Abstract
Prostaglandin A2 (PGA2), an experimental chemotherapeutic agent, causes growth arrest associated with decreased cyclin D1 expression in several cancer cell lines. Here, using human non-small-cell lung carcinoma H1299 cells, we investigated the mechanisms whereby PGA2 down-regulates cyclin D1 expression. Transcription rates of the cyclin D1 gene, studied using a cyclin D1 promoter-luciferase construct and nuclear run-on assays, were not affected by PGA2 treatment. Instead, the cyclin D1 mRNA was rendered unstable after exposure to PGA2. Since the stability of labile mRNA is modulated through binding of proteins to specific mRNA sequences, we sought to identify protein(s) recognizing the cyclin D1 mRNA. In electrophoretic mobility-shift assays using radiolabeled RNA probes derived from different regions of cyclin D1 mRNA, we observed that (i) lysates prepared from PGA2-treated cells exhibited enhanced protein-cyclin D1 RNA complex formation; (ii) the kinetics of complex formation correlated closely with that of cyclin D1 mRNA loss; and (iii) binding occurred within a 390-base cyclin D1 3′ untranslated region (UTR) (K12). This binding activity could be cross-linked, revealing proteins ranging from 30 to 47 kDa. The RNA-binding protein AUF1, previously associated with the degradation of target mRNAs, bound cyclin D1 mRNA, because anti-AUF1 antibodies were capable of supershifting or immunoprecipitating cyclin D1 mRNA-protein complexes. Finally, insertion of K12 in the 3′UTR of reporter genes markedly reduced the expression and half-life of the resulting chimeric mRNAs in transfected, PGA2-treated cells. Our data demonstrate that PGA2 down-regulates cyclin D1 expression by decreasing cyclin D1 mRNA stability and implicates a 390-base element in the 3′UTR in this regulation.
Progression of eucaryotic cells through the division cycle is a highly ordered process involving the sequential activation of cyclin-dependent kinases (cdks) (22, 37). This process is regulated in large part through their interaction with specific cyclins, which function during different phases of the cell cycle. D-type cyclins and cyclin D1 in particular play a critical role in regulating G1 progression (4, 43, 54) as cyclin D1-cdk4 complexes phosphorylate and thereby inactivate the retinoblastoma protein, a critical event required for G1-S transition (60). Cyclin D1 is present in low abundance in quiescent cells, but it rapidly accumulates after stimulation with serum or mitogens. Inhibition of cyclin D1 expression prevents transition of cells from G1 into S phase, while ectopic expression of cyclin D1 shortens the G1 interval in many cell types (40, 43, 45, 46). Although cyclin D1 levels remain relatively constant in normally growing cells, withdrawal of serum results in the rapid disappearance of the protein as cells enter a state of quiescence (56). In addition to serum withdrawal, treatment of cells with other agents known to induce growth arrest (e.g., retinoic acid) likewise results in reduced cyclin D1 expression (31). Support for the importance of cyclin D1 in regulating cell proliferation in vivo and contributing to tumorigenesis has come from observations that cyclin D1 is overexpressed in many different tumor types and that its transgenic overexpression in breast tissue leads to development of mammary tumors in mice (13, 25, 41, 57).
Consistent with its critical function in regulating cell proliferation, expression of cyclin D1 protein appears to be tightly regulated and subject to control at multiple levels. A number of studies have provided evidence that mitogen stimulation up-regulates cyclin D expression through effects on transcription. Transcriptional activation by serum appears to be mediated largely through events regulated by Ras and extracellular signal-regulated kinase, but the c-Jun N-terminal kinase (JNK) and STAT5 pathways have also been implicated in the regulation of cyclin D1 by mitogens (1, 5, 32, 36). Serum stimulation also elevates cyclin D1 expression by enhancing the translation of cyclin D1 mRNA through a mechanism involving the phosphatidylinositol 3-kinase/Akt signaling pathway (38). Treatments that result in the down-regulation of cyclin D1 expression also appear to act through multiple mechanisms, but these are poorly understood. The stress-activated kinase p38 has been shown to inhibit the activity of the cyclin D promoter, suggesting a mechanism through which stresses might down-regulate the transcriptional activity of the gene (32). Rapamycin and retinoic acid have been shown to enhance proteolysis of the protein, while rapamycin also appears to decrease the stability of cyclin D1 mRNA (23, 31).
Although the importance of mRNA stability in regulating gene expression has long been appreciated (3, 48, 49), it is only recently that the mechanisms contributing to such regulation have begun to be identified. The expression of many genes associated with growth regulation, including cytokines (interferon, interleukin-1, interleukin-2, tumor necrosis factor α) growth factors (granulocyte-macrophage colony-stimulating factor), proto-oncogenes (c-fos, c-myc), and cell cycle-regulatory genes (p21, cyclin A, cyclin B1, and cdc25), is regulated through alterations in mRNA stability (6, 7, 18, 19, 34, 48, 58, 59). This regulation is largely exerted through the adenosine and uridine (AU)-rich elements present in their 3′ untranslated regions (UTRs), which often encompass reiterations of an AUUUA motif, and through the 3′ poly(A) tail (6, 8, 9, 17, 19, 30, 50, 53). Several classes of RNA-binding proteins that are capable of interacting with AU-rich elements and influencing mRNA stability have been identified (9–11, 61, 64). While the functional significance of the associations between these RNA-binding proteins and target transcripts remains largely unknown, some of them, such as the Elav family of RNA-binding proteins, appear to enhance mRNA stability (14, 15, 44, 58, 59), while others, such as AUF1, appear to increase mRNA degradation (11, 35).
The cyclopentenone prostaglandin A2 (PGA2) is a potent inhibitor of growth of cultured cells and exhibits antitumor activity in vivo (16, 20, 28, 29, 42, 51, 52). Growth arrest following PGA2 treatment occurs primarily in the G1 phase of the cell cycle, but the mechanisms responsible for eliciting this effect are not well understood. Along with several growth-regulatory genes whose expression is altered following treatment with PGA2 or with related prostaglandins (2, 24, 27), we previously reported that PGA2-mediated growth inhibition was associated with a rapid and dramatic loss in cyclin D1 expression (20). Here, we have investigated the mechanisms contributing to this down-regulation. We show that PGA2 acts primarily by decreasing the stability of cyclin D1 mRNA transcripts through a specific 390-base region in the 3′UTR and provide biochemical and functional evidence for how this element may influence cyclin D1 mRNA turnover.
MATERIALS AND METHODS
Cell culture, treatment, constructs, transfections and luciferase/galactosidase assays.
The human lines H1299 (non-small-cell lung carcinoma), MCF-7, and MDA-MB-453 (breast carcinoma) were obtained from American Type Culture Collection. H1299 and MCF-7 cells were maintained in RPMI 1640 medium and MDA-MB-453 cells in McCoy's 5A medium (GIBCO-BRL, Gaithersburg, Md.). Media were supplemented with 10% fetal calf serum (Hyclone) and antibiotics. All experiments were performed using exponentially growing cells. Transfections were carried out using standard calcium phosphate precipitation methods. For the study of cyclin D1 promoter activity, H1299 cells were transfected with plasmids pD1Luc (a kind gift from H. Müller [39]) and pTK-Hyg (Clontech, Palo Alto, Calif.), selected in the presence of 250 μg of hygromycin B per ml, and stable transfectants were used for luciferase assays. To assess the influence of specific regions of the cyclin D1 mRNA on the expression of the luciferase reporter gene, PCR-amplified DNA fragments CR (encompassing nucleotides 640 through 1030 of the cyclin D1 cDNA) and K12 (described below) were subcloned into the XbaI site of the plasmid pGL3 promoter to generate pGL3-CR and pGL3-K12, respectively. Cotransfection with a β-galactosidase reporter construct served as an internal control for normalization of transient transfections. For direct assessment of the influence of specific regions of the cyclin D1 mRNA on the half-life of chimeric mRNAs, PCR-amplified fragments CR and K12 were subcloned into the XbaI site of pTRE-d2EGFP (Clontech). For this experiment, H1299 cells were initially transfected with the pTet-Off plasmid (Clontech) and selected in the presence of 500 μg of neomycin per ml; out of ∼60 stably transfected clones tested, clone H2 exhibited low background expression of reporter constructs and >20-fold repression of reporter constructs upon addition of doxycycline (1 μg/ml) and was thus chosen for subsequent transfection with either pTRE-d2EGFP, pTRE-d2EGFP-CR, or pTRE-d2EGFP-K12. Following transfection of these constructs into H2, enhanced green fluorescent protein (EGFP) mRNA or the chimeric mRNAs EGFP-CR and EGFP-K12 were transiently expressed. Transcription of these mRNAs was shut off 20 h later through the addition of doxycycline (1 μg/ml), and the rate of elimination of each transcript was measured in cells that had either been treated with PGA2 or left untreated.
Northern and Western blot analyses.
Total RNA was isolated using STAT-60 reagent (Tel-Test B; Friendswood, Tex.) according to the manufacturer's protocol and Northern blot analysis carried out as previously described (20). Human cyclin D1 mRNA, p21 mRNA, 18S rRNA, and EGFP-containing transcripts were detected with oligomers GAGGTTGGCATCGGGGTACGCGCGGCGGATGGTTTCCACTTCGCAGCACAGGAGCTGGTG, CTGGGCCGAAGAGGCGGCGGCAGGCCTTGCTGCCGCATGGGTTCTG, ACGGTATCTGATCGTCTTCGAACC, and TGGCATCGCCCTCGCCCTCGCCGGACACGCTGAACTTGTG, respectively, that were 3′-end labeled with [α-32P]dATP using terminal transferase (Boehringer Mannheim). Signals were visualized and quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). For Western blot analysis, 40 μg of cell lysate was resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes. Cyclin D1 was detected with monoclonal anti-cyclin D1 antibody (Santa Cruz Biotech, Santa Cruz, Calif.), and AUF1 was detected with a polyclonal antibody (64). For the detection of p45AUF1, the peptide SPRHSEAATAQRE, encoded by exon 2 of the human AUF1 gene, was synthesized with an N-terminal cysteine residue by Genemed Synthesis, Inc. (South San Francisco, Calif.), its purity assessed by high-performance liquid chromatography, and its identity verified by mass spectrometry. A keyhole limpet hemocyanin conjugate of this peptide was used to immunize and boost rabbits for production of antisera. Signals were detected with the ECL reagent (Amersham).
Nuclear run-on analysis.
Assays were performed as previously described (21). Briefly, 5 μg of DNA from either the pBlueScript plasmid or plasmid pRSV-D1 harboring cyclin D1 (a kind gift from C. J. Sherr) or a plasmid containing β-actin was linearized and blotted onto nitrocellulose membranes. Nuclei from 2 × 107 H1299 cells were collected from each treatment group (i.e., untreated or PGA2-treated) and nascent RNA was isolated using the STAT-60 reagent. Membranes were blocked with 100 μg of tRNA for 4 h, then hybridized with 4 × 106 cpm of nascent 32P-labeled RNA in 2 ml of hybridization buffer for 72 h at 65°C, and washed extensively at 65°C with wash buffer. Signals were visualized with a PhosphorImager.
Transcription shut-off assays for measuring mRNA half-life.
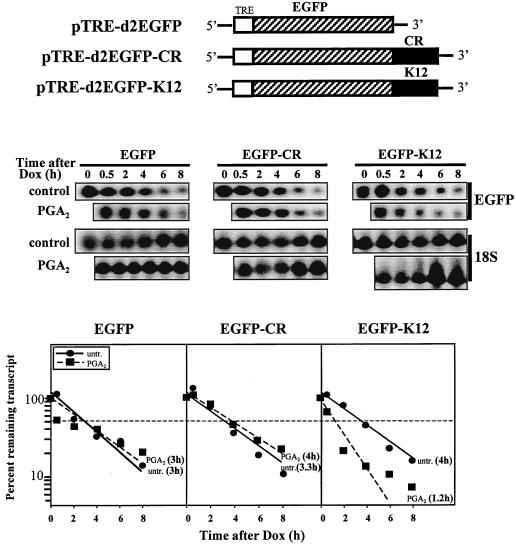
Assays were carried out essentially as described before (63), except that doxycycline was used to shut off transcription and the sequences of interest (CR and K12) were subcloned into pTRE-d2EGFP (Clontech), and thus the chimeric transcript contained the EGFP-coding region. H2 cells plated at a density of 200,000 cells in 100-mm dishes were transfected by calcium phosphate precipitation using 5 μg each of pTRE-d2EGFP, pTRE-d2EGFP-CR, or pTRE-d2EGFP-K12 plasmids. Twenty hours later, cells were treated either with a combination of doxycycline (1 μg/ml) and PGA2 (30 μM) or with doxycycline alone, whereupon RNA was collected at the times indicated and analyzed for expression of EGFP-containing transcripts, cyclin D1 mRNA (not shown), and 18S rRNA.
Preparation of cell fractions.
Cells (2 × 106 cells per sample) were harvested by trypsinization, collected by low-speed centrifugation, resuspended into 200 μl of cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, and 0.5 mM phenylmethylsulfonyl fluoride) and incubated for 15 min on ice. After addition of 25 μl of buffer A containing 2.5% NP-40, lysed cells were centrifuged (1,500 × g, 6 min, 4°C) to separate nuclei; supernatants (cytoplasmic fraction) were collected and stored at −80°C. Nuclear pellets were resuspended into buffer C (20 mM HEPES [pH 7.9], 0.45 M NaCl, and 1 mM EDTA plus protease inhibitors), and the suspensions were subjected to constant shaking for 20 min at 4°C, followed by centrifugation (20,800 × g, 10 min, 4°C) and collection of the supernatants (nuclear fraction). Whole-cell lysates were obtained after collection of cells in lysis buffer (20 mM HEPES [pH 7.4], 50 mM β-glycerolphosphate, 1% Triton X-100, 2 mM EGTA, and 10% glycerol plus protease inhibitors), centrifugation, and collection of supernatants.
Preparation of radiolabeled RNA transcripts.
Eight micrograms of total RNA from H1299 cells was used for reverse transcription using SuperScript reverse transcriptase (GIBCO-BRL), following the manufacturer's protocol. The cDNAs generated were used as templates in PCRs to amplify various regions of cyclin D1 cDNA. All of the 5′ primers used in this study contain the sequence of the T7 RNA polymerase promoter (T7): CCAAGCTTCTAATACGACTCACTATAGGGAGA. The 5′ primers used were as follows: A, (T7)TAGCAGCGAGCAGCAGAGT; B, (T7)ACATCTGAGGGCGCCAGG; C, (T7)AGATGTTTCACACCGGAAGG; H, (T7)GTACTTGTTTCTCTGTTGTAA; I, (T7)GACCTGTTTATGAGATGCTGG; J, (T7)GCTTTTCCTGATAAAGCACAG; K, (T7)CGTGGCCGTGTGCATGTC; L, (T7)GTTGTGTGTGCAGGGAGG; M, (T7)GGTTTGCATTCTCACATTGC; N, (T7)CAGCGACAAACCATCCAGTG; O, (T7)CGCTACTATAAAGAGAAGAC; P, (T7)CAGGTTCAACCCACAGCTAC; and Q, (T7)CCAATAGGTGTAGGAAATAG. The 3′ primers used were as follows: 1, CTCAGATGTCCACGTCCCG; 2, TTCCGGTGTGAAACATCTAAG; 3, CAAGAATTACATAGCCAAGATG; 4, CCACCTCCCTTCAACACTTC; 8, CTTACAACAGACAAACAAGTAC; 9, CCAGCATCTCATAAACAGGTC; 10, CTGTGCTTTATCAGGAAAAGC; 11, GACATGCACACGGCCACG; 12, CCTCCCTGCACACACAAC; 13, GCAATGTGAGAATGCAAACC; 14, GTAGCTGTGGGTTGAACCTG; and 15, CTATTTCCTACACCTATTGG.
PCR-amplified products were resolved on agarose gels, purified to serve as templates for RNA synthesis, and incubated at 37°C for 90 min in transcription buffer supplemented with 10 mM dithiothreitol, 2 mM rATP, 2 mM rCTP, 2 mM rGTP, 1 mM UTP, 80 U of RNasin, 40 U of T7 RNA polymerase, and 50 μCi of [α-32P]UTP in a 50-μl reaction volume. RNA transcripts were purified through spin columns, and 1 μl of each RNA transcript was resolved on formaldehyde-agarose gels, vacuum-transferred onto GeneScreen membranes, and examined to determine the integrity, quality, and specific activity of radiolabeled RNA probes. Nonradiolabeled RNAs for in vitro binding-competition assays were synthesized similarly, except that the final concentration of unlabeled UTP was 2 mM.
Binding assays.
Electrophoretic mobility-shift assays (EMSA) were carried out to detect the formation of complexes between cellular proteins and various cyclin D1 transcripts. Ten-microgram aliquots of either cytoplasmic or nuclear fractions were incubated with 100,000 cpm of RNA transcript on ice for 20 min in a 10-μl mixture containing 15 mM HEPES [pH 7.9], 10 mM KCl, 10% glycerol, 5 mM MgCl2, 1 mM dithiothreitol, and 1 μg of tRNA. After addition of RNase T1 (1,000 U/reaction mixture), reaction mixtures were incubated at 37°C for 20 min and electrophoresed through 7% (or 5%, for supershift analysis) nondenaturing polyacrylamide gels containing 0.25× Tris-borate-EDTA at 150 V for 2 h at 4°C. For cross-linking experiments, RNase T1-treated RNA-protein mixtures were exposed to UV using a Stratalinker (Stratagene), then electrophoresed through SDS-polyacrylamide gels. Gels were dried and radioactive signals visualized with a PhosphorImager. For supershift analysis, 2.5-μg cytoplasmic protein aliquots were incubated with 1 μg each of polyclonal antibodies recognizing AUF1 (64), JNK1, p27Kip1, or p53 (Santa Cruz) on ice for 30 min, then incubated with 100,000 cpm of radiolabeled transcripts for 15 min on ice. RNase T1 digestion and native gel electrophoresis were then performed as described above.
In vitro binding assays were carried out essentially as previously described (61), except that the binding buffer used is the same as that used for EMSA analysis.
Immunoprecipitation.
The coating of protein A beads with anti-AUF1 antibody (Sigma) was performed as previously described (64). For immunoprecipitation of the RNA-protein complexes, in vitro RNA-protein binding reaction mixtures were digested with RNase T1 and subjected to UV cross-linking as described above. Each reaction mixture was then mixed with 10 μl of either antibody-coated or uncoated protein A beads in 100 μl of NET (64)-gel buffer for 2 h at 4°C, centrifuged, and washed three times. The pellets were then mixed with 20 μl of gel-loading buffer, boiled, and resolved on SDS–12% polyacrylamide gels. Signals on dried gels were visualized with a PhosphorImager.
RESULTS
PGA2 treatment decreases cyclin D1 expression in H1299 cells in a dose- and time-dependent manner associated with growth arrest.
We have previously shown that PGA2 causes growth arrest of a variety of human cell lines, concomitant with alterations in the expression of cell cycle genes (20). Consistent with these previous observations, growth of PGA2-treated H1299 cells was quickly and strongly inhibited (Fig. 1). Most of PGA2-treated cells acquired a flattened morphology within 24 h, and after 48 h, a small proportion of treated cells detached from the surface (data not shown). PGA2 treatment also led to the loss of cyclin D1 expression (Fig. 2). This reduction was specific for cyclin D1, as PGA2 treatment led to the elevated expression of other transcripts, such as that encoding the cdk inhibitor p21. Dose-response studies revealed that treatment with 30 to 40 μM PGA2 results in a maximal decrease (80%) in cyclin D1 mRNA levels by 24 h; hence 30 μM PGA2 was selected for use in all further studies. Kinetic analyses revealed a marked reduction in cyclin D1 mRNA expression by 8 h with a maximum reduction achieved by 16 h after addition of the drug (Fig. 2B). The decline in cyclin D1 mRNA expression likewise correlated with a loss in cyclin D1 protein expression (Fig. 2C).
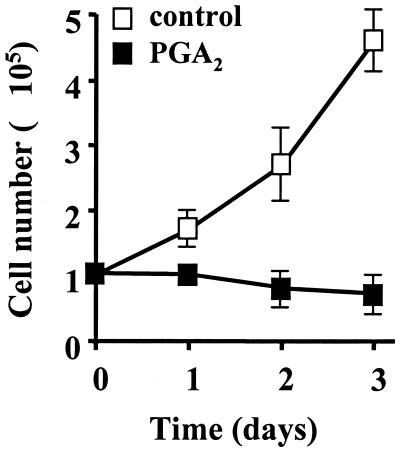
FIG. 1.
PGA2 treatment results in cell growth arrest. H1299 cells were either left untreated or were treated with 30 μM PGA2. Cell numbers were determined every 24 h using a hemacytometer. Open squares, untreated cells; filled squares, PGA2-treated cells. Values shown are the means ± SEM from three independently performed experiments.
FIG. 2.
Cyclin D1 expression after exposure to PGA2. (A) Dose-response analysis of PGA2 treatment on cyclin D1 expression. H1299 cells were treated for the times shown with the indicated doses of PGA2. RNA was then isolated, and the expression of cyclin D1 and p21 was assessed by Northern blot analysis. (B) Kinetics of cyclin D1 mRNA down-regulation. Cyclin D1 mRNA levels in H1299 cells that were either treated with the indicated doses of PGA2 for 16 h (right) or treated with 30 μM PGA2 for the times shown (left) were examined by Northern blot analysis. Cyclin D1 mRNA signal was normalized to 18S rRNA signal. Data represent the means ± SEM from three independently performed experiments. (C) Western blot analysis of cyclin D1 expression was carried out using 40 μg of whole-cell lysates prepared from either untreated or PGA2-treated H1299 cells, as described in Materials and Methods.
PGA2 reduces cyclin D1 expression through changes in mRNA stability.
To investigate the mechanism of the PGA2-triggered decrease in cyclin D1 mRNA levels, we first examined the effect of PGA2 on cyclin D1 promoter activity. H1299 cells stably transfected with a plasmid harboring a luciferase reporter under control of the cyclin D1 promoter were examined for changes in luciferase activity after treatment with PGA2. PGA2 treatment did not result in any substantial change in luciferase activity at either 8 or 12 h following treatment, indicating a lack of effect of PGA2 on cyclin D1 promoter activity (Fig. 3A).
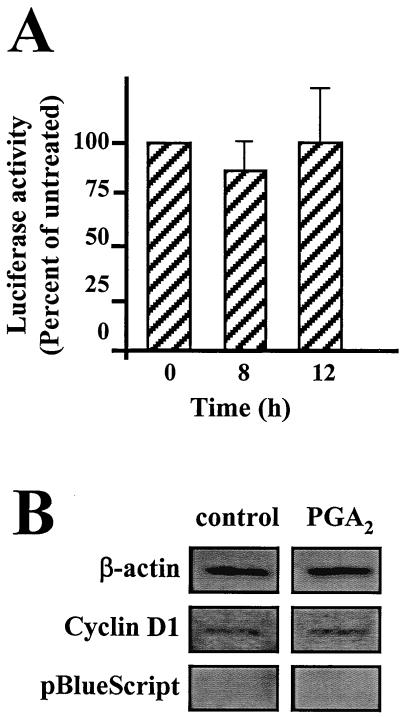
FIG. 3.
Influence of PGA2 on transcriptional activation of the cyclin D1 gene. (A) Effect of PGA2 on cyclin D1 promoter activity. Cells stably transfected with a plasmid harboring a luciferase reporter gene driven by the human cyclin D1 promoter (mg37) were either left untreated or were treated with 30 μM PGA2 for the times indicated. Luciferase activity was then determined on cell lysates (30 to 50 μg of protein) and expressed as percentages relative to luciferase activity in untreated cells. (B) Effect of PGA2 on the transcription rate of cyclin D1. Cells that were either left untreated or treated with 30 μM PGA2 for 8 h were subjected to nuclear run-on assay. Membranes were blotted with cDNAs encoding β-actin, cyclin D1, and a control plasmid without an insert (pBlueScript). The signals were visualized with a PhosphorImager.
The results from the cyclin D1 promoter-luciferase studies suggested that changes in transcriptional regulation are unlikely to account for the marked loss of cyclin D1 mRNA after PGA2 exposure (Fig. 2A and B). To further examine whether there was a transcriptional component in the PGA2-regulated cyclin D1 expression, we performed nuclear run-on assays to compare the transcription rates of the cyclin D1 gene in untreated and PGA2-treated cells. As shown in Fig. 3B, we observed no differences between the rate of cyclin D1 transcription in untreated and PGA2-treated cells. Given the lack of influence of PGA2 on transcription of the cyclin D1 gene, we hypothesized that the loss of cyclin D1 expression was due to enhanced degradation of its mRNA. Attempts to study the half-life of the cyclin D1 mRNA using standard actinomycin D-based methods were unsuccessful due to its artifactual stabilization of cyclin D1 mRNA. Thus, we set out to investigate the influence of PGA2 on cyclin D1 mRNA stability by other methods.
Identification of a PGA2-inducible cyclin D1 RNA-protein binding activity and its correlation with the elimination of cyclin D1 mRNA.
The process of mRNA turnover involves RNA-binding proteins that recognize specific RNA sequences on target transcripts. Therefore, we sought to identify the RNA-protein-binding activities involved in the turnover of cyclin D1 mRNA. Cell lysates prepared from untreated or PGA2-treated cells were fractionated into nuclear and cytoplasmic components and subsequently incubated with radiolabeled RNA transcripts corresponding to different regions of the cyclin D1 mRNA encompassing the 5′UTR, coding region, and 3′UTR (Fig. 4A; see Materials and Methods). As shown, abundant proteins in both cytoplasmic and nuclear lysates bound to cyclin D1 RNA probes (Fig. 4B), but proteins in each fraction displayed very different binding patterns. Nuclear proteins formed complexes with all transcripts tested (A1, B2, B3, and C4), but the binding patterns remained unchanged with PGA2 treatment. These observations suggested that complexes forming between nuclear proteins and cyclin D1 transcripts were relatively nonspecific, and nuclear fractions were not studied further. By contrast, cytoplasmic proteins exhibited distinct binding patterns. A transcript encompassing the coding region (A1) did not result in significant complex formation with cytoplasmic proteins, whether derived from untreated or from PGA2-treated cells. On the other hand, B2 and B3 transcripts generated significant RNA-protein complexes, whose intensities were significantly enhanced by PGA2 treatment. Transcript C4, which contains six AUUUA motifs, also bound to cytoplasmic proteins. Unexpectedly, however, this binding activity was relatively low when compared with that seen with transcripts B2 and B3 and was not affected by PGA2 treatment. These findings suggest that a responsive element involved in PGA2-mediated RNA-protein binding is located within the B2 region of the cyclin D1 mRNA.
FIG. 4.
Binding to cyclin D1 transcripts. (A) Schematic representation of the full-length cyclin D1 cDNA and various transcripts derived from the 5′UTR, coding region, and 3′UTR used in this study. Transcripts were synthesized after preparation of DNA templates by RT-PCR, as described in the Materials and Methods section. The 5′ end of each fragment contains the T7 RNA polymerase promoter sequence. (B) Detection of RNA-protein binding activity by EMSA analysis. PCR-amplified cDNA fragments were used as templates to synthesize 32P-radiolabeled RNA probes (A1, B2, B3, and C4). Following treatment of H1299 cells with 30 μM PGA2 for the times indicated, cytoplasmic and nuclear fractions (10 μg each) were assayed for binding to the indicated RNA probes. Reaction mixtures were resolved on 7% native polyacrylamide gels. Signals were visualized with a PhosphorImager. Brackets indicate complexes forming with B2 and B3 fragments in a PGA2-dependent fashion. f, radiolabeled RNA digested with RNase T1 without incubation with cell lysate. (C) Correlation between PGA2-mediated degradation of cyclin D1 mRNA and PGA2-induced RNA-protein-binding activity. Cyclin D1 mRNA levels in H1299 cells that were either left untreated or treated with 30 μM PGA2 for the times indicated were determined by Northern blot analysis (top); lysates (cytoplasmic fraction) prepared from H1299 cells treated in parallel were incubated with 32P-radiolabeled B2 transcript and then subjected to EMSA (bottom). Radioactive signals were visualized with a PhosphorImager.
To further characterize the formation of cyclin D1 RNA-protein complexes, we studied the kinetics of B2-protein complex formation following exposure to PGA2. As shown in Fig. 4C, untreated cells exhibited a basal level of RNA-protein binding by EMSA analysis. An increase in this RNA-protein binding was evident as early as 2 h after treatment and further increased with time (Fig. 4C). Parallel examination of cyclin D1 levels by Northern blot analysis showed a concomitant reduction in the levels of cyclin D1 mRNA after exposure to PGA2 (Fig. 4C). Thus, the PGA2-induced decrease in cyclin D1 mRNA correlated well with the increase in cyclin D1 RNA-protein binding. These findings provide further evidence that the formation of RNA-protein complexes may be important for cyclin D1 mRNA turnover in PGA2-treated cells, and they support the notion that the B2 region of cyclin D1 mRNA plays a critical role in this post-transcriptional regulation process.
Further characterization of binding region(s) in the cyclin D1 3′UTR.
In order to identify the region(s) within the B2 segment of cyclin D1 mRNA that was recognized by cytoplasmic proteins, we examined a panel of transcripts encompassing the entire B2 region by EMSA analysis (Fig. 5A). As shown in Fig. 5B, proteins specifically bound to the K9 and I12 probes, indicating that these two adjacent regions comprise the PGA2-inducible protein-binding site. We therefore combined K9 and I12 to generate a 390-base sequence (K12), which displayed a pattern of complex formation identical to that seen with the B2 transcript (Fig. 5B). Competition assays revealed that an excess of unlabeled K12 transcript was capable of blocking PGA2-triggered binding to B2 transcript (Fig. 5C): a 50-fold molar excess of cold K12 completely inhibited the formation of protein-B2 RNA complexes, while a 50-fold molar excess of A1 probe, which does not form PGA2-induced RNA-protein complexes, failed to compete with B2 for protein binding. These results further support the hypothesis that the K12 region contains the site responsive to PGA2-triggered protein binding observed with the B2 transcript. Given the complexity of binding between cellular proteins and K12, further testing of smaller transcripts generally resulted in the loss of one or several radiolabeled bands (Fig. 5D). These results indicate that the majority of the K12 region is important for binding, and we used K12 in subsequent experiments.
FIG. 5.
Binding region. (A) Schematic representation of regions within the B2 fragment of the cyclin D1 mRNA assayed for protein binding. (B) Binding of cytoplasmic proteins (10 μg) derived from either PGA2-treated or untreated cells, to various RNA transcripts encompassing the B2 region of cyclin D1 mRNA. Complex formation was assayed by EMSA as described in the legend of Figure 4. Note that fragment K12 is composed of fragments K9 and I12. f, radiolabeled transcript not incubated with protein lysate, then digested with RNase T1. (C) Competition assays. Cytoplasmic lysates from PGA2-treated cells (24-h exposure to the drug) were incubated with a 5-, 10-, or 50-fold molar excess of either cold K12 or cold A1 RNA on ice for 20 min, then with radiolabeled B2 RNA on ice for an additional 20 min. After RNase T1 digestion, reactions were analyzed by EMSA. (D) Schematic representation of regions within the K12 fragment (left) and transcripts assayed by EMSA after incubation either without (−) or with protein lysates (lysate) from PGA2-treated (30 μM, 8 h) H1299 cells (right). Transcripts not incubated with protein lysate, then subjected to RNase T1 digestion.
RNA-protein-binding complexes contain AUF1 protein, and AUF1 expression is induced by treatment with PGA2.
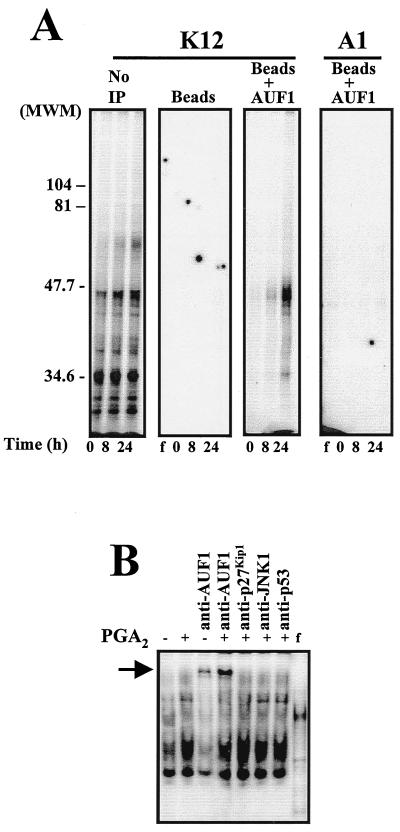
To further investigate the nature of the proteins forming complexes with the K12 RNA transcript, reaction mixtures were subjected to analysis by UV cross-linking followed by SDS-polyacrylamide gel electrophoresis. Our results revealed the presence of multiple K12 RNA-protein-binding complexes with molecular sizes ranging from 30 to 47 kDa (Fig. 6A). Since the RNA-binding protein AUF1 exists as four isoforms of 37, 40, 42, and 45 kDa, we directly examined whether AUF1 is part of the RNA-protein complexes. We allowed protein-K12 complexes to form, then cross-linked the complexes and subjected them to immunoprecipitation using an anti-AUF1 antibody. Subsequent analysis by SDS-polyacrylamide gel electrophoresis revealed that the anti-AUF1 antibody immunoprecipitated RNA-protein complexes of about 40 to 45 kDa (Fig. 6A). This observation constitutes strong evidence that AUF1 binds to the K12 region of cyclin D1 mRNA. To further investigate whether AUF1 binds to the K12 transcript, we examined whether the anti-AUF1 antibody could supershift the protein-K12 RNA complexes. As shown in Fig. 6B, PGA2-induced complexes were supershifted by anti-AUF1 antibody, but not by irrelevant antibodies recognizing JNK1, p27Kip1, or p53. Because the AUF1-binding activity was increased by PGA2 treatment, we performed Western blot analysis to determine whether PGA2 also induces AUF1 expression. Expression of the 45-kDa isoform was absent in whole-cell lysates from untreated cells, but it was markedly induced by PGA2 (Fig. 7A), while expression of the other isoforms remained unchanged. Further confirmation for the specific elevation in expression of this isoform was obtained through use of a recently available antibody (described in the Materials and Methods section) capable of recognizing the p40 and p45 AUF1 isoforms. The inducible expression of a 45-kDa species was detected using this antibody (Fig. 7B). Taken together, our data indicate that PGA2 treatment induced binding of cellular proteins to the K12 region of cyclin D1 mRNA and strongly suggest that at least one such binding protein is the AUF1 isoform p45.
FIG. 6.
Detection of AUF1 in the cyclin D1 RNA-protein complexes. (A) Detection of AUF1-cyclin D1 associations by immunoprecipitation. Cytoplasmic lysates from cells that were either left untreated (0 h) or treated with 30 μM PGA2 for 8 or 24 h were first allowed to bind radiolabeled RNA (either K12 or A1), and then the RNA-protein complexes were UV cross-linked. Complexes were either resolved directly by electrophoresis through SDS–12% polyacrylamide gels or first immunoprecipitated using protein A beads (Beads) or protein A beads coated with anti-AUF1 antibody (Beads+AUF1) or after no immunoprecipitation (No IP), and then resolved by electrophoresis through SDS–12% polyacrylamide gels. f, free radiolabeled RNA, not incubated with protein lysate. (B) Supershift analysis. Cytoplasmic proteins isolated from cells treated with PGA2 for 24 h were preincubated with anti-AUF1, anti-p27Kip1, anti-JNK1, or anti-p53 antibodies for 30 min on ice, prior to addition of radiolabeled K12 RNA. Complexes were subjected to EMSA analysis through 5% native gels. Native and SDS-containing polyacrylamide gels were dried and signals visualized with a PhosphorImager. Arrow indicates the band supershifted by the AUF1 antibody. f, radiolabeled transcript not incubated with protein lysate but digested with RNase T1.
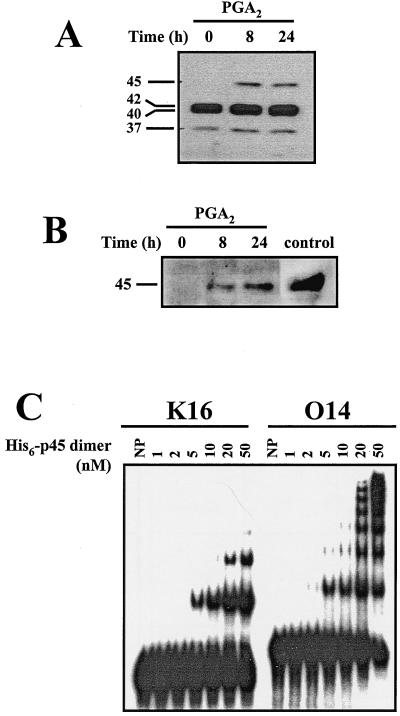
FIG. 7.
PGA2-induced AUF1 expression. (A) Forty-microgram aliquots of whole-cell lysate prepared from H1299 cells that were either left untreated (0 h) or treated with PGA2 for 8 or 24 h were subjected to Western blot analysis using anti-AUF1 antibody (64). Molecular weights of AUF1 isoforms are shown. (B) Forty-microgram aliquots of cytoplasmic lysates from cells treated as described in panel A were examined for the expression of p45AUF1 using an antibody that specifically recognizes a unique 19-amino-acid region present in the p45 isoform. This antibody also recognizes p40AUF1 (not shown). control, lysate from K562 cells. (C) Gel mobility shift of recombinant p45AUF1 (1 to 50 nM) binding either K16 or O14 radiolabeled transcripts. NP, no protein.
Additional evidence that p45AUF1 binds the cyclin D1 mRNA came from in vitro studies. As we needed to use a fragment smaller than K12, we decided to employ the 128-base fragment encompassing the O14 region (Fig. 5D), whose binding to H1299 cytoplasmic proteins exhibited a pattern that was almost identical to that seen with full-length K12 (with the exception of the uppermost band). Fragment K16 was employed as a negative control. As shown in Fig. 7C, incubation of each transcript with purified recombinant p45AUF1 revealed binding in vitro, although O14 was clearly the preferred substrate. In addition, the highly cooperative association exhibited by p45AUF1 and O14 could allow for significant changes in mRNA decay rates even if changes in its concentration are modest.
Influence of K12 on the expression of chimeric transcripts.
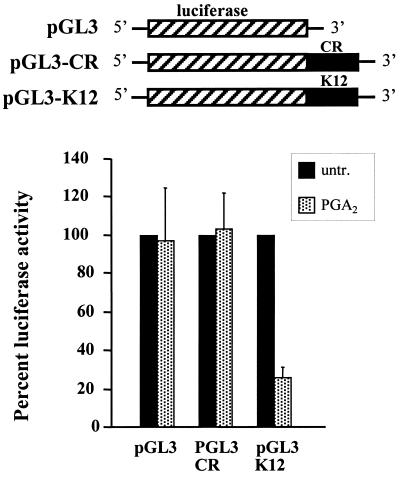
To investigate the functional role of the cyclin D1 K12 region in regulating gene expression, we carried out two series of experiments using chimeric RNAs. First, we constructed pGL3-derived plasmids in which either K12 or an equivalent-length fragment from the cyclin D1 mRNA-coding region (CR) was placed 3′ to the coding region of a luciferase reporter gene. The plasmids were cotransfected with a β-galactosidase reporter plasmid into H1299 cells, and the cells were subsequently examined for their responsiveness to PGA2 treatment. In each transfection group, luciferase activities were normalized to β-galactosidase activities, and the relative luciferase activity in PGA2-treated cells was compared with that seen in untreated cells (100%). Luciferase activities from either the empty pGL3 vector or the chimeric construct containing CR linked to luciferase (pGL3-CR) were unchanged by PGA2 treatment; by contrast, insertion of K12 into the 3′UTR of the luciferase gene (pGL3-K12) resulted in a significant decrease (approximately 70%) in luciferase activity of the chimeric gene following treatment with PGA2. These results provide functional evidence that the K12 sequence acts in a PGA2-dependent manner to reduce expression of an unrelated reporter gene, presumably by altering the stability of the chimeric mRNA.
Second, we employed a transcriptional pulsing strategy based on the Tet-regulatory system (similar to that previously reported [63]) to monitor the influence of various cyclin D1 sequences on the mRNA turnover rates. Briefly, a stably transfected H1299 clonal cell line (designated H2) that constitutively expresses the tetracycline-controlled transactivator was established. H2 cells express high levels of transfected, Tet-regulated reporter genes, and addition of doxycycline potently suppresses this activity, as they exhibit >20-fold-lower gene expression by 12 h in the presence of 1 μg of doxycycline per ml. To test the effect of the K12 region of cyclin D1 mRNA on mRNA stability, the Tet-regulated construct pTRE-d2EGFP-K12 was prepared and transfected into H2 cells. Control transfection groups included populations transfected with either the unmodified Tet-controlled vector pTRE-d2EGFP (expressing the EGFP transcript) or the construct pTRE-d2EGFP-CR, expressing a chimeric transcript bearing a sequences of the coding region of cyclin D1 mRNA (EGFP-CR). Following a 20-h transcriptional pulse, the Tet promoter-driven transcription was effectively shut off by the addition of doxycycline (1 μg/ml), and the rate of clearance of the three transcripts (EGFP, EGFP-CR, or EGFP-K12) was monitored by Northern blot analysis (Fig. 9) in cells that were either treated with PGA2 at the time of doxycycline addition or left untreated. As shown in Fig. 9, the stability of all three transcripts was comparable (with half-lives of 3 to 4 h) in untreated populations; importantly, in the PGA2-treated populations, only the K12-containing chimeric transcripts exhibited a reduction in half-life (1.2 h), while the half-lives of both the EGFP and EGFP-CR mRNAs were similar to that seen in each untreated population (3 or 4 h, respectively).
FIG. 9.
Influence of the K12 region of the cyclin D1 mRNA on the expression of a chimeric EGFP reporter construct after PGA2 treatment. Five micrograms of each pTRE-d2EGFP, pTRE-d2EGFP-CR, or pTRE-d2EGFP-K12 plasmid was transiently transfected into H2 cells (previously transfected with pTet and selected based on its strong induced and high doxycycline-dependent repression of reporter gene expression), along with 1 μg of β-galactosidase reporter as a control. Twenty hours after transfection, cells were treated either with doxycycline alone (control) or with a combination of doxycycline and PGA2 (30 μM, PGA2). At the times indicated following addition of the drug(s), RNA was prepared for Northern blot analysis; assessment of the expression levels of the EGFP, EGFP-CR, or EGFP-K12 transcripts was followed by that of cyclin D1 expression (not shown) and 18S rRNA, through sequential rounds of stripping and hybridization. Representative Northerns are shown. Graphs depict the relative abundance of each EGFP-derived transcript at various times after addition of doxycycline (time 0, 100%), represented on a logarithmic scale. Dashed line, 50% mRNA, which served to obtain the half-life values for each transcript.
Together, our results support the notion that PGA2-induced events resulting in decreased cyclin D1 mRNA half-life are mediated through the K12 region. We further propose that binding of proteins such as AUF1 to the K12 region of cyclin D1 mRNA may decrease its stability.
3′UTR-deleted cyclin D1 mRNA is resistant to PGA2-mediated down-regulation.
Further evidence that the 3′UTR of cyclin D1 mRNA is critical for PGA2-mediated down-regulation of its mRNA was obtained using MDA-MB-453 cells. This cell line expresses high levels of a mutant cyclin D1 mRNA bearing a large deletion in its 3′UTR. The resulting ∼1.2-kb transcript lacks the putative target sequence for PGA2-mediated destabilization. A comparative analysis of the PGA2-triggered down-regulation of cyclin D1 mRNA was then undertaken to compare the rate of cyclin D1 mRNA loss in MDA-MB-452 cells with that seen in another breast carcinoma cell line, MCF-7, which expresses the normal, 4.2-kb cyclin D1 transcript. As shown in Fig. 10, PGA2-treated MCF-7 cells exhibited approximately a 70% decrease in cyclin D1 mRNA levels by 8 h posttreatment. By contrast, the amount of truncated cyclin D1 mRNA transcript in MDA-MB-453 cells was essentially unaltered by PGA2 treatment. These results lend further support to our hypothesis that the 3′UTR of cyclin D1 mRNA is critical for modulating cyclin D1 expression during exposure to PGA2.
FIG. 10.
Expression of cyclin D1 mRNA in MDA-MB-453 and MCF-7 cells following PGA2 treatment. (A) MDA-MB-453 and MCF-7 cells were treated with PGA2 for the times indicated, and Northern blot analysis was used to assess cyclin D1 expression. (B) Quantitation of cyclin D1 mRNA signals after normalization to 18S rRNA signals on the same blot. Values shown are the means ± SEM from three independently performed experiments.
DISCUSSION
Cyclin D plays a critical role in regulating cell proliferation and mediating progression of cells through G1 into S phase. It is not surprising, therefore, that changes in the extracellular environment can greatly influence cyclin D expression. We had previously observed that treatment of several different types of mammalian cells with PGA2 leads to growth arrest in G1 associated with a rapid and marked reduction in cyclin D1 mRNA expression (20). In the present study we investigated the mechanisms contributing to the loss in cyclin D1 expression following PGA2 treatment. Our results indicate that PGA2 reduces cyclin D1 expression by decreasing the stability of cyclin D1 mRNA transcripts and have identified a region within the cyclin D1 3′UTR that is critical for regulating mRNA turnover.
The lack of involvement of transcriptional events in regulating the PGA2-mediated decline in cyclin D1 expression was supported by two critical findings: expression of a cyclin D1 promoter-reporter construct was not down-regulated in response to PGA2 treatment, and no repression of cyclin D1 transcription was evident by nuclear run-on assay (Fig. 3). While several other studies have likewise suggested that cyclin D1 expression can be regulated at the level of mRNA stability (23, 26, 33, 47), none of these examined the mechanisms contributing to this effect. Using gel mobility-shift analysis, we have demonstrated that lysates prepared from PGA2-treated cells contain proteins capable of binding to the 3′UTR of cyclin D1 mRNA. One of these segments, a 390-base region designated K12, was of particular interest as the relative amount of protein binding to it increased following treatment with PGA2. Indeed, when assaying this fragment, the kinetics of complex formation coincided with the decrease in cyclin D1 mRNA. That this region and the protein(s) capable of interacting with it are important in regulating the stability of cyclin D1 mRNA was further supported by two additional experiments. First, linking of this sequence to the coding sequence of the luciferase gene led to reduced luciferase expression after treatment with PGA2, presumably due to destabilization of the mRNA. Second, transcriptional-pulse strategies revealed that the half-life of a chimeric transcript containing K12 was considerably shorter in PGA2-treated populations than in untreated groups, while the half-life of control transcripts was unaffected by treatment with the drug.
Based on immunoprecipitation experiments and supershift analysis, our studies have identified the RNA-binding protein AUF1 as one component of the complexes interacting with K12. AUF1 has been implicated in the destabilization of labile mRNAs through its interaction with 3′UTR regions (11, 35). Although the precise sequence requirements for AUF1 binding have not been determined, binding generally occurs in regions which are AU-rich and harbor reiterations of AUUUA pentamers, such as those found in the c-myc, c-fos, and tumor necrosis factor mRNAs, all established targets of AUF1 in vitro (11, 12, 35, 64). While the findings in our study could support a function for AUF1 in regulating cyclin D1 mRNA decay in cells treated with PGA2, the 390-base sequence encompassing the cyclin D1 mRNA K12 region is unique in that it does not contain any AUUUA sequences. Several AUUUA sequences are present in the cyclin D1 3′UTR downstream of K12, but they do not appear to influence the binding to K12. Moreover, the cyclin D1 3′UTR segment encompassing six AUUUA elements (designated C4 [Fig. 4]), does not seem to be a major target for binding by other proteins in either untreated or PGA2-treated H1299 cells, as only weak bands appear on gel mobility shift assays (Fig. 4). Among other RNA-binding proteins that could potentially interact with these AU-rich sequences and/or other segments of the cyclin D1 3′UTR, we tested the elav-related protein HuR and hnRNP C and A1. However, in no instance did we obtain any evidence that these proteins bound the cyclin D1 mRNA (data not shown).
The AUF1 gene gives rise to four distinct protein isoforms through alternative splicing of the pre-mRNAs that also influences the relative expression of each isoform (55, 62). The functional distinctions between the four isoforms, with apparent molecular masses of 37, 40, 42, and 45 kDa, are unclear, but the p37 and p42 isoforms appear to display the most profound destabilizing effect on c-fos mRNA in K562 cells (35). Although we cannot say with absolute certainty which isoform is responsible for binding to K12, our findings indicate that expression of p45AUF1 is specifically elevated after PGA2 treatment and in a time frame consistent with enhanced protein-RNA complex formation, while none of the other isoforms were altered with PGA2 treatment. Purified recombinant p45AUF1 was indeed found to bind, in a highly cooperative fashion, a fragment of the K12 transcript in vitro (Fig. 7C), although p37AUF1 and p40AUF1 were also capable of binding this sequence (not shown). Finally, this study provides, to our knowledge, the first evidence that the endogenous p45AUF1 isoform is preferentially induced under certain conditions and provides further support for the emerging view that the various isoforms display different RNA-binding patterns and may have different functions. Nevertheless, definitive demonstration that AUF1 regulates the turnover of cyclin D1 mRNA awaits the development of additional experimental systems in which the expression of specific AUF1 isoforms can be overexpressed or inhibited.
It is known that, in addition to PGA2, other stresses and/or growth-arresting treatments can result in reduced cyclin D1 expression. It remains to be determined whether regulated mRNA turnover is involved in the down-regulation of cyclin D1 expression in a general sense by other stresses; however, it is worth noting that, in preliminary studies, we have seen evidence of enhanced protein binding to the K12 region using lysates from cells treated with various stress agents. For example, exposure to brefeldin A, which interferes with protein trafficking resulting in stress to the endoplasmic reticulum, led to both increased binding to cyclin D1 mRNA on gel mobility shift assays (with patterns of binding identical to those seen after PGA2 treatment) and reduced cyclin D1 mRNA expression by Northern blotting. Thus, the down-regulation of cyclin D1 mRNA by K12-dependent turnover may not be limited to PGA2 treatment.
Finally, cyclin D1 has been shown to be overexpressed in a number of different tumors. The elevated levels of cyclin D1 are believed to contribute directly to tumorigenicity (13, 25, 41, 57), and therapeutic approaches targeting cyclin D1 have been described (31). Although many different mechanisms are believed to contribute to the enhanced cyclin D1 expression, it is interesting to note that a number of studies have reported cyclin D1 gene mutations that result in truncated mRNAs with greater stability than that of wild-type full-length mRNA (26, 33, 47; Fig. 10). The region we have implicated in down-regulating cyclin D1 mRNA stability is likewise absent in these truncated mRNAs. Indeed, we had the opportunity to test a cell line which harbored one such mutant cyclin D1 mRNA and found that PGA2 failed to down-regulate its expression (Fig. 10). Thus, our studies provide additional support for the view that mutations that alter the posttranscriptional control of cyclin D1 expression could play an important role in tumorigenesis. Better understanding of these processes could lead to improved treatment strategies.
FIG. 8.
Influence of the K12 region of the cyclin D1 mRNA on the expression of a chimeric luciferase reporter construct after PGA2 treatment. Three micrograms of each pGL3-promoter (pGL3), pGL3-K12, and pGL3-CR plasmid was transiently transfected into H1299 cells along with 1 μg of β-galactosidase reporter as a control. Transfected cells were either treated with PGA2 for 24 h or left untreated. Luciferase activities were determined and normalized against β-galactosidase measurements. Values, shown as the means ± SEM from five independently performed experiments, represent the luciferase values in PGA2-treated populations relative to those measured in untreated cells (to which a value of 100% was assigned).
ACKNOWLEDGMENT
This study was supported by NIH R01 grant no. CA52443 to G.B.
REFERENCES
- 1.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 2.Amici C, Palamara A T, Santoro M G. Induction of thermotolerance by prostaglandin A in human cells. Exp Cell Res. 1993;207:230–234. doi: 10.1006/excr.1993.1188. [DOI] [PubMed] [Google Scholar]
- 3.Atwater J A, Wisdom R, Verma I M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- 4.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 5.Beier F, Lee R J, Taylor A C, Pestell R G, LuValle P. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chrondrocytes. Proc Natl Acad Sci USA. 1999;96:1433–1438. doi: 10.1073/pnas.96.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer G. An A + U-rich element RNA binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991;11:2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzby J S, Lee S M, Van Winkle P, DeMaria C T, Brewer G, Cairo M S. Increased GM-CSF mRNA stability in cord vs. adult mononuclear cells is translation dependent and associated with increased levels of A+U-rich element binding factor. Blood. 1996;88:2889–2897. [PubMed] [Google Scholar]
- 8.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-Y A, Shyu A-B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 10.Chung S L, Jiang L, Cheng S, Furneaux H. Purification and properties of HuD, a neuronal RNA-binding protein. J Biol Chem. 1996;271:11518–11524. doi: 10.1074/jbc.271.19.11518. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria C T, Brewer G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J Biol Chem. 1996;271:12179–12184. doi: 10.1074/jbc.271.21.12179. [DOI] [PubMed] [Google Scholar]
- 12.DeMaria C T, Sun Y, Long L, Wagner B J, Brewer G. Structural determinants in AUF1 required for high affinity binding to A + U-rich elements. J Biol Chem. 1997;272:27635–27643. doi: 10.1074/jbc.272.44.27635. [DOI] [PubMed] [Google Scholar]
- 13.Dickson C, Fantl V, Gillett C, Brookes S, Bartek J, Smith R, Fisher C, Barnes D, Peters G. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995;90:43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- 14.Fan X C, Steitz J A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford L P, Watson J, Keene J D, Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188–201. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima M, Sasaki H, Fukushima S. Prostaglandin J2 and related compounds: mode of action in G1 arrest and preclinical results. Ann N Y Acad Sci. 1994;744:161–165. doi: 10.1111/j.1749-6632.1994.tb52733.x. [DOI] [PubMed] [Google Scholar]
- 17.Gao F-B, Keene J D. Hel-N1/Hel-N2 proteins are bound to poly(A)+ mRNA in granular RNP structures and are implicated in neuronal differentiation. J Cell Sci. 1996;109:579–589. doi: 10.1242/jcs.109.3.579. [DOI] [PubMed] [Google Scholar]
- 18.Gorospe M, Kumar S, Baglioni C. Tumor necrosis factor increases the stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993;268:6214–6220. [PubMed] [Google Scholar]
- 19.Gorospe M, Baglioni C. Degradation of unstable interleukin-1 alpha mRNA in a rabbit reticulocyte cell-free system. Localization of the instability determinant to a cluster of AUUUA motifs. J Biol Chem. 1994;269:11845–11851. [PubMed] [Google Scholar]
- 20.Gorospe M, Liu Y, Xu Q, Chrest F J, Holbrook N J. Inhibition of G1 cyclin-dependent kinase activity during growth arrest of human breast carcinoma cells by prostaglandin A2. Mol Cell Biol. 1996;16:762–770. doi: 10.1128/mcb.16.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorospe M, Wang X, Holbrook N J. p53-dependent elevation of p21Waf1 expression by UV light is mediated through mRNA stabilization and involves a vanadate-sensitive regulatory system. Mol Cell Biol. 1998;18:1400–1407. doi: 10.1128/mcb.18.3.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grana X, Reddy E P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 23.Hashemolhosseini S, Nagamine Y, Morley S J, Desrivieres S, Mercep L, Ferraie S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook N J, Carlson S G, Choi A M, Fargnoli J. Induction of HSP70 gene expression by the antiproliferative prostaglandin PGA2: a growth-dependent response mediated by activation of heat shock transcription factor. Mol Cell Biol. 1992;12:1528–1534. doi: 10.1128/mcb.12.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosokawa Y, Arnold A. Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression. Genes Chr Cancer. 1998;22:66–71. doi: 10.1002/(sici)1098-2264(199805)22:1<66::aid-gcc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Hosokawa Y, Suzuki R, Joh T, Maeda Y, Nakamura S, Kodera Y, Arnold A, Seto M. A small deletion in the 3′-untranslated region of the cyclin D1/PRAD1/bcl-1 oncogene in a patient with chronic lymphocytic leukemia. Int J Cancer. 1998;76:791–796. doi: 10.1002/(sici)1097-0215(19980610)76:6<791::aid-ijc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Hughes-Fulford M. Cell cycle arrest by prostaglandin A1 at the G1/S phase interface with up-regulation of oncogenes in S-49 cyc- cells. J Cell Biochem. 1994;54:265–272. doi: 10.1002/jcb.240540302. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi Y, Kita T, Miyauchi M, Hirata J, Sasa H, Nagata I, Fukushima M. Adjuvant effects of antineoplastic prostaglandins to cisplatin in nude mice bearing human ovarian cancer cells. J Cancer Res Clin Oncol. 1992;118:453–457. doi: 10.1007/BF01629429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi Y, Kita T, Hirata J, Kuki E, Nagata I, Fukushima M. Modulation of human lymphocyte response to phytohemagglutinin by antineoplastic prostaglandins. Int J Immunopharmacol. 1992;14:105–110. doi: 10.1016/0192-0561(92)90110-7. [DOI] [PubMed] [Google Scholar]
- 30.Lagnado C A, Brown C Y, Goodall G J. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A) Mol Cell Biol. 1994;14:7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langenfeld J, Kiyokawa H, Sekula D, Boyle J, Dmitrovsky E. Posttranslational regulation of cyclin D1 by retinoic acid: a chemoprevention mechanism. Proc Natl Acad Sci USA. 1997;94:12070–12074. doi: 10.1073/pnas.94.22.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavoie J N, L'Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 33.Lebwohl D E, Muise-Helmericks R, Sepp-Lorenzino L, Serve S, Timaul M, Bol R, Borgen P, Rosen N. A truncated cyclin D1 gene encodes a stable mRNA in a human breast cancer cell line. Oncogene. 1997;9:1925–1929. [PubMed] [Google Scholar]
- 34.Liebhaber S A. mRNA stability and the control of gene expression. Nucleic Acid Symp Ser. 1997;36:29–32. [PubMed] [Google Scholar]
- 35.Loflin P, Chen C Y, Shyu A-B. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13:1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell R G, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan D O. Cell cycle control in normal and neoplastic cells. Curr Opin Genet Dev. 1992;2:33–37. doi: 10.1016/s0959-437x(05)80318-1. [DOI] [PubMed] [Google Scholar]
- 38.Muise-Helmericks R C, Grimes H L, Bellacosa A, Malstrom S E, Tsichlis P N, Rosen N. Cyclin D1 expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 39.Müller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci USA. 1994;91:2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musgrove E A, Lee C S L, Buckley M F, Sutherland R L. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura S, Yatabe Y, Seto M. Cyclin D1 overexpression in malignant lymphomas. Pathol Int. 1997;47:421–429. doi: 10.1111/j.1440-1827.1997.tb04519.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohno K, Fujiwara M, Fukushima M, Narumiya S. Metabolic dehydration of prostaglandin E2 and cellular uptake of the dehydration product: correlation with prostaglandin E2-induced growth inhibition. Biochem Biophys Res Commun. 1986;139:808–815. doi: 10.1016/s0006-291x(86)80062-6. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian cells. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 44.Peng S S, Chen C Y, Xu N, Shyu A B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quelle D E, Ashmun R A, Shurtleff S A, Kato J, Bar-Sagi D, Russel M, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 46.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rimokh R, Berger F, Bastard C, Klein B, French M, Archimbaud E, Rouault J P, Santa Lucia B, Duret L, Vuillaume M, et al. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood. 1994;83:3689–3696. [PubMed] [Google Scholar]
- 48.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross J. A hypothesis to explain why translation inhibitors stabilize mRNAs in mammalian cells: mRNA stability and mitosis. BioEssays. 1997;9:527–529. doi: 10.1002/bies.950190612. [DOI] [PubMed] [Google Scholar]
- 50.Sachs A B. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki H, Fukushima M. Prostaglandins in the treatment of cancer. Anticancer Drugs. 1994;5:131–138. doi: 10.1097/00001813-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki H, Takada K, Terashima Y, Ekimoto H, Takahashi K, Tsuruo T, Fukushima M. Human ovarian cancer cell lines resistant to cisplatin, doxorubicin, and L-phenylalanine mustard are sensitive to delta 7-prostaglandin A1 and delta 12-prostaglandin J2. Gynecol Oncol. 1991;41:36–40. doi: 10.1016/0090-8258(91)90251-y. [DOI] [PubMed] [Google Scholar]
- 53.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 54.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 55.Wagner B J, DeMaria C T, Sun Y, Wilson G M, Brewer G. Structure and genomic organization of the human AUF1 gene: alternative pre-mRNA splicing generates four protein isoforms. Genomics. 1998;48:195–202. doi: 10.1006/geno.1997.5142. [DOI] [PubMed] [Google Scholar]
- 56.Wang M B, Billings K R, Venkatesan N, Hall F L, Srivatsan E S. Inhibition of cell proliferation in head and neck squamous cell carcinoma cell lines with antisense cyclin D1. Otolaryngol Head Neck Surg. 1998;119:593–599. doi: 10.1016/S0194-5998(98)70017-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Furneaux H, Cheng H, Caldwell C, Hutter D, Liu Y, Holbrook N J, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Lin S, Caldwell C M, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during the cell division cycle. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 61.Wilson G M, Brewer G. Identification and characterization of proteins binding A+U-rich elements. Methods. 1999;17:74–83. doi: 10.1006/meth.1998.0709. [DOI] [PubMed] [Google Scholar]
- 62.Wilson G M, Sun Y, Sellers J, Lu H, Penkar N, Dillard G, Brewer G. Regulation of AUF1 expression via conserved alternatively spliced elements in the 3′ untranslated region. Mol Cell Biol. 1999;19:4056–4064. doi: 10.1128/mcb.19.6.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu N, Loflin P, Chen C-Y A, Shyu A-B. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 1998;26:558–565. doi: 10.1093/nar/26.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang W, Wagner B J, Ehrenman K, Schaefer A W, DeMaria C T, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]