Abstract
Several observational studies suggested that gut microbiome-affecting-medication impairs the effectiveness of immunotherapy in patients with metastatic non-small-cell lung cancer (NSCLC). We postulated that if the effectiveness of immunotherapy is affected by drug-related changes of the microbiome, a stronger association between the use of co-medication and overall survival (OS) will be observed in patients treated with immunotherapy as compared to patients treated with chemotherapy. In a retrospective matched cohort study, immunotherapy patients were matched (1:1) to patients treated with chemotherapy in the pre immunotherapy era. The association between the use of antibiotics, opioids, proton pump inhibitors, metformin and other antidiabetics on OS was assessed with multivariable cox-regression analyses. Interaction tests were applied to investigate whether the association differs between patients treated with immuno- or chemotherapy. A total of 442 patients were studied. The use of antibiotics was associated with worse OS (adjusted Hazard Ratio (aHR) 1.39, p = 0.02) independent of the type of therapy (chemotherapy or immunotherapy). The use of opioids was also associated with worse OS (aHR 1.33, p = 0.01). The other drugs studied showed no association with OS. Interaction term testing showed no effect modification by immuno- or chemotherapy for the association of antibiotics and opioids with OS. The use of antibiotics and opioids is similarly associated with worse outcomes in both chemotherapy and immunotherapy treated NSCLC patients. This suggests that the association is likely to be a consequence of confounding rather than disturbing the composition of the microbiome.
Subject terms: Epidemiology, Cancer immunotherapy
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. In 2012, the World Health Organisation (WHO) estimated that of the 1.6 million lung cancer related deaths, non-small-cell lung cancer (NSCLC) is the most frequent histological type, representing 85% of cases1.
Since the beginning of systemic treatment of stage III and IV NSCLC patients, platinum-doublet chemotherapy has been the first-choice treatment. With the development of new therapies, more effective treatment options have become available in addition to the platinum-doublet therapy2,3. For patients without driver mutations, immunotherapies targeting immune checkpoints, such as the PD-1/PD-L1 pathway, have become an important new treatment option. Currently, four PD-1/PD-L1 inhibitors (pembrolizumab, nivolumab, durvalumab and atezolizumab) have been approved for treatment of patients with advanced NSCLC.
The approval of immunotherapy, with or without chemotherapy, is based on several phase III studies demonstrating superior efficacy in a population meeting the strict in- and exclusion criteria4–6. For example, patients with present active infections, autoimmune conditions, and other comorbidities often requiring concomitant medication are not included in these trials. Therefore, the impact of these factors, including the use of concomitant medication on the efficacy of immunotherapy, cannot be studied from clinical trial data.
Recent studies demonstrate that the microbial flora plays an important role in modulation of immunotherapy effectiveness by affecting the tumor immuno-microenvironment7. It is well known that the microbiome can vary significantly from one individual to another, which has been proposed as an explanation of the variability of response to immunotherapy8. Routy et al. showed that changes in composition of the gut microbiome negatively influence the outcome of PD-1 inhibition by immunotherapy in mice and patients. Additionally, the study found that the replacement of deleterious microbial flora by a favorable one, can restore the efficacy of the immunotherapy response in mice9. The composition of the human microbiome is influenced by several factors such as host genetics, lifestyle factors and the use of antibiotics. Besides antibiotics, recent microbiome studies showed that many other drugs can also alter the composition of the microbiome, as investigated with high-throughput drug screens and metagenomics analyses10,11.
Accumulating observational studies show that antibiotic treatment associates significantly with attenuated clinical outcomes in NSCLC patients treated with immunotherapy9,12,13. However, the majority of these studies analyzed the effects of antibiotics on survival outcomes in cohort studies including only immunotherapy patients. This makes the results susceptible for bias due to confounding by indication, a situation in which patient characteristics—rather than the presence of concomitant medication- are independent predictors of clinical outcomes. Therefore, it is still an active area of debate whether or not there is a causal link between antibiotic related changes of the microbiome and the decreased effectiveness of immunotherapy. Assuming that a prospective clinical trial randomizing based on concomitant medication will not conducted, additional observational studies with alternative designs are needed to resolving this matter, for example a study design including a control group not treated with immunotherapy. If the association is causal one would expect the association to be more pronounced with immunotherapy compared to chemotherapy. The aim of the present study is to test this hypothesis.
Patients and methods
Study design
This is a retrospective matched cohort study using clinical data from six hospitals of the Santeon hospital network.
Data sources
The Santeon hospital network consists of seven large teaching hospitals geographically spread across the Netherlands. The Netherlands Cancer Registry (NCR) was used for identifying all patients diagnosed with NSCLC in a Santeon hospital, and for obtaining information on the date of diagnosis and the vital status. Individual patients are assigned a unique anonymous identifier, which enables them to be tracked in the Santeon Farmadatabase (SFD). The information of the SFD was used for collection of detailed information about the systemic treatments14. Finally, the patients’ medical records were used to complement the database with detailed information about the clinical and demographic characteristics, the use of concomitant medication and the treatment response. All data were gathered and stored at a Research Electronic Data Capture database (REDcap)15.
Study population
Patients diagnosed with stage IV NSCLC between 1st January 2015 to 1st January 2019 and who started first-, second- or third-line immunotherapy before the 1st of January 2020 were assigned to the immunotherapy group. We matched every patient in the immunotherapy group to a patient with stage IV NSCLC who received conventional chemotherapy in the pre-immunotherapy era, and has been diagnosed before 1st January 2015 (see publication of Cramer et al. for more details about this cohort)15. Patients were (1:1) matched on gender, age groups (< 50 year, 50–60 year, 61–70 year, 71–80 year, > 80 year) and line of treatment (first, second or third).
Clinical characteristics
The patient characteristics and demographics were collected manually from the patients’ medical records, including age, gender, body mass index (BMI), Eastern Cooperative Oncology Group-Performance status (ECOG-PS), the histology subtype, brain metastases and lines of systemic treatments. First-line treatment (1L) was defined as the initial systemic therapy used in the treatment of NSCLC. Second-line and third-line treatment (2L and 3L) were defined as the therapy given after discontinuation due to disease progression or completion of first or second-line treatment, respectively. Additionally, oncogenic driver mutation status (e.g. EGFR, ALK, ROS-1) and PD-L1 expression were collected if available.
Concomitant medication
Patient records were reviewed to collect information on the use of concomitant medication potentially affecting the microbiome10,11. Out of the drugs classes known to alter the composition of the microbiome, most commonly used classes in NSCLC patients were selected. The drug classes selected were antibiotics, proton-pump inhibitors, metformin, antidiabetics and opioids. Medication reconciliation for NSCLC patients was performed by the treating oncologist of assisting nurse prior to the start of the systemic treatment and was reported in the medical health record. Exposure was defined if any information on the use of these drugs was reported within a timeframe of 30 days before and 30 days after the start of either immunotherapy or chemotherapy. This timing was chosen because there is evidence that the effects are the strongest when used shortly before and after the start of immunotherapy and consequently, we would expect the largest effect modification by chemotherapy vs immunotherapy (if any)16. The 30 days timeframe is also in line with many previous reports on the topic9,13,17.
Clinical outcomes
OS was defined as the time from the start of systemic therapy to death. Patients still alive at the end of follow-up on 1 January 2020 were censored at this date.
Statistical analysis
Statistical Software (SPSS version 26 for Windows: IBM) was used for statistical analysis. Categorical and continuous variables were summarized using descriptive statistics. To compare the immunotherapy and chemotherapy group, we used chi-squared tests (categorical variables) and independent t- test (continuous variables). The potential impact of concomitant medication on OS was analyzed through multivariable cox regression analyses. Possible factors associated with OS were first identified using a univariable analysis. All univariate predictors with a p-value ≤ 0.15 and three other relevant variables—type of treatment, the ECOG-PS and the use of antibiotics—were used to construct the multivariable model. In the final models, backward selection was applied to eliminate non-significant variables (p-value ≤ 0.10). Finally, the models were examined for the existence of effect modification by statistical testing of an interaction term between concomitant drugs of interest and the type of treatment (chemotherapy or immunotherapy). In order to investigate the difference between the lines of treatment, an exploratory analysis was performed for 1L patients and for 2L + 3L patients using the same approach described above. Survival curves using the Kaplan–Meier method were constructed to visualize—where considered of relevance- contrast between the use of concomitant medication and the type of treatment (chemotherapy or immunotherapy).
Ethical statement
All methods were carried out in accordance with relevant guidelines and regulations. Our ethics committee—the Santeon Institutional Review Board—approved the study (SDB 2019–013) and waived the need for informed consent because of anonymous data handling and the retrospective nature of the study. The study was performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Baseline characteristics
A total of 221 immunotherapy patients could be matched to patients treated with chemotherapy in the pre-immunotherapy era resulting in a total of 442 patients available for our primary analysis. The baseline characteristics of the immunotherapy and chemotherapy group are summarized in Table 1. Both immuno- and chemotherapy groups had an average age of 64 years. The majority of patients was male (59.2%) and received immunotherapy as second or higher line of treatment (62%). The demographic and baseline characteristics of the patients were well balanced between the two treatment groups, with the exception of the ECOG-PS. In the immunotherapy group, there was a significantly higher proportion of patients with an ECOG -PS1 (62.4% versus 39.8%) as compared to the chemotherapy group. In both groups, the most common histology subtype was adenocarcinoma, although there were fewer patients in the immunotherapy group with squamous tumor histology than in the chemotherapy group (10.9% versus 18.1% respectively). Table 2 provides a list of concomitant medication in use at the time of start systemic treatment. The most commonly used drugs in both groups were PPIs (43.4% vs 45.7% for the immuno- and chemotherapy group). Patients who received immunotherapy used significantly less opioids (26.7% vs 37.6% p = 0.02) and used more antibiotics as compared to the patients who received chemotherapy (15.8% vs 19.5%, p = 0.32). Only a small percentage of patients in the immunotherapy and chemotherapy group used metformin (3.6% and 9.5% respectively) or other antidiabetics (2.3% and 3.2% respectively).
Table 1.
Patient characteristics of stage IV NSCLC patients treated with immunotherapy and chemotherapy.
| Characteristics | Immunotherapy (n = 221) | Chemotherapy (n = 221) | p-value |
|---|---|---|---|
| Age (median ± SD) | 64.0 ± 9.0 | 64.1 ± 8.9 | 0.83 |
| Gender | 131 (59.2%) | 131 (59.2%) | 1.0 |
| BMI (median ± SD) | 25.0 ± 6.3 | 25.0 ± 4.1 | 0.82 |
| Line of treatment | 1.0 | ||
| 1 | 84 (38.0%) | 84 (38.0%) | |
| 2 | 120 (54.3%) | 120 (54.3%) | |
| 3 | 17 (7.7%) | 17 (7.7%) | |
| ECOG PS | < 0.001 | ||
| 0 | 70 (31.7%) | 104 (47.1%) | |
| 1 | 138 (62.4%) | 88 (39.8%) | |
| 2 | 10 (4.5%) | 19 (8.6%) | |
| ≤ 3 | 2 (0.9%) | 4 (1.8%) | |
| Unknown | 1 (0.5%) | 6 (2.7%) | |
| Immunotherapy | |||
| Nivolumab | 121 (54.8%) | ||
| Pembrolizumab | 93 (42.1%) | ||
| Atezolizumab | 7 (3.0%) | ||
| Chemotherapy | |||
| Cisplatin/pemetrexed | 14 (6.3%) | ||
| Cisplatin/gemcitabine | 5 (2.3%) | ||
| Carboplatin/pemetrexed | 57 (31.3%) | ||
| Carboplatin/gemcitabine | 17 (7.7%) | ||
| Carboplatin/paclitaxel/bevacizumab | 21 (9.5%) | ||
| Pemetrexed monotherapy | 46 (20.8%) | ||
| Docetaxel monotherapy | 58 (26.2%) | ||
| Other | 3 (1.4%) | ||
| Histology | < 0.001 | ||
| Adenocarcinoma | 176 (79.6%) | 132 (59.7%) | |
| Squamous | 24 (10.9%) | 40 (18.1%) | |
| Large cell carcinoma | 10 (4.5%) | 17 (7.7%) | |
| Other | 11 (4.9%) | 32 (14.5%) | |
| PD-L1 expression | |||
| < 1% | 34 (15.4%) | ||
| 1–50% | 28 (12.7%) | ||
| > 50% | 100 (45.2%) | ||
| Unknown | 60 (27.1%) | ||
| Targetable mutations | 0.58 | ||
| EGFR | 5 (2.3%) | 6 (2.7%) | |
| ALK | 0 | 1 (0.5%) | |
| Other | 5 (2.3%) | 0 | |
| Brain metastasis (yes) | 49 (22.2%) | 43 (19.5%) | 0.48 |
Abbreviations: ECOG PS, Eastern Cooperative Group performance status; BMI, Body mass index; PD-L1, programmed death ligand-1.
Table 2.
Concomitant medication exposure according to immunotherapy of chemotherapy treatment.
| Immunotherapy (n = 221) | Chemotherapy (n = 221) | p-value | |
|---|---|---|---|
| Antibiotics | 35 (15.8%) | 43 (19.5%) | 0.32 |
| Antidiabetics | 5 (2.3%) | 7 (3.2%) | 0.56 |
| Metformin | 8 (3.6%) | 21 (9.5%) | 0.12 |
| PPI | 96 (43.4%) | 101 (45.7%) | 0.63 |
| Opioids | 61 (27.6%) | 83 (37.6%) | 0.02 |
Abbreviations: PPI, proton pump inhibitor.
Overall survival outcomes in the total population
The results of the univariable and multivariable analysis for OS are summarized in Table 3. The multivariable model showed that the use of antibiotics and opioids was significantly associated with shorter OS, with a corresponding HR of 1.39 (95%CI 1.06–1.81) and a HR 1.33 (95%CI 1.07–1.66) respectively. Proton pump inhibitors, metformin and other antidiabetics were not associated with OS. Overall, the strongest factor associated with OS was the type of systemic treatment (immunotherapy vs chemotherapy). Other factors identified as independently associated with OS in the multivariable model were type of histology (squamous vs. non-squamous) and line of treatment (≥ 2L vs. 1L).
Table 3.
Univariable and multivariable model for overall survival in the total population.
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Overall survival for the total population (n = 442) | ||||||
| Treatment immunotherapy (vs chemotherapy) | 0.45 | 0.36–0.56 | < 0.01 | 0.46 | 0.37–0.58 | < 0.01 |
| Histology squamous (vs non-squamous) | 1.38 | 1.04–1.84 | 0.03 | 1.33 | 0.10–1.79 | 0.05 |
| ECOG PS | ||||||
|
1 (vs 0) ≥ 2 (vs 0) |
1.00 1.40 |
0.80–1.25 0.95–2.06 |
0.99 0.09 |
1.08 1.24 |
0.86–1.35 0.84–1.83 |
0.52 0.28 |
| Gender female (vs. male) | 0.97 | 0.78–1.19 | 0.75 | |||
| Age ≥ 75 year ( vs. < 75 year) | 0.80 | 0.55–1.16 | 0.24 | |||
| BMI ≥ 25 (vs. < 25) | 0.99 | 0.76–1.28 | 0.92 | |||
| Brain metastases yes (vs. no) | 1.08 | 0.84–1.39 | 0.53 | |||
| Line of treatment ≥ 2 (vs. 1) | 1.45 | 1.17–1.80 | < 0.01 | 1.44 | 1.16–1.78 | < 0.01 |
| Antibiotics use (vs. no use) | 1.32 | 1.01–1.71 | 0.04 | 1.39 | 1.06–1.81 | 0.02 |
| Antidiabetics use (vs. no use) | 1.57 | 0.86–2.87 | 0.14 | 1.37 | 0.74–2.52 | 0.32 |
| Metformin use (vs. no use) | 1.33 | 0.89–1.98 | 0.17 | |||
| PPI use (vs. no use) | 1.16 | 0.94–1.42 | 0.17 | |||
| Opioid use (vs. no use) | 1.48 | 1.19–1.84 | < 0.01 | 1.33 | 1.07–1.66 | 0.01 |
Abbreviations: ECOG PS, Eastern Cooperative Group performance status; BMI, body mass index PPI, proton pump inhibitor; HR, Hazard ratio; CI, confidence interval.
For both antibiotics and opioids, the tests for interaction with treatment type were insignificant, both with a p value of 0.50. The distinctive effects of antibiotics and opioids on OS for the immuno- and chemotherapy cohort were summarized in Table 4a. The calculated point estimates for the hazard ratio for use of antibiotics towards OS were similar with immunotherapy (HR 1.20 95% CI 0.79–1.85) and chemotherapy (HR 1.46 95%CI 1.04–2.05). The same was observed for the use of opioids (HR 1.44 (95%CI 1.01–2.06) in immunotherapy group and a HR of 1.24 (95%CI 0.94–1.63) in chemotherapy group).
Table 4.
Multivariable model for OS within the total population (a) and the 1L (b) for both antibiotic and opioid exposure according to type of treatment (chemotherapy or immunotherapy).
| HR (95% CI) for antibiotic use (vs no use) according to the type of treatment | p-value | |
|---|---|---|
| a. OS total population | ||
| Antibiotic usea,1 | ||
| Chemotherapy cohort | 1.46 (1.04–2.05) | 0.03 |
| Immunotherapy cohort | 1.20 (0.79–1.85) | 0.39 |
| HR (95% CI) for opioid use (vs no use) according to the type of treatment | p-value | |
|---|---|---|
| Opioid usea,2 | ||
| Chemotherapy cohort | 1.24 (0.94–1.63) | 0.13 |
| Immunotherapy cohort | 1.44 (1.01–2.06) | 0.04 |
| HR (95% CI) for antibiotic use (vs no use) according to the type of treatment | p-value | |
|---|---|---|
| b. OS first line | ||
| Antibiotic useb.3 | ||
| Chemotherapy cohort | 1.54 (0.86–2.76) | 0.15 |
| Immunotherapy cohort | 1.45 (0.75–2.77) | 0.27 |
| HR (95% CI) for opioid use (vs no use) according to the type of treatment | p-value | |
|---|---|---|
| Opioid useb.4 | ||
| Chemotherapy cohort | 1.30 (0.80–2.09) | 0.29 |
| Immunotherapy cohort | 2.27 (1.24–4.16) | < 0.01 |
The multivariable model includes the variables:
aTreatment (immunotherapy vs chemotherapy), histology (squamous vs non-squamous), line of treatment (≥ 2 vs. 1), antibiotic (use vs no use), opioids (use vs no use).
1Interaction between antibiotic use and type of treatment.
2Interaction between opioid use and type of treatment.
bTreatment (immunotherapy vs chemotherapy), brain metastases (yes vs no), antibiotic (use vs no use),opioids (use vs no use).
3Interaction between antibiotic use and type of treatment.
4Interaction between opioid use and type of treatment.
Overall survival outcomes per treatment line
Restriction of the cohort to patients with 1L treatment yielded similar HRs in that setting. The HRs were 1.49 (95% CI 0.97–2.29) for antibiotic use and 1.58 (95% CI 1.08–2.32) for opioid use (Supplementary Table S1). Also in this setting there was no association between OS and proton pump inhibitors, metformin and other antidiabetics. Factors significantly associated with OS in the multivariable model were the type of treatment (immunotherapy vs chemotherapy) and the presence of brain metastases. The tests for interaction between treatment type and the use of antibiotics and opioids were both not statistically significant, with corresponding p values of 0.89 and 0.15 respectively. The negative effects of antibiotics and opioids on OS were observed in patients treated with chemotherapy as well as in patients treated with immunotherapy (Table 4b), with HRs of similar magnitude between the immuno- and chemotherapy group, although not all were statistically significant. In a second line setting, the use of antibiotics and the use opioids were not statistically significantly associated with OS. The multivariable model for OS showed that only the type of treatment (immunotherapy vs chemotherapy) was associated with a lower risk of death (Supplementary Table S2). Figures 1a and b demonstrate the Kaplan–Meier survival curves for patients treated with immuno- versus chemotherapy, and using/not using antibiotics (1a) or using/not using opioids (1b) in the 1L.
Figure 1.
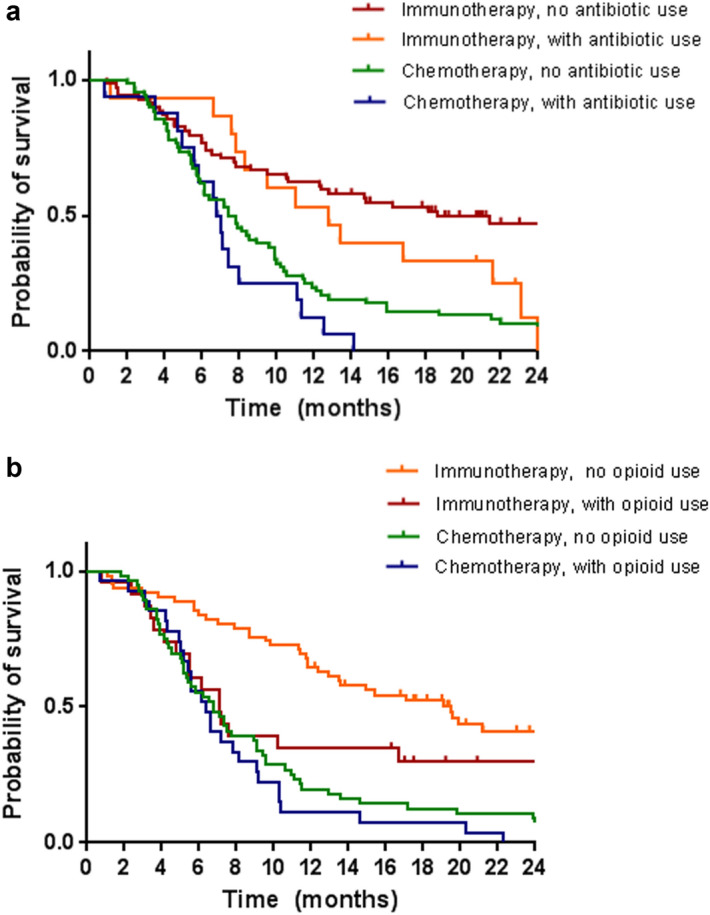
Kaplan–Meier curves showing overall survival for first-line chemo- and immunotherapy with and without the use of antibiotics (a) and with and without the use of opioids (b).
Discussion
In this historically matched cohort study of 442 stage IV NSCLC patients, the use of antibiotics and opioids were shown to be independently associated with worse survival outcomes after adjusting for other prognostic factors associated with a poorer prognosis. Moreover, the negative influence of antibiotics and opioids on OS was observed both for treatment with chemotherapy as well as with immunotherapy. This finding suggests that the association between worse survival outcomes and the use of antibiotics and opioids more likely originates from its use being linked with confounding factors rather than the disturbing effect these drugs have on the gut microbiome.
We believe that our historically matched cohort study design adds to what has been published about the topic so far because all previously published reports on the association of antibiotics with immunotherapy effectiveness are retrospective single arm cohort studies. Lurienne et al. (2020) summarized these cohort studies (n = 21) in a meta-analysis and reported a pooled HR for OS of 1.69 (95% CI 1.25–2.29) for NSCLC patients exposed to antibiotics when starting immunotherapy16. Our observed HR of 1.39 is in line with this pooled number but appears of similar magnitude in patients treated with chemotherapy in the years that immunotherapy was not yet available. This finding argues against causality because an effect of antibiotics on chemotherapy effectiveness as a result of microbiome disturbance is unlikely. Thus, we consider that residual confounding by indication bias plays a role in previous reports. An exception is the recent study of Chalabi et al. In a post hoc analysis of a randomized clinical trial of atezolizumab versus docetaxel in a second line setting, a larger association between antibiotics and worse OS was observed in the atezolizumab study arm compared to chemotherapy17. However, also in that study residual confounding cannot be excluded. It is also conceivable that symptomatic rapid progressive disease represented by antibiotic use, prevented patients randomized to immunotherapy from early disease control resulting in earlier death eventually18. Finally, Cortellini et al. recently reported a non-significant HR of 1.42 for antibiotics in patients treated with the combination of immunotherapy and chemotherapy in first line. This HR aligns very much with our observed HR in patients receiving chemotherapy (1.54), further supporting the hypothesis that recent antibiotic use is primarily a prognostic factor instead of an etiological factor19.
For opioid use our findings align with what has been studied by Zheng et al.20. The authors conducted a cohort analysis (n = 203 patients) and a meta-analysis (n = 26 articles) to investigate the impact of opioids use on survival of cancer patients. The results of their analyses showed a negative association between cancer-specific survival and the use of opioids explained cancer-related pain treated with opoids. Furthermore, other studies have reported that opioids may negatively affect cancer patients’ survival through respiratory depression, delirium, addiction or directly by acting on tumor cells21,21. Even though these authors did not investigate whether the negative effects of opioids on survival outcomes differ between patients treated with immunotherapy or chemotherapy, it supports our observed association between opioids and survival irrespective of the systemic treatment applied.
Noteworthy is that the use of PPIs, metformin and other antidiabetics were not associated with poor survival outcomes in our cohort. This might indicate that both the microbiome-hypothesis and the hypothesis of confounding by indication should be rejected for these drugs. Interesting, however, is that some other studies observed associations between PPI use and worse outcomes15. That we were unable to replicate this might be explained by the very high prevalence of PPI use in our cohort that could have resulted in overshadowing an assumed proxy of PPI use for worse clinical condition. In the Netherlands, PPIs are recommended for every adult > 65 years and using anti-platelet drugs, NSAIDS or corticosteroids. Nevertheless, our data suggest no effect of PPIs on effectiveness of immunotherapy in NSCLC patients. The frequency of patients using metformin (6.5%) and antidiabetics (2.7%) in our cohort we consider too small to conclude on any association between these drugs and survival outcomes.
Strengths and weaknesses
Our study has a number of notable strengths. The main strength is its matched cohort study design. This design enabled us to investigate the possibility of concomitant medication-use having a prognostic impact on patients’ clinical survival, regardless of the treatment given, under the assumption that unmeasured confounding factors will be balanced between the two groups. That we captured chemotherapy patients from a period that immunotherapy was not yet available maximized the potential for balance because after market access of immunotherapy patient characteristics are more likely to guide the decision for either treatment. Nevertheless, the distribution of the ECOG status appeared imbalanced in our study. The most likely explanation for this is that the milder toxicology from immunotherapy has led to patients who would not qualify for toxic chemotherapy starting with immunotherapy anyway. By fixing the ECOG status in all our multivariable regression models we think that adjustment for this imbalance has been maximized. Another positive aspect is that we were able to evaluate not only the effects of antibiotics on the therapeutic outcomes of NSCLC patients but also the effects of other drugs, known to be associated with changes of the microbiome composition. There are numerous observational studies investigating the impact of antibiotics, however, studies regarding the effects of other drugs are scarce. Lastly, compared to previous reports, we used a relatively large cohort with advanced NSCLC patients treated in six different hospitals across the Netherlands.
Limitation of our study is the small number of patients using some of the drugs under study. This might explain some null findings due to limited statistical power. Another limitation, in hindsight, is that we did not collect data about the use of corticosteroids in our patients. Corticosteroids have been linked to altered immunotherapy effectiveness as well and inclusion of this variable might have resulted in other HRs with our variables of interest23,24. Some co-linearity of corticosteroids and antibiotics or PPIs cannot be ruled out. Another potential drawback is that we examined concomitant medication when used within a relative short time frame of 30 days before up to 30 days after the start of the systemic therapy. Although the decision for this time frame was well-thought-out (see Methods), we acknowledge that this focus prevented us from studying time varying associations. Finally, our study shares all limitations linked to retrospective studies, at least information bias. For example, the use of concomitant medication was fully based on the information recorded in the patients’ health records and may not be completely comprehensive. On the other hand, both patients with chemotherapy and with immunotherapy were captured from the same hospitals, making the risk of misclassification bias, if any, being non-differential.
Conclusion
In conclusion, we observed that the use of antibiotics and opioids is associated with shorter survival similarly in chemotherapy and immunotherapy treated NSCLC patients. This suggests that the association is likely to be a consequence of confounding rather than disturbing the composition of the microbiome.
Supplementary Information
Author contributions
M.V.V, B.J.M.P. and E.M.W.v.d.G. were involved in creating the research question, study design and hypotheses. E.M.W.v.d.G. contributed to study supervision. M.V.V, M.T. and C.M.C.v.d.W., were responsible for acquisition of data and data analysis. M.V.V, C.M.C.v.d.W., M.T, B.J.M.P., F.M.N.H.S., and E.M.W.v.d.G. contributed to interpretation of data. M.V.V wrote the main manuscript text. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02598-0.
References
- 1.Union for International Cancer Control. Non-small cell lung cancer - 2014 Review of Cancer Medicines on the WHO List of Essential Medicines. https://www.who.int/selection_medicines/committees/expert/20/applications/NonSmallCellLungCancer.pdf?ua=1 (2014)
- 2.Chen R, Manochakian R, James L, Azzouqa AG, Shi H, Zhang Y, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J. Hematol. Oncol. 2020;13(1):1–23. doi: 10.1186/s13045-019-0838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastelijn EA, de Langen AJ, Peters BJM. Treatment of oncogene-driven non-small cell lung cancer. Curr. Opin. Pulm. Med. 2019;25(3):300–307. doi: 10.1097/MCP.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 10.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020;11(1):1–11. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van De Garde E, Plouvier B, Fleuren H, et al. Pharmacotherapy within a learning healthcare system: Rationale for the Dutch Santeon Farmadatabase. Eur. J. Hosp. Pharm. 2019;26(1):46–50. doi: 10.1136/ejhpharm-2017-001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providingtranslational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer-Van Der Welle CM, Peters BJM, Schramel FMNH, Klungel OH, Groen HJM, Van De Garde EMW. Systematic evaluation of the efficacy-effectiveness gap of systemic treatments in metastatic nonsmall cell lung cancer. Eur. Respir. J. 2018;52(6). [DOI] [PMC free article] [PubMed]
- 17.Lurienne L, Cervesi J, Duhalde L, de Gunzburg J, Andremont A, Zalcman G, et al. NSCLC immunotherapy efficacy and antibiotic use: A systematic review and meta-analysis. J. Thorac. Oncol. 2020;15(7):1147–1159. doi: 10.1016/j.jtho.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 19.van de Garde EMW, van Bedaf LR, Hurkmans DP, van den Heuvel MM. Antibiotic use and reduced effectiveness of second-line immunotherapy for lung cancer: All the time or just at the start of treatment? Ann. Oncol. 2020;31(12):1779–1780. doi: 10.1016/j.annonc.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Cortellini A, Ricciuti, B, Facchinetti F, et al. Antibiotic-exposed patients with non-small-cell lung cancer preserve efficacy outcomes following first-line chemo-immunotherapy. Ann. Oncol. [Preprint] 2021. Available from: 10.1016/j.annonc.2021.08.1744 10. [DOI] [PubMed]
- 21.Zheng J, He J, Wang W, Zhou H, Cai S, Zhu L, et al. The impact of pain and opioids use on survival in cancer patients: Results from a population-based cohort study and a meta-analysis. Med (United States). 2020;99(9). [DOI] [PMC free article] [PubMed]
- 22.Hasegawa T, Oguri T, Osawa T, Sawa T, Osaga S, Okuyama T, et al. Opioid dose and survival of patients with incurable nonsmall cell lung cancer: A prospective cohort study. J. Palliat. Med. 2018;21(10):1436–1441. doi: 10.1089/jpm.2018.0044. [DOI] [PubMed] [Google Scholar]
- 23.Singleton PA, Moss J, Karp DD, Atkins JT, Janku F. The mu opioid receptor: A new target for cancer therapy? Cancer. 2015;121(16):2681–2688. doi: 10.1002/cncr.29460. [DOI] [PubMed] [Google Scholar]
- 24.Drakaki A, Dhillon PK, Wakelee H, Chui SY, Shim J, Kent M, et al. Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothel. Oncoimmunology. 2020;9(1):1–9. doi: 10.1080/2162402X.2020.1824645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mountzios G, de Toma A, Economopoulou P, Friedlaender A, Banini M, Lo Russo G, et al. Steroid use independently predicts for poor outcomes in patients with advanced NSCLC and high PD-L1 expression receiving first-line pembrolizumab Mmonotherapy. Clin. Lung Cancer. (2020). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


