Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and is strongly associated with obesity-related ectopic fat accumulation in the liver. Hepatic lipid accumulation encompasses a histological spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma. Given that dysregulated hepatic lipid metabolism may be an onset factor in NAFLD, understanding how hepatic lipid metabolism is modulated in healthy subjects and which steps are dysregulated in NAFLD subjects is crucial to identify effective therapeutic targets. Additionally, hepatic inflammation is involved in chronic hepatocyte damage during NAFLD progression. As a key immune signaling hub that mediates NF-κB activation, the IκB kinase (IKK) complex, including IKKα, IKKβ, and IKKγ (NEMO), has been studied as a crucial regulator of the hepatic inflammatory response and hepatocyte survival. Notably, TANK-binding kinase 1 (TBK1), an IKK-related kinase, has recently been revealed as a potential link between hepatic inflammation and energy metabolism. Here, we review (1) the biochemical steps of hepatic lipid metabolism; (2) dysregulated lipid metabolism in obesity and NAFLD; and (3) the roles of IKKs and TBK1 in obesity and NAFLD.
Subject terms: Obesity, Chronic inflammation
Obesity: tracing the inflammation underlying liver disease
A deeper understanding of the molecular processes underlying dysfunctional fatty-acid metabolism in nonalcoholic fatty liver disease (NAFLD) could help protect patients from severe liver damage. NAFLD is a common consequence of obesity, and severe disease can ultimately give rise to liver cancer or cirrhosis. Alan Saltiel of the University of California, San Diego, USA, and Jin Young Huh of Seoul National University, South Korea, have reviewed current knowledge of the mechanisms governing fatty acid metabolism in the liver, and how they are disturbed in obesity and NAFLD. The various IκB kinase (IKK) complexes and a protein known as TANK-binding kinase 1 (TBK1) play particularly prominent roles in the inflammatory response associated with NAFLD pathogenesis. Better insights into IKK- and TBK1-associated regulatory pathways could inform the development of new interventions for this currently untreatable condition.
Introduction
The prevalence of obesity (excess fat storage) due to high-calorie food intake and a sedentary lifestyle is greatly increasing. Obesity is closely related to the prevalence of metabolic disorders, such as type 2 diabetes, cardiovascular disease, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD)1,2. As obesity progresses, excess fat builds up in organs other than adipose tissue, and chronic hepatic fat accumulation induces fatty liver, which is currently the most common cause of obesity-related NAFLD3,4.
NAFLD is typically classified into nonalcoholic fatty liver (NAFL, simple steatosis) and nonalcoholic steatohepatitis (NASH)5. Hepatic steatosis is defined as an increase in hepatic fat that exceeds 5% of the liver’s weight seen on imaging or histology in the absence of alcohol intake, long-term steatogenic medication intake, or certain genetic diseases5. NASH is characterized by steatosis with hepatocyte injury in the form of ballooning hepatocytes, which is associated with hepatocyte death, apoptosis, and inflammation5. NASH patients can develop advanced liver disease, leading to cirrhosis and hepatocellular carcinoma (HCC)6. The global prevalence of NAFLD is over 25%, with the highest prevalence in the Middle East and South America and the lowest in Africa3. In the United States, the overall NAFLD prevalence in the adult population is projected to be 33% in 20307. As there is still no efficient therapy for NAFLD, liver transplantation is currently the only treatment for advanced cirrhosis with liver dysfunction, which highlights the importance of understanding NAFLD development to find effective preventive and therapeutic strategies.
In this review, we summarize the molecular mechanism of hepatic fatty acid (FA) metabolism regulation under normal and NAFLD pathophysiological conditions. As hepatic inflammatory signaling is also a well-known factor regulating NAFLD progression, the role of the IκB kinase (IKK) complex, which is an inflammatory signaling hub, will be reviewed. In particular, the role of the IKK-related kinase TANK-binding kinase 1 (TBK1) in the regulation of lipid metabolism will be discussed.
Hepatic FA metabolism
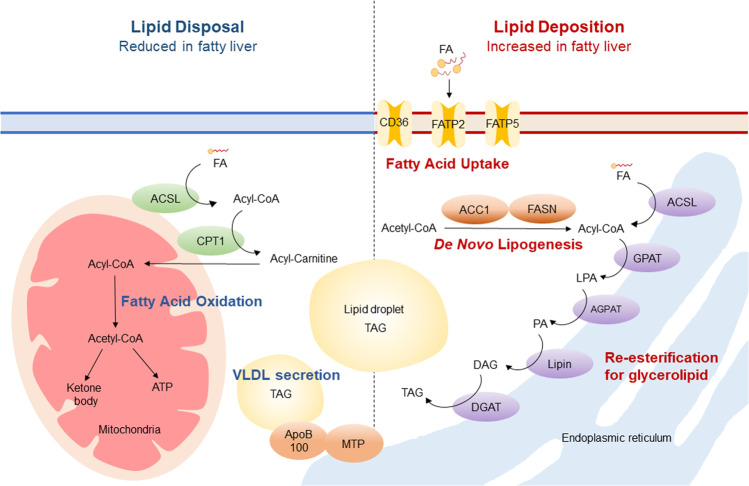
Hepatic steatosis results from an imbalance in lipid metabolism in hepatocytes, which is a major parenchymal cell type in the liver. Hepatocytes can maintain a steady lipid content of <5% of inflowing dietary or endogenous FAs8. This is attributable to the fact that FA uptake from the blood and de novo lipogenesis in the liver are counterbalanced by FA oxidation and release into the plasma as triglyceride (TG)-enriched very-low-density lipoprotein (VLDL)9. However, a preference for FA acquisition over lipid consumption via FA oxidation or VLDL secretion can lead to an increase in TG accumulation in cytosolic lipid droplets, which can promote steatosis (Fig. 1)9,10. Nonetheless, the molecular mechanisms driving abnormal hepatic lipid accumulation in NAFLD subjects remain elusive. As the regulation of hepatic lipid homeostasis is complex, we will first glance at some of the key regulators of NAFLD progression.
Fig. 1. Hepatic lipid metabolism in fatty liver.
Circulating FAs are imported via FATPs, including CD36, FATP2, and FATP5. ACSL converts the FAs taken up by cells to acyl-CoA, which is further reesterified to produce glycerolipids, mainly in the ER. In addition, FAs can be synthesized via DNL using acetyl-CoA. These intracellular FAs can be consumed to generate VLDL by forming a complex with ApoB100 via MTP. Furthermore, FAs are cleaved to generate acetyl-CoA through FA oxidation in the mitochondria to produce ATP and ketone bodies. An imbalance between lipid disposal and storage promotes fatty liver disease.
FA uptake
Long-chain FA uptake is largely dependent on plasma membrane-associated FA transporters, with a minor contribution of passive diffusion11. Plasma membrane FA-binding protein (FABPpm), caveolin-1, CD36, and FA transporter protein are known to be involved in FA uptake12,13. The roles of FABPpm and caveolin-1 in the progression of steatosis through FA uptake have not been fully elucidated. Liver-specific CD36 knockout (KO) mice showed less steatosis upon high-fat diet (HFD) feeding, indicating that hepatic CD36 is involved in FA uptake14. In addition, CD36 and PPARγ expression levels were similarly elevated in obese rats with hepatic steatosis15,16.
Among the six FATP isoforms, FATP2 and FATP5 are the major FATPs in hepatocytes17. While FATP4 is not highly expressed in normal hepatocytes, its expression is induced in steatosis18. FATP2 overexpression in HepG2 cells elevated their FA uptake19. FATP5-deficient mice showed reduced progression of steatosis20, indicating a crucial role of FA transporters in hepatic lipid accumulation. To date, it is known that many FA transporters are involved in governing hepatic lipid levels, however, further investigation, including of the FA species transported by each transporter and the regulators of FA transporter activity, is needed.
Acyl-CoA synthesis
FA uptake activity is closely related to acyl-CoA synthetase (ACS) activity because ACS-mediated consumption of intracellular FAs provides the driving force for FA uptake21,22. Intracellular FAs, including FAs taken up from the extracellular space, are conjugated with CoA by ACS, which makes them more hydrophilic and thus traps them inside the cells. Therefore, this conjugation is an important step for further FA metabolism23. ACS can be classified into very-long-chain ACS, long-chain ACS (ACSL), medium-chain ACS (ACSM), and short-chain ACS (ACSS), which have different substrate preferences but show significant overlap in chain-length specificity23,24. ACSL mainly catalyzes acyl-CoA synthesis with chain lengths of 12–20 carbons and comprises five isoforms (ACSL1, ACSL3, ACSL4, ACSL5, and ACSL6)23,25. While hepatocytes mainly express ACSL1, ACSL4, and ACSL5, ACSL1 has been suggested to contribute ~50% of hepatic ACSL activity26,27. ACSL1 has a substrate preference for palmitic acid and oleic acid, which are FA metabolites enriched in circulation25. Notably, liver-specific ACSL1 KO mice did not show significant changes in hepatic lipid levels, which may be due to diminished levels of FA re-esterification into triacylglycerol and FA oxidation27. ACSL1 in the liver can localize to both the endoplasmic reticulum (ER) and mitochondria, and various interacting proteins have been identified through mitochondrial or ER-targeted ACSL1 overexpression28,29. Based on the differential enrichment of FA oxidation-related proteins in the mitochondria and re-esterification enzymes in the ER, it has been suggested that the localization of ACSL1 can determine the metabolic fate of fatty acyl-CoA toward oxidation or re-esterification for lipid synthesis28,29; acyl-CoA produced by mitochondrial ACSL1 is oxidized, whereas acyl-CoA produced by ER ACSL1 is mostly used for re-esterification to diacylglycerol (DG) or TG. Consistent with this suggestion, adenovirus-mediated ACSL1 overexpression in rat primary hepatocytes increased FA reacylation and channeled FA toward DG and phospholipid synthesis, with elevated levels of ER-localized ACSL130. Furthermore, ACSL1 directly interacts with carnitine palmitoyltransferase 1A in the rat liver31, which supports the crucial role of mitochondria-localized ACSL1 in FA oxidation. Nonetheless, the molecular mechanism by which ACSL1 localization in hepatocytes is regulated remains to be investigated.
Re-esterification for lipid synthesis
Activated acyl-CoA can be reesterified for lipid synthesis32. The enzymes catalyzing re-esterification, including glycerol-3-phosphate acyltransferase (GPAT), 1-acylglycerol-3-phosphate acyltransferase (AGPAT), phosphatidic acid (PA) phosphohydrolase (PAPases/lipins), and diacylglycerol acyltransferase (DGAT), are mainly localized in microsomes and the ER29. The key rate-limiting step for re-esterification is lysophosphatidic acid (LPA) synthesis by mitochondrial and microsomal GPAT22,33. LPA can be converted to PA through AGPAT activity. PA can then be used as a precursor of phospholipids, such as phosphatidylethanolamine or phosphatidylcholine9,29. In addition, PA can be converted to DG via lipins, which have PAPase activity. DGAT activity esterifies additional FAs into DG to synthesize TG. Reesterified FAs can subsequently be transported to the cytosol and stored as cytosolic lipid droplets or form and secrete VLDL complexed with apoproteins into the circulation9,29.
Loss- and gain-of-function studies of these key enzymes have shown the crucial regulatory role of re-esterification in NAFLD progression. GPAT is responsible for the first step in re-esterification. There are four isoforms, GPAT1, GPAT2, GPAT3, and GPAT4, which differ in subcellular localization and substrate preferences34. GPAT1 and GPAT2 are localized in the mitochondria and account for 30–50% of total hepatic GPAT activity34. GPAT1 expression is elevated in response to insulin but is reduced upon fasting-related cAMP-dependent signaling35,36. GPAT1-deficient mice showed increased hepatic FA oxidation with enhanced circulating levels of ketone bodies37. Concomitantly, hepatic TG levels declined by 60%, which revealed the pivotal role of GPAT1 in the management of hepatic TG synthesis. In contrast, GPAT3 and GPAT4 are involved in microsomal GPAT activity in the liver38. Although GPAT4-deficient mice show reduced hepatic TG levels39, the roles of GPAT3 and GPAT4 in the regulation of hepatic lipid metabolism and their roles in NAFLD progression remain to be further elucidated.
DGAT1 is primarily involved in TG synthesis in conjunction with microsomal luminal activity for VLDL formation, whereas DGAT2 utilizes cytosol-accessible activity to store TG in cytosolic lipid droplets40. Intracellular TG levels increased when DGAT1 was overexpressed in rat hepatoma cells41, and HFD-induced steatosis was reduced in DGAT1 KO mice42. Furthermore, liver-specific DGAT2 KO mice showed suppressed steatosis upon HFD feeding43. Accordingly, DGAT2 inhibitor administration reduced hepatic steatosis in healthy adults44. These studies suggest that increased hepatic DGAT activity may be a crucial factor in promoting steatosis.
De novo lipogenesis
In both NAFLD patients and the HFD-fed mouse model, newly synthesized FAs resulting from DNL have been reported to increase45,46. DNL is initiated by ATP-citrate lyase (ACLY), which converts citrate to acetyl-CoA47. Acetyl-CoA carboxylase (ACC) synthesizes malonyl-CoA, which is a substrate for FA synthase (FASN) to generate palmitic acid. ACC has two isoforms, ACC1 in the cytoplasm and ACC2 in the mitochondria. ACC1 facilitates the synthesis of cytosolic malonyl-CoA, which is used as a substrate for FASN47. ACC2 mediates malonyl-CoA synthesis in the mitochondria, which inhibits carnitine palmitoyltransferase 1 (CPT1), the key enzyme of FA oxidation48. While ACC1 whole-body KO mice are embryonic lethal49, liver-specific ACC1 KO mice showed 70% reduced malonyl-CoA levels and significantly decreased hepatic TG levels50. FASN produces palmitate by affixing malonyl-CoA to acetyl-CoA in a series of condensation events, which are key rate-limiting steps in DNL51. FASN whole-body KO mice are embryonic lethal52, but liver-specific FASN KO mice showed attenuated hepatic DNL activity, resulting in the deposition of hepatic malonyl-CoA53. Interestingly, liver-specific FASN KO mice fed a zero-fat diet to stimulate DNL showed exacerbated hypoglycemia and hepatic steatosis, which were rescued upon administration of a PPARα agonist (WY-14.643)53. These studies indicate that newly synthesized FAs mediated by FASN act as PPARα ligands to regulate hepatic FA oxidation and gluconeogenesis. In NAFLD patients, 10-day administration of TVD-2640, a FASN inhibitor, resulted in significant reductions in ALT levels and DNL54. Therefore, the functional modulation of regulatory enzymes mediating the DNL pathway, such as ACC and FASN, has an important role in steatosis development.
Hepatic DNL is primarily controlled by transcriptional regulation55. ACC and FASN expression levels are synergistically controlled by carbohydrate-responsive element-binding protein (ChREBP) and sterol regulatory element-binding protein (SREBP-1c)56. SREBP-1c, a key transcriptional modulator of lipogenic enzymes, is activated by insulin in response to feeding, thereby increasing the expression of DNL-related genes57. SREBP-1c expression is regulated by the liver X receptor (LXR)58. ChREBP expression is strongly reduced under fasting conditions, whereas it is activated by a high-glucose or high-carbohydrate diet, increasing the expression of DNL-related genes59. ChREBP whole-body KO mice showed reduced mRNA levels of hepatic lipogenic enzymes, such as ACLY, ACC1, and FASN60. Furthermore, the expression of lipogenic genes, such as LXRα, SREBP-1c, ACC1, and FASN, is increased in the liver tissues of NAFLD patients61.
VLDL secretion
Hepatic TG can be derived from circulating lipids, such as albumin-bound FAs, VLDL remnants, chylomicrons, and DNL in the liver62. Hepatic TG is released into the bloodstream primarily in the form of VLDL, which contains both lipids and apoproteins, including apoB10063. Secreted VLDLs provide an energy source to other tissues, such as muscle and adipose tissues64. VLDL is formed in two steps. The first step is lipidation of apoB100 catalyzed by microsomal TG transfer protein (MTP), which is processed in the ER. The nascent VLDL particles are subsequently transported to the Golgi apparatus, where they are further lipidated until they grow into mature VLDL particles65. Thus, apoB100 and MTP are vital for hepatic VLDL secretion and lipid homeostasis. In obese subjects, altered hepatic TG levels affect plasma VLDL levels by augmenting the amount of substrate, enhancing MTP activity, or influencing apolipoprotein B synthesis or breakdown62,66. In nondiabetic obese NAFLD patients, VLDL secretion is doubled compared to that in normal subjects67. However, even with a substantial amount of hepatic lipids, steatosis can worsen if secretion is hampered. For example, whereas ob/ob animals show steatosis, their VLDL secretion is lowered68. Thus, maintaining a balance between hepatic TG and VLDL secretion can be an important aspect of managing hepatic lipid levels.
FA oxidation
Hepatic FA oxidation occurs in the mitochondria, peroxisomes, and ER, depending on the FA species. Very long-chain (C20–C26) and branched-chain FAs are mainly oxidized in peroxisomes, whereas β-oxidation of long-chain FAs primarily occurs in mitochondria9. Long-chain fatty acyl-CoA, synthesized by ACS using ATP and CoA, can be oxidized in the mitochondria to be cleaved into acetyl-CoA. Acetyl-CoA then enters the tricarboxylic acid (TCA) cycle to produce NADH and FADH223, which serve as substrates for electron transfer chains in the mitochondrial inner membrane, exploiting proton gradients in the intermembrane space to generate ATP. As acyl-CoA requires a carnitine shuttle to enter the mitochondria, it is converted to palmitoyl carnitine by CPT1 at the mitochondrial outer membrane69 and then enters the mitochondrial inner membrane matrix via carnitine-acylcarnitine translocase (CAT) and is converted back to acyl-CoA by CPT229. Acyl-CoA then undergoes β-oxidation through four enzymatic reactions catalyzed by acyl-CoA dehydrogenase, 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase70.
In response to starvation, FA oxidation and ketogenesis are actively induced in the liver69. The substrate concentration and/or affinity of an enzyme for its substrates, such as carnitine and long-chain acyl-CoAs, affect the activity of mitochondrial FA oxidation23,71. In particular, CPT1 is a key rate-limiting enzyme involved in mitochondrial FA oxidation72. CPT1 is regulated at multiple levels, with PPARα promoting Cpt1 mRNA expression during fasting, which leads to enhanced CPT1 enzyme activity73–75. In addition, alterations in tissue malonyl-CoA levels and in enzyme susceptibility to malonyl-CoA inhibition modulate hepatic CPT1 activity76,77. This feedback mechanism can effectively govern the fate of FAs toward oxidation or synthesis, considering that malonyl-CoA is an intermediate metabolite of DNL.
Obesity-associated adipose tissue dysfunction and NAFLD
In NAFLD patients, the majority (59%) of hepatic fat is derived from nonesterified FAs (NEFAs), 26% from DNL, and 15% from diet78. In particular, plasma FAs are the major source of hepatic TGs during fasting. Considering the increased blood NEFA level due to elevated lipolysis in the adipose tissue of obese subjects79, proper modulation of circulating NEFA levels may be used to avoid NAFLD development. The obesity-induced inflammatory response in adipose tissue is well known to contribute to enhanced basal lipolysis80. During excessive energy accumulation in obese adipose tissue, inflammatory factor expression is induced by hypoxia, ER stress, and enlarged adipocytes79,80. This not only stimulates proinflammatory responses in adipose tissue macrophages but also leads to increased infiltration of circulating monocytes into obese adipose tissue81–83. In addition to macrophages, immune cells (such as neutrophils, Th1 T cells, and CD8 T cells) increase, while anti-inflammatory immune cells (such as M2 macrophages, regulatory T cells, and invariant natural killer T cells) decrease, ultimately exacerbating adipose tissue inflammation79,84. Moreover, proinflammatory cytokines, such as TNFα and IL-1β, are elevated in obese adipose tissue and stimulate various inflammatory signaling pathways, including JNK and NF-κB, accelerating insulin resistance85. This eventually suppresses the crucial function of adipocytes as an energy reservoir and leads to increased basal lipolysis, which mediates increased blood NEFA levels. This may also drive ectopic fat accumulation in other organs, such as the liver, muscle, and heart, and may lead to steatosis of NAFLD (Fig. 2).
Fig. 2. Obesity-induced adipose tissue inflammation and NAFLD.
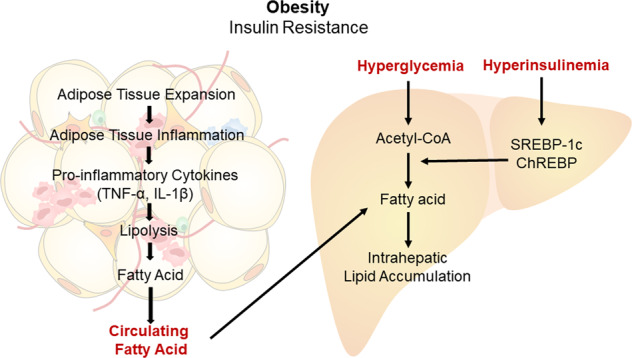
Excess energy intake promotes adipose tissue expansion, which is closely associated with elevated pro-inflammatory responses in adipose tissue through the recruitment of various immune cells. In turn, these cells enhance the levels of proinflammatory cytokines, such as TNF-α and IL-1β. Inflammatory signaling stimulates adipocyte lipolysis, thereby increasing FA influx into the liver. Moreover, insulin resistance in obese subjects mediates hyperglycemia and hyperinsulinemia, which stimulates DNL by supplying substrates and inducing the activation of key lipogenic transcription factors (SREBP-1c and ChREBP). All of these factors (increased plasma FA levels, hyperglycemia, and hyperinsulinemia) exacerbate lipid accumulation in the liver.
Insulin resistance caused by obesity can result in hyperinsulinemia, which can enhance DNL via transcriptional activation of SREBP-1c or ChREBP86,87. Furthermore, increased blood glucose levels, which can be induced by inefficient insulin-mediated suppression of hepatic glucose production, contribute to the supply of substrates for DNL88. In addition to the rise in basal lipolysis caused by inflammation, the suppression of lipolysis by insulin is impeded in the setting of insulin resistance80, resulting in increased circulating FA levels. Furthermore, circulating FAs can increase hepatic acetyl-CoA levels through enhanced FA uptake into the liver. Inefficient hepatic lipid disposal eventually leads to the accumulation of acetyl-CoA, promoting DNL and conversion to FA metabolites, including DG, which may suppress insulin receptor signaling through PKCε activation (Fig. 2)11,89. Therefore, deteriorating hepatic steatosis resulting from enhanced lipid anabolism and reduced lipid catabolism, which can be accelerated by various stress responses and insulin resistance, can progress to NAFLD.
Liver inflammation and IκB kinases
Short-term inflammation induced by an acute stimulus in the liver can have beneficial effects on hepatocyte survival and wound healing; however, chronic hepatic inflammation accelerates hepatocyte dysfunction and fibrosis by inducing hepatocyte injury and inflammatory responses90. Of note, NF-κB is one of the primary regulators of liver inflammation, and its activity is upregulated, which is an important factor leading to NAFLD91.
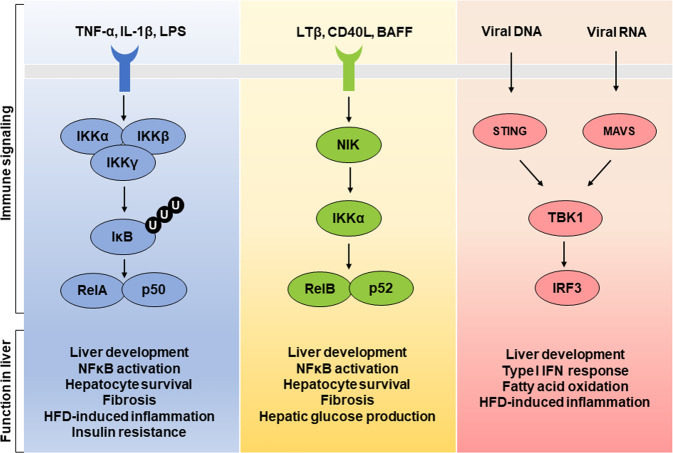
There are five NF-κB family members: NF-κB1 (p105 and p50), NF-κB2 (p100 and p52), c-Rel, RelA, and RelB. Each has a Rel homology domain, which is involved in DNA binding, dimerization, and interactions with IκB92. NF-κB signaling can be classified into canonical and noncanonical pathways. Canonical pathways are activated by stimuli such as lipopolysaccharides or inflammatory cytokines, including TNF-α and IL-1β. Initiation of the canonical pathway via Toll-like receptor or cytokine receptor signaling depends on the IKK complex formed by IKKα, IKKβ, and IKKγ (NEMO) to mediate IκB phosphorylation-mediated degradation, which liberates NF-κB and stimulates nuclear localization to induce target gene expression (inflammatory cytokines, growth factors, and cell adhesion molecules) (Fig. 3)93. Noncanonical NF-κB signaling is driven by ligands such as BAFF, CD40L, LTα1β2/LIGHT, and RANKL94. Ligand stimulation activates an IKKα homodimer via NF-κB-inducing kinase (NIK), which cleaves p100 to p52, triggering transcription via the p52:RelB complex. Noncanonical NF-κB signaling plays an important role in the development of B cells and lymphoid organs (spleen, lymph nodes, mucosal lymphoid tissue)94. Given NF-κB signaling is closely associated with hepatic inflammation, the roles of IKKs in hepatocyte survival, inflammation, and energy metabolism have been investigated using diverse genetic animal models.
Fig. 3. IKK and TBK1 functions in the liver.
Canonical NF-κB signaling is mediated by the IKKα/IKKβ/IKKγ complex upon proinflammatory cytokine (TNF-α or IL-1β) or lipopolysaccharide stimulus. This complex induces the phosphorylation-mediated degradation of IκB, which leads to NF-κB activation. Furthermore, LTβ, CD40L, and BAFF stimulate NIK phosphorylation, thereby promoting IKKα phosphorylation and downstream signaling via the RelB:p52 complex. On the other hand, TBK1 is phosphorylated via STING or MAVS activation upon viral infection, which promotes IRF3 activation for the type I IFN response. Additionally, inactive TBK1 promotes FA oxidation in hepatocytes.
Hepatic IKKβ
IKK is the major signaling factor mediating the inflammatory response, and it could impact NAFLD progression. Whole-body IKKβ KO mice are embryonic lethal due to massive liver apoptosis95,96. IKKβ deficiency diminishes not only basal NF-κB activation but also TNFα-induced activation, which suggests a key role for IKKβ in liver development and NF-κB signaling. Liver-specific IKKβ KO mice exhibited normal liver function with little residual NF-κB activity97. However, hepatic apoptosis was elevated in liver-specific IKKβ KO mice upon concanavalin A administration, increasing circulating TNFα levels. Furthermore, in a DEN (diethylnitrosamine)-induced HCC model, liver-specific IKKβ KO increased hepatocyte death, demonstrating that IKKβ has antiapoptotic activity in the liver98. On the other hand, HFD-fed liver-specific IKKβ KO mice showed improved glucose intolerance and insulin-mediated hepatic glucose production suppression compared to control mice99. Concomitantly, HFD-induced inflammatory gene expression was reduced in the KO mice. Also, transgenic mice with liver-specific constitutively active IKKβ developed insulin resistance and showed elevated secretion of hepatic cytokines, such as IL-1 and TNF-α91. These data suggest that hepatic IKKβ mediates HFD-induced insulin resistance and inflammation. Hepatic IKKβ has also been shown to promote intracellular lipidation by activating ChREBP, which can lead to enhanced VLDL secretion and, consequently, higher plasma TG levels100,101. Furthermore, liver-specific constitutively active IKKβ transgenic mice demonstrated significant increases in NF-κB signaling, inflammatory cell recruitment, and fibrosis102. These findings indicate that hepatic IKKβ inhibition may be a therapeutic target for NAFLD treatment. For example, in high sucrose diet-induced NASH model mice, administration of a pharmacological IKKβ inhibitor (AS602868) reduced weight gain, adipose tissue weight, and inflammatory cytokine production103.
Hepatic NEMO
NEMO binds to IKKα and IKKβ as a noncatalytic scaffolding subunit to mediate IκB phosphorylation, which has an essential role in canonical NF-κB signaling104,105. Like IKKβ KO mice, whole-body NEMO KO mice are embryonic lethal due to severe apoptosis-mediated liver damage, along with compromised IκB phosphorylation and NF-κB activity106. Complete NF-κB inactivation with increased hepatocyte death in hepatocyte-specific NEMO-deficient mice demonstrated that NEMO-mediated NF-κB signaling is essential for hepatocyte survival107. Of note, liver-specific NEMO KO mice gained less body weight but had exacerbated hepatic macrovascular steatosis with elevated apoptosis and proinflammatory cytokine expression108. These mice also showed increased spontaneous tumorigenesis, which reveals the important interaction between hepatocyte lipid metabolism and inflammation and their contribution to the regulation of tumorigenesis. However, the detailed mechanisms underlying these interactions have not yet been elucidated.
Hepatic NIK
NIK has a key role in noncanonical NF-κB signaling via IKKα phosphorylation. IKKα phosphorylation is increased in liver tissues from leptin-deficient ob/ob mice109. Whole-body NIK KO mice show lowered blood glucose levels and reduced pyruvate tolerance, which may indicate suppressed gluconeogenesis109. Mechanistically, hepatic NIK enhances the phosphorylation-mediated stability of CREB, which is one of the key transcription factors regulating fasting-induced gluconeogenic genes. In the context of increased IKKα activity in obese liver tissue, IKKα is involved in increasing blood glucose levels by stimulating hepatic glucose production. On the other hand, hepatocyte-specific NIK-overexpressing transgenic mice exhibit massive liver inflammation, oxidative stress, hepatocyte apoptosis, and liver fibrosis110. Overexpression of a mutant NIK (G885R) lacking catalytic activity for noncanonical NF-κB activation promoted hepatocyte apoptosis to the same extent as wild-type NIK overexpression, suggesting noncanonical NF-κB-independent regulation by NIK. Liu et al. assessed the roles of NIK in different cell types using hepatocyte-specific KO and myeloid cell-specific KO mice111. Both NIK-deficient mouse models showed comparable levels of hepatic steatosis and inflammation. Interestingly, Mx1-cre driver-mediated NIK deficiency in both hepatocytes and immune cells diminished hepatic inflammation and steatosis with reduced expression of lipogenic genes, such as FASN, ACC1, and SREBP-1c111. These data reveal that NIK mediates the interaction between hepatocytes and immune cells in the modulation of steatosis. However, further studies are needed to determine whether NIK exerts this function via the noncanonical NF-κB response and to unravel the mechanism controlling hepatic inflammation.
IKK-related kinase TBK1
TBK1 is an IKK-related kinase along with IKKε, and they exhibit 61% sequence identity112. TBK1, also known as NAK or T2K, is structurally similar to IKKβ. However, as TBK1 lacks the NEMO-binding domain that allows IKKβ to bind NEMO to form the IKK complex for IκB phosphorylation, it has been speculated that TBK1 has a distinct substrate preference112,113. TBK1 is composed of an N-terminal kinase domain (KD), a ubiquitin-like domain (ULD), and a C-terminal scaffolding/dimerization domain (SDD), and this domain arrangement appears to be shared among IKK family members93,114. These three domains combine to form a tripartite architecture upon dimerization, allowing the active kinase sites to face away from one another114,115. Upon upstream signaling, TBK1 is recruited to the signaling complex for activation116. A high local concentration of TBK1 allows interdimer KD interactions that lead to trans-autophosphorylation114,115. Once activated, phosphorylated TBK1 can rapidly phosphorylate the remaining TBK1 pool to form fully activated kinase dimers115. Both adaptor protein recruitment upon upstream signaling and the KD back-to-back structure of the TBK1 dimer provides fine-tuning regulatory mechanisms for TBK1 activity.
TBK1 substrate specificity appears to be regulated within the KD because removal of the ULD and SDD does not alter the specificity of TBK1114. In addition, subcellular localization may be a key determinant of TBK1 substrate specificity115,116. As the adaptors have unique binding partners, they recruit TBK1 to distinct signaling complexes and cellular locations, thereby directing TBK1 activity toward specific downstream pathways115. Thus, TBK1 can modulate various downstream substrates, which support the diverse functions of TBK1 through the formation of several different complexes. TBK1 has critical roles in the regulation of immune responses, autophagy, and energy metabolism.
Innate immune responses
TBK1 and IKKε have been explored as mediators of innate immune responses that mediate antiviral responses through interferon regulatory factor 3 (IRF3) phosphorylation117,118. The two IKK-related kinases have similar enzymatic features and require Ser172 phosphorylation for kinase activity119. TBK1 and IKKε have a redundant function in the antiviral response, although they are expressed at different levels, depending on the cell type120. Upon viral or bacterial infection, cytoplasmic DNA is sensed by cyclic GMP-AMP synthase, which synthesizes cGAMP, which can oligomerize stimulator of interferon genes (STING), which recruits and activates TBK1. Activated TBK1 phosphorylates STING, which leads to the phosphorylation of IRF3 and IRF7 to induce the expression of type I interferons and other cytokines (Fig. 3)121. In addition, TBK1 mediates antiviral responses by interacting with mitochondrial antiviral signaling protein (MAVS) in mitochondria122. Thus, downstream TBK1 signaling may be affected by its localization.
Autophagy
Autophagy is a self-digestion mechanism responsible for the removal of damaged organelles, malformed proteins, and nonfunctional long-lived proteins via lysosomal degradation of autophagosomes123. TBK1 is known as a key regulator of autophagy. In the context of innate immune response regulation, TBK1 has been shown to promote lysosomal degradation of ubiquitinated bacteria via interaction with NDP52 or optineurin (OPTN)124–126. In addition, phosphorylation of p62, known as an autophagic cargo adaptor protein, at Ser403 is critical for autophagic maturation, and TBK1 has been demonstrated to induce p62 Ser403 phosphorylation127,128.
Furthermore, impaired lipophagy, which is a selective degradation of intracellular lipid droplets by lysosomes, has been suggested to be linked to NAFLD progression129–131. Recently, it has been reported that increased SREBP-1c expression in response to an HFD suppresses cystathionine gamma-lyase-mediated ULK1 sulfhydration, which can exacerbate hepatic steatosis via reduced lipophagy132. However, whether TBK1 can modulate lipophagy in hepatocytes has not yet been elucidated.
Mitophagy is a type of selective autophagic regulation that removes depolarized or damaged mitochondria. If mitophagy is not properly coordinated, it can augment damaged mitochondrial accumulation, cellular dysfunction, mitochondria-mediated ATP deprivation, and oxidative stress responses133–135. TBK1 binds to polyubiquitinated mitochondria and promotes autophagosome engulfment via p62 Ser403 phosphorylation to mediate PINK/PARKIN-dependent mitophagy136. TBK1 autophosphorylation upon mitophagy induction is reported to occur first, followed by the recruitment of adaptor proteins, such as OPTN, NDP52, and p62, suggesting that TBK1 has key regulatory functions in the early phases of mitophagy137,138. PINK1/PARKIN-dependent and PINK1/PARKIN-independent pathways have been investigated as mitophagy regulatory mechanisms139. In C2C12 skeletal muscle cells, treatment with a mitochondrial depolarization reagent (carbonyl cyanide m-chlorophenyl hydrazone) induced AMPK activation along with TBK1 activation via the PINK1/PARKIN-independent pathway, which led to autophagosomal engulfment140. Although mitochondrial numbers have been recently found to be increased in liver-specific TBK1 KO mice, more research is needed to evaluate whether the alterations are driven by impaired mitophagy141.
Energy metabolism
TBK1 has a ubiquitous tissue distribution, while IKKε is more strongly expressed in immune cells120,142. IKKε is involved in obesity-related energy metabolism. IKKε expression has been shown to be higher in the adipose tissue and liver of obese mice, and whole-body IKKε KO mice showed reduced HFD-induced body weight gain and adipose tissue expansion and thus lower adipose inflammatory responses143. In addition, increased activities of IKKε and TBK1 can lead to obesity-mediated catecholamine resistance, primarily in adipocytes, by mediating PDE3B phosphorylation to restrict cAMP levels142. However, the specific role of hepatic IKKε in the regulation of lipid metabolism needs to be further investigated.
To clarify the function of TBK1 in the regulation of energy metabolism and obesity-induced inflammation in adipocytes and hepatocytes in vivo, adipocyte- and hepatocyte-specific TBK1 KO mice have been studied. Adipocyte-specific TBK1 KO mice gained less body weight due to increased energy expenditure but showed elevated adipose tissue inflammation with increased insulin resistance upon HFD feeding144. Mechanistically, adipocyte TBK1 mediates the inhibitory phosphorylation of AMPK and phosphorylates NIK, which results in increased energy expenditure with enhanced adipose inflammation in these mice.
In contrast, hepatic TG accumulation is significantly increased in liver-specific TBK1 KO (LTKO) mice upon both a normal chow diet and HFD feeding, with comparable body weights141. In particular, after a 16-h fast, hepatic TG accumulation in LTKO mice was increased due to impaired fasting-stimulated FA oxidation. Fasting-stimulated mitochondrial localization of ACSL1 was suppressed, thereby diminishing FA channeling for subsequent FA oxidation in LTKO mice. Conversely, ER-localized ACSL1 was enriched in the LTKO mouse liver, with enhanced re-esterification activity for TG synthesis. The suppression of FA oxidation with enhanced re-esterification activity in LTKO mice supports the important regulatory role of TBK1 in ACSL1 localization in the two organelles. Notably, fasting increased nonphosphorylated (inactive) TBK1 in hepatocytes. In addition, kinase-dead TBK1 (K38A) showed a higher binding affinity for ACSL1. Moreover, kinase-dead TBK1 more efficiently restored FA oxidation activity in TBK1-deficient primary hepatocytes than WT TBK1, supporting the role of inactive TBK1 in fasting-induced FA oxidation as a scaffolding protein for ACSL1. Although it has been suggested that inactive TBK1 interacts with ACSL1 with greater binding affinity, more research is needed to determine how mitochondrial localization is governed by TBK1. Furthermore, hepatic lipid accumulation has been observed in LTKO mice without inflammatory gene activation during NCD feeding; however, the increase in inflammation-related marker gene expression in HFD-fed LTKO mice may indicate that hepatic steatosis can precede inflammation in NAFLD progression.
Conclusion
Obesity-related inflammation and insulin resistance are associated with hepatic lipid accumulation and systemic inflammation, which contribute to NAFLD development. NAFLD occurs when FA oxidation and lipid export fail to compensate for increased lipid uptake and synthesis. In addition to hepatic lipid accumulation, the inflammatory response is a crucial factor in NAFLD pathogenesis. In particular, IKKs and TBK1 play crucial roles in hepatic inflammation and lipid homeostasis, consequently modulating NAFLD progression. Nonetheless, the question of how inflammatory signaling affects lipid metabolism and vice versa is largely unanswered. Given that, emerging evidence supports the pivotal functions of TBK1 in governing the inflammatory response, energy metabolism, and autophagy, TBK1 might serve as a signal integration point in response to various environmental stimuli. Notably, considering the regulatory function of TBK1 in the autophagy process, its effects on lipid metabolism, including lipophagy, remain to be determined in future studies. In addition, to understand NAFLD pathogenesis, it is necessary to unravel the molecular mechanisms underlying the cooperative regulation of lipid metabolism and inflammation, thus identifying potential therapeutic targets for improving NAFLD progression.
Acknowledgements
We thank Sang Mun Han for help in creating figures. This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) 2021R1C1C2010446 to J.Y.H. and US National Institutes of Health grants R01DK117551, 1R01DK125820, and R01DK076906 to A.R.S.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am. Heart J. 2005;149:33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Gruben N, Shiri-Sverdlov R, Koonen DP, Hofker MH. Nonalcoholic fatty liver disease: a main driver of insulin resistance or a dangerous liaison? Biochim. Biophys. Acta. 2014;1842:2329–2343. doi: 10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 9.Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr. Physiol. 2017;8:1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013;19:210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv. Nutr. 2013;4:697–710. doi: 10.3945/an.113.004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim. Biophys. Acta. 1999;1441:4–13. doi: 10.1016/s1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- 13.Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem. Biophys. Res. Commun. 1999;255:34–39. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- 14.Wilson CG, et al. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology. 2016;157:570–585. doi: 10.1210/en.2015-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 16.Buque X, et al. A subset of dysregulated metabolic and survival genes is associated with severity of hepatic steatosis in obese Zucker rats. J. Lipid Res. 2010;51:500–513. doi: 10.1194/jlr.M001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab. 2001;12:266–273. doi: 10.1016/s1043-2760(01)00427-1. [DOI] [PubMed] [Google Scholar]
- 18.Seessle J, Liebisch G, Schmitz G, Stremmel W, Chamulitrat W. Palmitate activation by fatty acid transport protein 4 as a model system for hepatocellular apoptosis and steatosis. Biochim. Biophys. Acta. 2015;1851:549–565. doi: 10.1016/j.bbalip.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Krammer J, et al. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int. J. Med. Sci. 2011;8:599–614. doi: 10.7150/ijms.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doege H, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130:1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Milger K, et al. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 22.Digel M, Ehehalt R, Stremmel W, Fullekrug J. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol. Cell. Biochem. 2009;326:23–28. doi: 10.1007/s11010-008-0003-3. [DOI] [PubMed] [Google Scholar]
- 23.Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 2014;34:1–30. doi: 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp. Biol. Med. (Maywood) 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Lewin TM, Coleman RA. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 2001;276:24667–24673. doi: 10.1074/jbc.M010793200. [DOI] [PubMed] [Google Scholar]
- 27.Li LO, et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009;284:27816–27826. doi: 10.1074/jbc.M109.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young PA, et al. Long-chain acyl-CoA synthetase 1 interacts with key proteins that activate and direct fatty acids into niche hepatic pathways. J. Biol. Chem. 2018;293:16724–16740. doi: 10.1074/jbc.RA118.004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman RA. It takes a village: channeling fatty acid metabolism and triacylglycerol formation via protein interactomes. J. Lipid Res. 2019;60:490–497. doi: 10.1194/jlr.S091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LO, et al. Overexpression of rat long chain acyl-coa synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J. Biol. Chem. 2006;281:37246–37255. doi: 10.1074/jbc.M604427200. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Kerner J, Hoppel CL. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. Biol. Chem. 2011;286:25655–25662. doi: 10.1074/jbc.M111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev. 2011;111:6359–6386. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, et al. Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance. Nutr. Diabetes. 2018;8:34. doi: 10.1038/s41387-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 36.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Hammond LE, et al. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- 38.Nagle CA, et al. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6-/- mice. J. Lipid Res. 2008;49:823–831. doi: 10.1194/jlr.M700592-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergnes L, et al. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J. Lipid Res. 2006;47:745–754. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen MR, Corstorphine CC, Zammit VA. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 1997;323:17–21. doi: 10.1042/bj3230017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnato C, Igal RA. Overexpression of diacylglycerol acyltransferase-1 reduces phospholipid synthesis, proliferation, and invasiveness in simian virus 40-transformed human lung fibroblasts. J. Biol. Chem. 2003;278:52203–52211. doi: 10.1074/jbc.M305760200. [DOI] [PubMed] [Google Scholar]
- 42.Chen HC, et al. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gluchowski NL, et al. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology. 2019;70:1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin NB, et al. Targeting diacylglycerol acyltransferase 2 for the treatment of nonalcoholic steatohepatitis. Sci. Transl. Med. 2019;11:eaav9701. doi: 10.1126/scitranslmed.aav9701. [DOI] [PubMed] [Google Scholar]
- 45.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 47.Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 49.Abu-Elheiga L, et al. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl. Acad. Sci. USA. 2005;102:12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao J, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 52.Chirala SS, et al. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proc. Natl. Acad. Sci. USA. 2003;100:6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravarthy MV, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Syed-Abdul MM, et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Kim JB, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita H, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higuchi N, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 62.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shelness GS, Sellers JA. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001;12:151–157. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Perla FM, Prelati M, Lavorato M, Visicchio D, Anania C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Child. (Basel) 2017;4:46. doi: 10.3390/children4060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin. Chim. Acta. 2006;368:1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 67.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Grundy SM, Patel SB. Obesity in db and ob animals leads to impaired hepatic very low density lipoprotein secretion and differential secretion of apolipoprotein B-48 and B-100. J. Lipid Res. 1997;38:1277–1288. [PubMed] [Google Scholar]
- 69.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010;11:380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 70.Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernandez-Fernandez C, Mourino-Bayolo D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion. 2019;46:73–90. doi: 10.1016/j.mito.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rognstad R. Rate-limiting steps in metabolic pathways. J. Biol. Chem. 1979;254:1875–1878. [PubMed] [Google Scholar]
- 73.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 74.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 75.Mascaro C, et al. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 1998;273:8560–8563. doi: 10.1074/jbc.273.15.8560. [DOI] [PubMed] [Google Scholar]
- 76.Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim. Biophys. Acta. 1981;665:628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- 77.Saggerson ED, Carpenter CA. Effects of fasting, adrenalectomy and streptozotocin-diabetes on sensitivity of hepatic carnitine acyltransferase to malonyl CoA. FEBS Lett. 1981;129:225–228. doi: 10.1016/0014-5793(81)80170-6. [DOI] [PubMed] [Google Scholar]
- 78.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 84.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010;12:83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 87.Hodson L, Gunn PJ. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nat. Rev. Endocrinol. 2019;15:689–700. doi: 10.1038/s41574-019-0256-9. [DOI] [PubMed] [Google Scholar]
- 88.Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016;91:452–468. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumashiro N, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luedde T, Schwabe RF. NF-kappaB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J. Clin. Immunol. 2005;25:541–550. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]
- 93.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 94.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka M, et al. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 97.Maeda S, et al. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 98.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 99.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 100.van Diepen JA, et al. Hepatocyte-specific IKK-beta activation enhances VLDL-triglyceride production in APOE*3-Leiden mice. J. Lipid Res. 2011;52:942–950. doi: 10.1194/jlr.M010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 102.Sunami Y, et al. Hepatic activation of IKK/NFkappaB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology. 2012;56:1117–1128. doi: 10.1002/hep.25711. [DOI] [PubMed] [Google Scholar]
- 103.Beraza N, et al. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut. 2008;57:655–663. doi: 10.1136/gut.2007.134288. [DOI] [PubMed] [Google Scholar]
- 104.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 105.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 106.Rudolph D, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 107.Beraza N, et al. Hepatocyte-specific IKK gamma/NEMO expression determines the degree of liver injury. Gastroenterology. 2007;132:2504–2517. doi: 10.1053/j.gastro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 108.Wunderlich FT, et al. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc. Natl. Acad. Sci. USA. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheng L, et al. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 2012;18:943–949. doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen H, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–2076. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, et al. Liver NF-kappaB-inducing kinase promotes liver steatosis and glucose counterregulation in male mice with obesity. Endocrinology. 2017;158:1207–1216. doi: 10.1210/en.2016-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tojima Y, et al. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 113.Bonnard M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma X, et al. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc. Natl. Acad. Sci. USA. 2012;109:9378–9383. doi: 10.1073/pnas.1121552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 116.Goncalves A, et al. Functional dissection of the TBK1 molecular network. PLoS One. 2011;6:e23971. doi: 10.1371/journal.pone.0023971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 118.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 119.Kishore N, et al. IKK-i and TBK-1 are enzymatically distinct from the homologous enzyme IKK-2: comparative analysis of recombinant human IKK-i, TBK-1, and IKK-2. J. Biol. Chem. 2002;277:13840–13847. doi: 10.1074/jbc.M110474200. [DOI] [PubMed] [Google Scholar]
- 120.Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J. Exp. Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang C, et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–398. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 123.Namkoong S, Cho CS, Semple I, Lee JH. Autophagy dysregulation and obesity-associated pathologies. Mol. Cells. 2018;41:3–10. doi: 10.14348/molcells.2018.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. USA. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- 126.Wild P, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell. 2011;44:279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 128.Pilli M, et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity. 2012;37:223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carotti S, et al. Lipophagy impairment is associated with disease progression in NAFLD. Front. Physiol. 2020;11:850. doi: 10.3389/fphys.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin DW. Lipophagy: molecular mechanisms and implications in metabolic disorders. Mol. Cells. 2020;43:686–693. doi: 10.14348/molcells.2020.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen TTP, et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice. Mol. Cell. 2021;81:3820–3832.e7. doi: 10.1016/j.molcel.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 133.Wallace DC. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pickles S, Vigie P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lou G, et al. Mitophagy and neuroprotection. Trends Mol. Med. 2020;26:8–20. doi: 10.1016/j.molmed.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Matsumoto G, Shimogori T, Hattori N, Nukina N. TBK1 controls autophagosomal engulfment of polyubiquitinated mitochondria through p62/SQSTM1 phosphorylation. Hum. Mol. Genet. 2015;24:4429–4442. doi: 10.1093/hmg/ddv179. [DOI] [PubMed] [Google Scholar]
- 137.Cho DH, Kim JK, Jo EK. Mitophagy and innate immunity in infection. Mol. Cells. 2020;43:10–22. doi: 10.14348/molcells.2020.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zachari M, et al. Selective autophagy of mitochondria on a ubiquitin-endoplasmic-reticulum platform. Dev. Cell. 2019;50:627–643.e5. doi: 10.1016/j.devcel.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 140.Seabright AP, et al. AMPK activation induces mitophagy and promotes mitochondrial fission while activating TBK1 in a PINK1-Parkin independent manner. FASEB J. 2020;34:6284–6301. doi: 10.1096/fj.201903051R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huh JY, et al. TANK-binding kinase 1 regulates the localization of Acyl-CoA synthetase ACSL1 to control hepatic fatty acid oxidation. Cell Metab. 2020;32:1012–1027.e7. doi: 10.1016/j.cmet.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mowers J, et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKepsilon and TBK1. Elife. 2013;2:e01119. doi: 10.7554/eLife.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao P, et al. TBK1 at the crossroads of inflammation and energy homeostasis in adipose. Tissue Cell. 2018;172:731–743.e2. doi: 10.1016/j.cell.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]