Abstract
Objectives
Documented experiences of relocating hospital pharmacies are rare, but adequate preparation is vital to ensuring smooth pharmacy operation and patient safety. In the autumn of 2019, the Pharmacy of Eastern Vaud Hospitals, composed of four units (Logistics, Manufacturing, Clinical Pharmacy, and Nursing Home Supply), was relocated to a new hospital in just a few days. In this context, a failure modes, effects and criticality analysis (FMECA) was carried out before the relocation in order to anticipate any failure modes likely to affect the pharmacy’s missions or patient safety during the move.
Methods
The FMECA was performed by a multidisciplinary team (pharmacists and logisticians) which analysed the complete upcoming process of relocating the pharmacy and its implications. Criticality indices (CIs) were defined based on the matrix developed by Williams et al, which sets a maximum score of 810. Every potential failure mode identified was analysed, and mitigation measures were proposed for each one.
Results
The analysis identified 86 potential failures. The mean initial CI calculated for the entire pharmacy relocation was 177 (min 4–max 567), but this was estimated to be reduced to 39 (−78%) after mitigation measures were identified. Within the whole pharmacy, the failures with the highest CIs were identified in the Logistics unit. Among these, the time necessary to transfer the pharmacy’s drugs from their traditional alphabetical storage location to their new location using robotic, chaotic storage principles was identified as the riskiest potential failure. Indeed, the rapid availability of emergency medicines would have to be guaranteed at all times.
Conclusions
The present study highlighted the relevance of using an FMECA-type evaluation to anticipate the impact of a hospital pharmacy relocation. This tool enabled pharmacy professionals to structure their potential relocation problems and reflect on mitigation measures in order to provide concerted, realistically applicable solutions before the move.
Keywords: pharmacy service, hospital, quality of health care, safety, total quality management, medication systems, hospital
Introduction
The Pharmacy of Eastern Vaud Hospitals (PHEL) is an interhospital pharmacy now located in the Riviera-Chablais Hospital, Vaud-Valais (HRC), in Rennaz, Switzerland. It comprises four units: Logistics, Manufacturing, Clinical Pharmacy, and Nursing Home Supply (NHS). The Logistics unit ensures drug supplies for about 870 hospital beds, including 410 acute beds, which corresponds to approximately 3000 order lines per week. The Manufacturing unit is responsible for preparing unlicensed drugs for specific patients or in small series (sterile or not sterile). In addition, chemotherapies prescribed to oncology patients are prepared individually on a day-to-day basis. The Clinical Pharmacy unit provides pharmaceutical support to medical teams in eastern Vaud’s hospitals, including operating a pharmaceutical helpline during pharmacy working hours, and it sends clinical pharmacists on clinical rounds several times a week. The NHS unit manages drug supplies for about 830 beds in several private and public nursing homes and has a robot that produces their patients’ weekly treatments (pillboxes) automatically. The PHEL’s mission is to provide quality pharmaceutical assistance, medicines and related products safely at all times.
The main site of the HRC itself is a newly constructed 360 bed hospital whose various former clinical wards (six general medicine, five surgical, two paediatric, including neonatology, three intensive care, two gynaecology and obstetrics, and three rehabilitation) were previously spread across six different geographical sites. All those sites, including the one in which the PHEL’s head office was based, were planned to relocate, at 1 week intervals, into a single, new building in November 2019. The PHEL’s head office site, from which it provided direct drug supply to the various hospital sites and services, was planned to move 1 month before the hospital wards (ie, in October 2019). Three satellite pharmacies were located in three other hospital buildings in the eastern Vaud region. Before the move, traditional drug storage shelves were used for alphabetical storage. The PHEL acquired a dispensing robot for its new site and could thus stock its drugs using a chaotic storage principle.
Hospital pharmacy relocations are complex and often unique events and there are only rare reports of such experiences in the literature1 2 and one guideline on developing and moving to new pharmacy facilities, but published in the 80s.3 Some articles have recounted experiences of whole hospital or care units relocations, but few have underlined the need for specific tools and preparedness for hospital moves or clinical wards relocations.4–10 These experiences were reported as the lessons learnt after moves, but they included no prospective risk analysis that could have been used to develop standardised tools. Prospective, targeted strategies to ensure patient safety during unique events such as a hospital or hospital pharmacy relocation are thus individually needed.4–12
Among the many different risk management approaches available, failure modes, effects and criticality analysis (FMECA) is a qualitative methodology used to systematically identify potential failures in a process and their effects.13 FMECA is a prospective tool for theoretically reducing process-related risks and is based on proactive, structured process analysis by a multidisciplinary team. The ultimate objective of its iterative approach is to improve the quality and safety of complex processes.
FMECA determines a score—its criticality index (CI)—that enables risk prioritisation. The method was initially developed by the US Army13 and then used to reduce risks in hazardous industrial processes.14 15 More recently, it has been successfully used to evaluate risks on hospital wards12 16–19 and in pharmacy processes such as chemotherapy manufacturing.20
The present study’s objective was thus to perform an FMECA-type risk analysis of our hospital pharmacy relocation in order to identify risks within the framework of that process and propose solutions to mitigate them. This would ensure that the pharmacy could pursue its mission during this specific, critical, real-life situation and therefore maintain the highest levels of patient safety.
Method
Failure modes, effects, and criticality analysis (FMECA)
An FMECA-type analysis was performed on the complete process and implications of relocating the PHEL. A multidisciplinary team of six professionals was created, composed of the four pharmacists responsible for each pharmacy unit, a coordinator, and a specialised logistician. Four meetings were organised: the first was a brainstorming session to review process mapping and determine failure modes; the second involved performing the criticality analysis; and the third and fourth meetings were used to identify improvements. A head pharmacy technician participated in the last two meetings to provide her practical vision of the pharmacy’s operation.
The full process was split into three formal stages: old pharmacy (departures from original sites), move (relocation), and new pharmacy (establishment in the new building). Some failures could affect every stage, and some could specifically affect one stage.
Determination of failure modes
First, the complete multidisciplinary team’s brainstorming session determined the failure modes for the overall relocation process, and failures common to all units were grouped into a general subtask. Second, the pharmacists responsible for the four units worked individually to determine failure modes linked to their unit’s specificities. Lastly, the moderator aggregated similar topics, the team analysed and discussed all the propositions, and a final list of failure modes was drawn up.
Criticality analysis
Based on the grid developed by Williams et al, the likelihood of occurrence of each mode of failure was rated from 1 (‘no known previous occurrence’) to 10 (‘documented previous occurrences, almost certain error’); the mode of failure’s potential severity for patients was rated from 1 (‘slight annoyance’) to 9 (‘terminal injury or death’); and the chance of detecting the mode of failure before it affected patient safety was rated from 1 (‘error always detected’) to 9 (‘detection impossible’).21 Rating estimations of all the failure modes were finalised by team consensus, considering the specific environment in which this hospital relocation might occur. Each mode of failure’s CI score was calculated by multiplying its occurrence, severity and detection scores (min 1–max 810).
Safety improvement analysis
The potential failure modes identified were analysed, discussed and prioritised based on their CI scores. Mitigation measures were proposed for each one taking into consideration feasibility and costs. Moreover, to ensure that mitigation measures would be applicable in practice by all pharmacy team members, the head pharmacy technician joined the FMECA team to provide her practical vision of pharmacy operations. The mitigation measures were classified according to the type of activity they were linked to. Finally, a second CI score was calculated based on the hypothetical implementation of all the proposed mitigation measures as an evaluation of their impact.
Statistics
Raw data were exported into Microsoft Excel 2010 software (Microsoft Corporation, Redmond, WA, USA) to calculate descriptive statistics (sum, mean, min, max).
Preparation for pharmacy relocation
The four pharmacists responsible for the four pharmacy units used implementation schedules to ensure their proper deployment of mitigation measures. This system made it possible to prioritise implementation measures requiring significant amounts of time and to develop and monitor their progress.
Results
Determination of failure modes and criticality index analysis
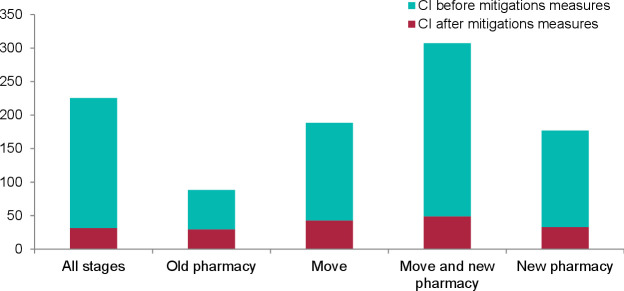
The 3 hour brainstorming session in January 2019 identified 86 potential failures, with 27 considered general, 26 specific to Logistics, 11 specific to Manufacturing, five specific to Clinical Pharmacy, and 17 specific to NHS. The 2 hour meeting for criticality analysis enabled the team to calculate an initial overall mean CI of 177 (min 4–max 567). Figure 1 shows the mean CI scores for each pharmacy unit, and figure 2 shows the mean CI scores by stage in the relocation.
Figure 1.

Mean CI by pharmacy unit. CI, criticality index; NHS, Nursing Home Supply.
Figure 2.
Mean CI by stage in the relocation. CI, criticality index.
Safety improvement analysis
Two 2 hour meetings allowed team members to propose mitigation measures for all the failures identified up to April 2019. Evaluations of these mitigation measures showed that their CIs had decreased across all four pharmacy units. The mean CI score after mitigation measures was 39, corresponding to a mean reduction of 78%. The biggest reduction after mitigation measures was calculated for the Logistics unit, which showed an 85% reduction in its mean CI. The summed CI for the whole pharmacy dropped from 15 246 to 3389 after mitigation measures.
Table 1 summarises the CI evaluations for each pharmacy unit before and after mitigation measures. Table 2 lists the failure modes identified as having the greatest potential risk during the hospital pharmacy relocation.
Table 1.
CI before and after mitigation measures
| Unit | Number of potential failures | Mean initial CI (min–max) | Mean final CI (min–max) | Decrease in CI |
| General | 27 | 132 (4–448) | 31 (1–168) | 77% |
| Logistics | 26 | 264 (36–567) | 41 (3–168) | 85% |
| Manufacturing | 11 | 163 (28–294) | 35 (5–147) | 78% |
| NHS | 17 | 158 (27–448) | 60 (12–256) | 62% |
| Clinical pharmacy | 5 | 70 (12–105) | 19 (4–28) | 73% |
| Whole pharmacy | 86 | 177 (4–567) | 39 (1–256) | 78% |
CI, criticality index; NHS, Nursing Home Supply.
Table 2.
Top 10 failures with the highest CI and mitigation measures proposed
| Unit | Failure mode | Cause(s) | Mitigation measures | Initial CI | Final CI | CI reduction |
| Logistics | Antidotes are not found or inaccessible | Unknown/unlisted storage locations, robotic/non-robotic storage, relocation to other hospital units, etc | Maintain continuous availability of a stock of antidotes for quick delivery at the old site and inform clinical wards about their location and accessibility | 567 | 72 | 87% |
| Logistics | Essential drugs are not found or inaccessible | Unknown/unlisted storage locations, robotic/non-robotic storage, service relocation, etc |
|
567 | 72 | 87% |
| Logistics | Errors in deliveries to clients | Lack of knowledge about wards’ supply channels, changes to pharmacy ordering software identification codes, changed or unknown procedures, wards at old site ordering goods for reception at new hospital |
|
512 | 32 | 94% |
| Logistics | Drugs not found in the new pharmacy but listed in the ERP software’s stock module | Inventory errors | Plan to take an inventory at merchandise departure and arrival | 504 | 28 | 94% |
| NHS | Patients do not receive their medication on time | Prescriptions changed during relocation, needs for urgent medication, etc |
|
448 | 256 | 43% |
| Logistics | Lack of or unavailability of other medication or urgent information | Urgent medical or educational need for an inpatient, a nursing home patient or a patient newly admitted to the hospital during the relocation |
|
448 | 168 | 63% |
| Logistics | Drugs not identifiable by location because of mixed storage (robotic and non-robotic) | Change in type of storage from manual to robotic |
|
441 | 54 | 88% |
| General | Lack of coordination and leadership, unit heads absent from one or two sites (at departure or arrival) | Responsibilities insufficiently defined, unclear delegation of responsibilities |
|
343 | 15 | 96% |
| General | Absence of key staff, including IT support staff | Accident, illness |
|
343 | 35 | 90% |
| General | Unresolved or unaddressed problems | Lack of information, coordination, communication |
|
315 | 270 | 86% |
CI, criticality index; ERP, enterprise resource planning; NHS, Nursing Home Supply.
Mitigation measures identified as having the potential to reduce the risks related to the hospital pharmacy relocation classified into 11 groups according to the activities they were linked to. They are presented in table 3. The head of the four units of the pharmacy were responsible for planning or implementing them between April and October 2019.
Table 3.
List of the most important mitigation measures classified by group
| Operation | Mitigation measures |
| Pharmaceutical support |
|
| Emergency situations |
|
| Communication for clients and healthcare professionals |
|
| Internal communication for pharmacy staff |
|
| Drug management |
|
| Manufacturing |
|
| Information technology systems |
|
| Logistical support |
|
| Quality systems |
|
| Human resources |
|
| Hazardous materials |
|
ERP, enterprise resource planning; IT, information technology; NHS, Nursing Home Supply.
Hospital pharmacy relocation
The pharmacy’s head office was moved 1 month before other hospital wards and departments. In contrast, the eastern Vaud hospitals’ satellite pharmacies moved at the same time as the clinical wards situated in their respective buildings. Finally, most of the drug manufacturing facilities moved 3 months later. The distances between the old sites and the new hospital were around 20 km (approximately 20 min driving time).
In addition to the challenge of ensuring drug supply and patient safety throughout the pharmacy and hospital relocation, the PHEL’s pharmacists had to face other challenges linked to new technologies and infrastructure: the acquisition of two identical dispensing robot, its installation and testing; acquisition of new drug manufacturing equipment (chemotherapy isolators), their qualification and validation; dismantling the robot used to prepare the weekly treatment pillboxes for the NHS unit’s patients and reassembling it in its new environment.
The new facilities were made available at the end of May 2019 to allow for the installation of the robotic stockroom system. Two weeks were necessary to assemble the dispensing robot and 4 weeks to test it.
The hospital pharmacy relocated to its new site between 3 and 7 October 2019. The pharmacy was closed at both the old and new sites, starting from 2 pm on 3 October, and, exceptionally, was inaccessible for 4 days. During this closure period, an emergency service was provided using a specific procedure for quickly finding the drugs needed for patients.
During the move, 1588 references were relocated from the old main site to the new site in 2.5 days, plus an additional 5 days afterwards for relocating from the different satellite pharmacies. The relocation of all the PHEL’s drugs and equipment required 184 pallets (282 m3), corresponding to 11 round trips with a lorry plus 10 with a van.
Stocking 1037 drug references (66% of the stock, corresponding to 40 000 drug packages) in the dispensing robot took 3 days, of which 3000 packages had to be introduced manually.
Problems experienced
The pharmacy experienced some problems during preparation and the relocation itself. Table 4 summarises the main challenges encountered.
Table 4.
Main challenges during the relocation and emergency solutions proposed
| Pharmacy unit | Type of challenge | Problems experienced | Antici-pated by FMECA | Consequences | Solutions |
| Logistics | Ensuring drug availability at all times | Refusal by some suppliers to deliver new orders to the new pharmacy’s address before full hospital relocation | No | Significant time required to negotiate with suppliers, explain the situation and find alternative solutions | Some orders still had to be delivered to the old pharmacy and then transferred directly to the new one by own means |
| Clinical Pharmacy | Changes to phone technology and numbers | New phones were not delivered on time | Yes | Fail-safe procedure used | Calls were transferred from original phone lines to a temporary working phone at the new site |
| Manufacturing | Preliminary qualification and validation of the manufacturing facilities by the authorities (especially clean rooms) | Measures could not be taken in time | Yes | ‘No go’ situation Drug manufacturing facilities moved 3 months later |
A fail-safe procedure was applied to enable safe production at the old facility and the delivery of preparations to the new site |
| Nursing Home Supply | Ensuring drug availability for nursing home patients at all times | Time needed to pack and transfer machinery used to prepare weekly treatment pillboxes was underestimated | Yes | Fail-safe procedure used | An extra week of treatments was produced, allowing time to install the machine and ensure quality before restarting production |
| General | Communication with partners and clients | Insufficient mobile phone network coverage in the first days after the move | Yes | Interruptions to phone calls and slow internet connections | Interventions by the hospital’s technical support staff and mobile phone company solved the problems |
| General | Operational alarm systems on the day of the move | False fire alarm signal activation | Yes | Auditory disturbances caused by false alarms | Interventions by the hospital’s technical support staff fixed the alarm system |
| General | Operational equipment on the day of the move | Delays in the delivery of some equipment | Yes | Some equipment missing on the day of the move (cold room storage shelves, bench-tops for Manufacturing unit) | Using alternative equipment until the new equipment arrived |
FMECA, failure modes, effects and criticality analysis.
Discussion
Determining failure modes and CIs
An FMECA was performed on the complete process of a planned hospital pharmacy relocation. It was the first risk analysis performed for such a relocation. All 86 failure modes identified were studied, and mitigation measures were proposed for each of them in order to maximise the chances of a safe and successful relocation. Most of the failure modes applied to all four of the pharmacy units (considered potential general failures); the Logistics unit also had a significant number of potential failure modes. The mean initial CI was highest for the Logistics unit, identifying it as the unit most at risk. Among the failures identified, the stock transfer step—moving from traditional alphabetical storage (manual) to using a chaotic storage principle (mainly robotic)—was identified as the riskiest. Indeed, throughout the entire hospital relocation, the rapid availability of emergency medicines had to be ensured at all times, taking into account that the pharmacy would move into the new hospital 1 month before the institution’s other clinical wards and departments. On the one hand, this time difference allowed the pharmacy to plan its early relocation with a view to providing better support to the clinical wards during their own moves. On the other hand, this required the pharmacy to be fully operational rapidly because the hospital’s normal activities continued at that time.
The pharmacy relocation stages identified as being the riskiest were the move itself and establishment in the new pharmacy. This could be explained by the intimate knowledge that staff had of their original pharmacy compared with the new one, considering that any modifications to their working environment (eg, transportation) might increase the risk of failing to anticipate changes.
Safety improvement analysis
The implementation of planned mitigation measures reduced all the CI scores. The greatest decrease was observed for the Logistics unit (85%), followed by the Manufacturing unit (78%). The FMECA undoubtedly highlighted the importance of preparedness.
All the mitigation measures proposed by the pharmacy’s multidisciplinary team were implemented and ready on the day of the hospital pharmacy’s relocation. Specific checklists and procedures were developed by dedicated staff and used to support and structure key stages of the relocation. In addition, fail-safe procedures were developed for issues that the FMECA identified as potential failure modes. Concerning the days surrounding the relocation, a precise action plan was put in place involving detailed planning of tasks and responsibilities for each staff member. The analysis also highlighted the need for careful upstream planning (timing, human resources, etc). Staff were also given a full briefing a few days before the move.
Maintaining an ‘emergency stock’ of drugs (including antidotes) was considered costly because it represented a partial duplicate of the main stock but reduced the potential risk of treatment interruptions for patients. This temporary stock of medicines was kept on the old pharmacy premises, whose location was known and accessible to the hospital’s healthcare professionals at all times.
In addition, communication about upcoming changes and preparing adequate support for partners (nursing homes, clinical wards of the HRC, other hospitals, etc), some of whom would also be moving 1 month later, had to be done effectively during this transition. A special newsletter was created for the relocation event and was published 2 weeks before the pharmacy’s move. Its main contents were information on the pharmacy’s moving schedule, opening hours, what to do in case of an emergency outside those opening hours, the principal changes related to the move itself (eg, robotic storage and its consequences), and the important pharmacy phone numbers.
The newsletter was distributed via different communication channels, such as the pharmacy’s website, the hospital’s intranet, email groups, and direct distribution on the wards by pharmacy staff. One important point regarding internal communication was nominating a staff member responsible for each unit’s old pharmacy departure and new pharmacy arrival. These individuals were in direct communication with each other during the move and ensured good overall coordination for each unit. Finally, each pharmacy employee was aware of the mitigation measures implemented and was trained in advance on how to use the available tools.
Problems experienced
Fortunately, no major human resources problems were encountered during the moving stage. All the key people or their deputies were present on the day of the relocation. Planning had made it possible to apply the fail-safe procedures without delays and to see the benefits of solutions implemented upstream to limit relocation-related risks (eg, a ‘no go’ situation in the Manufacturing unit, or advanced production in the NHS unit). Six out of seven problems were anticipated in the FMECA, which demonstrate that performing this risk analysis was beneficial for the safety of the relocation operation.
One problem which was not anticipated by the FMECA was the refusal by some suppliers to deliver new drug orders to the new pharmacy’s address before the full hospital relocation had been completed. However, after significant time spent negotiating with those suppliers, and with the support of the regional health authorities, those orders were eventually delivered to the old pharmacy and then transferred immediately to the new one.
Unfortunately, phone numbers and technology in the new pharmacy were different. This made the move a bit problematic, first because the new technology should have been available and functional immediately, which was not entirely the case, and second because customers should have known those new phone numbers in advance so that contact with the pharmacy was never lost. Contact with clients was maintained throughout the move, however, because the old pharmacy phone line was kept available and calls were transferred.
Limitations
Although FMECA enables problems to be anticipated and potential solutions to be implemented, it remains a subjective methodology depending on the number of team members participating and its interdisciplinary nature. FMECA also aims to be iterative—applying mitigation measures and then carrying out a new round of analysis—but this could not be the case in the present study due to the single hospital pharmacy relocation conducted. The post-mitigation measure CI scores are therefore presented based solely on the perceptions of the FMECA team’s participants.
Moreover, it was impossible for our study to quantitatively measure the mitigation measures’ impacts on the running of the pharmacy and patient safety. Although it is difficult to estimate the cost-effectiveness of such an analysis, we consider that the planning and preparedness that it enabled more than compensated for this limitation.
Conclusion
FMECA is a simple and efficient methodology, which the present study used to evaluate the potential risks to patient safety during the relocation of a hospital pharmacy. The analysis highlighted that moving a hospital pharmacy is a challenging event, but a structured risk analysis can proactively limit risks and make this type of process safer. Analysis of the implementation of mitigation measures put forward through this approach suggested that the risks associated with the relocation had been very significantly reduced. Thus, FMECA made it possible to anticipate most of the problems actually encountered on moving days and to put in place rapidly deployable solutions. Unanticipated problems could, therefore, be managed more smoothly without disturbing the overall management of the move.
The present work demonstrated the usefulness of risk analysis methods for improving preparedness and patient safety when relocating a major healthcare facility.
What this paper adds.
What is already known on this subject
Failure modes, effects and criticality analysis (FMECA) is a risk analysis instrument used to identify potential failures in complex processes and their effects, thus enabling the reduction of any associated risks.
Hospital relocations are complex events requiring specific risk management approaches, but few reports in the literature have described hospital pharmacy relocations.
What this study adds
Performing an FMECA-type analysis before a hospital pharmacy relocation can help to anticipate many of the associated risks, thus improving preparedness and ultimately patient safety.
Acknowledgments
The authors are thankful to Ms Florence Baeriswyl and Mr Dario Celis for their participation and contribution to the FMECA sessions. Mr Dario Celis was the designated relocation manager.
Footnotes
Correction notice: This paper has been updated since it was published online. There have been some small changes to the first two subtitles of the Methods section.
Contributors: LS: Conceptualisation, data curation, formal analysis, investigation, methodology, writing – original draft. MD: Formal analysis, investigation, writing – original draft. SK: Formal analysis, investigation, writing – original draft. CP: Formal analysis, investigation, writing – original draft. MLB: Formal analysis, investigation, writing – original draft. ALB: Formal analysis, investigation, writing – original draft. FR: Investigation, writing – review and editing. NW: Supervision, writing – review and editing. CB: Conceptualisation, methodology, supervision, writing – review and editing.
Funding: The first author was financed by the Specialised Centre for Emergency and Disaster Pharmacy, Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Raw data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Burns D. How proper planning helped us greatly in moving our pharmacy twice. Pharm Times 1980;46:90–3. [PubMed] [Google Scholar]
- 2. Crawley T. Hospital pharmacy relocation plan moves forward. Design contract awarded to relocate existing pharmacy department at regional hospital. BC locale news 2019. [Google Scholar]
- 3. Moore TD. Developing new pharmacy facilities. Top Hosp Pharm Manage 1983;3:17–26. [PubMed] [Google Scholar]
- 4. Francoeur C, Shea S, Ruddy M, et al. It takes a village to move a hospital: simulation improves intensive care team preparedness for a move to a new site. Hosp Pediatr 2018;8:148–56. 10.1542/hpeds.2017-0112 [DOI] [PubMed] [Google Scholar]
- 5. Gignon M, Amsallem C, Ammirati C. Moving a hospital: simulation - a way to co-produce safety healthcare facilities. Int J Occup Saf Ergon 2017;23:589–91. 10.1080/10803548.2016.1270543 [DOI] [PubMed] [Google Scholar]
- 6. Comeau OY, Armendariz-Batiste J, Baer JG. Preparing critical care and medical-surgical nurses to open a new hospital. Crit Care Nurs Q 2017;40:59–66. 10.1097/CNQ.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 7. Lin FF, Foster M, Chaboyer W, et al. Relocating an intensive care unit: an exploratory qualitative study. Aust Crit Care 2016;29:55–60. 10.1016/j.aucc.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Grier S, Gough CJ, Wrathall GJ. The relocation and road transfer of intensive care patients to a new hospital in Bristol: our experiences. J Intensive Care Soc 2016;17:326–31. 10.1177/1751143716644460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halfer D, Rosenheck M. Virtual education: is it effective for preparing nurses for a hospital move? J Nurs Adm 2014;44:535–40. 10.1097/NNA.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 10. Jen HC, Shew SB, Atkinson JB, et al. Creation of inpatient capacity during a major hospital relocation: lessons for disaster planning. Arch Surg 2009;144:859–64. 10.1001/archsurg.2009.146 [DOI] [PubMed] [Google Scholar]
- 11. Gowing JR, Walker KN, Elmer SL, et al. Disaster preparedness among health professionals and support staff: what is effective? An integrative literature review. Prehosp Disaster Med 2017;32:321–8. 10.1017/S1049023X1700019X [DOI] [PubMed] [Google Scholar]
- 12. Duffy K, Pearson A, Waters M. Moving a hospital--a once in a lifetime experience. Aust Health Rev 2002;25:155–61. 10.1071/AH020155 [DOI] [PubMed] [Google Scholar]
- 13. US Army . Procedure for performing a failure mode effect and criticality analysis. United States: Military Procedure, 1949. [Google Scholar]
- 14. Society for Automotive Engineers . Design analysis procedure for failure modes, effects and criticality analysis (FMECA), 1967. [Google Scholar]
- 15. International Electrotechnical Commission . Analysis techniques for system reliability – procedure for failure mode and effects analysis (FMEA) 1985.
- 16. Rodriguez-Gonzalez CG, Martin-Barbero ML, Herranz-Alonso A, et al. Use of failure mode, effect and criticality analysis to improve safety in the medication administration process. J Eval Clin Pract 2015;21:549–59. 10.1111/jep.12314 [DOI] [PubMed] [Google Scholar]
- 17. Asgari Dastjerdi H, Khorasani E, Yarmohammadian MH, et al. Evaluating the application of failure mode and effects analysis technique in hospital wards: a systematic review. J Inj Violence Res 2017;9:51–60. 10.5249/jivr.v9i1.794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vélez-Díaz-Pallarés M, Delgado-Silveira E, Carretero-Accame ME, et al. Using healthcare failure mode and effect analysis to reduce medication errors in the process of drug prescription, validation and dispensing in hospitalised patients. BMJ Qual Saf 2013;22:42–52. 10.1136/bmjqs-2012-000983 [DOI] [PubMed] [Google Scholar]
- 19. É D, Collin-Lévesque L, Boulé M. Analyse des modes de défaillance, de LeuRS effets et de leur criticité dans Le circuit Du médicament: revue de littérature. Can J Hosp Pharm 2018;71:376–84. [PMC free article] [PubMed] [Google Scholar]
- 20. Bonnabry P, Cingria L, Ackermann M, et al. Use of a prospective risk analysis method to improve the safety of the cancer chemotherapy process. Int J Qual Health Care 2006;18:9–16. 10.1093/intqhc/mzi082 [DOI] [PubMed] [Google Scholar]
- 21. Williams E, Talley R. The use of failure mode effect and criticality analysis in a medication error subcommittee. Hosp Pharm 1994;29:331–2. 4-6, 9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available upon reasonable request.