Summary
The frontal cortex is essential for organizing voluntary movement. The secondary motor cortex (MOs) is a frontal subregion thought to integrate internal and external inputs before motor action. However, how excitatory and inhibitory synaptic inputs to MOs neurons are integrated preceding movement remains unclear. Here, we address this question by performing in vivo whole-cell recordings from MOs neurons of head-fixed mice moving on a treadmill. We find that principal neurons produce slowly increasing membrane potential and spike ramps preceding spontaneous running. After goal-directed training, ramps show larger amplitudes and accelerated kinetics. Chemogenetic suppression of interneurons combined with modeling suggests that the interplay between parvalbumin-positive (PV+) and somatostatin-positive (SOM+) interneurons, along with principal neuron recurrent connectivity, shape ramping signals. Plasticity of excitatory synapses on SOM+ interneurons can explain the ramp acceleration after training. Altogether, our data reveal that local interneurons differentially control task-dependent ramping signals when MOs neurons integrate inputs preceding movement.
Keywords: frontal cortex, motor preparation, in vivo patch clamp, parvalbumin-expressing interneuron, somatostatin-expressing interneuron, virtual reality, go/no-go task
Graphical abstract

Highlights
-
•
MOs principal neurons show slow membrane potential and spike ramps before running
-
•
Membrane potential and spike ramps are faster and larger after goal-directed training
-
•
Inactivation of PV+ and SOM+ interneurons differentially affects ramping signals
-
•
Modeling suggests that external inputs and local inhibition shape prerunning ramps
The frontal cortex is essential for organizing voluntary movement. Zhang et al. show that frontal principal neurons produce slowly increasing ramps of subthreshold and spiking activity before running onset. Learning a task accelerates these signals. Specific subpopulations of interneurons play distinct roles in controlling the ramps and shaping their task dependence.
Introduction
The secondary motor cortex (MOs or M2) is thought to be an essential hub for guiding motor action (Barthas and Kwan, 2017; Erlich et al., 2011; Murakami et al., 2014). It receives inputs from numerous sensory cortical and thalamic sources and projects along the corticospinal tract to the spinal cord and superior colliculus to drive movement output (Donoghue and Wise, 1982; Gabbott et al., 2005). Network connectivity, inactivation experiments, and population recordings suggest that MOs integrates multisensory inputs and organizes motor output during voluntary action (Barthas and Kwan, 2017; Coen et al., 2021).
What are the neuronal correlates of this integration process? In several neural systems, ramp-like signals have been proposed to represent a typical signature of slow integration processes (Mehta et al., 2002; Schmidt-Hieber and Nolan, 2017; Yartsev et al., 2018). Neurons in several fronto-parietal brain regions of rodents and primates, including MOs, also show gradually increasing ramps of spiking activity that reach a threshold level just before the onset of movement (Chen et al., 2017; Hanes and Schall, 1996; Inagaki et al., 2019; Li et al., 2015; Maimon and Assad, 2006; Quintana and Fuster, 1999; Roitman and Shadlen, 2002; Thura and Cisek, 2014). It can occur concurrently in multiple areas within the fronto-parietal cortices (Erlich et al., 2015; Goard et al., 2016). Interactions between several brain regions, including thalamus and cerebellum, contribute to producing and maintaining this ramping neural activity in frontal cortices (Dacre et al., 2021; Gao et al., 2018; Tanaka, 2007).
What is the synaptic basis of these integration processes in frontal cortices preceding motor action? Although their circuit mechanisms have been extensively studied by extracellular recordings, it is unclear how excitatory and inhibitory synaptic inputs are integrated by individual neurons preceding motor action (Goldman-Rakic, 1995, Verduzco-Flores et al., 2009). The frontal cortex is characterized by a layered structure containing numerous types of excitatory and inhibitory neurons (DeFelipe and Fariñas, 1992; Kawaguchi and Kubota, 1997). Parvalbumin-positive (PV+) and somatostatin-positive (SOM+) interneurons are two principal subtypes of cortical γ-aminobutyric acid-ergic (GABAergic) neurons that differ in morphology, physiological properties, and targeting of principal neurons (Hangya et al., 2014; Hu et al., 2014; Rudy et al., 2011). Recent intracellular recordings have provided evidence that the interplay of inhibition from these interneurons, excitatory synaptic inputs, and intrinsic membrane properties governs the subthreshold membrane potential (ΔVm) dynamics during different locomotor states in several neocortical regions (Gentet et al., 2010; Polack et al., 2013; Schneider et al., 2014). These findings point toward a critical role for synaptic integration of excitatory and inhibitory inputs in shaping neuronal signals preceding motor action in the frontal cortex.
Here we sought to identify how synaptic inputs are integrated during premovement neuronal activity by performing in vivo whole-cell patch-clamp recordings from MOs principal neurons in awake mice during resting and running on a treadmill. We find that MOs neurons exhibit slowly depolarizing membrane potential ramps (∼10 s) preceding the onset of spontaneous movement in both superficial and deep neurons. In spiking neurons, membrane potential ramps are accompanied by slow firing rate ramps with similar dynamics. In animals trained in a goal-directed go/no-go task in a virtual-reality (VR) environment, these membrane potential and spike rate ramps are accelerated preceding movement onset. To assess the role of different interneuron subpopulations involved in premovement activity in MOs, we chemogenetically suppressed the activity of local PV+ or SOM+ cells in MOs while recording from principal neurons during spontaneous movement periods, unveiling distinct roles for different subtypes of interneurons in shaping task-dependent membrane potential and firing rate ramps preceding the onset of movement.
Results
To explore neuronal dynamics preceding movement onset across different behavioral tasks, we used a setup adapted for rodent head-fixed navigation. Two groups of mice were subject to different behavioral paradigms: a control group of animals performed self-paced spontaneous movement on a treadmill in a dark environment after a brief habituation period, whereas another group of animals was trained in a goal-directed behavioral task in a VR environment (Figure 1A; see STAR Methods). This latter group of animals learned to stop in a reward zone at the end of a linear VR corridor within ∼6 days of training, as quantified, for example, by increased reward and success rates (Figures 1B and 1C). To establish the role of MOs in this goal-directed task, we inactivated MOs by bilateral local infusion of muscimol (Figures 1E–1L; see STAR Methods) and found that muscimol application significantly and reversibly reduced behavioral performance, such as reward and success rates, suggesting a specific role for MOs in the goal-directed behavioral task (Figures 1D, 1E, 1G, and 1H). We further analyzed the motor behavior by comparing the durations and frequencies of running and resting periods. Inactivation of MOs led to longer resting periods, whereas running periods were reduced in frequency, but not in duration (Figures 1I–1L). These results are consistent with the interpretation that MOs controls running initiation during the goal-directed task (Barthas and Kwan, 2017; Coen et al., 2021).
Figure 1.
Role of MOs in a goal-directed behavioral VR task
(A) Top, illustration of the VR environment. Bottom, schematic drawing of the goal-directed go/no-go task.
(B and C) Training performance quantified as the reward rate (B), or dispensed rewards per completed lap, and the success rate (C), or successful licks (hits) per dispensed reward. In each panel, the left graph shows training performance across days, and the right graph compares training performance on day 1 versus day 6 (reward rate: day 1, 0.31 ± 0.04 rewards/lap, versus day 6, 0.47 ± 0.05 rewards/lap; success rate: day 1, 0.31 ± 0.05 hits/reward, versus day 6, 0.55 ± 0.05 hits/reward; n = 20 mice).
(D and E) Example training sessions from the same mouse under control conditions (D) and after muscimol application the next day (E). Top traces show animal speed, bottom traces show animal position on the virtual-reality track, green drops indicate dispensed rewards, and blue triangles indicate licks. The reward zone is located between 1.0 and 1.1 m along the track (green-shaded region).
(F) Fluorescent marker (bodipy, 350 nL per site) was injected bilaterally at the same coordinates as muscimol (0.6 μg/μL, 350 nL per site). Left, coronal sections at different rostro-caudal levels from an example animal. Right, cannula tip positions (bilateral injections into n = 5 mice, indicated in a single hemisphere as red circles) and example coronal section showing fluorescence signal where bodipy and muscimol were injected.
(G and H) Summary of performance in the goal-directed task before, during, and after inactivation of MOs, as quantified by the reward rate (G) and the success rate (H) (reward rate: day before, 0.46 ± 0.14 rewards/lap, versus muscimol, 0.07 ± 0.02 rewards/lap, versus day after, 0.37 ± 0.17 rewards/lap; success rate: day before, 0.49 ± 0.14 hits/reward, versus muscimol, 0.07 ± 0.07 hits/reward, versus day after, 0.48 ± 0.12 hits/reward; n = 5 mice).
(I–L) Summary of the running period frequency (I) and duration (J) and the resting period frequency (K) and duration (L) before, during, and after inactivation of MOs by local muscimol application (running frequency: day before, 2.1 ± 0.4 periods/min, versus muscimol, 1.0 ± 0.2 periods/min, versus day after, 2.3 ± 0.2 periods/min; running duration: day before, 7.0 ± 1.2 s, versus muscimol, 16.0 ± 6.9 s , versus day after, 7.8 ± 0.9 s; resting frequency: day before, 4.3 ± 0.6 periods/min, versus muscimol, 4.2 ± 0.3 periods/min, versus day after, 4.8 ± 0.6 periods/min; resting duration: day before, 4.0 ± 0.3 s, versus muscimol, 7.9 ± 0.5 s, versus day after, 4.7 ± 0.3 s).
Error bars represent SEM. Statistical significance was assessed using repeated-measures ANOVA (B, C, and G–L) and Wilcoxon signed rank tests (B and C) for day 1 versus day 6. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Statistic values are provided in Table S1.
To characterize intrinsic membrane properties of MOs principal neurons, we performed in vivo whole-cell patch-clamp recordings from head-fixed mice of the control group during resting states (Figures 2A–2C). Neurons that met basic recording criteria (n = 47) (see STAR Methods) were split into superficial (150–420 μm) and deep (430–850 μm) recordings according to their depth in MOs (Franklin and Paxinos, 2019) (Figures 2B–2D; see STAR Methods). Consistent with previous in vivo recordings from other neocortical regions (Zhao et al., 2016), superficial MOs neurons (mean recording depth, 303 ± 16 μm; n = 24) differed significantly from deep neurons (547 ± 24 μm; n = 23) in intrinsic membrane properties, with deep neurons showing more depolarized baseline membrane potentials and higher excitability (Figures 2E–2G; details in Table S1).
Figure 2.
Distinct intrinsic membrane properties of superficial and deep MOs principal neurons in vivo
(A) Schematic drawing of the recording setup.
(B) Recording coordinates.
(C) Left, coronal section of frontal cortex indicating the recording region in MOs (labeled by extracellular injection of tdTomato). Right, biocytin-filled MOs principal neurons.
(D) Distribution of recording depths (n = 24 superficial neurons, n = 23 deep neurons).
(E) Deep MOs principal neurons show a more depolarized baseline membrane potential (Vm) compared with superficial principal neurons.
(F) No significant difference in input resistance between deep and superficial MOs principal neurons.
(G) Left, example Vm responses to sustained current injections. Right, relationship between firing rate and current injection (f-I curve). Deep neurons are more excitable than superficial neurons when large currents are injected (comparison between superficial and deep groups: F = 9.94, p = 0.002; comparison of two groups at 300 pA: p = 0.01).
Error bars represent SEM. Statistical significance was assessed using Mann-Whitney tests (E and F) and two-way ANOVA with Bonferroni post hoc tests (G). ns, not significant; ∗p < 0.05; ∗∗p < 0.01. Mean values, SEM, and statistic details are provided in Table S1.
Motion dependence of membrane potential and firing in MOs principal neurons
How does goal-directed behavior affect membrane potential and firing rate dynamics during resting and running states? To address this question, we recorded from MOs neurons in both groups of mice during locomotor behavior (Figure 3A). 29 of the 47 recordings from the control group and all 18 recordings from the trained group reached the criteria for further analysis of motion-related membrane potential dynamics and firing patterns (see STAR Methods). During electrophysiological recordings, our analysis of motor behavior revealed that trained animals ran more frequently and at higher running speeds (Figures 3B–3F). During spontaneous movement, we observed two populations of MOs neurons with distinct firing rate patterns during resting and running periods: most principal neurons (20 of 29 neurons, 70%) exhibited higher firing rates during resting periods, whereas a smaller group of neurons (7 of 29 neurons, 24%) showed higher firing rates during running periods (Figure 3G, left) (resting, 0.50 ± 0.08 Hz, versus running, 0.54 ± 0.24 Hz; n = 29; 2 of 29 neurons were not firing). Notably, in animals trained in the goal-directed task, we observed the opposite trend: more neurons (9 of 18 neurons, 50%) showed higher firing rates during running periods, whereas 8 of 18 neurons (44%) showed lower firing rates during running than resting periods (Figure 3G, right) (resting, 1.21 ± 0.33 Hz, versus running, 2.42 ± 0.80 Hz; n = 18; 1 of 18 neurons was not firing). Detailed analysis of membrane potential dynamics revealed that mean membrane potential was more depolarized during running than resting periods in both groups of animals (Figure 3H). The membrane potential increase between resting and running periods was significantly larger during the goal-directed task (Figure 3I). Across neurons, we did not observe consistent correlation between membrane potential change and animal speed, indicating that state-dependent membrane potential changes cannot be explained by a simple linear relationship with animal speed (Figure S1).
Figure 3.
Differences in subthreshold membrane potential and firing rates between resting and running periods in MOs neurons
(A) Experimental timeline for spontaneously running control mice (top) and mice trained in the goal-directed task (bottom).
(B–D) Comparison of the mean running speeds (B), duration of individual running periods (C), and rate of running periods (D) between recordings from the control and trained groups (mean speed: control, 13.1 ± 1.4 cm/s, versus trained, 21.8 ± 1.7 cm/s; running period duration: control, 10.8 ± 1.4 s, versus trained, 7.7 ± 0.9 s; running frequency: control, 0.6 ± 0.1 periods/min, versus trained, 1.1 ± 0.3 periods/min; n = 29 for control and n = 18 for trained groups). Symbols represent individual recordings.
(E) Example whole-cell recording from a superficial MOs neuron during goal-directed behavior. Traces show (from top) animal speed, Vm, Vm after blanking action potentials, and Vm variance. Vertical dashed lines indicate movement onset.
(F) Same recording as in (E), with data aligned to the onset of running periods at t = 0 s. Traces show (from top) animal speed, Vm, low-pass filtered Vm after blanking action potentials, and Vm variance. Thin traces represent individual running periods, and thick traces represent the mean across running periods. The red trace on top of Vm (second from top) represents the mean of low-pass filtered Vm after blanking action potentials.
(G–J) Summary of firing rates (G), mean Vm (H), membrane potential difference (ΔVm) between running and resting periods (I), and Vm variance (J) during resting (blue) and running periods (red) for recordings from the control group (n = 29 recordings) and from the group of mice trained (n = 18 recordings) in a goal-directed task (H, left, control group: resting, −58.0 ± 1.5 mV, versus running, −56.0 ± 1.5 mV; H, right, trained group: resting, −57.0 ± 1.7 mV, versus running, −51.9 ± 1.8 mV; I: control group, 1.98 ± 0.65 mV, versus trained group, 5.04 ± 0.87 mV; J, left: resting, 26.5 ± 3.0 mV2, versus running, 9.5 ± 2.2 mV2; J, right: resting, 32.2 ± 6.5 mV2, versus running, 9.0 ± 1.2 mV2).
Error bars represent SEM. Statistical significance was assessed using Wilcoxon signed rank tests (G, H, and J) for paired groups and Mann-Whitney tests (B–D and G–J) for unpaired groups. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Mean values, SEM, and statistic details are provided in Table S1. See also Figures S1–S6.
Several previous studies have revealed that the amplitude of membrane potential fluctuations in neocortical neurons decreases promptly upon changes of behavioral states, reflecting a rapid transition from a synchronized to a desynchronized cortical state (Bennett et al., 2013; Churchland et al., 2010a; Eggermann et al., 2014; Polack et al., 2013; Poulet and Petersen, 2008; Poulet et al., 2012; Schiemann et al., 2015; Schneider et al., 2014; Zagha et al., 2013; Zhou et al., 2014). In agreement with these findings, we found that MOs neurons displayed larger subthreshold membrane potential fluctuations during resting periods compared with running periods in the control group (Figure 3J). This decrease in membrane potential fluctuation amplitude was also observed during goal-directed running, similar to the untrained group of animals (Figures 3E, 3F, and 3J). These fluctuations contained broadband frequency components without any obvious peak in the spectrum before and during movement (Figure S2). Thus, our data indicate that membrane potential fluctuations in MOs neurons rapidly transition from large to small amplitudes, reflecting a change to a desynchronized low-variability state upon movement onset (Churchland et al., 2010a) (Figures 3E, 3F, 3J, and S2).
Temporal dynamics of ramping signals preceding the onset of movement
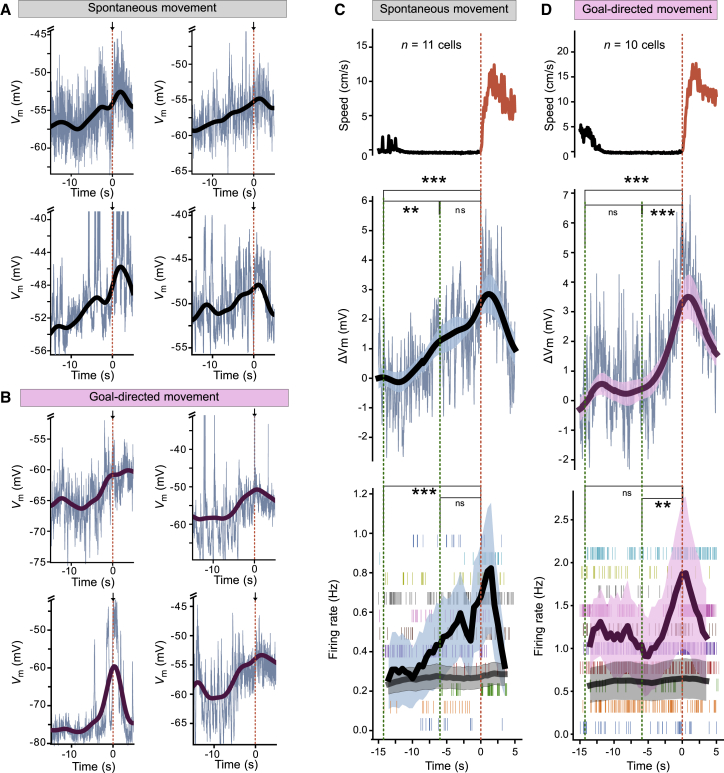
To characterize the membrane potential dynamics underlying premovement spiking activity, we analyzed recordings with sufficiently long recording periods before and after onset of movement from neurons spontaneously spiking preceding running periods (n = 11 of 29 recordings matching criteria) (Figure 4; see STAR Methods). Changes in ΔVm and firing rates were aligned to the onset of spontaneous running periods of untrained animals (Figures 4A and 4C). This analysis revealed that during spontaneous movement, subthreshold membrane potential displayed gradual depolarization (∼10 s) preceding running onset (Figure 4A, individual examples; Figure 4C, summary data). Simultaneously, firing rates averaged across different animals slowly and gradually increased preceding onset of movement (Figure 4C). To probe whether signatures of these depolarizing membrane potential ramps could also be found in firing patterns of larger neuronal populations preceding motor action, we performed extracellular recordings of MOs population activity with a Neuropixels probe from one untrained mouse (Figures S3A and S3B). These recordings revealed firing rate ramps in putative principal neurons preceding spontaneous movement with temporal dynamics similar to those observed in our whole-cell recordings (Figures S3C and S3D). In particular, a steady increase in premotion firing rates could be observed in sparsely firing neurons (Figure S3E) that matched the mean firing rates observed during whole-cell recordings (Figure 4C).
Figure 4.
Task dependence of membrane potential and firing rate dynamics preceding movement onset
(A and B) Example whole-cell recordings from different MOs neurons preceding movement onset at t = 0 s (indicated by a red vertical dashed line and an arrow). Thin blue traces represent raw membrane potential, and thick traces represent low-pass filtered membrane potential after blanking action potentials. Note slower dynamics of depolarizing ramps in control animals (A) compared with trained animals (B).
(C and D) Summary of speed, membrane potential, and firing rate dynamics preceding movement onset across control animals (C, n = 11 recordings) and trained animals (D, n = 10 recordings). Data are aligned to running onset at t = 0 s (indicated by a red vertical dashed line). Top, mean animal speed. Middle, mean membrane potential (thin traces) and mean low-pass filtered membrane potential (thick traces). Bottom, thick traces represent mean spike firing rates (black) and mean of shuffled firing rates (gray, n = 100 shuffles). Shaded regions represent mean ± SEM. Ticks represent spike firing, with all running periods of a single recording shown in a single row.
Statistical significance was assessed by Spearman’s correlation (C and D). ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Statistic details are provided in Table S1. See also Figures S3, S4, and S6.
To probe how goal-directed training affects synaptic integration during the transition from resting to running, we also analyzed membrane potential and firing rates preceding movement onset in whole-cell recordings from animals trained in the goal-directed task (n = 10 of 18 recordings matching criteria) (see STAR Methods). Strikingly, after training, membrane potential ramps were accelerated (∼6 s) (Figure 4B, individual examples; Figure 4D, summary data). Simultaneously, we observed faster spike ramps with a larger amplitude preceding movement onset in these recordings (Figure 4D). Thus, the dynamics of both sub- and suprathreshold ramping activity appears to depend on the nature of the behavioral task.
Previous work has suggested that MOs integrates multisensory information before motor action (Barthas and Kwan, 2017; Coen et al., 2021). Whisker motion constitutes a readily observable manifestation of sensory integration. We therefore captured whisker movements of animals across several sessions during goal-directed training in the VR environment (Figure S4A). Inspection of the training videos showed that whisker movement preceded ∼50%–60% of running periods (Figures S4B and S4C; see STAR Methods). Detailed quantification of these whisker precession times revealed that they depended on the training state of the animal, with longer whisker precession times in untrained compared with trained animals (Figure S4D). Across ∼6 days of training sessions, whisker precession times decreased from ∼9 to ∼6 s (Figure S4E), comparable to the temporal dynamics of membrane potential and firing rate ramps preceding running onset (Figure 4). To explore the role of MOs in processing whisker signals, we analyzed whisker movement when MOs was bilaterally inactivated by muscimol application (Figure 1F). Inspection of the training videos revealed that gross whisker movement was intact when MOs was inactivated (Figures S4F–S4H; see also Figures 1E–1L). Thus, although parts of MOs contribute to controlling whisker movement (Ebbesen et al., 2018), our observations are consistent with the view that the ramps represent integration of multisensory information, including from the whiskers, preceding running onset.
Previous studies have revealed differences in neuronal activity between neurons in superficial and those in deep layers of the frontal motor cortex in a whisker-based motor planning task (Chen et al., 2017; Wagner et al., 2019). Because recordings from superficial and deep neurons in our dataset showed differences in baseline membrane potential and intrinsic membrane properties (Figures 2E–2G), we analyzed superficial and deep recordings separately (Figures S5 and S6). This analysis revealed more pronounced changes in membrane potential and firing rates after behavioral training in deep compared with superficial neurons (Figures S6A–S6C), whereas premovement membrane potential dynamics were comparable across layers (Figures S6D–S6I).
Local inhibitory neurons disinhibit principal neurons and shape membrane potential ramps
Emerging evidence suggests that disinhibition plays a critical role in controlling neuronal activity during diverse behavioral functions (Letzkus et al., 2015; Wolff et al., 2014). To test the role of disinhibition in shaping the membrane potential dynamics of MOs neurons, we chemogenetically suppressed the activity of local PV+ or SOM+ interneurons in MOs (see STAR Methods) while recording from principal neurons during spontaneous movement periods (Figures 5A–5C). In line with previous reports (Jackson et al., 2018), chemogenetic inactivation of PV+ interneurons, but not of SOM+ interneurons, resulted in an increase of basal firing rates and membrane potential fluctuations of MOs principal neurons without affecting the mean membrane potential when animals were resting on the treadmill (Figures 5D–5F). Chemogenetic inactivation of PV+ interneurons, but not SOM+ interneurons, increased the movement-related firing rates of MOs principal neurons during running periods (Figure 5G). When we inactivated PV+ interneurons, we observed that 9 of 14 principal neurons (64%) exhibited higher firing rates during running compared with resting periods (6 of 9 superficial recordings and 3 of 5 deep recordings; details in Table S1). During inactivation of SOM+ interneurons, 6 of 12 principal neurons (50%) exhibited higher firing rates during running periods (6 of 9 superficial recordings and 0 of 3 deep recordings; details in Table S1). However, larger depolarization of principal neurons during transition between resting and running periods was only observed after inactivation of PV+ interneurons, not of SOM+ interneurons (Figures 5H–5I). By contrast, we still observed a decrease of membrane potential fluctuations when either PV+ or SOM+ interneurons were inactivated (Figure 5J). Thus, our results suggest that PV+ and SOM+ interneurons produce differential effects on membrane potential and firing rates of principal neurons during different locomotor states.
Figure 5.
Inactivation of local PV+ and SOM+ interneurons has differential effects on movement-related membrane potential and firing rate dynamics
(A) Schematic drawing of the experimental paradigm. Control recordings are the same as those shown in Figure 3.
(B) Fluorescence image of H4MDi-mCherry-expressing SOM+ interneurons in a coronal slice of MOs.
(C) Example intracellular recording from a MOs neuron during chemogenetic inactivation of PV+ interneurons. Traces show (from top) animal speed, Vm, and Vm variance.
(D–F) Comparison of firing rates (D), mean membrane potential (E), and Vm variance (F) under control conditions (n = 29) and during chemogenetic inactivation of PV+ interneurons (n = 28) or SOM+ interneurons (n = 34) during resting periods. (D) Control conditions represent the same data as shown in Figure 3G (left, rest) (during PV+ inactivation, 0.82 ± 0.11 Hz, versus during SOM+ inactivation, 0.63 ± 0.17 Hz). (E) Control conditions represent the same data as shown in Figure 3H (left, rest) (during PV+ inactivation, −58.1 ± 1.3 mV, versus during SOM+ inactivation, −60.6 ± 1.1 mV). (F) Control conditions represent the same data as shown in Figure 3J (left, rest) (during PV+ inactivation, 45.1 ± 4.3 mV2, versus during SOM+ inactivation, 35.6 ± 3.6 mV2).
(G–J) Comparison of firing rates (G), membrane potential (H), changes in membrane potential (I), and Vm variance (J) during resting periods (blue) and running periods (red) under control conditions (n = 29) and during chemogenetic inactivation of PV+ interneurons (n = 14) or SOM+ interneurons (n = 12). Recordings in (G)–(J) represent recordings in (D)–(F) with running periods. (G) Control conditions represent the same data as in Figure 3G (left) (during PV+ inactivation: resting, 0.86 ± 0.14 Hz, versus running, 2.77 ± 1.02 Hz; during SOM+ inactivation: resting, 0.71 ± 0.33 Hz, versus running, 2.07 ± 1.10 Hz). (H) Control conditions represent the same data as shown in Figure 3H (PV+ inactivation: resting, −60.3 ± 1.4 mV, versus running, −53.0 ± 2.2 mV; SOM+ inactivation: resting, −61.8 ± 1.7 mV, versus running, −58.3 ± 2.7 mV). (I) Control conditions represent the same data as shown in Figure 3I (left) (PV+ inactivation, 7.3 ± 1.4 mV, versus SOM+ inactivation, 3.5 ± 2.0 mV). (J) Control conditions represent the same data as shown in Figure 3J (left) (PV+ inactivation: resting, 39.9 ± 7.2 mV2, versus running, 15.9 ± 4.0 mV2; SOM+ inactivation: resting, 37.2 ± 5.7 mV2, versus running, 23.1 ± 5.8 mV2).
Error bars represent ± SEM. Statistical significance was assessed using Kruskal-Wallis tests (D–F and I), Wilcoxon signed rank tests (G–I and K) for paired groups, and Mann-Whitney tests (G) for unpaired groups. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Mean values, SEM, and statistic details are provided in Table S1. See also Figure S7.
We next sought to assess the differential roles of SOM+ and PV+ cells in shaping membrane potential ramp dynamics preceding movement onset. To consistently compare the effects of inactivation of different interneurons with our control data, we focused only on recordings from spontaneously spiking principal neurons with sufficiently long recording durations before and after running periods (Figure 6) (n = 8 spiking recordings during inactivation of PV+, depth: 338 ± 48 μm; n = 7 spiking recordings during inactivation of SOM+, depth: 359 ± 41 μm) (details in Table S1). We observed that during inactivation of PV+ interneurons, both subthreshold membrane potential and firing rate ramps were preserved without substantial changes in their duration (∼10 s) (Figures 6A and 6C). By contrast, membrane potential and firing rate ramps were abolished by inactivation of SOM+ interneurons (Figures 6B and 6D). When we compared membrane potential ramps between inactivation of interneurons and control recordings, we found that inactivation of PV+ cells increased the amplitude of ramps (Figures 6C and S7A), whereas inactivation of SOM+ interneurons depressed the ramps (Figures 6D and S7B). Notably, before the onset of movement, we observed sustained depolarization of membrane potential in MOs principal neurons during inactivation of PV+ interneurons, but not of SOM+ interneurons, compared with control recordings (Figures S7C and S7D). Thus, our data show that inactivation of PV+ interneurons leads to depolarized, large-amplitude membrane potential ramps during spontaneous running periods that partly resemble those observed after goal-directed training (Figures 4C, 4D, 6C, S7A, and S7C). Moreover, inactivation of SOM+ cells abolishes slow membrane potential ramps (Figure 6D and S7B) without changing the baseline membrane potential (Figure S7D). In addition, extracellular recordings from putative interneurons revealed specific changes in their firing activity both before and after the onset of spontaneous running periods (Figures S3F and S3G). Although these recordings do not distinguish between PV+ and SOM+ interneurons, they generally support the view that interneurons play specific roles in shaping ramping signals during the transition between resting and running states.
Figure 6.
Activity of local PV+ and SOM+ interneurons in MOs differentially shapes subthreshold membrane potential ramps
(A and B) Example whole-cell recordings from different MOs neurons preceding movement onset at t = 0 s (indicated by a red vertical dashed line and an arrow) during chemogenetic inactivation of PV+ interneurons (A) or SOM+ interneurons (B). Thin blue traces represent raw membrane potential, and thick traces represent low-pass filtered membrane potential.
(C and D) Summary of speed, membrane potential, and firing rate dynamics preceding movement onset during chemogenetic inactivation of PV+ interneurons (C, n = 8 recordings) or SOM+ interneurons (D, n = 7 recordings). Data are aligned to running onset at t = 0 s (indicated by a red vertical dashed line). Top, mean animal speed. Middle, mean membrane potential (thin traces) and mean low-pass filtered membrane potential (thick traces). The black dash traces represent the control recordings (n = 11 spiking neurons, same as shown in Figure 4C). Bottom, thick traces represent mean spike firing rates (orange and brown) and mean of shuffled firing rates (gray, n = 100 shuffles). Shaded regions represent mean ± SEM. Ticks represent spike firing, with each row showing a different recording.
Statistical significance was assessed by Spearman’s correlation. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Statistic details are provided in Table S1. See also Figure S7.
Concerted action of external inputs and local inhibition drives task-dependent ramping signals
In cortical circuits, PV+ interneurons mainly exert fast and powerful perisomatic inhibition on principal neurons and other PV+ cells, whereas SOM+ interneurons mainly form inhibitory synapses onto distal dendrites of principal neurons and PV+ cells (Cottam et al., 2013; Hangya et al., 2014; Hu et al., 2014; Pfeffer et al., 2013; Rudy et al., 2011). To obtain a better understanding of the differential role of SOM+ and PV+ interneurons in shaping neuronal dynamics preceding movement onset, we developed a simple model of the local MOs circuit (Figure 7; see details in Table S2). PV+, SOM+, and principal neurons were modeled as rate-based variables. All 3 types of neurons received external thalamocortical input (Ährlund-Richter et al., 2019) that increased step-like in activity preceding movement onset. The SOM+ cell inhibited both the PV+ interneuron and the principal neuron, whereas the PV+ cell mainly inhibited the principal neuron. The principal neuron excited both interneuron units and itself in a recurrent manner. As has previously been shown (Chaudhuri and Fiete, 2016; Economo et al., 2018; Gao et al., 2018; Li et al., 2016), the recurrent connectivity of the principal neuron, in concert with inhibition from PV+ and SOM+ cells, resulted in a conversion of the step-like external input into a slowly increasing and sustained ramp of activity (Figure 7A), which is consistent with our experimental observations (Figures 4 and S6). Inactivation of the SOM+ cell led to disinhibition of the PV+ interneuron and consequently to silencing of the principal neuron, thereby abolishing the ramp (Figure 7B). By contrast, inactivation of the PV+ cell led to disinhibition of the principal neuron, resulting in a ramp with a steeper slope and in increased baseline activity (Figure 7C). Both of these simulation results were qualitatively consistent with our experimental observations of membrane potential dynamics during inactivation of PV+ or SOM+ interneurons (Figures 6 and S7). Our simulations further revealed that increasing excitatory synaptic weights on the SOM+ cell could accelerate the temporal dynamics and amplitude of the ramping activity by changing the SOM+/PV+ activity balance over time (Figure 7D). These simulations show that the interplay between SOM+ and PV+ interneuron activities can explain their roles in shaping the ramping dynamics in principal neurons, in agreement with our experimental findings (Figures 6 and S7). Moreover, our model reveals that potentiation of excitatory synapses onto SOM+ cells changes the balance between SOM+ and PV+ interneuron activities, leading to decreased PV+ activity preceding movement onset and during the running period (Figure 7D). These dynamics can thereby contribute to the acceleration of the ramp that we observe after goal-directed training (Figures 4 and S6).
Figure 7.
Concerted action of external inputs and local SOM+ and PV+ interneurons drives task-dependent ramping signals in a computational model of the local MOs circuit
(A) Top, simple computational model of the local MOs circuit with 3 units: SOM+ interneurons, PV+ interneurons, and principal neurons (PNs). All units receive the same external inputs. Bottom, simulation results. Activity in arbitrary units is plotted against time. External inputs (top) increase step-like at t = −10 s before the onset of movement. Because of their recurrent connectivity in conjunction with inhibitory inputs (middle), pyramidal cells (bottom) respond with a graded slow increase of activity.
(B) Simulation of chemogenetic inactivation of SOM+ interneurons. Absence of SOM+-mediated inhibition of PV+ interneurons leads to an increase in PV+ activity, thereby abolishing the ramp in pyramidal neurons.
(C) Simulation of chemogenetic inactivation of PV+ interneurons. Absence of inhibition from PV+ interneurons leads to an acceleration of ramping activity and to an increase of baseline activity.
(D) Simulation of increasing excitatory synaptic weights on SOM+ interneurons. Increased activation of SOM+ interneurons leads to a change in SOM+/PV+ activity balance over time in favor of SOM+ interneurons, thereby accelerating the ramping activity in principal neurons.
See also Table S2.
Discussion
Using in vivo whole-cell patch-clamp recordings from principal neurons in the secondary motor cortex, we explore how excitatory and inhibitory synaptic inputs are integrated preceding running onset. We observe that both superficial and deep neurons display different neuronal activity dynamics depending on whether the animal was trained to perform a behavioral task: untrained animals show slow (∼10 s) ramps of membrane potential and spike rates preceding spontaneous movement periods, whereas in animals trained to perform a goal-directed task, the dynamics of both membrane potential and spike ramps are faster (∼6 s) and larger in amplitude. At the same time, membrane potential fluctuations rapidly decrease in amplitude upon onset of running, independent of the training state of the animal. To understand how these dynamics are generated at the cellular and circuit levels, we manipulated the activity of specific interneuron subpopulations using chemogenetic tools. Inactivation of PV+ interneurons disinhibits MOs principal neurons and increases the amplitude of membrane potential ramps, whereas inactivation of SOM+ cells abolishes membrane potential ramps. However, local inactivation of PV+ or SOM+ interneurons does not affect the running-related decrease in membrane potential fluctuation amplitude. Therefore, our results suggest that the concerted action of external inputs and local inhibition shapes premovement ramping signals in MOs.
Circuit mechanisms underlying motion-related transitions in subthreshold membrane potential fluctuations
Several mammalian brain regions transition between synchronized and desynchronized regimes when adapting to changes between different behavioral states or responding to different stimuli (Buzsáki and Draguhn, 2004; Churchland et al., 2010a; Harris and Thiele, 2011; Lee and Dan, 2012). Slow subthreshold fluctuations during synchronized states have previously been observed during resting periods in somatosensory, visual, and auditory cortices of head-restrained mice. When animals start to move or attend to a stimulus, these cortical neurons rapidly transition to a desynchronized low-variability state (Bennett et al., 2013; Churchland et al., 2010a; Eggermann et al., 2014; Polack et al., 2013; Poulet and Petersen, 2008; Poulet et al., 2012; Schiemann et al., 2015; Schneider et al., 2014; Zagha et al., 2013; Zhou et al., 2014). Consistently, we observed that most MOs principal neurons showed large subthreshold fluctuations during resting periods and transitioned to a low-fluctuation state during running periods (Figures 3J and S6C). Thus, our results suggest that neurons in MOs evolve from a synchronized to a desynchronized state upon running onset.
Which mechanisms can explain membrane potential fluctuation variability? Membrane potential fluctuations are governed by the interplay between intrinsic membrane properties and excitatory and inhibitory synaptic inputs. Excitatory and inhibitory neurons in the barrel cortex have been shown to affect subthreshold fluctuations during quiet wakefulness (Gentet et al., 2010). In our experiments, chemogenetic inactivation of PV+ interneurons, but not of SOM+ interneurons, results in higher firing rates and increased subthreshold membrane potential fluctuations during resting periods (Figures 5D–5F). Local PV+ cells in the auditory cortex have been suggested to play a role in controlling motion-related membrane potential fluctuation amplitudes (Schneider et al., 2014). However, we observed that chemogenetic suppression of local PV+ interneurons reversed the motion-related firing pattern of principal neurons (Figure 5G) but left the decrease of subthreshold fluctuations upon movement onset unaffected (Figure 5J). Thus, our results support the view that the motion-related decrease in membrane potential fluctuations is driven by a desynchronization of external inputs (Churchland et al., 2010a), rather than by increased activity of local interneurons.
What could be the source of these external inputs? It has been suggested that coordinated activity in a multiregional loop spanning cerebellum, thalamus, and frontal cortex is required for motor action (Gao et al., 2018; , 2017; Wagner et al., 2019). At the anatomical level, frontal cortex projects to the cerebellum via the basal pontine nucleus, and cerebellum projects back to the frontal cortex via the thalamus (Ährlund-Richter et al., 2019; Economo et al., 2018; Gao et al., 2018; Guo et al., 2017; Li et al., 2015). Therefore, thalamic projections to the neocortex play a crucial role in driving cortical sensory processing (Fuster and Alexander, 1971). How do these thalamocortical synapses modulate movement-related membrane potential dynamics? In a goal-directed motor task, silencing thalamic inputs to primary motor cortex blocks characteristic movement-related membrane potential dynamics and movement initiation (Dacre et al., 2021). Similarly, during whisking, membrane potential depolarization and desynchronization of the cortical state are driven by increased thalamic activity (Dacre et al., 2021; Poulet et al., 2012). These previous studies suggest that thalamic inputs may also drive the premovement ramping activity that we observe in MOs as part of a feedback loop, and the concerted action of these external inputs with local inhibition shapes the ramping signals in MOs before onset of movement (Figure 7A).
Information flow in the MOs circuit preceding movement
It has been proposed that MOs is important for integration of multisensory inputs before motor action (Barthas and Kwan, 2017; Coen et al., 2021). Because previous studies have used extracellular recordings, how synaptic inputs are integrated by MOs neurons preceding onset of movement has not been revealed yet. Our intracellular recordings from silent and firing MOs neurons show that both types of cells integrate synaptic inputs to produce slowly depolarizing membrane potential ramps preceding the onset of movement (Figures 4 and S6). In the neocortex, information from the thalamus and cortical areas is transmitted directionally from superficial to deep layers (Bureau et al., 2006; Lübke and Feldmeyer, 2007; Otsuka and Kawaguchi, 2008). Selective activation of deep layer 5 cells by superficial layer 2/3 neurons may facilitate this directional transfer of information (DeFelipe and Fariñas, 1992; Kampa et al., 2006; Otsuka and Kawaguchi, 2008). Superficial layers are thought to be the principal recipients for sensory information (Mao et al., 2011). By contrast, deep neurons in layer 5 of frontal areas produce movement output signals. For example, deep neurons from the anterior-lateral motor region play a distinct role in initiating ramping activity in preparation of movement (Chen et al., 2017, Li et al., 2015). These movement signals are then sent along the thalamus and the corticospinal tract to the spinal cord and superior colliculus to drive movement output in coordination with the cerebellum (Donoghue and Wise, 1982; Economo et al., 2018; Gabbott et al., 2005). We find that both superficial and deep MOs neurons display slowly depolarizing membrane potential ramps preceding movement onset by several seconds (Figures S6D–S6I). However, deep neurons are more depolarized and excitable than superficial neurons (Figures 2E–2G), rendering them more sensitive to changes in their inputs. Accordingly, neuronal spiking dynamics are more strongly affected by the training state of the animal in deep cells compared with superficial cells, because we observe a pronounced increase of firing rates only in deep neurons after behavioral tasks (Figure S6A). Altogether, our results support the view that excitatory synaptic inputs are processed in a feedforward manner from superficial to deep layers, resulting in task-dependent output from deep cortical neurons that exerts top-down influences in information processing (Gilbert and Sigman, 2007).
Inhibitory role of PV+ interneurons in premovement ramping signals
After goal-directed training, MOs neurons were more depolarized before and after onset of movement compared with neurons from spontaneously running animals (Figures S6F and S6I). At the same time, the mean membrane potential during resting periods was not significantly different between the two groups (Figure 3H). How can we explain the sustained seconds-long depolarization preceding onset of movement? Our chemogenetic experiments indicate that inactivation of PV+ interneurons, but not of SOM+ interneurons, results in large and persistent depolarization of principal neurons preceding movement onset and during running (Figure S7C), without changing the mean membrane potential during resting periods (Figure 5E). In addition, we find that inactivation of PV+ cells substantially increases membrane potential fluctuation amplitudes and firing rates during resting periods (Figures 5D and 5F). Furthermore, inactivation of PV+ interneurons, but not of SOM+ interneurons, drives membrane potential toward the threshold before the onset of running (Figures 5H and 5I), resulting in a higher firing rate during the subsequent running periods (Figure 5G). Thus, a reduction of PV+ interneuron activity during running periods in trained animals can explain several key aspects of our data. Studies in other brain regions support our conclusions: in the barrel cortex, the firing of PV+ cells dominates during quiet wakefulness (Gentet et al., 2010). Similarly, in other neocortical regions, a reduction of PV+ interneuron activity has been shown to affect membrane potential dynamics and neuronal firing during running states (Polack et al., 2013; Schneider et al., 2014). By contrast, inactivation of SOM+ cells leads to more heterogeneous effects on principal neurons: overall, membrane potential and firing rates during movement show fewer changes than during PV+ interneuron inactivation (Figures 5D, 5E, and 5G–5I).
Disinhibitory role of SOM+ interneurons in shaping membrane potential ramps
PV+ interneurons densely target the perisomatic domains of principal neurons across cortical areas and layers (Packer and Yuste, 2011). By contrast, SOM+ cells densely target the tuft, apical, and basal dendrites of principal neurons in a layer-specific manner (Fino and Yuste, 2011; Wang et al., 2004). Converging evidence suggests that PV+ cells strongly inhibit one another without inhibiting other interneuron subtypes, whereas SOM+ strongly inhibit PV+ interneurons across all layers without inhibiting themselves (Ährlund-Richter et al., 2019; Cottam et al., 2013; Pfeffer et al., 2013). Such a pattern of connectivity might define their distinct roles in shaping neural dynamics in the MOs neuronal network: a general and unimodal inhibitory role for PV+ interneurons and a specific and cross-modal role in experience-dependent plasticity for SOM+ cells (Figure 7). For example, recent evidence has shown that such SOM+ interneuron-mediated inhibition of PV+ cells may be important to disinhibit MOs principal neurons during encoding of cue associations in an associative fear learning task (Cummings and Clem, 2020) and to synchronize network activity in the prefrontal cortex during fear expression and social discrimination (Courtin et al., 2014; Scheggia et al., 2020). If activation of SOM+ cells was exclusively and unimodally providing dendritic inhibition to principal neurons, their inactivation should result in depolarization of excitatory neurons. By contrast, we observe that inactivation of SOM+ interneurons abolishes membrane potential ramps without affecting the mean baseline membrane potential (Figures 6D, S7B, and S7D). We therefore suggest that SOM+ cells disinhibit principal neurons via inhibition of PV+ interneurons, resulting in a slow depolarizing membrane potential ramp preceding the onset of movement (Figure 7). In agreement with our suggestion, other studies have shown that during maintenance of working memory, the activity of PV+ interneurons in medial prefrontal cortex is reduced during delay periods and strongly inhibited during reward-taking periods. By contrast, SOM+ cells show strong activation during delay periods (Kim et al., 2016a). The role of additional types of interneurons, such as vasoactive intestinal peptide (VIP)-expressing cells, shown to act mainly through indirect disinhibition of principal neurons via inhibition of SOM+ and PV+ cells (Koukouli et al., 2017; Lee et al., 2013; Pi et al., 2013), remains to be explored.
SOM+ plasticity can explain ramp acceleration after learning a goal-directed task
Our experiments and computational modeling suggest that PV+ and SOM+ interneurons play a key role in producing faster membrane potential ramps with larger amplitudes preceding onset of goal-driven movement (Figure 4D). Inactivation of PV+ interneurons during spontaneous movement results in depolarized membrane potential ramps with larger amplitudes, thereby reproducing some features of the ramps after goal-directed training. However, PV+ inactivation fails to reproduce the ramp acceleration that we observe before onset of goal-directed movements (Figures 6C and S7A). To fully capture all dynamics of ramping premovement signals after goal-directed training, we therefore suggest that excitatory inputs to SOM+ interneurons undergo task-specific experience-dependent plasticity . In agreement with this suggestion, other studies have shown that excitatory synaptic inputs targeting prefrontal SOM+ cells are potentiated after cue fear acquisition, thereby boosting their efficacy of disinhibition of principal neurons via potent inhibition of PV+ cells (Cummings and Clem, 2020). A similar mechanism could explain our observations: our modeling suggests that after goal-directed tasks, potentiated activity of SOM+ cells might exert more efficient inhibition of PV+ interneurons just before and during running periods, resulting in faster and larger depolarizing membrane potential ramps (Figure 7D).
Temporal dynamics of membrane potential ramp signals preceding motor action
As neural transmission within isolated neurons and circuits shows intrinsic time constants on the scale of milliseconds, how can ramping activity persist during several seconds preceding movement onset? Experiments and computational modeling have shown that sustained ramping activity can result from neuronal modules that integrate transient inputs (Murakami et al., 2014). Furthermore, the robustness of the ramping activity to large transient perturbations suggests that the network dynamics of these integrator modules in the cerebellar-thalamic-frontal network is independent, redundant, and coupled through feedback connections during motor preparation (Chaudhuri and Fiete, 2016; Economo et al., 2018; Gao et al., 2018; Inagaki et al., 2019; Li et al., 2016; Murakami et al., 2014). Preparatory ramping signals are thought to be initiated in frontal motor regions, from which they enter the loop and evolve during several seconds preceding motor action (Churchland et al., 2010b; Gao et al., 2018; Li et al., 2015), regardless of how movements are initiated (Lara et al., 2018). Altogether, these studies propose that multicircuit mechanisms for maintaining ramping activity underlie the role of motor-associated cortices in the temporal organization of motor behaviors (Svoboda and Li, 2018). Consistently, our computational modeling shows that recurrent connectivity of MOs principal neurons, in concert with inhibition from PV+ and SOM+ interneurons, can convert a step-like external input into a slowly increasing and sustained ramp of activity (Figures 4, 6, and 7).
Evidence suggests that MOs acts as a multisensory integration hub for adaptive choice behavior (Barthas and Kwan, 2017). The depolarizing ramp that we observe may represent integration of multisensory information predicted by a recent model of the MOs circuit (Coen et al., 2021). Our whole-cell recordings, which sample from neurons without bias for their firing rates, reveal that the synaptic integrative processes underlying the depolarizing ramp may occur at a slower rate than was previously expected, particularly preceding spontaneous running periods. Such slow integration processes may have important implications for the synaptic and circuit basis of decision making.
Limitations of the study
In the present study, we propose a circuit basis for ramping signals preceding movement and hypothesize that they represent integration of external and internal inputs by MOs neurons that control movement onset once a threshold is reached. However, we do not provide causal evidence for this hypothesis. Such a demonstration would require specifically blocking ramping signals during movement preparation and assessing the impact of this manipulation on behavioral performance. A further limitation of our study is that we do not unambiguously record the activity of identified interneuron subpopulations during movement onset. Such recordings could further support the roles that we propose for PV+ and SOM+ interneurons in shaping ramping signals. Furthermore, preparatory ramping signals have traditionally been studied in the context of delayed discrimination tasks. To test whether the proposed integration processes may underlie motor preparation signals more generally, it would be necessary to assess how membrane potential and spike ramping signals evolve during training in a go/no-go task and how they depend on specific interneuron subpopulations (Gao et al., 2018; Inagaki et al., 2019; Murakami et al., 2014). Finally, to understand the unique contribution of MOs to multisensory integration processes preceding motion, it would be interesting to compare premotor signals in MOs with those in neighboring regions, such as medial prefrontal cortex or primary motor cortex.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| AAV5.hSyn.DIO.hM4D(Gi).mcherry | Addgene | 44362-AAV5 |
| AAV1-CAG-FLEX-tdTomato-WPRE | Addgene | 28306-AAV1 |
| AAV1-CAG-tdTomato-WPRE | Addgene | 59462-AAV1 |
| Chemicals, peptides, and recombinant proteins | ||
| Biocytin | Sigma-Aldrich | B4261 |
| Potassium methanesulfonate | Sigma-Aldrich | 83000 |
| Potassium chloride | Sigma-Aldrich | P9333 |
| Sodium chloride | Sigma-Aldrich | S9888 |
| Magnesium chloride solution | Sigma-Aldrich | M1028 |
| Calcium chloride solution | Sigma-Aldrich | 21115 |
| HEPES | Sigma-Aldrich | H3375 |
| EGTA | Sigma-Aldrich | E3889 |
| Adenosine 5′-triphosphate disodium salt hydrate | Sigma-Aldrich | A26209 |
| Guanosine 5′-triphosphate sodium salt hydrate | Sigma-Aldrich | G8877 |
| Phosphocreatine disodium salt hydrate | Sigma-Aldrich | P7936 |
| BODIPY TMR-X | Invitrogen | D6117 |
| Clozapine N-oxide dihydrochloride | Tocris Biosciences | Cat. No. 6329 |
| Muscimol | Tocris Biosciences | Cat. No. 0289 |
| Deposited data | ||
| Analyzed data | This paper | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | Janvier Labs | SC-C57J-M4S |
| Mouse: PV-CRE | Jackson Laboratories | 013044 |
| Mouse: SOM-CRE | Jackson Laboratories | 008069 |
| Software and algorithms | ||
| Python | https://www.python.org/ | N/A |
| Blender | https://www.blender.org/ | N/A |
| Prism | https://www.graphpad.com/ | N/A |
| SpikeGLX | https://billkarsh.github.io/SpikeGLX/ | N/A |
| Kilosort 2 | https://github.com/MouseLand/Kilosort | N/A |
| DeepLabCut | https://github.com/DeepLabCut/DeepLabCut | |
| Phy | https://github.com/cortex-lab/phy | N/A |
| Simulation code | https://zenodo.org/record/5625822 | 10.5281/zenodo.5625822 |
| Other | ||
| Head post for head-fixed animals | Luigs & Neumann | 200-200 500 2133-1-11-20 |
| Neuropixel 1.0 | IMEC (https://www.neuropixels.org/) | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Christoph Schmidt-Hieber (christoph.schmidt-hieber@pasteur.fr).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Mice
All procedures were carried out in accordance with the national guidelines on the ethical use of animals of EU Directive 2010/63/EU and were approved by the Ethics Committee CETEA of the Institut Pasteur (APAFIS#7771-2016112516084126 v1 and APAFIS#6349-2016052416089693 v2). 6- to 12-week-old wild-type (WT) C57BL/6J and transgenic mice were maintained at our animal facility on a regular 12/12h light-dark cycle with ad libitum access to food and water. The following Cre mouse lines were obtained from the Jackson Laboratories: SST-Cre (no: 013044) and PV-Cre (no: 008069). These mice were backcrossed onto a C57BL/6J background. Stereotaxic injections were performed in 6- to 8-week-old male mice.
Method details
Surgical procedures and viral vector transduction
Surgeries were performed under continuous anesthesia with isoflurane (5% for induction, 1%–3% for maintenance, vol/vol). Preceding the surgery, mice were treated with buprenorphine (0.1 mg/kg i.p.) and lidocaine (0.4 ml/kg of a 1% solution, local application). Mice were positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A half-circle stainless steel headpost (Luigs & Neumann) was fixed to the mouse skull using dental cement (Super-Bond, Sun Medical Co. Lt). Animals were allowed to recover for 2 weeks after head post implantation. Body temperature was monitored and maintained at 37°C by placing the animals on a heating pad during and after the surgery. Animals were treated with metacam (1 mg/kg i.p.) before returning them to their home cages.
Circular craniotomies (0.5 mm diameter) were performed above the MOs under isoflurane anesthesia 1h before the onset of recordings using a dental drill (stereotaxic coordinates from Bregma, anteroposterior [AP] +2.7-3.1 mm, mediolateral [ML] ± 0.4-1.0 mm). Animals were treated with an injection of metacam (1 mg/kg i.p.) at the end of the procedure, and then transferred to the recording setup.
To suppress the activity of PV+ or SOM+ interneurons, an adeno-associated viral vector (AAV5-hSyn-DIO-hM4D(Gi)-mCherry, ref Addgene-44362, 7E12 vector genomes (vg)/ml) was injected into the MOs of either PV-Cre or SOM-Cre mice. The adeno-associated viral vectors (AAV1-CAG-FLEX-tdTomato-WPRE, ref Addgene-28306, 1E13 vector genomes (vg)/ml) and (AAV1-CAG-tdTomato-WPRE, ref Addgene-59462, 5E12 vector genomes (vg)/ml) were used as control viruses. 6- to 8-week-old mice were injected with vectors (300∼400 nl per site) into the MOs region (stereotaxic coordinates from Bregma, anteroposterior [AP] +2.7∼3.1 mm, mediolateral [ML] ± 0.4∼0.6 mm, 2 injections at 300 μm and 500 μm depth from dura). The virus was bilaterally pressure-injected through glass pipettes (Drummond Wiretrol 10μl) using an oil-hydraulic micromanipulator (MO-10, Narishige, Japan) at a rate of 100 nl/min. Headpost implantation was performed 3 weeks after the injections. Before the recordings were performed, CNO (Tocris Biosciences; 5 mg/kg i.p.) was administered to activate the hM4D receptor 30 min prior to recordings (Jackson et al., 2018). All recordings were performed within 3 hours after CNO injection.
Behavioral training and analysis
Two weeks after the headpost implantation, mice were handled 10 minutes per day for 3 days. After this period, head-fixed mice were placed on a cylindrical polystyrene treadmill (20 cm diameter) supported by pressurized air bearings. Cylinder rotation associated with animal locomotion was read out from the surface of the treadmill with a computer mouse (G700s, Logitech, used in wired mode) at a poll rate of 1 kHz. As shown in Figure 1A, all mice were habituated to the treadmill 10-20 min per day for 2 consecutive days. Following habituation, mice were trained 30-45 min per day for 2-3 days to perform self-paced voluntary movement on the treadmill in a dark environment.
Another group of mice was trained in a goal-directed task in a virtual-reality environment. The virtual reality setup was implemented as described previously (Schmidt-Hieber and Häusser, 2013). Briefly, motion on the treadmill was read out as described above and linearly converted to one-dimensional movement along the virtual reality corridor. The virtual environment was projected onto a spherical dome screen (120 cm diameter), covering nearly the entire field of view of the animal, using a quarter-sphere mirror (45 cm diameter) and a projector (Casio XJ-A256) located below the mouse. The virtual linear corridor was 1.2 m long, enriched with objects placed along the linear track and vertical or oblique grating textures on the walls. A reward zone was located near the end (1.0-1.1 m) of the corridor. The Blender Game Engine (https://www.blender.org/) was used in conjunction with the Blender Python API to drive the virtual reality system. Controlled water delivery was used to improve animal motivation during the goal-directed task. At the beginning of experiments, mice were placed under controlled water supply (0.5 g of hydrogel per day, Clear H2O, BioService) and maintained at ∼85% of their initial body weight over the course of behavioral training and electrophysiology experiments. The welfare and weight of animals were checked and documented on a daily basis. After habituation and water deprivation, mice underwent 6 training sessions, 30-45 min each, over the course of 1 week before recordings (Figures 1A–1C). A drop of sugar water (10 μl, 8 mg/mL sucrose) was dispensed by a spout as a reward if they spent 2 s or more within the reward zone. Animal licking was detected with a piezo element attached to the reward spout. A “hit” was detected when the animal performed at least two licks within a period of 2 s before or 2 s after the reward delivery. When the animals reached the end of the linear track, they were “teleported” back to the start of the virtual corridor after crossing a black frontal wall, indicating the end of a lap and the onset of the subsequent one. Behavioral performance of the training group was comparable between different sessions (Figures 1A–1C). Locomotor behavior was analyzed during muscimol application (Figures 1E–1L) and comparable between the trained group and the spontaneously running group (Figures 3B–3D).
To detect whisker and body motion (Figure S4), we filmed the animal using an infrared-sensitive camera (Point Grey CM3-U3-13Y3M-CS) operating at a frame rate of 200 Hz. The animal was illuminated by an array of infrared LEDs. Whisker movements were analyzed with the markerless pose estimation software DeepLabCut (Mathis et al., 2018). The bases and tips of 4 whiskers, i.e., a total of 8 labels, were identified across all captured frames (Figure S4A). To quantify whisker movement, across all captured frames we computed the sum of the Euclidean distances, in units of pixels, that were covered by each of the 8 whisker labels between adjacent frames. A whisker motion index was obtained by z-scoring the data, subtracting any offset, and low-pass filtering at fc ∼0.1 Hz. Onset of whisker movement preceding onset of running were detected where the whisker motion index continuously exceeded 10% of its maximal value within a time window of 15 s before running onset (Figure S4C). During muscimol application, the whisker movement period frequency per second was analyzed (Figures S4G and S4H).
Cannula implantation for muscimol Infusion
To infuse muscimol into MOs, two stainless steel guide cannulae (26 gauge; PlasticsOne, Roanoke, VA) were bilaterally implanted above the MOs (from Bregma position, anteroposterior [AP] +2.6–3.1 mm, mediolateral [ML] ± 0.8–1.3 mm, angled at 25-30° toward medial from vertical; dorsoventral, 0.5 mm). Cannulae were anchored to the skull with dental cement (Super-Bond, Sun Medical Co. Lt). Body temperature was monitored and maintained at 37°C by placing the animals on a heating pad during and after the surgery, and the guides were covered with a dummy cannula to reduce the risk of infection. Mice were allowed to recover during 3-4 weeks from surgery before the start of water restriction, and their well-being and weight were assessed on a daily basis.
An infusion cannula (33 gauge; connected to a 1 μL Hamilton syringe via polyethylene tubing) was inserted through the guide cannula, protruding 0.5 mm, to target the MOs. Muscimol (0.6 μg/μL in saline, 350 nL per site) was infused bilaterally at a rate of 100 nL per min using a motorized pump (Legato 100, Kd Scientific Inc., Hilliston, MA), 45–60 min before behavioral testing. To allow for penetration of the drug, the injector was maintained in position for an additional 3 min after the end of the infusion. Mice were placed back in their home cages at the end of the injection procedure.
To analyze the location and extent of the injections, we injected the fluorophore BODIPY TMR-X into MOs (Invitrogen; 5 mM in PBS 0.1 M, DMSO 40%). After 3 hours, animals were deeply anaesthetized and brains were fixed by intracardiac perfusion with paraformaldehyde (4% in PBS). Slices (50 μm) were cut with a vibratome and imaged using a confocal microscope (Opterra, Bruker). Mice were considered for further analysis if fluorescence signals could be confirmed in MOs (Figure 1F).
In vivo patch-clamp electrophysiology
Whole-cell patch-clamp recordings were performed from head-fixed mice placed on the treadmill as described above. Glass pipettes were pulled from borosilicate glass (∼5 MΩ pipette resistance) and filled with internal solution containing (in mM) 130 potassium methanesulphonate, 7.0 KCl, 0.3 MgCl2, 0.1 EGTA, 10 HEPES, 1 sodium phosphocreatine, 3.0 Na2ATP, 0.3 NaGTP. 5 mg/ml biocytin was added to the internal solution for staining purposes. pH was adjusted to 7.2 with KOH. Osmolarity was 289 mOsm. Whole-cell patch-clamp recordings were obtained using a standard blind-patch approach (Margrie et al., 2002; Schmidt-Hieber and Häusser, 2013). In brief, a high positive air pressure (∼1000 mbar) was applied to the pipettes before slowly lowering them into the dorsal part of the MOs region (Figure 2B) via a small craniotomy (∼500 μm) using a micromanipulator (Luigs & Neumann Mini In Vivo). Recordings were obtained at a depth of 150-420 μm (superficial neurons; typically layers 2/3) or 430-850 μm (deep neurons; typically layers 4–6) from the pial surface (Figure 2D). At a depth of ∼150 μm from the brain surface, the air pressure was decreased to 50∼80 mbar. Seal resistances were always ≫1 GΩ, and access resistances were typically 25∼70 MΩ, with recordings terminated when access resistance exceeded 100 MΩ. Recordings were made in current-clamp mode, and no holding current was applied during recordings.
Membrane potential was low-pass filtered at 10 kHz and acquired at 50 kHz (Intan Technologies CLAMP system). During recordings, a silver/silver chloride reference electrode (0.3 mm diameter) was positioned in an additional small craniotomy close to lambda. An external solution containing (in mM) 150 NaCl, 2.5 KCl, 10 HEPES, 2 CaCl2, and 1 MgCl2 (pH 7.2, 289 mOsm) was perfused on top of the craniotomy through a round plastic chamber (4 mm diameter).
In vivo extracellular electrophysiology
Extracellular recordings of population activity were made using Neuropixels probes (Jun et al., 2017). Probes were lowered into the dorsal part of the MOs region to a depth of ∼2.0 mm measured from the brain surface via a small craniotomy (∼500 μm) at an angle of 15° from vertical using a micromanipulator (Sensapex uMp-4). A silver/silver chloride wire, which was soldered to the external reference of the probes and connected to ground, was positioned in an additional small craniotomy. Recordings were performed from head-fixed mice placed on the treadmill as described above. Electrophysiological data were recorded with SpikeGLX (https://billkarsh.github.io/SpikeGLX/) and signals from the AP channels (0.3-10 kHz bandwidth, sampling rate of 30 kHz) were used for further processing. Spike sorting for unit identification was performed with Kilosort 2 (https://github.com/MouseLand/Kilosort). Identified units were manually curated using Phy (https://github.com/cortex-lab/phy). Putative cell types were assessed based on peak-to-valley ratio and half-valley width of the spike waveforms for each neuron by fitting a Gaussian mixture model (Kim et al., 2016b; Stark et al., 2013). Units with low classification confidence (p < 0.95) were unassigned. The interneuron population was further split based on average firing rate: interneurons with a mean firing rate > 10 Hz were classified as fast-spiking interneurons (Figure S3).
Immunohistochemistry and cell identification
At the end of some recordings, mice were deeply anesthetized with an overdose of ketamine/xylazine (100 mg/kg and 10 mg/kg i.p.) and quickly perfused transcardially with 0.1 M phosphate-buffered saline followed by paraformaldehyde (4% in PBS). Brains were removed from the skull and kept in PFA for at least 24 h. We stained 50-μm-thick parasagittal slices with Alexa Fluor 488–streptavidin to reveal biocytin-filled neurons and patch pipette tracts. We identified neurons as principal cells according to their characteristic electrophysiological signature (Figure 2G), including the presence of frequency adaptation during spike trains, and absence of pronounced afterhyperpolarizations following action potentials (Zhao et al., 2016). Whenever the morphological recovery of recorded neurons was successful, we confirmed this classification using the shape and position of biocytin-filled neurons. In addition, the pipette tract was confirmed to terminate in MOs (Figure 2C).
Quantification and statistical analysis
In vivo whole-cell electrophysiology data analysis
Input resistance was calculated from the steady-state voltage response to a small hyperpolarizing 500-ms current pulse from baseline membrane potential (Figure 2G). Only data from mice that were resting during this period were used (speed < 0.5 cm/s). Baseline membrane potential was measured before current pulse injections at the beginning of the recording. Spontaneous firing rate and membrane potential were measured across recordings with durations exceeding 60 s. To analyze subthreshold membrane potential and its variance, traces were digitally low-pass filtered at 5 kHz and resampled at 10 kHz. Action potentials were then removed by thresholding to determine action potential times and then masking values 2 ms before and 10-20 ms after the action potential peak. Membrane potential oscillations were analyzed by bandpass-filtering membrane potential traces after removal of action potentials (Figure S2). Power spectra were computed from subthreshold membrane potential traces using Hanning windowing over data windows of ∼328 ms. Membrane potential variance time series were computed in rolling time windows with a width of 1000 ms.
Changes in subthreshold membrane potential (ΔVm) were computed by subtracting the mean of subthreshold membrane potential traces after spike removal (see above). To compute movement-aligned mean traces of ΔVm (Figures 4, 6, S6, and S7), we aligned traces to movement periods spanning 15 s before movement onset until 5 s after movement onset. After alignment, we computed the mean of each trace between 15 and 10 s before the onset of movement, and subtracted this baseline from each trace.
Data inclusion criteria
For the analysis of intrinsic membrane properties (Figures 2E–2G), cells that met basic recording criteria (initial access resistance < 70 MΩ, initial baseline membrane potential < –50 mV) from the control group of mice were included (47 neurons). For the analysis of firing rate and Vm dynamics (Figures 3, 4, 5, and 6, and S5–S7), only cells with recording durations exceeding 60 s were included. Typical recordings with animals resting and running on the treadmill lasted 5–10 min, and longer recordings (∼30 min) were occasionally achieved.
Running periods were defined as time intervals when the running speed continuously exceeded 1 cm/s during at least 2 s. This criterion was confirmed by visual inspection of each recording to avoid inclusion of short or fractionated running periods. When we aligned data to the onset of movement (Figures 4, 6, S6, and S7), running periods had to be preceded by resting periods with a duration > 10 s to ensure that the pre-movement period was not contaminated by movement. Furthermore, the recording duration after movement onset had to exceed 5 s. These criteria were applied to 29 recordings during spontaneous movement and 18 recordings during goal-directed movement (Figures 3 and S5), reducing the number of recordings to 23 (12 superficial and 11 deep recordings) for the control group, and 12 (5 superficial and 7 deep recordings) for the trained group of animals (Figure S6). In our analysis of pre-movement spike firing (Figures 4 and 6), additional criteria were applied as we only selected neurons that spontaneously fired spikes during the movement-aligned time period. This selection further reduced the number of recordings to 11 of 23 during spontaneous movement, and 10 of 12 during goal-directed movement (Figure 4). In Figures 6 and S7, we selected recordings with resting periods > 6 s preceding movement onset and recording durations > 5 s after movement onset. This selection further reduced the number of recordings to 8 of 14 during PV+ interneurons inactivation, and 7 of 12 during SOM+ interneurons inactivation (Figure 5).
Spike rates in whole-cell recordings were computed from the time points of action potential peaks (see above) in time bins of 500 ms and filtered using a sliding average with a width of 3 s. Shuffled spike rate data were obtained by generating 100 artificial datasets. Each artificial dataset was created from the original dataset by shifting it circularly by a random number of sampling points. To quantify membrane potential ramps, we computed the mean of ΔVm values for each recording in time bins of 1 s in movement-aligned ΔVm traces (see above). Across all binned values of all recordings, we then computed the Spearman rank correlation to test for significant monotonous increases in membrane potential. To quantify the temporal dynamics of membrane potential and spike ramps, Spearman rank correlations were computed for different time periods preceding onset of movement and during movement, as indicated in the figures and figure legends (Figures 4, 6, S6, and S7).
Statistical analysis
Wilcoxon signed-rank or Mann-Whitney U tests were used to assess the statistical significance of paired or unpaired data as appropriate. For multiple comparisons, we performed Kruskal-Wallis tests and adjusted using Dunn’s correction. In Figures 1B, 1C, 1G–1L, S4D, and S4H, repeated-measures ANOVA was applied. In Figures 2G, S6F, S6I, S7C, and S7D, a two-way ANOVA with factors’ interactions and Bonferroni post hoc tests were used. Spearman rank correlations were computed to assess the significance of ramping dynamics (see above; Figures 4, 6, S6, and S7). Statistics details are listed in Table S1. Tests were considered significant if the p value was < 0.05, otherwise “n.s.” denotes “not significant.” Bar graphs and error bars show mean ± s.e.m..
Computational modeling
To simulate neuronal activity preceding onset of movement, we developed a reduced model of the local MOs circuit consisting of 3 neurons: a SOM+ cell, a PV+ cell, and a principal neuron. The dynamics of the rate-based model neurons was defined by
where τi is the time constant of neuronal integration of neuron i, si and sj are the activities of neurons i and j, dt is the simulation time step, and wji is the synaptic weight of inputs from neuron j to neuron i. f(x) is a threshold function: f(x) = x for x > 0, and f(x) = 0 otherwise. All 3 neurons received input from an external source representing thalamocortical inputs. Weights of synaptic connections between neurons were adjusted to reflect our experimental results (see Table S2). Time constants for neuronal integration τi were set to 20 ms for both interneurons and to 40 ms for the principal neuron. The simulation time step was set to 0.5 ms. Ramping activity preceding the onset of running (t = 0 s) was simulated by increasing the activity of the external inputs in a step-like fashion at t = –10 s. Chemogenetic inactivation of PV+ or SOM+ interneurons were simulated by fixing the activities of the corresponding model neurons to 0 throughout the simulation. Plasticity of SOM+ interneurons was simulated by increasing the excitatory synaptic weights to the SOM+ model interneuron (see Table S2).
Acknowledgments
We thank Ian Duguid and Josh Dacre for comments on the manuscript. We thank Lucile Le Chevalier-Sontag and Claire Lecestre for their technical support. This work was supported by grants from the ERC (StG 678790 NEWRON to C.S.-H., MSCA 800027 FindMEMO to M.A., Human Brain Project SGA2 785907 to J.-P.C. and F.K., and Human Brain Project SGA3 945539 to J.-P.C.), the Pasteur Weizmann Council (to C.S.-H.), the INCEPTION program (Investissement d’Avenir grant ANR-16-CONV-0005 to C.O.), the Fondation pour la Recherche Médicale (EQUIPE 2019 to U.M.), ERA-NET Neuron (iPS&BRAIN to U.M.), and a Pasteur-Roux fellowship to M.A. Schematic drawings in the graphical abstract and in Figure 2 were adopted from https://scidraw.io/ under a creative commons license (CC-BY).
Author contributions
Conceptualization, C.-L.Z., J.-P.C., and C.S.-H.; methodology, C.-L.Z., C.S.-H., F.K., and M.A.; experiments, C.-L.Z.; data analysis and investigation, C.-L.Z., H.-L.K., C.O., and C.S.-H.; computational modeling, C.S.-H.; resources, U.M. and C.S.-H.; writing – original draft, C.-L.Z. and C.S.-H.; writing – review & editing, C.-L.Z., F.K., M.A., C.O., U.M., J.-P.C., and C.S.-H.; supervision, C.S.-H.; funding acquisition, C.S.-H., M.A., J.-P.C., and F.K.
Declaration of interests
The authors declare no competing interests.
Published: November 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.110035.
Contributor Information
Chun-Lei Zhang, Email: chunlei.zhang@pasteur.fr.
Christoph Schmidt-Hieber, Email: christoph.schmidt-hieber@pasteur.fr.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. The DOI is listed in the Key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ährlund-Richter S., Xuan Y., van Lunteren J.A., Kim H., Ortiz C., Pollak Dorocic I., Meletis K., Carlén M. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci. 2019;22:657–668. doi: 10.1038/s41593-019-0354-y. [DOI] [PubMed] [Google Scholar]
- Barthas F., Kwan A.C. Secondary Motor Cortex: Where ‘Sensory’ Meets ‘Motor’ in the Rodent Frontal Cortex. Trends Neurosci. 2017;40:181–193. doi: 10.1016/j.tins.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C., Arroyo S., Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I., von Saint Paul F., Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4:e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R., Fiete I. Computational principles of memory. Nat. Neurosci. 2016;19:394–403. doi: 10.1038/nn.4237. [DOI] [PubMed] [Google Scholar]
- Chen T.-W., Li N., Daie K., Svoboda K. A Map of Anticipatory Activity in Mouse Motor Cortex. Neuron. 2017;94:866–879.e4. doi: 10.1016/j.neuron.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Churchland M.M., Yu B.M., Cunningham J.P., Sugrue L.P., Cohen M.R., Corrado G.S., Newsome W.T., Clark A.M., Hosseini P., Scott B.B., et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat. Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M.M., Cunningham J.P., Kaufman M.T., Ryu S.I., Shenoy K.V. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen P., Sit T.P.H., Wells M.J., Carandini M., Harris K.D. Mouse frontal cortex mediates additive multisensory decisions. bioRxiv. 2021 doi: 10.1016/j.neuron.2023.05.008. 2021.04.26.441250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam J.C.H., Smith S.L., Häusser M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 2013;33:19567–19578. doi: 10.1523/JNEUROSCI.2624-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J., Chaudun F., Rozeske R.R., Karalis N., Gonzalez-Campo C., Wurtz H., Abdi A., Baufreton J., Bienvenu T.C.M., Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Cummings K.A., Clem R.L. Prefrontal somatostatin interneurons encode fear memory. Nat. Neurosci. 2020;23:61–74. doi: 10.1038/s41593-019-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacre J., Colligan M., Clarke T., Ammer J.J., Schiemann J., Chamosa-Pino V., Claudi F., Harston J.A., Eleftheriou C., Pakan J.M.P., et al. A cerebellar-thalamocortical pathway drives behavioral context-dependent movement initiation. Neuron. 2021;109:2326–2338.e8. doi: 10.1016/j.neuron.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J., Fariñas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog. Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Donoghue J.P., Wise S.P. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J. Comp. Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Ebbesen C.L., Insanally M.N., Kopec C.D., Murakami M., Saiki A., Erlich J.C. More than Just a “Motor”: Recent Surprises from the Frontal Cortex. J. Neurosci. 2018;38:9402–9413. doi: 10.1523/JNEUROSCI.1671-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo M.N., Viswanathan S., Tasic B., Bas E., Winnubst J., Menon V., Graybuck L.T., Nguyen T.N., Smith K.A., Yao Z., et al. Distinct descending motor cortex pathways and their roles in movement. Nature. 2018;563:79–84. doi: 10.1038/s41586-018-0642-9. [DOI] [PubMed] [Google Scholar]
- Eggermann E., Kremer Y., Crochet S., Petersen C.C.H. Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep. 2014;9:1654–1660. doi: 10.1016/j.celrep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Erlich J.C., Bialek M., Brody C.D. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich J.C., Brunton B.W., Duan C.A., Hanks T.D., Brody C.D. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. eLife. 2015;4:e05457. doi: 10.7554/eLife.05457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E., Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams. Academic Press; 2019. [Google Scholar]
- Fuster J.M., Alexander G.E. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gabbott P.L.A., Warner T.A., Jays P.R.L., Salway P., Busby S.J. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gao Z., Davis C., Thomas A.M., Economo M.N., Abrego A.M., Svoboda K., De Zeeuw C.I., Li N. A cortico-cerebellar loop for motor planning. Nature. 2018;563:113–116. doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet L.J., Avermann M., Matyas F., Staiger J.F., Petersen C.C.H. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gilbert C.D., Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Goard M.J., Pho G.N., Woodson J., Sur M. Distinct roles of visual, parietal, and frontal motor cortices in memory-guided sensorimotor decisions. eLife. 2016;5:e13764. doi: 10.7554/eLife.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Guo Z.V., Inagaki H.K., Daie K., Druckmann S., Gerfen C.R., Svoboda K. Maintenance of persistent activity in a frontal thalamocortical loop. Nature. 2017;545:181–186. doi: 10.1038/nature22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes D.P., Schall J.D. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hangya B., Pi H.-J., Kvitsiani D., Ranade S.P., Kepecs A. From circuit motifs to computations: mapping the behavioral repertoire of cortical interneurons. Curr. Opin. Neurobiol. 2014;26:117–124. doi: 10.1016/j.conb.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.D., Thiele A. Cortical state and attention. Nat. Rev. Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Gan J., Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Inagaki H.K., Fontolan L., Romani S., Svoboda K. Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature. 2019;566:212–217. doi: 10.1038/s41586-019-0919-7. [DOI] [PubMed] [Google Scholar]
- Jackson J., Karnani M.M., Zemelman B.V., Burdakov D., Lee A.K. Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron. 2018;99:1029–1039.e4. doi: 10.1016/j.neuron.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.J., Steinmetz N.A., Siegle J.H., Denman D.J., Bauza M., Barbarits B., Lee A.K., Anastassiou C.A., Andrei A., Aydın Ç., et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551:232–236. doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa B.M., Letzkus J.J., Stuart G.J. Cortical feed-forward networks for binding different streams of sensory information. Nat. Neurosci. 2006;9:1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim D., Jeong H., Lee J., Ghim J.-W., Her E.S., Lee S.-H., Jung M.W. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron. 2016;92:902–915. doi: 10.1016/j.neuron.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Kim H., Ährlund-Richter S., Wang X., Deisseroth K., Carlén M. Prefrontal Parvalbumin Neurons in Control of Attention. Cell. 2016;164:208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukouli F., Rooy M., Tziotis D., Sailor K.A., O’Neill H.C., Levenga J., Witte M., Nilges M., Changeux J.-P., Hoeffer C.A., et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat. Med. 2017;23:347–354. doi: 10.1038/nm.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara A.H., Elsayed G.F., Zimnik A.J., Cunningham J.P., Churchland M.M. Conservation of preparatory neural events in monkey motor cortex regardless of how movement is initiated. eLife. 2018;7:e31826. doi: 10.7554/eLife.31826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kruglikov I., Huang Z.J., Fishell G., Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus J.J., Wolff S.B.E., Lüthi A. Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Li N., Chen T.-W., Guo Z.V., Gerfen C.R., Svoboda K. A motor cortex circuit for motor planning and movement. Nature. 2015;519:51–56. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Li N., Daie K., Svoboda K., Druckmann S. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532:459–464. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke J., Feldmeyer D. Excitatory signal flow and connectivity in a cortical column: focus on barrel cortex. Brain Struct. Funct. 2007;212:3–17. doi: 10.1007/s00429-007-0144-2. [DOI] [PubMed] [Google Scholar]
- Maimon G., Assad J.A. A cognitive signal for the proactive timing of action in macaque LIP. Nat. Neurosci. 2006;9:948–955. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- Mao T., Kusefoglu D., Hooks B.M., Huber D., Petreanu L., Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie T.W., Brecht M., Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Mathis A., Mamidanna P., Cury K.M., Abe T., Murthy V.N., Mathis M.W., Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018;21:1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- Mehta M.R., Lee A.K., Wilson M.A. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Murakami M., Vicente M.I., Costa G.M., Mainen Z.F. Neural antecedents of self-initiated actions in secondary motor cortex. Nat. Neurosci. 2014;17:1574–1582. doi: 10.1038/nn.3826. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J. Neurosci. 2008;28:11186–11195. doi: 10.1523/JNEUROSCI.1921-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A.M., Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J. Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer C.K., Xue M., He M., Huang Z.J., Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H.-J., Hangya B., Kvitsiani D., Sanders J.I., Huang Z.J., Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P.-O., Friedman J., Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet J.F.A., Petersen C.C.H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Poulet J.F.A., Fernandez L.M.J., Crochet S., Petersen C.C.H. Thalamic control of cortical states. Nat. Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035. [DOI] [PubMed] [Google Scholar]
- Quintana J., Fuster J.M. From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cereb. Cortex. 1999;9:213–221. doi: 10.1093/cercor/9.3.213. [DOI] [PubMed] [Google Scholar]
- Roitman J.D., Shadlen M.N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B., Fishell G., Lee S., Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D., Managò F., Maltese F., Bruni S., Nigro M., Dautan D., Latuske P., Contarini G., Gomez-Gonzalo M., Requie L.M., et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat. Neurosci. 2020;23:47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- Schiemann J., Puggioni P., Dacre J., Pelko M., Domanski A., van Rossum M.C.W., Duguid I. Cellular mechanisms underlying behavioral state-dependent bidirectional modulation of motor cortex output. Cell Rep. 2015;11:1319–1330. doi: 10.1016/j.celrep.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C., Häusser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 2013;16:325–331. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C., Nolan M.F. Synaptic integrative mechanisms for spatial cognition. Nat. Neurosci. 2017;20:1483–1492. doi: 10.1038/nn.4652. [DOI] [PubMed] [Google Scholar]
- Schneider D.M., Nelson A., Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E., Eichler R., Roux L., Fujisawa S., Rotstein H.G., Buzsáki G. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–1276. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr. Opin. Neurobiol. 2018;49:33–41. doi: 10.1016/j.conb.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Cognitive signals in the primate motor thalamus predict saccade timing. J. Neurosci. 2007;27:12109–12118. doi: 10.1523/JNEUROSCI.1873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thura D., Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron. 2014;81:1401–1416. doi: 10.1016/j.neuron.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Verduzco-Flores S., Bodner M., Ermentrout B., Fuster J.M., Zhou Y. Working memory cells’ behavior may be explained by cross-regional networks with synaptic facilitation. PLoS ONE. 2009;4:e6399. doi: 10.1371/journal.pone.0006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M.J., Kim T.H., Kadmon J., Nguyen N.D., Ganguli S., Schnitzer M.J., Luo L. Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell. 2019;177:669–682.e24. doi: 10.1016/j.cell.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Toledo-Rodriguez M., Gupta A., Wu C., Silberberg G., Luo J., Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J. Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S.B.E., Gründemann J., Tovote P., Krabbe S., Jacobson G.A., Müller C., Herry C., Ehrlich I., Friedrich R.W., Letzkus J.J., Lüthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Yartsev M.M., Hanks T.D., Yoon A.M., Brody C.D. Causal contribution and dynamical encoding in the striatum during evidence accumulation. eLife. 2018;7:e34929. doi: 10.7554/eLife.34929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E., Casale A.E., Sachdev R.N.S., McGinley M.J., McCormick D.A. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-J., Kremkow J., Poulet J.F.A. Translaminar Cortical Membrane Potential Synchrony in Behaving Mice. Cell Rep. 2016;15:2387–2399. doi: 10.1016/j.celrep.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Liang F., Xiong X.R., Li L., Li H., Xiao Z., Tao H.W., Zhang L.I. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat. Neurosci. 2014;17:841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. The DOI is listed in the Key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.