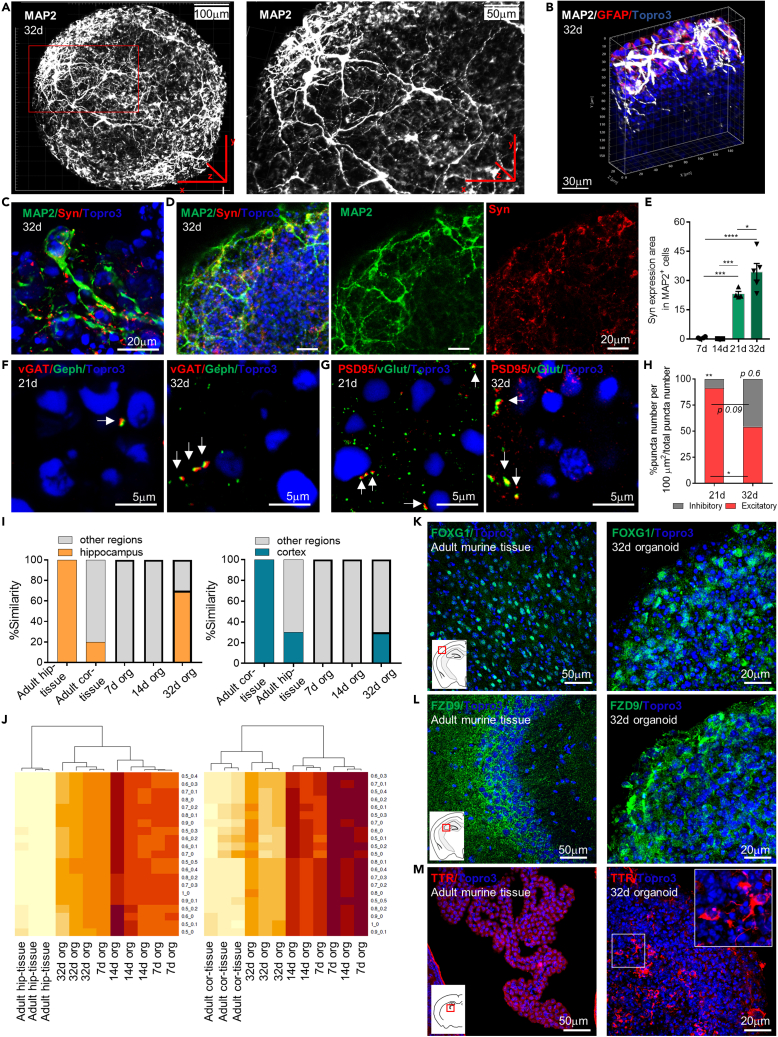
Figure 4.
Neuronal network formation and synapse development within mature murine brain organoids
(A) Representative maximum Z-projection of confocal images of whole mount immunostaining performed at 32 d for the MAP2 marker, showing the formation of an extended neuronal network on the organoids surface, with the characteristic process elongation. The red box highlights the magnification of an organoid region.
(B) Representative 3D reconstruction of confocal image of a mature organoid (32d) region using IMARIS software showing the distribution of neuronal cells (MAP2+, in white) in all the spatial three-dimensions.
(C) High magnification confocal maximum intensity z stack projections of image showing a mature neuronal cell (MAP2) inside the organoid at 32d with the characteristic process elongation and the expression of synaptic density marker (Syn).
(D) Representative maximum intensity z stack projection of confocal images of whole mount immunostaining performed at 32d for the Syn and the MAP2 markers, showing colocalization between neuronal cells and the synaptic marker.
(E) Graph showing the synaptic density (% Syn+ area in MAP2+ cells area, in 100 μm2) at different time points. Data are expressed as mean ± SEM. Statistical differences in graphs were calculated by ordinary one-way ANOVA. ∗∗∗p < 0.001; ∗p < 0.05.
(F and G) Representative confocal immunofluorescence maximum intensity z stack projections images of sliced murine brain organoids at 21 and 32 d for the detection of inhibitory vGat-Gephyrin (F) and excitatory PSD95-vGlut (G) synapses. White arrows indicate the co-localization of PSD95-vGlut and vGat-Gephyrin in double-positive cells, able to generate excitatory and inhibitory synaptic contacts, respectively.
(H) Graph showing the average inhibitory and excitatory synaptic puncta colocalization in 100 μm2. Note the general synaptic decrement, mainly the excitatory synapses reduction, matched with the increment of inhibitory synapses at 32 d. Analysis performed on n≥3 different organoids and at least 3 entire sections for each organoid. Data in all graphs are showed as mean ± SEM. Statistical differences in graphs were calculated by ordinary one-way ANOVA. ∗∗p < 0.01; ∗p < 0.05.
(I) Graphs showing the percentage of similarity of adult murine hippocampal tissue (adult hip-tissue), adult cortical tissue (adult cor-tissue), 7d organoids (7d org), 14d organoids (14d org), and 32d organoids (32d org) to hippocampal (left graph) or cortical (right graph) tissues.
(J) Heatmaps showing the degree of similarity between adult murine hippocampal tissue (adult hip-tissue) or adult cortical tissue (adult cor-tissue) and 7d organoids (7d org), 14d organoids (14d org), and 32d organoids (32d org) considering different proportions of hippocampal and cortical components, according to gene expression profiles of selected genes.
(K–M) Representative confocal immunofluorescence maximum intensity z stack projections images of sliced adult (8 weeks) murine brain tissue and mature (32d) murine brain organoids showing the expression of FOXG1-cortical (K), FZD9-hippocampal (L) and TTR-choroid plexus (M) markers. White insets represent a sketch of coronal mouse brain sections while red boxes highlight the brain region of the murine tissue staining.