Abstract
Functional gastrointestinal disorders (FGIDs), including functional abdominal pain (FAP), account for a large portion of conditions seen by paediatric gastroenterologists. Despite the commonality of FGIDs, there remains significant stigma around these diagnoses among medical providers, patients and families. This is due to the absence of easily identifiable biological markers in FGIDs and the overlay with psychological and social factors contributing to symptom onset and maintenance. As such, the biopsychosocial model is essential in conceptualising, evaluating and treating FGIDs. The way in which medical providers explain FGIDs and the manner in which they collaborate with other specialists (eg, psychologists, dieticians, physical therapists, school nurses) is paramount to the patient and family acceptance of an FGID diagnosis and the success of subsequent treatment. The following review outlines paediatric FGIDs with a focus on FAP in adolescents, in particular within the context of the biopsychosocial approach to pathophysiology, diagnosis and treatment.
Keywords: functional bowel disorder, psychological stress, irritable bowel syndrome, abdominal pain, functional dyspepsia
Introduction
To paraphrase Dwight Schrute, the fictional character on the television show The Office,1 who loves to enunciate axioms with supreme confidence, the following are undisputable facts: (1) the vast majority of patients seen by primary care physicians present with problems that are due to functional disorders. In a study of adults presenting with chest pain, fatigue, dizziness, headache, oedema, back pain, dyspnoea, insomnia, abdominal pain, numbness, impotence, weight loss, cough and constipation (pretty much every symptom that one can experience), an organic aetiology was demonstrated in only 16%2; (2) in a Canadian population-based survey, at least one functional gastrointestinal disorder (FGID) occurred in 61% out of 1149 respondents,3 suggesting that it is more normal for individuals to have rather than not have an FGID; (3) FGIDs dominated (not just won, they dominated) the type of conditions with which new paediatric referrals present to paediatric gastrointestinal clinics4; (4) the care of children with FGIDs is very costly.5
Yet, despite all these facts, there continues to be pervasive stigma associated with FGIDs6 and many physicians struggle to provide optimal care to such patients. In a study evaluating gastroenterology fellows on-call, physicians carried a lower perception of the importance of telephone requests and of the impact of the disorder when the calls were made by patients with FGIDs compared with those with organic diseases.7 In another study giving different clinical vignettes to a general population, there were higher levels of enacted stigma towards patients with irritable bowel syndrome (IBS) compared with both patients with inflammatory bowel disease and those with asthma.8 The discomfort that many medical providers perceive when taking care of children or adults with FGIDs may be partially explained by deficiencies in their training. In a recent survey of paediatric gastroenterologists in training, 53.1% of the fellows reported an interest in neurogastroenterology; however, less than 25% believed they had been adequately trained in this subspecialty during their fellowship.9 Only approximately one-third of them felt very comfortable dealing with patients with functional dyspepsia or IBS, two of the most common paediatric FGIDs.
What follows is a clinical vignette of a typical patient which every busy paediatric gastroenterologist is slated to meet in clinic almost every day.
Case
EM is a fictional but typical 14-year-old girl who presents with abdominal pain that has been present for the previous 4 months. The pain is localised to the periumbilical area and does not radiate to the back or the chest. The pain episodes have been occurring daily and last for 30–60 min at a time. She describes the pain as dull and uncomfortable in nature, and severe enough to make her double over at times. The pain seems more severe in the morning, there are no recognised triggers, and nothing appears to alleviate her pain. She does not endorse having nausea, vomiting, constipation or diarrhoea, anorexia, blood in the stool, weight loss, recurrent fevers or skin rashes. EM continues to sleep well and does not wake up at night due to the pain. She has missed 10 days of school because of her symptoms and is concerned about falling behind in her school assignments. She has also found herself unable to participate in physical activities as much as she used to. EM is described as an anxious child and a high achiever. She also likes to keep her room very organised and clean. She has a few close friends, and parents are not aware of any bullying or problems with peers. There is no family history of coeliac disease or inflammatory bowel disease, but there is a family history of IBS in both her mother and maternal grandmother.
Pathophysiology
The pathophysiology of FGIDs is not well understood but considered to be multifactorial in nature. The gut–brain axis and its disruption contribute to the pathogenesis. Genetic predisposition, early life events, medical and psychosocial events contribute to changes in pain processing and visceral hypersensitivity leading to the development of functional abdominal pain (FAP). The sensitising medical events include intestinal dysmotility, inflammation and distention, while depression, anxiety, family stress, coping style, secondary gains and abuse history are psychosocial factors that are equally important and may heighten disability.10 Previously healthy children often develop FGIDs following an infectious bacterial gastroenteritis.11 Pensabene et al reported in a prospective and multicentre study, that close to half of the children with acute infectious gastroenteritis, develop pain predominant FGIDs 6 months later compared with about 15% in a control group.12 Disturbances in intestinal microbiota, low-grade mucosal inflammation, immune activation and altered intestinal permeability combined with visceral hypersensitivity have been reported to play a role in the pathophysiology of FGIDs.13
Psychosocial factors, such as stress and anxiety, are purported to be predisposing factors for FGIDs among children and adolescents.14 15 Such stress can include singular, yet highly distressing events such as loss of family members, change in school or living circumstances or hospitalisations.14 16 17 Specific traumatic events, including physical or sexual abuse, are also risk factors for development of FGID.18 19 Numerous studies show that children and adolescents with FGIDs are more likely than children without gastrointestinal symptoms to have comorbid diagnoses of anxiety and depression.20–22 Functional MRI studies in adults and animal models show changes in brain functioning that lead to increased visceral hypersensitivity associated with major stressors. These functional brain alterations are theorised as a predisposing mechanism for increased nerve sensitivity that is then associated with FGIDs symptoms (eg, pain, nausea, constipation, diarrhoea),23–25 otherwise known as the brain–gut interaction.26
Social and environmental factors can also exacerbate FGIDs symptoms.27 For instance, many children with FGIDs tend to miss school as a result of their symptoms leading to stress due to school assignments that must be made-up. This is particularly true given that many patients with FGIDs are described as ‘Type A’, perfectionistic and highly self-motivated, as in the case example of EM. Missed school also frequently results in missing out on sports and other social activities, which can lead to feelings of isolation and frustration. Parents’ response to their child’s problems of pain can also impact overall severity of symptoms and related functional disability.28 29 Typically, when parents are highly attentive to their child’s pain problems, pain responses are then reinforced often leading to increased pain frequency and severity. Parents with higher self-somatisation are also more likely to have children with higher somatisation, suggesting both genetic and social learning factors are predisposing to FGIDs as well.30
It is important to note that having chronic illness, particularly illness associated with chronic pain, tends to lead to increases in stress, anxiety and depression.31–33 However, this could include initial onset of such mental health problems occurring after FGIDs symptoms begin. Indeed, many patients deny having premorbid anxiety or depression, identifying these symptoms as occurring only after FGIDs symptoms began. Whether this is true is up for debate, but patient perception of symptoms is just as important (if not more than) as when the symptoms truly began, and which may have come first. As such, the relationship between FGIDs and psychological symptoms is very cyclical.
Diagnosis
The diagnosis of FGIDs is primarily a clinical diagnosis that is dependent on detailed history and thorough physical exam. The symptom-based diagnostic approach embodied by the Rome criteria enables providers to make a positive diagnosis without unnecessary testing. In order to do so, a provider has to extract information from the patient and family and establishing an effective patient–provider relationship is of great importance. In the absence of objective biomarkers, the medical history is key at generating a thoughtful differential and ultimately establishing a diagnosis. Children presenting with symptoms suggestive of an FGID are not different than any other patient who presents with any other problem. As in any medical encounter, a differential is formulated at the end of the interaction and followed by a discussion about the most likely diagnosis and the need for testing and treatment. It is important that the discussion regarding potential testing only comes after a conversation about the diagnosis of an FGID and the therapeutic plan. The provider can use the multidimensional clinical profile (MDCP) approach introduced in Rome IV to help organise and establish a therapy plan.34 The MDCP in EM’s case as formulated based on the detailed history and physical exam is as follows.
MDCP categories for the clinical case described above
Categorical diagnosis: FAP-not otherwise specified (FAP-NOS).
Clinical modifiers: none.
Impact on daily activities: severe.
Psychosocial modifiers: known to be anxious and preoccupation on organisation and cleanliness.
Physiological features and biomarkers: none known.
FAP-NOS as a diagnosis for EM is very appropriate based on history of presentation, and the diagnosis should be thoroughly discussed and explained in an age-appropriate manner with the patient. It is also important to consider and discuss a differential diagnosis that may include coeliac disease, inflammatory bowel disease and peptic disease. It is also important that the patients is asked about her sexual and gynaecological history. If indicated, a urine pregnancy test and testing for sexually transmitted disease should be considered. Having a differential diagnosis that is very well thought out leads to a limited testing which should be done with a clear explanation of its purpose. The yield of diagnostic testing in children suspected to have a diagnosis of an FGID is very low and therefore exams should be ordered judiciously.35 36 A strong family history of IBS as in EM’s case is supportive of a FGID diagnosis in the right clinical presentation. On the other hand, the presence of alarm features such as family history of organic diseases (eg, inflammatory bowel disease or coeliac disease), signs and symptoms such as dysphagia, gastrointestinal blood loss, perirectal disease, involuntary weight loss and unexplained fever makes a diagnosis of an FGID less likely and should lead to more testing. In the above vignette, non-invasive tests such as obtaining coeliac serology, complete blood count with differential, sedimentation rate or C-reactive protein and stool testing for calprotectin are very reasonable to consider even if they are very unlikely to be abnormal.
The provider has to be thoughtful with the diagnostic workup not only because of its low yield but also because of its impact on the therapeutic plan, including its acceptance by the patient and family. There are many disadvantages to conducting testing in children with FGIDs without a clear explanation of its purpose. Some of these disadvantages include the never-ending desire to keep pursuing further testing, potential harm derived from incidental findings and financial burden. When done correctly, a limited workup can help reassure the patient, alleviate anxiety, and in the process be part of therapy.
Medical treatment
A psychologist breaks her leg in a ski accident. She goes to the local emergency department, where an orthopaedic surgeon is consulted. He visits her and says “Nothing to worry about. It is all in your body”.
This short, apocryphal, story emphasises the ubiquitous dichotomy between body and mind. Such old fallacious point of view (“the problem it is either in your head or in your body”), far from being forgotten with the demise of Descartes, is still contributing to the challenge to provide holistic treatment to patients with FGIDs. Patients, and often their parents, are frequently resistant to interventions that target either the brain or the environment when the symptom appears to be originating in the body, usually the abdomen. They frequently fail to understand how anxiety, stress and emotional arousal have a tremendous influence on generating physical symptoms and heighten disability. In reality, there are medical treatments for FGIDs that either target the gastrointestinal tract or the brain, but the best evidence for their efficacy comes from holistic, non-medical interventions which will be described later.
Targeting the gut: there have been multiple studies evaluating the efficacy of different probiotics in paediatric IBS. A recent review concluded that there is preliminary evidence for use of some probiotics, particularly Lactobacillus rhamnosus GG, in reducing abdominal pain in children with IBS. The positive effects of other probiotics are more inconsistent.37 One cross-over trial in paediatric IBS supports the efficacy of the probiotic VSL#3.38 Other treatments that affect the microbiome involve dietary changes and bile acids manipulation and faecal microbiota transplantation. Among these three interventions, the best paediatric data relate to the benefit deriving from a diet that restricts the intake of fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) in children with IBS. Interestingly, there are intriguing data suggesting that composition of the gut microbiome may predict the FODMAP diet efficacy.39 There are few data on herbal treatments in children. A systematic review concluded that there is some evidence for peppermint oil to decrease duration, frequency and severity of pain in children suffering from undifferentiated FAP.40 The addition of dietary fibre has been a controversial topic in adult and paediatric IBS. Psyllium fibre seems to reduce pain frequency in children with IBS.41 In contrast, glucomannan, a water soluble hemicellulose polysaccharide, was no more effective than the placebo in achieving therapeutic success in the management of FGIDs in children.42
Despite the widespread use of antispasmodics in children with pain-predominant FGID, there have been remarkably few studies evaluating their efficacy: mebeverine was not significantly better than placebo in one study,43 while trimebutine was beneficial in children with IBS in a study from Turkey.44 There is some evidence from a small randomised, double-blind trial45 and a larger open-label study46 that cyproheptadine is beneficial in treating abdominal pain in children with FAP or IBS. A double-blind placebo controlled trial of rifaximin47 showed that there was no significant difference in symptom improvement between groups, while an open-label trial48 suggested that rifaximin was effective and safe in treating bacterial overgrowth and IBS symptoms in children. A variety of different secretagogues have been recently approved by the Food and Drugs Administration for use in adults with IBS,49 but their efficacy has not been demonstrated in children yet.
Targeting the brain: three randomised, double-blind trials have assessed the efficacy of antidepressants in children with FGIDs. A small trial with amitriptyline50 found benefits; however, a larger multicentre study found no superiority of the drug over placebo.51 A study of citalopram found a trend towards the effectiveness of this medication in the treatment of children with FAP.52 It is possible that antidepressants benefit a particular subset of children with FGIDs.
Other treatments
Given the pathophysiological underpinnings, a biopsychosocial approach to treatment is particularly useful in conceptualising and treating patients with FGID. This framework acknowledges and seeks to address a number of factors that are impacting pain and related disability, including biological, psychological, social and environmental factors53 (see figure 1 for biopsychosocial framework for case example). Using the biopsychosocial model in treatment allows for therapies beyond medications to address symptoms.
Figure 1.
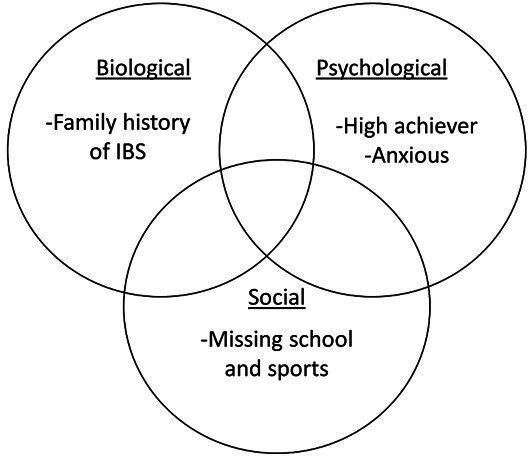
Biopsychosocial model for case example of EM. IBS, irritable bowel syndrome.
Cognitive–behavioural therapy (CBT) is a widely accepted form of treatment for FGIDs that addresses many layers within a biopsychosocial framework. Goals of CBT are to treat underlying anxiety, stressors (social and environmental) and/or depression that may be predisposing patients to FGIDs or worsening already present FGIDs symptoms.54 CBT includes identifying pain-related triggers, implementation of relaxation strategies (ie, diaphragmatic breathing, muscle relaxation, guided imagery) and altering or reframing maladaptive patterns of thinking related to pain.55 Biofeedback therapy is also often incorporated into CBT as an effective means of treating pain-related symptoms and anxiety.56 57 Evaluations of effectiveness of CBT in treating FGIDs show marked improvements in quality of life, decreases in abdominal pain intensity and frequency, and return to more optimal daily functioning and activities (school, sports, social activities).58–60
Hypnosis, sometimes included as part of CBT, is a form of mindfulness-based relaxation therapy that is useful for treatment of FGIDs.61 Hypnosis is described as a way of teaching children self-regulation through relaxation and modulation of physical and psychological symptoms.62 Studies have shown hypnosis to reduce hypersensitivity and related pain including decreases in pain frequency, severity and duration as well as decreased stress responses.61 63 64 These improvements have also been found to be sustained through long-term follow-up65 (5 years).
Other complementary and alternative therapies, such as massage, acupuncture and yoga, are often incorporated in treating FGID.66 67 While there is less research on such complementary interventions in children and adolescents, there is some evidence that they improve quality of life and decrease pain-related functional disability.
It should be noted that patients with pain, including abdominal pain, who engage in multidisciplinary care, including medical, psychological, other complementary therapies, have better outcomes when compared with those who engage in medical therapy alone.68–70 As such, a patient such as the one described in the case example above would be likely to benefit from CBT and other therapies in addition to medical interventions for her abdominal pain. This is particularly true given the noted anxiety in EM’s case. Importantly, the way in which the psychosocial factors and treatment are discussed by physicians with patients can determine the course of their care. If a physician simply suggests the patient should see a psychologist to help with their symptoms, patients and families are more likely to hear ‘it’s all in your head’, leading to resistance to diagnosis and rejection of the psychosocial treatment or alternative therapies altogether.27 70 71 Instead, the physician should take ownership and take the time to explain the FGID diagnosis in a manner that is understood by both the child and the parent. The discussion about the diagnosis should be followed by a comprehensive plan and the referral to psychology should be part of the plan and not the singular plan. One of the provider’s goal is to establish a working and candid patient provider relationship. A good relationship may help prevent the need for excessive testing and patients seeking care from multiple providers which is not uncommon in children with FGIDs.
Conclusion
FGIDs which have recently been renamed as disorders of gut–brain interactions are very prevalent in the paediatric population and represent some of the most common reasons for referral to paediatric gastroenterology clinic. These disorders and in particular the pain predominant have significant impact on the child’s quality of life and ability to function. They should be approached using a biopsychosocial model and addressed on a timely manner. It has been well reported that children and families respond best when providers take the problems seriously, listen carefully, explain the diagnosis thoroughly and present a comprehensive therapeutic plan.
Footnotes
Collaborators: None.
Contributors: All authors met and planned the review paper and tasks were divided equally amongst all three authors. Authors submitted their assigned sections to DY who compiled them together. DY and AMKVD generated the generic case. CDL was responsible for writing the abstract, introduction and a portion of the medical treatment section. DY was responsible for a portion of the pathophysiology and wrote the diagnosis and conclusion sections of the paper. AMKVD was responsible for the other portion of the pathophysiology section, the section titled as “other treatment” and creating the figure. All participated in reviewing the draft and editing. DY was responsible for submitting the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Dwight Schrute. Available: https://en.wikipedia.org/wiki/Dwight_Schrute
- 2. Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med 1989;86:262–6. 10.1016/0002-9343(89)90293-3 [DOI] [PubMed] [Google Scholar]
- 3. Thompson WG, Irvine EJ, Pare P, et al. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci 2002;47:225–35. 10.1023/A:1013208713670 [DOI] [PubMed] [Google Scholar]
- 4. Rouster AS, Karpinski AC, Silver D, et al. Functional gastrointestinal disorders dominate pediatric gastroenterology outpatient practice. J Pediatr Gastroenterol Nutr 2016;62:847–51. 10.1097/MPG.0000000000001023 [DOI] [PubMed] [Google Scholar]
- 5. Hoekman DR, Rutten JMTM, Vlieger AM, et al. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr 2015;167:1103–8. 10.1016/j.jpeds.2015.07.058 [DOI] [PubMed] [Google Scholar]
- 6. Hearn M, Whorwell PJ, Vasant DH. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol 2020;5:607–15. 10.1016/S2468-1253(19)30348-6 [DOI] [PubMed] [Google Scholar]
- 7. Dalton CB, Drossman DA, Hathaway JM, et al. Perceptions of physicians and patients with organic and functional gastrointestinal diagnoses. Clin Gastroenterol Hepatol 2004;2:121–6. 10.1016/S1542-3565(03)00319-7 [DOI] [PubMed] [Google Scholar]
- 8. Taft TH, Bedell A, Naftaly J, et al. Stigmatization toward irritable bowel syndrome and inflammatory bowel disease in an online cohort. Neurogastroenterol Motil 2017;29. 10.1111/nmo.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graham K, Belkind-Gerson J, Darbari A, et al. Barriers in neurogastroenterology and motility training experience for pediatric gastroenterology fellows. J Pediatr Gastroenterol Nutr 2019;68:806–10. 10.1097/MPG.0000000000002282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyams JS, Di Lorenzo C, Saps M, et al. Functional disorders: children and adolescents. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 11. Saps M, Pensabene L, Di Martino L, et al. Post-Infectious functional gastrointestinal disorders in children. J Pediatr 2008;152:812–6. 10.1016/j.jpeds.2007.11.042 [DOI] [PubMed] [Google Scholar]
- 12. Pensabene L, Talarico V, Concolino D, et al. Postinfectious functional gastrointestinal disorders in children: a multicenter prospective study. J Pediatr 2015;166:903–7. 10.1016/j.jpeds.2014.12.050 [DOI] [PubMed] [Google Scholar]
- 13. Shin A, Preidis GA, Shulman R, et al. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol 2019;17:256–74. 10.1016/j.cgh.2018.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boey CC, Goh KL. Stressful life events and recurrent abdominal pain in children in a rural district in Malaysia. Eur J Gastroenterol Hepatol 2001;13(4):401-404. [DOI] [PubMed] [Google Scholar]
- 15. Jones MP, Van Oudenhove L, Koloski N, et al. Early life factors initiate a ‘vicious circle’ of affective and gastrointestinal symptoms: A longitudinal study. United European Gastroenterology Journal 2013;1:394–402. 10.1177/2050640613498383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devanarayana NM, Mettananda S, Liyanarachchi C, et al. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. J Pediatr Gastroenterol Nutr 2011;53:659–65. [DOI] [PubMed] [Google Scholar]
- 17. Devanarayana NM, de Silva DGH, de Silva HJ. Recurrent abdominal pain syndrome in a cohort of Sri Lankan children and adolescents. J Trop Pediatr 2008;54:178–83. 10.1093/tropej/fmm114 [DOI] [PubMed] [Google Scholar]
- 18. Devanarayana NM, Rajindrajith S, Perera MS, et al. Association between functional gastrointestinal diseases and exposure to abuse in teenagers. J Trop Pediatr 2014;60:386–92. 10.1093/tropej/fmu035 [DOI] [PubMed] [Google Scholar]
- 19. Koloski NA, Talley NJ, Boyce PM. A history of abuse in community subjects with irritable bowel syndrome and functional dyspepsia: the role of other psychosocial variables. Digestion 2005;72:86–96. 10.1159/000087722 [DOI] [PubMed] [Google Scholar]
- 20. Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics 2004;113:817–24. 10.1542/peds.113.4.817 [DOI] [PubMed] [Google Scholar]
- 21. Ramchandani PG, Hotopf M, Sandhu B. The epidemiology of recurrent abdominal pain from 2 to 6 years of age: results of a large, population-based study. Pediatrics 2005;116:46–50. 10.1542/peds.2004-1854 [DOI] [PubMed] [Google Scholar]
- 22. Youssef NN, Atienza K, Langseder AL, et al. Chronic abdominal pain and depressive symptoms: analysis of the National longitudinal study of adolescent health. Clin Gastroenterol Hepatol 2008;6:329–32. 10.1016/j.cgh.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 23. Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011;140:761–5. 10.1053/j.gastro.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol 2002;282:G307–16. 10.1152/ajpgi.00240.2001 [DOI] [PubMed] [Google Scholar]
- 25. mayer ea, aziz q, coen s, et al. Brain imaging approaches to the study of functional Gi disorders: a Rome working team report. Neurogastroenterol Motil 2009;21:579–96. 10.1111/j.1365-2982.2009.01304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiou FK, How CH, Ong C. Recurrent abdominal pain in childhood. Singapore Med J 2013;54:195–200. quiz 200. 10.11622/smedj.2013072 [DOI] [PubMed] [Google Scholar]
- 27. Galdston MR, John RM. Mind over gut: psychosocial management of pediatric functional abdominal pain. J Pediatr Health Care 2016;30:535–45. 10.1016/j.pedhc.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 28. Levy RL. Exploring the intergenerational transmission of illness behavior: from observations to experimental intervention. ann. behav. med. 2011;41:174–82. 10.1007/s12160-010-9254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levy RL, van Tilburg MAL. Functional abdominal pain in childhood: background studies and recent research trends. Pain Res Manag 2012;17:413–7. 10.1155/2012/960104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psychol 1991;19:379–94. 10.1007/BF00919084 [DOI] [PubMed] [Google Scholar]
- 31. Forgeron PA, Evans J, McGrath PJ, et al. Living with difference: exploring the social self of adolescents with chronic pain. Pain Res Manag 2013;18:e115–23. 10.1155/2013/120632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jastrowski Mano KE, O'Bryan EM, Gibler RC, et al. The co-occurrence of pediatric chronic pain and anxiety: a theoretical review of a developmentally informed shared vulnerability model. Clin J Pain 2019;35:989–1002. 10.1097/AJP.0000000000000763 [DOI] [PubMed] [Google Scholar]
- 33. Soltani S, Kopala-Sibley DC, Noel M. The co-occurrence of pediatric chronic pain and depression: a narrative review and conceptualization of mutual maintenance. Clin J Pain 2019;35:633–43. 10.1097/AJP.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 34. Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil 2017;23:151–63. 10.5056/jnm16214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr 2010;51:579–83. 10.1097/MPG.0b013e3181de0639 [DOI] [PubMed] [Google Scholar]
- 36. Alioto A, Di Lorenzo C, Montgomery ML, et al. High cost and low yield: the diagnostic evaluation of Rumination syndrome in pediatrics. J Pediatr 2017;185:155–9. 10.1016/j.jpeds.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 37. Ding FCL, Karkhaneh M, Zorzela L, et al. Probiotics for paediatric functional abdominal pain disorders: a rapid review. Paediatr Child Health 2019;24:383–94. 10.1093/pch/pxz036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guandalini S, Magazzù G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010;51:24–30. 10.1097/MPG.0b013e3181ca4d95 [DOI] [PubMed] [Google Scholar]
- 39. Chumpitazi BP. The gut microbiome as a predictor of low fermentable oligosaccharides disaccharides monosaccharides and polyols diet efficacy in functional bowel disorders. Curr Opin Gastroenterol 2020;36:147–54. 10.1097/MOG.0000000000000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anheyer D, Frawley J, Koch AK, et al. Herbal medicines for gastrointestinal disorders in children and adolescents: a systematic review. Pediatrics 2017;139:e20170062. 10.1542/peds.2017-0062 [DOI] [PubMed] [Google Scholar]
- 41. Shulman RJ, Hollister EB, Cain K, et al. Psyllium fiber reduces abdominal pain in children with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol 2017;15:712–9. 10.1016/j.cgh.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horvath A, Dziechciarz P, Szajewska H. Glucomannan for abdominal pain-related functional gastrointestinal disorders in children: a randomized trial. WJG 2013;19:3062–8. 10.3748/wjg.v19.i20.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pourmoghaddas Z, Saneian H, Roohafza H, et al. Mebeverine for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Biomed Res Int 2014;2014:1–6. 10.1155/2014/191026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karabulut GS, Beşer Ömer F, Erginöz E, et al. The incidence of irritable bowel syndrome in children using the Rome III criteria and the effect of trimebutine treatment. J Neurogastroenterol Motil 2013;19:90–3. 10.5056/jnm.2013.19.1.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sadeghian M, Farahmand F, Fallahi GH, et al. Cyproheptadine for the treatment of functional abdominal pain in childhood: a double-blinded randomized placebo-controlled trial. Minerva Pediatr 2008;60:1367–74. [PubMed] [Google Scholar]
- 46. Madani S, Cortes O, Thomas R. Cyproheptadine use in children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr 2016;62:409–13. 10.1097/MPG.0000000000000964 [DOI] [PubMed] [Google Scholar]
- 47. Collins BS, Lin HC. Double-Blind, placebo-controlled antibiotic treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr 2011;52:382–6. 10.1097/MPG.0b013e3181effa3b [DOI] [PubMed] [Google Scholar]
- 48. Scarpellini E, Giorgio V, Gabrielli M, et al. Rifaximin treatment for small intestinal bacterial overgrowth in children with irritable bowel syndrome. Eur Rev Med Pharmacol Sci 2013;17:1314–20. [PubMed] [Google Scholar]
- 49. Black CJ, Burr NE, Quigley EMM, et al. Efficacy of Secretagogues in Patients With Irritable Bowel Syndrome With Constipation: Systematic Review and Network Meta-analysis. Gastroenterology 2018;155:1753–63. 10.1053/j.gastro.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 50. Bahar RJ, Collins BS, Steinmetz B, et al. Double-Blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr 2008;152:685–9. 10.1016/j.jpeds.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 51. Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137:1261–9. 10.1053/j.gastro.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roohafza H, Pourmoghaddas Z, Saneian H, et al. Citalopram for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Neurogastroenterol. Motil. 2014;26:1642–50. 10.1111/nmo.12444 [DOI] [PubMed] [Google Scholar]
- 53. Carter BD, Threlkeld BM. Psychosocial perspectives in the treatment of pediatric chronic pain. Pediatric Rheumatology 2012;10:15. 10.1186/1546-0096-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lynch-Jordan AM, Sil S, Peugh J, et al. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain 2014;155:1955–61. 10.1016/j.pain.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levy RL, Langer SL, Walker LS, et al. Cognitive-Behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol 2010;105:946–56. 10.1038/ajg.2010.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Robins PM, Smith SM, Glutting JJ, et al. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. J Pediatr Psychol 2005;30:397–408. 10.1093/jpepsy/jsi063 [DOI] [PubMed] [Google Scholar]
- 57. Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr 2000;31:47–51. 10.1097/00005176-200007000-00011 [DOI] [PubMed] [Google Scholar]
- 58. Youssef NN, Rosh JR, Loughran M, et al. Treatment of functional abdominal pain in childhood with cognitive behavioral strategies. J Pediatr Gastroenterol Nutr 2004;39:192–6. 10.1097/00005176-200408000-00013 [DOI] [PubMed] [Google Scholar]
- 59. Groß M, Warschburger P. Evaluation of a Cognitive–Behavioral pain management program for children with chronic abdominal pain: a randomized controlled study. Int J Behav Med 2013;20:434–43. 10.1007/s12529-012-9228-3 [DOI] [PubMed] [Google Scholar]
- 60. van der Veek SMC, Derkx BHF, Benninga MA, et al. Cognitive behavior therapy for pediatric functional abdominal pain: a randomized controlled trial. Pediatrics 2013;132:e1163–72. 10.1542/peds.2013-0242 [DOI] [PubMed] [Google Scholar]
- 61. Vlieger AM, Rutten JMTM, Govers AMAP, et al. Long-Term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol 2012;107:627–31. 10.1038/ajg.2011.487 [DOI] [PubMed] [Google Scholar]
- 62. Kohen D, Kaiser P. Clinical hypnosis with children and Adolescents—What? why? how?: origins, applications, and efficacy. Children 2014;1:74–98. 10.3390/children1020074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gulewitsch MD, Müller J, Hautzinger M, et al. Brief hypnotherapeutic–behavioral intervention for functional abdominal pain and irritable bowel syndrome in childhood: a randomized controlled trial. Eur J Pediatr 2013;172:1043–51. 10.1007/s00431-013-1990-y [DOI] [PubMed] [Google Scholar]
- 64. van Tilburg MAL, Chitkara DK, Palsson OS, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 2009;124:e890–7. 10.1542/peds.2009-0028 [DOI] [PubMed] [Google Scholar]
- 65. Schlarb AA, Gulewitsch MD, Bock Genannt Kasten I, et al. Recurrent abdominal pain in children and adolescents - a survey among paediatricians. Psychosoc Med 2011;8:Doc02. 10.3205/psm000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Evans S, Lung KC, Seidman LC, et al. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. J Pediatr Gastroenterol Nutr 2014;59:244–53. 10.1097/MPG.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vlieger AM, Blink M, Tromp E, et al. Use of complementary and alternative medicine by pediatric patients with functional and organic gastrointestinal diseases: results from a multicenter survey. Pediatrics 2008;122:e446–51. 10.1542/peds.2008-0266 [DOI] [PubMed] [Google Scholar]
- 68. Cushing CC, Friesen CA, Schurman JV. Collaboration with medical professionals in clinical practice: pediatric abdominal pain as a case example. Fam Syst Health 2012;30:279–90. 10.1037/a0030465 [DOI] [PubMed] [Google Scholar]
- 69. Odell S, Logan D. Pediatric pain management: the multidisciplinary approach. J Pain Res 2013;6:785–90. 10.2147/JPR.S37434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schurman JV, Friesen CA. Integrative treatment approaches: family satisfaction with a multidisciplinary paediatric abdominal pain clinic. Int J Integr Care 2010;10. 10.5334/ijic.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Di Lorenzo C, Colletti RB, Lehmann HP, et al. Chronic abdominal pain in children: a clinical report of the American Academy of pediatrics and the North American Society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr 2005;40:245–8. 10.1097/01.MPG.0000155367.44628.21 [DOI] [PubMed] [Google Scholar]


