Abstract
Fibrosis and cancer is described by some epidemiological studies as chronic stages of different disease conditions typically characterized by uncontrolled accumulation of extra-cellular matrix (ECM), thereby leading to inflammation of tissues and organ (lungs, heart, liver and kidney) dysfunction. It is highly prevalent, and contributes to increased mortality rate worldwide. Currently, the therapeutical approaches involving selected medications (bemcentinib, pirfenidone and nintedanib) obtained synthetically, and used in clinical practices for fibrosis and cancer management and treatment has shown to be unsatisfactorily, especially during progressive stages of the disease. With regards to finding a more potent, effective, and promising curative for fibrosis and cancer, there is need for continuous experimental studies universally. However, phytochemical constituents’ particularly phenolic compounds [Chlorogenic acid (CGA)] obtained from coffee, and coffee beans have been predominantly utilized in experimental studies, due to its multiple pharmacological properties against various disease forms. Considering its natural source alongside minimal toxicity level, CGA, a major precursor of coffee have gained considerable attention nowadays from researchers worldwide, owing to its wide, efficacious and beneficial action against fibrosis and cancer. Interestingly, the safety of CGA has been proven. Furthermore, numerous experimental studies have also deduced massive remarkable outcomes in the use of CGA clinically, as a potential drug candidate against treatment of fibrosis and cancer. In the course of this review article, we systematically discussed the beneficial contributions of CGA with regards to its source, absorption, metabolism, mechanistic effects, and molecular mechanisms against different fibrosis and cancer categorization, which might be a prospective remedy in the future. Moreover, we also highlighted CGA (in vitro and in vivo analytical studies) defensive effects against various disorders.
Keywords: Chlorogenic acid, Natural drugs, Fibrosis, Cancer, Epithelial-mesenchymal transition
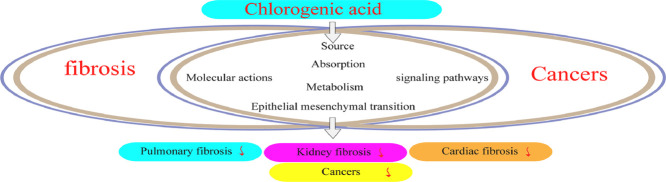
Graphical abstract
Fibrosis and cancer is described by some epidemiological studies as chronic stages of different disease conditions typically characterized by uncontrolled accumulation of extra-cellular matrix (ECM), thereby leading to inflammation of tissues and organ (lungs, heart, liver and kidney) dysfunction. It is highly prevalent, and contributes to increased mortality rate worldwide. With regards to finding a more potent, effective, and promising curative for fibrosis and cancer, there is need for continuous experimental studies universally. However, numerous experimental studies have deduced massive remarkable outcomes in the use of CGA clinically, as a potential drug candidate against treatment of fibrosis and cancer. In the course of this review article, we systematically discussed the beneficial contributions of CGA with regards to its source, absorption, metabolism, mechanistic effects, and molecular mechanisms against different fibrosis and cancer categorization, which might be a prospective remedy in the future. Moreover, we also highlighted CGA (in vitro and in vivo analytical studies) defensive effects against various disorders.
Introduction
Some analytical and clinical investigations stipulates that fibrosis and cancer intertwine and share distinctly overlapping characteristics. With respect to injury (deregulated response) that occurs in all tissues of the body, fibrosis is indicated via immune cells and fibroblast activation, contributing to continuous inflammation and deposition of extracellular matrix (ECM). Cancers are usually driven through genetic alterations emanating from dissemination, dysregulated cell survival, and proliferation. In addition, non-cancerous constituents of malignant tissues such as ECM, inflammatory cells, and fibroblasts play significant roles in progression of cancer, and oncogenesis by yielding a pro-mutagenic surrounding where cancer cells could thrive, contributing to their invasiveness, survival, and growth. Fibrosis and cancer are reportedly known to possess similar pathophysiological pathway commonalities involving inflammation, cellular senescence, epithelial mesenchymal transition (EMT), hippo mechanism activation, ECM modification, genetic alterations, TGF-β overproduction, fibroblast proliferation and differentiation, elevating invasiveness and stiffness respectively [1], [2], [3], [4], [5], [6]. Numerous causative risk factors such as diet, excessive alcohol consumption, smoking tobacco, genetic predisposition, radiation, and reproductive behavior, occupational and environmental pollutants (asbestos fibers, dust, silica, birds and animal droppings) are considered to be associated with fibrosis and cancer in human population [7], [8], [9], [10], [11], [12], [13], [14], [15]. As reported by World health organization (WHO), fibrosis and cancer, still remains one of the leading cause of deaths affecting millions of individual universally. Currently, the therapeutical approaches involving selected medications (bemcentinib, pirfenidone and nintedanib) obtained synthetically, and used in clinical practices for fibrosis and cancer management and treatment has shown to be unsatisfactorily, especially during progressive stages of the disease [16], [17], [18], [19], [20], [21], [22], [23]. With regards to finding a more potent, effective and promising curative for fibrosis and cancer, there is need for continuous experimental studies universally
However, phytochemical constituents particularly phenolic compounds [Chlorogenic acid (CGA)] obtained from coffee and coffee beans have been predominantly utilized in experimental studies, due to its multiple pharmacological properties (anti-metastatic, anti-oxidative, nephroprotective, anti-inflammatory, anti-diabetic, anti-hypertensive, hepatoprotective, anti-bacterial, neuroprotective, anti-proliferative, central nervous system stimulator, anti-obesity, cardioprotective, anti-pyretic, anti-viral, anti-angiogenic etc.) against various diseases forms [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. Considering its natural source alongside minimal toxicity level, CGA, a major precursor of coffee have gained considerable attention nowadays from researchers worldwide, owing to its wide, efficacious and beneficial action against fibrosis and cancer [40, 41]. Interestingly, the safety of CGA has been proven [42, 43]. Furthermore, numerous experimental studies have also deduced massive remarkable outcomes in the use of CGA clinically, as a potential drug candidate against treatment of fibrosis and cancer.
In the course of this review article, we systematically discussed the beneficial contributions of CGA with regards to its source, absorption, metabolism, mechanistic effects, and molecular mechanisms against different fibrosis and cancer categorization, which might be a prospective remedy in the future. Appropriate databases and archives like Embase, Hindawi, Springer, Google scholar, and PubMed etc., were applied in this literature.
Chlorogenic acid
Source, absorption and metabolism
Chlorogenic acid (CGA), otherwise known as 3-caffeoylquinate (3-CQA) or chlorogenate is a biologically active polyphenolic compound that represents an entire ester-hydroxycinnamic and quinic acid group involving dicaffeoyl, caffeoyl, coumaroylquinic and feruloyl acids respectively [44]. It portrays numerous therapeutical effects and properties such as minimal oral absorption rate and soluble in ethanol and acetone. Table 1 highlights the CGA (in vitro and in vivo analytical studies) defensive effects against other various disorders or conditions. Notably, most authors nowadays still have misconception regarding CGA (Fig. 1), due to its nomenclatural divergences [45], [46], [47]. CGA is usually marketed as svetol, widely obtained and distributed in herbs, foods, dicotyledonous ferns and plants species namely berry fruits, tea, apple, cocoa, coffee, citrus fruits, roasted bean, pears, carrots, wormwood, artichoke, potatoes, eggplant, betel, kiwi fruits, tobacco leaves, burdock, eucommia, coffee beans, tomatoes, honeysuckle, and grapes [48], [49], [50], [51], [52], [53], [54], [55], [56]. With regards to its health boosting attributes, CGA is also significantly applied clinically, particularly against fibrosis and cancer and serves as the main constituent in traditional herbal medicine (THM) formulations for detoxification, and heat clearance [57], [58], [59], [60], [61]. Furthermore, the excretion, utilization and bioavailability of CGA is still yet unclear. In humans, around one-third of chlorogenic acid ingested are absorbed via the small intestine, whereas absorbed in the stomach of mice through prototype [62], [63], [64], [65]. Following absorption, CGA is further metabolized into metabolites of sulfate, glycosides and glucuronic acid.
Table 1.
In-vivo and in-vitro protective actions associated with chlorogenic acid.
| No | Conditions | Details of Assay | Biological Sex | Application | Analytical Findings | Refs |
|---|---|---|---|---|---|---|
| 1 | Metabolic syndrome | High carbohydrate, high fat diet induced model | Male wistar mice | In-vivo | Alleviates high-fat-diet, high carbohydrates triggered liver, cardiovascular and metabolic alterations. | [66] |
|
- |
Male tsumura suzuki obese diabetes rats (TSOD) | In-vivo | Ameliorates the disrupted plasma short-chain fatty acids (SCFA) and gut microbiome. | [67] | ||
| High fat/high fructose fed model | Male sprague-dawley mouse | In-vivo | Diminishes food intake, weight gain, circulating triglycerides and their accumulation in the liver (liver steatosis). | [68] | ||
| 2 | Obesity | High-fat-diet (HFD) induced model | ICR male mice | In-vivo | Stimulates body loss and altered mRNA expressions of lipolysis and lipogenesis associated genes in the adipose tissue. Reverses the HFD triggered gut microbiota dysbiosis, and also suppressing plasma lipid levels, growth of Desulfovibrionaceae, Ruminococcaceae, Lachnospiraceae, Erysipelotrichaceae, and elevating the growth of Bacteroidaceae, Lactobacillaceae. |
[69] |
| HFD induced model | Male sprague-dawley rats | In-vivo | Decreases serum insulin level, abnormal islet hyperplasia, and blood glucose. | [70] | ||
| HFD induced model | C57BL/6 J rats | In-vivo | Modulates body weight, food intake, energy balance shift and enhanced body temperature, thermal dissipation, and brown adipose tissue activity. | [71] | ||
| HFD induced model | Female ICR rats | In-vivo | Suppresses intraperitoneal adipose tissue weight, body weight gain, hepatic TC and TG level, IL −6 concentrations, Leptin, serum LDL-c, FFA, expressions of transcriptional regulators (SREBP-1c and LXRα), HMGR, FAS and improved the phosphorylation of AMPKα. | [72] | ||
| Monosodium glutamate induced model and oleic acid induced model | Mouse and human fatty liver in HepG2 cells | In-vivo and in-vitro | Down-regulates fats deposition in the liver, blood lipid levels, mRNA and protein expressions of uncoupling protein-1 (UCP1) and peroxisome proliferator activated receptor gamma, coactivator 1α (PGC-1α). | [73] | ||
| Perfluorooctanoic acid exposure induced model |
ICR mice | In-vivo | Attenuates obesity, disruption of gut barrier integrity, lipid metabolism disorders, and hepatic inflammation. | [74] | ||
| HFD induced obesity and insulin resistance model | Male C57BL/6 J mice | In-vivo | Alters body weight gain, insulin resistance, evaluated via hyperglycemia, glucose and insulin intolerance. | [75] | ||
| 3 | Hyperlipidemia | High fat diet induced model | Male Sprague-dawley rats | In-vivo | Represses triglycerides, acetyl-CoA carboxylase (ACC), plasma free fatty acids (FFA) and increased carnitine palmitoyltransferase-1 (CPT-1) via activation of AMPK mechanism. |
[76] |
| 4 | NAFLD and atherosclerosis | High fat diet induced model | C57BL/6 rats | In-vivo | Diminishes RAS component expression, triglycerides, cholesterol, LDL and enhanced HDL plasma levels. | [77] |
| 5 | Diabetic nephropathy | High fat diet induced model | Male sprague-dawley mice | In-vivo | Potentiates heme oxygenase-1 expression (HO-1), and nuclear translocation of nuclear factor erythroid-derived-2-related factor 2 (Nrf2); repressed nuclear translocation of nuclear factor kappa beta (NF-kB) and IKB phosphorylation. | [78] |
| Streptozotocin induced model | Male sprague-dawley rats | In-vivo | Decreases levels of lipid peroxidation malondialdehyde, cyclooxygenase-2 protein, serum creatinine, blood urea nitrogen, and blood glucose; enhances the effects of catalase (CAT), glutathione peroxidase (GSH-px) and superoxide dismutase (SOD); obstructs the expression of activating transcription factor-6, C/EBP homology protein and the phosphorylation of eukaryotic initiation factor 2α and double stranded RNA-activated protein kinase-like endoplasmic reticulum kinase. | [79] | ||
| 6 | Diabetes | HFD and streptozotocin (STZ) induced model | Female sprague-dawley rats | In-vivo | Hampers insulin concentration, serum glucose, diabetes onset, mRNA levels of hepatic G-6-Pase, and ameliorated mRNA levels of skeletal muscle GLUT4, serum triglyceride, low density lipoprotein levels, total cholesterol, visceral fat weight, body weight and glucose tolerance. | [80] |
| HFD and induced model | Female db/db mice | In-vivo | Attenuates level of fasting blood glucose (FBG), body fat, glycosylated hemoglobin (HbA1c), TGF-β1 protein expression, aldose reductase (AR), and up-regulated the protein expression of adiponectin receptors (ADPNRs), AMPK phosphorylation, and the mRNA and protein levels of peroxisome proliferator activated receptor alpha (PPAR-α). | [81] | ||
| Streptozotocin induced model | Male wistar rats and L6 cell line | In-vivo and In-vitro | Promotes glucose tolerance and impaired basal hyperglycemia. | [82] | ||
| Streptozotocin induced model | Adult male wistar rats | In-vivo | Alleviates platelet aggregation and increased adenosine monophosphate (AMP) hydrolysis in the cerebral cortex | [83] | ||
| HFD and STZ mice | Male ICR mice | In-vivo | Down-regulates fasting blood glucose (FBG), fasting serum insulin, glycosylated serum protein levels, and also improved antioxidative effects. | [84] | ||
| 7 | Hypertension |
- |
Male SHR and wistar-Kyoto rats | In-vivo | Suppresses oxidative stress (ROS), vascular hypertrophy, endothelial dysfunction, and hypertension; improved bioavailability of nitric oxide (NO). | [85] |
| Cyclosporine induced model | Male wistar rats | In-vivo | Study 1: Impairs systolic blood pressure, heart rates, angiotensin-1 converting enzyme (ACE), arginase, butrylcholinesterase (BChE), acetylcholinesterase (AChE), GSH content, and MDA level; promotes bioavailability of NO and CAT activity. Study 2: Alters the activities of ACE, e-nucleotide triphosphate dephosphorylase (e-NTPDase), adenosine deaminase (ADA), 5ʹ nucleotidase and MDA level. |
[86, 87] | ||
| 8 | Neuropathic pain | Chronic constrictive nerve injury (CCI) induced model | Male sprague-dawley mice | In-vivo | Study 1: Prevents the occurrence of mechanical hyperalgesia. Study 2: Alleviates cold and mechanical hyperalgesia partly via triggering GABAergic transmission in the spinal cord. |
[88, 89] |
Fig. 1.
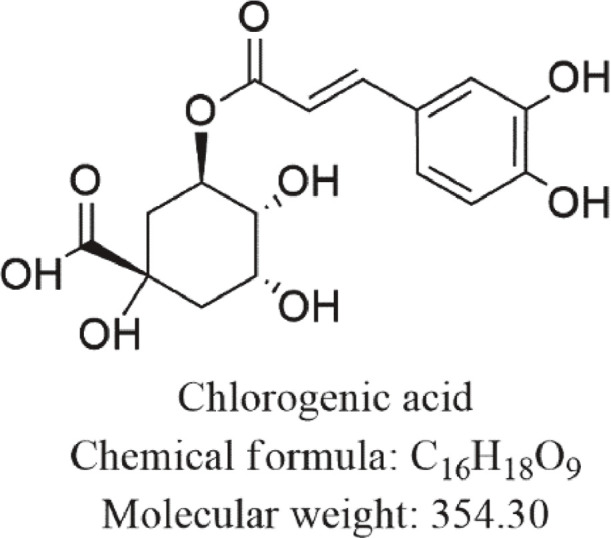
Chemical structure of CGA.
Mechanistic actions of CGA on fibrosis
A great number of experimental studies conducted by most researchers for over a decade has revealed positive significant actions of CGA use against treatment of chronic disease conditions such as fibrosis. Table 2 outlines the pharmacological activities (including assay models and signaling pathways) of CGA in various forms of fibrosis.
Table 2.
Molecular actions of chlorogenic acid on various forms of fibrosis.
| No | Types of Fibrosis Disease | Details of Assay | Biological sex | Application | Analytical Findings | Refs |
|---|---|---|---|---|---|---|
| 1 | Liver fibrosis | CCI4 induced model | Male sprague-dawley mice and LX2 cells line | In-vitro and In-vivo | Significantly down-regulates the protein expression of α-SMA, TGF-β1, p-smad2/3, p-smad3, p-smad2, TIMP-1, and CTGF and mRNA expression of α-SMA, TGF-β1, TIMP-1, CTGF, and miR-21 levels. Promotes protein and mRNA levels of MMP-9, and Smad-7; Decreases the expression of COL-I and α-SMA in liver tissue, degree of liver fibrosis and TGF-β1 in serum. | [90] |
| Schistosoma japonicum cercaria model | Male BALB/c mice and LX2 cell line | In-vitro and In-vivo | Diminishes the mRNA expression of CTGF and miR-21, and protein expressions of α-SMA, TGF-β1, p-smad2/3, p-smad3, p-smad2, and CTGF. Increases mRNA and protein expression of Smad-7. Modulates the in-vivo interaction of IL-13/miR-21/Smad7 signaling pathway | [91] | ||
| CCI4 induced model |
Male Sprague-dawley rats | In-vivo | Attenuates the expression of COL-I, TIMP-1, α-SMA, COL-III, degree of liver fibrosis, hydroxyproline content; CYP2E1, MDA, hepatic stellate cells proliferation, p38 and ERK1/2 phosphorylation, ROS production, levels of profibrotic genes and NOX subunits (p47phox and gp91phox). Potentiates the expression of SOD, CAT, and GSH in liver tissues, Nrf2 and Nrf2 modulated anti-oxidative genes (NQO1, GCLC and HO-1). | [92] | ||
| CCI4 induced model | Male sprague-dawley mice | In-vivo | Inhibits the mRNA expressions of COL-I, COL-III, VEGF, bcl-2, Bax, and TGF-β1, protein level of α-SMA, GRP78 and GRP94, and degree of liver fibrosis. | [93] | ||
| CCI4 induced and inflammation model | Male sprague-dawley mice | In-vivo | Suppresses the levels of α-SMA, COL-I, serum transaminase, degree of fibrosis, iNOS, TLR4, COX-2, MyD88, NF-κB activation, serum and mRNA expression of TNF-α, IL-1β and IL-6. Elevates the expression of bone morphogenetic protein and activin membrane-bound inhibitor. | [94] | ||
| Non-alcoholic steatohepatitis induced model | Male C57BL/6 mice | In-vivo | Alleviates the serum levels of hepatic hydroxyproline, aspartate aminotransferase, triglycerides, alanine aminotransferase, and cholesterol, hepatic stellate cells activation, hepatic genes expressions (involving MCP-1, TIMP-1, COL1α1, TGF-β1, and LOX), and oxidative stress via Nrf2 signaling pathway. Reverses the reduced levels of miR-122 and hepatic HIF-1α over-expression. | [95] | ||
| 2 | Pulmonary fibrosis | Bleomycin induced model | Male BALB/C rats | In-vivo | Reduces expression levels of GRP78, α-SMA, CHOP, and COL-I in dose-dependent manner, caspase-3, caspase-9, caspase-12, PERK phosphorylation, and cleaved ATF-6. Promotes uncleaved PARP level, and proliferation of RLE-6TN triggered via TGFβ1. | [96] |
| 3 | Kidney fibrosis | – | Male Swiss background rats | In-vivo | Represses myofibroblast and macrophage number, mRNA level of NF-ΚB, TNF-α, TLR-4, and MCP-1. | [97] |
| – | Adult male Swiss webster rats | In-vivo | Decreases α-SMA. Improves mRNA expressions of bone morphogenetic protein-7, and hepatocytes growth factor. | [98] | ||
| – | Male Swiss rats | In-vivo | Hinders the inflammatory response via decreasing TLR4, COX-2, TNFα expressions, and NF-κB action. Suppresses levels of creatinine, and BUN (blood urea nitrogen) to effect kidney optimal activities. | [99] | ||
| – | Adult Wistar mice | In-vivo | Diminishes the creatinine, BUN, proteinurea, oxidation stress, COL-IV, fibronectin, p-smad2 and TGF-β1 expressions in kidney tissues. | [100] | ||
| 4 | Cardiac fibrosis | Transverse aortic constriction induced heart failure | Male C57BL/6 N mice | In-vivo | Reverses TNF-α triggered cellular injuries. Ameliorates cell viability, mitochondrial membrane potential, ERK1/2, and attenuates cardiomyocytes apoptosis and c-Jun N-terminal kinase. Hampers NF‐κB signal via inhibiting NF‐κB/p65 phosphorylation. | [101] |
| coronary artery ligation-induced model | Male Sprague-dawley mice | In-vivo | Alleviates weight gain, plasma level of myocardial markers, myocardial injury, fibrosis, and pro-inflammatory factor expressions of IL-6, TNF-α, INF-γ, and IL-1β. Upregulates actions of IL-10 and IL-4 anti-inflammatory cytokines, including CAT and SOD enzymatic antioxidants. | [102] | ||
| Hyperglycemia induced model | Male C57BL/6 N mouse | In-vivo | Activates the cyclic GMP/protein kinase G pathway to obstruct hyperglycemia triggered nuclear translocation of p-smad2/3. Attenuates pro-fibrotic gene expression in cardiac fibroblasts. Potentiates cGMP level and induced PKG in cardiac fibroblasts via increasing NO production and endothelial nitric oxide synthase (eNOS). | [103] |
Chlorogenic acid and liver fibrosis
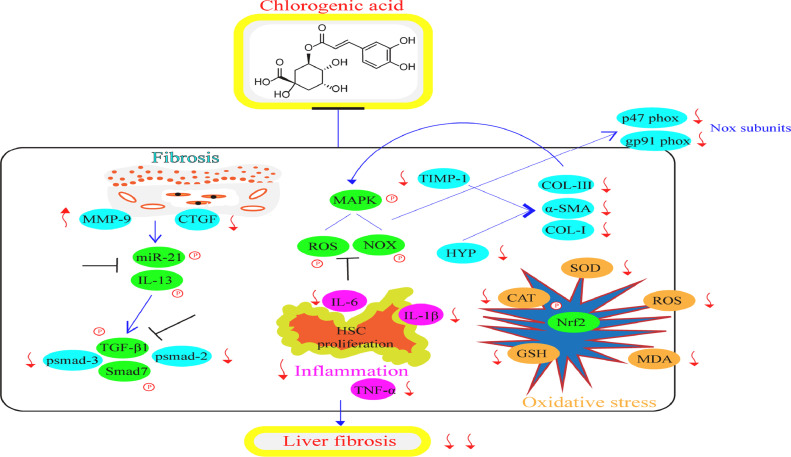
Liver fibrosis is described as uncontrolled deposition of ECM in liver tissue that leads to its functional and structural changes [104, 105]. It is mainly caused by various factors involving nonalcoholic steatohepatitis (NASH), autoimmune hepatitis, cholestatic liver disease, alcohol consumption, nonalcoholic fatty liver disease (NAFLD), and viral hepatitis [106]. Liver fibrosis still remains a significant health issue globally. Numerous studies by researchers regarding CGA potency on liver fibrosis has been reported and also continuously ongoing. Fig. 2 shows the diagrammatic illustration for the mechanistic effects and signaling pathways of CGA in ameliorating liver fibrosis.
Fig. 2.
Mechanistic effects and signaling pathways of CGA in ameliorating liver fibrosis.
CGA hampers liver fibrosis via obstructing the miR-21-regulated Smad7 or transforming growth factor beta-1 (TGF-β1) or interleukin-13 (IL-13) mechanism [90, 91]. CGA defends against CCL4 triggered liver fibrosis (in-vitro and in-vivo) via suppression of the oxidative stress [92], activation of HSCs and the production of vascular endothelial growth factor (VEGF) and TGF-β1 [93]. In addition, CGA diminishes fibrosis and inflammation via suppression of toll-like receptor 4 (TLR-4) mechanism [94]. CGA also exerts protective actions on fibrosis in nonalcoholic steatohepatitis through down-regulating multiple pro-fibrogenic factors and oxidative stress via HIF-α/miR-122 and Nrf2 pathways respectively [95]. Collectively, CGA prevents the oxidative stress, inflammation, and fibrosis in HSCs and fibroblast cells during liver fibrosis through inhibition of miR-21/Smad7/TGF-β1/IL-13/TLR-4/HIF-α/miR-122 and Nrf2 signaling pathways.
Chlorogenic acid and other fibrosis
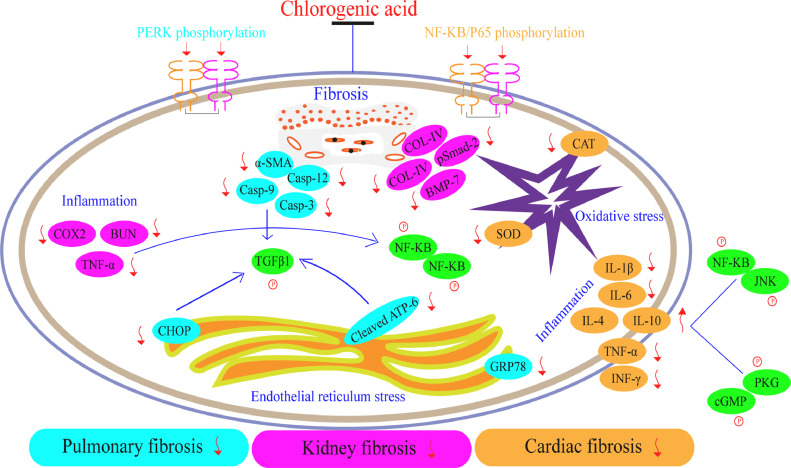
The capability and efficacy of CGA could also be indicated in other fibrosis forms consisting of pulmonary fibrosis, kidney fibrosis, and cardiac fibrosis (Fig. 3). Pulmonary fibrosis is referred as a disease condition indicated through atypical accumulation of ECM resulting to damage, scarring and sclerosis of lung tissues [107]. It usually occurs in different forms such as idiopathic pulmonary fibrosis (IPF) etc. [108], [109], [110]. Till-date, the life expectancy associated with IPF after detection is short and as well as possess unknown pathogenesis [111]. The existing drugs utilized in the treatment of these aforementioned class of fibrosis lacks potentiality, especially in chronic stages. Therefore, further discovering of more potent drugs is required. It has been reported by few experimental studies that CGA promotes BLM-activated pulmonary fibrosis through inhibition of endoplasmic reticulum stress [96].
Fig. 3.
Mechanistic effects and signaling pathways of CGA in ameliorating pulmonary fibrosis, kidney fibrosis, and cardiac fibrosis.
In kidney fibrosis, Arfian et al. found that CGA alleviates kidney ischemic/reperfusion injury through inhibiting inflammation, myofibroblast formation and tubular injury [97]. CGA also attenuated kidney fibrosis via regulating antifibrotic effects of hepatocyte growth factor (HGF) and bone morphogenetic protein-7 (BMP-7) [98]; suppressed the inflammatory response in kidney disease (ischemic reperfusion injury) through decreasing the production of pro-inflammatory cytokines [99]; as well as inhibits renal fibrosis and proteinuria via anti-oxidation and attenuating deposition of ECM [100].
On the other hand, regarding cardiac fibrosis, some studies proved that CGA protects cardiomyocytes from TNF-α activated injury through suppressing JNK and NF-kB actions [101]; inhibits acute myocardial infarction via diminishing oxidative stress and inflammatory damage [102], and attenuated hyperglycemia triggered cardiac fibrosis via activating the NO/cGMP/PKG mechanism [103].
In summary, CGA, inhibits the fibrosis associated with pulmonary fibrosis through down-regulation of endoplasmic reticulum stress; suppresses the oxidative stress, inflammation and fibrosis associated with kidney fibrosis via inhibiting the production of pro-inflammatory cytokines and TLR-4/BMP-7/NF-kB/HGF mechanism; and impairs the oxidative stress and inflammation linked to cardiac fibrosis through diminishing ECM accumulation and JNK/NF-kB/PKG/NO/cGMP signaling pathways.
Potential utilization of CGA for the therapeutics of cancer
The application of CGA in cancer treatment (Table 3) has been enormously reported and demonstrated in cell lines, preclinical and clinical assays, owing to its outstanding and strong anti-cancerous effects [112], [113], [114], [115], [116]. As stated by the National cancer institute, cancer is referred as group of disease condition that involves atypical and excessive growth of cells, basically driven through a genetic process indicated via genome instability and mutations observed at a cellular level [117, 118]. It is commonly classified or categorized as breast cancer, endometrial cancer, prostate cancer, pancreatic cancer, cervical cancer, brain cancer, colon or colorectal cancer, bladder cancer, gastric cancer, kidney cancer, stomach cancer, skin cancer, lung cancer, bone cancer (osteosarcoma) and blood cancer (leukemia) respectively [119]. The metastasizing signs and symptoms known to be associated with cancer includes lumps, unexplained weight loss, persistent cough or heavy breathing, abnormal bleeding, and skin changes. Interestingly, these fundamental manifestations frequently occurs at early and late stages of the disease, and triggered by some certain risk factors such as excessive alcohol intake, high body mass index (obesity), low vegetables and fruits (poor diet), lack of physical activity, biological carcinogens (parasites, bacteria or viruses), physical carcinogens (ionizing and ultraviolet radiation), and chemical carcinogens (aflatoxin, tobacco smoke, and arsenic) [120]. Moreover, cancer still poses a significant economic, social and clinical burden worldwide.
Table 3.
Molecular actions of chlorogenic acid on various forms of cancer.
| No | Types of Cancer | Details of Assay | Application | Analytical Findings | Ref. |
|---|---|---|---|---|---|
| 1 | Lung cancer | Male BALB/c nude mice and human lung cancer A549 cell | In-vivo and in-vitro | Effectively diminishes the binding of annexin A2 to p50 subunits, and expression of downstream anti-apoptotic genes cIAP1 and cIAP2 via NF-kB signaling pathway. | [121] |
| A549 human lung cancer cell | In-vitro | Attenuates cell proliferation, expression levels of BCL2, and stem cell-related markers (SOX2, POU5F1, and NANOG); triggered JNK and p38 MAPK gene expression; and elevated expressions of CASP3, BAX, and annexin V. | [122] | ||
| Human lung cancer A549 cell | In-vitro | Induces DNA damage, high level of topoisomerase-I and topoisomerase-II DNA complexes in cells. | [123] | ||
| Male BALB/c nude mice and human lung cancer A549 cell | In-vivo and In-vitro | Suppresses the expressions of VEGF, HEY1, HES1, Delta4, cell proliferation, and mRNA of Notch1; improved p-AKT, p-PTEN and PTEN in tumor tissues. | [124] | ||
| Human lung cancer A549 cell | In-vitro | Down-regulates migration of A549 cells, Ac-NF-kB expression, matrix metalloproteinase-2 (MMP-2) and histone deacetylase-6 (HDAC6) activities. | [125] | ||
| 2 | Breast cancer | Mouse 4T1 breast cancer cell | In-vivo | Hampers the expression of CD206 triggered by IL-13, M2 related gene Ym1 and metastatic nodes in the lungs. | [126] |
| Mouse 4T1 breast cancer cell | In-vivo | Hinders the viability, proliferation, migration and invasion in breast cancer cells, NF-kB p65 nuclear translocation, EMT and NF-kB mechanism | [127] | ||
| Mouse 4T1, EMT6, BT-549, and MDA-MB-231 cell and EMT6 xenograft model | In-vivo and In-vitro | Inhibits cell viability, tumor volume and weight, and expressions of EGF, TGF-β, VEGF, CD34, and IL-10; activates apoptosis in a dose dependent manner. | [128] | ||
| MCF-7 breast cancer cell | In-vitro | Promotes abundant nuclear condensation, morphological changes, alters the expression of p53 and caspase-3 mRNA, and diminished Bcl-2 protein as well as the acidic autophagosomal vacuolization. | [129] | ||
| Mouse 4T1 breast cancer cell | In-vivo | Decreases tumor weight and volume, elevates Bcl-2/Bax expression ratio, caspase-3 and p53 gene expression. | [130, 131] | ||
| MCF-7 breast cancer cell | In-vitro | Up-regulates STAT5B protein level and inhibits cyclin D1 levels. | [132] | ||
| 3 | Colon cancer | HT-29 colon cancer cell | In-vitro | Suppresses cell viability, G1 cell cycle arrest and apoptotic cell death. | [132] |
| CT-26 colon cancer cell | In-vitro | Decreases ERK phosphorylation, NF-kB and AP-1 transactivation, mitogen-activated MEK1 and TOPK activities, and EGF-, TPA-, and H-Ras-triggered neoplastic transformation of JB6 P+ cells. | [133] | ||
| Human HT-29 colon cancer Caco-2 cell | In-vitro | Reduces cell proliferation, and activated caspase-3 and cell cycle arrest at the S-phase | [134] | ||
| Human HT-29 colon adenocarcinoma cell | In-vitro | Potentiates specific changes in the cell cycle, rate of apoptosis and repressed HT-29 cell viability. | [135] | ||
| Human HCT-15 and CO-115 colon adenocarcinoma cells | In-vitro | Inhibits cell proliferation, BRAF, phospho-ERK expression, Akt phosphorylation, and activated caspase-dependent apoptosis, p38, JNK, S and G2/M phase cell cycle arrest. | [136] | ||
| N-methyl-N-nitro-N-nitrosoguanidine induced male wistar mice | In-vivo | Significantly alleviates the level of expressions of malondialdehyde, glutathione, cyclooxygenase-2, α-tocopherol and DNA damage intensity. | [137] | ||
| Human HCT-116 and HT-29 colon cancer cells | In-vitro | Attenuates the activation of extracellular signal related kinase, cell viability, and triggered S-phase cell cycle arrest and ROS production. | [138] | ||
| Human HCT-116 colon adenocarcinoma cell | In-vitro | Activates apoptosis via induction of PARP-1 cleavage, DNA fragmentation, caspase-9, decreases anti-apoptotic protein Bcl-2 and increased pro-apoptotic protein Bax. | [139] | ||
| 4 | Liver cancer | HepG2 human hepatocarcinoma cell | In-vitro | Enhances the apoptotic action of regorafenib via triggering pro-apoptotic annexin V, Bax, and caspase 3/7, and suppressed anti-apoptotic Bcl2 and Bcl-xL, cell motility, MAPK and PI3K/Akt/mTOR mechanism. | [140] |
| HepG2 and Hep3B human hepatocarcinoma cells | In-vitro | Causes cell proliferation, ERK1/2 inactivation and promotes production of reactive oxygen species (ROS). | [141] | ||
| HepG2 cell and HepG2 xenograft tissue | In-vitro | Down-regulates the expressions of MMP-2, MMP-9, cell proliferation, and triggered the inactivation of ERK1/2. | [142] | ||
| HepG2 human hepatocarcinoma cell | In-vitro | Inhibits the cellular proliferation, colony formation, invasion, and metastasis, MMP-2 and MMP-9 expressions, up-regulates p53 and p21 activity, and inactivates ERK1/2. | [143] | ||
| HepG2 human hepatocarcinoma cell | In-vitro | Promotes nuclear translocation of Nrf2, ARE reporter gene activity, and downstream antioxidant proteins (involving sestrin2, hemeoxygenase-1, glutamate cysteine ligase and NAD(P)H quinone oxidoreductase-1) | [144] | ||
| 5 | Blood cancer | Human U937 leukemia cell | In-vitro | Activates apoptosis via increasing ROS production, expression of caspase-3, 7, 8, 9, and decreasing the mitochondrial membrane potential (ΔΨm). | [145] |
| Human HL-60 leukemia cell | In-vitro | Increases the level of apoptosis in a dose-dependent manner, and halts G0/G1 phase cell cycle and proliferation. | [146] | ||
| Bcr-Abl(+) chronic myeloid leukemia and K562 xenograft nude rats | In-vivo | Potentiates death receptor DR5; triggered deposition of intracellular reactive oxygen species, loss of mitochondrial membrane potential, caspase-8 cleavage, and partially impaired apoptosis. | [147] | ||
| Bcr-Abl(+) chronic myeloid leukemia and K562 xenograft nude rats | In-vivo | Suppresses Bcr-Abl kinase resulting to activation of p38 MAPK. | [148] | ||
| Human K-562 and CCRF-CEM and A549 lung adenocarcinoma cells | In-vitro | Attenuates cell viability in a concentration dependent manner, and decreases mitochondria membrane potential via up-regulating mitochondrial DNA lesions in ND1 and ND5 genes and causing nuclear DNA damage in TP53 gene. | [149, 150] | ||
| 6 | Brain cancer | Human glioma cell | In-vitro | Reduces cell proliferation and triggered apoptosis in a dose dependent manner. Promotes the pro-apoptotic Bax protein, p53 protein level and inhibits Bcl-2 protein and mitochondrial membrane potential. | [151, 152] |
| Human glioma cell | In-vitro | Hinders colony formation. Activates apoptosis via enhancing ROS leading to a disruption of mitochondrial membrane potential. Improved S and G2/M phase cell cycle, and mRNA levels of the apoptotic factors such as p53, caspase-3, caspase-8, caspase-9, Tp53, and Bax. | [153, 154] | ||
| U87MG and patients-derived IV grade glioma cells | In-vitro | Alleviates UHRF1 and DNMT1. Activates double strand DNA damage via promoting the number of phosphorylated H2A.X and cleaved PARP1. | [155, 156] | ||
| 7 | Bone cancer | U2OS, Saos-2, and MG-63 OS cells | In-vitro | Diminishes cell proliferation via activation of apoptosis, inhibits ERK1/2, and altered cell cycle. | [157] |
| 8 | Skin cancer | Human melanoma (SK-MEL-2) cell | In-vitro | Mediates apoptosis via suppression of MEK/ERK mechanism and enhanced caspase-3 activity. | [158] |
| 9 | Kidney cancer | A498 human kidney cancer cell | In-vitro | Activates proliferation via induction of caspase protein and up-regulating pro-apoptotic protein Bax ratio to anti-apoptotic protein Bcl-2. | [159] |
| 10 | Pancreatic cancer | Human pancreatic cancer PANC-1 cell | In-vitro | Hampers cellular proliferation, causes cell cycle arrest, triggers apoptosis and loss in the mitochondrial membrane potential. | [160] |
Chlorogenic acid for the therapeutics of lung cancer
It was revealed by few experimental studies that CGA suppresses the growth of adenocarcinomic human alveolar basal epithelial cells (A549 cells) via targeting the annexin A2 (in vivo and in vitro) [121], and modulates the gene expression of stem cell associated markers and apoptosis [122]. CGA also activates cellular DNA damage and formation of topoisomerase-I and II DNA complexes [123]. In addition, CGA regulates apoptosis in non-small cell lung carcinoma (NSCLC) via Notch1-signaling pathway [124], and modulating histone deacetylase-6 [125].
Chlorogenic acid for the therapeutics of breast cancer
Some studies proved that the combination of CGA and lapatinib significantly represses metastasis via inhibiting macrophage M2 polarization in breast cancer [126]. CGA triggers apoptosis, obstructs metastasis, and enhanced anti-tumor immunity through the NF-kB signaling pathway [127]. An herbal medicine containing CGA and astragaloside as its active constituents proved to have remarkable anti-cancerous effects against breast cancer [128]. Also, the combination of CGA (aqueous extracts of Coffea arabica) and vitamin C elicits MCF-7 apoptosis or cell death [129]. Furthermore, CGA activates 4T1-breast cancer tumor's apoptosis through regulating Bcl-2, caspase-3, Bax, and p53 mechanism [130, 131].
Chlorogenic acid for the therapeutics of colon cancer
Several assays demonstrated that CGA, an essential component of coffee phenolic phytochemicals altered the levels of ATF-2 modifying cyclin D1 and STAT5B expression [132], and inhibits metastasis via targeting MEK and TOPK mechanism in colon cancer cells [133]. CGA, and its microbial metabolites also exerts S-phase cell cycle arrest, anti-proliferative actions, and apoptosis in human colon cancer Caco-2 cells [134]. The combination of CGA and caffeic acid elicits positive inhibitory actions on cellular uptake and cell viability in human colon adenocarcinoma cells [135]. Additionally, CGA, the principal phenolic compound obtained from water extract of Hypericum androsaemum, hinders the proliferation in human colorectal cancer cells via acting on phosphoinositide 3-kinase (PI3K) or protein kinase B (Akt) and mitogen-activated protein kinase (MAP kinase) signaling pathways [136]. CGA plays a chemoprotective role against direct carcinogen in the colon of wistar mice [137], triggers reactive oxygen species (ROS) generation and decreased the viability of human colon cancer cells [138]. Moreover, CGA complex exerts significant anti-cancer actions in cultured HCT-116 cells [139].
Chlorogenic acid for the therapeutics of liver cancer
It was reported that the addition of CGA potentiates regorafenib actions in human hepatocellular carcinoma (HCC) cells [140]. CGA also promotes 5-fluorouracil effect in HCC cells via attenuating the extracellular signal regulated kinases (ERKs) [141], exerts positive inhibitory effects in HCC (in vitro and in vivo) cells [142], and reduced malignant attributes of HCC cells via suppressing DNMT1 expression [143]. Additionally, CGA enhanced the oxidative stress-mediated apoptosis via activating the nuclear factor erythroid 2-related factor 2 (Nrf2) in hepatocytes [144].
Chlorogenic acid for the therapeutics of blood cancer
Another studies indicated that CGA triggers apoptotic cell death in U937 leukemia cells via mitochondria and caspase dependent mechanism [145], and in human acute promyelocytic leukemia cells (HL-60 cells) through suppressing the proliferation [146]. CGA also activated the apoptosis of Bcr-Abl (+) chronic myeloid leukemia cell and clinical leukemic samples via suppressing the Bcr-Abl phosphorylation [147], decreasing Bcr-Abl tyrosine kinase and inducing p38 mitogen triggered protein kinase-dependent apoptosis [148]. In addition, the metamorphosed root extract of Rhaponticum carthamoides and Leonurus sibiricus L., highly rich in caffeoylquinic acid derivatives (CGA), exerts strong anti-cancer effects in human leukemia and lung adenocarcinoma cells [149, 150].
Chlorogenic acid for the therapeutics of brain cancer
In recent years, CGA, obtained from normal and transformed roots of herbal plant known as Rhaponticum carthamoides and Leonurus sibiricus L., suppresses the proliferation of human glioma cell (HGC) via altering the Bax/Bcl-2-p53 expression and apoptotic activation [151, 152], activates apoptosis in HGC lines of different grades via reactive oxygen species-mediated mitochondrial mechanism and caspase induction [153, 154], and impaired HGC viability through activation of double strand DNA damage, H2AX phosphorylation, and Poly [ADP-ribose] polymerase 1 (PARP-1) cleavage [155]. Moreover, CGA with Arabidopsis thaliana production of anthocyanin pigment 1 (AtPAP1) transcriptional factor triggered apoptosis via DNA damage and inhibition of selected epigenetic factors in HGC [156].
Chlorogenic acid for the therapeutics of other cancer
Other form of cancers in which CGA was found to have potent effects includes blood cancer, skin cancer, kidney cancer and pancreatic cancer. In bone cancer, CGA reportedly induced extracellular-signal-regulated kinase1/2, and inhibits the growth of osteosarcoma cells [157]. However, for skin cancer, the fruit extracts of an herbal plant known as Sorbus commixta, constituting of CGA as its major phytochemical ingredient indicated certain anti-melanoma effects [158]. On the other hand, CGA also suppresses proliferation and triggers apoptosis in human kidney cancer cells (A498 cells) through inactivation of PI3K/Akt/mTOR mechanism [159]. Finally, the combination of CGA, polyphenols and epigallocatechin gallate portrays synergistic anti-cancerous effects against human pancreatic cancer cells (PANC-1 cell) [160].
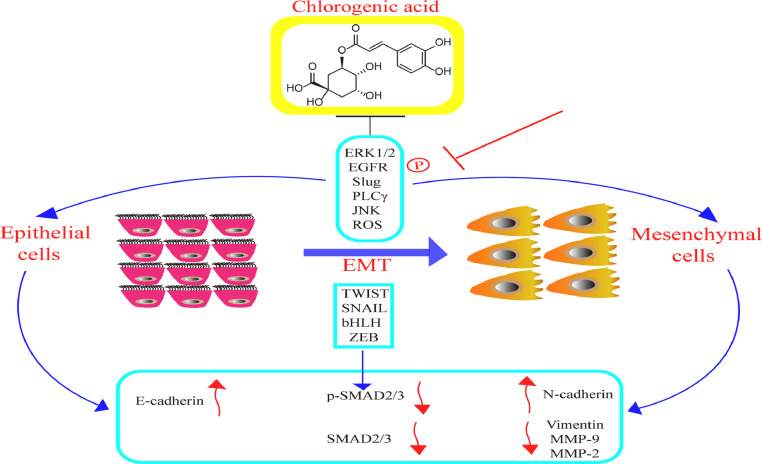
Inhibition of cancer-related epithelial mesenchymal transition by CGA
The preservation of homoeostasis and architecture in a healthy tissue is usually accompanied or characterized through epithelial integrity and inhibition of plasticity. Epithelial-mesenchymal transition (EMT), an essential cellular mechanism in cancer progression, is a process whereby cells go through functional and phenotypical changes resulting to a mesenchymal-like function and state. EMT, as a physiological process is generally involved in the regeneration of tissue, wound healing and embryogenesis. Numerous factors and conditions such as TGF-β, oxidative stress, tissue injury and inflammation, hippo pathway stimulation, hypoxia and HIFα release as well as improved biochemical stress have been reported to trigger EMT [161], [162], [163]. The significant EMT-associated transcription factors, ZEB, TWIST, bHLH, and SNAIL, inhibits the level of epithelial proteins involving E-cadherin, and activate protein expression related with mesenchymal phenotypes like N-cadherin, Vimentin, MMP9, Fibronectin, and MMP2 [164, 165]. For over a decade, experimental studies have shown that effectual suppression of EMT plays a crucial part in treating cancer metastasis. Fig. 4 demonstrates CGA effects in the inhibition of cancer-related EMT.It has been reported that CGA, an essential component of Annurca apple polyphenol extract, potentiates EMT and suppressed migration in MDA-MB-231 and MDA-MB-468 triple-negative breast cancer cells (TNBC), and inhibits metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) via JNK/ROS mechanism. In addition, CGA also downregulates the expressions of phospho-Smad-2/3 (p-SMAD-2/3) and Smad-2/3, upregulates N-cadherin/E-cadherin protein ratio, triggered the switch from N-cadherin to E-cadherin expression and significantly diminished vimentin levels [166]. Furthermore, an in-vitro studies found that CGA derivative (isochlorogenic acid C) reverses EMT through inhibition of Epithelial growth factor receptor (EGFR)/Phospholipase Cγ (PLCγ)/Extra cellular regulated protein kinase 1 or 2 (ERK1/2)/Slug signaling pathway in MDA-MB-231 cells [167]. These few analysis suggested that CGA possess a down-regulatory action against EMT and could be applied in treating cancer. The EMT reversal might have the significance of improving the regeneration of dispersed cancer cells [168]. Therefore, for better understanding regarding cell plasticity, further in-vivo and in-vitro studies should be carried-out to analyze the combined outcomes of CGA for anti-EMT remedy.
Fig. 4.
CGA effects in the inhibition of cancer-related EMT.
Conclusion and future perspective
Conclusively, CGA could serve as prospective drug candidate utilized in fibrosis and cancer treatment. Reports have shown that CGA portrays anti-fibrotic and anticancer effects through suppression of inflammation, cellular senescence, epithelial mesenchymal transition (EMT), hippo mechanism activation, ECM modification, genetic alterations, TGF-β overproduction, fibroblast proliferation and differentiation, elevating invasiveness and stiffness respectively. As a phenolic compound sourced naturally, it is known to possess minimal toxicity level as well as multiple pharmacological attributes such as anti-metastatic, anti-oxidative, nephroprotective, anti-inflammatory, anti-diabetic, anti-hypertensive, hepatoprotective, anti-bacterial, neuroprotective, anti-proliferative, central nervous system stimulator, anti-obesity, cardioprotective, anti-pyretic, anti-viral, and anti-angiogenic respectively. Presently, although CGA has been recognized and approved for phase-I (NCT02728349, Apr. 2016) and phase-II (NCT03758014, Nov. 2018) clinical trials by the China Food and Drug Administration (CFDA) as a possible drug for cancer in glioma patients. Yet, its molecular mechanism still remains unclear. Numerous experimental studies carried-out by researchers regarding CGA, most especially through the use of cell or animal model have demonstrated its great significance and positive therapeutical effects against fibrosis and cancer. Moreover, with regards to finding a more potent drugs and ascertaining a clinical breakthrough in fibrosis and cancer therapeutics universally, more studies using human model needs to be conducted in the future. In addition, the metabolism, excretion, utilization and bioavailability of CGA also requires further experimentation.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgments
Consent
This literature review does not contain studies with human participants or animals performed by any of the authors.
Credit authorship contribution statement
Ebuka Olisaemeka Nwafor conceived the manuscript and figures; Ebuka Olisaemeka Nwafor and Peng Lu wrote the manuscript; Ebuka Olisaemeka Nwafor made the figures; Ying Zhang reviewed and edited the manuscript; Zhidong Liu supervised and edited the manuscript.
Acknowledgment
This study was financially supported by Scientific Research Project of Tianjin Municipal Education Commission (Number: 2019KJ083).
References
- 1.Landolt L., Spagnoli G.C., Hertig A., Brocheriou I., Marti H.P. Fibrosis and cancer: shared features and mechanisms suggest common targeted therapeutic approaches. Nephrol. Dial. Transpl. 2020:gfaa301. doi: 10.1093/ndt/gfaa301. [DOI] [PubMed] [Google Scholar]
- 2.Rybinski B., Franco-Barraza J., Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol. Genomics. 2014;46(7):223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grande M.T., Sánchez-Laorden B., López-Blau C., De Frutos C.A., Boutet A., Arévalo M., Rowe R.G., Weiss S.J., López-Novoa J.M., Nieto M.A. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 2015;21(9):989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh M. Involvement of partial EMT in cancer progression. J. Biochem. 2018;164(4):257–264. doi: 10.1093/jb/mvy047. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi S., Saito A. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int. J. Mol. Sci. 2018;19(11):3674. doi: 10.3390/ijms19113674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herranz N., Gil J. Mechanisms and functions of cellular senescence. J. Clin. Invest. 2018;128(4):1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowska A.M., Rudzki M., Rudzki S., Lewandowski T., Laskowska B. Environmental risk factors for cancer - review paper. Ann. Agric. Environ. Med. 2019;26(1):1–7. doi: 10.26444/aaem/94299. [DOI] [PubMed] [Google Scholar]
- 8.Meijers W.C., de Boer R.A. Common risk factors for heart failure and cancer. Cardiovasc. Res. 2019;115(5):844–853. doi: 10.1093/cvr/cvz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dydjow-Bendek D., Zagożdżon P. Selected dietary factors and breast cancer risk. Przegl. Epidemiol. 2019;73(3):361–368. doi: 10.32394/pe.73.29. [DOI] [PubMed] [Google Scholar]
- 10.Lizama N., Jongenelis M. Awareness of cancer risk factors and protective factors among Australian adults. Health Promot. J. Austr. 2020;31(1):77–83. doi: 10.1002/hpja.248. [DOI] [PubMed] [Google Scholar]
- 11.Schuller H.M. The impact of smoking and the influence of other factors on lung cancer. Expert Rev. Respir. Med. 2019;13(8):761–769. doi: 10.1080/17476348.2019.1645010. [DOI] [PubMed] [Google Scholar]
- 12.Wilson K.M., Mucci L.A. Diet and lifestyle in prostate cancer. Adv. Exp. Med. Biol. 2019;1210:1–27. doi: 10.1007/978-3-030-32656-2_1. [DOI] [PubMed] [Google Scholar]
- 13.Steck S.E., Murphy E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020;20(2):125–138. doi: 10.1038/s41568-019-0227-4. [DOI] [PubMed] [Google Scholar]
- 14.Pelusi S., Cespiati A., Rametta R., Pennisi G., Mannisto V., Rosso C., Baselli G., Dongiovanni P., Fracanzani A.L., Badiali S., Maggioni M., Craxi A., Fargion S., Prati D., Nobili V., Bugianesi E., Romeo S., Pihlajamaki J., Petta S., Valenti L. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin. Gastroenterol. Hepatol. 2019;17(11):2310–2319. doi: 10.1016/j.cgh.2019.01.027. e2316. [DOI] [PubMed] [Google Scholar]
- 15.Roerecke M., Vafaei A., Hasan O.S.M., Chrystoja B.R., Cruz M., Lee R., Neuman M.G., Rehm J. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am. J. Gastroenterol. 2019;114(10):1574–1586. doi: 10.14309/ajg.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Portal J.A. Efficacy and safety of nintedanib for the treatment of idiopathic pulmonary fibrosis: an update. Drugs R. D. 2018;18(1):19–25. doi: 10.1007/s40268-017-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richeldi L., Kolb M., Jouneau S., Wuyts W.A., Schinzel B., Stowasser S., Quaresma M., Raghu G. Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis. BMC Pulm. Med. 2020;20(1):3. doi: 10.1186/s12890-019-1030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela C., Torrisi S.E., Kahn N., Quaresma M., Stowasser S., Kreuter M. Ongoing challenges in pulmonary fibrosis and insights from the nintedanib clinical programme. Respir. Res. 2020;21(1):7. doi: 10.1186/s12931-019-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seibold J.R., Maher T.M., Highland K.B., Assassi S. Safety and tolerability of nintedanib in patients with systemic sclerosis-associated interstitial lung disease: data from the SENSCIS trial. Ann. Rheum. Dis. 2020;79(11):1478–1484. doi: 10.1136/annrheumdis-2020-217331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniou K., Markopoulou K., Tzouvelekis A., Trachalaki A. Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study. ERJ Open Res. 2020;6(1):00172–02019. doi: 10.1183/23120541.00172-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster L.H., de Andrade J.A., Zibrak J.D., Padilla M.L., Albera C., Nathan S.D., Wijsenbeek M.S., Stauffer J.L., Kirchgaessler K.U., Costabel U. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017;26(146) doi: 10.1183/16000617.0057-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren H., Wang K., Yang H., Gao L. Efficacy and adverse events of pirfenidone in treating idiopathic pulmonary fibrosis. Saudi Med. J. 2017;38(9):889–894. doi: 10.15537/smj.2017.9.19349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcos Ribes B, Sancho-Chust J.N., Talens A., Arlandis M., Herraiz P., Chiner E., Aznar T. Effectiveness and safety of pirfenidone for idiopathic pulmonary fibrosis. Eur. J. Hosp. Pharm. 2020;27(6):350–354. doi: 10.1136/ejhpharm-2018-001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y., Zhou X., Guo K., Zhou F., Yang H. Use of Chlorogenic acid against diabetes mellitus and its complications. J. Immunol. Res. 2020;2020 doi: 10.1155/2020/9680508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermawati E., Arfian N. Chlorogenic acid ameliorates memory loss and hippocampal cell death after transient global ischemia. Eur. J. Neurosci. 2020;51(2):651–669. doi: 10.1111/ejn.14556. [DOI] [PubMed] [Google Scholar]
- 26.Hong B.N., Nam Y.H., Woo S.H., Kang T.H. Chlorogenic acid rescues sensorineural auditory function in a diabetic animal model. Neurosci. Lett. 2017;640:64–69. doi: 10.1016/j.neulet.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Han D., Chen W., Gu X., Shan R., Zou J., Liu G., Shahid M., Gao J., Han B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget. 2017;8(9):14680–14692. doi: 10.18632/oncotarget.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preetha Rani M.R., Anupama N., Sreelekshmi M., Raghu K.G. Chlorogenic acid attenuates glucotoxicity in H9c2 cells via inhibition of glycation and PKC α upregulation and safeguarding innate antioxidant status. Biomed. Pharmacother. 2018;100:467–477. doi: 10.1016/j.biopha.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Pimpley V., Patil S., Srinivasan K., Desai N., Murthy P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020;50(10):969–978. doi: 10.1080/10826068.2020.1786699. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y., Wang J., Ballevre O., Luo H., Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012;35(4):370–374. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.J., Lu X.W., Song N., Kou L., Wu M.K., Liu F., Wang H., Shen J.F. Chlorogenic acid alters the voltage-gated potassium channel currents of trigeminal ganglion neurons. Int. J. Oral Sci. 2014;6(4):233–240. doi: 10.1038/ijos.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., He J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Al-Megrin W.A., Metwally D.M., Habotta O.A., Amin H.K., Abdel Moneim A.E. Nephroprotective effects of chlorogenic acid against sodium arsenite-induced oxidative stress, inflammation, and apoptosis. J. Sci. Food Agric. 2020;100(14):5162–5170. doi: 10.1002/jsfa.10565. [DOI] [PubMed] [Google Scholar]
- 34.Park J.J., Hwang S.J., Park J.H., Lee H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 2015;38(2):111–118. doi: 10.1007/s13402-014-0216-2. [DOI] [PubMed] [Google Scholar]
- 35.Dungubat E., Watabe S., Togashi-Kumagai A., Watanabe M., Kobayashi Y., Harada N., Yamaji R. Effects of caffeine and chlorogenic acid on nonalcoholic steatohepatitis in mice induced by choline-deficient, L-amino acid-defined, high-fat diet. Nutrients. 2020;12(12):3886. doi: 10.3390/nu12123886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y., Yu Y.H., Wang S.T., Ren J., Camer D., Hua Y.Z., Zhang Q., Huang J., Xue D.L., Zhang X.F., Huang X.F., Liu Y. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016;54(6):1027–1034. doi: 10.3109/13880209.2015.1093510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao W., Wang C., Yu L., Sheng T., Wu Z., Wang X., Zhang D., Lin Y., Gong Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/6769789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong F., Tan J., Zheng Y. Chlorogenic Acid alleviates allergic inflammatory responses through regulating Th1/Th2 balance in ovalbumin-induced allergic rhinitis mice. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.923358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan Z., Luo M., Yang J., Cheng Y., Huang J., Lu C., Song D., Ye M., Dai M., Gonzalez F.J., Liu A., Guo B. Chlorogenic acid inhibits cholestatic liver injury induced by α-naphthylisothiocyanate: involvement of STAT3 and NFκB signalling regulation. J. Pharm. Pharmacol. 2016;68(9):1203–1213. doi: 10.1111/jphp.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glei M., Kirmse A., Habermann N., Persin C., Pool-Zobel B.L. Bread enriched with green coffee extract has chemoprotective and antigenotoxic activities in human cells. Nutr. Cancer. 2006;56(2):182–192. doi: 10.1207/s15327914nc5602_9. [DOI] [PubMed] [Google Scholar]
- 41.Palmioli A., Ciaramelli C. Natural compounds in cancer prevention: effects of coffee extracts and their main polyphenolic component, 5-O-caffeoylquinic acid, on Oncogenic Ras Proteins. Chem. Asian J. 2017;12(18):2457–2466. doi: 10.1002/asia.201700844. [DOI] [PubMed] [Google Scholar]
- 42.Liu B., Cao L., Zhang L., Yuan X., Zhao B. Preparation, phytochemical investigation, and safety evaluation of chlorogenic acid products from Eupatorium adenophorum. Molecules. 2016;22(1):67. doi: 10.3390/molecules22010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amano Y., Honda H. Safety pharmacological evaluation of the coffee component, caffeoylquinic acid, and its metabolites, using ex vivo and in vitro profiling assays. Pharmaceuticals. 2019;12(3):110. doi: 10.3390/ph12030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upadhyay R., Mohan Rao L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013;53(9):968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- 45.Naso L.G., Valcarcel M., Roura-Ferrer M., Kortazar D., Salado C., Lezama L., Rojo T., González-Baró A.C., Williams P.A., Ferrer E.G. Promising antioxidant and anticancer (human breast cancer) oxidovanadium(IV) complex of chlorogenic acid. Synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem. 2014;135:86–99. doi: 10.1016/j.jinorgbio.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Wang L.N., Wang W., Hattori M., Daneshtalab M., Synthesis Ma C.M. Anti-HCV, Antioxidant and reduction of intracellular reactive oxygen species generation of a chlorogenic acid analogue with an amide bond replacing the ester bond. Molecules. 2016;21(6):737. doi: 10.3390/molecules21060737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kremr D., Bajer T., Bajerová P., Surmová S., Ventura K. Unremitting problems with chlorogenic acid Nomenclature: a review. Quím. Nova. 2016;39:530–533. [Google Scholar]
- 48.Jacobo-Velázquez D.A., Martínez-Hernández G.B., del C., Rodríguez S., Cao C.-.M., Cisneros-Zevallos L. Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011;59(12):6583–6593. doi: 10.1021/jf2006529. [DOI] [PubMed] [Google Scholar]
- 49.Becerra-Moreno A., Redondo-Gil M., Benavides J., Nair V., Cisneros-Zevallos L., Jacobo-Velázquez D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015;6:837. doi: 10.3389/fpls.2015.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López-Martínez J.M., Santana-Gálvez J., Aguilera-González C., Santacruz A., Amaya-Guerra C.A., Jacobo-Velázquez D.A. Effects of carrot puree with enhanced levels of chlorogenic acid on rat cognitive abilities and neural development. J. Food. 2020;18(1):68–75. [Google Scholar]
- 51.Viacava F., Santana-Gálvez J., Heredia-Olea E., Pérez-Carrillo E., Jacobo-Velázquez D.A. Combined application of wounding stress and extrusion as an innovative tool to obtain carrot powders with modified functional properties. J. Food. 2019;17(1):613–621. [Google Scholar]
- 52.Torres-Contreras A.M., Nair V., Cisneros-Zevallos L., Jacobo-Velázquez D.A. Effect of exogenous amylolytic enzymes on the accumulation of chlorogenic acid isomers in wounded potato tubers. J. Agric. Food Chem. 2014;62(31):7671–7675. doi: 10.1021/jf5026983. [DOI] [PubMed] [Google Scholar]
- 53.Tajik N., Tajik M., Mack I., Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur. J. Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 54.Park H.J. Chemistry and pharmacological action of caffeoylquinic acid derivatives and pharmaceutical utilization of chwinamul (Korean Mountainous vegetable) Arch. Pharm. Res. 2010;33(11):1703–1720. doi: 10.1007/s12272-010-1101-9. [DOI] [PubMed] [Google Scholar]
- 55.Mills C.E., Oruna-Concha M.J., Mottram D.S., Gibson G.R., Spencer J. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013;141(4):3335–3340. doi: 10.1016/j.foodchem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Yun N., Kang J.W., Lee S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012;23(10):1249–1255. doi: 10.1016/j.jnutbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Aseervatham G.S., Suryakala U., Doulethunisha Sundaram S., Bose P.C., Sivasudha T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharmacother. 2016;82:54–64. doi: 10.1016/j.biopha.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 58.Bisht A., Dickens M., Rutherfurd-Markwick K., Thota R. Chlorogenic acid potentiates the anti-inflammatory activity of curcumin in LPS-stimulated THP-1 cells. Nutrients. 2020;12(9):2706. doi: 10.3390/nu12092706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian L., Su C.P., Wang Q., Wu F.J., Bai R., Zhang H.M., Liu J.Y., Lu W.J., Wang W., Lan F. Chlorogenic acid: a potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J. Cell. Mol. Med. 2019;23(7):4666–4678. doi: 10.1111/jcmm.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karcheva-Bahchevanska D.P., Lukova P.K., Nikolova M.M., Mladenov R.D., Iliev I.N. Effect of extracts of bilberries (Vaccinium myrtillus L.) on amyloglucosidase and α-glucosidase activity. Folia Med. 2017;59(2):197–202. doi: 10.1515/folmed-2017-0028. [DOI] [PubMed] [Google Scholar]
- 61.Lukitasari M., Nugroho D.A., Widodo N. Chlorogenic acid: the conceivable chemosensitizer leading to cancer growth suppression. J. Evid. Based Integr. Med. 2018;23 doi: 10.1177/2515690X18789628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olthof M.R., Hollman P.C., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131(1):66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 63.Stalmach A., Steiling H., Williamson G., Crozier A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010;501(1):98–105. doi: 10.1016/j.abb.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Lafay S., Morand C., Manach C., Besson C., Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006;96(1):39–46. doi: 10.1079/bjn20061714. [DOI] [PubMed] [Google Scholar]
- 65.Azuma K., Ippoushi K., Nakayama M., Ito H., Higashio H., Terao J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000;48(11):5496–5500. doi: 10.1021/jf000483q. [DOI] [PubMed] [Google Scholar]
- 66.Bhandarkar N.S., Brown L., Panchal S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019;62:78–88. doi: 10.1016/j.nutres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Nishitsuji K., Watanabe S., Xiao J., Nagatomo R., Ogawa H., Tsunematsu T., Umemoto H., Morimoto Y., Akatsu H., Inoue K., Tsuneyama K. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018;8(1):16173. doi: 10.1038/s41598-018-34571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shokouh P., Jeppesen P.B. Effects of unfiltered coffee and bioactive coffee compounds on the development of metabolic syndrome components in a high-fat-/high-fructose-fed rat model. Nutrients. 2018;10(10):1547. doi: 10.3390/nu10101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z., Lam K.L., Hu J., Ge S., Zhou A., Zheng B., Zeng S., Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7(2):579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo C.C., Zhang X.Y., Wang Y.X., Xie L., Chang C.Q. Effects of chlorogenic acid on glucose tolerance and its curve characteristics in high-fat diet-induced obesity rats. J. Peking Univ. Health Sci. 2020;52(2):269–274. doi: 10.19723/j.issn.1671-167X.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He X., Zheng S., Sheng Y., Miao T., Xu J., Xu W. Chlorogenic acid ameliorates obesity by preventing energy balance shift in high-fat diet induced obese mice. J. Sci. Food Agric. 2021;101(2):631–637. doi: 10.1002/jsfa.10675. [DOI] [PubMed] [Google Scholar]
- 72.Xu M., Yang L., Zhu Y., Liao M., Chu L., Li X., Lin L., Zheng G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019;10(11):7489–7497. doi: 10.1039/c9fo00502a. [DOI] [PubMed] [Google Scholar]
- 73.Zhong Y., Ding Y., Li L., Ge M., Ban G., Yang H., Dai J., Zhang L. Effects and mechanism of chlorogenic acid on weight loss. Curr. Pharm. Biotechnol. 2020;21(11):1099–1106. doi: 10.2174/1389201021666200318124922. [DOI] [PubMed] [Google Scholar]
- 74.Shao W., Xu J., Xu C., Weng Z., Liu Q., Zhang X., Liang J., Li W., Zhang Y., Jiang Z., Gu A. Early-life perfluorooctanoic acid exposure induces obesity in male offspring and the intervention role of chlorogenic acid. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.115974. [DOI] [PubMed] [Google Scholar]
- 75.Ghadieh H.E., Smiley Z.N., Kopfman M.W., Najjar M.G., Hake M.J., Najjar S.M. Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice. Nutr. Metab. 2015;12:19. doi: 10.1186/s12986-015-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.VS H., K V., Patel D., K S. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complement. Altern. Med. 2016;16:274. doi: 10.1186/s12906-016-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amato A., Caldara G.F., Nuzzo D., Baldassano S., Picone P., Rizzo M., Mulè F., Di Carlo M. NAFLD and atherosclerosis are prevented by a natural dietary supplement containing curcumin, silymarin, guggul, chlorogenic acid and inulin in mice fed a high-fat diet. Nutrients. 2017;9(5):492. doi: 10.3390/nu9050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bao L., Li J., Zha D., Zhang L., Gao P., Yao T., Wu X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018;54:245–253. doi: 10.1016/j.intimp.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 79.Ye H.Y., Li Z.Y., Zheng Y., Chen Y., Zhou Z.H., Jin J. The attenuation of chlorogenic acid on oxidative stress for renal injury in streptozotocin-induced diabetic nephropathy rats. Arch. Pharm. Res. 2016;39(7):989–997. doi: 10.1007/s12272-016-0771-3. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Peng S., Mei Z., Jin C., Kang J., Xiang M., Wang Z., Hu Y. Chlorogenic acid inhibits forming of diabetes mellitus in rats induced by high-fat high-sucrose and streptozotocin. Pak. J. Pharm. Sci. 2020;33(3):1063–1072. [PubMed] [Google Scholar]
- 81.Jin S., Chang C., Zhang L., Liu Y., Huang X., Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrare K., Bidel L.P.R., Awwad A., Poucheret P., Cazals G., Lazennec F., Azay-Milhau J., Tournier M., Lajoix A.D., Tousch D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018;215:241–248. doi: 10.1016/j.jep.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 83.Stefanello N., Schmatz R., Pereira L.B., Cardoso A.M., Passamonti S., Spanevello R.M., Thomé G., de Oliveira G.M.T., Kist L.W., Bogo M.R., Morsch V.M., Schetinger M.R.C. Effects of chlorogenic acid, caffeine and coffee on components of the purinergic system of streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2016;38:145–153. doi: 10.1016/j.jnutbio.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 84.Yin X.L., Xu B.Q., Zhang Y.Q. Gynura divaricata rich in 3, 5-/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr. Metab. 2018;15:73. doi: 10.1186/s12986-018-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki A., Yamamoto N., Jokura H., Yamamoto M., Fujii A., Tokimitsu I., Saito I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006;24(6):1065–1073. doi: 10.1097/01.hjh.0000226196.67052.c0. [DOI] [PubMed] [Google Scholar]
- 86.Agunloye O.M., Oboh G., Ademiluyi A.O., Ademosun A.O., Akindahunsi A.A., Oyagbemi A.A., Omobowale T.O., Ajibade T.O., Adedapo A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019;109:450–458. doi: 10.1016/j.biopha.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 87.Agunloye O.M., Oboh G., Bello G.T., Oyagbemi A.A. Caffeic and chlorogenic acids modulate altered activity of key enzymes linked to hypertension in cyclosporine-induced hypertensive rats. J. Basic Clin. Physiol. Pharmacol. 2020;32(3):169–177. doi: 10.1515/jbcpp-2019-0360. [DOI] [PubMed] [Google Scholar]
- 88.Bagdas D., Cinkilic N., Ozboluk H.Y., Ozyigit M.O., Gurun M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2013;67(4):698–704. doi: 10.1007/s11418-012-0726-z. [DOI] [PubMed] [Google Scholar]
- 89.Hara K., Haranishi Y., Kataoka K., Takahashi Y., Terada T., Nakamura M., Sata T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014;723:459–464. doi: 10.1016/j.ejphar.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 90.Yang F., Luo L., Zhu Z.D., Zhou X., Wang Y., Xue J., Zhang J., Cai X., Chen Z.L., Ma Q., Chen Y.F., Wang Y.J., Luo Y.Y., Liu P., Zhao L. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017;8:929. doi: 10.3389/fphar.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Yang F., Xue J., Zhou X., Luo L., Ma Q., Chen Y.F., Zhang J., Zhang S.L., Zhao L. Antischistosomiasis liver fibrosis effects of chlorogenic acid through IL-13/miR-21/Smad7 signaling interactions in vivo and in vitro. Antimicrob. Agents Chemother. 2017;61(2):e01347. doi: 10.1128/AAC.01347-16. -16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi H., Shi A., Dong L., Lu X., Wang Y., Zhao J., Dai F., Guo X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016;35(6):1366–1373. doi: 10.1016/j.clnu.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Shi H., Dong L., Bai Y., Zhao J., Zhang Y., Zhang L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur. J. Pharmacol. 2009;623(1–3):119–124. doi: 10.1016/j.ejphar.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 94.Shi H., Dong L., Jiang J., Zhao J., Zhao G., Dang X., Lu X., Jia M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology. 2013;303:107–114. doi: 10.1016/j.tox.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 95.Liu X., Huang K., Niu Z., Mei D., Zhang B. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin. Pharmacol. Toxicol. 2019;124(2):144–153. doi: 10.1111/bcpt.13122. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y.C., Dong J., Nie J., Zhu J.X., Wang H., Chen Q., Chen J.Y., Xia J.M., Shuai W. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis. 2017;22(9):1147–1156. doi: 10.1007/s10495-017-1393-z. [DOI] [PubMed] [Google Scholar]
- 97.Arfian N., Wahyudi D.A.P., Zulfatina I.B., Citta A.N., Anggorowati N., Multazam A., Romi M.M., Sari D.C.R. Chlorogenic acid attenuates kidney ischemic/reperfusion injury via reducing inflammation, tubular injury, and myofibroblast formation. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/5423703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yunus J., Salman M., Lintin G.B.R., Muchtar M., Sari D.C.R., Arfian N., Romi M.M. Chlorogenic acid attenuates kidney fibrosis via antifibrotic action of BMP-7 and HGF. Med. J. Malaysia. 2020;75(Suppl 1):5–9. [PubMed] [Google Scholar]
- 99.Ali Multazam S.L.S.R., Arfian Nur. Atlantis Press; 2017. Chlorogenic Acid Attenuated Inflammatory Response in Kidney Disease with Ischemic Reperfusion Injury; p. 2. [Google Scholar]
- 100.Jingqui Lou X.G., Yinghua X., Jing C., Lv Wei, Yan Z. Chlorogenic acid slows down proteinuria and renal fibrosis in 5/6 nephrectomized rats by antioxidation and inhibiting accumulation of extracellular matrix. Int. J. Clin. Exp. Med. 2016;8:15719–15727. [Google Scholar]
- 101.Tian L., Su C.P., Wang Q., Wu F.J., Bai R., Zhang H.M., Liu J.Y., Lu W.J., Wang W., Lan F., Guo S.Z. Chlorogenic acid: a potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. J. Cell. Mol. Med. 2019;23(7):4666–4678. doi: 10.1111/jcmm.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang D., Tian L., Lv H., Pang Z., Li D., Yao Z., Wang S. Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. Biomed. Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110773. [DOI] [PubMed] [Google Scholar]
- 103.Qin L., Zang M., Xu Y., Zhao R., Wang Y., Mi Y., Mei Y. Chlorogenic acid alleviates hyperglycemia-induced cardiac fibrosis through activation of the NO/cGMP/PKG pathway in cardiac fibroblasts. Mol. Nutr. Food Res. 2020 doi: 10.1002/mnfr.202000810. [DOI] [PubMed] [Google Scholar]
- 104.Šmíd V. Liver fibrosis. Vnitr. Lek. 2020;66(4):61–66. [PubMed] [Google Scholar]
- 105.Aydın M.M., Akçalı K.C. Liver fibrosis. Turk. J. Gastroenterol. 2018;29(1):14–21. doi: 10.5152/tjg.2018.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parola M., Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Chioma O.S., Drake W.P. Role of Microbial Agents in Pulmonary Fibrosis. Yale J. Biol. Med. 2017;90(2):219–227. [PMC free article] [PubMed] [Google Scholar]
- 108.Wakwaya Y., Brown K.K. Idiopathic pulmonary fibrosis: epidemiology, diagnosis and outcomes. Am. J. Med. Sci. 2019;357(5):359–369. doi: 10.1016/j.amjms.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 109.Hewitt R.J., Maher T.M. Idiopathic pulmonary fibrosis: new and emerging treatment options. Drug Aging. 2019;36(6):485–492. doi: 10.1007/s40266-019-00647-y. [DOI] [PubMed] [Google Scholar]
- 110.King T.E., Jr., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 111.Meyer K.C. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017;11(5):343–359. doi: 10.1080/17476348.2017.1312346. [DOI] [PubMed] [Google Scholar]
- 112.Hayakawa S., Ohishi T. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules. 2020;25(19):4553. doi: 10.3390/molecules25194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dominianni C., Huang W.Y., Berndt S., Hayes R.B., Ahn J. Prospective study of the relationship between coffee and tea with colorectal cancer risk: the PLCO Cancer Screening Trial. Br. J. Cancer. 2013;109(5):1352–1359. doi: 10.1038/bjc.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ismail T., Donati-Zeppa S., Akhtar S. Coffee in cancer chemoprevention: an updated review. Expert Opin. Drug Metab. Toxicol. 2021;17(1):69–85. doi: 10.1080/17425255.2021.1839412. [DOI] [PubMed] [Google Scholar]
- 115.Gaascht F., Dicato M., Diederich M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015;10(6):51. doi: 10.1007/s12263-015-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajagopal C., Lankadasari M.B., Aranjani J.M., Harikumar K.B. Targeting oncogenic transcription factors by polyphenols: a novel approach for cancer therapy. Pharmacol. Res. 2018;130:273–291. doi: 10.1016/j.phrs.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 117.Mattiuzzi C., Lippi Current cancer epidemiology. J. Epidemiol. Glob. Health. 2019;9(4):217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krieghoff-Henning E., Folkerts J., Penzkofer A., Weg-Remers S. Cancer – an overview. Med. Monatsschr. Pharm. 2017;40(2):48–54. [PubMed] [Google Scholar]
- 119.Theodoratou E., Timofeeva M., Li X., Meng X., Ioannidis J.P.A. Nature, nurture, and cancer risks: genetic and nutritional contributions to cancer. Annu. Rev. Nutr. 2017;37:293–320. doi: 10.1146/annurev-nutr-071715-051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jayasekara H., MacInnis R.J., Room R., English D.R. Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: a systematic review and meta-analysis. Alcohol Alcohol. 2016;51(3):315–330. doi: 10.1093/alcalc/agv110. [DOI] [PubMed] [Google Scholar]
- 121.Wang L., Du H., Chen P. Chlorogenic acid inhibits the proliferation of human lung cancer A549 cell lines by targeting annexin A2 in vitro and in vivo. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110673. [DOI] [PubMed] [Google Scholar]
- 122.Yamagata K., Izawa Y., Onodera D., Tagami M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2018;441(1–2):9–19. doi: 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
- 123.Burgos-Morón E., Calderón-Montaño J.M., Orta M.L., Pastor N., Pérez-Guerrero C., Austin C., Mateos S., López-Lázaro M. The coffee constituent chlorogenic acid induces cellular DNA damage and formation of topoisomerase I- and II-DNA complexes in cells. J. Agric. Food Chem. 2012;60(30):7384–7391. doi: 10.1021/jf300999e. [DOI] [PubMed] [Google Scholar]
- 124.Li W., Liu X., Zhang G., Zhang L. Mechanism of chlorogenic acid in apoptotic regulation through Notch1 pathway in non-small cell lung carcinoma in animal level. Chin. J. Lung Cancer. 2017;20(8):555–561. doi: 10.3779/j.issn.1009-3419.2017.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hongtao L., Xiaoqi G., Junni L., Feng X., Guodong B., Liang Y. Chlorogenic-induced inhibition of non-small cancer cells occurs through regulation of histone deacetylase 6. Cell. Mol. Biol. 2018;64(10):134–139. [PubMed] [Google Scholar]
- 126.Zhang J.Q., Yao Z.T., Liang G.K., Chen X., Wu H.H., Jin L., Ding L. Combination of lapatinib with chlorogenic acid inhibits breast cancer metastasis by suppressing macrophage M2 polarization. J. Zhejiang Uni. Med. Sci. 2015;44(5):493–499. doi: 10.3785/j.issn.1008-9292.2015.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeng A., Liang X., Zhu S., Liu C., Wang S., Zhang Q., Zhao J., Song L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF‑κB signaling pathway. Oncol. Rep. 2021;45(2):717–727. doi: 10.3892/or.2020.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y., Li X. Study on the anticancer effect of an astragaloside- and chlorogenic acid-containing herbal medicine (RLT-03) in breast cancer. Evid. Based Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/1515081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Garawani I.M., El-Nabi S.H., El-Shafey S., Elfiky M., Nafie E. Coffea arabica bean extracts and vitamin C: a novel combination unleashes MCF-7 cell death. Curr. Pharm. Biotechnol. 2020;21(1):23–36. doi: 10.2174/1389201020666190822161337. [DOI] [PubMed] [Google Scholar]
- 130.Changizi Z., Moslehi A. Chlorogenic acid induces 4T1 breast cancer tumor's apoptosis via p53, Bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J. Biochem. Mol. Toxicol. 2020;35(2):e22642. doi: 10.1002/jbt.22642. [DOI] [PubMed] [Google Scholar]
- 131.Changizi Z., Moslehi A., Rohani A.H., Eidi A. Chlorogenic acid inhibits growth of 4T1 breast cancer cells through involvement in Bax/Bcl2 pathway. J. Cancer Res. Ther. 2020;16(6):1435–1442. doi: 10.4103/jcrt.JCRT_245_19. [DOI] [PubMed] [Google Scholar]
- 132.Oleaga C., Ciudad C.J., Noé V., Izquierdo-Pulido M. Coffee polyphenols change the expression of STAT5B and ATF-2 modifying cyclin D1 levels in cancer cells. Oxid. Med. Cell Longev. 2012;2012 doi: 10.1155/2012/390385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kang N.J., Lee K.W., Kim B.H., Bode A.M., Lee H.J., Heo Y.S., Boardman L., Limburg P., Lee H.J., Dong Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011;32(6):921–928. doi: 10.1093/carcin/bgr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sadeghi Ekbatan S., Li X.Q., Ghorbani M., Azadi B., Kubow S. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Int. J. Mol. Sci. 2018;19(3):723. doi: 10.3390/ijms19030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murad L.D., Soares Nda C., Brand C., Monteiro M.C., Teodoro A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer. 2015;67(3):532–542. doi: 10.1080/01635581.2015.1004736. [DOI] [PubMed] [Google Scholar]
- 136.Xavier C.P., Lima C.F., Fernandes-Ferreira M., Pereira-Wilson C. Hypericum androsaemum water extract inhibits proliferation in human colorectal cancer cells through effects on MAP kinases and PI3K/Akt pathway. Food Funct. 2012;3(8):844–852. doi: 10.1039/c2fo10226a. [DOI] [PubMed] [Google Scholar]
- 137.Soares P.V., Kannen V., Jordão Junior A.A., Garcia S.B. Coffee, but neither decaffeinated coffee nor caffeine, elicits chemoprotection against a direct carcinogen in the colon of wistar rats. Nutr. Cancer. 2019;71(4):615–623. doi: 10.1080/01635581.2018.1506489. [DOI] [PubMed] [Google Scholar]
- 138.Hou N., Liu N., Han J., Yan Y., Li J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs. 2017;28(1):59–65. doi: 10.1097/CAD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 139.Gouthamchandra K., Sudeep H.V., Venkatesh B.J. Shyam Prasad K. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Well. 2017;6(3):147–153. [Google Scholar]
- 140.Refolo M.G., Lippolis C., Carella N., Cavallini A., Messa C., D'Alessandro R. Chlorogenic acid improves the regorafenib effects in human hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018;19(5):1518. doi: 10.3390/ijms19051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yan Y., Li J., Han J., Hou N., Song Y., Dong L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anticancer Drugs. 2015;26(5):540–546. doi: 10.1097/CAD.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yan Y., Liu N., Hou N., Dong L., Li J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017;46:68–73. doi: 10.1016/j.jnutbio.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y., Feng Y., Li Y., Hu Y., Zhang Q., Huang Y., Shi K., Ran C., Hou J., Zhou G., Wang X. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020;11:867. doi: 10.3389/fphar.2020.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen X., Yang J.H., Cho S.S., Kim J.H., Xu J., Seo K., Ki S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020;58(1):999–1005. doi: 10.1080/13880209.2020.1818791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang J.S., Liu C.W., Ma Y.S., Weng S.W., Tang N.Y., Wu S.H., Ji B.C., Ma C.Y., Ko Y.C., Funayama S., Kuo C.L. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. In Vivo (Brooklyn) 2012;26(6):971–978. [PubMed] [Google Scholar]
- 146.Liu Y.J., Zhou C.Y., Qiu C.H., Lu X.M., Wang Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL‑60 cells. Mol. Med. Rep. 2013;8(4):1106–1110. doi: 10.3892/mmr.2013.1652. [DOI] [PubMed] [Google Scholar]
- 147.Rakshit S., Mandal L., Pal B.C., Bagchi J., Biswas N., Chaudhuri J., Chowdhury A.A., Manna A., Chaudhuri U., Konar A., Mukherjee T., Jaisankar P., Bandyopadhyay S. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem. Pharmacol. 2010;80(11):1662–1675. doi: 10.1016/j.bcp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 148.Bandyopadhyay G., Biswas T., Roy K.C., Mandal S., Mandal C., Pal B.C., Bhattacharya S., Rakshit S., Bhattacharya D.K., Chaudhuri U., Konar A., Bandyopadhyay S. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood. 2004;104(8):2514–2522. doi: 10.1182/blood-2003-11-4065. [DOI] [PubMed] [Google Scholar]
- 149.Skała E., Synowiec E. Rhaponticum carthamoides transformed root extract has potent anticancer activity in human leukemia and lung adenocarcinoma cell lines. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/8198652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sitarek P., Synowiec E., Kowalczyk T. An In vitro estimation of the cytotoxicity and genotoxicity of root extract from Leonurus sibiricus L. overexpressing AtPAP1 against different cancer cell lines. Molecules. 2018;23(8):2049. doi: 10.3390/molecules23082049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skała E., Sitarek P., Toma M., Szemraj J., Radek M., Nieborowska-Skorska M., Skorski T., Wysokińska H., Śliwiński T. Inhibition of human glioma cell proliferation by altered Bax/Bcl-2-p53 expression and apoptosis induction by Rhaponticum carthamoides extracts from transformed and normal roots. J. Pharm. Pharmacol. 2016;68(11):1454–1464. doi: 10.1111/jphp.12619. [DOI] [PubMed] [Google Scholar]
- 152.Sitarek P., Skała E., Toma M., Wielanek M., Szemraj J., Nieborowska-Skorska M., Kolasa M., Skorski T., Wysokińska H., Śliwiński T. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2-p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumour Biol. 2016;37(7):8753–8764. doi: 10.1007/s13277-015-4714-2. [DOI] [PubMed] [Google Scholar]
- 153.Skała E., Kowalczyk T., Toma M., Szemraj J., Radek M., Pytel D., Wieczfinska J., Wysokińska H., Śliwiński T., Sitarek P. Induction of apoptosis in human glioma cell lines of various grades through the ROS-mediated mitochondrial pathway and caspase activation by Rhaponticum carthamoides transformed root extract. Mol. Cell. Biochem. 2018;445(1–2):89–97. doi: 10.1007/s11010-017-3254-z. [DOI] [PubMed] [Google Scholar]
- 154.Sitarek P., Skała E., Toma M., Wielanek M., Szemraj J., Skorski T., Białas A.J., Sakowicz T., Kowalczyk T., Radek M., Wysokińska H., Śliwiński T. Transformed root extract of leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol. Oncol. Res. 2017;23(3):679–687. doi: 10.1007/s12253-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 155.Skała E., Toma M., Kowalczyk T., Śliwiński T., Sitarek P. Rhaponticum carthamoides transformed root extract inhibits human glioma cells viability, induces double strand DNA damage, H2A.X phosphorylation, and PARP1 cleavage. Cytotechnology. 2018;70(6):1585–1594. doi: 10.1007/s10616-018-0251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sitarek P., Kowalczyk T., Santangelo S., Białas A.J., Toma M., Wieczfinska J., Śliwiński T., Skała E. The extract of Leonurus sibiricus transgenic roots with AtPAP1 transcriptional factor induces apoptosis via DNA damage and down regulation of selected epigenetic factors in human cancer cells. Neurochem. Res. 2018;43(7):1363–1370. doi: 10.1007/s11064-018-2551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sapio L., Salzillo A., Illiano M., Ragone A., Spina A., Chiosi E., Pacifico S., Catauro M., Naviglio S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020;235(4):3741–3752. doi: 10.1002/jcp.29269. [DOI] [PubMed] [Google Scholar]
- 158.Jin S., Kim K.C., Kim J.S. Anti-melanoma activities and phytochemical compositions of Sorbus commixta fruit extracts. Plants. 2020;9(9):1076. doi: 10.3390/plants9091076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X., Liu J., Xie Z., Rao J., Xu G., Huang K., Li W., Yin Z. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019;71(7):1100–1109. doi: 10.1111/jphp.13095. [DOI] [PubMed] [Google Scholar]
- 160.Lu C.H., Chen W.T., Hsieh C.H., Kuo Y.Y., Chao C.Y. Thermal cycling-hyperthermia in combination with polyphenols, epigallocatechin gallate and chlorogenic acid, exerts synergistic anticancer effect against human pancreatic cancer PANC-1 cells. PLoS ONE. 2019;14(5) doi: 10.1371/journal.pone.0217676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. CellCell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 164.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. NatureNature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 165.Coffman L.G., Pearson A.T., Frisbie L.G., Freeman Z., Christie E., Bowtell D.D., Buckanovich R.J. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells. 2019;37(2):257–269. doi: 10.1002/stem.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vuoso D.C., D'Angelo S., Ferraro R., Caserta S., Guido S., Cammarota M., Porcelli M., Cacciapuoti G. Annurca apple polyphenol extract promotes mesenchymal-to-epithelial transition and inhibits migration in triple-negative breast cancer cells through ROS/JNK signaling. Sci. Rep. 2020;10(1):15921. doi: 10.1038/s41598-020-73092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu J.K., Yue C.H., Pan Y.R., Chiu Y.W., Liu J.Y., Lin K.I., Lee C.J. Isochlorogenic acid C reverses epithelial-mesenchymal transition via down-regulation of EGFR pathway in MDA-MB-231 cells. Anticancer Res. 2018;38(4):2127–2135. doi: 10.21873/anticanres.12453. [DOI] [PubMed] [Google Scholar]
- 168.Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22(6):699–701. doi: 10.1016/j.ccr.2012.11.009. [DOI] [PubMed] [Google Scholar]