Abstract
Objectives
In recent years, hospital pharmacists have gained more importance in the clinical support of patients. However, most of the studies evaluating the impact of clinical pharmacy have only studied patients’ adherence or satisfaction. The aim of this study was to evaluate the direct clinical outcomes of a pharmacist-led educational intervention in patients with chronic disease.
Methods
We conducted a randomised, controlled, parallel, physician-blinded study in a day hospital and a consultation unit of a French teaching hospital over a 1-year period. Patients with hypertension, type 2 diabetes or hypercholesterolaemia who did not reach their therapeutic goals despite drug therapy were eligible. Patients in the intervention group received an intervention from a hospital pharmacist who provided patient education on pathology and drug management. The primary outcome was the proportion of patients reaching their therapeutic goals for blood pressure, glycated haemoglobin level or low-density lipoprotein cholesterol level at the 3-month follow-up consultation.
Results
From January to December 2015, 89 patients were included and 73 completed the study. In the intervention group, 61.7% (21/34) of the patients reached their therapeutic goals compared with 33.3% (13/39) in the control group (p=0.015). The intervention was significantly more effective in polypharmacy patients (60.0% (12/20) vs 16.7% (4/24); p=0.005), in those aged >60 years (57.9% (11/19) vs 26.1% (6/23); p=0.037) and in patients with a high education level (68.8% (11/16) vs 29.4% (5/17); p=0.024).
Conclusion
A single pharmacist-led educational intervention has a clinical impact, doubling the proportion of patients reaching their therapeutic goals at 3 months, especially in polypharmacy patients and those aged >60 years. This study confirms the value of clinical involvement of hospital pharmacists in patient care in a consultation unit and day hospital.
Keywords: clinical pharmacy, education, pharmacy, pharmacy service, hospital, hypertension, education, pharmacy, continuing
Introduction
In recent years, hospital pharmacists have gained more and more importance in the clinical support of patients,1 particularly through the advent of clinical pharmacy.2 Pharmacists’ interventions in clinical services are valued by healthcare providers3 4 and result in reducing medication errors,5 6 drug-related hospitalisation,7 healthcare cost8 9 and even mortality.10 When addressed to patients, pharmacist interventions improved their satisfaction and decreased non-adherence to medication.11–13
Non-adherence to medication has become a new public health burden. The WHO estimates that 50% of patients with chronic disease do not take their treatment properly,14 which has been confirmed by epidemiological and experimental studies.15 Besides increasing morbidity and mortality,16 non-adherence has been estimated to cost 100–300 billion dollars annually of avoidable healthcare costs in the USA.15
By increasing patients’ abilities to understand and live with their treatment, an educational intervention should promote behaviour changes in patients and therefore increase their adherence to medication. Thus, through this increased adherence, a pharmacist-led educational intervention should increase clinical outcomes. But is it really the case? All adherence evaluating methods have limitations,17 and an increase in the adherence score does not necessarily mean an improvement in the patient's clinical condition. Moreover, improving adherence to a treatment is not a final goal but only a way to ultimately improve the patient's clinical condition. To date, few studies have investigated the impact of a pharmacist-led educational intervention on the clinical condition of patients. A recent exhaustive review of randomised controlled studies evaluating general practice‐based pharmacist interventions on pathology parameters in patients with hypertension, type 2 diabetes or dyslipidaemia identified only 21 studies, which were of variable quality.18 Despite the small number of these studies, there are very encouraging results in decreasing pathology parameters. However, the decrease in a pathology parameter does not mean the patient will reach the recommended therapeutic target, and to date few studies have focused on the achievement of therapeutic goals set by physicians or recommendations. Moreover, to our knowledge, no such study has been conducted in France.
In this context, we conducted a randomised controlled physician-blinded clinical study to evaluate the impact in terms of clinical outcomes of a pharmacist-led educational intervention in patients treated for hypertension, hypercholesterolaemia or type 2 diabetes but not reaching their therapeutic goals.
Methods
Screening and recruitment
Patients were recruited between 1 January and 31 December 2015 in the Hypertension and Cardiovascular Prevention Unit, Diagnosis and Therapeutic Centre, Hôtel-Dieu Hospital, Assistance Publique des Hôpitaux de Paris (AP-HP), Paris. The inclusion period was limited due to human resource limitations and was set before the study started.
Inclusion criteria were: patients being regularly followed by a physician of the unit, having at least one chronic pathology among hypertension, hypercholesterolaemia and type 2 diabetes, and not reaching their therapeutic goals despite drug treatment. Patients aged <18 years, pregnant women and non-French speakers were non-eligible for inclusion.
Study design
This was a single-site, single-blind, randomised, controlled, parallel study. Eligible patients were included by the physician at the end of their medical consultation. The pharmacist randomised the patients into the control group or the intervention group using a 1:1 ratio with a randomisation list not seen by the physicians. The physician was not aware of the inclusion group. The intervention group received a single pharmacist intervention at the hospital, directly after the inclusion, just after leaving the physician’s office. The outcomes were measured at 3 months, during a follow-up medical consultation as part of their usual medical care. For ethical reasons, the control group also received a pharmacist intervention after the 3-month follow-up consultation (figure 1).
Figure 1.
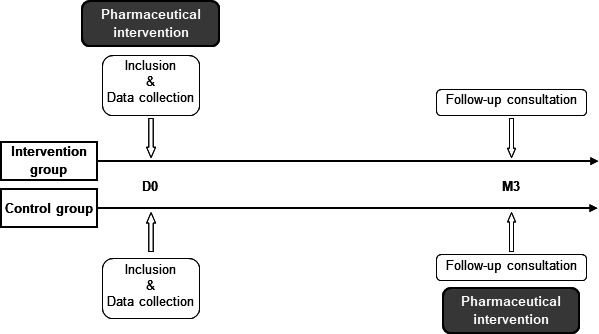
Study design. Patients in the intervention group had a pharmacist intervention on the day of inclusion (D0; day 0). Both groups had a follow-up consultation at 3 months (M3) after the inclusion, and the control group had a pharmacist intervention after the follow-up consultation.
Data collection and treatments
Glycated haemoglobin (HbA1c) levels, blood pressure (BP), plasma low-density lipoprotein cholesterol (LDL-c) levels and other information such as the number of drugs prescribed, other chronic diseases, age and kidney and liver functions were obtained from the patients’ medical records. The presence of side effects and the information used to estimate the social level were collected during the inclusion interview. The adherence score was calculated using a six-item yes/no questionnaire. Each item scored one point, and a high score meant low adherence. Questions were asked by the pharmacist during the inclusion visit. Polypharmacy was defined as a daily intake of five different drugs or more, following the methodology of previous studies.19 20 The social level was estimated based on the education level and the Bachelor’s Degree: 1=education level below high school diploma, 2=education level equivalent to high school diploma, 3=education level higher than high school diploma.
Intervention
The same hospital pharmacist (CD) performed all the interventions. The intervening pharmacist received 40 hours of training in therapeutic education as required by French legislation to lead a therapeutic patient education programme. Interventions took place in the Cardiology Day Hospital or in the Diagnosis and Therapeutic Centre in the Hôtel-Dieu Hospital, Paris. The intervention consisted of providing patient education on pathology management and advice on how to deal with the pathology on a daily basis. The intervention frame for hypertension was set following the French Society of Hypertension recommendations for the “information and announce consultation”.21 The hypertension frame was adapted for hypercholesterolaemia and type 2 diabetes. The following items were discussed using open questions:
Pathology: definition, origins, consequences, follow-up, non-drug treatment and nutritional hygienic rules
Drug mechanisms of action
Posology and drug intake modalities
Adherence and behaviour in case of missed dose
Drug side effects and their handling
Drug interactions and self-medication
Medication plan elaboration (if necessary)
Temporality and chronic aspects
Patient feelings and perception
The discussion was an open interaction with the patient and all topics on which he/she had questions were addressed. The point of this intervention was to determine the patient’s knowledge and capabilities regarding his/her pathology in order to focus on lacking knowledge.
Data and statistical analysis
Number of subjects needed
Based on the physicians’ experience, outcomes were empirically estimated at 25% of patients reaching therapeutic goals in the control group versus 50% in the intervention group. With a power of 90% and a one-sided error of 5%, the estimated number of patients to be included in the study was 126.
Descriptive analysis
We compared the intervention and control group populations based on age, body mass index, sex, social level, kidney function, liver function, number of chronic diseases, number of diseases involved in the study, number of different drugs prescribed, side effects at inclusion, adherence score at inclusion, pathology used for primary outcome and number lost to follow-up.
Primary outcome
The primary outcome was the proportion of patients reaching their therapeutic goals set by the physician at inclusion: HbA1c for type 2 diabetes, LDL-c blood levels for hypercholesterolaemia and systolic BP for hypertension. The objectives were set individually for each patient by the physician in accordance with French or international recommendations for hypertension,21 type 2 diabetes22 and hypercholesterolaemia23 depending on individual parameters (eg, LDL-c goals <1.15 g/L in patients at moderate cardiovascular risk and <1 g/L in patients at high cardiovascular risk). If the patient had more than one pathology, only the achievement of the therapeutic goal for the main pathology was considered for the primary outcome. This main pathology was designated by the physician prior to inclusion as the one to be treated as a priority in terms of cardiovascular prevention. As all patients randomised in the intervention group received the intervention, we performed intention to treat (ITT) analysis.24
Main analysis
As the intervention was not a drug administration, patients lost to follow-up did not leave the study because of side effects or ineffectiveness of the medication. We can therefore assume that missing data are missing at random. We thus performed a main analysis, excluding missing values.
Sensitivity analysis
To take into account missing data due to drop-out of patients, we also performed a sensitivity analysis according to the ‘best case/worst case scenario’ principle. In the ‘best case scenario’ analysis we considered all drop-outs as they reached their therapeutic goals in the intervention group and as they did not in the control group. In the ‘worst case scenario’ we considered drop-outs as they reached therapeutic goals in the control group and as they did not in the intervention group.25
We proceeded to subgroup analysis of the number of patients reaching their therapeutic goals depending on total drugs prescribed, main pathology involved, age and social level.
Statistical analysis
To compare the participants and their distribution in the two different groups, we used a Student t-test, a χ2 test or a Fisher test. A Pearson correlation test was used to determine the correlation between age and the number of drugs. Statistical tests were performed with GraphPad Prism 5. P values <0.05 were considered significant.
Results
Study population
Between 1 January and 31 December 2015, 89 patients were included. The follow-up period ended on 30 April 2016. Baseline characteristics were similar across the groups and did not differ significantly on any criteria (table 1).
Table 1.
Patient characteristics at baseline
| Intervention group (n=45) |
Control group (n=44) |
P value (Student t-test or χ² test) |
|
| Age (years) | 59.4±3.9 | 61.7±4.0 | 0.407* |
| Body mass index (kg/m2) | 27.2±1.4 | 28.3±1.7 | 0.323* |
| No of men (n) | 30 (66.7%) | 25 (56.8%) | 0.339† |
| Social level (n) | |||
| 1 | 14 (31.1%) | 19 (43.2%) | 0.419† |
| 2 | 11 (24.4%) | 7 (15.9%) | |
| 3 | 20 (44.4%) | 18 (40.9%) | |
| Kidney failure (n) | 6 (13.3%) | 8 (18.2%) | 0.530† |
| Liver failure (n) | 0 (0%) | 1 (2.3%) | NA |
| No of total chronic diseases (n) | |||
| 1 | 11 (24.4%) | 8 (18.2%) | 0.613† |
| 2 | 14 (31.1%) | 12 (27.3%) | |
| >2 | 20 (44.4%) | 24 (54.5%) | |
| No of diseases involved in the study (n) | |||
| 1 | 37 (82.2%) | 36 (81.8%) | 0.960† |
| Hypertension | 24 (53.3%) | 22 (50.0%) | 0.613† |
| Type 2 diabetes | 5 (11.1%) | 7 (15.9%) | |
| Hypercholesterolaemia | 8 (17.8%) | 7 (15.9%) | |
| 2 | 7 (15.6%) | 8 (18.2%) | 0.777† |
| Hypertension + type 2 diabetes | 6 (13.3%) | 6 (13.6%) | 1† |
| Hypertension + hypercholesterolaemia | 1 (2.2%) | 0 (0.0%) | NA |
| Type 2 diabetes + hypercholesterolaemia | 0 (0.0%) | 2 (4.5%) | NA |
| 3 | 1 (2.2%) | 0 (0.0%) | NA |
| No of different drugs prescribed | 5.0±0.8 | 6.2±1.2 | 0.118* |
| Adjusted no of different drugs prescribed (n) | |||
| <5 | 18 (40.0%) | 18 (40.9%) | 0.930† |
| ≥5 | 27 (60.0%) | 26 (59.1%) | |
| Side effects (n) | 9 (20%) | 11 (25.0%) | 0.572† |
| Adherence score | 2.0±0.4 | 2.2±0.4 | 0.501* |
| Patients with hypertension (n) | 32 (71.1%) | 28 (63.6%) | 0.452† |
| Used as primary outcome (n) | 27 (60.0%) | 28 (63.6%) | 0.724† |
| Patients with type 2 diabetes (n) | 12 (26.7%) | 15 (34.1%) | 0.446† |
| Used as primary outcome (n) | 9 (20.0%) | 7 (15.9%) | 0.615† |
| Patients with hypercholesterolaemia (n) | 10 (22.2%) | 9 (20.5%) | 0.839† |
| Used as primary outcome (n) | 9 (20%) | 9 (20.5%) | 0.957† |
| Lost to follow-up (n) | 11 (24.4%) | 5 (11.4%) | 0.108† |
Data are expressed as proportion of patients and number of patients for dichotomous variables, and as mean±95% CI for continuous variables.
*Student t-test.
†χ2 test.
Sixteen patients were lost to follow-up (figure 2). Baseline characteristics were not significantly different between drop-out patients and those who completed the study (online supplemental file). However, trends emerged in some subpopulations which seemed more prone to loss to follow-up including low social level, women or patients with type 2 diabetes.
Figure 2.
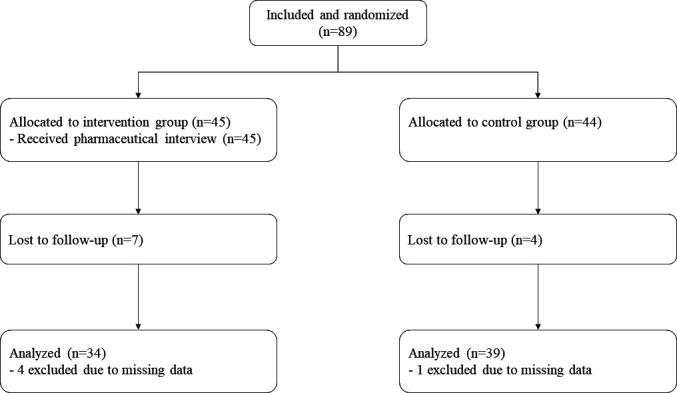
Number of patients included in the study and those lost to follow-up.
ejhpharm-2021-002787supp001.pdf (102.4KB, pdf)
Duration
Interventions lasted for 10–90 min, with an average of 36.1 min (95% CI 31.6 to 40.6) and a median of 35 min (first–last quartile: 25–45 min).
Primary outcome
The main analysis reported a significantly higher proportion of patients reaching their therapeutic goals in the intervention group than in the control group (61.7% (21/34) vs 33.3% (13/39); p=0.015) (table 2).
Table 2.
Intention to treat main and sensitivity analysis, and subgroup analysis at the 3-month follow-up consultation
| Intervention group % (N/n) |
Control group % (N/n) |
P value | |
| Primary outcome | |||
| Main analysis | 61.7% (21/34) | 33.3% (13/39) | 0.015*‡ |
| Sensitivity analysis | |||
| ‘Best case scenario’ | 71.1% (32/45) | 29.5% (13/44) | <0.001†‡ |
| ‘Worst case scenario’ | 46.7% (21/45) | 40.9% (18/44) | 0.584‡ |
| Subgroup analysis | |||
| No of chronic diseases | |||
| 1 | 77.7% (7/9) | 50.0% (4/8) | 0.335§ |
| 2 | 70.0% (7/10) | 33.3% (3/9) | 0.179§ |
| >2 | 46.7% (7/15) | 27.3% (6/22) | 0.225‡ |
| No of prescribed drugs | |||
| <5 | 64.3% (9/14) | 60.0% (9/15) | 0.812‡ |
| ≥5 | 60.0% (12/20) | 16.7% (4/24) | 0.005*§ |
| Social level | |||
| 1 | 37.5% (3/8) | 37.5% (6/16) | 1§ |
| 2 | 70.0% (7/10) | 33.3% (2/6) | 0.302§ |
| 3 | 68.8% (11/16) | 29.4% (5/17) | 0.024*‡ |
| Age (years) | |||
| <60 | 66.7% (10/15) | 50.0% (7/14) | 0.362‡ |
| ≥60 | 57.9% (11/19) | 26.1% (6/23) | 0.037*‡ |
| Study disease | |||
| Hypertension | 62.5% (15/24) | 38.5% (10/26) | 0.089‡ |
| Type 2 diabetes | 33.3% (3/9) | 10.0% (1/10) | 0.303§ |
| Hypercholesterolaemia | 80.0% (4/5) | 37.5% (3/8) | 0.266§ |
Data are expressed as percentage of patients reaching their therapeutic goals.
*p<0.05.
†p<0.001.
‡χ2 test.
§Fisher test.
N, number reaching therapeutic goal.
In the sensitivity analysis, the difference between the two groups was higher in the ‘best case scenario’, with 71.1% (32/45) of patients reaching their therapeutic objectives in the intervention group compared with 29.5% (13/44) in the control group (p<0.0001). This difference was lower in the ‘worst case scenario’, with 46.7% (21/45) of patients in the intervention group and 40.9% (18/44) in the control group reaching their therapeutic objectives (p=0.584) (table 2).
Subgroup analysis
Number of prescribed drugs
The number of patients reaching their therapeutic goal varied depending on the number of drugs prescribed. In patients taking <5 different drugs, no difference was observed between the two groups with 64.3% (9/14) in the intervention group and 60.0% (9/15) in the control group (p=0.812). However, in patients taking ≥5 different drugs, 60.0% (12/20) reached their therapeutic objectives in the intervention group compared with 16.7% in the control group (4/24) (p=0.05) (table 2).
Age
The proportion of patients reaching their therapeutic goals did not differ in patients aged <60 years, with 66.7% (10/15) in the intervention group and 50.0% (7/14) in the control group (p=0.362). In patients aged >60 years the difference was significant, with 57.9% (11/19) in the intervention group and 26.1% (6/23) in the control group (p=0.037) (table 2).
Age and number of drugs prescribed
The number of prescribed drugs was correlated to the age in our sample (r=0.42; p<0.00001) (data not shown). After adjusting for the number of prescribed drugs, the proportion of patients reaching their therapeutic goals was the same, whatever their age or study group for patients who took <5 different drugs (table 3). In patients who took ≥5 different drugs, the proportion was higher in the intervention group in patients aged >60 years (57.1% (8/14) vs 15.8% (3/19); p=0.024). In patients aged <60 years the difference was not significant (66.7% (4/6) vs 20.0% (1/5); p=0.242).
Table 3.
Proportion and number of patients achieving their therapeutic goals by age and number of drugs prescribed
| Control group | Intervention group | P value | |
| <5 drugs prescribed | |||
| Age <60 years | 60.0% (6/10) | 66.7% (6/9) | 1† |
| Age ≥60 years | 60.0% (3/5) | 60.0% (3/5) | 1† |
| ≥5 drugs prescribed | |||
| Age <60 years | 20.0% (1/5) | 66.7% (4/6) | 0.242† |
| Age ≥60 years | 15.8% (3/19) | 57.1% (8/14) | 0.024*† |
*p<0.05.
†Fisher test.
Social level
In the control group, the proportion of patients achieving their therapeutic goals decreased as the social level increased, from 37.5% (6/16) to 29.4% (5/17) (table 2). On the other hand, in the intervention group the proportion increased with the social level, from 37.5% (3/8) to 68.8% (11/16) (table 2). There was no difference between the two groups for lower education levels, with 37.5% in both groups (6/16 and 3/8). The difference appeared for middle education levels, with 70.0% (7/10) in the intervention group and 33.3% (2/6) in the control group (p=0.302), and became significant for higher education levels with 68.8% (11/16) in the intervention group and 29.4% (5/17) in the control group (p=0.024).
Main pathology involved
The proportion of patients reaching their therapeutic goals seemed to vary depending on the pathology involved, with a higher proportion for hypertension and hypercholesterolaemia both in the control and in the intervention group (table 2). However, the small sample sizes do not allow us to come to a definite conclusion on an effect in these subpopulations.
Discussion
The main analysis revealed a clinical impact of a single pharmacist-led educational intervention in chronic patients with hypertension, type 2 diabetes and hypercholesterolaemia. The proportion of patients reaching the therapeutic goals set by the physician almost doubled at 3 months (from 33.3% to 61.7%), which is consistent with some previous studies.26–28 The population lost to follow-up did not differ from the rest of the patients included, confirming the suitability of an ITT main analysis excluding the missing data. This difference was also seen in the sensitivity analysis, logically higher and significant in the ‘best case scenario’. In the ‘worst case scenario’, the difference was no more significant but still suggested a positive effect of the intervention.
Given the small sample sizes, it is difficult to interpret the results of subgroup analysis, which are more exploratory. According to a previous study, the benefit of a pharmacist intervention seemed greater in polypharmacy patients.29 In the control group, the number of patients achieving their therapeutic goals decreased conversely with the total number of drugs prescribed. This result is consistent with the literature, which reports a decrease in compliance with an increase in the number of daily doses,30 31 and an increase in the risk of drug interaction and side effects in polypharmacy patients.32 The benefit of the intervention was also greater in older patients. However, in our study the number of drugs prescribed was correlated with the age of the patients, as previously described.33 Samples are too small to state if one of these two parameters is more predictive of a larger effect than the other. Thus, it would be interesting in further studies to investigate if age might be a confounding factor.34 The intervention appeared to be more effective in patients with a higher social level as no effect was seen in patients with a low social level. However, due to the small samples, we cannot conclude with certainty the absence of effect in this subpopulation.
Drop-out patients were not significantly different from those who completed the study. However, there was a strong trend that drop-outs in the intervention group were female diabetics of lower socioeconomic class. It would be interesting to confirm these trends and to identify the specific characteristics of drop-out patients in larger studies. This could then allow adaptation of interventions to apply specifically and more effectively to these populations.
The time required to discuss all topics with the patient was 35 min. In France, the average duration of a consultation with a general practitioner is 10–16 min.35 Unfortunately, even if physicians are aware of the importance of listening to and educating patients about their drug management, they cannot afford to spend 20 min with all their patients in addition to their regular consultations. This implies the need to have these interventions performed by a practitioner other than the physician, with a good knowledge of medicines, whose activity would be at least partially devoted to these interventions. This finding also highlights the difficulty of offering this kind of intervention to all chronic patients in the course of their routine care. It would therefore be necessary to target these pharmacist interventions to specific patient populations where they are most effective and/or necessary (eg, polypharmacy and older patients).
Limitations of the study
The time limitation of the study did not allow us to include the estimated number of patients. However, the measured effect of the intervention (61.7%) was higher than the empirical estimation (50%) we used to determine the number of patients to include. Thus, despite these small samples, we still found a significant difference between groups.
The same pharmacist performed all the interventions, which gave a better reproducibility of interventions during the study. However, this makes it difficult to dissociate the effect related to the interpersonal relationship between the pharmacist and the patient from the effect of the content of the information transmitted. This should be taken into account in potential future studies if they involve several different pharmacists, all of whom should have the same patient education training prior to these studies.
We explored the effect of the pharmacist intervention over a period of only 3 months. However, some studies have reported a temporary and short-term effect of this kind of intervention.11 36 Thus, further studies should focus on the long-term effect, as well as the need for repeated interventions several times a year, in person or by telephone.12 29
Conclusion
This study proved the effectiveness of a pharmacist-led educational intervention in patients with hypertension, type 2 diabetes or hypercholesterolaemia by doubling the proportion of patients reaching their therapeutic goals after 3 months of drug therapy. It also defined the populations most likely to benefit from these interventions, such as those on polypharmacy and older patients. It confirmed the clinical implications of involving hospital pharmacists in patient care in a hospital unit and also in a consultation unit and day hospital.
However, further studies with larger samples are needed to confirm the trends observed in the subpopulations. Long-term multicentre studies should also be conducted to refine our results. These studies, supplemented by economic studies, would then provide a comprehensive view of the full consequences of routinely implementing such practices.
What this paper adds.
What is already known on this subject?
Adherence is a major health issue as an estimated 50% of chronic patients are non-adherent
A pharmacist intervention seems to increase the satisfaction and adherence to treatments of patients with chronic conditions
There is a need for proof of the value of these interventions on direct clinical and public health outcomes
What this study adds
Evidence of improved clinical outcomes for patients with pharmacist care
A single pharmacist intervention doubled the number of patients reaching their therapeutic goals in hypertension, type 2 diabetes and hypercholesterolaemia
Patients on polypharmacy and those aged >60 years are preferential target populations
Acknowledgments
The authors thank all the investigators of the present study: Sandrine Kretz, Hélène de Champs Léger, Alexandra Yannoutsos, Céline Dreyfuss and Caroline Touboul. The authors would also like to thank Rémi Corne for editorial assistance and all the patients who agreed to participate in this study.
Footnotes
Twitter: @ClementDelage
Contributors: CD, HL, FB and JB designed the study. CD analysed the data and wrote the manuscript with support from HL. All authors discussed the results and contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data available on request due to privacy/ethical restrictions.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by an Ethics Committee (Comité de Protection des Personnes d’Île-de-France no 1) in agreement with the European Medicines Agency Guidelines for Good Clinical Practice, and declared to the ANSM, the French Medicines Agency (no. 2015-A00228-41). Each patient was informed of the purpose of the study, received an information letter and signed a consent form before inclusion.
References
- 1. Barnett NL. Opportunities for collaboration between pharmacists and clinical pharmacologists to support medicines optimisation in the UK. Br J Clin Pharmacol 2019;85:1666–9. 10.1111/bcp.13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Clinical Pharmacy . The definition of clinical pharmacy. Pharmacotherapy 2008;28:816–7. 10.1592/phco.28.6.816 [DOI] [PubMed] [Google Scholar]
- 3. Treu CN, Llamzon JL, Acquisto NM, et al. The impact of an emergency medicine clinical pharmacist on nursing satisfaction. Int J Clin Pharm 2019;41:1618–24. 10.1007/s11096-019-00927-y [DOI] [PubMed] [Google Scholar]
- 4. Hampson N, Ruane S. The value of pharmacists in general practice: perspectives of general practitioners-an exploratory interview study. Int J Clin Pharm 2019;41:496–503. 10.1007/s11096-019-00795-6 [DOI] [PubMed] [Google Scholar]
- 5. Bedouch P, Charpiat B, Conort O, et al. Assessment of clinical pharmacists’ interventions in French hospitals: results of a multicenter study. Ann Pharmacother 2008;42:1095–103. 10.1345/aph.1L045 [DOI] [PubMed] [Google Scholar]
- 6. Naseralallah LM, Hussain TA, Jaam M. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin Pharm 2020:1–16. [DOI] [PubMed] [Google Scholar]
- 7. Sloeserwij VM, Hazen ACM, Zwart DLM, et al. Effects of non-dispensing pharmacists integrated in general practice on medication-related hospitalisations. Br J Clin Pharmacol 2019;85:2321–31. 10.1111/bcp.14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Renaudin P, Coste A, Audurier Y, et al. Clinical, economic, and organizational impact of the clinical pharmacist in an orthopedic and trauma surgery department. J Patient Saf 2018;Publish Ahead of Print. 10.1097/PTS.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 9. Jourdan J-P, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm 2018;40:1474–81. 10.1007/s11096-018-0733-6 [DOI] [PubMed] [Google Scholar]
- 10. Zhai X-B, Gu Z-C, Liu X-Y. Clinical pharmacist intervention reduces mortality in patients with acute myocardial infarction: a propensity score matched analysis. Eur J Hosp Pharm 2019;26:248–52. 10.1136/ejhpharm-2017-001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296:2563–71. 10.1001/jama.296.21.joc60162 [DOI] [PubMed] [Google Scholar]
- 12. Abughosh SM, Wang X, Serna O, et al. A pharmacist telephone intervention to identify adherence barriers and improve adherence among nonadherent patients with comorbid hypertension and diabetes in a Medicare advantage plan. J Manag Care Spec Pharm 2016;22:63–73. 10.18553/jmcp.2016.22.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilhelmsen NC, Eriksson T. Medication adherence interventions and outcomes: an overview of systematic reviews. Eur J Hosp Pharm 2019;26:187–92. 10.1136/ejhpharm-2018-001725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Adherence to long-term therapies – evidence for action, 2003. Available: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1
- 15. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 16. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772–9. 10.1016/j.ahj.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 17. Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm 2014;36:55–69. 10.1007/s11096-013-9865-x [DOI] [PubMed] [Google Scholar]
- 18. Alshehri AA, Jalal Z, Cheema E, et al. Impact of the pharmacist-led intervention on the control of medical cardiovascular risk factors for the primary prevention of cardiovascular disease in general practice: a systematic review and meta-analysis of randomised controlled trials. Br J Clin Pharmacol 2020;86:29–38. 10.1111/bcp.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiapella LC, Montemarani Menna J, Mamprin ME. Assessment of polypharmacy in elderly patients by using data from dispensed medications in community pharmacies: analysis of results by using different methods of estimation. Int J Clin Pharm 2018;40:987–90. 10.1007/s11096-018-0663-3 [DOI] [PubMed] [Google Scholar]
- 20. Pazan F, Petrovic M, Cherubini A, et al. Current evidence on the impact of medication optimization or pharmacological interventions on frailty or aspects of frailty: a systematic review of randomized controlled trials. Eur J Clin Pharmacol 2021;77:1–12. 10.1007/s00228-020-02951-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blacher J, Halimi J-M, Hanon O, et al. Prise en charge de l’hypertension artérielle de l’adulte. Recommandations 2013 de la Société française d’hypertension artérielle. La Presse Médicale 2013;42:819–25. 10.1016/j.lpm.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 22. French High Health Authority (HAS) and French Medicine Agency (ANSM) . Therapeutic strategy for glycemic control of type 2 diabetes, 2013. Available: https://www.has-sante.fr/portail/upload/docs/application/pdf/2013-02/10irp04_reco_diabete_type_2.pdf
- 23. Mach F, Baigent C, Catapano AL, De Backer G, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 24. White IR, Carpenter J, Horton NJ. Including all individuals is not enough: lessons for intention-to-treat analysis. Clin Trials 2012;9:396–407. 10.1177/1740774512450098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sackett D. Decide how to handle missing data in the analysis. In: Haynes R, Sackett D, Guyatt G, et al., eds. Clinical epidemiology: how to do clinical practice research. Lippincott Williams & Wilkins, 2012: 128–9. [Google Scholar]
- 26. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–67. 10.1001/jama.299.24.2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cripps RJ, Gourley ES, Johnson W, et al. An evaluation of diabetes-related measures of control after 6 months of clinical pharmacy specialist intervention. J Pharm Pract 2011;24:332–8. 10.1177/0897190010392235 [DOI] [PubMed] [Google Scholar]
- 28. Cazarim MdeS, de Freitas O, Penaforte TR, et al. Impact assessment of pharmaceutical care in the management of hypertension and coronary risk factors after discharge. PLoS One 2016;11:e0155204. 10.1371/journal.pone.0155204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Odeh M, Scullin C, Fleming G, et al. Ensuring continuity of patient care across the healthcare interface: telephone follow-up post-hospitalization. Br J Clin Pharmacol 2019;85:616–25. 10.1111/bcp.13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296–310. 10.1016/S0149-2918(01)80109-0 [DOI] [PubMed] [Google Scholar]
- 31. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009;15:e22–33. [PubMed] [Google Scholar]
- 32. Hohl CM, Dankoff J, Colacone A, et al. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med 2001;38:666–71. 10.1067/mem.2001.119456 [DOI] [PubMed] [Google Scholar]
- 33. Huon J-F, Lenain E, LeGuen J, et al. How drug use by French elderly patients has changed during the last decade. Drugs Real World Outcomes 2015;2:327–33. 10.1007/s40801-015-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herr M, Robine J-M, Pinot J, et al. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf 2015;24:637–46. 10.1002/pds.3772 [DOI] [PubMed] [Google Scholar]
- 35. Kandel O, Ripault A, Jourdain M, et al. Does the duration of medical consultations have an impact on the prescription of psychotropic drugs? Cross-sectional study carried out in general practice on 2,896 procedures. Rev Prat 2008;58:19–24. [PubMed] [Google Scholar]
- 36. Mehuys E, Van Bortel L, De Bolle L, et al. Effectiveness of a community pharmacist intervention in diabetes care: a randomized controlled trial. J Clin Pharm Ther 2011;36:602–13. 10.1111/j.1365-2710.2010.01218.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2021-002787supp001.pdf (102.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data available on request due to privacy/ethical restrictions.


