Abstract
Background
A gluten-free (GF) diet is the only treatment for coeliac disease (CD), non-adherence to the diet is associated with greater morbidity. The study aimed to examine the effect of a telephone clinic, designed to increase GF dietary knowledge and adherence, in adults with CD.
Methods
A prospective study of 125 patients with histologically confirmed CD. Patients, not adhering to a GF diet (n=30), engaged in a personalised telephone clinic. Validated questionnaires were used to assess GF dietary adherence (Coeliac Disease Adherence Test; CDAT), knowledge of GF foods and CD-related quality of life (QoL). GF dietary adherence was assessed up to 12 months post telephone clinic. The control group completed the questionnaires only.
Results
GF dietary adherence (CDAT) median scores significantly improved at 3 and 6 months after the telephone clinic compared with baseline (16, 13 and 13, respectively, p<0.01). Reassuringly, the dietary burden QoL score remained similar to baseline values. No change in CDAT scores were observed in the control group. Change in GF dietary knowledge score was associated with improved GF dietary adherence CDAT score (r=−0.22; p=0.039). At 9 and 12 months, CDAT scores were similar to baseline values.
Conclusions
Telephone clinics have a positive impact on dietary knowledge and GF dietary adherence in adults with CD, promoting health-benefitting behaviours in those previously not adhering to a GF diet. The study highlights the need for patients to have regular follow-up, with targeted reviews for those not adhering to a GF diet.
Keywords: coeliac disease, gluten free diet, celiac disease, nutrition
Significance of this study.
What is already known on this topic?
A gluten-free (GF) diet is the only treatment for coeliac disease, non-adherence to the diet is associated with greater morbidity.
National Institute for Health and Care Excellence (NICE) guidelines recommend adults with coeliac disease receive annual reviews. In 2020, due to the COVID-19 pandemic, there was a rapid shift towards telephone and online clinic appointments.
What this study adds?
Robust evidence that a telephone clinic provides an effective format to improve GF dietary adherence in adults who were not previously adhering to a GF diet.
Regular follow-up is important to maintain dietary adherence, particularly for those new to adhering to a GF diet.
How might it impact on clinical practice in the foreseeable future?
Telephone clinics can be confidently used as a format to improve GF dietary adherence in adults with coeliac disease.
Telephone clinics should be offered to patients beyond the current COVID-19 pandemic, as they reduce patient burden in attending outpatient or general practitioner appointments and are time, space and cost effective for the health service.
Introduction
Coeliac disease (CD) is an autoimmune disease with a worldwide prevalence of approximately 1%.1 Untreated, it is associated with inflammation within the small intestine and villous atrophy leading to malabsorption,2 anaemia,3 osteoporosis4 and malignancy.5 The only treatment available is adhering to a gluten-free diet (GFD).6 A GFD diet excludes wheat, barley and rye; grains commonly used in breads, pasta, breakfast cereals, cakes, biscuits and pastries. Adherence to a GFD is widely accepted to be challenging7; it requires knowledge, motivation and modified behaviours. Adherence to a GFD ranges between 42% and 91%8 dependent on patient group and method of recruitment.7 GF dietary adherence can be influenced by many factors including, coexisting depression, symptoms on ingestion of gluten, knowledge of GF foods, understanding of food labels, cost and availability of GF foods including receiving GF foods on prescription, and membership of a coeliac society.9 10 International guidelines advocate long-term follow-up to facilitate dietary adherence.11
To date, only four intervention studies in children or adults with CD have significantly improved GF dietary adherence, predominately in participants who were already adhering to a GFD and none undertaken in the UK. Interventions have included dietary and psychological counselling, and the use of online courses, follow-up appointments and text messages with mixed success reported. In India, adult patients with CD (n=92) who attended follow-up appointments had an improved GF dietary adherence, based on dietary history.12 Likewise, an Italian study demonstrated that the improving psychological well-being of patients (n=33) through counselling was associated with improved self-reported adherence to GFD.13 In contrast to this, an American study used repeated text messages to promote GF dietary adherence in children and young adults with CD (n=30), this had no impact on GF dietary adherence.14 Whereas an online educational programme in Australian improved GF dietary adherence in adults with CD (n=46) at 3 months, although only a small number (n=18) were not adhering to the diet at baseline15
Globally, a range of methods are used to enable clinicians to follow-up and review patients, including one-to-one appointments, group sessions, telephone clinics and online interfaces.16 17 Kurien et al 11 highlighted that work is required to establish a cost-effective way of delivering CD follow-up care, which is acceptable to both patients and healthcare professionals. Additionally, the demands on healthcare professionals within the health service are increasing and future follow-up outpatient care needs to be more flexible and informed by patients.18 Qualitative interviews with 34 adults with CD explored patient preference for the design of an intervention aiming to improve dietary adherence.19 Participants perceived telephone clinics as easy, flexible and convenient, as well as being considered as useful as a face-to-face clinic when considering GF dietary adherence.
Surprisingly, there is little research evaluating interventions to promote GF dietary adherence, and none evaluating the use of telephone clinics. While ‘Telehealth’ has been used sporadically in other areas with varying success,20 the current COVID-19 pandemic has resulted in many face-to-face clinics being undertaken via the telephone.21 There hitherto remain gaps in our evidence base as to whether such an intervention is effective in CD management. This study aims to evaluate the effect of a telephone clinic on GFD knowledge and GF dietary adherence in adults with CD.
Methods
A prospective controlled study using a telephone clinic, with an accompanying leaflet, targeting both CD-related knowledge and gluten consumption behaviour was undertaken by patients with histologically confirmed CD. The choice of intervention was informed from qualitative interviews with adults with CD, 30 of whom were not adhering to a GFD.19
Participants were recruited from Dudley General Hospital, identified through a database of patients with CD held within the Dietetic Department. Inclusion criteria required patients to be over the age of 18 years, resident in Dudley, and have histologically confirmed CD. All eligible patients with CD (n=195) were approached via postal invitation. All patients gave their informed consent for inclusion before they participated in the study. The control group were all adhering to the GF diet, thus not matched to the intervention group, all intervention participants were not adhering to the GF diet at baseline, it would have been unethical to allocate patients not adhering into a control group.
Primary outcome was a change in GF dietary adherence assessed by Coeliac Disease Adherence Test (CDAT) score (table 1).22 A score of 13 or less was previously determined to accurately predict an adequate adherence taking the assessment of an expert dietitian.23 Twenty-one patients in each group were calculated to be required to have a 90% chance of detecting a two point change in CDAT score (based on data from Sainsbury et al 15). Exclusion criteria were diagnosis of depression, as determined by Depression, Anxiety Stress Scale (DASS) questionnaire,24 as an association between depression and dietary adherence has been reported, though direction of causation remains unknown25
Table 1.
Coeliac Disease Adherence Test22
| Questions | 1 | 2 | 3 | 4 | 5 |
| Have you been bothered by low energy level during the past 4 weeks? | None of the time | A little of the time | Some of the time | Most of the time | All of the time |
| Have you been bothered by headaches during the past 4 weeks? | None of the time | A little of the time | Some of the time | Most of the time | All of the time |
| I am able to follow a gluten-free diet when dining outside my home | Strongly agree | Somewhat agree | Neither agree nor disagree | Somewhat disagree | Strongly disagree |
| Before I do something I carefully consider the consequences | Strongly agree | Somewhat agree | Neither agree nor disagree | Somewhat disagree | Strongly disagree |
| I do not consider myself a failure | Strongly agree | Somewhat agree | Neither agree nor disagree | Somewhat disagree | Strongly disagree |
| How important to your health are accidental gluten exposures? | Very important | Somewhat important | Neutral/unsure | A little important | Not at all important |
| Over the past 4 weeks, how many times have you eaten foods containing gluten on purpose? | 0–never | 1–2 | 3–5 | 6–10 | >10 |
The sum of the numeric values provide a score ranging from 7 to 35; lower scores reflect better adherence to a GFD.
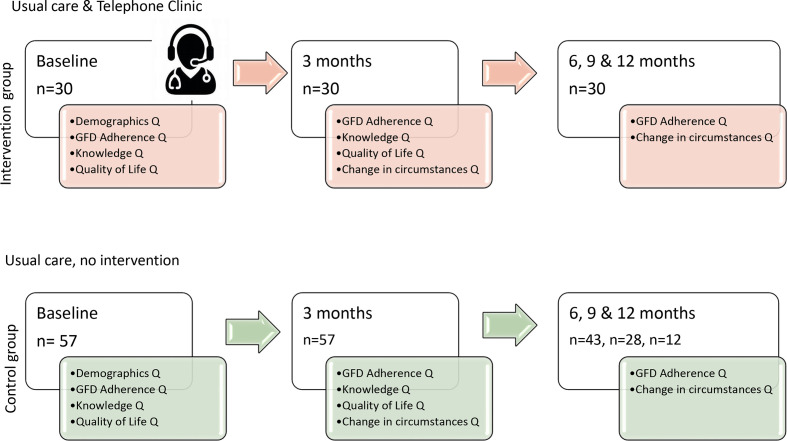
At baseline, and 3 months post intervention, information on demographics and circumstances relevant to CD were collected. GF dietary adherence, as per CDAT questionnaire,22 GFD knowledge and Coeliac Disease Quality of Life (Coeliac Disease Assessment Questionnaire (CDAQ)) validated questionnaires were completed by participants26 27 (figure 1). Thereafter, the CDAT and change in circumstances questionnaires were collected at 6, 9 and 12 months. Silvester et al’s GF knowledge questionnaire26 consisted of 17 foods, participants indicated if the food was ‘allowed’, ‘to question’ or ‘not allowed’. The GF knowledge score was cumulative and ranged from 0 to 17, with 17 the most knowledgeable in relation to a GFD. The CDAQ measured health-related QoL over the preceding 4 weeks, with 32 items, inclusive of dietary burden (8 items), scores were transformed to a 0–100 scale, where 100 is the highest QoL.
Figure 1.
Study protocol. GFD, gluten-free diet.
Telephone clinic intervention
The study leaflet on CD and GFD was posted to participants ahead of, and accompanied the telephone clinic. The leaflet contained images relating to knowledge, motivation and behaviour change to facilitate discussion, giving the clinic a standardised structure, agreed by a registered dietitian. A consultant gastroenterologist, with a Clinical Nutrition MSc and expertise in CD led the telephone consultations. The telephone clinic was a personalised intervention focusing on areas of knowledge or behaviour that was important to the participant (figure 2). The mean call duration was 49 min (SD 7.2, range 33–63 min).
Figure 2.
Telephone clinic content, with the time spent on topics dependent on the individual participants needs. CD, coeliac disease; GF, gluten free.
Data were analysed using SPSS statistical package V.26 (IBM Corp.). Data were assessed for normality. χ2 test, McNemar test, Mann-Whitney U, Wilcoxon signed-rank and Kruskal-Wallis tests were used to compare groups. Friedman test compared CDAT scores across time points, revealed with post hoc analysis using Wilcoxon Signed Rank Test using a Bonferonni adjusted alpha value significance at p=0.017. Data presented as median and IQR.
Results
Demographics and baseline data
One hundred and twenty-five adult patients with CD participated in the study (a 64% return rate; responders and non-responders were similar in age, sex and ethnicity: NS). All participants who had a CDAT score of ≥13 were classified as not adhering to the GF diet and were included in the intervention group (n=30) and all patients with a CDAT score of <13 formed the control group (n=95). The intervention group had a 100% completion rate at 3M, 6M, 9M and 12M. From the control group, 60% (n=57) completed all the questionnaires at baseline and 3 months.
The intervention and control groups were of similar age (mean 52 (range 35–70) years and 51 (32-94) years, respectively (p=0.64)) and with a similar predominance of female participants (77% and 68%, respectively; p=0.58). The majority of participants were Caucasian (100% and 82%) with 18% South Asian in the control group. The depression scores from the DASS questionnaire were similar for both groups (9 (7–11) and 9(7-10)respectively; p=0.6).
At baseline, participants adhering to the GF diet (control group) had a higher score for GF dietary knowledge compared with intervention group, in particular they correctly identified a higher number of GF foods (7 (6–7) compared with 6(5-6) for the intervention group (max score 7, p<0.001; table 2). The groups scored similarly on gluten-containing foods to avoid (3 (3–3) and 3(3-3)3 respectively (max score 3, p=0.89). Higher GF dietary knowledge scores were associated with better GF dietary adherence CDAT scores (r=−0.43, p<0.01, n=87).
Table 2.
Gluten-free (GF) dietary adherence, knowledge of GF foods and Quality of Life scores at baseline and 3 months for control and telephone clinic intervention groups
| Telephone clinic intervention | Control group | |||
| Baseline | 3 months F’up | Baseline | 3 months F'up | |
| Adherence, CDAT score | 16.0*† 14.0–17.3 |
13.0† 12.0–14.0 |
9.0* (8–11) |
9.0 (8–10) |
| Knowledge: total score | 13.5*† 12.0–14.0 |
15.0† 14.0–16.0 |
15*‡ (14–16) |
16‡ (15–16) |
| GF foods only knowledge score | 6.0*† (5.0–6.0) |
6.5† (6.0–7.0) |
7.0* (6.0–7.0) |
7.0 (6.0–7.0) |
| Quality of Life: CDAQ score | 49.0 (49.8–61.9) |
50.3 (48.0–55.4) |
51.5 (46.8–79.5) |
61.7 (54.7–76.7) |
| CDAQ: stigma | 50.0 (37.5–58.6) |
46.9 (39.1–59.4) |
43.8 (31.3–78.1) |
56.3 (40.6–65.6) |
| CDAQ: dietary burden | 50.0 (40.6–68.8) |
56.3 (50.0–68.8) |
50.0 (43.8–71.9) |
65.6 (53.1–71.9) |
| CDAQ: symptoms | 50.0* (43.8–56.3) |
50.0 (40.0–60.0) |
70.0* (55.0–82.5) |
70.0 (60.0–80.0) |
| CDAQ; social isolation | 50.0 40.0–73.8) |
50.0 (40.0–65.0) |
55.0 (45.0–82.5) |
65.0 (55.0–80.0) |
| CDAQ; worries and concerns | 56.3 (49.0–67.7) |
58.3 (45.9–75.0) |
58.3 (47.9–77.1) |
62.5 (50.0–75.0) |
Data presented as median (IQR).
Telelphone clinic intervention group n=30; control group n=57, Quality of Life questionnaire responses n=55.
CDAT score range 7–35. Knowledge score range 1–17; GF foods only scores 0–7, higher scores indicate better knowledge. CDAQ score range 1–100, with a higher score reflecting better quality of life.
*Significant difference between intervention and control group at baseline; Mann-Whitney U analysis.
†Significant difference between baseline and 3-month data.intervention group; Wilcoxon Signed Rank Test,
‡Significant difference between baseline and 3-month data. Control group; Wilcoxon Signed Rank Test, significant determined as p<0.05.
CDAQ, Coeliac Disease Assessment Questionnaire; CDAT, Coeliac Disease Adherence Test.
Response to the telephone clinic
The majority of the intervention group (90%; n=27) reported an ‘excellent’ or ‘high’ level of satisfaction with the telephone clinic, with the remainder of neutral opinion (10%: n=3). During the telephone clinic, 11 participants (37%) reported habitually consuming gluten-containing foods. Lack of motivation to adhere to a GF diet was reported as an issue by 70% (n=21) of participants.
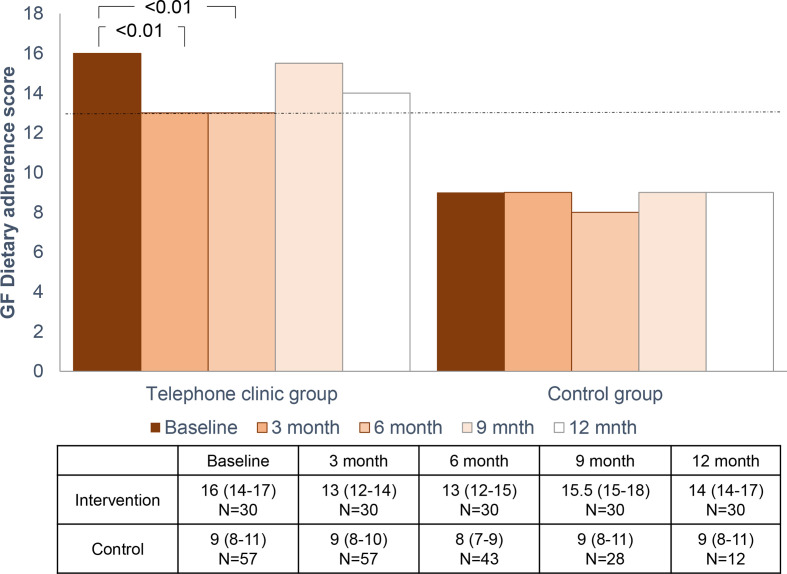
A significant improvement in GF dietary adherence scores was observed at 3 and 6 months after the telephone clinic compared with baseline scores (figure 3). Accidental gluten ingestion was considered as ‘somewhat/ very important’ in 87% and 83% of the intervention group at 3 and 6 months post telephone clinic compared with just 47% at baseline (p<0.001). Additionally, 87% and 84% of the intervention group reported ‘never consuming gluten over the previous 4 weeks’ 3 and 6 months post telephone clinic, compared with 63% at baseline (p=0.016). GF dietary adherence score improved in 83% of participants at 3 months compared with baseline values; the score remained over 13 in 36% of participants. GF dietary adherence score remained similar over time in the control group (figure 3).
Figure 3.
Median gluten-free (GF) dietary adherence score (Coeliac Disease Adherence Test (CDAT)) for intervention and control group at baseline, up to 12 months after the telephone clinic. At 3 months, CDAT scores improved from 16 to 13 (z=−4.162, p<0.01, with a large effect size (r=0.54), with no significant difference between 3-month and 6-month CDAT scores (p=0.39)). Friedman test, post hoc analysis using Wilcoxon Signed Rank Test using a Bonferonni adjusted alpha value. Whereas there was no significant difference in scores across time in the control group.
There was no detrimental change in QoL score in the intervention group (table 2), nor dietary burden subscore (50 (41–69) and 56 (50–69) at baseline and 3 months, respectively; p=0.25), QoL data not collected at 6 months. Likewise, the QoL scores remained similar for the control group.
GF knowledge score was significantly better at 3 months compared with baseline for intervention and control groups (z=−4.234, p<0.001, (r=0.55) and z=−3.849, p<0.001, (r=0.30) respectively, table 2). There was a greater change in GF knowledge score in the intervention group (2 (1–3) compared with the control group (0 (0–1); p<0.001). Half of the intervention group (50%) correctly identified all seven GF foods at 3 months, compared with just 13% at baseline. Change in GF dietary knowledge score was associated with change in GF dietary adherence CDAT score (r=−0.22; p=0.039, n=87).
Review of GF dietary adherence scores up to 12 months
At 9 and 12 months post telephone clinic, the median GF dietary adherence CDAT scores for the intervention group was 15.5 (15.0–18.8) and 14.0 (13.8–17), respectively, which did not differ significantly from baseline values.
Discussion
This is the first prospective controlled study to demonstrate a telephone clinic intervention improves GF dietary adherence, and remained effective for 6 months. The participant’s dietary knowledge improved, in particular their awareness of the GF foods they can consume. The study has also highlighted that patients, not adhering to a GF diet, need regular follow-up, as the changes were most effective for 6 months. This study provides clinically useful data to demonstrate the effectiveness of using such an intervention in clinic, as globally the use of telemedicine and video conferencing to replace face-to-face consultations has become more commonplace due to the COVID-19 pandemic.27
The telephone clinic improved dietary knowledge and this change in knowledge was associated with a positive change in GF dietary adherence. There are no directly comparable studies, although one online educational programme improved GF dietary adherence in adults with CD; however, this was not associated with a change in dietary knowledge.15 The telephone clinic had a personalised approach, with greater similarity to an outpatient clinic. A study which involved adults with CD who attended follow-up appointments demonstrated improved GF dietary adherence over a 6-month period; however, they did not collect data on dietary knowledge.12 A small study in the Netherlands, online consultations were compared with face-to-face appointments for children and young adults with CD, while they were unable to assess the effect on dietary adherence, they reported that online appointments improved CD-related QoL.28 Kallos and Jeanes17 reported a diverse range of dietetic annual review provision for adults with CD within the UK. It is likely that there are more offerings by telephone or online due to the lack of movement allowed during the COVID-19 pandemic. Croker et al 29 explored patient preferences for CD annual reviews; however, the delivery structure was not part of their study. Pritchard et al 30 reported CD follow-up provision by face-to-face appointment with a general practitioner (GP) compared with a nurse-led telephone clinic. Of the adults who were offered a telephone clinic, they were more likely to receive an annual review, have their symptoms assessed and their diet reviewed compared with those who had GP follow-up provision. Patients are diverse in their needs and preferences; thus, a telephone clinic is not suitable for all. However, several studies have shown patient preference for a telephone clinic to save them time and money compared with a conventional outpatient or GP appointment.19 30 A recent survey of Italian adults with CD (n=276) explored the impact of COVID-19 on the management of CD; they report 86% of the participants were happy with telemedicine as an alternative to face-to-face clinics.31
It is widely recognised that assessing dietary adherence is challenging.7 A limitation of the current study, and the majority of published studies in this area, is the lack of data on histological changes to the villi; this is predominately due to the invasive nature of the procedure. A proportion of the intervention participants dietary adherence CDAT scores did not go below 13; this could be partly reflective of the difficulty in quantifying adherence. Lau et al 32 have reported the superiority of combining two questionnaires, compared with serology, in detecting villous atrophy. We propose future studies should include a dietetic assessment of adherence within the telephone clinic and an additional validated questionnaire. Our study does have a number of strengths, all the participants had serology and biopsy-proven CD diagnosis, the intervention focused on those who were not adhering to a GF diet and while there was high attrition in the control group, the attrition rate for the intervention group was zero over a 12-month follow-up period.
Further study is required to compare face-to-face appointments with telephone clinics. While our intervention included discussions around behaviour change and motivation, no measures of these were undertaken. Future studies would benefit form a broader range of measurements, while continuing to take into consideration participant burden.
Within the UK, there is a broad range of follow-up provision,17 and a recent study highlighted the allocated time for clinics to be insufficient compared with time advocated in guidelines.33 Rather than guidance for a standard follow-up system for all adults with CD, we propose a system whereby patients who are not adhering to a GFD engage in a telephone clinic every 6 months, until adherence is sustained. Economic analysis of telephone clinics would be highly beneficial; there would be initial cost implications in areas where current provision is minimal, however, long-term savings due to reduced morbidity associated with gluten ingestion by patients with CD. The authors promote the use of a flexible approach to allow patients to choose whether they would prefer a telephone clinic rather than an outpatient appointment. The importance of personalised advice by a healthcare professional with expertise in CD has been re-enforced in this study. Telephone clinics can be confidently used as a format to improve dietary adherence in adults with CD.
Conclusion
Globally, there is a lack of robust studies exploring interventions to promote GF dietary adherence in adults with CD. Our study provides data for evidence-based practice, to enable clinicians to embrace telephone clinics as part of their follow-up and/or annual review provision for patients with CD.
Footnotes
Twitter: @DrYJeanes
Contributors: HM, SR and YMJ conceived the study. SI and JFM substantially contributed to the design of the work. HM collected the data. Data analysis was performed by HM, SR and YMJ. The manuscript was written by YMJ, HM and SR. All authors assisted in the review and approval of the final manuscript.
Funding: The study was part funded by a DSI International Nutrition Award; they had no input into the study design, analysis nor reporting of the findings.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Deidentified participant data are available upon reasonable request to y.jeanes@roehampton.ac.uk.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the procedures of the University of Roehampton Ethics Board and Health Research Authority approval REC number 17/EM/0056.
References
- 1. Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:823–36. 10.1016/j.cgh.2017.06.037 [DOI] [PubMed] [Google Scholar]
- 2. Reilly NR, Fasano A, Green PHR. Presentation of celiac disease. Gastrointest Endosc Clin N Am 2012;22:613–21. 10.1016/j.giec.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 3. Mahadev S, Laszkowska M, Sundström J, et al. Prevalence of celiac disease in patients with iron deficiency Anemia-A systematic review with meta-analysis. Gastroenterology 2018;155:374–82. 10.1053/j.gastro.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meyer D, Stavropolous S, Diamond B, et al. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 2001;96:112–9. 10.1016/S0002-9270(00)02300-5 [DOI] [PubMed] [Google Scholar]
- 5. Emilsson L, Semrad C, Lebwohl B, et al. Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Gastroenterology 2020. 10.1053/j.gastro.2020.07.007. [Epub ahead of print: 15 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 6. Rubio-Tapia A, Hill ID, Kelly CP, et al. Acg clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76. 10.1038/ajg.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muhammad H, Reeves S, Jeanes YM. Identifying and improving adherence to the gluten-free diet in people with coeliac disease. Proc Nutr Soc 2019;78:418–25. 10.1017/S002966511800277X [DOI] [PubMed] [Google Scholar]
- 8. Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther 2009;30:315–30. 10.1111/j.1365-2036.2009.04053.x [DOI] [PubMed] [Google Scholar]
- 9. Hanci O, Jeanes YM. Are gluten-free food staples accessible to all patients with coeliac disease? Frontline Gastroenterol 2019;10:222–8. 10.1136/flgastro-2018-101088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muhammad H, Reeves S, Ishaq S, et al. Adherence to a gluten free diet is associated with receiving gluten free foods on prescription and understanding food labelling. Nutrients 2017;9:705. 10.3390/nu9070705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurien M, Trott N, Sanders DS. Long-Term care for patients with coeliac disease in the UK: a review of the literature and future directions. J Hum Nutr Diet 2016;29:617–23. 10.1111/jhn.12379 [DOI] [PubMed] [Google Scholar]
- 12. Rajpoot P, Sharma A, Harikrishnan S, et al. Adherence to gluten-free diet and barriers to adherence in patients with celiac disease. Indian J Gastroenterol 2015;34:380–6. 10.1007/s12664-015-0607-y [DOI] [PubMed] [Google Scholar]
- 13. Ring Jacobsson L, Friedrichsen M, Göransson A, et al. Does a coeliac school increase psychological well-being in women suffering from coeliac disease, living on a gluten-free diet? J Clin Nurs 2012;21:766–75. 10.1111/j.1365-2702.2011.03953.x [DOI] [PubMed] [Google Scholar]
- 14. Haas K, Martin A, Park KT. Text message intervention (teach) improves quality of life and patient activation in celiac disease: a randomized clinical trial. J Pediatr 2017;185:62–7. 10.1016/j.jpeds.2017.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sainsbury K, Mullan B, Sharpe L. A randomized controlled trial of an online intervention to improve gluten-free diet adherence in celiac disease. Am J Gastroenterol 2013;108:811–7. 10.1038/ajg.2013.47 [DOI] [PubMed] [Google Scholar]
- 16. Sainsbury K, Mullan B, Sharpe L. Predicting intention and behaviour following participation in a theory-based intervention to improve gluten free diet adherence in coeliac disease. Psychol Health 2015;30:1063–74. 10.1080/08870446.2015.1022548 [DOI] [PubMed] [Google Scholar]
- 17. Kallos S, Jeanes Y. Cross-Sectional survey of the dietetic provision for adults with coeliac disease in the UK. J Hum Nutr Diet 2020;33:6–15. [Google Scholar]
- 18. Royal Colege of Physicians (RCP) . Outpatients: the future – adding value through sustainability, 2018. Available: https://www.rcplondon.ac.uk/projects/outputs/outpatients-future-adding-value-through-sustainability[Accessed July, 2020].
- 19. Muhammad H, Reeves S, Jeanes Y. Qualitative interviews to explore patient preference for health care led interventions to promote gluten free dietary adherence. Coeliac UK, 2019. https://www.coeliac.org.uk/document-library/5691-delegate-brochure-2019/?preview=true. (Accessed July, 2020). [Google Scholar]
- 20. Cottrell MA, Hill AJ, O'Leary SP, et al. Clinicians’ Perspectives of a Novel Home-based Multidisciplinary Telehealth Service for Patients with Chronic Spinal Pain. Int J Telerehabil 2018;10:81–8. 10.5195/IJT.2018.6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 2020;26:309–13. 10.1177/1357633X20916567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol 2009;7:530–6. 10.1016/j.cgh.2008.12.032 [DOI] [PubMed] [Google Scholar]
- 23. Villafuerte-Galvez J, Vanga RR, Dennis M, et al. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther 2015;42:753–60. 10.1111/apt.13319 [DOI] [PubMed] [Google Scholar]
- 24. Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 2005;44:227–39. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- 25. Sainsbury K, Marques MM. The relationship between gluten free diet adherence and depressive symptoms in adults with coeliac disease: a systematic review with meta-analysis. Appetite 2018;120:578–88. 10.1016/j.appet.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 26. Silvester JA, Weiten D, Graff LA, et al. Is it gluten-free? relationship between self-reported gluten-free diet adherence and knowledge of gluten content of foods. Nutrition 2016;32:777–83. 10.1016/j.nut.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alrubaiy L. Gastroenterology services in the time of COVID-19 pandemic. Frontline Gastroenterol 2020;11:257–8. 10.1136/flgastro-2020-101509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vriezinga S, Borghorst A, van den Akker-van Marle E, et al. E-Healthcare for celiac Disease-A multicenter randomized controlled trial. J Pediatr 2018;195:154–60. 10.1016/j.jpeds.2017.10.027 [DOI] [PubMed] [Google Scholar]
- 29. Crocker H, Jenkinson C, Peters M. Quality of life in coeliac disease: item reduction, scale development and psychometric evaluation of the coeliac disease assessment questionnaire (CDAQ). Aliment Pharmacol Ther 2018;48:852–62. 10.1111/apt.14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pritchard L, Waters C, Murray IA, et al. Comparing alternative follow-up strategies for patients with stable coeliac disease. Frontline Gastroenterol 2020;11:93–7. 10.1136/flgastro-2018-101156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siniscalchi M, Zingone F, Savarino EV, et al. COVID-19 pandemic perception in adults with celiac disease: an impulse to implement the use of telemedicine. Dig Liver Dis 2020;52:1071–5. 10.1016/j.dld.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau MS, Mooney PD, Rees MA, et al. Gluten free diet adherence assessment using CDAT and BIAGI questionnaires in patients with coeliac disease. Gut 2018;67:A160–1. [Google Scholar]
- 33. Rej A, Buckle RL, Shaw CC, et al. National survey evaluating the provision of gastroenterology dietetic services in England. Frontline Gastroenterol 2020;31:flgastro-2020-101493. 10.1136/flgastro-2020-101493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data are available upon reasonable request to y.jeanes@roehampton.ac.uk.