Abstract
Background
Even with global efforts to prevent medication errors, they still occur and cause patient harm. Little systematic research has been done in Norway to address this issue.
Objectives
To describe the frequency, stage and types of medication errors in Norwegian hospitals, with emphasis on the most severe and fatal medication errors.
Methods
Medication errors reported in 2016 and 2017 (n=3557) were obtained from the Norwegian Incident Reporting System, based on reports from 64 hospitals in 2016 and 55 in 2017. Reports contained categorical data (eg, patient age, incident date) and free text data describing the incident. The errors were classified by error type, stage in the medication process, therapeutic area and degree of harm, using a modified version of the WHO Conceptual Framework for the International Classification for Patient Safety.
Results
Overall, 3372 reports were included in the study. Most medication errors occurred during administration (68%) and prescribing (24%). The leading types of errors were dosing errors (38%), omissions (23%) and wrong drug (15%). The therapeutic areas most commonly involved were analgesics, antibacterials and antithrombotics. Over half of all errors were harmful (62%), of which 5.2% caused severe harm, and 0.8% were fatal.
Conclusions
Medication errors most commonly occurred during medication administration. Dosing errors were the most common error type. The substantial number of severe and fatal errors causing preventable patient harm and death emphasises an urgent need for error-prevention strategies. Additional studies and interventions should further investigate the error-prone medication administration stage in hospitals and explore the dynamics of severe incidents.
Keywords: clinical pharmacy, organisation of health services, risk management, medication errors, quality management, medication safety
Introduction
Medication errors are recognised as a major patient safety problem. WHO has a goal of globally reducing avoidable harm related to medications by 50%, by 2022.1 2 Medication errors occur in all stages of the medication management process3 and may lead to patient harm, prolonged hospital stay, readmission or death.4 Based on data from error reporting systems, most medication errors occur in the administration stage, and the most common types of errors are wrong dosage errors.5 6
Measures to improve medication safety in hospitals have been taken, such as implementing computerised prescriber order entry, electronic medication administration record, bar code medication administration, automated dispensing devices and other clinical decision support systems.7 Despite such measures, medication errors still occur and cause significant patient harm and even death.4 5
There have been numerous case reports and media stories on medication errors in Norwegian hospitals,8 9 but little systematic research on medication errors has been done. A National Patient Safety Program was established in 2014, but medication errors were not among the target areas to improve patient safety.10 To be able to monitor safety in the medication management process, identify unsafe practice and implement safety measures, one has to learn from errors.4 The aim of this study is to describe the frequency, stage and error types, as well as analyse the harm caused by the medication errors reported to the mandatory Norwegian Incident Reporting System (NIRS).
Methods
Study design and data source
This was a retrospective study of medication errors reported to the NIRS, from 1 January 2016 to 31 December 2017. The NIRS, placed under the Norwegian Directorate of Health, was a mandatory, anonymous, electronic, reporting and learning system of incident reports from all hospitals across Norway. In the 2-year study period, 64 hospitals in 2016 and 55 hospitals in 2017 reported errors. The NIRS received approximately 10 000 incident reports yearly, of which about 20% were medication errors. The most frequently reported incident categories were clinical procedures, medication errors and patient accidents.
Data collection and processing
Incident reports consisted of categorical data (eg, patient age, incident date, day of the week, etc) and free-text data (eg, incident description, description of the cause, patient consequences, prevention measures, caseworker’s comments, etc). Some reports were short, whereas others had detailed free-text descriptions of the incidents. We thoroughly read all the reports. Employees (caseworkers) at the NIRS had classified two-thirds of the reports by error type and stage in the medication process in which the error occurred, we classified the remaining one-third of the reports. The classification system was a modified version of the Conceptual Framework for the International Classification for Patient Safety (WHO).11 The error types were as follows: wrong patient, wrong drug, wrong dose/strength or frequency, wrong route, wrong dispensing label or instruction, wrong storage, contraindication, omitted medicine or dose and adverse drug reaction. The stages in the medication process were as follows: prescribing (as well as transcribing, documenting and reconciliation failure), preparation/dispensing, administration and storage. The drugs involved in the errors were not dedicated to a specific field in the reporting form. However, when possible, we extracted the drug name and the therapeutic area at Anatomical Therapeutic Chemical (ATC) level 2.12 The statistical analysis was performed with IBM SPSS V.25.
Definitions and exclusion/inclusion process
We defined a medication error according to the National Coordinating Council for Medication Error Reporting and Prevention as ‘any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient or consumer’.13
Based on this definition, we excluded incident reports of suicide events and intentional overdoses, side effects or adverse drug reactions that occurred as a result of an appropriate medication process, as well as reports which were not from a hospital setting. We included adverse drug reactions caused by medication errors (eg, administering drugs to patients with known allergies). Both harmful errors and those not causing patient harm were included.
The degree of harm was classified according to the following five-point scale11: (1) no harm: an incident had the potential to cause harm, but was prevented (near miss) or ran to completion, but no harm occurred; (2) low harm: a patient required extra observation or minor treatment; (3) moderate harm: significant, but no permanent harm, where the patient required treatment measures; (4) severe harm: significant treatment/harm that required surgery, transfer to an intensive care unit, a prolonged hospital stay or permanent harm and (5) death: the error may have contributed to or resulted in a patient’s death. Incident reports with insufficient information to classify the degree of harm were coded as missing.
Understanding the context of the data
The data on medication errors provided a rich description of a large variety of medication errors from health personnel. For us, it was important to gain a broader understanding of the research questions, the nature of the reported incidents and the setting where they occurred. We therefore held several meetings with the NIRS employees who gave valuable insight into the classification of errors. We performed fieldwork observations of medication preparation, dispensing and administration, in two hospital wards by shadowing nurses on medication rounds. We also performed a semi-structured interview with two employees devoted to quality of care in the hospital.
Results
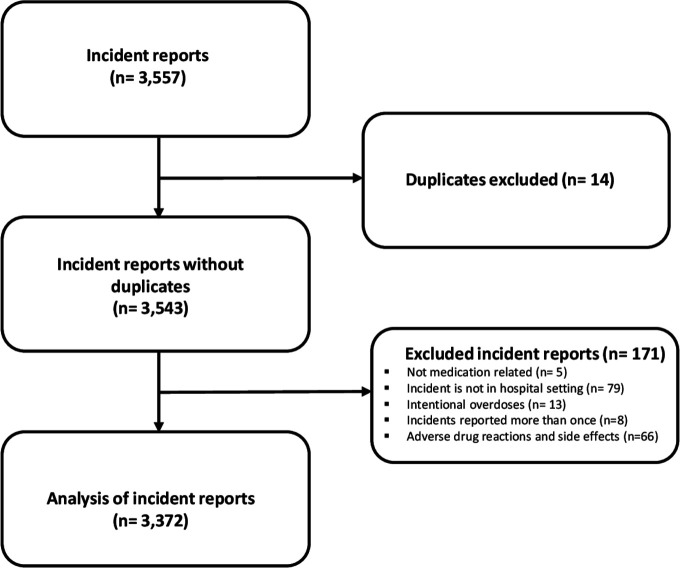
In total, 3557 medication errors were reported from Norwegian hospitals to the NIRS. Of these, 185 were excluded because they were side effects or adverse drug reactions, not from a hospital setting, not medication-related, intentional overdoses or duplicates (figure 1). The 3372 medication error incidents that met the inclusion criteria are shown in table 1. Errors were classified as originating in the administration stage (68%), prescribing stage (24%) or preparation/dispensing stage (6%) of the medication process. The most commonly reported error types were wrong dose/strength or frequency (38%), omissions (23%) and wrong drug (15%) (table 1).
Figure 1.
Inclusion and exclusion of reported incidents to the Norwegian Incident Reporting System in 2016 and 2017.
Table 1.
Medication error characteristics
| Characteristic | N | % |
| Year of reporting | ||
| 2016 | 1780 | 53.4 |
| 2017 | 1572 | 46.6 |
| Total | 3372 | 100.0 |
| Medication process stage* | ||
| Administration | 2544 | 67.8 |
| Prescribing | 888 | 23.7 |
| Preparation/Dispensing | 231 | 6.2 |
| Storage | 87 | 2.3 |
| Error type* | ||
| Wrong dose/strength or frequency (total) | 1354 | 37.5 |
| Omitted medicine or dose | 836 | 23.2 |
| Wrong drug | 548 | 15.2 |
| Wrong route | 198 | 5.5 |
| Contraindication | 191 | 5.3 |
| Wrong patient | 186 | 5.2 |
| Wrong formulation or presentation | 94 | 2.6 |
| Adverse drug reaction | 77 | 2.1 |
| Wrong dispensing label/instruction | 66 | 1.8 |
| Wrong storage | 60 | 1.7 |
| Reported by | ||
| Nurse | 2103 | 62.4 |
| Physician | 385 | 11.4 |
| Other staff | 169 | 5.0 |
| Leader | 71 | 2.1 |
| Bioengineer/Engineer | 43 | 1.3 |
| Midwife | 37 | 1.1 |
| Missing | 564 | 16.7 |
| Total | 3372 | 100.0 |
| Patient age (years) | ||
| 0–9 | 184 | 5.5 |
| 10–19 | 101 | 3.0 |
| 20–29 | 183 | 5.4 |
| 30–39 | 202 | 6.0 |
| 40–49 | 240 | 7.1 |
| 50–59 | 342 | 10.1 |
| 60–69 | 559 | 16.6 |
| 70–79 | 734 | 21.8 |
| 80–89 | 524 | 15.5 |
| 90–112 | 180 | 5.3 |
| Missing | 123 | 3.6 |
| Total | 3372 | 100.0 |
| Degree of harm | ||
| No harm | 1272 | 37.7 |
| Low harm | 1277 | 37.9 |
| Moderate harm | 538 | 16.0 |
| Severe harm | 177 | 5.2 |
| Death | 27 | 0.8 |
| Missing | 81 | 2.4 |
| Total | 3372 | 100.0 |
*The total number of error types and medication process stages was greater than the number of incidents because the classification system permitted more than one category to be selected for one incident.
In total, 39% (988/2544) of the errors in the administration stage were due to wrong dose/strength/frequency errors (further in the text referred to as wrong dose errors) (online supplementary appendix A). Within the prescribing stage, wrong dose errors account for 46% (410/888) of errors.
ejhpharm-2020-002298supp001.pdf (220.3KB, pdf)
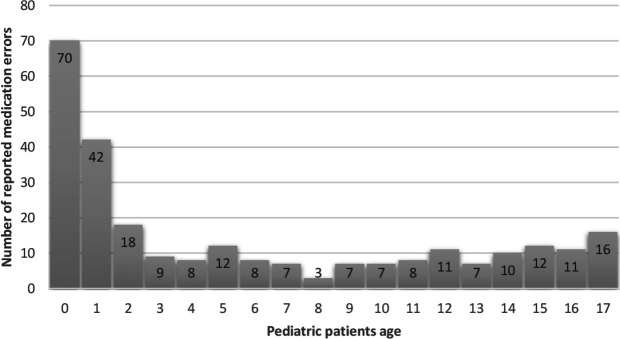
Patient age ranged from 0 to 112 years. The majority of patients who experienced medication errors were aged over 65 years (50.8%), while there were 266 children (<18 years) who experienced medication errors (figure 2). The majority of severe and fatal errors were reported for patients aged over 65 years (59%).
Figure 2.
Distribution of medication errors in the paediatric patients reported to the Norwegian Incident Reporting System in 2016 and 2017.
In the reported errors, 62% caused patient harm, of which 5.2% caused severe harm and 0.8% were fatal errors (n=27) (table 2). Most of the fatal errors were due to wrong dose errors, while the most severe harm errors were due to medication omissions (table 2).
Table 2.
Severe harm and fatal reports from the Norwegian Incident Reporting System in 2016 and 2017
| All reported errors n (%) | Severe n (%) | Death n (%) | |
| Total number of errors | 3372 | 177 (5.2) | 27 (0.8) |
| Error type* | |||
| Wrong dose/strength or frequency | 1354 (37.5) | 47 (27) | 13 (48) |
| Omitted medicine or dose | 836 (23.2) | 57 (32) | 6 (22) |
| Adverse drug reaction | 77 (2.1) | 24 (13.6) | 3 (11) |
| Wrong drug | 548 (15.2) | 20 (11.3) | 1 (3.7) |
| Wrong route | 198 (5.5) | 10 (5.6) | 1 (3.7) |
| Contraindication | 191 (5.3) | 24 (13.6) | 4 (14.8) |
| Other | 406 (11.3) | 14 (7.9) | 0 (N/A) |
| Medication process stage* | |||
| Administration | 2544 (68) | 96 (54) | 16 (59.3) |
| Prescribing | 888 (23.7) | 70 (39.5) | 11 (40.7) |
| Other | 118 (8.5) | 11 (6.5) | 0(N/A) |
| Health professionals reporting | |||
| Nurse | 2103 (62.4) | 48 (27.0) | 3 (11.0) |
| Physician | 385 (11.4) | 70 (40.0) | 16 (59.0) |
| Other health professionals | 884 (26.0) | 59 (33.0) | 8 (29.0) |
| Patient age (years) | |||
| 0–17 | 266 | 10 | 1 |
| 18–65 | 1283 | 63 | 5 |
| >65 | 1698 | 100 | 21 |
| Missing | 125 | 4 | 0 |
*The total number of error types and medication process stages was greater than the number of incidents because the classification system permitted more than one category to be selected for one incident.
The majority of errors were reported by nurses (62%) and physicians (11%). However, 42% of severe and fatal errors were reported by physicians, with only 25% reported by nurses (table 2).
The therapeutic areas (ATC level 2) most frequently involved in errors were analgesics (N02), antibacterials for systemic use (J01) and antithrombotic agents (B01) (online supplementary appendix B). Medications most commonly associated with death were analgesics and antithrombotic agents. Antithrombotic agents were most commonly associated with severe harm, including 25% of all fatal errors, making it the most harmful therapeutic area in the reported incidents (online supplementary appendix B).
ejhpharm-2020-002298supp002.pdf (225.5KB, pdf)
Table 3 exemplifies the richness of qualitative descriptions in the incident reports of five severe and three fatal cases, with the assigned medication process stage, error type and therapeutic area all specified.
Table 3.
Incident description of severe harm and fatal errors reported to the Norwegian Incident Reporting System in 2016 and 2017, with the assigned medication process stage, error type and therapeutic subgroup
| Incident information | Incident description |
| Error type: contraindication Degree of harm: severe Patient age (years): 18–65 Medication process: administration Antithrombotic agents (B01) |
A patient received his usual antithrombotic (apixaban) prior to surgery, although a contraindication existed. After surgery, the patient experienced bleeding in the throat and underwent another surgery to stop the bleeding. |
| Error type: wrong dose/strength/frequency Degree of harm: severe Patient age (years): 18–65 Medication process: administration Analgesics (N02) |
A patient has received 50 mg oxycodone, but was initially prescribed 5 mg. The 10-fold dose was incorrectly transcribed from the previous record in the commentary field, while the prescription was correct. The patient became drowsy and experienced apnoea episodes up to 30 s over several hours. The patient received naloxone antidote to reverse the opiate effect. |
| Error type: wrong dose/strength/frequency Degree of harm: severe Patient age (years): >65 Medication process: administration Intravenous solutions: electrolytes |
A patient with hypocalcaemia should have recieved 0.3 mmol/kg of CaCl according to his weight of 100 kg. The junior doctor showed the doctor in charge how she had calculated the dose, ie, 0.3 mmol/kg×100 kg=130 mmol. The doctor in charge did not spot the wrongly calculated dose of 130 mmol, instead of the correct 30 mmol. The patient became acutely ill, was moved to the intensive care unit and was given fluids to eliminate the calcium and continuous heart monitoring. |
| Error type: wrong storage Degree of harm: severe Patient age (years): 18–65 Medication process: administration Therapeutic subgroup: missing |
The patient was readmitted to the hospital 3 days after discharge, with a stomach ache. The CT scan revealed a foreign object in the small intestine. The next day, the patient had a tablet of an intact blister pack surgically removed from the small intestine; there was a rupture and suture of two areas within the damaged intestinal wall. The blister pack had not been removed when the tablet was administered/ingested. |
| Error type: wrong drug Degree of harm: severe Patient age (years): 18–65 Medication process: prescribing Psycholeptics (N05) |
The physician prescribed olanzapine even though the patient’s medical record stated a severe reaction to this type of neuroleptics, and that he should only receive quetiapine or clozapine. The patient developed the neuroleptic malignant syndrome, was in a life-threatening state and was hospitalised for several weeks with intensive monitoring. |
| Error type: wrong route Degree of harm: death Patient age (years): 0–17 Medication process: administration Antineoplastic agents and immunomodulating agents (L01–L04) |
The patient was prescribed two drugs, methotrexate (intrathecal) and vincristine (intravenous). During administration, the vincristine syringe was mixed up with the methotrexate syringe, and injected intrathecally. The error was intercepted after 25 min but it was too late. The child died due to the consequences of the histotoxic drug. Vincristine was delivered in a syringe similar to methotrexate. This was a well-known error and risk in hospitals. |
| Error type: omitted medicine or dose Degree of harm: death Patient age (years): 18–65 Medication process: prescribing Antithrombotic agents (B01) |
The patient had knee surgery previously and was discharged. The patient was readmitted to the hospital in a critical state. Tests showed multiple bilateral pulmonary embolisms. The patient was very obese and no thrombosis prophylaxis was stated on his discharge report. The patient died. |
| Error type: wrong dose/strength/frequency Degree of harm: death Patient age (years): >65 Medication process: prescribing Antibacterials for systemic use (J01) |
A patient with renal failure was to be prescribed vancomycin. The physician prescribed 3 g, while the nurse responded that the dose seemed very high. The physician however confirmed that the dose should be given. The patient died the day after the 3 g dose was administered. |
Discussion
Our data share similarities with the published literature, including the error type, stage of the medication process and therapeutic area involved in errors.3 5 In contrast to other incident reporting systems, we found a large proportion of harmful errors (62%) and a substantial number of errors associated with severe harm and death. This provides unique data to discuss error preventing strategies to target the most harmful medication errors, which are likely to have the highest impact on patient safety.14
Medication process stage and error type
Two-thirds of the reported errors occurred in the administration stage, and in line with other studies, the majority of severe and fatal errors occurred at this stage.3 6 15 16 The administration stage represents the last step in the medication process before the patient receives the drug, and therefore errors are less likely to be detected and intercepted by other health professionals.17 However, some studies, particularly from the USA, have found errors in the prescribing stage to be the most commonly reported.5 This contrast to our findings could be due to the high implementation grade of technologies in the USA to prevent administration errors, for example, bar code medication administration.7
Wrong dose was the most common error type in our study, accounting for 38% of all errors. One systematic review of medication administration errors found similar results,15 as did a review of prescribing errors in hospitals, which found that dosage errors were most commonly reported by a majority of studies.18 In our study, wrong dose errors were the error type associated with the highest severity of harm. Every fourth severe harm error and half of the fatal errors were dosage errors.
An interesting finding is that 73% of the dosing errors occurred during administration. One would normally expect the wrong dose errors to stem from the prescribing/preparation/dispensing stage. However, as the preparation, dispensing and administration are (usually) nurse’s tasks in Norwegian hospitals, it is possible that these processes are taking place simultaneously (eg, in the patient’s room), especially with intravenous medications (preparing and dispensing while administrating shortly after), and hence are all reported as administration errors. One study that compared medication errors from the UK (wrong dose—the most common error type) and USA (omission—the most common error type) incident reporting systems described the difference in the frequency of dosing errors as a reflection of the two countries’ different medication management practices.5 A plausible explanation could be that in the USA, pharmacists typically prepare and dispense unit doses, whereas in the UK, and Norway, those tasks are performed by nurses at the wards. More training and knowledge in handling drugs should be provided to nurses, as they are usually the last step in medication management. Changing the Norwegian hospital drug distribution systems could be an opportunity to reduce wrong dose errors.17 Technological improvements, an increase in ready-to-use medication and improved cooperation between the wards and the hospital pharmacy could reduce medication preparation and dispensing errors including wrong dose errors (ie, calculation errors).
In Norway, prescriptions issued by hospital physicians are not routinely reviewed by clinical pharmacists, and therefore prescribing errors are difficult to spot, despite that hospital physicians in Norway have been shown to make four times as many prescribing errors as general practitioners.19
The severity of harm
We found that 5.2% of all medication errors were associated with severe harm, and 0.8% were fatal. A study that compared the medication errors reported to the US and UK’s incident reporting systems from intensive care units showed that the percentage of events associated with severe harm was below 1%, and death below 0.1%, in both systems.5 We found a much higher rate of harmful errors compared with other incident reporting systems.3 5 6 16 However, it is difficult to make a clear judgement as to why our data differ. The high rate of harmful errors in our data say more about trends in reporting behaviour than they say about the true underlying rate of medication errors. Some health professionals reported directly to NIRS, while others reported via their local Patient Safety Department, which tends to report real events, and filter out near misses. This could lead to a lower number of non-harmful incidents and overestimate severe errors. Some incident reporting systems, such as the National Reporting and Learning System-UK, are criticised for being ‘wide and shallow’ and not ‘narrow and deep’, that is, lacking in detailed incidents, which are less common and more serious in harm.16
The process of reading and analysing detailed incident reports in the NIRS data, as illustrated in table 3, has given us a unique insight into the many pitfalls of the medication management process. This knowledge makes it difficult to comprehend that, as of today, hospitals in Norway do not employ Medication Safety Officers. This is in strict contrast to many hospitals worldwide, with dedicated full-time Medication Safety Officers,20 who lead the medication safety programme, develop protocols for high-risk medications and processes, perform root cause analysis and devise reporting systems. Why there is such a lack of overall political interest in the topic is not easy to comprehend.
Based on our analysis of a 2-year dataset on medication errors in Norwegian hospitals, we recommend introducing a medication safety programme to monitor and improve medication safety. Furthermore, a newly published National Action Plan in Patient Safety and Quality Improvement (2019–2023) has chosen medications as one of three target areas needing special attention.21 Hopefully, this will also contribute to prioritising medication safety.
Vulnerable patient groups
In our study, 50% of all errors were associated with patients aged over 65 years. This is as expected, because older people use more medicines. The elderly constitute the majority of cases involving severe and fatal medication errors,22 however, many elderly are also among the most frail and vulnerable patients. Interventions should focus on the safe administration of drugs to patients aged over 65 years, especially antithrombotics, as these drugs are associated with the most harm in this patient group.
A considerable proportion of incident reports involved children, and every fourth paediatric medication errors concerned the infant population (0–1 years). Errors could be due to the nature of medication preparation, dispensing and administration to children.23 In Norway, the National Competency Network for Medication to Children contributes with developing guidelines, distributing information and supporting research.24 However, in the 2-year study period, 10 children were severely harmed and one child died due to medication errors. This finding calls for urgent action.
Besides the integration of a clinical pharmacist in the clinical team, stronger emphasis on paediatric medication preparation23 and technology implementation are clearly needed. Another aspect of improving medication safety is to strengthen the partnership between the patients, their relatives and the healthcare providers.1 4
Therapeutic area
The top three therapeutic areas most frequently reported in our data were analgesics, antibacterials for systemic use and antithrombotic agents. These three medication groups were associated with 40% of all reported errors, 50% of severe harm errors and 60% of fatal errors, somewhat similar to other studies.3 5 15 22 Most fatal errors were associated with analgesics and antithrombotic agents. The majority of deaths in the analgesics group were associated with opioids. However, a patient with renal failure died after a high dose of paracetamol was given. Five of the six fatal errors involving antithrombotic agents were related to an intracranial haemorrhage that occurred when a thrombolytic tissue plasminogen activator (tPA) was administered to treat an acute heart attack or acute ischaemic stroke. TPA also caused severe errors, particularly when the incorrect dosage was given for the set bodyweight or if administrated before a CT head scan had ruled out a brain haemorrhage. Our five fatal tPA-related errors are in line with a tPA study on 131 patients, where 27 patients were exposed to overdosage errors, of which three were fatal.25 Hence, tPA errors in hospitals are common, severe, and in need of a more systematic approach and education in prescribing and administration to prevent patient harm.
Strengths and limitations
A major strength with our study is that we use a national database of medication errors from all types of hospital wards, populations, medications and harm scores, in contrast to other studies which have focused on specific hospital wards,5 populations,23 medications16 25 or harm scores.22 Under-reporting is a well-known limitation of the incident reporting systems.26 It is assumed that only one in five incidents are reported.16 High reporting rates may indicate an organisational culture committed to identifying and reducing errors rather than a truly high rate.20
It is important to recognise the challenge of harm score assignment if prevention strategies are to be focused on medication errors with significant harm scores.27
Despite these limitations, it is crucial to acknowledge the importance of incident reporting systems in medication error research. The incident reporting systems provide an effective and low-cost tool to detect risks, which can initiate improvements in medication safety.
The primary purpose of incident reports is identifying risks in the healthcare system and determining need for further investigating and analysis, while there remains little evidence to support the critical learning from these reports.28 Description of events and causes are often written from one person’s view of a complex clinical and organisational situation, and thus discussing underlying causes and revealing flaws in healthcare systems should rather be preformed by investigating relevant and severe incidents with system analysis tools.29 30
Recently, the Ministry of Health closed down the NIRS, and the final legislative decision was adopted in April 2019 by the Norwegian Parliament. This decision was made against international recommendations4; however, the Minister of Health argued that the NIRS had not sufficiently contributed to patient safety in hospitals. The incidents of errors are as of May 2019 only to be reported at a hospital or regional level, and therefore a national overview is no longer available, which makes our dataset unique.
Conclusions
This paper shows that errors most commonly occurred during medication administration and that dosing errors were the most common error type. The substantial number of severe and fatal errors causing preventable patient harm and death emphasises an urgent need for error-prevention strategies, which includes introducing a medication safety officer in hospitals as an essential measure to monitor, prevent and improve medication safety. Additional studies and interventions should further investigate the error-prone medication administration stage in hospitals and explore the dynamics of severe incidents, emphasising incidents associated with children and the safe administration of antithrombotics to patients over 65 years.
Key messages.
What is already known on this subject
Even with global efforts aimed to reduce medication errors, they continue to be the most frequent source of healthcare mishaps and continue to cause patient harm and death.
Little is known about medication errors in Norwegian hospitals.
Incident reports provide with sufficient data to describe the nature and type of medication errors.
What this study adds
This study comprehensively examined, through the Norwegian Incident Reporting System, medication errors reported in a 2-year period, 2016 and 2017.
Medication errors most commonly occurred during medication administration and involved most frequently dosing errors.
The substantial number of severe and fatal errors causing preventable patient harm and death stresses an urgent need for error-prevention strategies.
Acknowledgments
The authors would like to thank the employees at the Norwegian Incident Reporting System (Meldeordningen) for their contribution to this study and making the data available.
Footnotes
Contributors: AGG and AM conceived of the presented idea. AGG, KT and EH were involved in planning and supervising the work. AM took lead in writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
The data from the Norwegian Directorate of Health is not publicly available.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Donaldson LJ, Kelley ET, Dhingra-Kumar N, et al. Medication without harm: who's third global patient safety challenge. The Lancet 2017;389:1680–1. 10.1016/S0140-6736(17)31047-4 [DOI] [PubMed] [Google Scholar]
- 2. Sheikh A, Rudan I, Cresswell K, et al. Agreeing on global research priorities for medication safety: an international prioritisation exercise. J Glob Health 2019;9:010422. 10.7189/jogh.09.010422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cousins DH, Gerrett D, Warner B. A review of medication incidents reported to the National reporting and learning system in England and Wales over 6 years (2005-2010). Br J Clin Pharmacol 2012;74:597–604. 10.1111/j.1365-2125.2011.04166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Council of Europe . Creation of a better medication safety culture in Europe: building up safe medication practices. expert group on safe medication practices (P-SP-PH/SAFE). Available: http://optimiz-sih-circ-med.fr/Documents/Council_of_Europe_Medication_Safety_Report_19-03-2007.pdf [Accessed 23 Aug 2019].
- 5. Wahr JA, Shore AD, Harris LH, et al. Comparison of Intensive Care Unit Medication Errors Reported to the United States’ MedMarx and the United Kingdom’s National Reporting and Learning System. Am J Med Qual 2014;29:61–9. 10.1177/1062860613482964 [DOI] [PubMed] [Google Scholar]
- 6. Pham JC, Story JL, Hicks RW, et al. National study on the frequency, types, causes, and consequences of voluntarily reported emergency department medication errors. J Emerg Med 2011;40:485–92. 10.1016/j.jemermed.2008.02.059 [DOI] [PubMed] [Google Scholar]
- 7. Shah K, Lo C, Babich M, et al. Bar code medication administration technology: a systematic review of impact on patient safety when used with computerized prescriber order entry and automated dispensing devices. Can J Hosp Pharm 2016;69:394–402. 10.4212/cjhp.v69i5.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otterlei S. Same errors happen over and over again. We are extremely worried [Samme feil skjer om og om igjen på norske sykehus. Vi er svært bekymret]. NRK Hordaland, 2019. Available: https://www.nrk.no/hordaland/professor-ut-mot-helseministeren_-_-vi-mister-muligheten-til-a-laere-av-sykehusfeil-1.14482690 [Accessed 23 Aug 2019].
- 9. Skjetne O, Roed R, Rosenlund-Hauglid S, et al. Djabrail (6) died due to a medication error. Haukeland hospital recieves masssive criticism. [Djabrail (6) døde etter feilmedisinering. Haukeland sykehus får massiv kritikk], 2017. Available: https://www.vg.no/nyheter/innenriks/i/joeWA/djabrail-6-doede-etter-feilmedisinering-haukeland-sykehus-faar-massiv-kritikk [Accessed 23 Aug 2019].
- 10. The Norwegian patient safety programme . In safe hands, 2016. Available: https://www.pasientsikkerhetsprogrammet.no/om-oss/english/the-norwegian-patient-safety-programme-in-safe-hands [Accessed 13 Jun 2019].
- 11. World Health Organization . Conceptual framework for the International classification for patient safety, 2010. Available: https://www.who.int/patientsafety/implementation/taxonomy/ICPS-report/en/ [Accessed 10 May 2019].
- 12. WHO collaborating Centre . For drug statistics methodology anatomical therapeutic chemical (ATC) classification index with defined daily doses (DDD). Available: https://www.whocc.no/ [Accessed 23 Aug 2019].
- 13. The National coordinating Council for medication error reporting and prevention (NCC-MERP) about medication errors. Available: https://www.nccmerp.org/about-medication-errors [Accessed 08 May 2019]. [DOI] [PubMed]
- 14. Dean Franklin B, Vincent C, Schachter M, et al. The incidence of prescribing errors in hospital inpatients: an overview of the research methods. Drug Saf 2005;28:891–900. 10.2165/00002018-200528100-00005 [DOI] [PubMed] [Google Scholar]
- 15. Keers RN, Williams SD, Cooke J, et al. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother 2013;47:237–56. 10.1345/aph.1R147 [DOI] [PubMed] [Google Scholar]
- 16. Dabba K, Elswood M, Ameer A, et al. A mixed methods analysis of clozapine errors reported to the National reporting and learning system. Pharmacoepidemiol Drug Saf 2019;28:657–64. 10.1002/pds.4727 [DOI] [PubMed] [Google Scholar]
- 17. McLeod M, Barber N, Franklin BD. Facilitators and Barriers to Safe Medication Administration to Hospital Inpatients: A Mixed Methods Study of Nurses’ Medication Administration Processes and Systems (the MAPS Study). PLoS One 2015;10:e0128958. 10.1371/journal.pone.0128958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis PJ, Dornan T, Taylor D, et al. Prevalence, incidence and nature of prescribing errors in hospital inpatients. Drug Saf 2009;32:379–89. 10.2165/00002018-200932050-00002 [DOI] [PubMed] [Google Scholar]
- 19. Haavik S, Soeviknes S, Erdal H, et al. Prescriptions from general practitioners and in hospital physicians requiring pharmacists' interventions. Pharmacoepidemiol Drug Saf 2011;20:50–6. 10.1002/pds.1949 [DOI] [PubMed] [Google Scholar]
- 20. Larson CM, Saine D. Medication safety officer's Handbook. Bethesda, MD: American Society of Health-System Pharmacists, 2013. [Google Scholar]
- 21. National Action Plan strengthens the efford for Patient Safety [Nasjonal handlingsplan styrker innsatsen for pasientsikkerhet]. Available: https://www.pasientsikkerhetsprogrammet.no/aktuelt/nyheter/en-felles-innsats-for-pasientsikkerhet [Accessed 05 Jul 2019].
- 22. Härkänen M, Vehviläinen-Julkunen K, Murrells T, et al. Medication administration errors and mortality: incidents reported in England and Wales between 2007 – 2016. Res Social Adm Pharm 2019;15:858–63. 10.1016/j.sapharm.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 23. Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev 2015;3:Cd006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Competency Network for Medications to Children [Nasjonalt kompetansenettverk for legemidler til barn]. Available: https://www.legemidlertilbarn.no [Accessed 13 Jun 2019].
- 25. Chung LS, Tkach A, Lingenfelter EM, et al. Tissue plasminogen activator prescription and administration errors within a regional stroke system. J Stroke Cerebrovasc Dis 2016;25:565–71. 10.1016/j.jstrokecerebrovasdis.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franklin BD, Taxis K, Barber N. Parenteral drug errors. reported error rates are likely to be underestimation. BMJ 2009;338:b1814. 10.1136/bmj.b1814 [DOI] [PubMed] [Google Scholar]
- 27. Williams SD, Ashcroft DM. Medication errors: how reliable are the severity ratings reported to the National reporting and learning system? Int J Qual Health Care 2009;21:316–20. 10.1093/intqhc/mzp034 [DOI] [PubMed] [Google Scholar]
- 28. Macrae C. The problem with incident reporting: Table 1. BMJ Qual Saf 2016;25:71–5. 10.1136/bmjqs-2015-004732 [DOI] [PubMed] [Google Scholar]
- 29. Lawton R, Parker D. Barriers to incident reporting in a healthcare system. Qual Saf Health Care 2002;11:15–18. 10.1136/qhc.11.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor-Adams S, Vincent C. Systems analysis of clinical incidents: the London protocol. Clin Risk 2004;10:211–20. 10.1258/1356262042368255 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2020-002298supp001.pdf (220.3KB, pdf)
ejhpharm-2020-002298supp002.pdf (225.5KB, pdf)
Data Availability Statement
The data from the Norwegian Directorate of Health is not publicly available.