidase), tartaric, benzoic, oxalic, succinic acids, and volatile components (alcohol, aldehyde, ketones, esters, and hydrocarbons) (Sharma et al., 2017). The usual proximate composition of the citrus by-products has been summarized in Table 1.
Table 1.
Usual proximate content of citrus waste (% dry weight basis)
| Citrus waste | Moisture | Crude protein | Crude fibre | Crude fat | Crude ash | Pectin | Lignin | Cellulose | Hemicellulose | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Citrus sinensis seeds | – | 3.1 | 5.5 | 52 | 2.5 | – | – | – | – | Akpata and Akubor (1999) |
| Citrus sinensis peels | 75.3 ± 10.2 | 10.2 ± 3.70 | 57.0 ± 10.0 | 2.22 ± 6.10 | 3.33 ± 0.50 | – | – | – | – | Chau and Huang (2003) |
| Citrus sinensis peels | – | 9.10 | – | 4.00 | 2.60 | 23.02 | 7.52 | 37.10 | 11.04 | Marin et al. (2007) |
| Citrus limon pulp | – | 8.72 | – | 4.00 | 2.54 | 22.53 | 7.55 | 36.22 | 11.05 | Marin et al. (2007) |
| Citrus unshiu peel | 3.30 | 34.0 | 4.30 | 25.10 | 10.20 | Kim et al. (2015) | ||||
| Citrus by-productsa | – | – | – | – | 4.80 | 15.30 | 1.95 | 8.82 | 7.96 | Satari et al. (2017) |
aMixture of peels, leaf and seeds of C. sinensis and C. paradise after extraction of juice
Citrus peel
Citrus peels constitute the major citrus waste which accounts for 50–55% of the total fruit weight. Structurally, the citrus fruit peel has two layers i.e. flavedo and albedo. Citrus peels contain an abundance of nutrients and bioactive compounds such as polyphenolic compounds, flavonoids (polymethoxylated flavones- hesperidin, naringin, nobiletin, tangeretin), essential oils, pigments, and dietary fibers (Wang et al., 2015). Essential oils primarily D-limonene, α-pinene, and α-terpinolene obtained from the citrus peel have enormous market demand owing to their utilization in foods, beverages, perfumery, and cosmetic industry. Citrus peel also contains an appreciable amount of soluble sugars (glucose, fructose, and sucrose) which could be efficiently utilized for the production of bioethanol. The cellular component of peel consists of pectin, cellulose and hemicellulose-galacturonic acid, galactose, arabinose. The richness of both soluble and insoluble carbohydrates suggests the potent use of peel for value-added products by consequent biochemical processes (Rivas et al., 2008). On contrary, the use of pesticides to protect citrus fruits from unwanted molds or insects before and after harvest is the major constraining factor affecting the safe utilization of citrus waste for food applications (Ortelli et al., 2005). Citrus peels are usually exposed to higher concentration of fungicidal insecticides (4468 mg/kg) as compared to the whole fruit; however there is low penetration of the same from peel to pulp (Calvaruso et al., 2020).
Citrus seeds
Citrus seeds are the unusable part of the citrus fruit which are known for their rich oil content and accounts for 20–40% by weight (Rosa et al., 2019). Literature survey discovered the potential use of oil extracted from citrus seed in the production of biodiesel. The proximate study of unhulled and dehulled citrus seeds flour revealed that on dry weight basis it contains 52% crude fat, 28.5% carbohydrate, 5.5% crude fiber, 3.1% crude protein, and 2.5% crude ash content (Chavan et al., 2018). Besides, the citrus seeds contain a good quantity of protein, limonoids, and phenols (eriocitrin, hesperidin), subsequently expanding their reusability. Fatty acid profile of the citrus seed reflects the occurrence of good fats i.e. poly unsaturated fatty acids mainly linolenic acid and oleic acid (Ndayishimiye and Chun, 2017). In the past, citrus seeds have been economically used for oil production and meal that is additionally used for the development of soaps, cleansers, consumable oils, as well as biodiesel (Rosa et al., 2019). Certain recent studies also reported the pharmacological effects of limonoids and phenols in the form of functional foods (Sharma et al., 2017).
Citrus pomace
Citrus pomace is the by-product/residue which is left after the citrus fruit processing for production of juice or other products. Almost half of the citrus fruits are discarded during industrial citrus juice processing, and the gigantic measure of citrus pomace leads to deleterious ecological issues (Wang et al., 2018). Owing to the high moisture as well as sugar content, citrus pomace is at risk of uncontrolled fermentation and spoilage by microorganisms (Majerska et al., 2019). Hence the efficient management of pomace is necessary for preventing environmental hazards. In addition, citrus pomace is rich in polyphenols, pectin, dietary fibres, molasses as well as essential oil which could be utilized at the industrial level (Papoutsis et al., 2016a). Citrus pomace (dried) consists of moisture (10%), sugars (30–40%), fat (0.4–1.6%), micronutrients (0.5%), pectin (14–25%), cellulose and hemicellulose (13–17%) (Widmer et al., 2010).
Citrus wastewater
Citrus fruit product preparing businesses creates lot of citrus wastewater attributable to the unit operations of processing for example, fruit cleaning, washing, canning, apparatus cleaning and peel drying. Citrus wastewater contains parts of spoiled fruit, seeds, pulp, and peels (Zema et al., 2018). However, as a consequence of rich organic mass, lower pH, high suspended solids, the disposal of citrus waste water leads to risk for water bodies, soil condition, and the ecosystem. On the contrary, this citrus wastewater contains the richness of sugars, essential oils, bioactive compounds –polyphenols, flavonoids, and pectin which could be utilized in different ways for effective valorization. Recent research focused on the anaerobic digestion of wastewater obtain from citrus fruit processing for the on-site generation of energy in a citrus processing facility. The results showed that the digestion of citrus wastewater yields 2.1m3 of methane at STP/m3. This produced biogas could be used for supplying all the required energy in the form of electricity and fuel and meeting the needs of the plant (Koppar and Pullammanappallil, 2013).
Strategies for valorization of citrus peel
Citrus peels are loaded with ample quantities of bioactive compounds viz; polyphenols, pectin, essential oils, carotenoids, dietary fibre, and many more. These could be recovered by using efficient extraction technologies leading to their utilizations for food applications.
Source of bioactive compounds- polyphenols (flavonoids and phenols)
Bioactive compounds could be characterized as essential or non-essential phytochemicals, which can be extracted from food or its byproducts and are capable of regulating biological and metabolic processes (Galanakis and Kotsiou, 2017). The phenols, flavonoids, and carotenoids constitute the major bioactive compounds found in fruits, vegetables, and their by-products. Citrus waste contains plenty of naturally occurring bioactive compounds including polyphenols, predominantly phenolic acids, and flavonoids (Sharma et al., 2017). Interestingly, it is revealed that the citrus fruit by-products contain more polyphenol content than in edible portion of citrus fruit (Balasundram et al., 2006), therefore, instead of considering it as waste, these could be used for the recovery of significant valuable components, in this way encouraging the zero-waste theory.
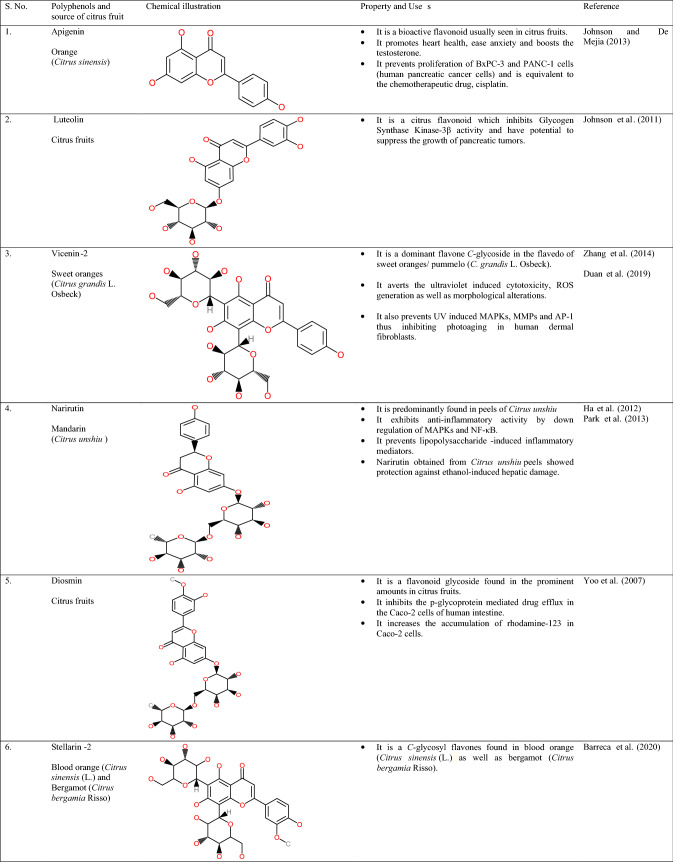
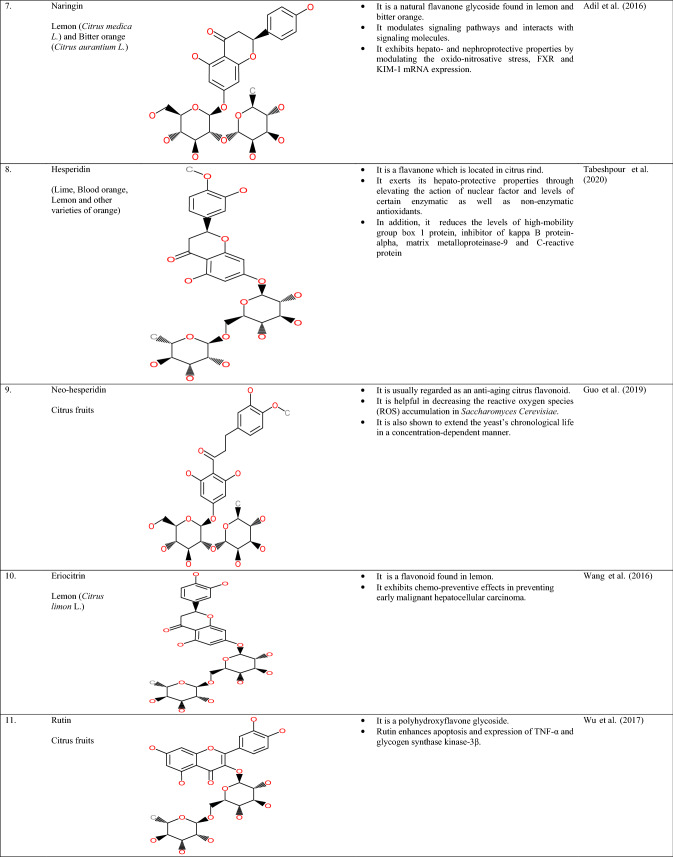
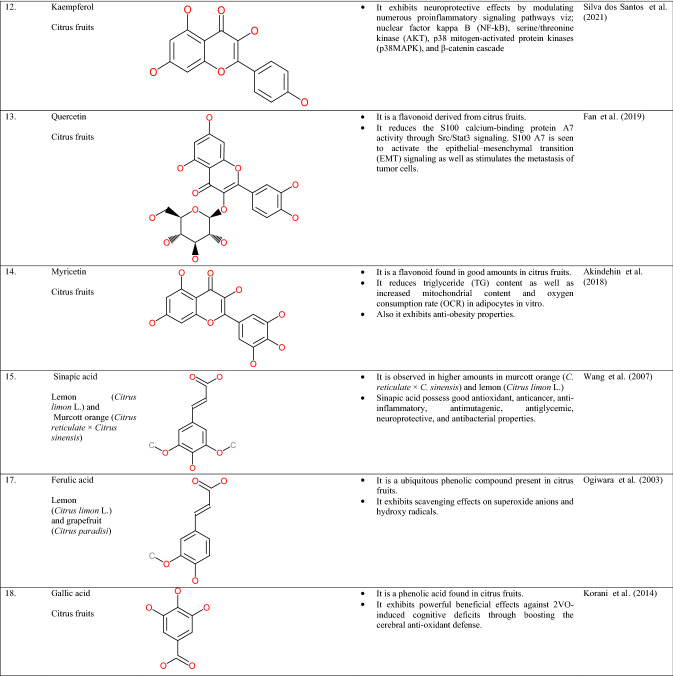
Citrus peel contains a wide array of polyphenols such as flavonoids, especially flavones, isoflavones, flavonones, flavonols, and anthocyanidins. Flavonoids are majorly located in the peel, pulp, and rag tissues of citrus waste. Major flavonoids observed in fruits belonging to citrus origin are naringin, hesperidin, eriocitrin, and narirutin (Schieber et al., 2001). Furthermore, these citrus flavonoids are categorized into 4 subdivisions, specifically flavanones (Narirutin, Hesperidin, Neo-hespiridin, and Naringin) present in higher amounts followed by flavones (Luteolin, Diosmin, and Apigenin), flavonols (Kaempferol, Rutin, and Quercetin) and anthocyanins (Sharma et al., 2017). On the other hand, the phenols present in the citrus peel are broadly categorized into 2 subdivisions, viz. hydroxybenzoic acid and hydroxycinnamic acid (Ignat et al., 2011). The chemical illustrations and properties/uses of the polyphenols found in citrus fruit waste have been shown in Table 2.
Table 2.
Chemical illustrations and properties/uses of polyphenols found in citrus waste
Extraction of bioactive compounds
Various researchers studied the polyphenol components of the citrus by-products by utilizing different extraction techniques, solvents, and process parameters as presented in Table 3. Microwave assisted extraction, ultrasound assisted extraction, pulsed electric field extraction, high voltage electric discharge technique, supercritical fluid extraction, and pressurized water extraction are some of the green extraction techniques utilized by the researchers.
Table 3.
Bioactive polyphenols extraction from citrus waste using different extraction methods
| Citrus by-products | Extraction parameters | Solvent | Bioactive compound yield | References |
|---|---|---|---|---|
| Citrus peel | ||||
| Microwave assisted extraction | ||||
| Citrus reticulata (Kinnow) peel |
Power:152 W Time:49 s |
Methanol concentration- 66% | Phenolic acid content: 1162.84 μg/g dry basis | Hayat et al. (2009) |
| Citrus inshiu peel |
Temperature:140 °C Time:7 min |
Ethanol concentration—70% |
Hesperidin: 5860 mg/100 g Narirutin: 1310 mg/100 g |
Inoue et al. (2010) |
| Citrus limon peel |
Power:400 W Time:120 s |
Ethanol concentration—48% | Total phenols: 1574 mg GAE/100 g | Dahmoune et al. (2013) |
| Citrus sinensis peel |
Power:500 W Temperature: ≤ 80 °C Time:122 s |
Aqueous acetone-51% | Total phenols: 12.20 mg GAE/g dry basis | Nayak et al. (2015) |
| Citrus sinensis peel |
Power:339 W Time:53 s |
– |
Total phenols: 0.86 mg GAE/g dry basis Total flavonoids: 0.24 mg CE/g dry basis |
Sahin (2015) |
| Citrus paradisi peel |
Power:275 W Time:45 s |
Ethanol |
Total phenols: 17.22 mg GAE/g dry basis Total flavonoids: 1.71 mg CE/g dry basis |
Nishad et al. (2019) |
| Conventional extraction method | ||||
| Citrus reticulate L. (Mandarin) peel | – | Acetone—70% |
Hesperidin (80.90 mg/g extract) Narirutin (15.30 mg/g extract) DPPH radical scavenging activity (EC50 = 0.179 mg/ml) Hydroxyl radical scavenging activity (EC50 = 0.415 mg/ml) |
Tumbas et al. (2010) |
| Supercritical fluid extraction | ||||
| Citrus unshiu peel |
Pressure:250ATM Temperature:60 °C Time: 1 h (Balanced time) 30 min (extraction time) |
CO2-methanol | Hesperidin 1.6–1.8% | Hayat et al. (2010a) |
| Citrus spp. (Mandarin, lemon, orange, and grapefruit) peel |
Pressure:160ATM Temperature:35 °C |
Ethanol concentration -40% |
Total phenols in lemon: 0.66 mg GAE/100 g Total phenols in tangerine: 0.38 mg GAE/100 g Total phenols in grapefruit: 0.67 mg GAE/100 g Total phenols in orange: 0.45 mg GAE/100 g |
Omar et al. (2013) |
| Citrus aurantium amara peel |
Pressure:170 bar Time: 50 min (balanced time) 1200 min (extraction time) |
CO2-Ethanol concentration -3% | Osthol 47% | Trabelsi et al. (2016) |
| High hydrostatic pressure (HHP) | ||||
| Citrus spp. (Orange and lemon) peel |
Pressure: 300 MPa Time:10 min Temperature: 10 °C and Pressure: 500 MPa Time:3 min Temperature—10 °C |
Aqueous ethanol-80% (Containing 1% conc. HCL) |
Total phenols in orange: 136.85 mg GAE/100 mL Total phenols in lemon: 344.53 mg GAE/100 mL |
Casquete et al. (2014) |
| Citrus spp. (Lemon, orange, lime, and mandarin) peel | Pressure: 300 MPa |
Aqueous ethanol-80% (Containing 1% conc. HCL) |
Total phenols in lemon: 266.23 mg GAE/100 g Total phenols in tangerine: 587.28 mg GAE/100 g Total phenols in lime: 397.21 mg GAE/100 g Total phenols in orange: 288.16 mg GAE/100 g |
Casquete et al. (2015) |
| High voltage electric discharge | ||||
| Navel Navelate (Mandarin peel) |
Current: 40 kV Time:10 μs |
N/A | Total phenols: 184.2 mg GAE/100 mL of extract | Buniowska et al. (2015) |
| Citrus spp. (Grapefruit) peel | Pulse:10–300 | Glicerol (20%) | Total phenols: 1880 mg GAE/100 g | El Kantar et al. (2019) |
| Ultrasound assisted extraction | ||||
| Citrus reticulate L. peel |
Power:56.71 W Temperature: 48 °C Time: 40 min |
Acetone-80% |
Total phenols: 15,263.32 mg Eq gallic/100 g dry basis Hesperidin: 6435.53 mg/100 g dry basis |
Nipornram et al. (2018) |
| Pulsed electric field extraction (PEF) | ||||
| Citrus spp. (Orange) peel |
Time:30 min Pressure:5 bars |
N/A |
Phenolic compounds yield at 1 kV/cm PEF: 20%, Phenolic compounds yield at 3 kV/cm PEF: 129% Phenolic compounds yield at 5 kV/cm PEF: 153% Phenolic compounds yield at 7 kV/cm PEF: 159% Antioxidant activity at 1 kV/cm PEF: 51% Antioxidant activity at 3 kV/cm PEF: 94% Antioxidant activity at 5 kV/cm PEF:148% Antioxidant activity at 7 kV/cm PEF:192% Naringin at 5 kV/cm PEF: 3.1 mg/100 g fresh weight basis Hesperidin at 5 kV/cm PEF:4.6 mg/100 g fresh weight basis |
Luengo et al. (2013) |
| Citrus spp. (Orange, grapefruit, and lemon peel) |
First treatment: 3 kV/cm Second treatment: 10 kV/cm |
Ethanol -50% |
Total phenols: 2200 mg GAE/100 g Hesperidin content in Orange peel (flavedo): 507 mg/100 g Hesperidin content in Orange peel (albedo): 482 mg/100 g Naringin content in Grapefruit peel (flavedo): 1036 mg/100 g Naringin content in Grapefruit peel (albedo): 2686 mg/100 g Eriocitrin content in Lemon (flavedo):144 mg/100 g Eriocitrin content in Lemon (albedo): 197 mg/100 g |
El Kantar et al. (2018) |
| Enzyme assisted extraction | ||||
| Citrus paradisi peel |
Temperature: 60 °C Time:4.81 h pH: 4.8 Enzyme concentration: 0.9% |
Ethanol |
Total phenols: 3170.35 mg/GAE/100 g dry basis Total flavonoids: 329.89 mg QE/100 g dry basis |
Nishad et al. (2019) |
| Citrus Pomace | ||||
| Microwave assisted extraction | ||||
| Citrus reticulate L. (Mandarin) pomace |
Microwave Treatment (Power:250 W; Time: 5, 10 and 15 min) (Power: 500 W; Time: 5 min) |
Methanol-80% |
Total phenols 116–1317 μg/g dry basis Total flavonoids: 4838 to 5801 μg/g dry basis |
Hayat et al. (2010b) |
| Hot water extraction | ||||
| Citrus limon pomace |
Temperature: 95 °C Time:15 min Sample to solvent ratio: 1:100 g/ml |
Distilled water |
Total phenols:13.79mgGAE/g Total flavonoids: 4.78mgCE/g DPPH activity: 0.17 mg TE/g ABTS activity: 0.40 mg TE/g FRAP activity: 8.05 mg TE/g CUPRAC activity: 34.18 mg TE/g |
Papoutsis et al. (2016b) |
| Electron beam irradiation | ||||
| Citrus unshiu pomace | Scan speeds: 420, 200, 100, 50 and 33 mm/revolution | Ethanol-70%, methanol, acetone, or distilled water |
Total polyphenols:6543.2 to 7405.4Μm Radical scavenging activity: 37.6 to 52.9% Reducing power: 0.64 to 0.90 |
Kim et al. (2008) |
| Citrus wastewater | ||||
| Aqueous extraction | ||||
| Citrus unshiu pomace waste water |
Temperature: 25 °C Time:24 h |
Distilled water |
DPPH IC50: 0.16 ± 0.00 mg/mL Alkyl IC50: 0.31 ± 0.01 mg/mL Hydroxyl radicals IC50: 0.86 ± 0.02 mg/mL |
Wang et al. (2018) |
Microwave assisted extraction
It involves the use of electromagnetic waves of frequency ranging from 0.3 to 300 GHz. It is commonly utilized method for recovery of bioactive compounds from citrus waste. The major advantage of using microwave over the other conventional as well as non-conventional extraction methods is its low energy consumption, lower extraction time, reduced utilization of solvents (Anticona et al., 2020). Inoue et al. (2010) examined the phenolic compounds present in Citrus inshiu peel extracted through microwave-assisted extraction. They reported 5860 mg/100 g extract of hesperidin and 1310 mg/100 g extract of narirutin in the peel extract. Dahmoune et al. (2013) reported total phenol content of 1574 mg GAE/100 g in Citrus limon peel extracted through microwave-assisted extraction. Besides, using the same extraction technique, Nayak et al. (2015) reported the phenolic content of 12.20 mg GAE/g DW, antioxidant activity by ORAC (482.27 ± 57.43 μM TE/g), and DPPH (IC50, 337.162 ± 8.45 mL extract/L) in the Citrus sinensis peel.
Ultrasound assisted extraction
It is another method for the extraction of biologically active compounds especially polyphenols (Anticona et al., 2020). Extraction of phenols from mandarine and lime peels through low power ultrasound technique resulted in recovery of 2800 mg GAE/100 g of total phenols and 440 mg catequin equivalent/100 g flavonoids in lime (Singanusong et al., 2015). Nipornram et al. (2018) studied the mandarins (Citrus reticulate L.) peel for its phenolic content. The low power ultrasound-assisted extraction (UAE) method was utilized for extraction. The study reported the extraction yield, total phenol, and hesperidin content of 26.52%, 6435.53 mg/100 g, and 15,263.32mgEq gallic acid/100 g, individually. The study also reported UAE as a better method considering its higher efficiency (1.77times) of extraction of peel, phenolics, and hesperidin in contrast to microwave-assisted extraction.
High voltage electric discharge
A number of researchers used high voltage electric discharge technique for recovery of essential compounds. Buniowska et al. (2015) examined the bioactive compounds present in orange peel obtained through high voltage electric discharge. The parameters used for extraction were voltage of 40 kV with discharge per 10 s in addition to two energy inputs of 55 kJ/kg and 364 kJ/kg. The study reported an increase in the bio-accessibility of carotenoids (82.5%) with an increase in the energy input from 55 kJ/kg to 364 kJ/kg. However maximum bio-accessibility of total phenol (40.7%) was detected at 55 kJ/kg. Another study done by El Kantar et al. (2019) pretreated the grapefruit peel with high voltage electric discharge before the diffusion of extract phenolic compounds. The study reported that upon treatment with high voltage electric discharge and at 10 min of diffusion there was an increase in total phenols of grapefruit peel from 1330 mg/100 g to 1880 mg/100 g with an increase in the energy input from 0 to 218 kJ/kg, respectively.
Pulsed electric field extraction
It is another extraction method which is based on inducting the electroporation of the cell membrane leading to enhancement of yield of extraction. In this technique, an electric potential is passed through the cellular membrane for a short duration of time (1–2500 μs) leading to the separation of molecules based on their charge. The repulsion causes the formation of pores in this manner expanding their porousness (Azmir et al., 2013). Generally, a pulsed electric field is applied for preservation of foods and extraction of intracellular constituents from plant matrix, food waste, and byproducts. Luengo et al. (2013) checked the effect of pulsed electric field on pressing extraction of total phenols and flavonoid content from orange peel. The highest disintegration index was achieved at a treatment time of 60 μs (20pulses of 3 μs). The study reported an increment in the extraction yield of total phenols by 20%, 129%, 153%, and 159% for the treatment of orange peel with pulse electric field at 1, 3, 5, and 7 kV/cm, individually. In addition, compared to the untreated sample, the pulsed electric field sample showed increased naringin, hesperidin, and total antioxidant activity. Besides, El Kantar et al. (2018) also treated the citrus fruits with pulsed electric field and compared the polyphenol content of juice and peels obtained from lemon, orange, and grapefruit. Primarily, whole fruit was pre-treated with a pulsed electric field (3 kV/cm) to facilitate comparison between treated and non-treated samples. They revealed an increment in the flavonoid content of pre-treated samples of orange, grapefruit, and lemons. In addition, total phenol content of 2200 mg GAE/100 g was noticed in the orange peel treated with a pulsed electric field (10 kV/cm). In general, the improvement in the performance of total phenols and flavonoids pre-treated through pulse electric field is attributed to the electroporation phenomenon in contrast to untreated samples.
Supercritical fluid extraction
It is one of the green alternatives to conventional extraction methods. Supercritical fluid extraction syndicates fluids at higher temperatures and pressures with a value near their critical points (Diaz-Reinoso et al., 2006). A study done by Cheigh et al. (2012) revealed greater recovery of flavonoids (hesperidin and naritunin) from Citrus unshiu peels through supercritical water extraction. Additionally, the study reported the key impact of extraction temperature plus time on the recovery of flavonoids. Hesperidin content of 7200 mg/100 g and narirutin content of 11,700 mg/100 g were obtained at temperature (160 °C), time (10 min), and pressure (100ATM). Pertaining to the efficiency of extraction and reduced environmental risks, supercritical water was recognized as an excellent substitute to the organic solvents for non-polar flavanones extraction from citrus waste. Trabelsi et al. (2016) used the supercritical carbon dioxide technique to obtain essential components from peel of bitter variety of citrus (Citrus aurantium amara). They reported the extraction yield of 1.07% on treatment with ethanol as co-solvent (3%) at CO2 flow rate (2.87 kg/h), pressure (170 bar), and static time (53 min). Osthol was the chief extracted compound from the peels. Hexadecane, squalene, and esters of fatty acids were also identified in the citrus peel waste. Lachos-Perez et al. (2018) extracted flavanones from defatted orange peel using a supercritical water extraction technique. They reported an increased yield of extraction with increased temperature. Yet the interaction between flow rate and temperature showed an influence on extraction yields. An extract yield of 10.63% was reported at temperature (150 °C) and water flow rate (10 mL/min). At these conditions, narirutin (2.33 mg/g) and hesperidin (20 mg/g) were observed, and total phenol content (31.70 mg GAE/g) was obtained from the defatted peel.
Pressurized water extraction
Some researchers elucidated the use of pressurized water extraction technique to obtain biologically active constituents from the citrus waste. In a study done by Casquete et al. (2014) a high-pressure extraction of phenolics from lemon and orange peel was carried out. Pressure-treated citrus peel trials (300 MPa, 10 min; 500 MPa, 3 min) exhibited greater phenolic content and antioxidant action contrasted with the control samples. The total phenolic content of fresh peel extract of lemon at 300 MPa and 500 MPa was 265.95 mg GAE/100 g and 344.53 mg GAE/100 g, respectively while for orange was 364.57 and 378.90 mg GAE/100 g, respectively.
Meanwhile, several kinds of research are currently being conducted for the efficient polyphenols recovery utilizing synchronous extraction through a blend of diverse procedures for the improved yield alongside decreased time consumption and extensive usage of these novel strategies is suggested. Moreover, a study done by Safdar et al. (2017) stated that the polyphenol extraction relies upon the procedure utilized and the phenol extraction from Citrus reticulate L. peel could be increased four times through ultrasound extraction as compared to the maceration technique. Jagannath and Biradar (2019) conducted a relative analysis of soxhlet and ultrasound extraction techniques on the morphology and bioactive compounds present in Citrus limon L. peel and revealed that ultrasound extraction was proved to be superior in comparison to soxhlet for bioactive compounds extraction. Ultrasound treatment at temperature (33 °C), time (40 min), and 80% duty cycle exhibited the maximum total phenols (88.06 mg GAE/100 g). A high total flavonoid content of 13.92 mg CE/100 g were found at 40 °C, 50 min, and 60% and vitamin C content of 58.87 mg/100 g at 40 °C, 50 min, and 100% duty cycle. In general, the effectiveness of extraction varies depending on the phase and concentration of food matrix, solvent used and extraction process (Sridhar et al., 2021).
Source of essential oils
Essential oils are the hydrophobic aromatic liquid comprising of volatile components that are found in oil sacs of citrus peel and cuticles (El Asbahani et al., 2015). The oils extracted from citrus waste are of great potential due to their potent antioxidant, anti-microbial, and anti-inflammatory properties. They can be used as additives and preservatives in the food industry; also they have their role in the pharmacological and cosmetic sectors (Sharma et al., 2017). Owing to their appealing fragrance, they also have a role in the perfumery industry. Essential oil comprises of terpenes mainly monoterpenes (ex-limonene), sesquiterpenes together with their oxygenated compounds (ex-alcohols, aldehydes, ketones, and esters) (Espina et al., 2011).
Literature survey revealed that a number of researchers utilized different extraction techniques for improving the essential oils yield obtained from citrus waste (Table 4). For instance, in a study, microwave-assisted extraction was utilized for essential oil extraction from Citrus sinensis L. peel; the study reported an oil yield of 0.42% at 100 °C temperature and 30 min time. Limonene and oxygenated monoterpenes found in the oil were 76.7% and 7.0%, respectively (Ferhat et al., 2006). Solvent-free microwave extraction has likewise been used for the recovery of essential oils from Citrus limon peels. The results revealed the presence of limonene (1.26%) (Golmakani and Moayyedi, 2016).
Table 4.
Essential oils extraction from citrus waste using different extraction methods
| Citrus by-products | Extraction parameters | Solvent | Bioactive compound yield | References |
|---|---|---|---|---|
| Citrus peel | ||||
| Microwave assisted extraction | ||||
| Citrus sinensis L. Osbeck peel |
Temperature:100 °C Time: 30 min |
– |
Essential oil yield: 0.42% Oxygenated monoterpenes: 7.00% Limonene: 76.70% |
Ferhat et al. (2006) |
| Citrus sinensis L. Osbeck peel |
Power:200 W Time:12 min |
– |
Essential oil yield: 1.54% Monoterpene hydrocarbons: 98.23% Oxygenated monoterpenes: 0.43% Limonene: 94.88% |
Farhat et al. (2011) |
| Citrus limon peel |
Temperature: 100 °C Time: 15 min Power:1000 W |
– | Limonene—1.26% | Golmakani and Moayyedi (2016) |
| Citrus grandis peel |
Time: 90 min Power:150 W |
– | Limonene—33.7% | Chen et al. (2016) |
| Citrus spp. (Orange) peel |
Power:250 W Time: 5 min |
– |
Essential oil yield: 1.16% Limonene: 95.20% Valencene: 0.20% |
Gonzalez-Rivera et al. (2016) |
| Citrus sinensis (Orange and lemon) peel |
Power:1000 W Time:10 min |
– |
d-limonene yield: 3.7% in orange 2.0% in lemon |
Auta et al. (2018) |
| Super critical fluid extraction | ||||
| Citrus medica peel |
Pressure:100 bar Temperature: 40 °C Time: 6 h |
CO2 |
Oil yield: 28% Citropten: 84.5% |
Menichini et al. (2011) |
| Citrus aurantium peel |
Temperature: 90 °C Time: 120 min Pressure:170 bar Static time:50 min CO2 flow rate-:2.7 kg/h |
CO2 + Ethanol | Limonene—47% | Trabelsi et al. (2016) |
| Citrus unshiu peel |
Temperature: 15 °C Equilibrium Time: 15 min |
CO2 | Essential oil yield: 0.85% | Tsitsagi et al. (2018) |
| Citrus limon peel |
Pressure:15 MPa Temperature: 40 °C Time: 40 min |
CO2 | d-limonene yield: 4.5% | Lopresto et al.(2019) |
| Combinational method (ultrasonic/microwave/hydro-distillation technique) | ||||
| Citrus medica peel |
Time:15.5 min Power:816 W |
– | Limonene—0.55% | Zhang et al. (2019) |
| Citrus seeds | ||||
| Soxhlet extraction | ||||
| Acid lime and sweet orange |
Temperature: 70 °C Time: 6 h |
n-hexane-150 ml |
Oil yield Acid lime: 31.90% Sweet orange: 33.32% |
Garrido et al. (2019) |
| Citrus maxima (pummelo) seeds |
Temperature: 78.94 °C Time: 4.62 h Solvent/solid ratio:29/1 ml/g |
Acetone-89.68 ml | Limonoids yield: 11.52 mg/g | Qin et al. (2018) |
| Flash extraction technique | ||||
| Citrus sinensis seeds |
Temperature: 4 °C Time: 1 h Solvent/solid ratio: 29/1 ml/g |
Ethanol- 72% | Limonin yield: 6.8 mg/g | Liu et al. (2012) |
Supercritical fluid technique for essential oil extraction from Citrus medica peels has also been studied. The study reported 28% yield of essential oils and the presence of citropten (84.5%) at pressure (100 bar), temperature (40 °C), and time (6 h) (Menichini et al., 2011). Tsitsagi et al. (2018) used supercritical carbon dioxide for bioactive compound extraction and reported the essential oil yield obtained from Citrus unshiu peels using supercritical fluid extraction was 0.85%. Besides, Lopresto et al. (2019) in their study recovered D-limonene (4.5%) by the processing of Citrus limon peels through supercritical fluid extraction at pressure (15 MPa), temperature (40 °C), and time (40 min).
Furthermore, the process of extraction of essential oil from citrus waste leads to the separation of some percentage of oil into distilled water (D'Amato et al., 2018). During the distillation process, the polar essential oil vapors from citrus peel come in contact with boiling water or steam, leading to the formation of hydrogen bonds, thus the formation of a complex mix of essential oils and volatile components. These secondary compounds that are formed in the course of recovery of essential oil from the citrus peel are referred to as Hydrosols. Owing to the presence of dissolved hydrophilic compounds and smaller amounts of essential oils imparting appealing aroma and flavor, these hydrosols are widely used in aromatherapy and cosmetics (Inouye, 2008). Hydrosols have a pH ranging from 3.5 to 6.5, containing less than 1 g/L of essential oils contributing to its organoleptic properties. The chemical morphology of hydrosols obtained from citrus byproducts suggests the presence of oxygenated compounds. For instance, Linalool, linalool oxide, α-terpineol, transcarveol, geraniol, citronellol, nerol and neral constitutes the major chemical components of hydrosol of Citrus reticulata, Citrus sinensis, Citrus aurantifolia and Citrus maxima peels (Ndiaye et al., 2017). Hydrosols have their role as an antioxidant, anti-microbial, and antifungal agent. For example, orange blossom hydrosol showed anti-microbial effects against Escherichia coli as well as Listeria monocytogens at minimum inhibitory concentration of 200 mg/L (Ait-Ouazzou et al., 2011). Similarly, the hydrosol obtained from steam distillation of Citrus aurantium exhibited potent anti-microbial and anti-oxidant properties (Degirmenci and Erkurt, 2020). Apart from this, Citrus hydrosols also exhibit effective enzyme inhibition properties, for example, a study done by Lante and Tinello (2015) evaluated the anti-tyrosinase activity of citrus hydrosols through the spectrophotometric method; along with the terpenes content through gas chromatography. They reported the impact of citrus hydrosols on mushroom tyrosinase at varying levels of 21.8 to 68.9%. Gas chromatography revealed the presence of tyrosinase inhibitors such as citral, myrcene, geraniol, and sabinene in the citrus waste hydrosol.
In addition, these citrus hydrosols are used in the food industry owing to their zero side effects on the human body. Indeed, a lot has to be done in this field to check the mechanism of action, toxic levels, and in vitro action of hydrosols.
Source of pectin
Pectin is a complex structural polysaccharide found in the cell walls of higher plants. The main component of pectin involves D-galacturonic acid which is a sugar acid obtained from galactose. Pectin has its role as a thickener, emulsifier, stabilizer, fat replacer, and gelling agent in dairy products, jam, jellies, and fruit juices. Commercially, pectin is usually obtained from apple and citrus processing waste, still the yield of citrus pectin is higher (20–30%) in contrast to the pectin obtained from apple by-products (10–15%) of whole dry weight (Kulkarni et al., 2002). Owing to good yield, citrus peel-based pectin have diverse food applications.
Literature survey revealed that many researchers utilized green extraction procedures for pectin extraction from the citrus wastes (Table 5). For instance, pectin extraction from grapefruit peel was carried out by using microwave-assisted extraction; the study reported a pectin yield of 27.81% (Bagherian et al., 2011). Another study revealed a 23.83% yield of pectin at power (660 W) and time (9 min) through microwave-assisted extraction from Citrus medica peels (Quoc et al., 2015). Besides, Liu et al. (2017) reported 29.16% pectin yield from Citrus maxima peels through Bronsted acidic ionic liquid-based microwave extraction. Su et al. (2019) utilized surfactant-based microwave extraction method pectin recovery from Citrus sinensis peels. The study reported a pectin yield of 28% at power (400 W), pH (1.2), and time (7 min).
Table 5.
Pectin extraction from citrus waste using different extraction methods
| Citrus by-products | Extraction parameters | Solvent | Pectin yield (%) | References |
|---|---|---|---|---|
| Citrus peel | ||||
| Microwave assisted extraction | ||||
| Citrus spp. (Grapefruit) peel |
Power: 900 W Time: 06 min |
Ethanol | 27.81 | Bagherian et al. (2011) |
| Citrus reticulate peel |
Power: 422 W Time: 169 s pH: 1.4 |
Distilled water | 19.19 | Maran et al. (2013) |
| Citrus sinensis L. Osbeck peel |
Power: 500 W Time: 03 min |
Distilled water | 24.20 | Boukroufa et al. (2015) |
| Citrus medica peel |
Time: 9 min pH: 1.5 Power: 660 W |
Tartaric acid solvent | 23.83 | Quoc et al. (2015) |
| Citrus aurantium L. peel |
Power: 700 W Time:3 min pH:1.5 |
Acidified distilled water | 29.10 | Hosseini et al. (2016) |
| Citrus maxima peel |
Temperature: 100 °C Time: 15 min pH:2.5 Power:331 W |
HO3S(CH2)4mim]HSO4 Ethanol |
29.16 | Liu et al. (2017) |
| Citrus sinensis peel |
Time: 7 min pH:1.2 Power:400 W |
Acidic solution | 28.00 | Su et al. (2019) |
| Ultrasound-assisted extraction | ||||
| Citrus aurantium peel |
Temperature: ≤ 30 °C Time: 10 min pH:1.5 Power:150 W |
Acidified distilled water | 28.07 | Hosseini et al. (2019) |
| Enzyme-assisted extraction | ||||
| Citrus aurantifolia peel |
Temperature:50 °C Time: 30 min pH:4.5 Pressure:100 Mpa Enzymes used: Cellulase, Xylanase |
Milli-Q water | 26.50 | Naghshineh et al. (2013) |
Moreover, ultrasound-assisted extraction was utilized for pectin extraction from Citrus aurantium peels. The study reported a 28.07% yield of pectin at power (150 W), temperature (≤ 30 °C), and time (10 min) (Hosseini et al., 2019). Besides, Naghshineh et al. (2013) in their study recovered pectin (26.50%) by the processing of Citrus aurantifolia peels through enzyme based extraction using cellulase and xylanase enzymes. Wang et al. (2014) reported the pectin yield of 21.95% extracted from the citrus peel using the supercritical water extraction method. Certainly, the use of green extraction methods for extraction of pectin is helpful in relation to good yield, energy, and time-saving process.
Source of dietary fibre
As per the American Association of Cereal Chemists (AACC) the dietary fibre is the remnants of the edible portion of the plant or analogous carbohydrate which is resistant to the process of digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. These fibres comprise of polysaccharides, oligosaccharides, cellulose, lignin, and related plant substances (AACC, 2001). Fibre-rich diet including fruits, vegetables, and cereals positively affects health and diminishes the rate of different diseases. They decrease the transit span of food in the intestine, reduces cholesterol, and glycemic level, increase the volume of fecal mass, traps the mutagenic as well as carcinogenic agents, etc. (Dhingra et al., 2012).
Citrus fruits contain good amounts of soluble (for example- pectin, gum, glucan, and other biological /synthetic polysaccharides) and insoluble dietary fibers (cellulose, hemicellulose, and lignin) (Yang et al., 2017). The fiber concentrates obtained through dehydration of fruits and vegetables contain good amounts of total dietary fibre content ranging from 25 to 60 g/100 g dry matter as well as improved soluble to insoluble dietary fibre ratio as compared to major cereal crops (Garau et al., 2007). In addition, the citrus waste comprises of dietary fibers having higher soluble fraction, good nutritional and bioactive components like flavonoids and carotenoids (Panwar et al., 2021). A study done by Figuerola et al. (2005) analyzed the dietary fibre content of citrus peel through enzymatic gravimetric method and reported a total dietary fibre content of 64.3%, 44.2–62.6%, and 60.1–68.3% in peels of orange, grapefruit, and limon, respectively. Besides, the inclusion of water-insoluble fibre obtained from Citrus sinensis peels at a level of 50 g/kg in the diet showed significant improvement in the intestinal function by increasing the activity of certain enzymes viz. serum alkaline phosphatase, intestinal maltase, and sucrose and reducing the activity of fecal β-D-glucosidase, β-D-glucuronidase and urease (Chau et al., 2005).
Citrus peels were utilized for extraction of dietary fibers using a number of different techniques including ultrasound, enzymes, acid/alkali treatment, dry/wet processing, and combinations (Yang et al., 2017). Supercritical water extraction via semi continuous flow extractor (at 160–320 °C temperature) was employed for dietary fibre extraction from Citrus junos (yuzu) peel waste (Tanaka et al., 2012). Besides, Wang et al., (2015) utilized combination of steam explosion plus dilute acid soaking technique for efficient dietary fibre extraction from Citrus sinensis peels. They reported an improved yield of soluble dietary fibre fraction (33.74%) and its improved use as a functional ingredient. A more recent study done by Karaman et al. (2017) utilized an ultrasound-assisted extraction technique for dietary fibre extraction from press meal of citrus (Citrus limon, Citrus paradisi and Citrus sinensis) seeds. They reported soluble dietary fibre content ranging from 4.59 to 7.95% and insoluble dietary fibre content ranging from 75.95–82.24%.
Source of carotenoids
Carotenoids are fat-soluble isoprenoid pigments that are commonly found in plants, vegetables, fungi, and flowers (Otles and Cagindi, 2007). The beneficial impacts of carotenoids are believed to be because of their antioxidant content. β-carotene, lutein, zeaxanthin, and lycopene are the extensively studied carotenoids owing to their various health benefits through the prevention of diseases (Krinsky and Johnson, 2005). Carotenoids such as xanthophylls (lutein and violaxanthin) and hydrocarbon carotenoids (β-carotene and lycopene) are the key carotenoids found in the citrus waste (Saini and Keum, 2018). These classes of carotenoids present in citrus waste add to their splendid and bright color. They impart yellow, orange, and red colors to the citrus fruits. Carotenoids are well known for their health-promoting effects owing to their anti-oxidative properties. They are also efficiently used in the food and beverage industry for enhancing their market value.
With the passing times, deliberate actions have been undertaken for the advancement of extraction strategies for carotenoids. However, the recovery from complex food network stays low, since the different physical and chemical boundaries present in the food framework prevent the mass exchange of carotenoids during extraction. Moreover, the factors such as diverse polarity of carotenoids and oxidation properties restrict exposure to excess heat, light, acids, and longer extraction times. Additionally, due to its hydrophobicity, organic solvents are used for its extraction (Saini and Keum, 2018). A number of green extraction methods viz; ultrasound, microwave, solvent, and pulse electric field have been proposed for efficient extraction of carotenoids from citrus waste. Sun et al. (2011) compared the conventional extraction method with ultrasound extraction of carotenoids from citrus peels. They reported enhanced extraction of β-carotene by using ethanol solvent with ultrasound extraction in contrast to conventional ones. Besides, Boukroufa et al. (2017) in their study recovered carotenoids through an ultrasound-assisted extraction of Citrus sinensis peels by using D-limonene as a solvent which was prior recovered from peels by solvent-free microwave extraction technology. They reported a high carotenoid yield (11.25 mg/L) at 208 W/cm2 power, 20 °C temperature and 5 min time. Murador et al. (2019) extracted carotenoids from orange peels by ultrasound-assisted extraction using ionic liquids as solvents. The study reported improved extract yield through ultrasound extraction for 5 min in contrast to the conventional method and acetone solvent. Also, β-carotene yield was reported to be 32.08 μg/g of total carotenoids through the use of chlorinated 1-Butyl-3-methylimidazolium as compared to the extraction yield of 7.88 μg/g of total carotenoids using acetone as solvent. Montero-Calderon et al. (2019) used the ultrasound extraction method for total carotenoid extraction from Citrus sinensis peels cultivated in Spain. The study reported a carotenoid yield of 0.63 mg/100 g peels by using 50% ethanol as a solvent for 30 min extraction time. Saini et al. (2020) utilized response surface methodology to optimize the process parameters (temperature, time, and amplitude) of ultrasound extraction of lutein from Citrus reticulata (Kinnow peels). They reported lutein yield of 2.97 mg/100 g which was similar to the predicted values (2.85 mg/100 g). Ndayishimiye and Chun (2017) utilized supercritical carbon dioxide technique for carotenoids extraction from peels as well as seeds of Citrus ichangensis × Citrus reticulate (papeda). The results revealed the carotenoid yield of 1.983 mg/g at optimum pressure (25.196 MPa) and temperature (44.88 °C).
Strategies for valorization of citrus seeds
Source of limonoids
Limonoids are the phytochemicals belonging to the class of extremely oxygenated and modified triterpenes that are biosynthesized through the acetate-mevalonate pathway in sweet or sour citrus fruits. Limonoids are present in prominent quantities in seeds and fruits of citrus fruits (Manners and Breksa, 2004). Two major classes of limonoids are seen in citrus fruits; namely limonoid aglycones (limonin and nomilin) and limonoid glucosides (limonin glucoside and nomilic acid glucoside). Citrus fruits consist of almost 36 limonoid aglycones and 17 limonoid glucosides (Hasegawa and Miyake, 1996). Limonin contributes to the bitter flavor to the citrus seeds. Citrus seeds are the only natural source of limonoid aglycones (Vikram et al., 2007). These limonoids have several health-promoting factors. Numerous studies reported health protective effects of limonoids for example in preventing the growth of human neuroblastoma and colonic adenocarcinoma (Poulose et al., 2005); suppressing the azoxymethane-induced colon cancer in rats (Vanamala et al., 2006); and in inhibition of human breast cancer (Tian et al., 2001). With the recent advances in research different bioactive properties of limonoids such as antioxidative, anti-inflammatory, anti-neurological, anti-bacterial, anti-viral, immunomodulatory, anti-viral, and anti-tumor effects has been discovered (Shi et al., 2020).
Literature survey revealed that several researchers quantified the limonoids present in the different classes of citrus fruits using chromatographic and spectroscopic methods. For instance, a study done by Vikram et al. (2007) quantified the limonoid aglycones and limonoid glucosides present in the citrus seeds using high-performance liquid chromatography (HPLC). The study reported the detection of seven limonoids namely limonin, limonin 17-β-d glucopyranoside, isolimonic acid, nomilin, ichangin, isoobacunoic acid, and deacetyl nomilinic acid 17-β-d glucopyranoside at 210 nm. Besides, Limonin and limonin glucoside were observed to be the principal limonoids found in citrus seeds. Likewise, Breksa III et al. (2011) analyzed the limonoid content of sour orange (Citrus aurantium) and reported the total limonoid aglycone concentration of 2.4 to 18.4 mg/L and total limonoid glucoside concentration of 149.0 to 612.3 mg/L. Besides, Russo et al. (2016) analyzed the limonoid content of Citrus bergamia Risso (Bergamot) fruit and reported high limonoid aglycone content in the seeds and peel. Furthermore, Qin et al. (2018) extracted limonoids from Citrus maxima (pummelo) seeds and reported the limonoids yield of 11.52 mg/g on treatment with acetone (89.68 mL) at temperature (78.94 °C), and time (4.62 h).
Several researchers also utilized modern extraction techniques for the recovery of limonoids from citrus seeds. Flash extraction technique was utilized for extraction of limonoids from Citrus sinensis seeds and the study results revealed a yield of 6.8 mg/g (Liu et al., 2012). Supercritical fluid extraction and hydrophobic extraction are some other methods for limonoids extraction. These methods help to save larger amounts of organic solvents thereby saving the environment but their higher cost limits the practical usage. Hydrotropic technique involves the use of hydrotropes which enhances the limonin solubility in water but it leaves behind a large amount of alkali-metal salts. In addition, solid–liquid extraction is also used for limonoid extraction. HPLC, column chromatography, and flash chromatography are some methods widely utilized for the isolation and purification of limonoids (Mahato et al., 2020).
Source of essential oils
Citrus fruit seeds are viewed as a potential source of oil because of their good fatty acid content and significant tocopherol content. Owing to the presence of high protein, minerals, and fiber content it may perhaps be utilized in food applications for the development of value-added items (Mahawar et al., 2020). A study done by Anwar et al. (2008) revealed that the citrus seeds contain oil (31.15%), protein (9.56%), fiber (6.50%), and ash (5.60%). Further investigation by same authors reported that the citrus seeds oil exhibit iodine value of 104.80 g Iodine/100 g of seed oil, density (25 °C) of 0.927 mg/mL, acid value of 1.30 mg KOH/g of seed oil, refractive index (40 °C) of 1.465, saponification value of 186 mg KOH/g of seed oil, unsaponifiable matter of 0.48% of seed oil, and color red units (2.50) plus yellow units (20.00). Besides the seed oil also exhibited remarkable oxidative stability checked through experiments at 232 and 270 nm (2.64 and 0.81), peroxide value (2.40 mequiv/kg of oil) and p-anisidine value (3.15). Another examination done by Garrido et al. (2019) studied the influence of Soxhlet and direct or indirect ultrasound-assisted extraction techniques on the oil yield, fatty acid composition, total phenols, as well as antioxidant activity of acid lime and sweet orange seed oil. They reported high oil yield through soxhlet extraction (acid lime = 31.90% and sweet orange = 33.32%). Nonetheless, the total phenol content in lime seed oil extracted through ultrasound-assisted extraction was higher. Citrus seed oil contains decent measure of unsaturated fatty acids such as linoleic acid (36.69%) (Table 4).
Strategies for valorization of citrus pomace
Source of polyphenols and antioxidants
In an examination of phenolic compounds among juice and pomace of mandarin, orange, lemon, and bergamot, the pomace contains 3-to fourfold greater phenolic compounds than the juices (Multari et al., 2020). Polyphenol extraction from citrus pomace has been broadly considered, incorporating with ongoing advancements utilizing "green" extraction methods (Table 3). Additionally, with the advancement in extraction methods, the emphasis has now been moved towards the optimization of extraction strategies to upgrade the polyphenol yield. For instance, the influence of various process parameters viz, extraction time, temperature, and sample-water ratio on the total phenols, flavonoids plus antioxidant activity of the lemon pomace was investigated using Box-Behnken design of response surface methodology. The optimized results (total phenols—13.79 mg GAE/g, total flavonoids—4.78 mg CE/g, DPPH activity—0.17 mg TE/g, ABTS—0.40 mg TE/g, FRAP—8.05 mg TE/g, and CUPRAC—34.18 mg TE/g) were obtained at extraction 95 °C temperature, 15 min time and 1:100 g/mL sample to solvent ratio. However the sample to solvent ratio was the only factor which showed influence on the phenols, flavonoids and antioxidant activity of the lemon pomace (Papoutsis et al., 2016b). Another study done by Kim et al. (2008) examined the influence of electron beam irradiation on the total phenols, reducing power and radical scavenging activity of citrus pomace. The study reported an increase in the polyphenol content from 6543.2 to 7405.4 Μm, radical scavenging from 37.6 to 52.9% and reducing power from 0.64 to 0.90 upon irradiation. Likewise, Hayat et al. (2010b) utilized the microwave technique for drawing out of phenolic compounds and antioxidants from the citrus mandarin pomace. The study reported a rise in the free phenols and improvement of antioxidant capacity of the citrus pomace extract upon microwave treatment. Another study focused on checking the influence of infrared drying and/or convective drying technique on the drying behavior of wine grape pomace as well as on the polyphenols and pro-anthocyanidins content. The study results showed that infrared drying have a high drying rate and led to least destruction to the polyphenols and pro-anthocyanidins (Sui et al., 2014).
Furthermore, a similar study investigated the effects of UV-C irradiation on the bioactive properties of aqueous extract of lemon pomace and reported enhancement in the bioactive component by application of UV-C irradiation at high doses (Papoutsis et al., 2016c). Likewise, polyphenol extraction from lemon pomace was done through different extraction methods viz; freeze drying, hot air-drying and vacuum drying at temperatures—70 °C, 90 °C and 110 °C, respectively. The results showed that high total phenols and antioxidant activity in the hot air or vacuum dried lemon pomace sample as compared to the freeze dried sample (Papoutsis et al., 2017). Notwithstanding, substantially more advancement is needed for investigation of the citrus pomace for its bioactive components. Additionally, further research in the field of effective valorization of citrus pomace for useful value added products is required.
Strategies for valorization of citrus waste water
Citrus wastewater is generally obtained from washing of fruit, cleaning of processing equipment, and during essential oil extraction and peel drying. This waste water also includes the effluents obtained through process of production of citric acid, pectin, citric molasses and peel oil (Sharma et al., 2017). Many researchers utilized citrus waste water for recovery of essential bioactive components (Table 3). Viuda‐Martos et al. (2011) studied the characterization of physiochemical and microbiological attributes of citrus waste water left after orange juice processing. They reported that citrus waste water contains good amounts of phenolic compounds namely narirutin and hesperidin. In addition it was revealed that citrus waste water leads to reduction in the residual nitrate levels thereby reducing chances of formation of nitrosamine and exhibiting eminent properties in reducing free radical formation. A recent study investigated the antioxidant potential of citrus waste water and reported that it exhibits great potential in scavenging 1, 1-diphenyl-2-picrylhydrazyl (IC50 0.16 ± 0.00), alkyl (IC50 0.31 ± 0.01), and hydroxyl radicals (IC50 0.86 ± 0.02 mg/ml). Moreover, the pomace wastewater enhanced the cell viability and play role in scavenging intracellular reactive oxygen species in AAPH-stimulated Vero cells in a dose-dependent manner (Wang et al., 2018).
Use of citrus waste for non-food items: biofuels-bioethanol, biodiesel, and biogas
Various non-food items that can be generated through bio-processing of citrus waste are listed below. Citrus waste can be used as a natural resource for providing various important products, for example, biofuel, bioethanol, biogas, bio-oil, natural acids, enzymes, etc. Different biological processes such as fermentation and anaerobic digestion are utilized for bio-transformation of citrus waste into biofuel. Henceforth, Citrus by products would generate an inexpensive and natural source of fuel that will be environment friendly.
Bio-fuel
Bio-fuels are the liquid as well as gaseous fuels that are derived from agro-waste or biomass. Bio-fuels are renewable source of fuel which could be produced in liquid forms such as bioethanol, biodiesel, methanol, Fischer–Tropsch diesel and gaseous forms for example methane as well as hydrogen (Demirbas, 2008). These renewable sources of energy have gained importance because of their biodegradable nature and reduced damage to environment. Studies have reported significance of anaerobic digestion plus fermentation for bio-fuel production by utilizing citrus peel waste. The abundance of carbohydrate along with low lignin content makes the citrus peel waste favorable substrate for the production of bio-fuel (Satari and Karimi, 2018). However the presence of fundamental oil components i.e. D-limonene in citrus waste is one significant restraining factor during biofuel production; hence, appropriate pretreatments are mandatory for the removal of oils, hence certain studies examined the effect of pretreatment in reducing the inhibiting action of D-limonene. Further, technologically advanced strategies including the use of bioreactors and fermentation process have been explored for acquiring greater productivity of biofuel from citrus waste.
Bioethanol
Bioethanol is a renewable source of bio-fuel. Combustion of bioethanol generates high thermal efficiency and power. This might be due to the greater heat of vaporization and higher octane numbers of bioethanol (Demirbas, 2008). Ethanol is widely utilized liquid fuel for transport purposes (Demirbas, 2005), also it could be utilized in enhancement of octane content and to supplant lead as an anti-knocking agent (Zema et al., 2018). Additionally, it is used as a replacer of gasoline. The significance of ethanol is expanding because of various reasons, for example, global warming and environmental change. Bioethanol has been getting far reaching interest at the global, national and state levels. The worldwide market for bioethanol has entered a period of rapid development. Numerous nations all throughout the planet are moving their focus toward renewable sources for power generation in view of draining crude oil reserves.
Grohmann and Baldwin firstly conducted the enzymatic hydrolysis of citrus waste for sugars which is later fermented for production of bioethanol (Grohmann and Baldwin, 1992), thereafter citrus waste including peels of lemon, mandarin, orange, and grapefruit have been widely considered for bioethanol production (Boluda-Aguilar and Lopez-Gomez, 2013; Wilkins et al., 2007; Talebnia et al., 2008). It is vital that presence of essential oils in citrus waste is one significant limiting component in bioethanol production; consequently, appropriate pre-treatments are needed for the elimination of citrus oils. Both laboratory and pilot studies on the ethanol production from orange peel waste is being explored (Pourbafrani et al., 2010; Zhou et al., 2008).
The process of bioethanol production chiefly includes three major steps viz. pretreatment, hydrolysis and fermentation. Pretreatment involves better discharge of fermentable polysaccharides, though in hydrolysis or saccharification process, complex polysaccharides are hydrolyzed into simple sugars. The last step of bioethanol production encompasses the fermentation process leading to production of ethanol from simple sugars (Zema et al., 2018).
Citrus waste is continuously considered for bioethanol production. Some of the recent researches employed different pretreatment steps such as, steam explosion, auto hydrolysis, dilute-acid and grinding, prior to enzyme/acid based hydrolysis and fermentation. For example, Boluda-Aguilar et al. (2010) examined the mandarin peel waste for ethanol production through pretreatment with steam explosion, enzymatic hydrolysis and fermentation with Saccharomyces cerevisiae. They stated ethanol content of 50–60 L/1000 kg mandarin peel waste. Oberoi et al. (2011) reported ethanol content of 42 g/L obtained through hydrothermal pretreatment of Kinnow (Citrus reticulata) by-product through synchronized saccharification plus fermentation. Awan et al. (2013) reported ethanol content of 0.8 g/g and yield of 4.7 from orange peel through separate hydrolysis and fermentation. Choi et al. (2013) utilized popping as pretreatment step for production of ethanol from mandarin peel following separate hydrolysis and fermentation. They reported an ethanol yield of 90.6% and productivity of 3.85 g/Lh. Santi et al. (2014) converted orange peel waste into bioethanol through simultaneous pretreatment with acid catalyzed steam-explosion and separate enzymatic hydrolysis plus fermentation with Saccharomyces cerevisiae F15. The study reported an ethanol yield of 0.495 g/g as well as productivity of 4.85 g/Lh. Joshi et al. (2015) utilized the orange peel waste as substrate for ethanol production through steam explosion pretreatment and separate hydrolysis and fermentation with Saccharomyces cerevisiae NCIM 3495 2877. They reported an ethanol content of 0.25 g/g. Lately, John et al. (2020) utilized musambi (Citrus limetta) peels as a substrate for bioethanol production through using acid catalyzed steam pretreatment followed by enzymatic hydrolysis and fermentation and reported ethanol yield of 64% obtained at pH 4 after 48 h treatment.
Bio-diesel
It is a non-toxic, biodegradable class of fuel acquired from inexhaustible sources. It is a favorable replacement for petrol and diesel. Biodiesel is a non-alkyl ester of long chain fats that adds to negligible amounts of net greenhouse gases, like CO2 and NO2 (Bouaid et al., 2007). Usually, plant based oils such as cottonseed oil, peanut oil, soybean oil, sunflower oil, coconut oil, and palm kernel oil as well as animal fats were used for production of biodiesel (Liang et al., 2010; Watanabe et al., 2002; Crabbe et al., 2001; Al-Widyan and Al-Shyoukh, 2002; De los Rios et al., 2011). Conversely, most of these vegetable oils used for biodiesel production are edible oils and are expensive therefore large scale production of biodiesel might not be beneficial (Yusuf et al., 2011). Biodiesel can likewise be acquired from citrus seeds and peel waste through trans-esterification of essential oil with alcohol (Taghizadeh-Alisaraei et al., 2017). Rashid et al. (2013) utilized mandarin (Citrus reticulate) seeds for production of biodiesel through methanol based alkali catalyzed trans-esterification process and reported Citrus reticulate seed oil as a potent non-food feedstock for production of biodiesel. Seed oil obtained from Citrus limetta seeds has additionally been utilized for production of biodiesel through trans-esterification at 60 °C temperature for 120 min at 6:1 oil to methanol molar ratio (Musthafa, 2016).
Likewise, biodiesel was synthesized from the oil extracted from Citrus sinensis seeds through methanoxide catalyzed trans-esterification process at 60 °C temperature for 60 min. The study reported a high quality biodiesel yield of 76.93% meeting the international standards for biodiesel (Ezekoye et al., 2019). Indeed, further investigations with respect to the production of biodiesel from agro-waste and conduct of biodiesel for its commercialization is to be explored.
Bio gas
Biogas is mainly grouping of methane and carbon dioxide obtained from anaerobic breakdown of organic compounds (Zema et al., 2018). It also contains fewer quantities of nitrogen, oxygen, hydrogen and hydrogen sulphite. Naturally, biogas is produced in paddy fields, rumen of ruminants, swamps and trash landfills, where oxygen usually depletes the nearby environment. Biogas could be produced through utilizing the agro-waste such as citrus waste which has high mineral content beneficial for boosting methane yield (Bozym et al., 2015). In addition, citrus waste is enriched with minerals like zinc, magnesium, iron, cobalt and nickel beneficial for functioning of methanogenic microorganism (Martin et al., 2010). An energy examination revealed that in a citrus processing plant dealing with 600 ton/day of citrus fruits yields 2.1m3 of methane at standard temperature- pressure/m3, the generated biogas is sufficient to meet the demand of power and fuel, the abundance power produced from biogas could likewise be sold (Koppar and Pullammanappallil, 2013). Certain factors such as temperature, pH, and substrate availability affect the methane yield from citrus waste. On the other hand, the presence of essential oil in citrus waste is a major challenge during anaerobic digestion. Ruiz and Flotats (2016) reported the inhibitory action of citrus essential oil component-limonene beyond the concentration of 200 mg/kg. Calabro et al. (2016) examined the effect of citrus peel based essential oil concentration on the methane yield. The study reported methane yield of 370 N mL methane per grams of volatile solids in mesophilic and 300 N mL methane/g of volatile solids in thermophilic environment. In addition, the biodegradation of D-limonene to p-cymene was also studied by the same authors. Consequently, some researchers studied the effect of steam explosion pretreatment in reducing the inhibiting action of D-limonene. A study reported 426% increment in methane production in an experiment involving steam explosion of substrate as pretreatment step at 150 °C temperature for 20 min as compared to non-treated one. Also, under ideal conditions, citrus waste weighing 100 kg was observed to produce ethanol (40 L), methane (45 m3), pectin (39 kg) and limonene (9 L) (Forgacs, 2012). Wikandari et al. (2014) utilized hydrophilic poly-vinylidine difluoride (PVDF) membrane based bioreactor for inhibition of d-limonene during methane production from citrus waste in thermophilic conditions. The study exhibited higher methane production of 0.33 Nm3/kg volatile solids in membrane bioreactor while a lower methane production of 0.05 Nm3/kg in traditional free cell reactor. Therefore, it is observed that the limonene removal before the digestion process lead to higher yields of methane from citrus waste.
In conclusion, this review summarizes the recent work done on the citrus fruit waste valorization for recovery of value-added compounds leading to zero wastage. The presence of plentiful amounts of chemical constituents makes the citrus waste the preeminent resource for value-added compounds which could be obtained by using different approaches for application in food, pharma, and cosmetic industries. Also in context to the bio-economy, citrus fruit waste could be beneficial for non-food applications such as for production of biofuel, bioethanol, biogas, biodiesel, and other commodities. Several green extraction technologies for the recovery of polyphenols, pectin, essential oils, dietary fiber, carotenoids, and limonoids from citrus processing waste have been developed. Numerous studies reported improved extract yield from citrus waste through use of green extraction methods like microwave, ultrasound, high pressure, supercritical fluid, and pulsed electric field-based extraction in contrast to the conventional methods. However, with the advanced research towards the effective valorization of citrus waste, an industrial-scale use of all such technologies has not been achieved so far. Therefore, industrially centered research is to be carried out for better valorization for economic growth as well as environmental sustainability.
Acknowledgements
The authors are grateful to the Ministry of Food Processing Industries (MOFPI), New Delhi, India for granting the financial support (FN Q-11/08/2021- R&D) and National Institute of Food Technology Entrepreneurship and Management, Sonipat, Haryana, India for the institutional facility.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shweta Suri, Email: shwetasuri94@gmail.com.
Anupama Singh, Email: asingh3niftem@gmail.com.
Prabhat K. Nema, Email: pknema@yahoo.co.in
References
- AACC American Association of Cereal Chemists. Dietary Fiber Technical Committee. The definition of dietary fiber. Cereal Foods World. 2001;46:112–126. [Google Scholar]
- Adil M, Kandhare AD, Ghosh P, Venkata S, Raygude KS, Bodhankar SL. Ameliorative effect of naringin in acetaminophen-induced hepatic and renal toxicity in laboratory rats: role of FXR and KIM-1. Renal Failure. 2016;38:1007–1020. doi: 10.3109/0886022X.2016.1163998. [DOI] [PubMed] [Google Scholar]
- Ait-Ouazzou A, Cherrat L, Espina L, Loran S, Rota C, Pagan R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innovative Food Science & Emerging Technologies. 2011;12:320–329. [Google Scholar]
- Akindehin S, Jung YS, Kim SN, Son YH, Lee I, Seong JK, Jeong HW, Lee YH. Myricetin exerts anti-obesity effects through upregulation of SIRT3 in adipose tissue. Nutrients. 2018;10:1962. doi: 10.3390/nu10121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpata MI, Akubor PI. Chemical composition and selected functional properties of sweet orange (Citrus sinensis) seed flour. Plant Foods for Human Nutrition. 1999;54:353–362. doi: 10.1023/a:1008153228280. [DOI] [PubMed] [Google Scholar]
- Al-Widyan MI, Al-Shyoukh AO. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresource Technology. 2002;85:253–256. doi: 10.1016/s0960-8524(02)00135-9. [DOI] [PubMed] [Google Scholar]
- Anticona M, Blesa J, Frigola A, Esteve MJ. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods. 2020;9:811. doi: 10.3390/foods9060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar F, Naseer R, Bhanger MI, Ashraf S, Talpur FN, Aladedunye FA. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. Journal of the American Oil Chemists' Society. 2008;85:321–330. [Google Scholar]
- Auta M, Musa U, Tsado DG, Faruq AA, Isah AG, Raji S, Nwanisobi C. Optimization of citrus peels d-limonene extraction using solvent-free microwave green technology. Chemical Engineering Communications. 2018;205:789–796. [Google Scholar]
- Awan AT, Tsukamoto J, Tasic L. Orange waste as a biomass for 2G-ethanol production using low cost enzymes and co-culture fermentation. RSC Advances. 2013;3:25071–25078. [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering. 2013;117:426–436. [Google Scholar]
- Bagherian H, Ashtiani FZ, Fouladitajar A, Mohtashamy M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical Engineering and Processing: Process Intensification. 2011;50:1237–1243. [Google Scholar]
- Balasundram N, Sundram K, Samman S. Phenolic Compounds in Plants and Agri-industrial By-products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chemistry. 2006;99:191–203. [Google Scholar]
- Banerjee S, Ranganathan V, Patti A, Arora A. Valorisation of pineapple wastes for food and therapeutic applications. Trends in Food Science & Technology. 2018;82:60–70. [Google Scholar]
- Barreca D, Mandalari G, Calderaro A, Smeriglio A, Trombetta D, Felice MR, Gattuso G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants (basel, Switzerland). 2020;9:288. doi: 10.3390/plants9030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluda-Aguilar M, Lopez-Gomez A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Industrial Crops and Products. 2013;41:188–197. [Google Scholar]
- Boluda-Aguilar M, Garcia-Vidal L, del Pilar Gonzalez-Castaneda F, Lopez-Gomez A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresource Technology. 2010;101:3506–3513. doi: 10.1016/j.biortech.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Bouaid A, Martinez M, Aracil J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel. 2007;86:2596–2602. [Google Scholar]
- Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrasonics Sonochemistry. 2015;24:72–79. doi: 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Boukroufa M, Boutekedjiret C, Chemat F. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resource-Efficient Technologies. 2017;3:252–262. [Google Scholar]
- Bozym M, Florczak I, Zdanowska P, Wojdalski J, Klimkiewicz M. An analysis of metal concentrations in food wastes for biogas production. Renewable Energy. 2015;77:467–472. [Google Scholar]
- Breksa AP, III, Kahn T, Zukas AA, Hidalgo MB, Yuen ML. Limonoid content of sour orange varieties. Journal of the Science of Food and Agriculture. 2011;91:1789–1794. doi: 10.1002/jsfa.4383. [DOI] [PubMed] [Google Scholar]
- Buniowska M, Carbonell-Capella J, Zulueta A, Frigola A, Esteve MJ. Bioaccessibility of bioactive compounds and antioxidant capacity from orange peel after pulsed electric fields and high voltage electrical discharges. MOJ Food Processing Technology. 2015;1:77–83. [Google Scholar]
- Calabro PS, Pontoni L, Porqueddu I, Greco R, Pirozzi F, Malpei F. Effect of the concentration of essential oil on orange peel waste biomethanization: Preliminary batch results. Waste Management. 2016;48:440–447. doi: 10.1016/j.wasman.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Calvaruso E, Cammilleri G, Pulvirenti A, Lo Dico GM, Lo Cascio G, Giaccone V, Vitale Badaco V, Cipri V, Alessandra MM, Vella A, Macaluso A, Di Bella C, Ferrantelli V. Residues of 165 pesticides in citrus fruits using LC-MS/MS: a study of the pesticides distribution from the peel to the pulp. Natural Product Research. 2020;34:34–38. doi: 10.1080/14786419.2018.1561682. [DOI] [PubMed] [Google Scholar]
- Casquete R, Castro SM, Martin A, Ruiz-Moyano S, Saraiva JA, Cordoba MG, Teixeira P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innovative Food Science & Emerging Technologies. 2015;31:37–44. [Google Scholar]
- Casquete R, Castro SM, Villalobos MC, Serradilla MJ, Queiros RP, Saraiva JA, Cordoba MG, Teixeira P. High pressure extraction of phenolic compounds from citrus peels. High Pressure Research. 2014;34:447–451. [Google Scholar]
- Chau CF, Huang YL. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv. Liucheng. Journal of Agricultural and Food Chemistry. 2003;51:2615–2618. doi: 10.1021/jf025919b. [DOI] [PubMed] [Google Scholar]
- Chau CF, Sheu F, Huang YL, Su LH. Improvement in intestinal function and health by the peel fibre derived from Citrus sinensis L cv Liucheng. Journal of the Science of Food and Agriculture. 2005;85:1211–1216. [Google Scholar]
- Chavan P, Singh AK, Kaur G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. Journal of Food Process Engineering. 2018;41:e12895. [Google Scholar]
- Cheigh CI, Chung EY, Chung MS. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. Journal of Food Engineering. 2012;110:472–477. [Google Scholar]
- Chen Q, Hu Z, Yao FYD, Liang H. Study of two-stage microwave extraction of essential oil and pectin from pomelo peels. LWT-Food Science and Technology. 2016;66:538–545. [Google Scholar]
- Chen X, Ding Y, Forrest B, Oh J, Boussert SM, Hamann MT. Lemon yellow# 15 a new highly stable, water soluble food colorant from the peel of Citrus limon. Food Chemistry. 2019;270:251–256. doi: 10.1016/j.foodchem.2018.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IS, Kim JH, Wi SG, Kim KH, Bae HJ. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Applied Energy. 2013;102:204–210. [Google Scholar]
- Crabbe E, Nolasco-Hipolito C, Kobayashi G, Sonomoto K, Ishizaki A. Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochemistry. 2001;37:65–71. [Google Scholar]
- Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K. Valorization of Citrus limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Industrial Crops and Products. 2013;50:77–87. [Google Scholar]
- D'Amato S, Serio A, Lopez CC, Paparella A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control. 2018;86:126–137. [Google Scholar]
- De los Rios AP, Fernandez FH, Gomez D, Rubio M, Víllora G. Biocatalytic transesterification of sunflower and waste cooking oils in ionic liquid media. Process Biochemistry. 2011;46:1475–1480. [Google Scholar]
- Degirmenci H, Erkurt H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. Journal of Infection and Public Health. 2020;13:58–67. doi: 10.1016/j.jiph.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Demirbas A. Bioethanol from cellulosic materials: A renewable motor fuel from biomass. Energy Sources. 2005;27:327–337. [Google Scholar]
- Demirbas A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Conversion and Management. 2008;49:2106–2116. [Google Scholar]
- Dhingra D, Michael M, Rajput H, Patil RT. Dietary fibre in foods: a review. Journal of Food Science and Technology. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Reinoso B, Moure A, Dominguez H, Parajo JC. Supercritical CO2 extraction and purification of compounds with antioxidant activity. Journal of Agricultural and Food Chemistry. 2006;54:2441–2469. doi: 10.1021/jf052858j. [DOI] [PubMed] [Google Scholar]
- Duan X, Wu T, Liu T, Yang H, Ding X, Chen Y, Mu Y. Vicenin-2 ameliorates oxidative damage and photoaging via modulation of MAPKs and MMPs signaling in UVB radiation exposed human skin cells. Journal of Photochemistry and Photobiology b: Biology. 2019;190:76–85. doi: 10.1016/j.jphotobiol.2018.11.018. [DOI] [PubMed] [Google Scholar]
- El Asbahani A, Miladi K, Badri W, Sala M, Addi EA, Casabianca H, El Mousadik A, Hartmann D, Jilale A, Renaud FNR, Elaissari A. Essential oils: from extraction to encapsulation. International Journal of Pharmaceutics. 2015;483:220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- El Kantar S, Boussetta N, Lebovka N, Foucart F, Rajha HN, Maroun RG, Louka N, Vorobiev E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innovative Food Science & Emerging Technologies. 2018;46:153–161. [Google Scholar]
- El Kantar S, Rajha HN, Boussetta N, Vorobiev E, Maroun RG, Louka N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chemistry. 2019;295:165–171. doi: 10.1016/j.foodchem.2019.05.111. [DOI] [PubMed] [Google Scholar]
- Espina L, Somolinos M, Loran S, Conchello P, Garcia D, Pagan R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22:896–902. [Google Scholar]
- Ezekoye V, Adinde R, Ezekoye D, Ofomatah A. Syntheses and characterization of biodiesel from citrus sinensis seed oil. Scientific African. 2019;6:e00217. [Google Scholar]
- Fan JJ, Hsu WH, Lee KH, Chen KC, Lin CW, Lee YLA, Ko TP, Lee LT, Lee MT, Chang MS, Cheng CH. Dietary flavonoids luteolin and quercetin inhibit migration and invasion of squamous carcinoma through reduction of Src/Stat3/S100A7 signaling. Antioxidants. 2019;8:557. doi: 10.3390/antiox8110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO STAT. Food and Agriculture Data. Food and Agriculture Organization. FAO, Rome. Available from: http://www.fao.org/faostat/en/?#data/QC (2019). Accessed June 01, 2021.
- Farhat A, Fabiano-Tixier AS, El Maataoui M, Maingonnat JF, Romdhane M, Chemat F. Microwave steam diffusion for extraction of essential oil from orange peel: kinetic data, extract’s global yield and mechanism. Food Chemistry. 2011;125:255–261. [Google Scholar]
- Ferhat MA, Meklati BY, Smadja J, Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. Journal of Chromatography a. 2006;1112:121–126. doi: 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Figuerola F, Hurtado ML, Estevez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chemistry. 2005;91:395–401. [Google Scholar]
- Forgacs G. Biogas production from citrus wastes and chicken feather: pretreatment and co-digestion. PhD thesis, Chalmers University of Technology, Goteborg, Sweden (2012)
- Fuertes MBR. Effect of limonene on anaerobic digestion of citrus waste and pretreatments for its improvement. PhD thesis, Valencia Polytechnic University, Valencia, Spain (2015)
- Galanakis CM. Kotsiou K. Recovery of bioactive compounds from olive mill waste. pp. 205–229. In: Olive Mill Waste. Academic Press (2017)
- Garau MC, Simal S, Rossello C, Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chemistry. 2007;104:1014–1024. [Google Scholar]
- Garrido G, Chou WH, Vega C, Goity L, Valdes M. Influence of extraction methods on fatty acid composition, total phenolic content and antioxidant capacity of Citrus seed oils from the Atacama Desert, Chile. Journal of Pharmacy & Pharmacognosy Research. 2019;7:389–407. [Google Scholar]
- Golmakani MT, Moayyedi M. Comparison of microwave-assisted hydrodistillation and solvent-less microwave extraction of essential oil from dry and fresh Citrus limon (Eureka variety) peel. Journal of Essential Oil Research. 2016;28:272–282. [Google Scholar]
- Gonzalez-Rivera J, Spepi A, Ferrari C, Duce C, Longo I, Falconieri D, Piras A, Tine MR. Novel configurations for a citrus waste based biorefinery: from solventless to simultaneous ultrasound and microwave assisted extraction. Green Chemistry. 2016;18:6482–6492. [Google Scholar]
- Grohmann K, Baldwin EA. Hydrolysis of orange peel with pectinase and cellulase enzymes. Biotechnology Letters. 1992;14:1169–1174. [Google Scholar]
- Guo C, Zhang H, Guan X, Zhou Z. The anti-aging potential of Neohesperidin and its synergistic effects with other citrus flavonoids in extending chronological lifespan of Saccharomyces Cerevisiae BY4742. Molecules. 2019;24:4093. doi: 10.3390/molecules24224093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SK, Park HY, Eom H, Kim Y, Choi I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food and Chemical Toxicology. 2012;50:3498–3504. doi: 10.1016/j.fct.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Miyake M. Biochemistry and biological functions of citrus limonoids. Food Reviews International. 1996;12:413–435. [Google Scholar]
- Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, Jia C, Zhang X, Xia W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Separation and Purification Technology. 2009;70:63–70. [Google Scholar]
- Hayat K, Zhang X, Chen H, Xia S, Jia C, Zhong F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Separation and Purification Technology. 2010;73:371–376. [Google Scholar]
- Hayat K, Zhang X, Farooq U, Abbas S, Xia S, Jia C, Zhong F, Zhang J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chemistry. 2010;123:423–429. [Google Scholar]
- Hosseini SS, Khodaiyan F, Yarmand MS. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydrate Polymers. 2016;140:59–65. doi: 10.1016/j.carbpol.2015.12.051. [DOI] [PubMed] [Google Scholar]
- Hosseini SS, Khodaiyan F, Kazemi M, Najari Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. International Journal of Biological Macromolecules. 2019;125:621–629. doi: 10.1016/j.ijbiomac.2018.12.096. [DOI] [PubMed] [Google Scholar]
- ICAR-Central Citrus Research Institute. Annual Report. Indian Council of Agricultural Research, Nagpur, Maharashtra, India (2019). Available from https://ccri.icar.gov.in/ccringp/. Accessed June 28, 2021.
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chemistry. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsubaki S, Ogawa K, Onishi K, Azuma JI. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chemistry. 2010;123:542–547. [Google Scholar]
- Inouye S. A comparative study on the composition of forty four hydrosols and their essential oils. International Journal of Essential Oil Therapeutics. 2008;2:89–104. [Google Scholar]
- Jagannath A, Biradar R. Comparative evaluation of soxhlet and ultrasonics on the structural morphology and extraction of bioactive compounds of lemon (Citrus limon L.) peel. Journal of Food Chemistry and Nanotechnology. 2019;5:56–64. [Google Scholar]
- John I, Pola J, Thanabalan M, Appusamy A. Bioethanol production from musambi peel by acid catalyzed steam pretreatment and enzymatic saccharification: Optimization of delignification using taguchi design. Waste and Biomass Valorization. 2020;11:2631–2643. [Google Scholar]
- Johnson JL, De Mejia EG. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κ B signaling cascade. Molecular Nutrition & Food Research. 2013;57:2112–2127. doi: 10.1002/mnfr.201300307. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Rupasinghe SG, Stefani F, Schuler MA, Gonzalez de Mejia E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. Journal of Medicinal Food. 2011;14:325–333. doi: 10.1089/jmf.2010.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SM, Waghmare JS, Sonawane KD, Waghmare SR. Bio-ethanol and bio-butanol production from orange peel waste. Biofuels. 2015;6:55–61. [Google Scholar]
- Karaman E, Yılmaz E, Tuncel NB. Physicochemical, microstructural and functional characterization of dietary fibers extracted from lemon, orange and grapefruit seeds press meals. Bioactive Carbohydrates and Dietary Fibre. 2017;11:9–17. [Google Scholar]
- Kim JW, Lee BC, Lee JH, Nam KC, Lee SC. Effect of electron-beam irradiation on the antioxidant activity of extracts from Citrus unshiu pomaces. Radiation Physics and Chemistry. 2008;77:87–91. [Google Scholar]
- Kim BS, Kim YM, Jae J, Watanabe C, Kim S, Jung SC, Kim SC, Park YK. Pyrolysis and catalytic upgrading of Citrus unshiu peel. Bioresource Technology. 2015;194:312–319. doi: 10.1016/j.biortech.2015.07.035. [DOI] [PubMed] [Google Scholar]
- Koppar A, Pullammanappallil P. Anaerobic digestion of peel waste and wastewater for on site energy generation in a citrus processing facility. Energy. 2013;60:62–68. [Google Scholar]
- Korani MS, Farbood Y, Sarkaki A, Moghaddam HF, Mansouri MT. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. European Journal of Pharmacology. 2014;733:62–67. doi: 10.1016/j.ejphar.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Molecular Aspects of Medicine. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kulkarni GT, Gowthamarajan K, Rao BG, Suresh B. Evaluation of binding properties of Plantago ovata and Trigonella foenum graecum mucilages. Indian Drugs. 2002;39:422–425. [Google Scholar]
- Lachos-Perez D, Baseggio AM, Mayanga-Torres PC, Junior MRM, Rostagno MA, Martinez J, Forster-Carneiro T. Subcritical water extraction of flavanones from defatted orange peel. The Journal of Supercritical Fluids. 2018;138:7–16. [Google Scholar]
- Lante A, Tinello F. Citrus hydrosols as useful by-products for tyrosinase inhibition. Innovative Food Science & Emerging Technologies. 2015;27:154–159. [Google Scholar]
- Ledesma-Escobar CA, de Castro MDL. Towards a comprehensive exploitation of citrus. Trends in Food Science & Technology. 2014;39:63–75. [Google Scholar]
- Liang JH, Ren XQ, Wang JT, Li ZJ. Preparation of biodiesel by transesterification from cottonseed oil using the basic dication ionic liquids as catalysts. Journal of Fuel Chemistry and Technology. 2010;38:275–280. [Google Scholar]
- Liu J, Liu C, Rong Y, Huang G, Rong L. Extraction of limonin from orange (Citrus Reticulata Blanco) seeds by the flash extraction method. Solvent Extraction Research & Development Japan. 2012;19:137–145. [Google Scholar]
- Liu Z, Qiao L, Gu H, Yang F, Yang L. Development of Brnsted acidic ionic liquid based microwave assisted method for simultaneous extraction of pectin and naringin from pomelo peels. Separation and Purification Technology. 2017;172:326–337. [Google Scholar]
- Lohrasbi M, Pourbafrani M, Niklasson C, Taherzadeh MJ. Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresource Technology. 2010;101:7382–7388. doi: 10.1016/j.biortech.2010.04.078. [DOI] [PubMed] [Google Scholar]
- Lopresto CG, Meluso A, Di Sanzo G, Chakraborty S, Calabro V. Process-intensified waste valorization and environmentally friendly D-limonene extraction. Euro-Mediterranean Journal for Environmental Integration. 2019;4:1–12. [Google Scholar]
- Lotito AM, De Sanctis M, Pastore C, Di Iaconi C. Biomethanization of citrus waste: Effect of waste characteristics and of storage on treatability and evaluation of limonene degradation. Journal of Environmental Management. 2018;215:366–376. doi: 10.1016/j.jenvman.2018.03.057. [DOI] [PubMed] [Google Scholar]
- Luengo E, Alvarez I, Raso J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innovative Food Science & Emerging Technologies. 2013;17:79–84. [Google Scholar]
- Mahato N, Sharma K, Sinha M, Baral ER, Koteswararao R, Dhyani A, Cho S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. Journal of Advanced Research. 2020;23:61–82. doi: 10.1016/j.jare.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawar MK, Jalgaonkar K, Bibwe B, Bhushan B, Meena VS, Sonkar RK. Post-harvest processing and valorization of Kinnow mandarin (Citrus reticulate L.): A review. Journal of Food Science and Technology. 2020;57:799–815. doi: 10.1007/s13197-019-04083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerska J, Michalska A, Figiel A. A review of new directions in managing fruit and vegetable processing by-products. Trends in Food Science & Technology. 2019;88:207–219. [Google Scholar]
- Mamma D, Christakopoulos P. Biotransformation of citrus by-products into value added products. Waste and Biomass Valorization. 2013;5:529–549. [Google Scholar]
- Manners GD, Breksa AP., III Identifying citrus limonoid aglycones by HPLC-EI/MS and HPLC-APCI/MS techniques. Phytochemical Analysis: an International Journal of Plant Chemical and Biochemical Techniques. 2004;15:372–381. doi: 10.1002/pca.790. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydrate Polymers. 2013;97:703–709. doi: 10.1016/j.carbpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- Marin FR, Soler-Rivas C, Benavente-Garcia O, Castillo J, Perez-Alvarez JA. By-products from different citrus processes as a source of customized functional fibres. Food Chemistry. 2007;100:736–741. [Google Scholar]
- Martin MA, Siles JA, Chica AF, Martin A. Biomethanization of orange peel waste. Bioresource Technology. 2010;101:8993–8999. doi: 10.1016/j.biortech.2010.06.133. [DOI] [PubMed] [Google Scholar]
- Menichini F, Tundis R, Bonesi M, De Cindio B, Loizzo MR, Conforti F, Statti GA, Menabeni R, Bettini R, Menichini F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Natural Product Research. 2011;25:789–799. doi: 10.1080/14786410902900085. [DOI] [PubMed] [Google Scholar]
- Montero-Calderon A, Cortes C, Zulueta A, Frigola A, Esteve MJ. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Scientific Reports. 2019;9:1–8. doi: 10.1038/s41598-019-52717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multari S, Carlin S, Sicari V, Martens S. Differences in the composition of phenolic compounds, carotenoids, and volatiles between juice and pomace of four citrus fruits from Southern Italy. European Food Research and Technology. 2020;246:1991–2005. [Google Scholar]
- Murador DC, Braga ARC, Martins PL, Mercadante AZ, de Rosso VV. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Research International. 2019;126:108653. doi: 10.1016/j.foodres.2019.108653. [DOI] [PubMed] [Google Scholar]
- Musthafa MM. Production of biodiesel from Citrus limetta seed oil. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2016;38:2994–3000. [Google Scholar]
- Naghshineh M, Olsen K, Georgiou CA. Sustainable production of pectin from lime peel by high hydrostatic pressure treatment. Food Chemistry. 2013;136:472–478. doi: 10.1016/j.foodchem.2012.08.036. [DOI] [PubMed] [Google Scholar]
- Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aoun O, Khodir M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chemistry. 2015;187:507–516. doi: 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Ndayishimiye J, Chun BS. Optimization of carotenoids and antioxidant activity of oils obtained from a co-extraction of citrus (Yuzu ichandrin) by-products using supercritical carbon dioxide. Biomass and Bioenergy. 2017;106:1–7. [Google Scholar]
- Ndiaye EHB, Gueye MT, Ndiaye I, Diop SM, Diop MB, Thiam A, Fauconnier ML, Lognay G. Chemical composition of distilled essential oils and hydrosols of four senegalese citrus and enantiomeric characterization of chiral compounds. Journal of Essential Oil Bearing Plants. 2017;20:820–834. [Google Scholar]
- Nipornram S, Tochampa W, Rattanatraiwong P, Singanusong R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chemistry. 2018;241:338–345. doi: 10.1016/j.foodchem.2017.08.114. [DOI] [PubMed] [Google Scholar]
- Nishad J, Saha S, Dubey AK, Varghese E, Kaur C. Optimization and comparison of non-conventional extraction technologies for Citrus paradisi L. peels: a valorization approach. Journal of Food Science and Technology. 2019;56:1221–1233. doi: 10.1007/s13197-019-03585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberoi HS, Vadlani PV, Nanjundaswamy A, Bansal S, Singh S, Kaur S, Babbar N. Enhanced ethanol production from Kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresource Technology. 2011;102:1593–1601. doi: 10.1016/j.biortech.2010.08.111. [DOI] [PubMed] [Google Scholar]
- Ogiwara T, Satoh K, Negoro T, Okayasu H, Sakagami H, Fujisawa S. Inhibition of NO production by activated macrophages by phenolcarboxylic acid monomers and polymers with radical scavenging activity. Anticancer Research. 2003;23:1317–1323. [PubMed] [Google Scholar]
- Omar J, Alonso I, Garaikoetxea A, Etxebarria N. Optimization of focused ultrasound extraction (FUSE) and supercritical fluid extraction (SFE) of citrus peel volatile oils and antioxidants. Food Analytical Methods. 2013;6:1244–1252. [Google Scholar]
- Ortelli D, Edder P, Corvi C. Pesticide residues survey in citrus fruits. Food Additives and Contaminants. 2005;22:423–428. doi: 10.1080/02652030500089903. [DOI] [PubMed] [Google Scholar]
- Otles S, Cagindi O. 2.2 Carotenoids as Natural Colorants. Vol. 2008, pp. 51–70. In: Food Colorants: Chemical and Functional Properties. CRC Press, Florida, United States (2007)
- Panwar D, Saini A, Panesar PS, Chopra HK. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends in Food Science & Technology. 2021;111:549–562. [Google Scholar]
- Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Scarlett CJ, Bowyer MC, Van Vuong Q. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Science and Biotechnology. 2016;25:971–977. doi: 10.1007/s10068-016-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ, Vuong QV. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. International Journal of Food Science & Technology. 2016;51:2009–2018. [Google Scholar]
- Papoutsis K, Vuong QV, Pristijono P, Golding JB, Bowyer MC, Scarlett CJ, Stathopoulos CE. Enhancing the total phenolic content and antioxidants of lemon pomace aqueous extracts by applying UV-C irradiation to the dried powder. Foods. 2016;5:55. doi: 10.3390/foods5030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ, Vuong QV. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. International Journal of Food Science & Technology. 2017;52:880–887. [Google Scholar]
- Park HY, Ha SK, Eom H, Choi I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food and Chemical Toxicology. 2013;55:637–644. doi: 10.1016/j.fct.2013.01.060. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Harris ED, Patil BS. Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. The Journal of Nutrition. 2005;135:870–877. doi: 10.1093/jn/135.4.870. [DOI] [PubMed] [Google Scholar]
- Pourbafrani M, Forgacs G, Horvath IS, Niklasson C, Taherzadeh MJ. Production of biofuels, limonene and pectin from citrus wastes. Bioresource Technology. 2010;101:4246–4250. doi: 10.1016/j.biortech.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Qin S, Lv C, Wang Q, Zheng Z, Sun X, Tang M, Deng F. Extraction, identification, and antioxidant property evaluation of limonin from pummelo seeds. Animal Nutrition. 2018;4:281–287. doi: 10.1016/j.aninu.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoc LPT, Huyen VTN, Hue LTN, Hue NTH, Thuan NHD, Tam NTT, Thuan NN, Duy TH. Extraction of pectin from pomelo (Citrus maxima) peels with the assistance of microwave and tartaric acid. International Food Research Journal. 2015;22:1637–1641. [Google Scholar]
- Rashid U, Ibrahim M, Yasin S, Yunus R, Taufiq-Yap YH, Knothe G. Biodiesel from Citrus reticulata (mandarin orange) seed oil, a potential non-food feedstock. Industrial Crops and Products. 2013;45:355–359. [Google Scholar]
- Rivas B, Torrado A, Torre P, Converti A, Dominguez JM. Submerged citric acid fermentation on orange peel autohydrolysate. Journal of Agricultural and Food Chemistry. 2008;56:2380–2387. doi: 10.1021/jf073388r. [DOI] [PubMed] [Google Scholar]
- Rosa A, Era B, Masala C, Nieddu M, Scano P, Fais A, Porcedda S, Piras A. Supercritical CO2 extraction of waste citrus seeds: Chemical composition, nutritional and biological properties of edible fixed oils. European Journal of Lipid Science and Technology. 2019;121:1800502. [Google Scholar]
- Ruiz B, Flotats X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochemical Engineering Journal. 2016;109:9–18. [Google Scholar]
- Russo M, Arigo A, Calabro ML, Farnetti S, Mondello L, Dugo P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: limonoids and flavonoids. Journal of Functional Foods. 2016;20:10–19. [Google Scholar]
- Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. Journal of Food and Drug Analysis. 2017;25:488–500. doi: 10.1016/j.jfda.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin S. A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean Journal of Chemical Engineering. 2015;32:950–957. [Google Scholar]
- Saini RK, Keum YS. Carotenoid extraction methods: A review of recent developments. Food Chemistry. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- Saini A, Panesar PS, Bera MB. Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Conversion and Biorefinery. 2020 doi: 10.1007/s13399-020-00605-4. [DOI] [Google Scholar]
- Santi G, Crognale S, D'Annibale A, Petruccioli M, Ruzzi M, Valentini R, Moresi M. Orange peel pretreatment in a novel lab-scale direct steam-injection apparatus for ethanol production. Biomass and Bioenergy. 2014;61:146–156. [Google Scholar]
- Santiago B, Moreira MT, Feijoo G, Gonzalez-Garcia S. Identification of environmental aspects of citrus waste valorization into D-limonene from a biorefinery approach. Biomass and Bioenergy. 2020;143:105844. [Google Scholar]
- Satari B, Karimi K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resources, Conservation and Recycling. 2018;129:153–167. [Google Scholar]
- Satari B, Palhed J, Karimi K, Lundin M, Taherzadeh MJ, Zamani A. Process optimization for citrus waste biorefinery via simultaneous pectin extraction and pretreatment. BioResources. 2017;12:1706–1722. [Google Scholar]
- Schieber A, Stintzing FC, Carle R. By-products of plant food processing as a source of functional compounds—recent developments. Trends in Food Science & Technology. 2001;12:401–413. [Google Scholar]
- Sharma K, Mahato N, Cho MH, Lee YR. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Shi YS, Zhang Y, Li HT, Wu CH, El-Seedi HR, Ye WK, Wang ZW, Li CB, Zhang XF, Kai GY. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. Journal of Functional Foods. 2020;75:104213. [Google Scholar]
- Silva dos Santos J, Goncalves Cirino JP, de Oliveira Carvalho P, Ortega MM. The pharmacological action of Kaempferol in central nervous system diseases: a review. Frontiers in Pharmacology. 2021;11:2143. doi: 10.3389/fphar.2020.565700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanusong R, Nipornram S, Tochampa W, Rattanatraiwong P. Low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) and lime (Citrus aurantifolia) peels and the antioxidant. Food Analytical Methods. 2015;8:1112–1123. [Google Scholar]
- Sridhar A, Ponnuchamy M, Kumar PS, Kapoor A, Vo DN, Prabhakar S. Techniques and modeling of polyphenol extraction from food: a review. Environmental Chemistry Letters. 1–35 (2021) [DOI] [PMC free article] [PubMed]
- Su DL, Li PJ, Quek SY, Huang ZQ, Yuan YJ, Li GY, Shan Y. Efficient extraction and characterization of pectin from orange peel by a combined surfactant and microwave assisted process. Food Chemistry. 2019;286:1–7. doi: 10.1016/j.foodchem.2019.01.200. [DOI] [PubMed] [Google Scholar]
- Sui Y, Yang J, Ye Q, Li H, Wang H. Infrared, convective, and sequential infrared and convective drying of wine grape pomace. Drying Technology. 2014;32:686–694. [Google Scholar]
- Sun Y, Liu D, Chen J, Ye X, Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrasonics Sonochemistry. 2011;18:243–249. doi: 10.1016/j.ultsonch.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Tabeshpour J, Hosseinzadeh H, Hashemzaei M, Karimi G. A review of the hepatoprotective effects of hesperidin, a flavanon glycoside in citrus fruits, against natural and chemical toxicities. DARU Journal of Pharmaceutical Sciences. 2020;28:305–317. doi: 10.1007/s40199-020-00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh-Alisaraei A, Hosseini SH, Ghobadian B, Motevali A. Biofuel production from citrus wastes: A feasibility study in Iran. Renewable and Sustainable Energy Reviews. 2017;69:1100–1112. [Google Scholar]
- Talebnia F, Bafrani MP, Lundin M, Taherzadeh M. Optimization study of citrus wastes saccharification by dilute acid hydrolysis. BioResources. 2008;3:108–122. [Google Scholar]
- Tanaka M, Takamizu A, Hoshino M, Sasaki M, Goto M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food and Bioproducts Processing. 2012;90:180–186. [Google Scholar]
- Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutrition and Cancer. 2001;40:180–184. doi: 10.1207/S15327914NC402_15. [DOI] [PubMed] [Google Scholar]
- Trabelsi D, Aydi A, Zibetti AW, Della Porta G, Scognamiglio M, Cricchio V, Langa E, Abderrabba M, Mainar AM. Supercritical extraction from Citrus aurantium amara peels using CO2 with ethanol as co-solvent. The Journal of Supercritical Fluids. 2016;117:33–39. [Google Scholar]
- Tsitsagi M, Ebralidze K, Chkhaidze M, Rubashvili I, Tsitsishvili V. Sequential extraction of bioactive compounds from tangerine (Citrus Unshiu) peel. Annals of Agrarian Science. 2018;16:236–241. [Google Scholar]
- Tumbas VT, Cetkovic GS, Dilas SM, Canadanovic-Brunet JM, Vulic JJ, Knez Z, Skerget M. Antioxidant activity of mandarin (Citrus reticulata) peel. Acta Periodica Technologica. 2010;41:195–203. [Google Scholar]
- Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–1265. doi: 10.1093/carcin/bgi318. [DOI] [PubMed] [Google Scholar]
- Vikram A, Jayaprakasha GK, Patil BS. Simultaneous determination of citrus limonoid aglycones and glucosides by high performance liquid chromatography. Analytica Chimica Acta. 2007;590:180–186. doi: 10.1016/j.aca.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Fernandez-Lopez J, Sayas-Barbera E, Sendra E, Perez-Alvarez JA. Physicochemical characterization of the orange juice waste water of a citrus by-product. Journal of Food Processing and Preservation. 2011;35:264–271. [Google Scholar]
- Wadhwa M, Bakshi MPS. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products. India: RAP Publication; 2013. pp. 1–67. [Google Scholar]
- Wang YC, Chuang YC, Ku YH. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chemistry. 2007;102:1163–1171. [Google Scholar]
- Wang X, Chen Q, Lu X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocolloids. 2014;38:129–137. [Google Scholar]
- Wang L, Xu H, Yuan F, Fan R, Gao Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chemistry. 2015;185:90–98. doi: 10.1016/j.foodchem.2015.03.112. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang H, Zhou J, Zhang X, Chen L, Chen K, Huang Z. Eriocitrin from lemon suppresses the proliferation of human hepatocellular carcinoma cells through inducing apoptosis and arresting cell cycle. Cancer Chemotherapy and Pharmacology. 2016;78:1143–1150. doi: 10.1007/s00280-016-3171-y. [DOI] [PubMed] [Google Scholar]
- Wang L, Lee WW, Yang HW, Ryu BM, Cui YR, Lee SC, Lee TG, Jeon YJ. Protective effect of water extract of citrus pomace against AAPH-induced oxidative stress in vitro in vero cells and in vivo in Zebrafish. Preventive Nutrition and Food Science. 2018;23:301–308. doi: 10.3746/pnf.2018.23.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Shimada Y, Sugihara A, Tominaga Y. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. Journal of Molecular Catalysis b: Enzymatic. 2002;17:151–155. [Google Scholar]
- Widmer W, Zhou W, Grohmann K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresource Technology. 2010;101:5242–5249. doi: 10.1016/j.biortech.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Wikandari R, Millati R, Cahyanto MN, Taherzadeh MJ. Biogas production from citrus waste by membrane bioreactor. Membranes. 2014;4:596–607. doi: 10.3390/membranes4030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MR, Widmer WW, Grohmann K, Cameron RG. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresource Technology. 2007;98:1596–1601. doi: 10.1016/j.biortech.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Wu F, Chen J, Fan LM, Liu K, Zhang N, Li SW, Zhu H, Gao HC. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Experimental and Therapeutic Medicine. 2017;14:127–130. doi: 10.3892/etm.2017.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YY, Ma S, Wang XX, Zheng XL. Modification and application of dietary fiber in foods. Journal of Chemistry. 2017;2017:9340427. [Google Scholar]
- Yoo HH, Lee M, Chung HJ, Lee SK, Kim DH. Effects of diosmin, a flavonoid glycoside in citrus fruits, on P-glycoprotein-mediated drug efflux in human intestinal Caco-2 cells. Journal of Agricultural and Food Chemistry. 2007;55:7620–7625. doi: 10.1021/jf070893f. [DOI] [PubMed] [Google Scholar]
- Yusuf NNAN, Kamarudin SK, Yaakub Z. Overview on the current trends in biodiesel production. Energy Conversion and Management. 2011;52:2741–2751. [Google Scholar]
- Zema DA, Calabro PS, Folino A, Tamburino V, Zappia G, Zimbone SM. Valorisation of citrus processing waste: A review. Waste Management. 2018;80:252–273. doi: 10.1016/j.wasman.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Zhang M, Nan H, Wang Y, Jiang X, Li Z. Comparison of flavonoid compounds in the flavedo and juice of two pummelo cultivars (Citrus grandis L. Osbeck) from different cultivation regions in China. Molecules. 2014;19:17314–17328. doi: 10.3390/molecules191117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lou Z, Chen X, Cui Y, Wang H, Kou X, Ma C. Effect of simultaneous ultrasonic and microwave assisted hydrodistillation on the yield, composition, antibacterial and antibiofilm activity of essential oils from Citrus medica L. var. sarcodactylis. Journal of Food Engineering. 2019;244:126–135. [Google Scholar]
- Zhou W, Widmer W, Grohmann K. Developments in ethanol production from citrus peel waste. Proceedings of the Florida State Horticultural Society. 2008;121:307–310. [Google Scholar]