Highlights
-
•
Brivaracetam has a more selective action to SV2A binding site than levetiracetam.
-
•
Neuropsychological adverse events improved in 76% of patients that switched.
-
•
An overnight switch is well-tolerated in our relatively small Spanish sample.
Keywords: Brivaracetam, Epilepsy, Levetiracetam, Safety profile, Tolerability, Antiseizure medication
Abstract
Brivaracetam is a newer antiseizure medication than levetiracetam. It has a more selective action on the synaptic vesicle glycoprotein 2A binding site, and it seems to provide a more favorable neuropsychiatric profile. The aim of this study was to assess the safety and tolerability of an overnight switch from levetiracetam to brivaracetam.
This was a retrospective descriptive study including patients with epilepsy treated with levetiracetam, who switched due to inefficacy or previous adverse events (AEs). In total, forty-one patients were included (mean age 40.9 ± 17.8 years, women 48.8%). Focal epilepsy represented 75.6% (n = 31) of patients (structural cause [n = 25], unknown cause [n = 6]). Four patients had idiopathic generalized epilepsy, two had developmental and epileptic encephalopathy and four patients were unclassified. The reason to start brivaracetam was inefficacy in 53.7% (n = 22), AEs in 65.9% (25/27 neuropsychiatric) and both in 19.5% (n = 8). Brivaracetam-related AEs were reported in 24.4%. Neuropsychological AEs associated with the previous use of levetiracetam improved in 76% of patients. Treatment was discontinued in 19.5% patients. Patients’ reported seizure frequency improved, worsened and remained stable in 26.8%, 12.2%, and 61.0% of the cases, respectively.
An overnight switching to brivaracetam is safe and well tolerated. This treatment can improve levetiracetam-related neuropsychiatric AEs.
Introduction
Brivaracetam (BRV) is a selective and high affinity synaptic vesicle 2A (SV2A) ligand, with a ten to thirty-fold increased affinity for SV2A compared to levetiracetam (LEV). BRV reveals efficacy and safety without titration period from the initial dose [1].
Previous studies revealed that 13% of patients treated with LEV presented neuropsychiatric adverse events (AEs) [2]. Commonly reported AEs of BRV include dizziness, nausea, and drowsiness. It seems that BRV provides a more favorable neuropsychiatric safety profile than LEV [3], [4]. Numerous studies have assessed the effect of this switch [4], [5], [6], [7], [8], [9], sometimes by means of an overlapping period of the two drugs. An overnight switch may improve therapeutic adherence and minimize AEs [10].
Materials and methods
Study design
This was a retrospective and descriptive study at the Vall d’Hebron University Hospital Epilepsy Unit of epilepsy outpatients, who underwent an overnight switch from LEV to BRV. From November 2016 to November 2017 all patients aged over 16 with all types of epilepsy were included. However, patients with major psychiatric pathology were excluded. The study was approved by the Vall d’Hebron University Hospital local ethics committee [PR(AG)163/2017].
The switch to BRV was performed in accordance with clinical practice on an outpatient basis at home. It was conducted immediately to avoid taking both drugs simultaneously. Since this study was performed in accordance with clinical practice, no assessment was made the first few days after the change. Therefore, patients were re-assessed at control follow-up visit 6 months after commencing the new treatment. Nevertheless, patients also reported and explained the adverse events at the beginning of the switch during the first few days or weeks.
Epilepsy variables such as seizure frequency, responder rate (≥50% reduction in frequency of seizures), AEs and retention rates of the antiseizure medication (ASM) were assessed. Specific tests or electroencephalogram were not performed to monitor the switch. Neuropsychiatric symptoms and AEs were recorded from the information reported by the patient. The frequency of seizures was obtained from either the participant’s reported outcomes or a seizure diary.
Data analysis
Continuous variables were expressed as a mean and standard deviation (SD). Categoric variables were shown as absolute and relative frequencies. Descriptive analysis was performed using the software package IBM SPSS Statistics, Version 24.0 (IBM Corp, Armonk, NY, USA). No comparative analysis was performed since this is a descriptive single-arm study.
Our aim was to assess the safety and tolerability of an overnight switch from LEV to BRV in a group of epilepsy patients.
Results
Demographic and clinical characteristics
In total, forty-one patients were included, with a mean follow-up period of 9.7 months (range: 6–15, Table 1). Focal structural epilepsy was the most common diagnosis (61.0%) followed by unknown cause (14.6%). The most common etiologies were vascular cause (24.4%) and tumors (19.5%). At the time of the switch, 36.6% were on monotherapy with LEV, and 63.4% were taking up to four drugs in addition to LEV. The most common ASMs were clobazam (n = 7), lamotrigine (n = 7), and oxcarbazepine (n = 6). The mean number of previously tested ASMs was 3.3 (SD: 2.3).
Table 1.
Demographic and clinical characteristics of patients with epilepsy who underwent an overnight switch.
| Characteristics | Patients (N = 41) |
|---|---|
| Age (years), mean (SD) | 40.9 (17.8) |
| Gender, n (%) | |
| Male | 21 (51.2) |
| Female | 20 (48.8) |
| Time since first seizure (years), mean (SD) | 17.0 (12.4) |
| Etiology of the epilepsy, n (%) | |
| Structural | 25 (61.0) |
| Vascular | 10 (24.4) |
| Neurodevelopmental disorders | 3 (7.3) |
| Tumors | 8 (19.5) |
| Infectious | 2 (4.9) |
| Tuberous sclerosis complex | 1 (2.4) |
| Mesial temporal sclerosis | 1 (2.4) |
| Unknown | 6 (14.6) |
| Generalized genetic | 4 (9.8) |
| Unclassified | 4 (9.8) |
| Developmental and epileptic encephalopathy | 2 (4.9) |
| Previous antiseizure medication used, mean (number, SD) | 3.3 (2.3) |
| Current treatment, n (%) | |
| levetiracetam monotherapy | 15 (36.6) |
| levetiracetam + 1 ASM | 12 (29.3) |
| levetiracetam + 2 ASMs | 11 (26.8) |
| levetiracetam + 3 ASMs | 1 (2.4) |
| levetiracetam + 4 ASMs | 2 (4.9) |
| Reason for switch, n (%) | |
| Poor seizure control | 22 (53.6) |
| Side effects | 27 (65.9) |
| Irritability/aggressiveness | 15 (36.6) |
| Depression | 10 (24.4) |
| Drowsiness | 5 (12.2) |
| Dizziness | 1 (2.4) |
| Both | 8 (19.5) |
| Levetiracetam dose at the time of the switch (mg/day), mean (SD) | 1761.0 (884.6) |
| Starting dose of brivaracetam (mg/day), mean (SD) | 142.0 (47.0) |
ASM = antiseizure medication.
The reason to start BRV was inefficacy (53.6%), AEs (65.9%) and both (19.5%). Irritability or aggressive behavior (36.6%), depressive symptoms (24.4%) and drowsiness (12.2%) were predominant.
The mean daily dose of LEV was 1761.0 mg/day (SD: 884.6). The mean starting dose of BRV was 142.0 mg/day (standard deviation, SD: 47.0). Most adjustments were performed in a dose ratio between 10:1 and 15:1 (LEV:BRV).
Safety and tolerability of the overnight switch
AEs were reported in 24.4% (n = 10) of patients. After one year of follow-up, 73.2% continued with BRV treatment (n = 30). In total, eleven patients (26.8%) discontinued BRV treatment due to inefficacy or AEs. Of them, seven patients recommenced LEV.
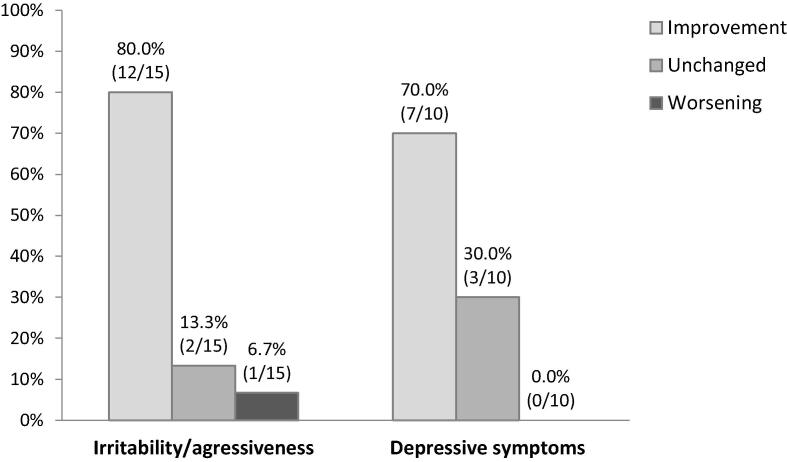
Psychiatric AEs were diagnosed in 25 patients before starting BRV. Irritability or aggressive behavior was present in 15 participants under LEV. Among them, 80.0% (n = 12) improved, 13.3% (n = 2) remained unchanged, and 6.7% (n = 1) worsened (Fig. 1). Depressive symptoms were reported in 10 patients with LEV, among which 7 patients improved.
Fig. 1.
Change in neuropsychiatric adverse events after switching from levetiracetam to brivaracetam.
Regarding other AEs, drowsiness improved in 2 out of 5 patients and dizziness did not change in the single patient.
Frequency of seizures
In total, 61.0% of patients (n = 25) reported the same frequency of seizures after starting BRV treatment. Of them, nine patients (22.0%) were previously seizure-free, and switching was due to previous AEs. Responder rate (≥50% reduction in frequency of seizures) was 26.8% (n = 11), and five patients (12.2%) reported a higher frequency of seizures.
Discussion
This study shows that the overnight switch from LEV to BRV is a safe and well tolerated therapeutic option.
Few studies have evaluated this switch [5], [6], [7], [8], [9], [10]. In a multicenter study, Steining et al. assessed the efficacy of BRV as add-on treatment in 262 patients with refractory epilepsy [5]. Of them, 105 patients who were on LEV, switched to BRV (dosing ratio of 10:1). There was an improvement in behavioral disorders (57%) and drowsiness (71%). Regarding studies with a Spanish population, Ortega et al. assessed state and trait anger with BRV, adjusted by several indicators [7]. In total, 39 patients underwent an overnight switch. Of them, 37% reported AEs and up to 60% restarted LEV treatment due to inefficacy. In addition, BRV increased anger measures less than LEV. Ours is the first Spanish study to evaluate both irritability/aggressiveness and depressive symptoms as adverse events in real-life practice when switching exclusively from LEV to BRV. Furthermore, the inclusion criteria were flexible and data was collected in a control visit, which reflects current practice.
Titration of ASMs is a critical period where the desire to attain effective range dose early may be balanced with the need to reduce the subsequent AEs. Subtherapeutic drug levels may result in seizures and rapid titration may cause the patient to discontinue the treatment [11]. Some situations, such as acute seizures, require fast administration of intravenous ASMs (LEV, phenytoin, lacosamide, valproic acid, and benzodiazepines). However, AEs and safety alerts are commonly reported for most ASMs [12]. LEV has a short titration period and is commonly used in emergency situations, but maximal therapeutic doses are not often used without titration, particularly in the outpatients’ clinic [2]. The dosing ratio varies among studies since there is no standardization. In the study performed by Yates et al. patients received 200 mg/day with dose adjustments around 50–200 mg/day if necessary [5]. Authors reported a reduction in the maximum intensity of primary nonpsychotic behavioral adverse events (93.1%). Steinhoff et al. used the ratio 20:1 for patients previously in treatment with 1,000 mg/day or 2,000 mg/day and 15:1 for 3,000 mg/day [6]. In total, 37% reported AEs (most commonly dizziness and somnolence). In the case of Hirsch et al. the ratios ranged from 10.1:1 to 15.6:1. The most common AEs were depressive symptoms, mood lability, or fear [8]. In our case, the therapeutic dose range was 10:1 and 15:1, and the maximal recommended doses were favourably tolerated, with an improvement in neuropsychiatric AEs.
There is no standardized method regarding the switch. In the study by Yates et al., the change was made in the same day [5]. Other authors retrospectively analyzed this switch and identified cases in which there was a cross-titration, BRV titration or direct switch [9]. Our study was performed with an abrupt overnight switch from LEV to BRV without any kind of titration.
There are some limitations to our analysis. First, this is a real-world retrospective open-label uncontrolled study and data are limited to the visits after six months. In addition, our study represents a small sample limiting confidence of the data.
Conclusions
The abrupt overnight switch from LEV to BRV (dosing ratio 15:1 and 10:1) is a safe and well tolerated therapeutic option, in our small sample. BRV treatment can improve LEV-related neuropsychiatric AEs, which could also increase retention rates. This retrospective Spanish study is representative of current clinical practice, and due to the limited evidence in real world-setting, it provides valuable information for physicians changing from LEV to BRV.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Ethical statement
The study was approved by the Vall d’Hebron University Hospital local ethics committee (PR(AG)163/2017). Patients were informed and agreed to switch immediately to BRV to avoid taking both drugs simultaneously.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: L. Abraira declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Sanofi Genzyme, GW Pharmaceuticals and Esteve laboratorios. J. Salas-Puig declares funding and honoraria from UCB Pharma, BIAL Pharmaceutical, EISAI Inc. and Esteve laboratorios. M. Quintana has no conflicts of interest to declare. I. M. Seijo-Raposo declares research funding from UCB Pharma, Neuraxpharm, and GW pharmaceuticals. E. Santamarina declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Arvelle, and Esteve laboratorios. E. Fonseca declares research funding and speaker fees from UCB Pharma, Esteve laboratorios, Eisai Inc, GW Pharmaceuticals, BIAL Pharmaceutical and Sanofi Genzyme. M. Toledo declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Sanofi, Arvelle, and Esteve laboratorios.
References
- 1.Klein P., Diaz A., Gasalla T., Whitesides J. A review of the pharmacology and clinical efficacy of brivaracetam. Clin Pharmacol. 2018;10:1–22. doi: 10.2147/CPAA.S114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harden C. Safety profile of levetiracetam. Epilepsia. 2001;42:36–39. doi: 10.1111/j.1528-1167.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 3.Toledo M., Whitesides J., Schiemann J., Johnson M.E., Eckhardt K., McDonough B., et al. Safety, tolerability and seizure control during long-term treatment with adjunctive Brivaracetam for partial seizures. Epilepsia. 2016;57:1139–1151. doi: 10.1111/epi.13416. [DOI] [PubMed] [Google Scholar]
- 4.Steinig I., von Podewils F., Möddel G., Bauer S., Klein K.M., Paule E., et al. Postmarketing experience with brivaracetam in the treatment of epilepsies: a multicenter cohort study from Germany. Epilepsia. 2017;58(7):1208–1216. doi: 10.1111/epi.13768. [DOI] [PubMed] [Google Scholar]
- 5.Yates S.L., Fakhoury T., Liang W., Eckhardt K., Borghs S., D’Souza J. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52:165–168. doi: 10.1016/j.yebeh.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Steinhoff B.J., Bacher M., Bucurenciu I., Hillenbrand B., Intravooth T., Kornmeier R., et al. Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy. A monocenter survey. Seizure. 2017;48:11–14. doi: 10.1016/j.seizure.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Ortega G., Abraira L., Martí G., Quintana M., Mazuela G., Santamarina E., et al. Anger assessment in patients treated with brivaracetam. Clin Neuropharmacol. 2018;41:6–9. doi: 10.1097/WNF.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch M., Hintz M., Specht A., Schulze-Bonhage A. Tolerability, efficacy and retention rate of Brivaracetam in patients previously treated with Levetiracetam: a monocenter retrospective outcome analysis. Seizure. 2018;61:98–103. doi: 10.1016/j.seizure.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Theochari E., Cock H., Lozsadi D., Galtrey C., Arevalo J., Mula M. Brivaracetam in adults with drug-resistant epilepsy and psychiatric comorbidities. Epilepsy Behav. 2019;90:129–131. doi: 10.1016/j.yebeh.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari C.M.M., de Sousa R.M.C., Castro L.H.M. Factors associated with treatment non-adherence in patients with epilepsy in Brazil. Seizure. 2013;22(5):384–389. doi: 10.1016/j.seizure.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Fishman J., Kalilani L., Song Y., Swallow E., Wild I. Antiepileptic drug titration and related health care resource use and costs. J Manag Care Spec Pharm. 2018;24(9):929–938. doi: 10.18553/jmcp.2018.17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilad R., Izkovitz N., Dabby R., Rapoport A., Sadeh M., Weller B., et al. Treatment of status epilepticus and acute repetitive seizures with i.v. valproic acid vs phenytoin. Acta Neurol Scand. 2008;118(5):296–300. doi: 10.1111/j.1600-0404.2008.01097.x. PMID: 18798830. [DOI] [PubMed] [Google Scholar]