Figure 6.
Calcium imaging, transsynaptic tracing and electrophysiology on intact organoids show neuronal synaptic connections and spontaneous activity
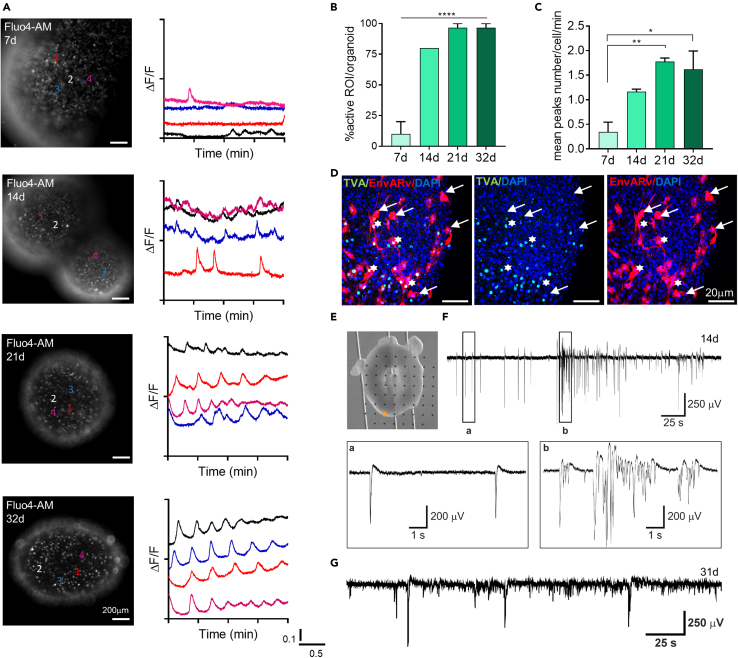
(A) Representative fluorescence images reported in greyscale of whole mount organoids at different time points during the time-lapse Fluo4-AM dye calcium imaging assay (left side), paired to graphs of calcium oscillations of representative cells analyzed (right side). It is possible to observe an increment of cellular activity over time, as well as major peaks synchronization, from early (7d) to mature (21-28d) organoids.
(B and C) Graphs showing (B) the percentage of active cells within the organoid at different time points (n = 3 organoids/time point; n = 10 cells selected in each organoid) and (C) the average number of the peaks counted for each cell per minute at different time points (n = 3 organoids/time point; n = 10 cells selected in each organoid). Data in all graphs are shown as mean ± SEM. Statistical differences in graphs were calculated by ordinary one-way ANOVA. ∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p< 0.05.
(D) Representative maximum intensity z stack projection of confocal images of Pseudotyped rabies-virus (EnvARv)–based transsynaptic tracing in neural cells of whole mount organoids at 32d. White asterisks indicate the “starter” neurons (yellow); white arrows indicate the projecting neurons (red) taking synaptic contact with TVA-neurons.
(E) Optical bright-field image of a 14d organoid laying on a planar MEA and held in place by a custom hold-down anchor.
(F) Electrical activity generated by the organoid in (E) and recorded by the electrode marked by the orange dot. The insets show a representative population spike (A) and a representative population burst (B) corresponding to the boxed signal portions.
(G) Electrical activity generated by a 31d organoid (different from E), showing the persistence of the population spikes and bursts resembling those observed at the earlier organoid developmental stage shown in (F).