Abstract
Glucokinase (GK) is an enzyme involved in synthesising glucose into glucose-6 phosphate and serves a crucial function in glucose sensing. Therefore, agents that induce GK activation could be used to treat T2DM. The present work has been carried out to investigate the GK activation potential of phytoconstituents of Enicostemma littorale through molecular docking. All the phytoconstituents have been screened through the Lipinski rule of 5, Veber’s rule, and ADMET properties. From these initial screening, only Apigenin, Ferulic acid, Genkwanin, p-coumaric acid, Protocatechuic acid, Syringic acid, and Vanillic acid have been selected to perform molecular docking studies. The binding free energy and binding mode of the native ligand in the allosteric site of the enzyme have been considered the reference for the other molecules' validation. The native ligand has exhibited − 7.2 kcal/mol binding free energy, whereas; it has formed four hydrogen bonds with THR-228, LYS-169, ASP-78, and GLY-81. Based on these findings, the interactions of phytoconstituents have been justified. Apigenin, genkwanin, and swertiamarin exhibited − 8.7, − 7.5, and − 8.3 kcal/mol binding free energy, respectively, which indicates better enzyme activation than the native ligand. Swertiamarin has formed 08 hydrogen bonds with allosteric amino acid residues, which confirms the excellent enzyme activation by these phytoconstituents. We concluded that if we can isolate and consume the exact active phytoconstituents (GK activators) from this plant, we can use them effectively to treat T2DM. More GK activators can be developed by considering them as a natural lead moiety.
Keywords: Enicostemma littorale, Glucokinase activators, Apigenin, Swertiamarin, Verticilliside, Betulin
Introduction
The Enicostemma littorale Blume (E. littorale) plays a critical role in human wellbeing. Parts of the plant E. littoral were used historically in therapeutic applications against malaria, skin disorders, leprosy, diabetes, etc. This plant's constituents were beneficial therapeutic compounds because they had low toxicity, environmental friendliness, a long shelf life, and no side effects (Murali et al. 2002; Upadhyay and Goyal, 2004; Vasu et al. 2005). It is a noble source of iron, potassium, sodium, calcium, magnesium, silica, chloride, sulphate, phosphate, and vitamins B and C (Maroo et al. 2003, 2002; Sonawane et al. 2010; Thirumalai et al. 2011).
Numerous phytoconstituents have been isolated from the plant, E. littorale. The aerial sections of the plant yielded 34% of the dry alcoholic extract and 15.7% of the ash (Patel et al. 2009; Sadique et al. 1987; Sanmugarajah 2013). It has been stated in the literature that this plant produces five alkaloids, two sterols, and volatile oils(Selvaraj et al. 2014; Vishwakarma et al. 2010). Another sapogenin, betulin, was also isolated from this plant (Indumathi et al. 2014). Monoterpene alkaloids such as enicoflavin, gentiocrucine and seven diverse flavonoids have been extracted from the alcoholic extract and the structures have been categorised as apigenin, genkwanin, isovitexin, wertisin, saponarin, 5-o glucosylwertisin and 5-o glucosylisowertisin have also been isolated by Goshal et al. (1974). For the first time in this species, the occurrence of catechins, saponins, steroids, sapogenin, triterpenoids, flavonoids, and xanthones and a new flavonous C-glucoside called verticilliside was isolated(Jahan et al. 2009). The compound swertiamarin was isolated from E. littoral by the alcoholic extract(Alam et al. 2011; Leong et al. 2016; Patel et al. 2013; Sonawane et al. 2010; Vaidya et al. 2009a; Vishwakarma et al. 2004). There have also been six phenolic acids identified: vanillic acid, syringic acid, p-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, and ferulic acid (Abirami and Gomathinayagam 2011; Rathod and Dhale, 2013; Srinivasan et al. 2005). The methanol extract contained numerous amino acids such as l-glutamic acid, tryptophan, alanine, serine, aspartic acid, l-proline, l-tyrosine, threonine, phenylalanine, l-histidine mono-hydrochloride, methionine (Nagarathnamma et al. 2010; Sawant et al. 2011). Diabetic patients are advised to consume 2g of fresh E. littorale leaves on daily basis (Upadhyay and Goyal 2004). Therefore, E. littorale has been selected to investigate the antidiabetic potential.
Diabetes mellitus is a metabolic condition that increases the body's blood glucose, also known as diabetes (Pal 2009; Zelent et al. 2005). The hormone insulin converts blood sugar into energy-saving cells. In diabetic conditions, either the body cannot produce enough insulin or the insulin it produces cannot be used efficiently (Grewal et al. 2014; Singh et al. 2016). Two significant forms of diabetes are present; type 1 diabetes mellitus (T1DM) is an autoimmune disorder in the pancreas, where insulin is produced, the immune system targets and kills cells. Type 2 diabetes mellitus (T2DM) happens as the body becomes insulin resistant and the blood accumulates sugar (Grewal et al. 2018; Fyfe and Procter 2009).
Glucokinase is an enzyme involved in synthesising glucose into glucose-6 phosphate and serves a crucial function in glucose sensing (Charaya et al. 2018; Grewal et al. 2019). Therefore, agents induce glucokinase activation to be used to treat T2DM. The several various groups of compounds that have been discovered to cause glucokinase activation, such as benzamides (Charaya et al. 2018; Grewal et al. 2019; Li et al., 2011; Park et al. 2015), acetamides (Agrawal et al. 2013; Grewal et al. 2014), carboxamides (Grewal et al. 2014), acrylamides (Sidduri et al. 2010), benzimidazoles (Ishikawa et al. 2009), quinazolines, thiazoles (Agrawal et al. 2013), pyrimidines (Pfefferkorn et al. 2011), and urea derivatives (Castelhano et al. 2005; Grewal et al. 2020; Houze et al. 2013; Kohn et al. 2016; Murray et al. 2005; Polisetti et al. 2004; Sarabu et al. 2008).
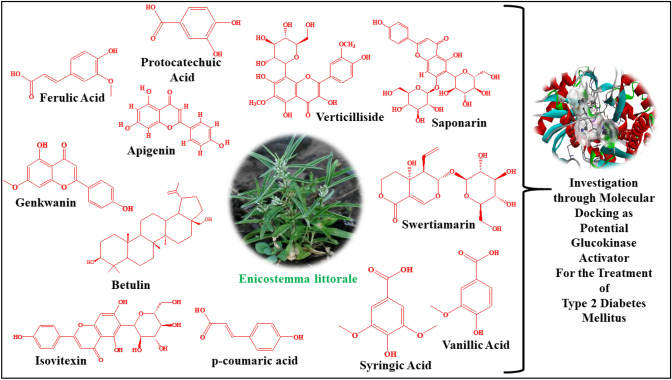
After knowing the essential value of the activators of glucokinase in the control of T2DM (Filipski et al. 2012; Grewal et al. 2017, 2019; Grimsby et al. 2003; Matschinsky 2004; Zhang et al. 2016), we investigated the effectiveness of phytoconstituents of E. littorale as glucokinase activators, as per the literature which reports the hypoglycemic activity of this plant(Babu and Prince 2004; Maroo et al. 2002; Murali et al. 2002; Patel et al. 2009, 2012; Sonawane et al. 2010; Thirumalai et al. 2011; Upadhyay and Goyal 2004; Vaidya et al. 2009b; Vasu et al. 2005; Vijayvargia et al. 2000; Vishwakarma et al. 2010). We tried to identify the potential natural lead compounds from E. littorale as glucokinase activators through their binding mode in the allosteric site of the enzyme. The structures of all the significant phytoconstituents of E. littorale are represented in Fig. 1.
Fig. 1.
The structures of all the significant phytoconstituents of E. littorale
Material and methods
Calculation of Lipinski's rule of five
In order to further optimize the molecules, all the phytoconstituents were tested for violating the Lipinski's rule of five, Veber’s rule and the pharmacokinetic (ADMET) characteristics. The properties of all the phytoconstituents were calculated from SwissADME online tool (http://www.swissadme.ch/index.php).
Molecular docking
We conducted molecular docking (MD) on Lenovo ThinkPad T440p using PyRx-Virtual Screening Tool (Dallakyan and Olson, 2015). The structures of all the phytoconstituents and native ligand (.sdf File format) were downloaded from the National Center for Biotechnology Information PubChem (https://pubchem.ncbi.nlm.nih.gov/). The energy minimization (optimization) was performed by Universal Force Field (UFF) (Rappé et al. 1992).
A crystalline human glucokinase structure was obtained as input 1V4S from the Protein Data Bank (PDB) of RCSB (https://www.rcsb.org/structure/1V4S). 1V4S also contained the native ligand 5-(1-methyl-1H-imidazol-2-ylthio)-2-amino-4-fluoro-N-(thiazol-2-yl)benzamide that was used as a reference molecule for MD. In PyRx 0.8, Autodock vina 1.1.2 was used to conduct MD analyses of both the phytoconstituents and native ligands against the crystal structure of glucokinase (Dallakyan and Olson, 2015). With the aid of Discovery Studio Visualizer 2019, the composition of the enzyme was refined, purified, and prepared for MD (San Diego: Accelrys Software Inc. 2012). The specifications of the crystal structure and input compositions of human glucokinase used (PDB ID-1V4S) are provided in Table 1 of the PDB X-ray Structure Validation Report released on 10 August 2020. There were only 5 specific molecules in this entry, and there was one chain (Chain A). The entry comprises 3690 atoms, including 0 hydrogens and 0 deuteriums, which illustrates the need to incorporate hydrogen atoms in protein preparation processes for MD.
Table 1.
The information of the crystal structure and input compositions of human glucokinase used (PDB ID-1V4S)
| The details of crystal structure (1V4S): | ||||||
| Title: | Crystal structure of human glucokinase | |||||
| DOI: | 10.2210/pdb1V4S/pdb | |||||
| Authors: | Kamata, K., Mitsuya, M., Nishimura, T., Eiki, J., Nagata, Y | |||||
| Deposited on: | 30-03-2004 | |||||
| Resolution: | 2.30 Å(reported) | |||||
| Classification: | Transferase | |||||
| Organism(s): | Homo sapiens | |||||
| Expression System: | Escherichia coli | |||||
| Method: | X-Ray diffraction | |||||
| Residues | Atoms | |||||
| The entry composition of 1V4S: | ||||||
| Total | C | N | O | S | Na/F | |
| Molecule 1 was a protein called glucokinase isoform 2 | ||||||
| 448 | 3505 | 2178 | 609 | 686 | 32 | 0 |
| Molecule 2 was alpha-D-glucopyranose (three-letter code: GLC) (formula: C6H12O6) | ||||||
| 1 | 12 | 6 | 0 | 6 | 0 | 0 |
| Molecule 3 was SODIUM ION (three-letter code: NA) (formula: Na) | ||||||
| 1 | 1 | 0 | 0 | 0 | 0 | 1 (Na) |
| Molecule 4 was native ligand 5-(1-methyl-1H-imidazol-2-ylthio)-2-amino-4-fluoro-N-(thiazol-2-yl)benzamide (three-letter code: MRK) (formula: C14H12FN5OS2) | ||||||
| 1 | 23 | 14 | 5 | 1 | 2 | 1 (F) |
| Molecule 5 was water | ||||||
| 149 | 149 | 0 | 0 | 149 | 0 | 0 |
Where, C carbon; N nitrogen; O oxygen; S sulphur; Na sodium; F fluorine
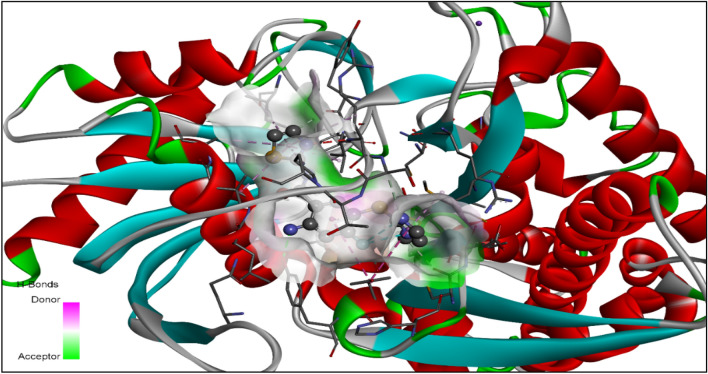
The MD was executed by using Vina Wizard Tool in PyRx 0.8. Molecules (PDBQT Files), both ligands and target (human glucokinase), were selected for MD. For the purpose of MD simulation, the three-dimensional grid box (size_x = 43.35 A0; Size_y = 59.36 A0; Size_z = 43.92 A0) was built using Autodock tool 1.5.6 with exhaustiveness value of 8 (Dallakyan and Olson, 2015). The active amino acids in the protein were analyzed and illuminated using Visualizer in BIOVIA Discovery Studio (version-19.1.0.18287) (San Diego: Accelrys Software Inc. 2012). The full MD process, the identification of cavity and active amino acid residues, were performed as defined by S. L. Khan et al. (Chaudhari et al. 2020; Khan and Siddiui 2020; Khan et al. 2020a, b, 2021; Siddiqui et al. 2021). The enzyme cavity is depicted in Fig. 2 with the co-crystallized ligand molecule.
Fig. 2.
The cavity of the enzyme is depicted with the co-crystallize ligand molecule (PDB ID: 1V4S)
Results
Pharmacokinetic characteristics are an important component of drug development because it enable researchers to assess the biological aspects of medication candidates. In order to establish whether or not the compound optimal for oral bioavailability, Lipinski's rule of five and Veber's rules was utilized (Table 2). All the phytoconstituents were studied for their ADMET characteristics better to grasp their pharmacokinetics profiles and drug-likeness qualities (Table 3). The ligand energies (kcal/mol), binding free energy (kcal/mol), root mean square deviation/upper bound (rmsd/ub), and root mean square deviation/lower bound (rmsd/lb) of the conformers generated of all the docked phytoconstituents are tabulated in Table 4. The active amino residues, reactive atom of ligands, bond length (A0), and type of interactions of phytoconstituents with glucokinase enzyme are depicted in Table 5. The 2D- and 3D-docking poses of all the docked molecules are represented in Figs. 3, 4, 5, 6.
Table 2.
The molecular formula, Lipinski rule of five and Vebers’s rule
| Molecule Name | Molecular Formula | Lipinski rule of 5 | Veber’s rule | |||||
|---|---|---|---|---|---|---|---|---|
| Mol. Wt.a | HBAa | HBDa | LogP | Violation | Total polar surface area (Å2) | No. of rotatable bonds | ||
| Native Ligand | C14H12FN5OS2 | 349 | 04 | 02 | 2.00 | 0 | 139.37 | 5 |
| Apigenin | C15H10O5 | 270.24 | 05 | 03 | 3.02 | 00 | 90.90 | 1 |
| Betulin | C30H50O2 | 442.72 | 02 | 02 | 8.28 | 01 | 40.46 | 2 |
| Ferulic acid | C10H10O4 | 194.18 | 04 | 02 | 1.51 | 00 | 66.76 | 3 |
| Genkwanin | C16H12O5 | 284.26 | 05 | 02 | 3.35 | 00 | 79.90 | 2 |
| Isovitexin | C21H20O10 | 432.38 | 10 | 07 | 0.21 | 01 | 181.05 | 3 |
| p-coumaric acid | C9H8O3 | 164.16 | 03 | 02 | 1.46 | 00 | 57.53 | 2 |
| Protocatechuic acid | C7H6O4 | 154.12 | 04 | 03 | 1.15 | 00 | 77.76 | 1 |
| Saponarin | C27H30O15 | 594.52 | 15 | 10 | − 1.60 | 03 | 260.20 | 6 |
| Swertiamarin | C16H22O10 | 374.34 | 10 | 05 | − 2.00 | 00 | 155.14 | 4 |
| Syringic acid | C9H10O5 | 198.17 | 05 | 02 | 1.04 | 00 | 75.99 | 3 |
| Vanillic acid | C8H8O4 | 168.15 | 04 | 02 | 1.43 | 00 | 66.76 | 2 |
| Verticilliside | C23H24O13 | 508.43 | 13 | 08 | 0.00 | 00 | 219.74 | 5 |
aMol. Wt. molecular weight; HBA hydrogen bond acceptor; HBD hydrogen bond donor
Table 3.
The pharmacokinetic and drug-likeness properties of selected phytoconstituents
| Parameters | Compound names | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native Ligand | Apigenin | Betulin | Ferulic acid | Genkwanin | Isovitexin | p-coumaric acid | Protocatechuic acid | Saponarin | Swertiamarin | Syringic acid | Vanillic acid | Verticilliside | |
| Pharmacokinetics | |||||||||||||
| GI absorption | Low | High | Low | High | High | Low | High | High | Low | Low | High | High | Low |
| BBB permeation | No | No | No | Yes | No | No | Yes | No | No | No | No | No | No |
| P-gp substrate | No | No | No | No | No | No | No | No | Yes | No | No | No | No |
| CYP1A2 inhibitor | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | Yes | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | Yes | Yes | No | No | Yes | No | No | Yes | No | No | No | No | No |
| Log Kp (skin permeation, cm/s) | − 6.59 | − 5.80 | − 3.12 | − 6.41 | − 5.66 | − 8.79 | − 6.26 | − 6.42 | − 11.06 | − 10.00 | − 6.77 | − 6.31 | − 9.40 |
| Drug-likeness | |||||||||||||
| Ghose | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No |
| Egan | No | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | No |
| Muegge | Yes | Yes | No | No | Yes | No | No | No | No | No | No | Yes | No |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.85 | 0.55 | 0.55 | 0.85 | 0.56 | 0.17 | 0.11 | 0.56 | 0.85 | 0.17 |
Table 4.
The ligand energies (kcal/mol), binding free energy (kcal/mol), rmsd/ub, and rmsd/lb of the conformers generated of all the docked phytoconstituents
| Compound name | Ligand energies (kcal/mol) | Binding free energies of conformers (kcal/mol) | rmsd/ub | rmsd/lb |
|---|---|---|---|---|
| Native Ligand | 689.51 | – 7.2 | 0 | 0 |
| – 7.1 | 11.569 | 9.551 | ||
| – 7 | 3.954 | 3.154 | ||
| – 6.9 | 14.845 | 11.338 | ||
| – 6.8 | 10.337 | 7.01 | ||
| – 6.7 | 1.683 | 1.445 | ||
| – 6.6 | 7.21 | 2.806 | ||
| – 6.5 | 17.14 | 14.594 | ||
| – 6.4 | 6.309 | 2.65 | ||
| Apigenin | 192.64 | – 8.7 | 0 | 0 |
| – 7.4 | 10.327 | 4.068 | ||
| – 7.3 | 10.821 | 4.626 | ||
| – 7.2 | 21.133 | 19.956 | ||
| – 6.9 | 35.515 | 34.274 | ||
| – 6.9 | 35.782 | 34.596 | ||
| – 6.8 | 33.332 | 31.774 | ||
| – 6.8 | 10.029 | 4.368 | ||
| – 6.7 | 9.059 | 6.294 | ||
| Ferulic Acid | 470.67 | – 6.8 | 0 | 0 |
| – 6.4 | 6.204 | 1.962 | ||
| – 5.9 | 19.764 | 19.019 | ||
| – 5.9 | 20.297 | 19.456 | ||
| – 5.6 | 39.562 | 37.223 | ||
| – 5.5 | 35.688 | 35.127 | ||
| – 5.4 | 36.304 | 35.659 | ||
| – 5.1 | 22.572 | 21.564 | ||
| – 5.1 | 20.025 | 18.771 | ||
| Genkwanin | 206.69 | – 7.5 | 0 | 0 |
| – 7.1 | 24.283 | 22.091 | ||
| – 7 | 37.334 | 34.875 | ||
| – 6.5 | 25.831 | 23.254 | ||
| – 6.5 | 25.252 | 22.573 | ||
| – 6.5 | 24.856 | 21.891 | ||
| – 6.5 | 25.269 | 23.189 | ||
| – 6.4 | 24.678 | 23.526 | ||
| – 6.2 | 25.372 | 23.061 | ||
| p-Coumaric acid | 86.43 | – 6.4 | 0 | 0 |
| – 6.2 | 5.781 | 1.352 | ||
| – 5.8 | 36.105 | 35.604 | ||
| – 5.6 | 19.899 | 19.203 | ||
| – 5.5 | 5.985 | 4.033 | ||
| – 5.3 | 20.45 | 19.509 | ||
| – 5.1 | 19.394 | 18.094 | ||
| – 5 | 46.168 | 46.046 | ||
| – 5 | 5.274 | 3.587 | ||
| Protocatechuic acid | 69.09 | – 5.9 | 0 | 0 |
| – 5.8 | 3.823 | 2.267 | ||
| – 5.7 | 20.234 | 19.688 | ||
| – 5.7 | 37.453 | 36.16 | ||
| – 5.3 | 37.892 | 36.5 | ||
| – 5.3 | 20.033 | 19.486 | ||
| – 5.1 | 34.46 | 34.289 | ||
| – 5 | 34.43 | 34.254 | ||
| – 5 | 45.895 | 45.58 | ||
| Syringic acid | 837.9 | – 5.7 | 0 | 0 |
| – 5.4 | 3.552 | 0.385 | ||
| – 5.3 | 5.175 | 2.812 | ||
| – 5.2 | 4.587 | 2.804 | ||
| – 5.2 | 15.57 | 13.004 | ||
| – 5.1 | 22.174 | 19.227 | ||
| – 5.1 | 15.49 | 13.102 | ||
| – 5.1 | 22.457 | 19.465 | ||
| – 5 | 3.615 | 3.153 | ||
| Vanillic acid | 85.01 | – 5.5 | 0 | 0 |
| – 5.5 | 28.978 | 28.041 | ||
| – 5.5 | 4.174 | 1.335 | ||
| – 5.3 | 28.558 | 27.034 | ||
| – 5.2 | 28.09 | 26.65 | ||
| – 5 | 21.792 | 20.446 | ||
| – 5 | 29.168 | 28.414 | ||
| – 5 | 22.059 | 20.823 | ||
| – 4.9 | 29.605 | 27.718 |
Table 5.
The active amino residues, reactive atom of ligands, bond length (A0), and type of interactions of phytoconstituents with glucokinase enzyme (1V4S)
| Active amino residue | Atom from ligand | Bond length (A0) | Bond category | Bond types |
|---|---|---|---|---|
| Native ligand | ||||
| ASP78 | H | 2.04258 | Hydrogen bond | Conventional hydrogen bond |
| LYS169 | F | 2.60065 | Hydrogen bond; halogen | Conventional hydrogen bond; halogen (Fluorine) |
| THR228 | S | 2.20855 | Hydrogen bond | Conventional hydrogen bond |
| THR228 | F | 3.75081 | Carbon hydrogen bond | |
| ARG85 | Pi-Orbitals | 3.56863 | Electrostatic | Pi-Cation |
| ASP205 | 3.9455 | Pi-Anion | ||
| ASP409 | 3.70544 | |||
| Apigenin | ||||
| TYR215 | O | 2.06298 | Hydrogen bond | Conventional hydrogen bond |
| TYR215 | O | 2.15918 | ||
| TYR214 | H | 3.00628 | Pi-Donor hydrogen bond | |
| VAL455 | Pi-Orbitals | 3.86479 | Hydrophobic | Pi-Sigma |
| TYR214 | 5.11899 | Pi-Pi T-shaped | ||
| PRO66 | 4.99697 | Pi-Alkyl | ||
| ILE211 | 5.46378 | |||
| VAL455 | 4.74718 | |||
| ILE211 | 4.92959 | |||
| VAL62 | 4.80355 | |||
| PRO66 | 5.06 | |||
| VAL452 | 5.03778 | |||
| ALA456 | 4.9239 | |||
| Ferulic acid | ||||
| TYR61 | H | 2.0266 | Hydrogen bond | Conventional hydrogen bond |
| ILE211 | Pi-Orbitals | 4.61546 | Hydrophobic | Pi-Alkyl |
| VAL452 | 4.8904 | |||
| VAL455 | 4.64867 | |||
| Genkwanin | ||||
| ILE159 | C-H | 3.78006 | Hydrophobic | Pi-Sigma |
| ILE159 | C-H | 3.77297 | ||
| VAL455 | C-H | 3.87537 | ||
| ALA456 | Pi-orbitals | 4.63516 | Pi-Alkyl | |
| ALA456 | 4.89221 | |||
| LYS459 | 5.06083 | |||
| VAL62 | 5.08219 | |||
| PRO66 | 5.00443 | |||
| ILE159 | 5.34614 | |||
| VAL452 | 5.4002 | |||
| ALA456 | 4.7483 | |||
| p-Coumaric acid | ||||
| TYR61 | H | 1.86828 | Hydrogen bond | Conventional hydrogen bond |
| TYR215 | O | 2.07668 | ||
| ILE211 | Pi-Orbitals | 4.58608 | Hydrophobic | Pi-Alkyl |
| VAL452 | 4.8732 | |||
| VAL455 | 4.66716 | |||
| Protocatechuic acid | ||||
| THR65 | O | 2.3016 | Hydrogen bond | Conventional hydrogen bond |
| TYR215 | O | 2.70823 | ||
| VAL452 | O | 3.71031 | Carbon hydrogen bond | |
| ILE211 | Pi-Orbitals | 3.47995 | Hydrophobic | Pi-Sigma |
| TYR214 | 5.12043 | Pi-Pi T-shaped | ||
| Syringic acid | ||||
| ASP205 | H | 2.69294 | Hydrogen bond | Conventional hydrogen bond |
| ARG85 | O | 2.33855 | ||
| ARG85 | O | 2.66692 | ||
| LYS169 | O | 2.52862 | ||
| ASP409 | Methyl C | 3.28918 | Carbon Hydrogen Bond | |
| ASN83 | 3.59997 | |||
| ASP78 | Pi-Orbitals | 3.71563 | Electrostatic | Pi-Anion |
| Vanillic acid | ||||
| LEU25 | O | 2.5621 | Hydrogen bond | Conventional hydrogen bond |
| SER373 | O | 1.95412 | ||
| THR376 | Pi-Orbitals | 3.61887 | Hydrophobic | Pi-Sigma |
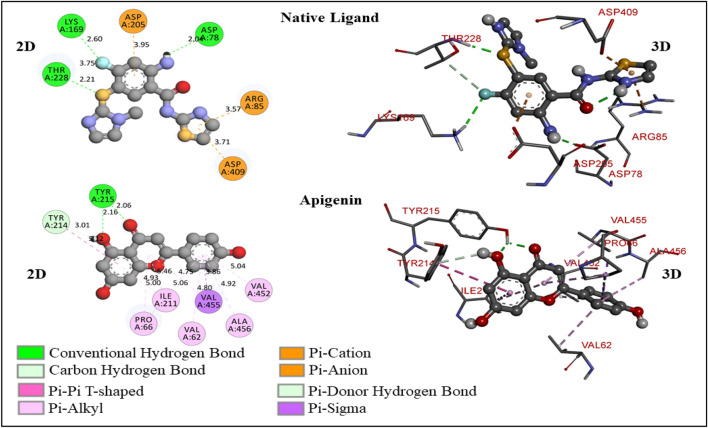
Fig. 3.
The 2D- and 3D-molecular interaction poses of native ligand and apigenin with the glucokinase enzyme
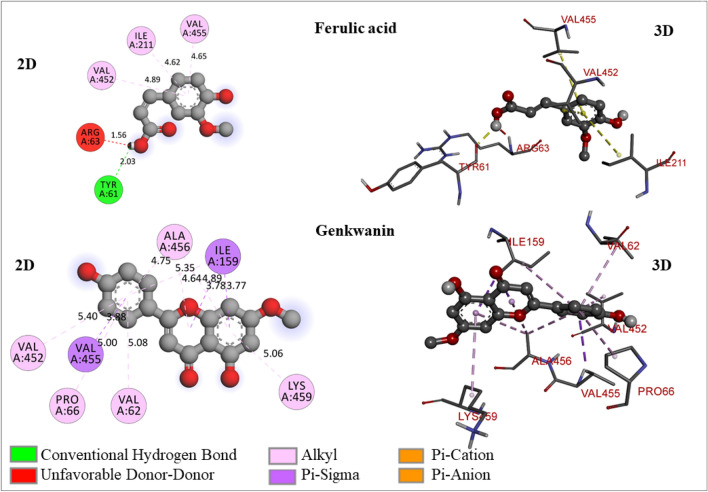
Fig. 4.
The 2D- and 3D-molecular interaction poses of ferulic acid and genkwanin with the glucokinase enzyme
Fig. 5.
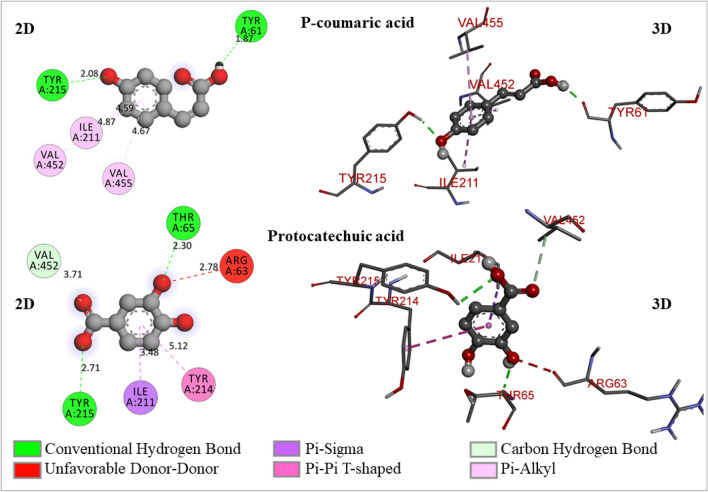
The 2D- and 3D-molecular interaction poses of p-coumaric acid and protocatechuic acid with the glucokinase enzyme
Fig. 6.
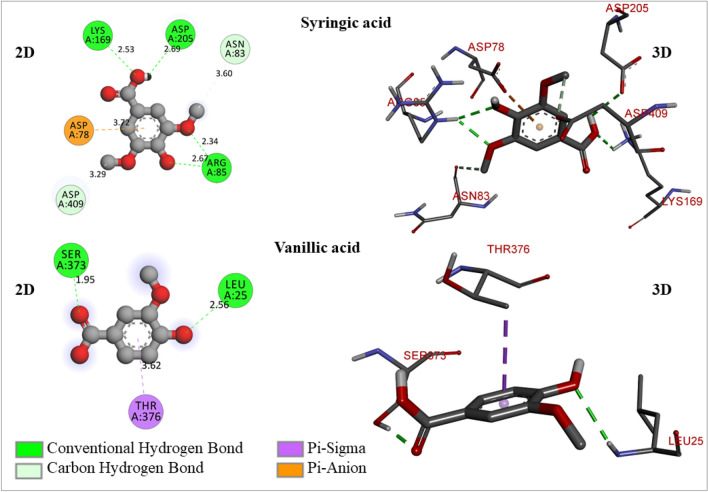
The 2D- and 3D-molecular interaction poses of syringic acid and vanillic acid with the glucokinase enzyme
Where: GI, gastrointestinal; BBB, blood brain barrier; P-gp, p-glycoprotein.
Discussion
We tried to identify the potential natural lead compounds from E. littorale as glucokinase activators through the binding mode in the enzyme's allosteric site and binding free energies. In accordance with Lipinski's and Veber's rules (Table 2), many of the phytoconstituents did not demonstrated the drug-likeness characteristics and violated both the rules. Amongst all the molecules, betulin has a log P value of 8.28, which violates the Lipinski rule of 5 and indicates poor lipophilicity. An essential aspect of the compound that influences its function in the human body is lipophilicity. The compound’s Log P value shows the permeability of the drugs in the body to enter the target tissue (Krzywinski and Altman 2013; Lipinski et al. 2012). Isovitexin was found to have 7 hydrogen bond donors, which violates the Lipinski rule of 5. Saponarin has a molecular weight of 594.52 Da, 15 hydrogen bond acceptors, and 10 hydrogen bond donors, with 3 violations of the Lipinski rule of 5. It is preferable to look for substances that exceed the Lipinski limit of 500 Da, since this will only boost absorption. Still, there are several reports of relatively more significant compounds that are transported effectively through the cells. The remaining phytoconstituents, including native ligand, had fortunately not violated the Lipinski rule of 5, indicating better absorption and/or lipophilicity of the molecules. Many phytoconstituents violated the Veber's rule with total polar surface area (TPSA, should be less than 140) values and the number of rotatable bonds (which should be less than 10) that do not fall within the acceptable range for oral availability. Isovitexin, Saponarin, Swertiamarin, and Verticilliside violated the Veber’s rule.
For further optimization, all the molecules have been subjected to calculations of pharmacokinetics and drug-likeness properties. All the molecules did not show BBB penetration potential which is not favorable property for the drugs to be targeted for central nervous system. Unfortunately, many molecules did exhibited optimum log Kp (skin permeation, cm/s) and bioavailability scores. Many molecules violated the Ghose, Egan, and Muegge filters (Table 3). The molecules which displayed low GI absorption and violations of Lipinski and Veber’s rules have been eliminated from further optimization. Also, native ligand displayed low GI absorption. Therefore, only Apigenin, Ferulic acid, Genkwanin, p-coumaric acid, Protocatechuic acid, Syringic acid, and Vanillic acid have been selected to perform molecular docking studies on the GK enzyme.
A total 9 conformers were generated through MD for each molecule (Table 4). The conformer with zero rmsd/ub and rsmd/lb values has been treated as the best fit model for the glucokinase enzyme activation. The binding free energy and binding mode of the native ligand in the allosteric site of the enzyme have been considered a reference for validating the other molecules (Table 5 and Figs. 3, 4, 5, 6). Native ligand has binding free energy of − 7.2 kcal/mol and has formed 4 hydrogen bonds (3 conventional and 1 carbon-hydrogen bond) with ASP78 (2.04258 A0), LYS169 (2.60065 A0), and THR228 (2.20855 A0, 3.75081 A0). The hydrogen of a free primary amino group from the native ligand has formed a hydrogen bond ASP78, and the fluorine atom has formed a hydrogen bond with LYS169. THR228 has reacted with sulfur and fluorine simultaneously with forming one conventional hydrogen bond and one carbon-hydrogen bond. Native ligand showed electrostatic interactions with ARG85 (3.56863 A0), ASP205 (3.9455 A0), and ASP409 (3.70544 A0) through Pi-orbitals of the aromatic ring system (Fig. 3).
Apigenin (4′,5-trihydroxyflavone), a flavonoid, falls under the flavone class that is the aglycone of many naturally-occurring glycosides [(Ali et al. 2017; Baumann 2008; Salehi et al. 2019; Shukla and Gupta 2010)]. It has shown − 8.7 kcal/mol of binding free energy and formed 3 hydrogen bonds (2 conventional and 1 Pi-donor hydrogen bond) with TYR215 (2.06298 A0, 2.15918 A0), and TYR214 (3.00628 A0) (Fig. 3). It has formed two hydrogen bonds with TYR215 through hydroxyl and carbonyl oxygen atoms. One free hydroxyl group in apigenin has formed one Pi-donor hydrogen bond with TYR214 through hydrogen atom. It has shown many hydrophobic interactions due to Pi-orbitals of aromatic ring systems with VAL455 (3.86479 A0), TYR214 (5.11899 A0), PRO66 (4.99697 A0), ILE211 (5.46378 A0), VAL455 (4.74718 A0), ILE211 (4.92959 A0), VAL62 (4.80355 A0), PRO66 (5.06 A0), VAL452 (5.03778 A0), and ALA456 (4.9239 A0).
Ferulic acid is an organic compound; chemically, it is 3-methoxy-4-hydroxycinnamic acid. In plant cell walls a rich phenolic phytochemical is present covalently attached to arabinoxyls as side chains (Mathew and Abraham 2006; Wu et al. 2018). It exhibited -6.8 kcal/mol of binding free energy, which is less than native ligand, and therefore, this molecule does not possess potential to activate glucokinase enzyme. It has also formed an unfavorable donor-donor bond with ARG63 (1.56 A0) through hydroxyl hydrogen atom (Fig. 4).
Genkwanin is a monomethoxyflavone, which is a derivative of apigenin. It has been biosynthesized by apigenin in plants by methylation of the hydroxyl group at 7th position (Lee et al. 2015; Nasr Bouzaiene et al. 2016). Genkwanin has shown − 7.5 kcal/mol of binding free energy with glucokinase enzyme and possesses stable ligand energy of 206.69 kcal/mol. It exhibited hydrophobic interactions (Pi-sigma and Pi-alkyl) with ILE159 (3.78006 A0, 3.77297 A0, 5.34614 A0), VAL455 (3.87537 A0), ALA456 (4.63516 A0, 4.89221 A0, 4.7483 A0), LYS459 (5.06083 A0), VAL62 (5.08219 A0), PRO66 (5.00443 A0), and VAL452 (5.4002 A0) (Fig. 4). As it has not formed any hydrogen bond, which may result in poor activation of the enzyme.
p-Coumaric acid is a hydroxyl derivative of cinnamic acid and widely distributed in many plant species(Pei et al. 2016). It has shown − 6.4 kcal/mol of binding free energy and formed 2 conventional hydrogen bonds with TYR61 (1.86828 A0), TYR215 (2.07668 A0), whereas hydrophobic interactions (Pi-alkyl) with ILE211(4.58608 A0), VAL452 (4.8732 A0), VAL455 (4.66716 A0) (Fig. 5).
Protocatechuic acid is a type of phenolic acid that is naturally present and over 500 plants have it or its derivatives (active constituents), and these substances have different therapeutic potential. It has structural similarities with gallic acid, caffeic acid, vanillic acid, and syringic acid, which are well-known antioxidants found in foods and other items (Kakkar and Bais 2014). Protocatechuic acid has shown − 5.9 kcal/mol of binding free energy and formed 3 hydrogen bonds (2 conventional and 1 carbon-hydrogen bond) with THR65 (2.3016 A0), TYR215 (2.70823 A0), and VAL452 (3.71031 A0). It has demonstrated 2 hydrophobic bonds (Pi-sigma and Pi-Pi T-shaped) with ILE211 (3.47995 A0) and TYR214 (5.12043 A0) (Fig. 5). From these results, it can be concluded that protocatechuic acid does not have much potential to activate the glucokinase enzyme.
Syringic acid is a phenolic substance that is mostly present in fruits and vegetables. This compound is made by the shikimic acid process and is found in plants. It shows a wide variety of clinical applications in preventing diabetes, coronary disorders, cancer, ischemic stroke, etc. It can shield brain tissue from free radical injury, delay the development of diabetes, and is hepatoprotective medicine (Srinivasulu et al. 2018). It has shown -5.7 kcal/mol of binding free energy and formed 6 hydrogen bonds (4 conventional and 2 carbon-hydrogen bonds) with ASP205 (2.69294 A0), ARG85 (2.66692 A0, 2.33855 A0), LYS169 (2.52862 A0), ASP409 (3.28918 A0), ASN83 (3.59997 A0). It has formed 1 electrostatic (Pi-anion) bond with ASP78 (3.71563 A0) (Fig. 6). It demonstrated less binding free energy but exhibited a good number of hydrogen bonds, which may effectively activate the glucokinase enzyme. Vanillic acid has exhibited − 5.5 kcal/mol of binding free energy and formed 2 conventional hydrogen bonds with LEU25 (2.5621 A0), and SER373 (1.95412 A0) (Fig. 6).
Conclusion
Glucokinase is an enzyme involved in synthesising glucose into glucose-6 phosphate and serves a crucial function in glucose sensing. Therefore, agents that induce glucokinase activation could be used to treat T2DM. The Enicostemma littorale Blume (E. littorale) plays a critical role in human wellbeing. Parts of the plant E. littoral, were used historically in therapeutic applications against malaria, skin disorders, leprosy, and mostly antidiabetic activity of this plant have been reported in many literatures as well as it has been recommended in diabetic patients in Ayurveda system of medicine. The present work has been carried out to investigate the glucokinase activation potential of phytoconstituents of E. littorale through MD. All the phytoconstituents have been screened through the Lipinski rule of 5, Veber’s rule, and ADMET properties. From this initial screening, only Apigenin, Ferulic acid, Genkwanin, p-coumaric acid, Protocatechuic acid, Syringic acid, and Vanillic acid have been selected to perform molecular docking studies on the GK enzyme.
MD is a computational research-based technique for exploring possible binding interfaces through the docking of proteins and drugs. A total of 9 conformers were generated through MD for each molecule. The conformer with zero rmsd/ub and rsmd/lb values has been treated as the best fit model for activating the glucokinase enzyme. The binding free energy and binding mode of the native ligand in the allosteric site of the enzyme have been considered the reference for the other molecules' validation. The native ligand has exhibited -7.2 kcal/mol binding free energy with useful binding mode into the enzyme's allosteric site, whereas; it has formed four hydrogen bonds with THR-228, LYS-169, ASP-78, and GLY-81. Based on these findings, the interactions of phytoconstituents have been justified. Apigenin, genkwanin, and swertiamarin exhibited − 8.7, − 7.5, and − 8.3 kcal/mol binding free energy, respectively, which indicates better enzyme activation than the native ligand. Swertiamarin has formed 08, whereas syringic acid exhibited − 5.7 kcal/mol binding affinity but has formed 06 hydrogen bonds with allosteric amino acid residues, which confirms the excellent enzyme activation by these phytoconstituents. Many antidiabetic Ayurvedic formulations contain E. littorale extract, which is already known to have therapeutic effects in diabetic patients. We identified and reported the lead phytoconstituent responsible for the antidiabetic potential. We have concluded that if we can isolate and consume the exact active phytoconstituents (glucokinase activators) from this plant, we can use them effectively to treat T2DM and by considering them as a natural lead compound, we can develop and validate more glucokinase activators.
Abbreviations
- E. littorale
Enicostemma littorale
- WHO
World Health Organization
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- MD
Molecular docking
- UFF
Universal Force Field
- PDB
Protein Data Bank
- RMSD/UB
Root mean square deviation/upper bound
- RMSD/LB
Root mean square deviation/lower bound
- Mol. Wt.
Molecular weight
- HBA
Hydrogen bond acceptor
- HBD
Hydrogen bond donor
Author contributions
All the authors have contributed equally.
Funding
Not applicable.
Availability of data and materials
The properties of all the phytoconstituents were calculated from SwissADME online tool (http://www.swissadme.ch/index.php). The structures of all the phytoconstituents and native ligand (.sdf File format) were downloaded from the National Center for Biotechnology Information PubChem (https://pubchem.ncbi.nlm.nih.gov/). A crystalline structure of human glucokinase was obtained from RCSB's Protein Data Bank (PDB) as entry 1V4S (https://www.rcsb.org/structure/1V4S).
Code availability
Not applicable.
Declarations
Conflict of interest
Declared none.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Altaf Khan, Email: altafzpatel@gmail.com.
Aziz Unnisa, Email: khushiazeez@yahoo.co.in.
Mo Sohel, Email: mssj9570@gmail.com.
Mohan Date, Email: datemoh98@gmail.com.
Nayan Panpaliya, Email: npanpaliya9595@gmail.com.
Shweta G. Saboo, Email: shweta.saboo1@gmail.com
Falak Siddiqui, Email: falakarjumand26@gmail.com.
Sharuk Khan, Email: sharique.4u4@gmail.com.
References
- Abirami P, Gomathinayagam M. A review on Enicostemmalittorale. Pharmacologyonline. 2011;1:75–83. [Google Scholar]
- Agrawal M, Kharkar P, Moghe S, Mahajan T, Deka V, Thakkar C, Nair A, Mehta C, Bose J, Kulkarni-Almeida A, Bhedi D, Vishwakarma RA. Discovery of thiazolyl-phthalazinone acetamides as potent glucose uptake activators via high-throughput screening. Bioorganic Med Chem Lett. 2013;23:5740–5743. doi: 10.1016/j.bmcl.2013.07.067. [DOI] [PubMed] [Google Scholar]
- Alam P, Ali M, Singh R, Shakeel F. A new HPTLC densitometric method for analysis of swertiamarin in Enicostemmalittorale and commercial formulations. Nat Prod Res. 2011;25:17–25. doi: 10.1080/14786411003754348. [DOI] [PubMed] [Google Scholar]
- Ali F, Rahul NF, Jyoti S, Siddique YH. Health functionality of apigenin: a review. Int J Food Prop. 2017 doi: 10.1080/10942912.2016.1207188. [DOI] [Google Scholar]
- Babu PS, Prince PSM. Antihyperglycaemic and antioxidant effect of hyponidd, an ayurvedic herbomineral formulation in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2004;56:1435–1442. doi: 10.1211/0022357044607. [DOI] [PubMed] [Google Scholar]
- Baumann S. Apigenin. Ski Allergy News. 2008;39:32. doi: 10.1016/s0037-6337(08)70149-9. [DOI] [Google Scholar]
- Castelhano AL, Dong H, Fyfe MCT, Gardner LS, Kamikozawa Y, Kurabayashi S, Nawano M, Ohashi R, Procter MJ, Qiu L, Rasamison CM, Schofield KL, Shah VK, Ueta K, Williams GM, Witter D, Yasuda K. Glucokinase-activating ureas. Bioorganic Med Chem Lett. 2005;15:1501–1504. doi: 10.1016/j.bmcl.2004.12.083. [DOI] [PubMed] [Google Scholar]
- Charaya N, Pandita D, Grewal AS, Lather V. Design, synthesis and biological evaluation of novel thiazol-2-yl benzamide derivatives as glucokinase activators. Comput Biol Chem. 2018;73:221–229. doi: 10.1016/j.compbiolchem.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Chaudhari RN, Khan SL, Chaudhary RS, Jain SP, Siddiqui FA. Β-Sitosterol: isolation from muntingia calabura linn bark extract, structural elucidation and molecular docking studies as potential inhibitor of SARS-CoV-2 Mpro (COVID-19) Asian J Pharm Clin Res. 2020;13:204–209. doi: 10.22159/ajpcr.2020.v13i5.37909. [DOI] [Google Scholar]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- Filipski KJ, Futatsugi K, Pfefferkorn JA, Stevens BD. Glucokinase activators. Pharm Pat Anal. 2012 doi: 10.4155/ppa.12.26. [DOI] [PubMed] [Google Scholar]
- Fyfe MCT, Procter MJ. Glucokinase activators as potential antidiabetic agents possessing superior glucose-lowering efficacy. Drugs Future. 2009 doi: 10.1358/dof.2009.034.08.1394557. [DOI] [Google Scholar]
- Ghosal S, Singh AK, Sharma PV, Chaudhuri RK. Chemical constituents of gentianaceae IX: natural occurrence of erythrocentaurin in Enicostemmahyssopifolium and Swertialawii. J Pharm Sci. 1974 doi: 10.1002/jps.2600630632. [DOI] [PubMed] [Google Scholar]
- Grewal A, Sekhon B, Lather V. Recent updates on glucokinase activators for the treatment of type 2 diabetes mellitus. Mini-Rev Med Chem. 2014;14:585–602. doi: 10.2174/1389557514666140722082713. [DOI] [PubMed] [Google Scholar]
- Grewal AS, Lather V, Pandita D, Bhayana G. Synthesis, docking and evaluation of phenylacetic acid and trifluoro-methylphenyl substituted benzamide derivatives as potential PPARδ agonists. Lett Drug Des Discov. 2017 doi: 10.2174/1570180814666170327164443. [DOI] [Google Scholar]
- Grewal AS, Sharma K, Singh S, Singh V, Pandita D, Lather V. Design, synthesis and antidiabetic activity of novel sulfamoyl benzamide derivatives as glucokinase activators. J Pharm Technol Res Manag. 2018;6:115–124. doi: 10.15415/jptrm.2018.62008. [DOI] [Google Scholar]
- Grewal AS, Kharb R, Prasad DN, Dua JS, Lather V. N-pyridin-2-yl benzamide analogues as allosteric activators of glucokinase: Design, synthesis, in vitro, in silico and in vivo evaluation. Chem Biol Drug Des. 2019;93:364–372. doi: 10.1111/cbdd.13423. [DOI] [PubMed] [Google Scholar]
- Grewal AS, Lather V, Charaya N, Sharma N, Singh S, Kairys V. Recent developments in medicinal chemistry of allosteric activators of human glucokinase for type 2 diabetes mellitus therapeutics. Curr Pharm Des. 2020;26:2510–2552. doi: 10.2174/1381612826666200414163148. [DOI] [PubMed] [Google Scholar]
- Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Allosteric activators of glucokinase: Potential role in diabetes therapy. Science (80-) 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- Houze JB, Dransfield P, Pattaropong V, Du X, Fu Z, Lai S, Park J, Jiao X, Kohn TJ, Aicher TD, Boyd SA, Bencsik J, Condroski KR, Hinklin RJ, Kraser CF, Pratt S, Singh A, Wenglowsky SM, Boys ML, Chicarelli MJ, Mohr PJ, Cardozo MG (2013) Urea compounds as GKa activators and their preparation. PCT Int Appl
- Indumathi C, Durgadevi G, Nithyavani S, Gayathri PK. Estimation of terpenoid content and its antimicrobial property in Enicostemma litorrale. Int J ChemTech Res. 2014;6:4264–4267. [Google Scholar]
- Ishikawa M, Nonoshita K, Ogino Y, Nagae Y, Tsukahara D, Hosaka H, Maruki H, Ohyama S, Yoshimoto R, Sasaki K, Nagata Y, Eiki J, Nishimura T. Discovery of novel 2-(pyridine-2-yl)-1H-benzimidazole derivatives as potent glucokinase activators. Bioorganic Med Chem Lett. 2009;19:4450–4454. doi: 10.1016/j.bmcl.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Jahan E, Perveen S, Malik A. Verticilliside, a new flavone C-glucoside from Enicostemma verticillatum. J Asian Nat Prod Res. 2009;11:257–260. doi: 10.1080/10286020802675019. [DOI] [PubMed] [Google Scholar]
- Kakkar S, Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:1–9. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SL, Siddiui FA. Beta-Sitosterol: as immunostimulant, antioxidant and inhibitor of SARS-CoV-2 spike glycoprotein. Arch Pharmacol Ther. 2020 doi: 10.33696/pharmacol.2.014. [DOI] [Google Scholar]
- Khan SL, Siddiqui FA, Jain SP, Sonwane GM. Discovery of potential inhibitors of SARS-CoV-2 (COVID-19) main protease (Mpro) from Nigella Sativa (black seed) by molecular docking study. Coronaviruses. 2020;2:384–402. doi: 10.2174/2666796701999200921094103. [DOI] [Google Scholar]
- Khan SL, Sonwane GM, Siddiqui FA, Jain SP, Kale MA, Borkar VS. Discovery of naturally occurring flavonoids as human cytochrome P450 (CYP3A4) inhibitors with the aid of computational chemistry. Indo Glob J Pharm Sci. 2020;10:58–69. doi: 10.35652/igjps.2020.10409. [DOI] [Google Scholar]
- Khan SL, Siddiqui FA, Shaikh MS, Nema NV, Shaikh AA. Discovery of potential inhibitors of the receptor-binding domain (RBD) of pandemic disease-causing SARS-CoV-2 Spike glycoprotein from Triphala through molecular docking. Curr Chinese Chem. 2021 doi: 10.2174/2666001601666210322121802. [DOI] [Google Scholar]
- Kohn TJ, Du X, Lai S, Xiong Y, Komorowski R, Veniant M, Fu Z, Jiao X, Pattaropong V, Chow D, Cardozo M, Jin L, Conn M, DeWolf WE, Kraser CF, Hinklin RJ, Boys ML, Medina JC, Houze J, Dransfield P, Coward P. 5-Alkyl-2-urea-substituted pyridines: identification of efficacious glucokinase activators with improved properties. ACS Med Chem Lett. 2016;7:666–670. doi: 10.1021/acsmedchemlett.6b00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Altman N. Points of significance: significance P values and t-tests. Nat Methods. 2013;10:1041–1042. doi: 10.1038/nmeth.2698. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim BG, Kim M, Ahn JH. Biosynthesis of two flavones, apigenin and genkwanin, in Escherichiacoli. J Microbiol Biotechnol. 2015;25:1442–1448. doi: 10.4014/jmb.1503.03011. [DOI] [PubMed] [Google Scholar]
- Leong XY, Thanikachalam PV, Pandey M, Ramamurthy S. A systematic review of the protective role of swertiamarin in cardiac and metabolic diseases. Biomed Pharmacother. 2016 doi: 10.1016/j.biopha.2016.10.044. [DOI] [PubMed] [Google Scholar]
- Li YQ, Zhang YL, Hu SQ, Wang YL, Song HR, Feng ZQ, Lei L, Liu Q, Shen ZF. Design, synthesis and biological evaluation of novel glucokinase activators. Chinese Chem Lett. 2011;22:73–76. doi: 10.1016/j.cclet.2010.07.023. [DOI] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Maroo J, Vasu VT, Aalinkeel R, Gupta S. Glucose lowering effect of aqueous extract of Enicostemmalittorale Blume in diabetes: a possible mechanism of action. J Ethnopharmacol. 2002;81:317–320. doi: 10.1016/S0378-8741(02)00095-8. [DOI] [PubMed] [Google Scholar]
- Maroo J, Vasu VT, Gupta S. Dose dependent hypoglycemic effect of aqueous extract of Enicostemmalittorale Blume in alloxan induced diabetic rats. Phytomedicine. 2003;10:196–199. doi: 10.1078/094471103321659933. [DOI] [PubMed] [Google Scholar]
- Mathew S, Abraham TE. Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit Rev Microbiol. 2006 doi: 10.1080/10408410600709628. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Glucokinase and glycemic disease: from basics to novel therapeutics. Front Diabetes Front Diabetes. 2004;16:169–179. doi: 10.1159/000079015. [DOI] [Google Scholar]
- Murali B, Upadhyaya UM, Goyal RK. Effect of chronic treatment with Enicostemmalittorale in non-insulin-dependent diabetic (NIDDM) rats. J Ethnopharmacol. 2002;81:199–204. doi: 10.1016/S0378-8741(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Murray A, Lau J, Jeppesen L, Vedso P, Ankersen M, Lundbeck JM, Kristiansen M, Valcarce-Lopez MC, Polisetti DR, Subramanian G, Andrews RC, Christen DP, Cooper JT, Santhosh KC (2005) Preparation of heteroaryl ureas and their use as glucokinase activators. PCT Int Appl
- Nagarathnamma M, Sudarshana MS, Niranjan MH, Pandurangamurthy, Rapid regeneration of enicostemma littorale blume from leaf and stem cultures. J Plant Interact. 2010;5:69–73. doi: 10.1080/17429140903353549. [DOI] [Google Scholar]
- Nasr Bouzaiene N, Chaabane F, Sassi A, Chekir-Ghedira L, Ghedira K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016;144:80–85. doi: 10.1016/j.lfs.2015.11.030. [DOI] [PubMed] [Google Scholar]
- Pal M. Medicinal chemistry approaches for glucokinase activation to treat type 2 diabetes. Curr Med Chem. 2009;16:3858–3874. doi: 10.2174/092986709789177993. [DOI] [PubMed] [Google Scholar]
- Park K, Lee BM, Hyun KH, Han T, Lee DH, Choi HH. Design and synthesis of acetylenyl benzamide derivatives as novel glucokinase activators for the treatment of t2dm. ACS Med Chem Lett. 2015;6:296–301. doi: 10.1021/ml5004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Shah RS, Goyal RK. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J Exp Biol. 2009;47:564–570. [PubMed] [Google Scholar]
- Patel P, Harde P, Pillai J, Darji N, Patel B. Antidiabetic herbal drugs a review. Pharmacophore. 2012;3:18–29. [Google Scholar]
- Patel TP, Soni S, Parikh P, Gosai J, Chruvattil R, Gupta S. Swertiamarin: an active lead from Enicostemmalittorale regulates hepatic and adipose tissue gene expression by targeting PPAR-γ and improves insulin sensitivity in experimental niddm rat model. Evid-Based Complement Altern Med. 2013 doi: 10.1155/2013/358673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei K, Ou J, Huang J, Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn JA, Guzman-Perez A, Oates PJ, Litchfield J, Aspnes G, Basak A, Benbow J, Berliner MA, Bian J, Choi C, Freeman-Cook K, Corbett JW, Didiuk M, Dunetz JR, Filipski KJ, Hungerford WM, Jones CS, Karki K, Ling A, Li JC, Patel L, Perreault C, Risley H, Saenz J, Song W, Tu M, Aiello R, Atkinson K, Barucci N, Beebe D, Bourassa P, Bourbounais F, Brodeur AM, Burbey R, Chen J, D’Aquila T, Derksen DR, Haddish-Berhane N, Huang C, Landro J, Lee Lapworth A, MacDougall M, Perregaux D, Pettersen J, Robertson A, Tan B, Treadway JL, Liu S, Qiu X, Knafels J, Ammirati M, Song X, Dasilva-Jardine P, Liras S, Sweet L, Rolph TP. Designing glucokinase activators with reduced hypoglycemia risk: discovery of N, N-dimethyl-5-(2-methyl-6-((5-methylpyrazin-2-yl)-carbamoyl)benzofuran-4- yloxy)pyrimidine-2-carboxamide as a clinical candidate for the treatment of type 2 diabetes mellitus. Medchemcomm. 2011;2:828–839. doi: 10.1039/c1md00116g. [DOI] [Google Scholar]
- Polisetti DR, Kodra JT, Lau J, Bloch P, Valcarce-Lopez MC, Blume N, Guzel M, Santhosh KC, Mjalli AMM, Andrews RC, Subramanian G, Ankersen M, Vedso P, Murray A, Jeppesen L (2004) Preparation of thiazolyl aryl ureas as activators of glucokinase. PCT Int Appl
- Rappé AK, Casewit CJ, Colwell KS, Goddard WA, Skiff WM. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc. 1992;114:10024–10035. doi: 10.1021/ja00051a040. [DOI] [Google Scholar]
- Rathod MC, Dhale DA. Pharmacognostic characterization of Enicostemmalittorale blume. Int J Res Ayurveda Pharm. 2013;4:893–898. doi: 10.7897/2277-4343.04624. [DOI] [Google Scholar]
- Sadique J, Chandra T, Thenmozhi V, Elango V. The anti-inflammatory activity of Enicostemmalittorale and Mollugocerviana. Biochem Med Metab Biol. 1987;37:167–176. doi: 10.1016/0885-4505(87)90023-5. [DOI] [PubMed] [Google Scholar]
- Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, Antolak H, Azzini E, Setzer WN, Martins N. The therapeutic potential of Apigenin. Int J Mol Sci. 2019 doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Diego: Accelrys Software Inc. Discovery Studio Modeling Environment Release 35. San Diego: Accelrys Softw Inc.; 2012. [Google Scholar]
- Sanmugarajah V. Phyto, physicochemical standardization of medicinal plant EnicostemmaLittorale. Blume IOSR J Pharm. 2013;3:52–58. doi: 10.9790/3013-32205258. [DOI] [Google Scholar]
- Sarabu R, Berthel SJ, Kester RF, Tilley JW. Glucokinase activators as new type 2 diabetes therapeutic agents. Expert Opin Ther Pat. 2008;18:759–768. doi: 10.1517/13543776.18.7.759. [DOI] [Google Scholar]
- Sawant LP, Prabhakar BR, Pandita NS. A validated quantitative HPTLC method for analysis of biomarkers in Enicostemmalittorale Blume. J Planar Chromatogr. 2011;24:497–502. doi: 10.1556/JPC.24.2011.6.8. [DOI] [Google Scholar]
- Selvaraj S, Selvaraj S, Chittibabu CV, Janarthanam B. Studies on phytochemical screening, antioxidant activity and extraction of active compound (Swertiamarin) from leaf extract of Enicostemma littorale. Asian J Pharm Clin Res. 2014;7:240–244. [Google Scholar]
- Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010 doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui FA, Khan SL, Marathe RP, Nema NV. Design, synthesis, and in silico studies of novel N-(2-Aminophenyl)-2,3- diphenylquinoxaline-6-sulfonamide derivatives targeting receptor- binding domain (RBD) of SARS-CoV-2 spike glycoprotein and their evaluation as antimicrobial and antimalarial agents. Lett Drug Des Discov. 2021;18:915–931. doi: 10.2174/1570180818666210427095203. [DOI] [Google Scholar]
- Sidduri A, Grimsby JS, Corbett WL, Sarabu R, Grippo JF, Lou J, Kester RF, Dvorozniak M, Marcus L, Spence C, Racha JK, Moore DJ. 2,3-Disubstituted acrylamides as potent glucokinase activators. Bioorganic Med Chem Lett. 2010;20:5673–5676. doi: 10.1016/j.bmcl.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Singh R, Lather V, Pandita D, Judge V, Arumugam K, Grewal A. Synthesis, docking and antidiabetic activity of some newer benzamide derivatives as potential glucokinase activators. Lett Drug Des Discov. 2016;14:540–553. doi: 10.2174/1570180813666160819125342. [DOI] [Google Scholar]
- Sonawane RD, Vishwakarma SL, Lakshmi S, Rajani M, Padh H, Goyal RK. Amelioration of STZ-induced type 1 diabetic nephropathy by aqueous extract of Enicostemma littorale Blume and swertiamarin in rats. Mol Cell Biochem. 2010;340:1–6. doi: 10.1007/s11010-010-0393-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Padmanabhan M, Prince PSM. Effect of aqueous Enicostemma littorale Blume extract on key carbohydrate metabolic enzymes, lipid peroxides and antioxidants in alloxan-induced diabetic rats. J Pharm Pharmacol. 2005;57:497–503. doi: 10.1211/0022357055722. [DOI] [PubMed] [Google Scholar]
- Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh Kumar C. Syringic acid (SA): a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother. 2018 doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Thirumalai T, Viviyan Therasa S, Elumalai EK, David E. Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume. Asian Pac J Trop Biomed. 2011;1:381–385. doi: 10.1016/S2221-1691(11)60084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay UM, Goyal RK. Efficacy of Enicostemma littorale in type 2 diabetic patients. Phyther Res. 2004 doi: 10.1002/ptr.1434. [DOI] [PubMed] [Google Scholar]
- Vaidya H, Rajani M, Sudarsanam V, Padh H, Goyal R. Swertiamarin: a lead from Enicostemmalittorale Blume. For anti-hyperlipidaemic effect. Eur J Pharmacol. 2009;617:108–112. doi: 10.1016/j.ejphar.2009.06.053. [DOI] [PubMed] [Google Scholar]
- Vaidya H, Rajani M, Sudarsanam V, Padh H, Goyal R. Antihyperlipidaemic activity of swertiamarin, a secoiridoid glycoside in poloxamer-407-induced hyperlipidaemic rats. J Nat Med. 2009;63:437–442. doi: 10.1007/s11418-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Vasu VT, Modi H, Thaikoottathil JV, Gupta S. Hypolipidaemic and antioxidant effect of Enicostemmalittorale Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol. 2005;101:277–282. doi: 10.1016/j.jep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Vijayvargia R, Kumar M, Gupta S. Hypoglycemic effect of aqueous extract of Enicostemmalittorale Blume (Chhotachirayata) on alloxan induced diabetes mellitus in rats. Indian J Exp Biol. 2000;38:781–784. [PubMed] [Google Scholar]
- Vishwakarma SL, Rajani M, Bagul MS, Goyal RK. A rapid method for the isolation of swertiamarin from Enicostemma littorale. Pharm Biol. 2004;42:400–403. doi: 10.1080/13880200490885095. [DOI] [Google Scholar]
- Vishwakarma SL, Sonawane RD, Rajani M, Goyal RK. Evaluation of effect of aqueous extract of enicostemma littorale blume in streptozotocin-induced type 1 diabetic rats. Indian J Exp Biol. 2010;48:26–30. [PubMed] [Google Scholar]
- Wu P, Li L, Du GH. Natural small molecule drugs from plants. Berlin: Springer; 2018. Ferulic acid; pp. 75–80. [Google Scholar]
- Zelent D, Najafi H, Odili S, Buettger C, Weik-Collins H, Li C, Doliba N, Grimsby J, Matschinsky FM. Glucokinase and glucose homeostasis: Proven concepts and new ideas. Biochem Soc Trans. 2005 doi: 10.1042/BST0330306. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu S, Lei L, Zhang Y, Zhang L, Song H, Shen Z, Feng Z. Design, synthesis and evaluation of novel derivatives of orotic acid amide as potent glucokinase activators. Lett Drug Des Discov. 2016;14:252–261. doi: 10.2174/1570180813666161013150056. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The properties of all the phytoconstituents were calculated from SwissADME online tool (http://www.swissadme.ch/index.php). The structures of all the phytoconstituents and native ligand (.sdf File format) were downloaded from the National Center for Biotechnology Information PubChem (https://pubchem.ncbi.nlm.nih.gov/). A crystalline structure of human glucokinase was obtained from RCSB's Protein Data Bank (PDB) as entry 1V4S (https://www.rcsb.org/structure/1V4S).
Not applicable.