Abstract
The nanofibrous nature and its intricate structural organization are the basis for the extraordinary ability of sound enamel to outlive masticatory forces at minimal failure rates. Apatite nanofibers of several hundreds of micrometers to possibly millimeters in length originate during the secretory stage of amelogenesis as 2-nm-thin and 15-nm-wide ribbons that develop and grow in length under the guidance of a dynamic mixture of specialized proteins, the developing enamel matrix (DEM). A critical role in the unidirectional and oriented growth of enamel mineral ribbons has been attributed to amelogenin, the major constituent of the DEM. This review elaborates on recent studies on the ability of ribbon-like assemblies of amelogenin to template the formation of an amorphous calcium phosphate precursor that transforms into apatite mineral ribbons similar to the ones observed in developing enamel. A mechanistic model of the biological processes that drive biomineralization in enamel is presented in the context of a comparative analysis of enamel mouse models and earlier structural data of the DEM emphasizing a regulatory role of the matrix metalloproteinase 20 in mineral deposition and the involvement of a process-directing agent for the templated mineral growth directed by amelogenin nanoribbons.
Keywords: biomineralization, matrix metalloproteinase 20, amorphous calcium phosphate, apatite, self-assembly, developing enamel matrix
Principles of Biomineralization
The past 2 decades have seen substantial progress in our understanding of molecular mechanisms for biomineralization. While some mechanistic aspects are debated, the development of an amorphous precursor that proceeds oriented crystallization on an organic framework has emerged as overarching phenomena in tissues that mineralize controlled by biology (George and Veis 2008; Gower 2008; Beniash et al. 2009; Nudelman et al. 2012; DeVol et al. 2015; Macias-Sanchez et al. 2017). In general, biomineralization involves specialized cells that exocytose a series of tissue-specific proteins competent in completing 4 essential steps:
Assembly of the main matrix component into a supramolecular structure that forms a continuous organic framework
Inhibition of nucleation of mineral by acidic/phosphorylated proteins that are instrumental in transporting mineral ions to the framework to induce formation of amorphous mineral precursor
Enzymatic processing of structural and/or acidic/phosphorylated proteins for temporospatial mineral nucleation in association with self-assembled framework
Guided transformation of amorphous phase into oriented mineral crystallites aligned to the organic framework (Saito et al. 2000; Veis 2011)
Examples of this process have been described for the development of nacre in abalone shells (Macias-Sanchez et al. 2017), in which the polysaccharide chitin and silk-like proteins build a framework, while proteins rich in aspartic acid (Gotliv et al. 2005) may act as ion carrier facilitating the formation of amorphous calcium carbonate, which transforms into oriented aragonite platelets in the brick wall structure of nacre (DeVol et al. 2015). In vertebrate animals, mineralization of bone and dentin follows a similar scheme, with assembled collagen fibrils providing the organic framework that becomes reinforced by intrafibrillar mineral through the interaction with phosphorylated proteins (Gower 2008; Deshpande et al. 2011). Activity of phosphoproteins appears to be triggered by enzymatic processing and the generation of cleavage products like the dentin matrix protein 1 (DMP-1) and osteopontin found in dentin and bone, as well as dentin phosphoprotein (DPP) specific to dentin. All are rich in phosphorylated residues or proteins with multiple aspartic acid domains that attract calcium and phosphate ions (Butler 1998; Gotliv et al. 2005; Rodriguez et al. 2014). Recent studies suggest that interaction of this protein-ion complex with collagen leads to infiltration of mineral ions into the lumen of collagen I fibrils possibly driven by osmotic pressure and/or capillary forces (Olszta et al. 2007; Niu et al. 2017). This process has been replicated in vitro and was first described using a biomineralization analog (Olszta et al. 2007; Nudelman et al. 2010). Comparable to the actions of phosphorylated proteins, the anionic polymer, polyaspartic acid (pAsp), was able to induce intrafibrillar mineralization in collagen through a mechanism termed the polymer-induced liquid precursor (PILP) process (Fig. 1) (Gower 2008). A critical component of the PILP mineralization process is the presence of PILP nanodroplets, which form when an ion cloud concentrates around the anionic macromolecules, creating nanometer-sized droplets (10–15 nm) (Fig. 1A). Only upon interaction with suitable substrates, mineral ions are released from the nanodroplets (Fig. 1B). In the presence of collagen fibrils, calcium and phosphate ions infiltrate into collagen fibrils and gradually solidify as amorphous calcium phosphate (ACP) in the gap zones and spaces between collagen molecules (Fig. 1C). Over a period of 2 to 4 d, ACP transforms into apatite crystallites oriented with their c-axes parallel to the fibril long axis (Fig. 1D).
Figure 1.
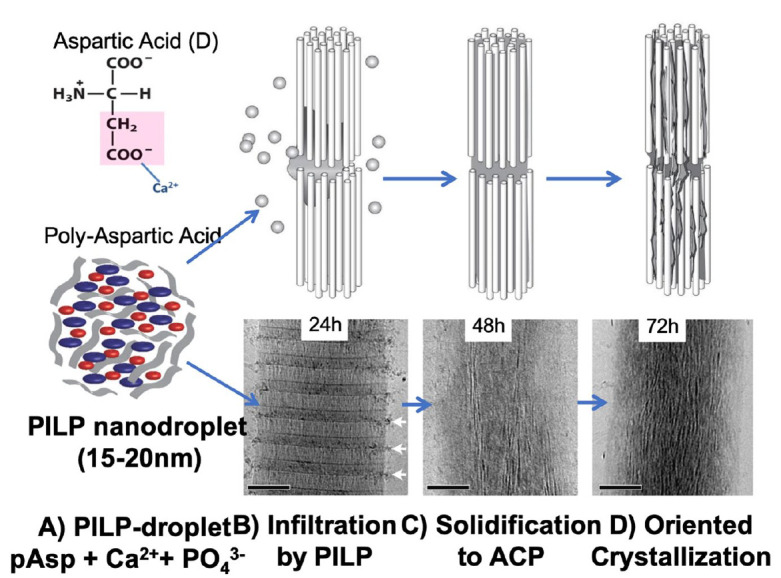
Schematic of the polymer-induced liquid precursor (PILP) process with transmission electron microscopy (TEM) evidence. (A) PILP nanodroplets of 15 to 20 nm form when polyaspartic acid (pAsp) is mixed with Ca2+ and PO43− ions in saturated aqueous solutions. (B) PILP-droplets attach to collagen fibrils comprising aligned collagen molecules with gap zones between them. Droplets attach to fibrils at the gap zones and mineral precursors infiltrate into the interstices. (C) Liquid precursors solidify into amorphous calcium phosphate (ACP), which gradually transforms into oriented apatite crystals (D) aligned with collagen molecules. Schematic adapted from Olszta et al. (2007); TEM images from Nudelman et al. (2010).
Do Overarching Principles of Biomineralization Also Translate to Enamel?
Enamel is the only epithelial-derived tissue that mineralizes in vertebrates. The hard tissue develops in an extracellular matrix secreted by a specialized cell, the ameloblast, which synthesizes unique matrix proteins with little homology to any other known proteins. Contrary to other mineralizing structures, enamel’s organic matrix is not permanent like in nacre and dentin, nor does it remodel like in bone. In order to provide maximum stiffness for mastication, enzymes degrade the protein matrix, which is almost completely removed by the end of tissue maturation. The foundation for the ultimate micro- and nanostructure of mature enamel, however, is laid down early in amelogenesis. During the secretory stage, thin mineral nanoribbons, about 15 nm wide and 1.5 to 2.0 nm thick (Daculsi and Kerebel 1978), develop from an amorphous mineral precursor (ACP) that gradually transforms into crystalline apatite in close proximity to the initial mineralization front at the Tomes’s process (Beniash et al. 2009; Shin et al. 2020). At this stage, the mineral phase makes up <20% of the tissue by volume, while matrix and liquid phase share the remaining space about equally (Smith 1998; Simmer et al. 2012). All major matrix components (e.g., amelogenin, enamelin, ameloblastin) are hydrolyzed by matrix metalloproteinase 20 (MMP20), producing a set of dynamic molecules that are known to interact with each other in order to develop a micro- and nanostructure that is optimized to the mechanics of mastication. However, only after the entire thickness of the enamel layer has been laid down, the mineralization process is fully turned on. Mineral ribbons have reached their full length by the end of the secretory stage and established enamel’s prismatic structure before matrix is removed by enzymatic degradation, allowing for lateral growth of apatite ribbons into nanofibers of 40 to 60 nm width (Smith 1998).
Identifying the Supramolecular Structure of the Organic Framework in the Developing Enamel Matrix
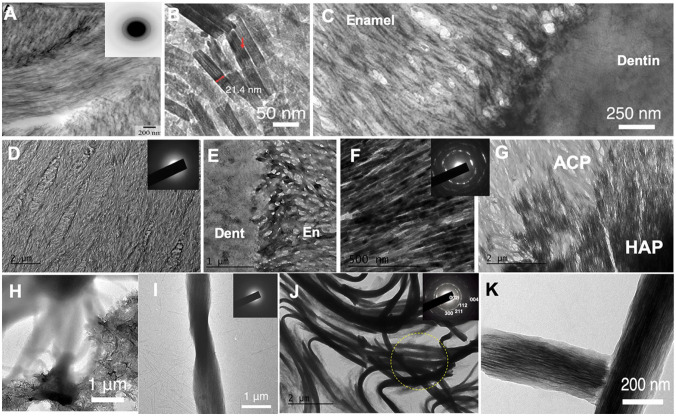
Controversy about the architecture of the organic matrix has subsisted in enamel research since the original studies on enamel structure by electron microscopy (Travis and Glimcher 1964). In part due to the transient and dynamic nature of the developing enamel matrix (DEM), reports of supramolecular matrix structures are manifold with ample varieties of secondary motifs, aggregates, and assemblies (Zhang et al. 2011; Moradian-Oldak 2012; Bidlack et al. 2014). Most published work on secretory stage enamel describes the presence of ribbons (Fig. 2A–C) (Diekwisch et al. 2002; Smith et al. 2016). These ribbons (~20 nm wide) have been termed enamel ribbons or enamel mineral ribbons under the assumption they are mineralized structures. Indeed, selected area electron diffraction suggested that oriented apatite crystals are present in these ultramicrotome sections (Travis and Glimcher 1964; Shin et al. 2020). However, analysis of the chemical components by weight using protein extraction, differential calorimetry, or ashing experiments established that the DEM consists of an equal amount of each: liquid, protein, and mineral. Hence, by volume, only 15% to 20% is inorganic mineral, whereas 40% should be associated with protein (Smith et al. 2011; Nanci and Ten Cate 2013). Nevertheless, most transmission electron microscopy (TEM) studies ignored indicating the organic components in their image analysis. A recent study (Pandya et al. 2017), nonetheless, acknowledged that most mineral appears to originate in close proximity to the ameloblast as thin stripes along the edges of ribbon-shaped assemblies of organic matter 15 to 20 nm wide (Fig. 2C) comparable to filamentous structures observed in the demineralized DEM (Travis and Glimcher 1964).
Figure 2.
Supramolecular structures of the developing enamel matrix (DEM). (A) Ribbon-like structures are predominant in secretory stage enamel in transmission electron microscopy (TEM) images and have been associated with apatite mineral (adapted from Diekwisch et al. 2002). (B) Scanning TEM analysis shows that ribbons initiate at dentin (De), which has a higher degree of mineralization compared to secretory stage enamel (En) (adapted from Smith et al. 2016). (C) TEM analysis indicated that mineral appears to form at the edges of protein ribbons at ameloblast (ambst) membrane (adapted from Pandya et al. 2017). (D) Demineralized and negatively stained section of klk4−/− enamel shows ribbon-like protein structures previously described as “crystal ghosts.” (E) Protein ribbons measure 16 to 20 nm in width (red arrows) and show a dark central line (D and E adapted from Bai et al. 2020). (F) Section of klk4−/− enamel-stained Congo Red for amyloid (adapted from Carneiro et al. 2016). (G) X-ray diffraction analysis of developing enamel showing reflection pattern characteristic of cross-β structure (adapted from Jodaikin et al. 1986). (H) TEM analysis of developing murine enamel stained with uranyl acetate showing electron-lucent spherical structures (black arrows) of 15 to 20 nm between fine mineral needles (adapted from Fincham et al. 1995). This figure is available in color online.
Further evidence for filamentous enamel proteins was provided in a recent study that showed an abundance of protein nanoribbons in Klk4−/− mice (Fig. 2D) (Bai et al. 2020). Due to the lack of the KLK4-protease, enamel does not mature in these animals and remains in a state similar to the secretory stage, with enamel largely comprising MMP20-cleavage products of amelogenin (Hu, Smith, Richardson, et al. 2016). Negative staining of the demineralized Klk4−/− enamel revealed an intricate network of ribbon-like structures matching the texture of mineral ribbons in prismatic enamel (Fig. 2D). Higher magnification showed that these sections comprise thin ribbons, 15 to 20 nm wide, with a high degree of parallel alignment matching the characteristics of enamel mineral ribbons (Fig. 2E). Therefore, protein nanoribbons should also exist in secretory stage enamel of wild-type mice. This hypothesis was successfully tested by TEM examination of the secretory stage of murine incisors after demineralization and staining. Protein nanoribbons were also abundant in the DEM of normal mice and match the protein nanoribbons observed in Klk4−/− enamel (Appendix Fig. 1). Moreover, an extensive body of literature already exists describing similar structures in the DEM. These fibrous structures were labeled “crystal ghosts” (Bai and Warshawsky 1985) and assumed to be imprints of the mineral ribbons in a gel-like substance of protein matrix in the DEM. Notably, additional methods, other than TEM, provided critical information on structural determinants of the DEM, suggesting the presence of filaments in form of amyloids in enamel. Staining of enamel from Klk4−/− mice was positive to amyloid-sensitive stains, like Congo Red (Fig. 2F) (Carneiro et al. 2016). The presence of amyloids was also supported by earlier studies that revealed characteristic cross-β patterns in demineralized enamel by X-ray diffraction (XRD) (Fig. 2G) (Glimcher et al. 1965; Jodaikin et al. 1986). These observations strongly contrast TEM studies from the 1990s showing radio-translucent spherical structures of approximately 20 nm diameter between dark lines of mineral in the DEM (Fig. 2H) (Fincham et al. 1995).
Supramolecular Structures of Amelogenin
It is well understood that folding and the secondary structure of proteins are critical for executing their function. Amelogenin is by far the most prevalent component and comprises about 90% of all enamel proteins in mammals. From genetic defects and knockout animal models, it is known that amelogenin is critical for prismatic enamel structure formation in conjunction with the development of thin apatite ribbons (1–2 nm thick, 10–15 nm wide) that can grow hundreds of micrometers in length during the secretory stage of amelogenesis (Daculsi and Kerebel 1978). Thus, amelogenin is the matrix component able to construct an organic framework for guided mineralization in enamel.
In vitro studies on the recombinant full-length amelogenin protein showed the formation of nanospheres (Fig. 3A) and stimulated the postulation of the nanosphere model of amelogenin-guided mineralization in enamel (Fincham et al. 1999). A vast number of in vitro studies followed and examined possible mechanisms for nanospheres to produce mineral ribbons but struggled to provide useful information to describe certain enamel phenotypes in a series of mouse models revealing a disconnect between in vitro and in vivo observations (Simmer et al. 2012). A key shortcoming of the nanosphere model is the instability of nanospheres in the presence of calcium and phosphate ions or in contact with apatite mineral (Tarasevich et al. 2009; Chen et al. 2011; Martinez-Avila et al. 2012). Moreover, the size of nanospheres varies substantially with pH or amelogenin concentration and between different MMP20-cleavage products of amelogenin (Moradian-Oldak et al. 1998; Moradian-Oldak 2001). The wide range of diameters reported and their instability led to the conclusion that amelogenin is intrinsically disordered and largely adapts random-coil structure with varying degrees of β-strands (Buchko et al. 2008; Ndao et al. 2011). The hypothesis that amelogenin is sensitive to its environment and significantly changes upon interaction with surfaces such as minerals and cells (Shaw et al. 2020) is more likely an indication that previous studies did not examine amelogenin in an environment that stabilized its structure, as they were conducted in the absence of calcium ions.
Figure 3.
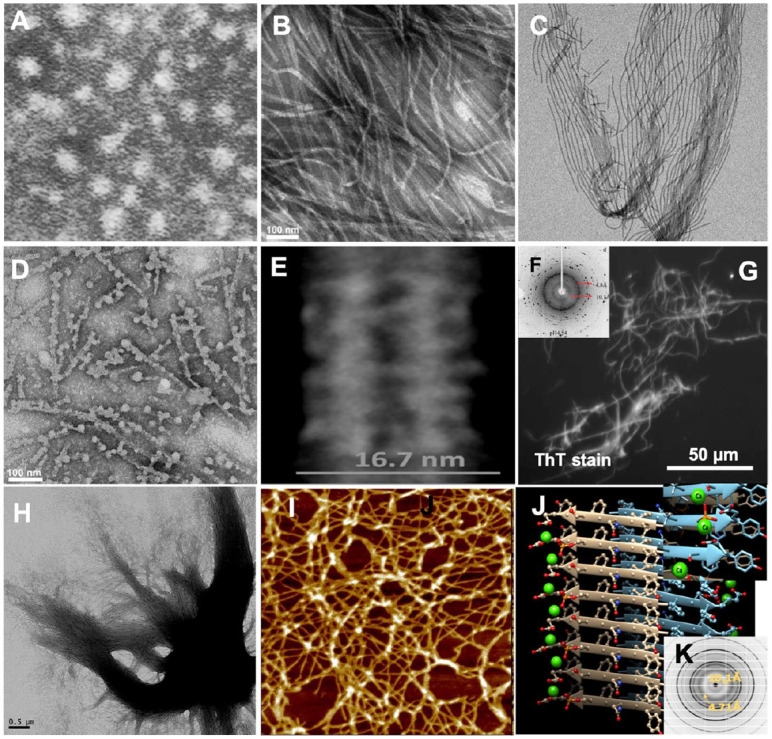
Supramolecular structures of amelogenin proteins and peptides. (A) Transmission electron microscopy (TEM) analysis of recombinant full-length murine amelogenin rM180 assembled in phosphate buffer showed nanospheres of 15 to 20 nm (adapted from Fincham et al. 1995). (B) TEM analysis of recombinant full-length human amelogenin rH174 showed the formation of nanoribbons when calcium and phosphate ions were present during assembly (adapted from Carneiro et al. 2016). (C) Nanoribbons have high propensity to self-align (adapted from He et al. 2011). (D) When exposed to chelating agent EDTA, nanoribbons disintegrate and form spherical aggregates (adapted from Martinez-Avila et al. 2012). (E) TEM analysis shows a dark central line and width of rH174 nanoribbons averages at 16.7 nm with only ±1 nm variation (adapted from Martinez-Avila et al. 2012). (F) X-ray diffraction (XRD) analysis of human full-length amelogenin assembled into ribbons showed characteristic of cross-β structure of the developing enamel matrix (DEM) (adapted from Zhang et al. 2020). (G) Staining of micrometer-sized aggregates of rH174 nanoribbons by ThT indicates their amyloid-like character (adapted from Carneiro et al. 2016). (H) Amelogenin nanoribbons tend to align in parallel and will aggregate into larger bundles of 1 to 2 μm in diameter over time (adapted from Martinez-Avila et al. 2012). (I) Atomic force microscopy analysis of 14P2 peptide derived from self-assembling domain at the N-terminus of amelogenin showing randomly oriented nanoribbons of about 7 nm in width (adapted from Carneiro et al. 2016). (J) Model of the cross-β structure adapted by amyloid domain P2 shows that phosphorylation site at serine residue is able to interact with glutamic acid residue from the neighboring β-sheet, forming intermolecular ion bridges and triggering self-assembly into nanoribbons (adapted from Carneiro et al. 2016). (K) XRD analysis of 14P2 nanoribbons also shows cross-β structure (adapted from Engelberth et al. 2018).
A different superstructural model of amelogenin emerged 10 y ago and is based on the discovery that recombinant amelogenin can self-assemble into nanoribbons in vitro (Fig. 3B) resembling the ribbon-like morphologies described in the DEM above (Fig. 2A–H) (He et al. 2011). The principal difference for assembling recombinant amelogenin into nanoribbons versus nanospheres is the addition of calcium and phosphate to the assembly solutions (Martinez-Avila et al. 2012; Engelberth et al. 2018). Ribbon-like assemblies of a few hundred nanometers form as early as 2 d and continue to grow in length. Nanoribbons tend to align themselves in parallel over time, reaching tens of micrometers within 2 wk (Fig. 3B, C). The critical importance of calcium ions in assembly was demonstrated by addition of EDTA, which disassembled the nanoribbons and allowed nanospheres to form upon extraction of calcium (Fig. 3D) (Martinez-Avila et al. 2012). In contrast to amelogenin nanospheres (Moradian-Oldak 2001), the width and thickness of amelogenin nanoribbons are consistent and do not vary significantly with time and pH, suggesting the presence of a superstructural motif that persists in changing environments. Nanoribbons of recombinant full-length human amelogenin (rH174) and its MMP20-cleavage product (rH146) are on average 3.1 ± 0.4 nm thick and measure 16.7 ± 1 nm and 14.2 ± 0.8 nm in width, respectively (Fig. 3E), sequences described in Appendix Figure 2. The reduced width of rH146 nanoribbons has been associated with the lack of the 28 residues at the C-terminus, in accordance to structural models predicting the hydrophilic tail being oriented toward the edges of the ribbons (Martinez-Avila et al. 2012). While their molecular structure has not been resolved yet, various characteristics suggest that nanoribbons comprise β-sheet rich domains with antiparallel cross-β orientation (Sanii et al. 2014; Engelberth et al. 2018). In its simplest form, nanoribbons may comprise antiparallel dimers (He et al. 2011; Martinez-Avila et al. 2012). Analogous to XRD data for the DEM (Fig. 2G), amelogenin nanoribbons showed amyloid characteristic reflections at 4.7 and 10.1 Å (Fig. 3F) (Zhang et al. 2020), providing further evidence for a connection between in vitro and in vivo data. The ability of amelogenin to adopt an amyloid structure was further supported by means of thioflavin T, a stain used for amyloid detection in neurodegenerative tissue. Large fibrillar aggregates of amelogenin stained positive for thioflavin T (Fig. 3G). These fibers consist of aligned nanoribbons that self-organize into micrometer-thick bundles of approximately parallel nanoribbons reaching over hundreds of micrometers in length (Fig. 3H) and resemble the organization of ribbons in secretory stage enamel rods.
Protein structure prediction gave high propensities of a N-terminal domain of amelogenin for adaptation of β-sheets and possible aggregation into amyloids (Carneiro et al. 2016). Indeed, an amelogenin-derived 14-mer peptide (14P2) (residues 8–21; see Appendix), with the sequence 8-GHPGYI NFSYEVLT-21, self-assembled into nanoribbons within minutes (Fig. 3I). In agreement with studies on the full-length protein, ribbon assembly was only achieved in the presence of both calcium and phosphate ions. Interestingly, addition of phosphate was no longer required when serine was phosphorylated, suggesting that the single phosphorylation site of amelogenin is involved in the self-assembly process, which may have been an overlooked contributing factor to the strong enamel phenotype described for the knock-in mouse lacking amelogenin phosphorylation (Shin et al. 2020). Modeling of the structure at this domain demonstrated that calcium ions sterically fit between 2 antiparallel dimers, triggering ribbon assembly via calcium ion bridges between phosphorylated serine 16 residues (Fig. 3J) (Carneiro et al. 2016). XRD analysis confirmed the cross-β structure of 14P2 ribbons (Fig. 3K).
Nanoribbons Template Amorphous Mineralization and Subsequent Oriented Crystallization
While biomineralization occurs in solutions saturated in calcium and phosphate ions, the classical pathway to apatite crystallization through nucleation and crystal growth is avoided (Colfen 2008). Instead, substantial evidence suggests that in most biological systems, mineral forms via an amorphous precursor phase (Rao and Colfen 2016). The role of so-called nucleation inhibitors is essential for the prevention of mineral precipitation, like glutamic acid–rich matrix-GLA protein that stabilizes blood serum in the vasculature (Luo et al. 1997). Their mechanism of action is most likely associated with the acid and anionic character and their ability to bind calcium ions, thus reducing the free calcium concentration and degree of saturation. A dual role for “inhibitors” has also been observed as they appear to direct mineralization when in contact with proper substrates (Saito et al. 2000). Examples, as described above, include DSPP, DMP-1, and OPN, which facilitate mineral formation when in contact with type I collagen fibrils (Deshpande et al. 2011) but not by contact with type II collagen (Habelitz et al. 2014). The polymer-induced liquid precursor process describes a possible mechanism (Fig. 1) by which these “inhibitors” could fulfill their dual role and not only prevent nucleation but also direct mineral formation. The PILP method gave rise to the term process-directing agents, defining molecules that are able to induce functional mineralization when in contact with a mineralizing template.
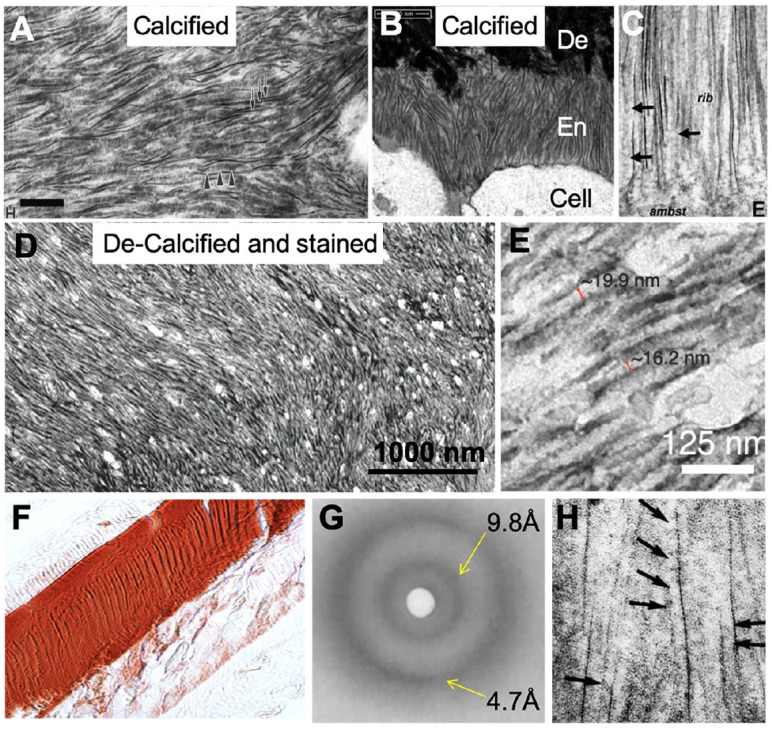
Enamel mineralization also follows the nonclassical pathway. TEM studies show that an amorphous precursor, ACP, forms in close proximity to matrix secretion sites at the Tomes’s process (Beniash et al. 2009). The early mineral ribbons remain amorphous within the first 6 to 8 µm into the mineralization front according to selected-area electron diffraction (SAED) (Fig. 4A) before oriented crystalline apatite is observed (Shin et al. 2020). In a recent study (Bai et al. 2020), the above-described process of enamel mineralization was in part replicated in vitro by application of the PILP method to demineralized sections of enamel. Klk4−/− enamel was sectioned (Fig. 4B) and subsequently demineralized and stained, revealing a pattern of filamentous, ribbon-like structures that originated at the surface of dentin (Fig. 4C). Demineralized sections of enamel from Klk4−/− mice were exposed to PILP treatments containing 0.1 mg/mL pAsp as previously described for collagen mineralization (Bai et al. 2020). After PILP treatment, a mineral layer formed following the texture of protein filaments, and the characteristic pattern of rod and interrod regions was observed (Fig. 4D). SAED analysis indicated an amorphous layer (Fig. 4D) that was also apparent at the dentinoenamel junction and primarily developed on the enamel side (Fig. 4E). In an attempt to promote phase transformation, sections were exposed to an elevated temperature at 50°C, which resulted in the formation of ribbon-like apatite crystals with defined edges and organized into the characteristic texture of prismatic enamel (Fig. 4F). PILP treatments remineralized the tissue sections and restored the structure of the Klk4−/− enamel. In some areas, the phase transformation was incomplete and showed defined and ordered transition zones illustrating that the underlying structure (e.g., the amelogenin nanoribbons) must be involved in guiding the crystallization process by directing apatite crystallization along their c-axes.
Figure 4.
Mineral formation in enamel tissue and on recombinant amelogenin nanoribbons. (A) Enamel also follows the nonclassical pathway and mineralizes into apatite ribbons from an amorphous precursor, amorphous calcium phosphate (ACP) (adapted from Beniash et al. 2009). (B) Transmission electron microscopy (TEM) analysis of section through enamel from klk4−/− mice shows apatite mineral ribbons of 20 to 23 nm in width with a dark central line. (C) Negative staining of demineralized enamel from klk4−/− mice revealed matrix was composed of nanoribbons that originated at the dentin, with collagen fibrils in dentin visible. (D) Polymer-induced liquid precursor (PILP) mineralization treatments of demineralized enamel from klk4−/− mice produced a coating of ACP on the surface of nanoribbons; enamel texture is visible. (E) Same treatment produced ACP coating at the dentinoenamel junction with only limited interaction and changes to dentin collagen. (F) Exposing PILP-treated specimens to a temperature of 50°C succeeded in phase transformation to oriented crystalline apatite, which aligned in the direction of protein ribbons. (G) TEM shows an area of incomplete phase transformation, and the transition zone indicates that ACP gradually transforms into crystalline apatite with guidance of the underlying amelogenin nanoribbons. (H) PILP treatment of nanoribbons from rH174 amelogenin did not facilitate ACP formation. (I) PILP treatment of nanoribbons from rH146 amelogenin resulted in aggregation of nanoribbons into bundles and formation of amorphous mineral rich in Ca and PO4. (J) PILP-treated rH146 nanoribbons, after heating to 80°C, formed tens of micrometer-long bundles with oriented apatite mineral; selected-area electron diffraction (SAED) shows the c-axis parallel to the nanoribbon long-axis. (K) Bundles show mineral fibers or ribbons separated by organic templates and amelogenin nanoribbons (B to K adapted from Bai et al. 2020).
In analogy to demineralized enamel, PILP treatments were conducted on recombinant amelogenin nanoribbons (Bai et al. 2020). Interestingly, efforts to mineralize nanoribbons from rH174 were unproductive. Mineral formation occurred, but crystalline apatite developed without association to the organic framework (Fig. 4H). In contrast, immersion of nanoribbons from rH146 into PILP solutions displayed an extraordinary ability to align and aggregate the nanoribbons into bundles. Elemental analysis revealed the bundles were infiltrated by calcium and phosphate ions (Bai et al. 2020), which solidified as amorphous mineral (Fig. 4I). Exposing these structures to temperatures around 80°C induced phase transformation from ACP to crystalline apatite. The protein bundles reached tens of micrometers in length and comprised protein and mineral ribbons with a high degree of orientation, akin to the ones described for developing enamel (Fig. 4J). The c-axes of apatite aligned to the long-axes of rH146 nanoribbons (Fig. 4K).
Critical Role of MMP20-Processing in Enamel Formation
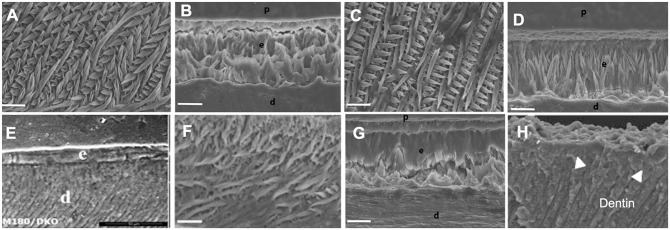
Over the past 2 decades, a number of knockout and transgenic mice were created that showed surprising enamel phenotypes useful in deciphering mechanisms of guided biomineralization (Hu et al. 2008; Pugach et al. 2010, 2013; Hu, Smith, Richardson, et al. 2016; Xia et al. 2016). For example,AmelX−/− mice were not able to form proper enamel microstructure as seen in wild-type enamel (Fig. 5A) but developed fan-like minerals of octacalcium-phosphate (Hu, Smith, Cai, et al. 2016) (Fig. 5B). A transgenic rescue of the AmelX−/− enamel using the full-length amelogenin gene (M180) was largely successful and restored enamel prism structure and properties (Fig. 5C), while inserting a transgene for amelogenin without its C-terminus did not provide any structural improvements (Fig. 5D); see Appendix for sequences. Concomitantly, a dual knockout of AmelX and Mmp20 could not be rescued by inserting the M180 gene (Fig. 5E), thus illustrating the importance of amelogenin processing by MMP20 for the formation of apatite nanofibers and their organization into rod and interrod enamel (Pugach et al. 2010, 2013). Another rescue attempt of the amelogenin knockout mouse relied on delivering the alternatively spliced transgenes for LRAP, in addition to amelogenin lacking the hydrophilic C-terminal. This rescue model recovered some of the enamel structure and its prismatic architecture (Xia et al. 2016) (Fig. 5F), while inserting the LRAP transgene by itself failed to rescue the phenotype (Fig. 5G). Since LRAP comprises the C-terminal region of amelogenin, cleavage by MMP20 releases C-terminal peptides. Thus, the model (Fig. 6) suggests that the Amel−/− enamel phenotype improves as MMP20-cleaved amelogenin self-assembles into nanoribbons, possibly with inferior alignment, and mineralizes through the interaction with C-terminal peptides derived from LRAP.
Figure 5.
Enamel microstructures in mouse models by scanning electron microscopy (SEM) analysis. (A) Characteristic pattern of decussated enamel rods in wild-type mouse incisor. (B) Amel–knockout (KO) mouse with sharply reduced thickness of enamel, absence of prismatic structure, and mineral mostly comprising octacalcium phosphate (OCP). (C) Rescue of Amel-KO mouse using M180 transgene results in characteristic prismatic structure with slightly reduced thickness. (D) Rescue of Amel-KO mouse using MMP20-cleavage product failed to produce prismatic structure. (E) Rescue of double KO-mouse lacking Amel and MMP20 by inserting M180 transgene failed to produce prismatic structure. (F) Rescue of Amel-KO mouse inserting transgene for LRAP and MMP20-cleavage product shows significant improvement of phenotype with enamel rods developing but lack of rod organization. (G) Rescue of Amel-KO mouse inserting LRAP transgene does not recover prismatic structure. (H) Enam-KO mouse shows only minimal layer of mineral on dentin, without structural organization (Fig. A–D, F, and G adapted from Xia et al. 2016; Fig. E adapted from Pugach et al. 2013; Fig. H adapted from Hu et al. 2008).
Figure 6.
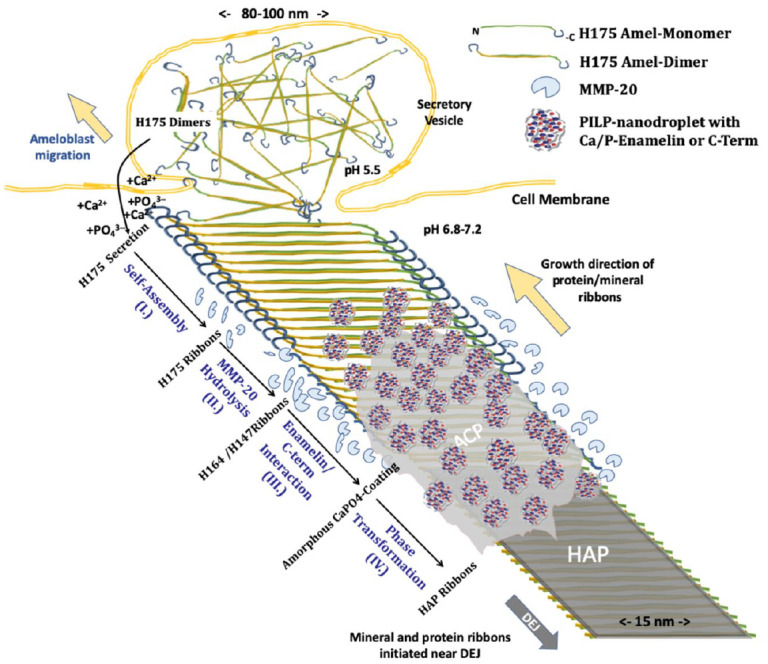
Mechanistic model of templated growth of apatite ribbons in enamel development. Full-length human amelogenin (H175) is exocytosed at Tomes’s process, possibly as an antiparallel dimer. Step I: Interaction with calcium and phosphate triggers self-assembly into nanoribbons. Step II: Matrix metalloproteinase 20 (MMP20) cleaves off hydrophilic C-termini at both edges of the nanoribbons. Step III: C-terminal peptide and/or MMP20-processed enamelin form aggregates with calcium and phosphate ions, for example, polymer-induced liquid precursor (PILP) nanodroplets, which interact with remaining nanoribbons comprising mostly H147 amelogenin. Unloading of calcium and phosphate ions from nanodroplets results in the deposition of amorphous mineral (amorphous calcium phosphate [ACP]) on the ribbon surface via the PILP process. Step IV: ACP transforms into crystalline apatite (hydroxyapatite [HAP]) directed by underlying amelogenin nanoribbons. HAP adopts ribbon-like morphology matching the approximate width of the protein template of 15 nm. Protein ribbon assembly and concomitant mineral growth advance as the ameloblasts recede and continue to exocytose matrix proteins; consequently, ribbons follow the cell pathway and create the intricate microstructure of prismatic enamel.
Other nonamelogenin proteins have also shown an essential role in enamel formation. The phenotype of the Enam−/− mice has been striking as ameloblasts were unable to form a substantial mineral layer on the dentin surface (Fig. 5H). In addition, many cases of amelogenesis imperfecta have been associated with ENAM mutations, indicating that enamelin is crucial for mineral induction at early stages of amelogenesis (Smith et al. 2016).
A Mechanistic Model of Amelogenin-Guided Mineralization in Enamel
Integrating the information from in vivo and in vitro studies, discussed above, a new mechanistic model of enamel biomineralization is proposed and described by the following sequence of extracellular events that determine protein structure and morphology of calcium phosphate mineral during the secretory stage of amelogenesis (Fig. 6):
Step I: Full-length amelogenin (H175) is exocytosed during the secretory stage and assembles into amyloid-like nanoribbons through calcium and phosphate ion bridges that connect β-sheet-rich domains in protein building blocks, creating a structural framework for subsequent mineralization. Due to the antiparallel arrangement, the C-termini are oriented toward the edges of nanoribbons and thus readily accessible to enzymatic hydrolysis.
Step II: An important model component is the passivating effect of full-length amelogenin to mineralization. Mineral in the form of ACP only forms on nanoribbons after the hydrophilic C-termini are cleaved off. Hence, MMP20 cleavage enables the remaining amelogenin nanoribbon to template the deposition of mineral.
Step III: Mineralization, however, is not initiated from exposure to saturated solutions directly but instead requires the interaction with a process-directing agent to initiate ACP formation. Phenotypic observations on mouse models suggest that enamelin and/or the cleaved C-termini of amelogenin may be able to act as such agents and form nanometer-sized aggregates similar to PILP nanodroplets (Khan et al. 2012). Such nanodroplets may be the origin of nanospherical structures observed by others in conjunction with filamentous protein (Bai and Warshawsky 1985; Pandya et al. 2017). Both enamelin and C-terminal peptides contain charged domains and are thus able to carry mineral ions to the proper organic framework, the enzymatically processed amelogenin nanoribbon. Upon interaction with PILP nanodroplets, calcium and phosphate ions are released and ACP deposits onto nanoribbons.
Step IV: ACP is metastable and transforms into crystalline apatite with time. This phase transformation results in 1- to 2-nm-thin and 10- to 20-nm-wide crystals with ribbon-like morphology as observed in secretory stage enamel and in vitro (Beniash et al. 2009; Bai et al. 2020). A variety of mechanisms have been proposed for oriented crystallization from amorphous precursors in biological systems. Epitaxial relationships between organic and inorganic phases appear pivotal to this process. Nonetheless, other pathways have been reported, for example, formation of mesocrystals through particle attachment in carbonate systems, suggesting structurally disordered proteins or the process-directing agents themselves direct the transformation into aligned single crystals (Rao and Colfen 2018).
While enamel mineral at the dentin surface may nucleate without amelogenin guidance (Hu, Smith, Cai, et al. 2016), the mechanism (Fig. 6) appears to be suited for the synthesis of enamel ribbons throughout the secretory stage. As the ameloblast retrieves and continues to exocytose matrix, amelogenin nanoribbons extend in length and allow for templating of mineral upon MMP20 hydrolysis. Ribbon orientation depends on the secretion site at the Tomes’s process and determines the organization into rod and interrod domains, as described previously (Nanci and Ten Cate 2013; Habelitz 2015).
Conclusions
Uniquely distinctive from abiotic mineralization, biomineralization is a complex, well-orchestrated event where mineral deposition, growth, and morphology are controlled from the nanometer to the macroscopic scale. Organic templates play a vital role in biomineralization, as demonstrated in dentin, bone, and shell formation, where collagen and chitin construct the structural foundation, respectively. This critical review concludes that similar mechanistic principles apply to enamel mineralization. The presence of calcium and phosphate is critical to proper folding of amelogenin and triggers its self-assembly into nanoribbons, which create an organic framework. In a regulatory manner, amelogenin processing by MMP20 activates the mineralization process. Deposition of ACP onto ribbon surfaces is facilitated with assistance by a yet to-be-determined, process-directing matrix component. Interaction between protein surface and amorphous mineral directs the transformation into oriented apatite ribbons. Continued secretion of extracellular matrix allows for extension of protein and mineral ribbons following the pathway of the ameloblasts, thus defining the foundations of the intricate architecture of dental enamel during the secretory stage, only to be finalized by protein removal and crystal expansion in the maturation stage of amelogenesis.
Author Contributions
S. Habelitz, Y. Bai, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211012925 for Mechanisms of Enamel Mineralization Guided by Amelogenin Nanoribbons by S. Habelitz and Y. Bai in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by the National Institutes of Health/National Institute of Dental and Craniofacial Research RO1-DE-027509.
References
- Bai P, Warshawsky H. 1985. Morphological studies on the distribution of enamel matrix proteins using routine electron microscopy and freeze-fracture replicas in the rat incisor. Anat Rec. 212(1):1–16. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yu Z, Ackerman L, Zhang Y, Bonde J, Li W, Cheng Y, Habelitz S. 2020. Protein nanoribbons template enamel mineralization. Proc Natl Acad Sci USA. 117(32):19201–19208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniash E, Metzler RA, Lam RS, Gilbert PU. 2009. Transient amorphous calcium phosphate in forming enamel. J Struct Biol. 166(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack FB, Huynh C, Marshman J, Goetze B. 2014. Helium ion microscopy of enamel crystallites and extracellular tooth enamel matrix. Front Physiol. 5:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchko GW, Tarasevich BJ, Bekhazi J, Snead ML, Shaw WJ. 2008. A solution NMR investigation into the early events of amelogenin nanosphere self-assembly initiated with sodium chloride or calcium chloride. Biochemistry. 47(50):13215–13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler WT. 1998. Dentin matrix proteins. Eur J Oral Sci. 106(Suppl 1):204–210. [DOI] [PubMed] [Google Scholar]
- Carneiro KM, Zhai H, Zhu L, Horst JA, Sitlin M, Nguyen M, Wagner M, Simpliciano C, Milder M, Chen CL, et al. 2016. Amyloid-like ribbons of amelogenins in enamel mineralization. Sci Rep. 6:23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Bromley KM, Moradian-Oldak J, DeYoreo JJ. 2011. In situ AFM study of amelogenin assembly and disassembly dynamics on charged surfaces provides insights on matrix protein self-assembly. J Am Chem Soc. 133(43):17406–17413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfen H. 2008. Single crystals with complex form via amorphous precursors. Angew Chem. 47(13):2351–2353. [DOI] [PubMed] [Google Scholar]
- Daculsi G, Kerebel B. 1978. High-resolution electron microscope study of human enamel crystallites: size, shape, and growth. J Ultrastruct Res. 65(2):163–172. [DOI] [PubMed] [Google Scholar]
- Deshpande AS, Fang PA, Zhang X, Jayaraman T, Sfeir C, Beniash E. 2011. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules. 12(8):2933–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVol RT, Sun CY, Marcus MA, Coppersmith SN, Myneni SC, Gilbert PU. 2015. Nanoscale transforming mineral phases in fresh nacre. J Am Chem Soc. 137(41):13325–13333. [DOI] [PubMed] [Google Scholar]
- Diekwisch TG, Berman BJ, Anderton X, Gurinsky B, Ortega AJ, Satchell PG, Williams M, Arumugham C, Luan X, McIntosh JE, et al. 2002. Membranes, minerals, and proteins of developing vertebrate enamel. Microsc Res Tech. 59(5):373–395. [DOI] [PubMed] [Google Scholar]
- Engelberth SA, Bacino MS, Sandhu S, Li W, Bonde J, Habelitz S. 2018. Progression of self-assembly of amelogenin protein supramolecular structures in simulated enamel fluid. Biomacromolecules. 19(10):3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Diekwisch TG, Lyaruu DM, Wright JT, Bringas P, Jr, Slavkin HC. 1995. Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J Struct Biol. 115(1):50–59. [DOI] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Simmer JP. 1999. The structural biology of the developing dental enamel matrix. J Struct Biol. 126(3):270–299. [DOI] [PubMed] [Google Scholar]
- George A, Veis A. 2008. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 108(11):4670–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher MJ, Daniel EJ, Travis DF, Kamhi S. 1965. Electron optical and x-ray diffraction studies of the organization of the inorganic crystals in embryonic bovine enamel. J Ultrastruct Res. 50(Suppl 7):1–77. [PubMed] [Google Scholar]
- Gotliv BA, Kessler N, Sumerel JL, Morse DE, Tuross N, Addadi L, Weiner S. 2005. Asprich: a novel aspartic acid-rich protein family from the prismatic shell matrix of the bivalve atrina rigida. Chembiochem. 6(2):304–314. [DOI] [PubMed] [Google Scholar]
- Gower LB. 2008. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 108(11):4551–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habelitz S. 2015. Materials engineering by ameloblasts. J Dent Res. 94(6):759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habelitz S, Hsu T, Hsiao P, Saeki K, Chien YC, Marshall SJ, Marshall GW. 2014. The natural process of biomineralization and in vitro remineralization of dentin lesions. In: McKittrick JM, Narayan R, editors. Advances in bioceramics and biotechnologies II ceramic transactions. Vol. 247. New York (NY): Wiley. p. 13–24. [Google Scholar]
- He X, Wu S, Martinez-Avila O, Cheng Y, Habelitz S. 2011. Self-aligning amelogenin nanoribbons in oil-water system. J Struct Biol. 174(1):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Hu Y, Smith CE, McKee MD, Wright JT, Yamakoshi Y, Papagerakis P, Hunter GK, Feng JQ, Yamakoshi F, et al. 2008. Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice.J Biol Chem. 283(16):10858–10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Smith CE, Cai Z, Donnelly LA, Yang J, Hu JC, Simmer JP. 2016. Enamel ribbons, surface nodules, and octacalcium phosphate in C57BL/6 Amelx–/– mice and Amelx+/– lyonization. Mol Genet Genomic Med. 4(6):641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Smith CE, Richardson AS, Bartlett JD, Hu JC, Simmer JP. 2016. MMP20, KLK4, and MMP20/KLK4 double null mice define roles for matrix proteases during dental enamel formation. Mol Genet Genomic Med. 4(2):178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodaikin A, Traub W, Weiner S. 1986. Protein conformation in rat tooth enamel. Arch Oral Biol. 31(10):685–689. [DOI] [PubMed] [Google Scholar]
- Khan F, Li W, Habelitz S. 2012. Biophysical characterization of synthetic amelogenin C-terminal peptides. Eur J Oral Sci. 120(2):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. 1997. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 386(6620):78–81. [DOI] [PubMed] [Google Scholar]
- Macias-Sanchez E, Willinger MG, Pina CM, Checa AG. 2017. Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre. Sci Rep. 7(1):12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Avila O, Wu S, Kim SJ, Cheng Y, Khan F, Samudrala R, Sali A, Horst JA, Habelitz S. 2012. Self-assembly of filamentous amelogenin requires calcium and phosphate: from dimers via nanoribbons to fibrils. Biomacromolecules. 13(11):3494–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian-Oldak J. 2001. Amelogenins: assembly, processing and control of crystal morphology. Matrix Biol. 20(5–6):293–305. [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J. 2012. Protein-mediated enamel mineralization. Front Biosci (Landmark Ed). 17:1996–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian-Oldak J, Leung W, Fincham AG. 1998. Temperature and pH-dependent supramolecular self-assembly of amelogenin molecules: a dynamic light-scattering analysis. J Struct Biol. 122(3):320–327. [DOI] [PubMed] [Google Scholar]
- Nanci A, Ten Cate AR. 2013. Ten cate’s oral histology: development, structure, and function. St. Louis (MO): Elsevier. [Google Scholar]
- Ndao M, Dutta K, Bromley KM, Lakshminarayanan R, Sun Z, Rewari G, Moradian-Oldak J, Evans JS. 2011. Probing the self-association, intermolecular contacts, and folding propensity of amelogenin. Protein Sci. 20(4):724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu LN, Jee SE, Jiao K, Tonggu L, Li M, Wang L, Yang YD, Bian JH, Breschi L, Jang SS, et al. 2017. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat Mater. 16(3):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman F, Bomans PH, George A, de With G, Sommerdijk NA. 2012. The role of the amorphous phase on the biomimetic mineralization of collagen. Faraday Discuss. 159:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, Hilbers PA, de With G, Sommerdijk NA. 2010. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 9(12):1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszta MJ, Cheng XG, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB. 2007. Bone structure and formation: a new perspective. Mater Sci Eng R Rep. 58(3–5):77–116. [Google Scholar]
- Pandya M, Lin T, Li L, Allen MJ, Jin T, Luan X, Diekwisch TGH. 2017. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Front Physiol. 8:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach MK, Li Y, Suggs C, Wright JT, Aragon MA, Yuan ZA, Simmons D, Kulkarni AB, Gibson CW. 2010. The amelogenin C-terminus is required for enamel development. J Dent Res. 89(2):165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach MK, Suggs C, Li Y, Wright JT, Kulkarni AB, Bartlett JD, Gibson CW. 2013. M180 amelogenin processed by MMP20 is sufficient for decussating murine enamel. J Dent Res. 92(12):1118–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Colfen H. 2016. Morphology control and molecular templates in biomineralization. In: Aparicio C, Ginebra M-P, editors. Biomineralization and biomaterials. Boston (MA): Elsevier. p. 51–93. [Google Scholar]
- Rao A, Colfen H. 2018. From solute, fluidic and particulate precursors to complex organizations of matter. Chem Rec [epub ahead of print 24 Mar 2018]. doi: 10.1002/tcr.201800003 [DOI] [PubMed] [Google Scholar]
- Rodriguez DE, Thula-Mata T, Toro EJ, Yeh YW, Holt C, Holliday LS, Gower LB. 2014. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 10(1):494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Yamauchi M, Abiko Y, Matsuda K, Crenshaw MA. 2000. In vitro apatite induction by phosphophoryn immobilized on modified collagen fibrils. J Bone Miner Res. 15(8):1615–1619. [DOI] [PubMed] [Google Scholar]
- Sanii B, Martinez-Avila O, Simpliciano C, Zuckermann RN, Habelitz S. 2014. Matching 4.7-Å XRD spacing in amelogenin nanoribbons and enamel matrix. J Dent Res. 93(9):918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WJ, Tarasevich BJ, Buchko GW, Arachchige RMJ, Burton SD. 2020. Controls of nature: secondary, tertiary, and quaternary structure of the enamel protein amelogenin in solution and on hydroxyapatite. J Struct Biol. 212(3):107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin NY, Yamazaki H, Beniash E, Yang X, Margolis SS, Pugach MK, Simmer JP, Margolis HC. 2020. Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor.J Biol Chem. 295(7):1943–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer JP, Richardson AS, Hu YY, Smith CE, Ching-Chun Hu J. 2012. A post-classical theory of enamel biomineralization . . . and why we need one. Int J Oral Sci. 4(3):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. 1998. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 9(2):128–161. [DOI] [PubMed] [Google Scholar]
- Smith CE, Hu Y, Hu JC, Simmer JP. 2016. Ultrastructure of early amelogenesis in wild-type, amelx–/–, and enam–/– mice: enamel ribbon initiation on dentin mineral and ribbon orientation by ameloblasts. Mol Genet Genomic Med. 4(6):662–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Hu Y, Richardson AS, Bartlett JD, Hu JC, Simmer JP. 2011. Relationships between protein and mineral during enamel development in normal and genetically altered mice. Eur J Oral Sci. 119(Suppl 1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasevich BJ, Lea S, Bernt W, Engelhard MH, Shaw WJ. 2009. Changes in the quaternary structure of amelogenin when adsorbed onto surfaces. Biopolymers. 91(2):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis DF, Glimcher MJ. 1964. The structure and organization of, and the relationship between the organic matrix and the inorganic crystals of embryonic bovine enamel. J Cell Biol. 23:447–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis A. 2011. Organic matrix-related mineralization of sea urchin spicules, spines, test and teeth. Front Biosci (Landmark Ed). 16:2540–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Ren A, Pugach MK. 2016. Truncated amelogenin and lrap transgenes improve amelx null mouse enamel. Matrix Biol. 52–54:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang J, Ma C, Lu J. 2020. Hydroxyapatite formation coexists with amyloid-like self-assembly of human amelogenin. Int J Mol Sci. 21(8):2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ramirez BE, Liao X, Diekwisch TG. 2011. Amelogenin supramolecular assembly in nanospheres defined by a complex helix-coil-PPII helix 3D-structure. PLoS One. 6(10):e24952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211012925 for Mechanisms of Enamel Mineralization Guided by Amelogenin Nanoribbons by S. Habelitz and Y. Bai in Journal of Dental Research