Abstract
With an increasing interest and demand for biotechnology crops in agriculture worldwide, genetically modified (GM) breeding stacks produced by conventional breeding of previously approved GM single events remain popular for farmers in GM crop cultivation countries. However, regulations on stacks vary in each country. Currently, Korea requires approval for all breeding stacks intended for cultivation. To determine whether the stack is subject to a full safety assessment as a new GM crop, molecular characterization, protein expression, composition analysis, and agronomic characterization data are required. Korea’s regulatory policy on stacks has not adopted the high-covers-low concept; therefore, subcombinations of already approved higher combination events are subject to breeding stack review if any subcombination was purposefully bred for cultivation use. This review will help promote the efficient management of GM breeding stacks in Korea in the future.
Keywords: GM breeding stack, Conventional breeding, GM single event, Regulatory policy, High covers low
Introduction
Agriculture has been one of the key areas strongly influenced by advances in modern biotechnology, and 25 years have passed since planting genetically modified (GM) crops on commercial scales. Since the first commercial cultivation of GM crops in 1996, its cultivation acreage worldwide increased from 1.7 million hectares in 1996–190.4 million hectares in 29 countries in 2019 (ISAAA, 2019). GM crops contain economically important traits, such as insect resistance, herbicide tolerance, and drought tolerance, and product quality traits, such as modified fatty acid content or non-browning. The meta-analysis provides significant evidence that farmers as first consumers benefit from GM crops through improved productivity and profitability (Klumper and Qaim, 2014) and the end consumer can be provided with improved quality. However, the perception of GM crops has not changed much after 20 years. According to a report by Wunderlich and Gatto (2015), consumer awareness of GM crops has not improved significantly, suggesting that efforts from the industry and the scientific community are needed. GM crops have been regulated for the past 20 years, and safety and risk assessments have been conducted. Not all but some countries regulating GM crops require a separate review of breeding stacks crossed between already approved parental lines.
According to the GM approval database of the International Service for the Acquisition of Agri-biotech Applications (ISAAA), 530 events of 32 plants worldwide are known to have completed safety evaluations for commercialization (ISAAA, 2021). Maize, soybean, cotton, and canola are the most cultivated GM plants more than 90% worldwide. In contrast, other plants, such as alfalfa, apple, bean, carnation, chicory, cowpea, creeping bentgrass, eggplant, eucalyptus, flax, melon, papaya, petunia, pineapple, plum, polish canola, poplar, potato, rice, rose, safflower, squash, sugar beet, sugarcane, sweet pepper, tobacco, tomato, and wheat, are being developed and utilized as needed (ISAAA, 2019, 2021).
Crossing different lines of plants to combine desirable traits or transfer traits to other genetic backgrounds has been one of the pivotal tools for conventional breeding that has a long history of safe use. Breeding stacks crossed between already authorized parental lines provide plants with seeds equipped with multiple times the traits of parental lines. The conventional breeding product between GM crops does not insert new recombinant DNA sequence and so-called “stacked GM event” (Isabel et al., 2008). Compared to single-trait crops, breeding stacks provide certain benefits to farmers who need to overcome diverse insect pests or weeds.
What is a GM breeding stack?
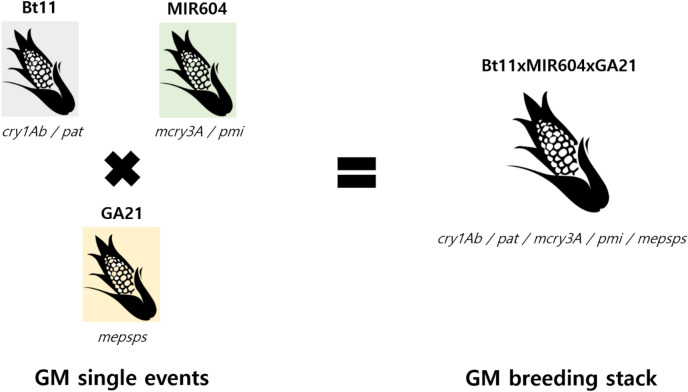
Breeding stacks are obtained by crossing parental lines containing GM traits that have been already approved. Representation for GM breeding stacks is differentiated from GM single events typically through a naming convention that combines the names of each GM single event using the “ × ” nomenclature typical for breeding crosses. In Fig. 1, the GM breeding stack event Bt11 × MIR604 × GA21 contains multiple traits possessed by each GM single event (Bt11 with glufosinate herbicide tolerance and lepidopteran insect resistance, MIR604 with coleopteran insect resistance, and GA21 with glyphosate herbicide tolerance). GM breeding stacks are designed to give farmers a better chance to overcome multiple problems in the field, such as pests, weeds, or environmental stress, so farmers can have more tools to cope with such pressures.
Fig. 1.
Imaging of GMO stack event (Bt11 × MIR604 × GA21)
Several concerns about the problem of GM breeding stacks are whether the breeding stack creates unintended effects and changes that require additional safety assessment. Two main concerns are discussed whether GM breeding stack can increase genomic instability and whether potential interactions between the products of the transgenes in stacked GMO impact safety (Steiner et al., 2013; Wang et al., 2019).
Recent reports have demonstrated that stacked trait products are not substantially different from their conventional comparator or the GM parent plants (Kramer et al., 2016; Wu et al., 2018).
Approval of breeding stacks has been consistent since 2008, when stacked trait approvals consistently outnumbered the single events, and this trend is expected to continue in the future (ISAAA, 2019; Schutte et al., 2017). The GM breeding stack is used in various terms, such as stacked GM event, stacked trait event, stacked trait product, combined trait product, stacked traits, stacked event, or GM stack. In this review, the term “breeding stack” is used to describe a GM breeding stack made by combining individual transformation events using conventional breeding.
Regulatory status of GM stacks in key countries
Given the demands and preferences of farmers for seeds containing various traits, the adoption rate of the stack is predicted to continue to increase in the future. Thus, it is expected that various stack products will be developed and commercialized in the future.
The regulatory principles and data requirements on the safety assessment of GM single events are well harmonized worldwide based on the guidelines published by the Codex Alimentarius Commission (CAC, 2003), the Food and Agricultural Organization/World Health Organization (FAO/WHO, 1996, 2000), the Organization for Economic Cooperation and Development (OECD, 2010), and others. However, there is no international consensus on the regulation of stacks that combines already approved GM events by conventional breeding, and regulatory positions on stacks differ between countries. There are three broad categories of national policies for stacks: (1) countries that do not require separate approvals for breeding stacks, (2) countries that require approval of the higher combination stack, and (3) countries that require approval for every commercialized stack (Table 1).
Table 1.
Difference in regulation status of GM breeding stack by country
| Category | Country | |
|---|---|---|
| Do not require separate approvals for breeding stacks | US | |
| Canada | ||
| Australia/ | ||
| New Zealand | ||
| Require separate approvals for breeding stacks | The approval of the higher combination stack covers all sub-combinations | Japan |
| Argentina | ||
| Brazil | ||
| Singapore | ||
| Philippines | ||
| EU | ||
| Require approval for every commercialized stack | Korea | |
| Taiwan | ||
First, the U.S. Food and Drug Administration, U.S. Department of Agriculture, Health Canada, and Food Standards Australia New Zealand do not separately regulate GM breeding stacks obtained by conventional breeding of single events that have already been approved (FDA, 2001; HC, 2006; ISAAA, 2017; Kramer et al., 2016; Pilacinski et al., 2011). The rationale for this policy is based on the history of the safe use of conventional breeding and the fact that the single events used as parents have already been proven safe and have been approved. That is, if single events are considered safe compared to their conventional counterparts, then the stack obtained by conventional breeding, using them as parents, is as safe as the combination of non-GM parents in conventional varieties.
However, some countries require separate approvals for stacks. Although data requirements vary slightly between countries, most countries that regulate stacks apply comparative safety assessment concept which focuses on the assessment of any differences compared to parental events and its conventional counterpart. Regulatory agencies in those countries request data on a) molecular characteristics that verifies the presence and stability of the traits after combining single events through conventional breeding; b) protein levels comparable to the single event parents; c) substantial equivalence based on composition or agronomic/phenotypic data compared to the conventional non-GM crop (CropLife International, 2015, 2017; Kramer et al, 2016; Pilacinski et al., 2011). Several countries, such as the European Union (EU), Japan, Taiwan, and the Philippines, also require explanations based on the modes of action of the each of traits (ie, logical explanations based on science) to evaluate potential interactions between the parental traits (Goodwin et al., 2021). There is also a difference among countries requiring separate approvals in terms of how to deal with their lower order combinations. In Japan, Argentina, Brazil, the European Union (EU), Singapore, and the Philippines, approval of higher combinations of events covers lower event combinations that can appear during cultivation due to natural genetic segregation (FSCJ, 2004; GMAC, 2020; Kramer et al., 2016; Philippines, 2013; Pilacinski et al., 2011). That is, if the higher combination stack is approved, all subcombinations, including the same events, are approved without any separate approval of the higher combination stack that covers approvals of all subcombinations. It is also called the high-covers-low policy.
In the EU, as described in paragraph 18 of No. 503/2013 of the EU’s regulations, grains harvested from crops where natural genetic segregation occurs include their subcombinations; thus, approval of a higher combination stack may cover subcombination stacks (EC, 2013). In Japan, the Ministry of Health, Labour and Welfare (MHLW) and the Ministry of Agriculture, Forestry and Fisheries (MAFF) concluded that there is no additional safety concern for the GM stack containing a transgene that does not interact with the host’s metabolic pathway, in addition to adopting the high-covers-low policy, based on the experience and knowledge of the stack evaluations accumulated from 2004 to 2014 (MHLW, 2014; USDA, 2016). Therefore, since June 2014, the majority of the breeding stacks are not subject to any additional safety assessment and approval procedures but need to be reported to the relevant authorities. For the stack containing the introduced genes interacting with the host’s metabolic pathway, only “protein expression or bioefficacy data” and “composition data” are required for stack evaluation. In Japan, the MAFF and the Ministry of Environment require approval for a GM breeding stack; it only requires an explanation for the lack of interaction rather than additional experimental data for evaluation. In Argentina, Ministry of Production and Labor recently simplified the regulation by published a new norm for stacked events breeding between single events previously evaluated (Goodwin et al., 2021).
In contrast, in Korea or Taiwan, even if a higher combination stack is approved, separate approvals are required for the subcombinations to be planted in GM cultivation countries, such as the United States, Canada, or Brazil.
GM breeding stack regulation policy and status in Korea
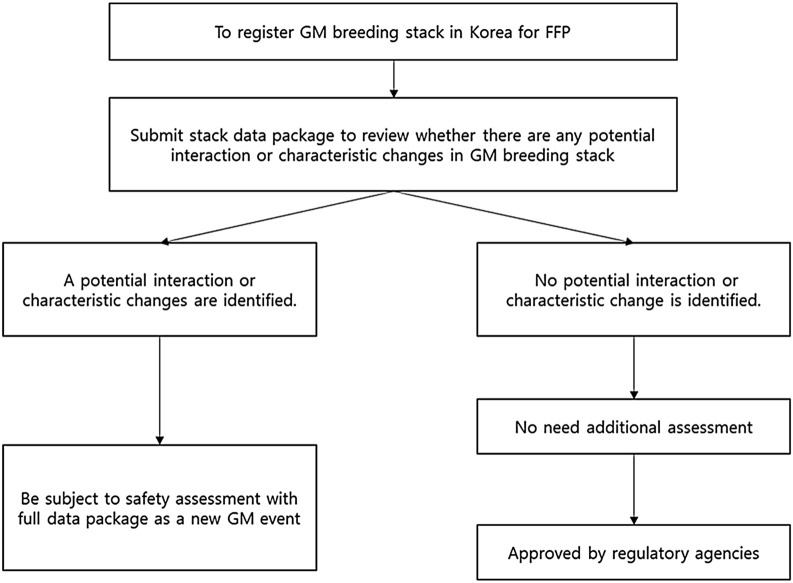
In Korea, GM soybean and GM corn, including GM breeding stacks are important crops used in the food industry. Regulatory policy for these GMOs is essential for food safety management. Currently, the GM breeding stack is subject to regulatory approval in Korea, and the related regulatory agencies are the Ministry of Food and Drug Safety (MFDS) and the Rural Development Administration (RDA). The MFDS conducts a safety assessment for food use in accordance with the Food Sanitation Act and the Act on Transboundary Movement, etc. of Living Modified Organisms (LMO Act). The RDA conducts a risk assessment for feed use according to the LMO Act. Breeding stack reviews by the MFDS and RDA require no change in traits inherited from parental lines. Therefore, applicants need to demonstrate the equivalency of the traits between the breeding stack and its parental lines. If a breeding stack fails to pass the MFDS or the RDA reviews, such a breeding stack will be subject to a risk review required for single GM events. Also, in the context of requiring approval for all stacks to be commercialized, both agencies take the same regulatory approach (Fig. 2).
Fig. 2.
The regulation process on GM breeding stack in Korea
Legal basis and regulation on GM stacks of the MFDS
According to Clauses b of Subparagraph 1 of Article 3 of the “Regulations on the Safety Assessment, etc. of Genetically Modified Foods, etc.” of the MFDS, safety assessments are necessary when the characteristics given from each parent before the breeding are changed, when the stacks are bred between different species, or when the stacks are different from their conventional varieties in the amount of intake, edible parts, and processing method (MFDS, 2021). The MFDS requires data demonstrating no change in the characteristics inherited from each single event, no crossing occurred between different species, and no changes in the amount of intake, edible parts, and processing methods in accordance with Annex 3 of the MFDS regulation to determine whether a breeding stack is subject to safety assessments.
Legal basis and regulation on GM stacks of the RDA
According to Article 3–2 of the “Consolidated Notice on Transboundary Movement, etc. of Living Modified Organisms,” the RDA requires that the following data are submitted to conduct a risk assessment on stacks: (1) data to determine the presence or absence of interactions between traits on inserted nucleotides in the parental lines, (2) information related to the characteristics of the stack, and (3) comprehensive assessment data based on information on parental lines. The RDA also lists the stack on the approval list if it is judged that there is no interaction or specificity as a result of the risk assessment; if not, the regulatory agency considers the stack a new GM crop and carries out the risk assessment according to the same procedure for a single.
Review procedure
Applicants are required to make applications to the MFDS and RDA, attaching experimental data satisfying respective regulations. Upon receiving breeding stack applications, the MFDS and RDA pass along the submission to the MFDS and RDA review committees. Subsequently, the review committee will review the submitted data. If the submitted data do not satisfy the committee, the committee asks the applicant for supplementary data. After the review, which can be up to multiple rounds due to the questions to applicants, the committee then decides whether to authorize the breeding stack. The result of the review will be announced on the website, and the applicant will be notified.
Data requirements for stack evaluation
The “Consolidated Notice on Transboundary Movement, etc. of Living Modified Organisms” is the legal ground of the RDA’s breeding stack review, but it does not specify the data needed for the breeding stack review by the RDA. In contrast, the MFDS specifies the data requirements in Annex Table 3 of the “Regulations on the Safety Assessment, etc. of Genetically Modified Foods, etc.” The data required by the MFDS and RDA for the breeding stack review are as follows: Southern blots and/or sequencing data as molecular characterization to confirm the integrity of the transgenic inserts compared to parental lines, protein expression measured by enzyme-linked immunosorbent assay for the comparison between the breeding stack and the parental lines, and composition analysis data comparing the breeding stack and the near isoline not containing transgenes. In addition, agronomic characteristic data comparing the breeding stack and its near isoline are required specifically for RDA review.
Approval status of GM stack events intended for commodity grain import for food and/or feed use in Korea
As of May 2021, a total of 101 GM breeding stacks have been approved by the MFDS or RDA for food or feed use, which covers five crops: maize, soybean, cotton, canola, and alfalfa (Table 2). Among the approved items were 64 maize products, which account for the largest portions, and most of the items are usually insect-resistant and herbicide-tolerant (KBCH, 2021). Of the approved breeding stacks, the largest event combination was crossed between six different parental lines, of which eight different subcombinations of the breeding stack have also been approved in Korea. Korea has accumulated considerable experience and knowledge in breeding stack review for more than 10 years since the early 2000s. There have been no significant differences in the stability of introduced genes, protein expression levels, composition, and agronomic performance in a large number of the breeding stacks reviewed to date, and there have been interactions or changes in the characteristics identified.
Table 2.
List of approved stacked event in Korea
| Classification | Stacked events (101) |
|---|---|
| Soybean (12) | DAS-68416-4xMON89788, DAS-81419-2xDAS-44406–6, DP-305423-1xGTS40-3–2, DP-305423-1xMON87708xMON89788, FG72xA5547-127, MON87701xMON89788, MON87705xMON87708xMON89788, MON87705xMON89788, MON87708xMON89788, MON87708xMON89788xA5547-127, MON87751xMON87701xMON87708xMON89788, MON87769xMON89788 |
| Maize (64) | TC1507xMON810xMIR162, 3272xBt11xMIR604xGA21, 3272xBt11xMIR604xTC1507 × 5307xGA21, Bt11xDAS-59122-7xMIR604xTC1507xGA21, Bt11xGA21, Bt11xMIR162, Bt11xMIR162xGA21, Bt11xMIR162xMIR604xTC1507 × 5307xGA21, Bt11xMIR162xMIR604xGA21, Bt11xMIR162xTC1507xGA21, Bt11xMIR604, Bt11xMIR604xGA21, Bt11xMIR604xTC1507 × 5307xGA21, Bt11xMIR162xMIR604xMON89034 × 5307xGA21, Bt11xMIR162xMON89034, Bt11xMIR162xMON89034xGA21, Bt11xTC1507xGA21, DAS-59122-7xNK603, DAS-59122-7xTC1507xNK603, DP-004114-3xMON810xMIR604xNK603, DP-004114-3xMON89034xMON87411xDAS-40278–9, GA21xT25, MIR604xGA21, MON810xGA21a, MON810xMON863xNK603, MON810xNK603, MON863xMON810, MON863xNK603, MON87427xMON87419xNK603, MON87427xMON87460xMON89034xTC1507xMON87411xDAS-59122–7, MON87427xMON89034xMIR162xMON87411, MON87427xMON89034xMIR162xNK603, MON87427xMON89034xMIR162xMON87419xNK603, MON87427xMON89034xMON810xMIR162xMON87411xMON87419, MON87427xMON89034xMON87419xNK603, MON87427xMON89034xMON88017, MON87427xMON89034xNK603, MON87427xMON89034xTC1507xMON87411xDAS-59122–7, MON87427xMON89034xTC1507xMON88017xDAS-59122–7, MON87460xMON89034xMON88017, MON87460xMON89034xNK603, MON87460xNK603, MON88017xMON810, MON89034xMIR162, MON89034xMON88017, MON89034xNK603, MON89034xTC1507xMIR162xNK603, MON89034xTC1507xMIR162xNK603xDAS-40278–9, MON89034xTC1507xMON88017xDAS-59122–7, MON89034xTC1507xMON88017xDAS-59122-7xDAS40278-9, MON89034xTC1507xNK603xDAS-40278–9, MON89034xTC1507xNK603, NK603xDAS-40278–9, NK603xT25, NK603xT25xDAS-40278–9, TC1507xDAS-59122–7, TC1507xDAS-59122-7xMON810xMIR604xNK603, TC1507xDAS-59122-7xMON810xNK603, TC1507xMIR604xNK603, TC1507xMON810, TC1507xMON810xMIR162xNK603, TC1507xMON810xMIR604xNK603, TC1507xMON810xNK603, TC1507xNK603 |
| Cotton (18) | 281/3006xCOT102xMON88913, 281/3006xCOT102xMON88913xDAS-81910–7, 281/3006xMON1445a, 281/3006xMON88913, COT102xMON15985xMON88913, COT102xMON15985xMON88913xMON88701, GHB614xLLCotton25, GHB614xLLCotton25xMON15985, GHB614xT304-40xGHB119, GHB614xT304-40xGHB119xCOT102, LLCotton25xMON15985, MON15985xMON1445, MON15985xMON88913, MON88701xMON88913xMON15985, T304-40xGHB119, T304-40xGHB119xCOT102b, MON531xMON1445, MON88701xMON88913 |
| Canola (6) | DP-073496-4xRF3, MON88302xMS8xRF3, MON88302xRF3, MS11xRF3, MS11xRF3xMON88302, MS8xRF3xRT73 |
| Alfalfa (1) | KK179xJ101 |
a only used for food, b only used for agricultural
Discussion
Global guidelines, such as the Codex guideline, have contributed to the globally aligned safety assessment for GM crops. However, countries regulating GM crops have had a range of different views for breeding stacks. Thus, not all countries regulating GM crops require the safety review of breeding stacks crossed between already approved parental lines. Also, there are no globally agreed safety assessment guidelines for breeding stacks.
Conventional plant breeding techniques have been used for breeding nonbiotechnology-derived traits for a long time to develop new varieties with improved productivity and quality (CropLife International, 2013; FAO/WHO, 2000; Hajjar and Hodgkin, 2007; Pilacinski et al., 2011; Powell et al., 2003), and conventionally bred crops between non-GM crops are not regulated separately (Bradford et al., 2005). Many scientists claimed that the GM breeding stack, obtained using the same techniques used safely for a long time, is not a new event. They argued that it could not be said that the GM breeding stack is more harmful than the stacking of conventional varieties; therefore, if the parental lines are approved, no additional approval is required for the breeding stack (Kok et al., 2014; Kramer et al., 2016; Pilacinski et al., 2011). The results of reviewing the safety of stacks in the European Food Safety Authority and other countries for more than 15 years, as well as the stack reviews conducted in Korea, confirmed that there was no significant change that might cause concerns over insert stability, protein expression, composition, and agronomy. Wang et al. (2019) reported that the development of stack events through conventional breeding of single events did not affect the safety of transfer DNA (T-DNA). In addition, assessments of single events that have already been approved as safe through comprehensive and robust assessments provide sufficient scientific evidence to determine whether the stack could potentially affect the safety of humans, animals, or the environment. Therefore, when GM events are combined by conventional breeding, the stack is considered as safe as the parental lines if the parental lines of the breeding stack are considered safe (CropLife International, 2015).
In Korea, the debate over GM organisms has become a social issue. Therefore, Korean Government authorities have approached the safety assessment of GM crops in a very conservative manner. Currently, under Korea’s regulatory policy, all commercialized stacks must be approved in Korea, and the high-covers-low policy is not yet applied. However, as genetic segregation of introduced events naturally occurs during the cultivation of segregating crops, such as maize, commodity grains harvested from the higher combination breeding stack contain the kernels of various subcombinations of GM events. This scientific fact of the occurrence of kernels containing subcombinations has been recognized by Korean authorities, but any subcombinations to be commercialized required separate authorization in Korea. It seems that Korean regulatory agencies are continuing to monitor international trends and scientific evidence for stack regulation and are making every effort to establish scientific and rational regulations.
Acknowledgements
This work was supported by a grant from the New breeding technologies development Program (Project No. PJ015190), Rural Development Administration, Republic of Korea.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bradford KJ, Van Deynze A, Gutterson N, Parrott W, Strauss SH. Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nature Biotechnology. 23: 439-444 (2005) [DOI] [PubMed]
- Codex Alimentarius Commission. Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants (CAC/GL 45–2003). Available from: http://www.fao.org/fileadmin/user_upload/gmfp/docs/CAC.GL_45_2003.pdf. Accessed June 30, 2003
- CropLife international. Position Paper: Compositional and nutritional safety assessment of stacked trait products and their lower order combinations. Available form: https://croplife.org/wp-content/uploads/2016/08/Composition-and-nutritional-safefty-assessment-of-stacked-trait-products.pdf. Accessed Feb 1, 2017
- CropLife International. Position Paper: Regulation of Plant Biotechnology-Derived Breeding Stacks. Available from: https://croplife.org/wp-content/uploads/pdf_files/CLI-STPT-Stack-Position-Paper-January-2015_FINAL.pdf. Accessed Jan 29, 2015
- CropLife International. Position paper: regulatory assessment of plant biotechnology-derived combined event products. Available from: https://croplife.org/wp-content/uploads/pdf_files/Regulatory-Assessment-of-Plant-Biotechnology-Derived-Combined-Event-Products.pdf. Accessed Jul 10, 2013
- FAO/WHO. Joint FAO/WHO Expert Consultation on Biotechnology and Food Safety. Available from: http://www.fao.org/ag/agn/food/pdf/biotechnology.pdf. Accessed Sep 30, 1996
- FAO/WHO. Safety aspects of genetically modified foods of plant origin. http://www.fao.org/fileadmin/templates/agns/pdf/topics/ec_june2000_en.pdf. Accessed May 29, 2000
- FDA. United States food and drug administration. Premarket notice concerning bioengineered foods. Federal Register. 66: 4706-4738 (2001)
- Food Safety Commission of Japan. Stance on the safety assessment of GM plants generated through cross-breeding Available from: https://www.fsc.go.jp/hyouka/index.data/GM_plants_through_cross-breeding.pdf. Accessed Jan. 29, 2004
- Genetic Modification Advisory Committee. Annex A - risk assessment for stacked events. Available from: https://www.gmac.sg/pdf/Risk%20Assessment%20for%20Stacked%20Events.pdf. Accessed Aug. 1 2020
- Goodwin L, Hunst P, Burzio L, Rowe L, Money S, Chakravarthy S. Stacked Trait Products Are As Safe As Non-Genetically Modified (GM) Products Developed By Conventional Breeding Practices. Journal of Regulatory Science. 9: 22-25 (2021)
- Hajjar R. Hodgkin T. The use of wild relative in crop improvement: a survey of developments over the last 20 years. Euphytica. 156: 1-13 (2007)
- Health Canada. Guidelines for the safety assessment of novel foods. Available from: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/hpfb-dgpsa/pdf/gmf-agm/guidelines-lignesdirectrices-eng.pdf. (2006)
- Korea Biosafety Clearing House (KBCH). Domestic event approval status. Available from: https://www.biosafety.or.kr/portal/index.do?pageid=f_02. Accessed Aug 3, 2021
- ISAAA. Global status of commercialized biotech/GM Crops in 2019. ISAAA Brief No. 55. ISAAA, Ithaca, NY, USA (2019)
- ISAAA. GM approval database. Available from: https://www.isaaa.org/gmapprovaldatabase/default.asp. (2021)
- ISAAA. Pocket K No. 42: Stacked traits in biotech crops. Available from: http://isaaa.org/resources/publications/pocketk/42/default.asp. (2017)
- Isabel T, Nina P, Yves B, Marc DL, Arne HJ. Gene stacking in transgenic plants: towards compliance between definitions, terminology, and detection within the EU regulatory framework. Environmental Biosafety Research. 7: 197-218 (2008) [DOI] [PubMed]
- Klumper W, Qaim M. A meta-analysis of the impacts of genetically modified crops. PLoS ONE. 9: e111629 (2014) [DOI] [PMC free article] [PubMed]
- Kok E, Pedersen J, Onori R, Sowa S, Schauzu M, De Schrijver A, Teeri T. Plants with stacked genetically modified events: to assess or not to assess? Trends in Biotechnology. 32: 70-73 (2014) [DOI] [PubMed]
- Kramer C, Brune P, McDonald J, Nesbitt M, Sauve A, Storck-Weyhermueller S. Evolution of risk assessment strategies for food and feed uses of stacked GM events. Plant Biotechnology Journal. 14: 1899-1913 (2016) [DOI] [PMC free article] [PubMed]
- Ministry of Food and Drug Safety. Regulations on the safety assessment, etc. of genetically modified foods, etc. Available from: https://www.mfds.go.kr/brd/m_211/view.do?seq=14615. Accessed Aug 6, 2021
- Ministry of Health, Labour, and Welfare. Partial revision of Standards for foods, additives, etc. and safety review procedures for Modified DNA Technology Applied Foods and Additives. Food safety 0627 No 4. Director, Food Safety Department, Ministry of Health, Labor and Welfare. Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-11130500-Shokuhinanzenbu/0000049695.pdf. Accessed Jun 27, 2014
- European Commission. Commission Implementing Regulation (EU) No 503/2013 of 3 April 2013 on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/2006. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:157:0001:0048:EN:PDF. Accessed Aug 6, 2013
- OECD. Consensus document on molecular characterisation of plants derived from modern biotechnology. Available from: https://biosafety.icar.gov.in/wp-content/uploads/2016/04/Molecular-characterization.pdf. (2010)
- Philippines. Coverage of biosafety approval of multiple-stacked events. memorandum order no. 8. republic of the philippines department of agriculture, Bureau of Plant Industry. Manila. (2013)
- Pilacinski W, Crawford A, Downey R, Harvey B, Huber S, Hunst P, Lahman LK, Macintosh S, Pohl M, Rickard C, Tagliani L, Weber N. Plants with genetically modified events combined by conventional breeding: an assessment of the need to additional regulatory data. Food and Chemical Toxicology. 49: 1-7 (2011) [DOI] [PubMed]
- Powell W, Waugh R, Bradshaw J, Russell J, Ramsay L, Forster BP. Introduction to classical genetics and plant breeding. vol. 1, pp. 3–29. In: Handbook of Plant Biotechnology. Wiley & Sons, Hoboken, NJ, USA (2003)
- Schutte G, Eckerstorfer M, Rastelli V, Reichenbecher W, Restrepo-Vassalli S, Ruohonen-Lehto M, Saucy AGW, Mertens M. Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environmental Sciences Europe. 29(5): 1-12 (2017) [DOI] [PMC free article] [PubMed]
- Steiner HY, Halpin C, Jez JM, Kough J, Parrott W, Underhill L, Weber N, Hannah LC. Editor’s Choice: Evaluating the potential for adverse interactions within genetically engineered breeding stacks. Plant Physicology. 161: 1587-1594 (2013) [DOI] [PMC free article] [PubMed]
- USDA Global Agricultural Information Network. 2016 Agricultural Biotechnology Annual. Available from: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=AGRICULTURAL%20BIOTECHNOLOGY%20ANNUAL_Tokyo_Japan_11-30-2016.pdf. Accessed Nov 18, 2016
- Wang X, Zhang X, Yang J, Liu X, Song Y, Wang Z. Genetic variation assessment of stacked-trait transgenic maize via conventional breeding. BMC Plant Biology. 19: 346 (2019) [DOI] [PMC free article] [PubMed]
- Wu AJ, Chapman K, Sathischandra S, Massengill J, Araujo R, Soria M, Bugas M, Bishop Z, Haas C, Holliday B, Cisneros K, Lor J, Canez C, New S, Mackie S, Ghoshal D, Pricalle L, Hunst P, Pallet K. GHB614 x T304-40 x GHB119 x COT102 Cotton: Protein expression analyses of field-grown samples. Journal of Agricultural and Food Chemistry. 61: 275-281 (2018) [DOI] [PubMed]
- Wunderlich S, Gatto KA. Consumer perception of genetically modified organisms and sources of information. American Society for Nutrition. 6: 842-851 (2015) [DOI] [PMC free article] [PubMed]