Abstract
Introduction:
Diabetic patients have a higher tendency of developing all infections, especially infections of the genitourinary tract. Urinary tract infections cause considerable disorders in diabetic patients, and if complicated, can cause renal failure. In Ethiopia, the magnitude of diabetes mellitus-associated urinary tract infections increased from 7.1% in 2005 to 33.9% in 2019. The successful management of patients suffering from urinary tract infections in diabetic patients depends upon the identification of risk factors. This study aimed to determine the magnitude and factors affecting the urinary tract infections among diabetic patients which enable professionals to prevent infections and manage them effectively.
Methods:
Hospital-based cross-sectional study was conducted with 365 diabetic patients selected by systematic sampling technique from March to April 2020. Data were collected by trained BSc nurses via face-to-face interview and patient chart review. Urine microscopy was done to diagnose urinary tract infections. Data were coded and entered using Epi data version 3.1 and exported to Statistical Package of Social Sciences version 26 for analysis. Variables with p-value < 0.25 in bivariable logistic regression were included multivariable logistic regression and variables with a p-value < 0.05 were considered statistically significant.
Results:
The magnitude of urinary tract infections was 22.3% (95% confidence interval: 18–27). The odds of being infected by urinary tract infections were significantly higher in diabetic females (adjusted odds ratio: 2.46; 95% confidence interval: 1.40–4.32), duration of diabetes mellitus diagnosis of ⩾5 years (adjusted odds ratio: 1.98; 95% confidence interval: 1.05–3.72), with comorbidity (adjusted odds ratio: 4.87; 95% confidence interval: 2.76–8.59) and khat chewer (adjusted odds ratio: 1.84; 95% confidence interval: 1.04–3.24) compared with their counter.
Conclusion and recommendation:
Urinary tract infections were high among diabetic patients. Predictors like sex, duration of diagnosis, comorbidity, and khat chewer were found to be associated with urinary tract infections. Improvement of the regular screening of patients with diabetes mellitus for urinary tract infections will provide more effective measures in prevention and management.
Keywords: Dessie Referral Hospital, diabetes mellitus, urinary tract infections
Introduction
Diabetes mellitus (DM) is a syndrome with disordered metabolism and hyperglycemia. 1 This resultant hyperglycemia impairs multiple immune pathways, including neutrophil activation and antibody function.2,3 All these changes make diabetic patients prone to have more infections, such as urinary tract infection (UTI), respiratory tract, soft tissue, and skin infection. 4 Bacterial infection is identified as the major problem in diabetics, and the hazard of acquiring an infection among patients with DM is higher than the normal individuals. 5 The correlation between the occurrence of UTIs and diabetic patients has been attributed to various impairments in the body defense mechanism, poor metabolic control of DM, and incomplete bladder emptying due to autonomic neuropathy. 6
Globally, 150 million people are diagnosed with UTIs yearly. 7 Among diabetic patients, 40% of the years of life lost can be attributed to nonvascular conditions like cancers, infections, and neurodegenerative disorders. 8 A matched cohort study conducted in Canadian primary care for 1-year follow-up on diabetic patients revealed an increased risk of genitourinary infections. 5 A comparative study conducted in Nigeria indicated that patients with DM have highly prone to develop UTIs. Diabetic patients had 70% of the isolated organisms, while non-diabetic patients had 56% of the isolated organisms. 9 The estimated magnitude of UTIs in Uganda was 13.3%. 10 The magnitude of UTIs among patients with DM in Sudan was 19.5%. 11 This illustrates that prevalence is high in Africa. The most commonly observed infectious disease among Ethiopian DM patients is UTIs. 12 In Ethiopia, the prevalence of UTIs among diabetic patients is estimated between 13.9% and 33.8%.13,14 Diabetes is also associated with prolonged hospitalization, septicemia, azotemia, and septic shock in patients with UTIs. 15 UTIs caused significant morbidity in patients with DM, and if complicated, can cause severe renal injury and serious infections. 16
UTIs are affected by factors like gender, educational status, and type of DM, duration of DM, insulin therapy, blood glucose level, and cigarette smoking. Behavioral factors like smoking and alcohol drinking have a vital effect on poor glycemic control which indirectly affects the occurrence of UTIs among those diabetics. 17 Females were more likely to develop UTIs than males due to anatomic and physiologic factors 10 ; diabetic patients with high blood glucose levels, type II DM, and patients who cannot read and write have more prone to be infected. 18 A study conducted in New York showed that participants with diabetes receiving insulin therapy experience an even higher risk of infection than those not receiving insulin.19,20
Even though some research was available in Ethiopia on the magnitude of UTIs among diabetic patients, there are limited studies on factors affecting UTIs. Especially, behavioral alcohol drinking, soft drink intake, perineal hygiene, cigarette smoking, and khat chewing, and clinical factors duration of DM diagnosis, comorbidity, and type of anti-diabetic medications were not addressed adequately which would enable nurses to prevent diabetic clients from UTIs by reducing those modifiable factors. Thus, this study had investigated the magnitude and associated factors of UTIs among patients attending the diabetic unit at Dessie Referral Hospital (DRH).
Methods
Study area
A study was conducted in DRH, Northeastern Ethiopia. Dessie town administration is the capital city of South Wollo zone of Amhara National Regional State in Ethiopia. It is found 472 km from Bahir Dar, the capital city Amhara region, and is 400 km from Addis Ababa, the capital of Ethiopia. The weather condition in north latitude and east longitude is 11.8ºC and 39ºC, respectively. DRH provides health care in different units, namely, inpatient (medical, surgical, pediatrics, orthopedics, and gynecology units), outpatients, including follow-up for chronic disorders, like DM, congestive heart failure (CHF), hypertension, acquired immunodeficiency syndrome (AIDS), and cancer chemotherapy. In a diabetic clinic, a total of 1850 clients visit this unit for follow-up per month.
Study design and period
A hospital-based cross-sectional study from 9 March to 8 April 2020 was conducted.
Sample size and sampling procedure
Sample size determination
The sample size was computed based on previous studies by taking prevalence for first and adjusted odds ratio (AOR) for the second objective of the study. After computing for both the first and second objectives with the consideration of 95% confidence interval (CI), 5% margin of error, the larger sample size was selected which was 365 (history of previous UTI). By adding a 10% non-response rate, the final sample size became 365.
Sampling procedure
Patients with both types I and II DM visit a diabetic unit of DRH for follow-up per month were 1850; among these, participants were selected by systematic random sampling with a sampling interval of five, and the first participant was selected by lottery method.
Data collection procedure
The participants were diabetic patients regardless of UTIs signs and symptoms and socio-demographic characteristics, clinical data, and behavioral factors were collected by two trained BSc nurses. A face-to-face interview and patients’ chart review for clinical data were done in alignment with the study participant’s follow-up data. 16
Data collection tool
Tools for socio-demographic characteristics,4,16,21 clinical data from client charts, and behavioral factors13,20 were adapted from different works of literature based on the objective of the study and reviewed by an expert. Initially, the tools were prepared in English, then translated into the local language (Amharic), and back to English by language experts to maintain its consistency.
Diagnosis of UTIs was confirmed by direct microscopy urine examination for pathogen and leukocytes.
Collection, handling, and transportation of urine specimen
Mid-stream urine samples were collected using aseptically transferred to conical tubes. On the urine sample bottles, the patient’s name and age were indicated. Female participants were informed to clean their hands with water and their genital area before collection of the clean catch mid-stream urine samples. A total of 10 mL of well-mixed urine was aseptically transferred to labeled conical tubes. The tubes were centrifuged at 1000 revolutions per minute. The supernatant fluid was transferred into another container. The sediment was re-mixed by tapping the bottom of the tube. One drop of the well-mixed sediment was transferred to a clean slide and covered with a coverslip. The preparation was examined microscopically using the X10 and X40 objective lenses. The urine microscopy was performed in the microbiology laboratory of DRH by the laboratory technician.
Operational definition
UTI positive. The presence of ⩾ 1 bacteria or ⩾ 10 pus cells or white blood cells per high-power field (HPF) of urine for microscopic examination. 22
Cigarette smoking. The inhalation of the smoke of one or more burning tobacco encased in cigarettes. 23
Khat chewer. An act of chewing the leaves of the stimulant East African plant, Khat edulis, in any amount by the patients with DM as current practice. 24
Study participants
All adult patients with DM who were visiting the diabetic unit of DRH for follow-up were the source population and randomly selected patients with DM aged ⩾ 18 years who were attending a clinical follow-up visit at the diabetic unit of the hospital during the data collection period were the study population.
Inclusion and exclusion criteria
All adult diabetic patients who had to follow-up at DRH during the data collection period were included and patients who were too ill to respond to the questions, pregnant women, or patients with longer than 2 days of catheterization, or clients who took antibiotics within the last 2 weeks were excluded from the study.
Study variables
Dependent variable
UTIs
Independent variables
Socio-demographic factors. Age, sex, educational level, occupational status, and residence.
Clinical factors. Type of DM, duration of diagnosis, type of medication, history of previous UTIs, comorbidity, and serum sugar level.
Behavioral factors. Perineum hygiene habit, alcohol drinking habit, cigarette smoking, khat chewing, and soft drink intake.
Data quality assurance
A pre-test was done with 5% of the sample size at diabetic patients attending Woldia General Hospital, Ethiopia before the actual data collection, and a correction was made based on the pre-test feedback. The data collectors and supervisor were trained for 3 days on how to collect the data from the selected participants. And the progress of data collection was monitored by the supervisor every other day. Model fitness was tested by Hosmer and Lemeshow’s test, and it was 0.391.
Statistical analysis
Data were coded, entered, cleaned, and edited, using Epi data version 3.1 and exported to Statistical Package of Social Sciences (SPSS) version 26 for analysis. Before conducting the multivariable logistic regression analysis, preliminary analyses were conducted to assess multicollinearity by a variance inflation factor (VIF) and tolerance test, and became 1.02 and 0.98, respectively. The crude odds ratio (COR) used in estimating association in the bivariable logistic regression analysis and variables with a p-value < 0.25 in the bivariable logistic regression analysis were included in the multivariable logistic regression analysis. AOR with a 95% CI was used to assess the strength of the association. A p-value < 0.05 was used to declare statistical significance. Finally, findings were presented in tables, frequency, percentages, charts, and texts.
Results
Socio-demographic characteristics of participants
From 365 patients with DM were attending their follow-up at DRH, 359 participants were responded with a 98.4% response rate. The majority of the respondents were males, 199 (55.4%) and only 14.2% were single. A large proportion, 122 (34%) of the participants were found to be in the age group between 46 and 55 years. The majority 82 (22.8%) were government employees, 75 (20.9%) farmers, and 60 (16.7%) merchants. The educational level of the majority (83.3%) of the respondents was above primary school level (Table 1).
Table 1.
Socio-demographic status of diabetic patients at DRH, 2020 (N = 359).
| Characteristics | Category | Frequency | % |
|---|---|---|---|
| Sex | Male | 199 | 55.4 |
| Female | 160 | 44.6 | |
| Marital status | Single | 51 | 14.2 |
| Married | 217 | 60.4 | |
| Widowed | 65 | 18.1 | |
| Divorced | 26 | 7.2 | |
| Age in years | ⩽ 20 | 30 | 8.4 |
| 21–35 | 55 | 15.3 | |
| 36–45 | 51 | 14.2 | |
| 46–55 | 122 | 34.0 | |
| ⩾ 56 | 101 | 28.1 | |
| Residence | Urban | 207 | 57.7 |
| Rural | 152 | 42.3 | |
| Educational status | Unable read and write | 60 | 16.7 |
| Primary school | 139 | 38.7 | |
| Secondary school | 94 | 26. | |
| College/university | 66 | 18.4 | |
| Occupation of participants | Government employee | 82 | 22.8 |
| Housewife | 53 | 14.8 | |
| Student | 38 | 10.6 | |
| Farmer | 75 | 20.9 | |
| Merchant | 60 | 16.7 | |
| Daily labor | 40 | 11.1 | |
| Others | 11 | 3.1 | |
| Type of DM | Type I | 100 | 27.9 |
| Type II | 259 | 72.1 | |
| Serum sugar level | < 126 mg/dL | 110 | 30.6 |
| ⩾ 126 mg/dL | 249 | 69.4 | |
| Duration of diagnosis | < 5 years | 140 | 39.0 |
| ⩾ 5 years | 219 | 61.0 | |
| Previous history of UTIs | No | 212 | 59.1 |
| Yes | 147 | 40.9 | |
| Comorbidity | No | 240 | 66.9 |
| Yes | 119 | 33.1 | |
| Type of anti-diabetic medication | Oral anti-diabetic agents | 204 | 56.8 |
| Insulin | 114 | 31.8 |
DM: diabetes mellitus; UTIs: urinary tract infections.
Clinical and behavioral characteristics of participants
The majority of respondents had type II DM 259 (72.1%), and the proportion with the last fasting blood glucose higher than 126 mg/dL was 249 (69.4%). For 219 (61%) of the respondents, the duration of DM diagnosis was ⩾ 5 years. The occurrence of UTIs among patients with a long duration of diabetes was 27.9% (Table 2).
Table 2.
Factors associated with UTIs among patients with DM attending DRH, Dessie Ethiopia, 2020 (N = 359).
| Variables | Urine microscopy | COR (95% CI) |
AOR (95% CI) |
p-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Sex | Male | 31 | 168 | 1 | 1 | |
| Female | 49 | 111 | 2.39 (1.43–3.98) | 2.46 (1.40–4.32)* | 0.02 | |
| Type of DM | Type I | 13 | 87 | 1 | 1 | |
| Type II | 67 | 192 | 1.91 (1.22–4.45) | 1.08 (0.34–3.42) | 0.21 | |
| Serum sugar level | < 126 mg/dL | 17 | 93 | 1 | 1 | |
| ⩾ 126 mg/dL | 63 | 186 | 1.85 (1.02–3.34 | 1.56 (0.74–3.29) | 0.238 | |
| Duration from DM diagnosis | < 5 years | 19 | 121 | 1 | 1 | |
| ⩾ 5 years | 61 | 158 | 2.46 (1.39–4.33) | 1.98 (1.05–3.72)* | 0.035 | |
| Previous history of UTIs | No | 37 | 175 | 1 | 1 | |
| Yes | 43 | 104 | 1.96 (1.18–3.23) | 1.18 (0.61–2.25) | 0.612 | |
| Comorbidity | No | 29 | 210 | 1 | 1 | |
| Yes | 51 | 68 | 5.43 (3.20–9.28) | 4.87 (2.76–8.59)* | < 0.001 | |
| Khat chewing | No | 38 | 174 | 1 | 1 | |
| Yes | 42 | 105 | 1.83 (1.11–3.02) | 1.84 (1.04–3.24)* | 0.034 | |
COR: crude odds ratio; CI: confidence interval; AOR: adjusted odds ratio; DM: diabetes mellitus.
Statistically significant.
Regarding behavioral characteristics of respondents, 147 (40.9%) were khat chewers, 237 (65.7%) had a habit of washing their perineum after excretion, 28 (7.8%) were smokers, 28 (7.8%) of the participants were alcohol drinkers, and 130 (36.2%) consume soft drinks.
Magnitude of UTIs
Of 359 respondents, 80 (22.3%) were positive for UTIs based on microscopic urine analysis (95% CI: 18–27; Figure 1). Of these, 49 (61.3%) were females, about two-third, 61 (76.3%), of the participants were with DM ⩾ 5 years, and 51 (63.75%) of respondents had comorbidity.
Figure 1.
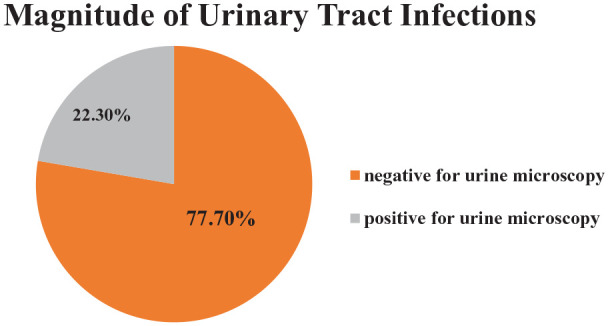
Graphic representation of microscopic urine analysis result among diabetic patients in DRH, Dessie, Ethiopia, 2020 (N = 359).
Factors associated with UTIs
Using the analysis of bivariable logistic regression and “enter” method, the variables sex, marital status, age, residence, educational status, occupation, type of DM, serum sugar level, duration of DM from diagnosis, previous history of UTIs, comorbidity, type of anti-diabetic medicine, khat chewing, smoke cigarette, and taking soft drink were statistically associated with UTIs at a p-value < 0.25, and were considered as candidates for multivariate logistic regression.
In multivariable logistic regression using the “Forward LR” method, the odds of UTIs were 2.4 times more likely among diabetic females than males (AOR: 2.46; 95% CI: 1.40–4.32). Diabetic patients who had greater than or equal to 5-year duration of DM diagnosis had two times the odds of UTIs than those who had less than 5-year duration (AOR: 1.98; 95% CI: 1.05–3.72). The odds of developing UTIs were considerably higher among DM patients who had comorbidity than those patients without comorbidity (AOR: 4.87; 95% CI: 2.76–8.59). The odds of being infected with UTIs were 1.8 times higher among diabetic patients who were khat chewers than those who are not khat chewers (AOR: 1.84; 95% CI: 1.04–3.24; Table 2).
Discussion
The current study’s findings revealed the severity of UTIs as well as socio-demographic, clinical, and behavioral aspects in diabetic individuals. In this study, the overall prevalence of UTIs was 22.3%. A report from studies conducted in Ethiopia, Saint Paul Specialized Hospital (22.6%), 25 Khartoum Hospital, Sudan (19.5%), 11 and Diabetes Center of King Saud University, Saudi Arabia (25.3%). 20
In contrast, the magnitude was lower than studies reported from Arba Minch Hospital (33.9%) in south Ethiopia, 13 Dasman Diabetes Institute (35%) in Kuwait, 26 Tertiary Care Center in Nepal (54.7%), 27 and tertiary hospital in Malaysia (40.2%). 4 The variation in magnitude might be due to differences in the study year, the host factor, and practices, such as social habits of the community and standards of personal hygiene.
In the meantime, the finding was higher in another study cited from Ethiopia Gondar University Hospital, Gondar (17.4%), Debre Tabor General Hospital (10.1%), five hospitals in Harar (15.4%), Hawassa University Referral Hospital (13.8%), Nekemte Referral Hospital (NRH; 16.1%), Metu Karl Heinz Referral Hospital (16.7%),14,16,21,28–30 and in abroad, Mbarara Regional Referral Hospital in Uganda (13.3%), 10 Nanjing First Hospital in China (11.2%), 31 Tamil Nadu, India (12.2%), 32 Timisoara Hospital of Romania (12%), 33 General Hospital in Pakistan (13.6%), 34 and a study conducted in Dodoma Regional Referral Hospital, Tanzania (15.5%). 35 The variation in magnitude might be due to differences in socio-demographic characteristics of the study setting.
In the current study, the magnitude of UTIs was 2.4 times higher among diabetic females than males. This was based on previous studies conducted in Harar, south Ethiopia, Debre Tabor General Hospital, Arba Minch Hospital, Metu Karl Heinz, and Hawassa University Referral Hospital,13,14,16,28,30 Malaysia, Pakistan, and Tanzania.4,33–35 The higher risk of infection in females might be due to the fact women are vulnerable to UTIs due to their anatomy and reproductive physiology. Short urethra and closer to a perirectal area where pathogen colonizes easier, absence of bacteriostatic prostatic secretions and sexual intercourse may force bacteria into the female bladder.
Based on this finding, patients with DM who had ⩾ 5-year duration of DM being infected by UTIs were two times higher than that of less than 5 years, in line with the study conducted in Romania. 33 This raised risk of UTIs is probably because of concurrent neuropathy. 20 In contrary to my finding, a study conducted in Mettu Karl in Ethiopia and Tanzania showed that the duration of DM diagnosis was not statistically associated with UTIs.16,35 UTIs were 4.8 times more likely in diabetics with comorbidities than in diabetics without comorbidities. This could be because the existence of comorbidities is a sign of illness progression, making patients unable to manage with infections.
According to the current study, khat chewer diabetic patients have a greater risk to get UTIs than those not. This is because khat chewers’ glycemic control is poor due to the effect of a chemical substance found in khat, cathinone on reduction of insulin secretion from beta cells of the pancreas which would elevate the glucose level in the blood and the elevation of glucose level in the blood by activation of glycogenolysis in liver and skeletal muscle. These all are the effect of sympathomimetic actions of cathinone would be expected to rise plasma catecholamine levels.11,36 Another reason may be because khat chewers are likely to sit for a long time and retain their urine for longer hours.
According to this finding, the prevalence of UTIs in diabetic patients was high, and females, diabetics who had been diagnosed for more than 5 years, patients with comorbidities, and diabetics who chewed khat were more likely to contract UTIs. Because DM patients are more prone to UTIs, it is critical to focus on early detection and treatment. Identifying such characteristics aids health care providers in providing better care to diabetes patients as a whole, as well as nurses in developing evidence-based nursing care that emphasizes prevention.
Limitation of the study
A causal relationship between the independent and dependent variables could not be demonstrated in this cross-sectional investigation. Because only those who visited the diabetes clinic as an outpatient throughout the study period were included in the study, it is possible that this study does not represent the overall population of the Dessie area. Self-reporting was used to collect data on substance usage, which could be skewed by social desirability bias.
Conclusion
UTIs were more recurrent and were likely to undergo a more problematical course in DM patients. According to this study, the magnitude of UTIs among diabetic patients was high. The magnitude of UTIs among DM patients was somewhat comparable with the prior studies conducted in Ethiopia and abroad. Like other similar studies, the increased occurrence of UTIs confirms that uncontrolled DM is significantly associated with more UTIs. Furthermore, this study found that sex, duration of DM diagnosis in years, comorbidity, and khat chewing habit were independent contributing factors for UTIs among diabetic patients. Describing the contributing factors can improve the quality of health care and enable diabetic patients to be mindful of the prevention of UTIs. Health care professionals who are assigned to in diabetic unit of DRH should work on risk factors identification, reduction, and emphasis should be given to diabetic individuals with comorbidity and increased duration of DM diagnosis as well. A further study that includes many hospitals with better study design is recommended to identify specific risk factors.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121211060614 for The occurrence of urinary tract infection and determinant factors among diabetic patients at Dessie Referral Hospital, South Wollo, Northeast Ethiopia by Betelhem Walelgn, Mehd Abdu and Prem Kumar in SAGE Open Medicine
Acknowledgments
The authors have great gratitude to their data collectors, supervisors, and study participants.
Footnotes
Author contributions: B.W. contributed to conceptualization, title and design selection, data analysis, interpretation, and article write-up. M.A. contributed to conceptualization, title and design selection, data collection, analysis, interpretation, and article write-up. P.K. contributed to conceptualization, data analysis, and interpretation. All authors read and approved the final article.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical clearance was obtained from the ethical review committee of the College of Medicine and Health Sciences, Wollo University, with the ethical approval number of CMHS/1291/013/12. A formal permission letter was obtained from the Dessie Referral Hospital administration.
Informed consent: Before data collection, the purpose and importance of the study were explained to each study participant. Informed verbal consent was obtained from the respondents or legally authorized representatives by the consent form approved by the Ethical Review Committee. The confidentiality and privacy of participants were secured by omitting any personal identifier and the participants had full right to withdraw from the study at any time of the study. The study had not any inhumane treatment of research participants and any physical harm, social discrimination, or psychological trauma. The laboratory findings of study participants were communicated with the responsible clinicians assigned at the diabetic clinic and treated accordingly.
ORCID iD: Mehd Abdu  https://orcid.org/0000-0003-4078-5757
https://orcid.org/0000-0003-4078-5757
Supplemental material: Supplemental material for this article is available online.
References
- 1. Papadakis MA, McPhee SJ, Rabow MW. Current medical diagnosis and treatment. 58th ed. San Francisco, CA: McGraw Hill Education, 2019. [Google Scholar]
- 2. Hine JL, de Lusignan S, Burleigh D, et al. Association between glycaemic control and common infections in people with type 2 diabetes: a cohort study. Diabet Med 2017; 34(4): 551–557. [DOI] [PubMed] [Google Scholar]
- 3. Hodgson K, Morris J, Bridson T, et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015; 144(2): 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah MA, Kassab YW, Anwar MF, et al. Prevalence and associated factors of urinary tract infections among diabetic patients. Heal Sci J 2019; 13(2): 1–5. [Google Scholar]
- 5. Abu-Ashour W, Twells LK, Valcour JE, et al. Diabetes and the occurrence of infection in primary care: a matched cohort study. BMC Infect Dis 2018; 18(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stephen SJ, Gaharwar R. Effect of glycemic control on the clinical and laboratory profile of UTI in patients with diabetes mellitus. Int J Contemp Med Res 2019; 6(6): 1–5. [Google Scholar]
- 7. Gebremariam G, Legese H, Woldu Y, et al. Bacteriological profile, risk factors and antimicrobial susceptibility patterns of symptomatic urinary tract infection among students of Mekelle University, northern Ethiopia. BMC Infect Dis 2019; 19(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Julka S. Genitourinary infection in diabetes. Ind J Endocrinol Metab 2013; 17(74): 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ifediora A, Obeagu EI. Prevalence of urinary tract infection in diabetic patients attending Umuahia health care facilities. J Bio Innov 2016; 5(1): 68–82. [Google Scholar]
- 10. Ampaire L, Butoto A, Orikiriza P, et al. Bacterial and drug susceptibility profiles of urinary tract infection in diabetes mellitus patients at Mbarara Regional Referral Hospital, Uganda. Br Microbiol Res J 2015; 9(4): 1–5. [Google Scholar]
- 11. Hamdan HZ, Kubbara E, Adam AM, et al. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob 2015; 14(26): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gebre MW. Diabetes mellitus and associated diseases from Ethiopian perspective: systematic review. Ethiop J Heal Dev 2013; 27(3): 249–253. [Google Scholar]
- 13. Mama M, Manilal A, Gezmu T, et al. Prevalence and associated factors of urinary tract infections among diabetic patients in Arba Minch Hospital, Arba Minch province, South Ethiopia. Turk J Urol 2019; 45(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nigussie D, Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turk J Urol 2017; 43(1): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitzan O, Elias M, Chazan B, et al. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 2015; 8: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutema T, Weldegebreal F, Marami D, et al. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among adult diabetic patients at Metu Karl Heinz Referral Hospital, Southwest Ethiopia. Int J Microbiol 2018; 2018: 7591259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fekadu G, Bula K, Bayisa G, et al. Challenges and factors associated with poor glycemic control among type 2 diabetes mellitus patients at Nekemte Referral Hospital, Western Ethiopia. J Multidiscip Healthc 2019; 12: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. John AS, Mboto CI, Agbo B. A review on the prevalence and predisposing factors responsible for urinary tract infection among adults. Eur J Exp Biol 2016; 6(4): 7–11. [Google Scholar]
- 19. Donnelly JP, Nair S, Griffin R, et al. Association of diabetes and insulin therapy with risk of hospitalization for infection and 28-day mortality risk. Clin Infect Dis 2017; 64(4): 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Rubeaan KA, Moharram O, Al-Naqeb D, et al. Prevalence of urinary tract infection and risk factors among Saudi patients with diabetes. World J Urol 2013; 31(3): 573–578. [DOI] [PubMed] [Google Scholar]
- 21. Yismaw G, Asrat D, Woldeamanuel Y, et al. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Eur J Exp Biol 2012; 2(4): 889–898. [Google Scholar]
- 22. Nwankwo UG, Ezeadila CU, Ezeadila JO, et al. Macroscopy and microscopy urinalysis: a vital screening procedure for urinary tract infections (UTIs) in a hospital in Awka, Nigeria. J Biol Life Sci 2020; 11(1): 143–153. [Google Scholar]
- 23. Chidozie NJ, Okorie EA, Chima OE, et al. Study on the effect of smoking on type 2 diabetic patients in Federal Medical Center Owerri, Southeastern Nigeria. Asian J Med Sci 2014; 5(3): 63–71. [Google Scholar]
- 24. Teni FS, Surur AS, Hailemariam A, et al. Prevalence, reasons, and perceived effects of khat chewing among students of a college in Gondar town, Northwestern Ethiopia: a cross-sectional study. Ann Med Heal Sci Res 2015; 5: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woldemariam HK, Geleta DA, Tulu KD, et al. Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Infect Dis 2019; 19(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sewify M, Nair S, Warsame S, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res 2016; 2016: 6573215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar Jha P, Baral R, Khanal B. Prevalence of uropathogens in diabetic patients and their susceptibility pattern at a tertiary care center in Nepal: a retrospective study. Int J Biomed Lab Sci 2014(2): 29–34. [Google Scholar]
- 28. Worku S, Derbie A, Sinishaw MA, et al. Prevalence of bacteriuria and antimicrobial susceptibility patterns among diabetic and nondiabetic patients attending at Debre Tabor Hospital, Northwest Ethiopia. Int J Microbiol 2017; 2017: 5809494. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Kebamo S, Dabsu R, Deressa A, et al. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending NRH. Am J Curr Microbiol 2017; 5(1): 19–32. [Google Scholar]
- 30. Abate D, Kabew G, Urgessa F, et al. Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr J Heal Biomed Sci 2017; 1(2): 11–20. [Google Scholar]
- 31. He K, Hu Y, Shi JC, et al. Prevalence, risk factors and microorganisms of urinary tract infections in patients with type 2 diabetes mellitus: a retrospective study in China. Ther Clin Risk Manag 2018; 14: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senthamarai S, Sivasankari S, Anitha C. A study on clinical presentation, bacterial profile and its antibiotic sensitivity pattern in urinary tract infections among diabetic patients attending tertiary care hospital, Tamilnadu. Infect J Appl Res 2016; 2(3): 157–159. [Google Scholar]
- 33. Chiţă T, Timar B, Muntean D, et al. Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Ther Clin Risk Manag 2017; 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sekhar VS, Prasad RR, Rajyalakshmi M. A clinical and microbiological profile of urinary tract infection in diabetes mellitus patients, a South India Perceptive. Int J Contemp Med Surg Radiol 2020; 5(1): 143–147. [Google Scholar]
- 35. Nassoro DD. Prevalence, aetiologies, sensitivity profile and factors associated with significant bacteriuria among diabetes mellitus patients attending Dodoma Regional Referral Hospital. Enugu, Nigeria: Afribary Limited, 2017. [Google Scholar]
- 36. Nigussie TF. The effect of Catha Edulis (khat) on blood pressure, pulse rate, and blood glucose concentration, in adult subjects of Arba Minch town, southern Ethiopia, 2020, https://www.globalscientificjournal.com/researchpaper/The_effect_of_Catha_Edulis_khat_on_blood_pressure_pulse_rate_and_blood_glucose_concentration_in_adult_subjects_of_Arba_Minch_town_southern_Ethiopia_.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121211060614 for The occurrence of urinary tract infection and determinant factors among diabetic patients at Dessie Referral Hospital, South Wollo, Northeast Ethiopia by Betelhem Walelgn, Mehd Abdu and Prem Kumar in SAGE Open Medicine


