Summary
Background
Allergen immunotherapy (AIT) is the only causal treatment for respiratory allergy. Long-term real-life effectiveness of AIT remains to be demonstrated beyond the evidence from randomised controlled trials (RCTs).
Methods
REACT (Real world effectiveness in allergy immunotherapy) is a retrospective cohort study using claims data between 2007 and 2017. Study eligibility was a confirmed diagnosis of allergic rhinitis (AR), with or without asthma, and AIT. To ensure comparable groups, AIT-treated subjects were propensity score matched 1:1 with control subjects, using characteristic and potential confounding variables. Outcomes were analysed as within (pre vs post AIT) and between (AIT vs control) group differences across 9 years of follow-up (ClinicalTrial.gov: NCT04125888).
Findings
46,024 AIT-treated subjects were matched with control subjects and 14,614 were included in the pre-existing asthma cohort. AIT-treated subjects were 29·5 (16·3) years and 53% were male. Compared to pre-index year, AIT was consistently associated with greater reductions compared to control subjects in AR and asthma prescriptions, including both asthma controller and reliever prescriptions. Additionally, the AIT group had significantly greater likelihood of stepping down asthma treatment (P <0·0001). In addition to the reduction in asthma treatment in the AIT group, a greater reduction in severe asthma exacerbations was demonstrated (P<0·05). Reductions in pneumonia with antibiotic prescriptions, hospitalisations, and duration of inpatients stays were all in favour of AIT.
Interpretation
The study extends the existing RCT evidence for AIT by demonstrating longer-term and sustained effectiveness of AIT in the real world. Additionally, in patients with concurrent asthma, AIT was associated with reduced likelihood of asthma exacerbations and pneumonia.
Funding
The study was funded by ALK A/S.
Key words: Allergic rhinitis, Allergy, Allergy immunotherapy, Asthma, Effectiveness, Real-world evidence, Retrospective cohort study
Abbreviations: AIT, allergy immunotherapy; AR, allergic rhinitis; FU, follow-up; HDM, house dust mite; HRU, health care resource utilisation; ICS, inhaled corticosteroids; INCS, intranasal corticosteroids; LABA, long-acting beta2-agonists; PSM, propensity score matching; RCT, randomised clinical trial; RWE, real world evidence; Rx, prescription; SABA, short-acting beta2-agonists; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy
Research in context.
Evidence before this study
In recent years, randomised controlled trials (RCTs) have confirmed the efficacy and safety of allergy immunotherapy (AIT) in subjects with allergic rhinitis (AR) with or without asthma, but real-world effectiveness data remain scarce. We searched PubMed using the search string (allergen immunotherapy OR allergy immunotherapy OR specific immunotherapy OR AIT OR SIT) AND (allergic rhinitis OR AR OR allergy OR asthma) AND (retrospective studies OR retrospective OR real-world evidence OR RWE OR cohort studies OR cohort stud* OR Follow-Up Studies). The search was limited to publications during the last 10 years and yielded 229 publications. Upon review of the publications, 19 real-world effectiveness studies in allergy immunotherapy (AIT) were identified. However, the 19 studies were limited to either a specific allergen or route of administration, by a short follow-period or focused on the impact of compliance on effectiveness.
Added value of this study
Based on the literature search, we conclude that this pre-specified propensity score matched retrospective cohort study is the most comprehensive real-world effectiveness study within AIT to date, looking at effect of AIT in AR and asthma. Since AIT is the only causal treatment for allergy, demonstrating sustained, long-term effects of AIT-treatment beyond the limited follow-up period in RCTs is highly relevant. Being a causal treatment for allergy also infers potential benefits on multiple manifestations e.g. allergic rhinitis and comorbid asthma. The results of the current study complement the existing RCT evidence for AIT, by demonstrating long-term and sustained reductions in prescriptions for symptom-relieving AR medication across nine years of follow-up. Additionally, the study provides novel long-term data in patients with asthma. In the study, AIT was found to be consistently associated with sustained, long-term reductions across clinically relevant outcomes, e.g. prescriptions of reliever and controller asthma medication, asthma exacerbations, pneumonia, and hospitalisations.
Implications of all the available evidence
While the study methodology addresses an important knowledge gap by providing high-quality real-world evidence in AIT, the findings of the study also add new information on how AIT works both long-term and in real life. The novel findings from the study are complementary to the evidence from RCTs and can further support clinical decision- making on AIT for the treatment and sustained control of both allergic rhinitis and asthma.
Alt-text: Unlabelled box
Introduction
Allergy is caused by an abnormal reaction of the immune system to otherwise harmless allergens and afflicts 10–40% of populations.1 Respiratory allergy is a heterogeneous disease causing allergic rhinitis (AR) and asthma.2 10–40% of patients with AR have allergic asthma and a large proportion (up to 60–80%) of asthmatic patients experience chronic symptoms of AR.3,4 AR is a well-known risk factor for poor asthma control,5,6 as patients with asthma and severe AR are up to 4–5 times more likely to have poor asthma control than those without AR.7 As AR is associated with a great disease burden,8 the frequent co-existence with asthma only increases the overall impact of the allergic disease.9,10 Consequently there is a need for treatment options that target the underlying allergy and benefit both diseases.
Allergy immunotherapy (AIT) is the only causal treatment for allergy. Increased tolerance towards a causal allergen is achieved by repeated allergen administrations, resulting in modulation of the immunological response.11. AIT can be administered sublingually (SLIT) as tablets or drops or subcutaneously (SCIT) for a minimum treatment period of three years.12 Long-term, randomised controlled trials (RCTs) for SQ grass SLIT-tablets and SCIT have confirmed that AIT can modify the allergic disease by demonstrating sustained reductions in symptoms and symptom-relieving medication use up to two years after treatment cessation in patients with allergic rhinitis.13,14 Despite AIT being the only causal treatment, long-term data are limited. Data on the efficacy of AIT in patients with comorbid asthma were demonstrated in house dust mite (HDM), grass, and tree pollen allergy.15 In RCTs, treatment with SQ HDM SLIT-tablets in subjects with asthma and AR provided reductions in inhaled corticosteroids (ICS) use and a 34% relative risk reduction in moderate-severe asthma exacerbations was demonstrated.16,17 However, whether AIT can prevent disease progression from mild to severe asthma needs to be further examined.
While the efficacy and safety of AIT administered as SLIT-tablets for the most common respiratory allergies, e.g. grass, tree, HDM, cedar, and ragweed have been demonstrated in RCTs,15 uncertainty around real-world effectiveness and across longer follow-up periods of AIT in the real world, in particular on asthma related outcomes, remains.18 There is a need for generating robust real world evidence (RWE) for AIT as treatment of respiratory allergies to provide complementary evidence for the existing RCTs.18,19 The aim of the REACT (Real world effectiveness in allergy immunotherapy) study was to assess the long-term effectiveness of AIT for the treatment of AR and asthma in the real world setting.
Methods
Study design and dataset
For this retrospective, observational, propensity score matched (PSM) cohort study, anonymised claims data during 2007 to 2017 from a German health insurance fund database (Betriebkrankenkasse (BKK)) were used. The BKK health insurance fund is a branch of the statutory health insurance and is the most representative of persons insured by the German statutory health insurance,20 covering approximately 90% of the German population.21 The real-world data used in this study comprised insurance claims of 5.9 million insured persons per year and included data on e.g. prescriptions, confirmed diagnosis codes, hospitalisations, specialist visits, and sick leave benefits.
Insurance data from January 1, 2007, through December 31, 2017 (study period) were used. Subjects were included in the dataset if they had an AIT prescription between January 1, 2008, and December 31, 2016, to allow for at least one-year pre and post AIT data availability. The study complied with European and German data protection regulations. Approval from the BKK database for the use of anonymized data was obtained. No institutional review board or ethical committee approval was needed. The study was pre-registered on clinicaltrials.gov (NCT04125888).
The study population consisted of subjects with AR with or without asthma and treated with AIT (AIT group) and a control group not treated with AIT. A confirmed diagnosis of AR had to be recorded during the study period for both groups. AIT-treated subjects were matched 1:1 using propensity score matching (PSM) to a control group of subjects with AR with or without asthma not treated with AIT (control group). The objective of PSM was to obtain an unbiased estimate of treatment effect adjusted for the impact of given confounding factors. The eligibility criteria for the AIT group were two consecutive prescriptions of the same AIT (index AIT) during the identification period (except for venom AIT); the first AIT prescription constituted the index date. The exclusion criterion for both groups was an AIT prescription during the pre-index period.
To evaluate the impact of AIT on asthma, two subcohorts, i.e. no asthma and pre-existing asthma, were specified from the main cohort (Suppl. Key definitions) before the index AIT. Hence, the study consisted of three cohorts (a main cohort, a pre-existing asthma cohort, and a no asthma cohort), each including AIT-treated subjects and their matched control subjects.
Outcomes
The primary outcome was AR prescriptions in each FU-year. Secondary outcomes were asthma prescriptions, severe asthma exacerbations, and change in asthma treatment steps (Suppl. Key definitions, Protocol). In the no asthma cohort, new onset of asthma was assessed as an outcome (Suppl. Key definitions). Diagnosis codes for asthma and use of controller medications mimicking the GINA (Global Strategy for Asthma Management) steps were used to assess changes in asthma treatments (Suppl. Protocol) 3. Short-acting beta-agonists (SABA) were reported separately. Key exploratory outcomes included health care resource utilisation (HRU), costs, sick leave, and respiratory tract infections (Suppl. Protocol). Events of anaphylaxis in relation to AIT index-date were assessed (Suppl. Protocol).
Subgroup analyses across different populations and treatment modalities were included and a sensitivity analysis was pre-specified for the assessment of asthma treatment steps. In the main analysis one prescription within a FU-year was sufficient to qualify for a treatment step. As asthma treatment may fluctuate, the asthma treatment steps were evaluated in a sensitivity analysis that required a confirmatory prescription (within the same drug class). A validation analysis was pre-specified to detect profound bias, i.e. reductions in AR medication previously demonstrated in multiple RCTs for SLIT-tablets22, 23, 24, 25 should be demonstrated for the SLIT-tablet subgroup to show consistency with the existing evidence base.22, 23, 24, 25
Statistical analysis
To mitigate the risk of confounding due to differences in subjects treated with AIT and control subjects, PSM was used to balance the groups with respect to known confounders. In PSM, the likelihood of being treated with AIT (i.e. the propensity score) is calculated based on a range of variables for both treated and untreated subjects. Treated subjects are then matched with untreated subjects with a very similar propensity score. In this study, a range of variables was used to estimate the propensity scores for each individual subject (Suppl. Protocol). Treated subjects were matched 1:1 with control subjects using the fitted value on the logit scale and matching using the nearest neighbour approach without replacement with a calliper of 0·01. For successful matching, all standardised mean differences for variables included in the matching had to be <10% for the main cohort. All modifications to the PSM to achieve all standardised mean differences <10% took place prior to cohort lock and outcomes analyses (Suppl. Protocol). In addition to successful matching of the main cohort, the pre-existing asthma cohort, the no asthma cohort, and the age subgroup (age: <18 years and ≥18 years) were matched using the same PSM. Per protocol, all cohorts were locked prior to initiating any analyses of outcome. An index event (index AIT prescription) could only be observed in the AIT group; therefore control subjects were assigned the same index date as the subjects in the AIT group to whom they were matched. (Suppl. Protocol). Since a control could receive AIT at a later timepoint, the matched control subjects were censored when they were prescribed AIT alongside the matched subject in the AIT group.
Outcomes were analysed as within group changes from pre-index year and between group differences i.e. AIT vs. control subjects. Continuous variables were reported as mean, absolute mean difference, relative mean difference, 95% confidence interval (CI) and p-value. For continuous variables, a paired t-test was used and for between groups differences an unpaired t-test was used as independent samples were assumed. Categorical variables were reported as odds ratio, 95% CI and p-value. For categorical variables, the Fisher's exact test was used to test for statistically significant differences. For variables with multiple categories, the Monte Carlo Simulation was used. Time-to-first analyses were summarised using Kaplan Meier (KM) curves. The median time to event was reported with 95% CI. The Cox Constant Proportional Hazards method was used to estimate the ratio of treatment effects and 95% CI. The proportional hazard assumption was tested using the Kolmogorov-type supremum test. For the time-to-any analysis including recurrent events, Cox proportional regression, i.e. the “The Prentice, Williams, and Peterson Total Time Model” (PWP total time model) was applied. (Suppl. Protocol, Statistical Analysis Plan).26
Role of the funding source: As employees at ALK A/S, SB, LE, MR, and JRL designed the study in collaboration with BF, CB, MC, and NF. Data retrieval, PSM, cohort lock, outcome analyses, and statistical reporting were provided by a third-party vendor. SB, LE, MR, and JRL assessed and interpreted the findings in collaboration with BF, CB, MC, and NF. All authors had full access to the results, contributed to the preparation of the manuscript and concurred to its submission for publication.
Results
From 2007 to 2017, a total of 115,098 subjects had at least one AIT prescription out of the 5,983,511 available subjects in the database. 47,440 subjects with an AIT prescription were eligible for the study and included in the PSM alongside 1,003,332 control subjects who had at least one AR diagnosis and no AIT prescription 12 months prior to index date (Suppl. Figure S1). Before the matching, AIT-treated subjects were younger, more had asthma, and the rate of AR related comorbidities was higher than untreated patients (Suppl. Table S1). The PSM resulted in well balanced cohorts meeting the pre-specified criterion of standardised mean differences <10% for all variables (Suppl., Table S1, Figure S2). The final AIT cohort included 46,024 subjects matched 1:1 with a control subject, resulting in a main cohort of 92,048 subjects. The pre-existing and no asthma cohort were matched separately, after the main cohort was matched. Subjects, for which no good match was found were lost. In the pre-existing asthma cohort, 4635 subjects could not be matched and therefore lost. Similarly, in the no asthma cohort 3911 subjects were not matched and therefore not included in the asthma subcohorts (AIT and control subjects). Hence, from the main cohort, 29,228 subjects were included in a pre-existing asthma cohort and 54,274 in a no asthma cohort (Suppl. Figure S3). Subgroups by route of administration comprised N = 36,927; N = 4816, and N = 3754 for SCIT, SLIT-drops, and SLIT-tablets, respectively. Subgroups by allergen comprised N = 11,713 on grass AIT, N = 7774 on HDM AIT, N = 11,897 on tree AIT, and N = 9726 on other allergens. In the main cohort, the AIT group had a mean age of 29·5 (16·3) years, 53% were males, and had a high prevalence of comorbidities. AIT-treated subjects were exposed to AIT treatment for a mean of 549 (284) days and had 1·084 (1·705) AR prescriptions. The demographics were overall similar across the main, pre-existing, and no asthma cohorts (Table 1, Suppl. Tables S2-S4).
Table 1.
Key demographics for main and pre-existing asthma cohort
| Main |
Pre-existing asthma |
|||||||
|---|---|---|---|---|---|---|---|---|
| AIT N=46024 | Control N=46024 | AIT N=14614 | Control N=14614 | |||||
| Age* (years) | 29·5 | 16·3 | 29·5 | 17·4 | 28·3 | 16·9 | 29·5 | 17·7 |
| Sex# (males) | 24410 | 53% | 24134 | 52% | 7919 | 54% | 7766 | 53% |
| Key comorbidities# | ||||||||
| Conjunctivitis | 10001 | 22% | 10126 | 22% | 3869 | 26% | 3898 | 27% |
| Eczema | 11909 | 26% | 12868 | 28% | 4418 | 30% | 4830 | 33% |
| Health care utilisation* | ||||||||
| Ambulatory care visits (visits) | 15·6 | 12·3 | 17·9 | 16·6 | 18·1 | 13·3 | 20·7 | 17·0 |
| Hospitalisations (days) | 0·2 | 0·6 | 0·2 | 0·7 | 0·2 | 0·7 | 0·2 | 0·7 |
| Sick leave (days) | 0·7 | 1·4 | 0·8 | 1·5 | 0·7 | 1·4 | 0·8 | 1·6 |
| Treatment with AIT* (days) | ||||||||
| Duration until first discontinuation | 216 | 118 | .. | .. | 218 | 120 | .. | .. |
| Total duration on index-AIT | 549 | 284 | .. | .. | 559 | 289 | .. | .. |
| AR prescriptions* | 1·084 | 1·705 | 1·034 | 1·878 | 1·415 | 1·985 | 1·416 | 2·258 |
| Antihistamine | 0·452 | 1·063 | 0·404 | 1·101 | 0·628 | 1·283 | 0·587 | 1·390 |
| INCS | 0·464 | 0·925 | 0·445 | 1·090 | 0·552 | 1·054 | 0·602 | 1·292 |
| Asthma prescriptions* | 0·996 | 2·199 | 0·986 | 2·367 | 2·465 | 3·022 | 2·495 | 3·308 |
| SABA | 0·398 | 1·033 | 0·393 | 1·169 | 0·964 | 1·489 | 0·980 | 1·715 |
| ICS | 0·221 | 0·724 | 0·209 | 0·767 | 0·565 | 1·095 | 0·540 | 1·166 |
| ICS/LABA | 0·261 | 0·901 | 0·267 | 0·980 | 0·650 | 1·359 | 0·679 | 1·462 |
| At least one severe asthma exacerbations in pre-index year* | 2756 | 6% | 2368 | 5% | 2329 | 16% | 2088 | 14% |
| Asthma treatment steps# | ||||||||
| Treatment step 1 | .. | .. | .. | .. | 5499 | 38% | 6074 | 42% |
| Treatment step 2 | .. | .. | .. | .. | 3289 | 23% | 2888 | 20% |
| Treatment step 3 | .. | .. | .. | .. | 4600 | 31% | 4434 | 30% |
| Treatment step 4 | .. | .. | .. | .. | 531 | 4% | 524 | 4% |
Continuous variables are reported as mean ± SD; # Categorical variables are reported as n (%); Prescriptions (Rx) are reported as Rx/ subject; Pre-existing asthma: At least one asthma diagnosis or two prescriptions of SABA/ICS within the pre-index year; Asthma treatment step [cross sectional]: Predefined categories based on asthma diagnoses codes and asthma medication prescriptions within pre-index year - Treatment step 1: Asthma diagnosis in the absence of controller asthma medication; Treatment step 2: Monotherapy with either low dose ICS or LTRA; Treatment step 3: Dual therapy or therapy with medium-high ICS, LABA, or methylxanthine; Treatment step 4: Triple therapy or therapy with anti-IgE or anti-IL-5;AIT: allergen immunotherapy; AR: allergic rhinitis; Rx: prescription; IL: interleukin; ICS: inhaled corticosteroids, LABA: long-acting beta-agonists; SABA: short-acting beta2-agonists, LTRA: leukotriene receptor antagonists; INCS: intranasal corticosteroids.
In the pre-existing asthma cohort, the AIT group had on average 2·465 (3·022) asthma prescriptions. Subjects with pre-existing asthma were mainly on asthma treatment step 1–3 (Table 1). Furthermore, 16% had at least one severe asthma exacerbation. The sample size declined gradually over time. At 9 years of follow-up (FU) the study populations consisted of 3692 subjects (main cohort) and 1142 subjects (pre-existing asthma cohort) (Suppl. Figure S4).
In the main cohort, both the AIT and control group had reductions in AR prescriptions per subject across the FU-years compared to the pre-index year, with effect sizes ranging from −0·140 to −0·646 and −0·155 to −0·522 for AIT-treated subjects and control subjects, respectively (Suppl. Table S6). AIT was associated with greater reductions over time compared to the control group, which was sustained for 9 years (Fig. 1, Table 2). The AIT group was also found to have fewer AR prescriptions compared to the controls, when analysed cross-sectionally year by year (Suppl. Table S6). The overall reductions were consistent across drug classes, but slightly greater for antihistamine compared to intranasal corticosteroids (INCS) (Table 2 and Suppl. Table S5-S6, Figure S6). Greater reductions in AR prescriptions per subject were seen in AIT-treated subjects, while a larger proportion of AIT-treated subjects received at least one AR prescription during year 1–4 (Table S5) and in the same period also had more ambulatory care visits (including specialist visits) compared to control subjects (Suppl. Figure S5).
Fig. 1.
Change from pre-index year in average Rx/subjects per follow-up year. AR prescriptions includes symptom-relieving allergic rhinitis medication, e.g. antihistamine and INCS.
Table 2.
AR and asthma prescriptions in key follow-up years
| MAIN COHORT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rx/subject | SD | Change from pre-index | 95% CI | Rx/subject | SD | Change from pre-index | 95% CI | P-value | |
| Year 3 | AIT (N= 32112) | Control (N= 32112) | AIT vs. Control | ||||||
| AR Rx* | 0·609 | 1·344 | -0·458 | -0·482;-0·434 | 0·629 | 1·544 | -0·379 | -0·406;-0·353 | 0·081 |
| Antihistamine | 0·240 | 0·788 | -0·217 | -0·232;-0·203 | 0·250 | 0·895 | -0·150 | -0·165;-0·134 | 0·14 |
| INCS | 0·292 | 0·843 | -0·155 | -0·168;-·0·141 | 0·291 | 0·970 | -0·143 | -0·159;-0·128 | 0·86 |
| Year 5 | AIT (N= 19783) | Control (N= 19783) | AIT vs. Control | ||||||
| AR Rx* | 0·471 | 1·191 | -0·571 | -0·600;-0·542 | 0·533 | 1·504 | -0·448 | -0·482;-0·415 | <0·0001 |
| Antihistamine | 0·179 | 0·695 | -0·282 | -0·300;-0·264 | 0·202 | 0·807 | -0·194 | -0·213;-0·175 | 0·0021 |
| INCS | 0·247 | 0·789 | -0·178 | -0·195;-0·162 | 0·278 | 1·034 | -0·143 | -0·164;-0·122 | 0·0008 |
| Year 9 | AIT (N= 1846) | Control (N= 1846) | AIT vs. Control | ||||||
| AR Rx* | 0·393 | 1·142 | -0·646 | -0·741;-0·551 | 0·384 | 1·156 | -0·522 | -0·617;-0·427 | 0·81 |
| Antihistamine | 0·175 | 0·787 | -0·333 | -0·397;-0·269 | 0·138 | 0·659 | -0·250 | -0·305;-0·194 | 0·12 |
| INCS | 0·200 | 0·733 | -0·183 | -0·235;-0·131 | 0·225 | 0·842 | -0·154 | -0·214;-0·095 | 0·34 |
| PRE-EXISTING ASTHMA COHORT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rx/subject | SD | Change from pre-index | 95% CI | Rx/subject | SD | Change from pre-index | 95% CI | P-value | |
| Year 3 | AIT (N=10370) | Control (N=10370) | AIT vs. Control | ||||||
| Asthma Rx* | 1·666 | 2·713 | -0·825 | -0·904;-0·746 | 1·880 | 3·308 | -0·630 | -0·718;-0·542 | <0·0001 |
| SABA | 0·618 | 1·289 | -0·361 | -0·400;-0·322 | 0·725 | 1·579 | -0·264 | -0·310;-0·219 | <0·0001 |
| ICS | 0·308 | 0·863 | -0·274 | -0·301;-0·247 | 0·316 | 0·920 | -0·250 | -0·279;-0·221 | 0·49 |
| ICS/LABA | 0·554 | 1·338 | -0·076 | -0·112;-0·039 | 0·613 | 1·387 | -0·031 | -0·070;0;007 | 0·0019 |
| Year 5 | AIT (N=6545) | Control (N=6545) | AIT vs. Control | ||||||
| Asthma Rx* | 1·445 | 2·490 | -1·022 | -1·119;-0·926 | 1·740 | 2·964 | -0·763 | -0·872;-0·653 | <0·0001 |
| SABA | 0·530 | 1·168 | -0·429 | -0·476;-0·382 | 0·657 | 1·517 | -0·320 | -0·377;-0·263 | <0·0001 |
| ICS | 0·249 | 0·789 | -0·347 | -0·381;-0·313 | 0·263 | 0·841 | -0·315 | -0·351;-0·278 | 0·33 |
| ICS/LABA | 0·502 | 1·227 | -0·095 | -0·139;-0·051 | 0·610 | 1·382 | -0·015 | -0·063;0·033 | <0·0001 |
| Year 9 | AIT (N=571) | Control (N=571) | AIT vs. Control | ||||||
| Asthma Rx* | 1·331 | 2·651 | -1·030 | -1·361;-0·699 | 1·511 | 2·735 | -1·063 | -1·433;-0·693 | 0·26 |
| SABA | 0·443 | 1·131 | -0·464 | -0·613;-0·316 | 0·532 | 1·340 | -0·501 | -0·684;-0·318 | 0·22 |
| ICS | 0·172 | 0·620 | -0·343 | -0·437;-0·247 | 0·198 | 0·782 | -0·452 | -0·577;-0·327 | 0·53 |
| ICS/LABA | 0·553 | 1·271 | -0·056 | -0·210;0·098 | 0·601 | 1·298 | 0·096 | -0·059;0·252 | 0·53 |
Continuous variables are reported as mean +/- SD. Changes from pre-index year are presented as the within group absolute change and 95% confidence intervals CI; P-values are between group comparison of AIT vs. control subjects; Rx prescriptions are reported as Rx/subject; Only selected years are presented: Year 3 = expected end of treatment; Year 5 = duration of long-term RCTs investigating disease-modification, Year 9 = last year of observation. Outcomes across all nine follow-up years are presented in Suppl. Table S6-S7. AIT: allergy immunotherapy; AR: allergic rhinitis; INCS: intranasal corticosteroids; SABA: short-acting beta2-agonists; ICS: inhaled corticosteroids; LABA: long-acting beta2-agonists.
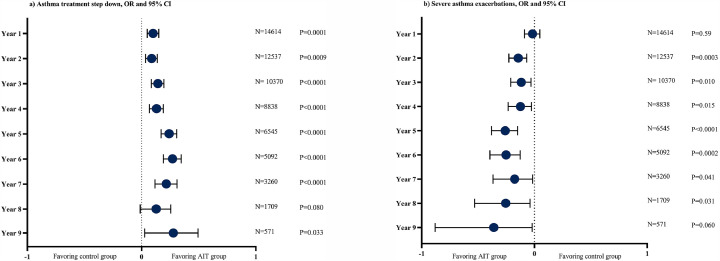
Similarly, in the pre-existing asthma cohort, both the AIT and the control group had reductions in asthma prescriptions per subject, with effect sizes ranging from −0·363 to −1·110 and −0·253 to −1·063 for AIT-treated subjects and control subjects, respectively (Suppl. Table S7). AIT was associated with greater reductions in asthma prescriptions over time compared to the control group, which was sustained for 9 years (Table 2, Suppl. Table S5). The AIT group was also found to have fewer asthma prescriptions compared to the controls, when analysed cross-sectionally (Suppl. Table S7). The between group differences in asthma prescriptions were mainly driven by SABA and ICS/long-acting beta2 agonists (LABA) prescriptions (Table 2, Suppl. Table S7, Figure S7). The greater reduction in both reliever and controller prescriptions for AIT-treated subjects, resulted in significantly greater odds of stepping down asthma treatment across all FU-years (Fig. 2, Table 3).
Fig. 2.
a and b): Odds ratio for stepping down asthma treatment (a) and severe asthma exacerbations (b) in pre-existing asthma cohort. Panel a): Odds ratio and 95%CI of stepping down in asthma treatment step compared to pre-index year per follow-up year. Panel b): Odds ratio and 95%CI of severe asthma exacerbation per follow-up year. Asthma treatment step [change]: The definition of improvement or worsening in asthma treatment step was met, if subjects with pre-existing asthma changed asthma treatment step compared to the pre-index year. The asthma treatment steps were pre-defined categories based on asthma diagnoses codes and asthma medication prescriptions during pre-index and post-index years. Treatment step 1: Asthma diagnosis in the absence of controller asthma medication; Treatment step 2: Monotherapy with either low dose ICS or LTRA; Treatment step 3: Dual therapy or therapy with medium-high ICS, LABA or methylxanthine; Treatment step 4: Triple therapy or therapy with anti-IgE or anti-IL-5; Severe asthma exacerbation: 3-point composite of either new systemic corticosteroid prescription, status asthmaticus recorded in ambulatory care or hospitalisation for asthma. AIT: allergen immunotherapy; CI: confidence interval; OR: odds ratio; IL: interleukin; SABA: short-acting beta2-agonists, ICS: inhaled corticosteroids; LABA: long-acting beta2-agonists; LTRA: leukotriene receptor antagonists.
Table 3.
Supportive secondary and explorative outcomes across key follow-up years in subjects with pre-existing asthma
| PRE-EXISTING ASTHMA COHORT | |||||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | OR | 95% CI | p-value | |
| Year 3 | AIT N=10370 | Control N=10370 | AIT vs. Control | ||||
| Asthma treatment step# | |||||||
| ≥1 step improvement | 4582 | 44% | 4225 | 41% | 1·15 | 1·09;1·22 | <0·0001 |
| ≥1 step worsening | 1449 | 14% | 1425 | 14% | 1·02 | 0·94;1·10 | 0·64 |
| Severe asthma exacerbations | 1001 | 10% | 1114 | 11% | 0·89 | 0·81;0·97 | 0·010 |
| Respiratory tract infection# | |||||||
| Pneumonia diagnosis | 147 | 1·4% | 204 | 2·0% | 0·72 | 0·58;0·89 | 0·0025 |
| Pneumonia with Rx antibiotic | 67 | 0·6% | 105 | 1·0% | 0·64 | 0·47;0·87 | 0·0045 |
| Hospitalisations# | 1792 | 17% | 1915 | 18% | 0·92 | 0·86;0·99 | 0·027 |
| Inpatient stay | 1091 | 11% | 1345 | 13% | 0·79 | 0·73;0·86 | <0·0001 |
| Outpatient stay | 911 | 9% | 851 | 8% | 1·08 | 0·98;1·19 | 0·14 |
| Ambulatory visits# | 10232 | 99% | 10096 | 97% | 2·01 | 1·64;2·47 | <0·0001 |
| Year 5 | AIT N=6545 | Control N=6545 | AIT vs· Control | ||||
| Asthma treatment step | |||||||
| ≥1 step improvement | 3391 | 52% | 3000 | 46% | 1·27 | 1·19;1·36 | <0·0001 |
| ≥1 step worsening | 918 | 14% | 947 | 14% | 0·96 | 0·87;1·06 | 0·48 |
| Severe asthma exacerbations | 571 | 9% | 724 | 11% | 0·77 | 0·69;0·86 | <0·0001 |
| Respiratory tract infection | |||||||
| Pneumonia diagnosis | 81 | 1·2% | 117 | 1·8% | 0·69 | 0·52;0·92 | 0·012 |
| Pneumonia with Rx antibiotic | 40 | 0·6% | 69 | 1·1% | 0·58 | 0·39;0·85 | 0·0068 |
| Hospitalisations | 1171 | 18% | 1256 | 19% | 0·92 | 0·84;1·00 | 0·059 |
| Inpatient stay | 739 | 11% | 867 | 13% | 0·83 | 0·75;0·93 | 0·0007 |
| Outpatient stay | 578 | 9% | 565 | 9% | 1·03 | 0·91;1·16 | 0·71 |
| Ambulatory visits | 6341 | 97% | 6343 | 97% | 0·99 | 0·81;1·21 | 0·96 |
| Year 9 | AIT N=571 | Control N=571 | AIT vs· Control | ||||
| Asthma treatment step | |||||||
| ≥1 step improvement | 320 | 56% | 283 | 50% | 1·30 | 1·03;1·64 | 0·032 |
| ≥1 step worsening | 79 | 14% | 108 | 19% | 0·69 | 0·50;0·94 | 0·025 |
| Severe asthma exacerbations | 41 | 7% | 60 | 11% | 0·66 | 0·44;1·00 | 0·060 |
| Respiratory tract infection | |||||||
| Pneumonia diagnosis | 9 | 1·6% | 12 | 2·1% | 0·75 | 0·31;1·78 | 0·66 |
| Pneumonia with Rx antibiotic | 4 | 0·7% | 9 | 1·6% | 0·44 | 0·14;1·44 | 0·26 |
| Hospitalisations | 93 | 16% | 121 | 21% | 0·72 | 0·54;0·98 | 0·040 |
| Inpatient stay | 61 | 11% | 88 | 15% | 0·66 | 0·46;0·93 | 0·022 |
| Outpatient stay | 42 | 7% | 49 | 9% | 0·85 | 0·55;1·30 | 0·51 |
| Ambulatory visits | 546 | 96% | 549 | 96% | 0·88 | 0·49;1·57 | 0·77 |
Categorical variables are reported as n % and changes as odds ratio (OR) and 95% confidence interval (CI) from pre-index year; P-values are between group comparison of AIT vs. control subjects; Asthma treatment step [change]: The definition of improvement or worsening in asthma treatment step was met, if subjects with pre-existing asthma changed asthma treatment step compared to the pre-index year. The asthma treatment steps were pre-defined categories based on asthma diagnoses codes and asthma medication prescriptions during pre-index and post-index years. Treatment step 1: Asthma diagnosis in the absence of controller asthma medication; Treatment step 2: Monotherapy with either low dose ICS or LTRA; Treatment step 3: Dual therapy or therapy with medium-high ICS, LABA or methylxanthine; Treatment step 4: Triple therapy or therapy with anti-IgE or anti-IL-5; Severe asthma exacerbation: 3-point composite of either new systemic corticosteroid prescription, status asthmaticus recorded in ambulatory care or hospitalisation for asthma. Only selected years are presented: Year 3 = expected end of treatment; Year 5 = duration of long-term RCTs investigating disease-modification, Year 9 = last year of observation.Rx: prescriptions; AIT: allergen immunotherapy; ICS: inhaled corticosteroids; LABA: long-acting beta2-agonists; LTRA: leukotriene receptor antagonists; IL: interleukin
This was supported by time-to-first and time-to-any stepping down of asthma treatment, both demonstrating a greater likelihood of stepping down asthma treatment in the AIT group (p<0·0001 for both) (Suppl. Table S8-S9, Figure S8). There was no increased risk of stepping up asthma treatment in the AIT group as the odds were similar between the AIT and the control group across most FU years (Table 3), and time-to-first stepping up of treatment showed a significantly lower risk of stepping up for the AIT group (P<0·0001) and time-to-any a similar risk (P = 0·42) (Suppl. Tables S10-S11, Figure S9).
Subjects treated with AIT had concurrent lower odds of experiencing a severe asthma exacerbation across the FU years (Fig. 2A) with significantly fewer events in years 2–7 (Suppl. Table S14). These findings were supported by time-to-first and time-to-any asthma exacerbation in the pre-existing asthma cohort, demonstrating a significantly reduced hazard ratio (HR) of 0·94 (95% CI: 0·90–0·98); p<0·05 and 0·92 (95%CI: 0·90–0·95); p <0·0001, respectively (Suppl. Tables S12-S13, Figure S9). Time-to-first and time-to-any asthma exacerbation were similar in the main cohort (Suppl. Table S10). In the no asthma cohort, the number of asthma prescriptions over time was low, however, while asthma prescriptions remained stable for the AIT group across the 9 FU years, prescriptions tended to increase in the control group (Suppl. Figure S12A). Opposing results were found in time-to-first onset of asthma, with an increased risk for the AIT group (HR: 1·22 (1·12–1·32); p<·0001) (Suppl. Table S15 and Figure S11) and no significant difference was found for the 3-point composite of time-to-worsening of asthma (Suppl. Tables S10-S11 and Figure S9). For the remaining survival analyses, the proportional hazard assumption was violated and only KM-curves and p-values were reported (Suppl. Figures S8-S10).
Key exploratory outcomes supported the secondary asthma outcomes (Table 3, Fig. 3). Compared to the control group, respiratory tract infections with antibiotic use, HRU with fewer hospital visits, hospital stays, and costs of inpatient hospitalisations were in favour of AIT (Table 3 for pre-existing asthma cohort, Suppl. Tables S16-S17 for main cohort). Similar to ambulatory care visits, increases in total health care costs were higher after 3 FU-years in the AIT group, while at 5-years of FU and 9-years of FU total health care costs increased more in the control group (Suppl. Table S18). Diagnosis codes for key comorbidities related to asthma such as bronchitis and chronic cough were also reduced for the AIT group (Suppl. Table S19).
Fig. 3.
a) and b): Odds ratios for pneumonia diagnosis (a) and inpatient hospitalisations (b) in pre-existing asthma cohort. Panel a) Odds ratio and 95%CI of pneumonia diagnosis per follow-up year. Panel b) Odds ratio and 95%CI of inpatient hospitalisations per follow-up year. AIT: allergen immunotherapy; CI: confidence interval; OR: odds ratio.
The number of anaphylaxis cases around index date were low (Suppl. Definitions). amongst AIT-treated subjects in total 33 cases were recorded; 30 of 36,927 subjects treated with SCIT; 2 of 4816 subjects treated with SLIT-drops; and 1 of 3754 subjects treated with SLIT-tablets.
The primary outcome, AR prescriptions, was consistently associated with greater reductions across all subgroups (Suppl. Table S20). The sensitivity analyses for asthma treatment steps were less robust, however, the likelihood of stepping down treatment remained significant in favour of AIT in FU-years 4–6 (Suppl. Table S21) and the validation analysis confirmed greater reduction in AR prescriptions for the SLIT-tablet subgroup (Suppl. Figure S13).
Discussion
This is the largest and most comprehensive AIT effectiveness study in the real world to date to our knowledge, demonstrating sustained, long-term reductions in both AR and asthma medication use, improved asthma control, prevention of asthma exacerbations, and respiratory tract infections.
The disease modifying effects of AIT were previously confirmed by sustained reductions in symptoms and symptom-relieving medication use two years post-treatment in RCTs, following three-years of treatment with once-daily SQ grass SLIT tablets 13. In this study the real world effectiveness of AIT was confirmed by greater reductions in AR medications in the AIT group across 9 years of FU, demonstrating long-term and sustained effects substantially beyond the observation period from RCTs 13. Since AIT is considered a causal therapy, long-term follow-up data are of fundamental importance.
To date, two large RCTs with SQ HDM SLIT-tablets have confirmed the efficacy of AIT for treatment of asthma. One study found significant reductions in daily ICS dose compared to placebo while another RCT demonstrated a significant risk reduction in moderate-severe asthma exacerbation.16,17 In the current study, AIT-treated subjects with AR and pre-existing asthma had significant reductions in both controller and reliever medication, suggesting improved asthma control. Concurrently to being more likely to step down asthma treatment, AIT reduced the odds of experiencing asthma exacerbations. As the findings were sustained across the nine years of FU, the study suggests prevention of asthma progression. Interestingly, the beneficial effects of AIT on asthma were found in a less severe asthmatic population, as only 4% were at treatment step 4, supporting the hypothesis that AIT may be able to prevent progression from mild to more severe asthma and should be considered earlier in the treatment of allergic patients with asthma.27 AIT-treated subjects were less likely to experience respiratory tract infections requiring antibiotics. Respiratory tract infections and allergy are known risk factors for asthma exacerbations, and the findings are further supported by another study that found AIT to be associated with a reduced risk of respiratory tract infections28 The clinical benefits of AIT seen in AR and asthma outcomes were supported by reductions in hospitalisations, length of stays and related costs. Ambulatory care visits and related costs, as well as specialist visits were increased, possibly due to subjects on SCIT having more HCP visits for AIT treatment. A low frequency of anaphylaxis cases was recorded.
RCTs have shown a beneficial effect of AIT on prevention of asthma.29 A few RWE studies have also assessed onset of asthma with conflicting results.30, 31, 32 Due to different methodologies and definitions used, and due to risk of bias in these studies, it is difficult to draw general conclusions. The finding herein may be explained by confounding. AIT-treated subjects were mainly treated with SCIT which is also administered in clinics, and consequently, the AIT group had more ambulatory, and specialists care visits, particularly during the expected 3-year AIT treatment period. As new onset of asthma required a confirmed asthma diagnosis, the higher frequency of specialist visits may have resulted in more asthma diagnoses being coded in the AIT group, despite asthma prescriptions remaining lower in the AIT group compared to the control subjects.
The study was pre-registered, and all outcomes and statistical analyses were pre-specified in the protocol. Further strengths include strong matching, documented locking of cohorts before outcome analyses, pre-defined validation step, large sample size, long-term follow-up, and robustness of results across FU years, outcomes, and subgroups.
In RCTs for AIT, patients are included based on diagnostic allergy testing, and symptoms and medication intake are recorded on a daily basis both to inform severity at baseline and efficacy of the treatment. In claims databases, such as the one used for this study, subjects' symptoms are not captured, and neither are results from diagnostic allergy testing captured to inform inclusion of subjects. Filled prescriptions, diagnosis codes and health care resource utilisation are used as proxies instead. This approach can cause some limitations. 1) When assessing the effectiveness of AIT using filled prescriptions, information on the subjects' symptom control is lacking. The less granularity of the available data makes it difficult to compare the effect size with RCTs. Instead, filled prescriptions are used as a proxy for symptom and medication scores, and a range of clinically relevant outcomes like confirmed diagnosis codes for known comorbidities and pneumonia, asthma exacerbations, hospitalisations, the number of ambulatory visits, and overall drug burden were used as markers of severity and included to further substantiate the clinical relevance of the findings. 2) Many AR medications are also available as over the counter medicines and are therefore not captured in a claims database unless they were prescribed by a physician. 3) Prescriptions do not necessarily translate into utilisation of medication. 4) PSM is restricted to variables which are observed in the database, which causes some limitations to matching subjects in terms of AR disease severity and allergic sensitisation. However, the PSM in this study has included a range of variables as proxy for disease severity. Still, socio-economic status and smoking may be confounding factors for asthma exacerbations as the PSM did not directly account for this beyond age, gender, insurance status, and regional distribution.
Despite all the efforts done with matching the cohorts, some small differences in the pre-index year may remain, which may affect outcomes reported as change from pre-index year (i.e. change in AR or asthma prescriptions). Although, the effect sizes of changes in prescriptions from pre-index year appear small, the differences were larger than the differences between prescriptions per subject seen in the pre-index year, inferring a true decline in prescriptions per subject over time amongst AIT-treated subjects. The results for AR and asthma prescriptions were consistent regardless of data being presented as a change from pre-index year or cross-sectional between group differences.
Subjects on AIT were seen more frequently by specialists, likely due to SCIT being an injection given repeatedly under medical supervision. The increased number of specialist visits may disfavour the AIT group and bias the results, leading to a higher proportion of AIT subjects having prescriptions in the first years of FU compared to control subjects. Although AIT-treated subjects were more likely to get AR prescriptions than control subjects they showed a larger reduction in AR prescriptions per subject.
The control group also experienced reductions in AR prescriptions per subject compared to the pre-index year with a progressive trend mimicking the AIT group, and likely explained by regression towards the mean, a statistical phenomenon that can make natural variation in repeated data look like real change. Of this large cohort, 3692 in the main and 1142 subjects in the pre-existing asthma cohort had the full 9 years of FU data available. However, despite these limitations, a larger sustained effect across all years for the majority of outcomes was found in the AIT group compared to control subjects.
With rigorous methodology, a large sample size, and long-term follow up the REACT study further substantiated the existing evidence from RCTs and added new information about the effects of AIT beyond the limited follow-up in RCTs. The effectiveness of AIT in real life was confirmed with sustained reductions in AR and asthma prescriptions, prevention of asthma exacerbations, and improved and sustained long-term asthma control. The findings emphasised AIT as a safe and effective treatment option for patients with respiratory allergies.
Contributors
Authors of the manuscript are: Benedikt Fritzsching (BF), Marco Contoli (MC), Celeste Porsbjerg (CB), Sarah Buchs (SB), Julie Rask Larsen (JRL), Lisa Elliott (LE), Mercedes Romano Rodriguez (MR), and Nick Freemantle (NF). Third party vendors include: Michael Schultze (MS), Kantar GmbH, Elena Georgiadou-Schmidt (EGS), Team Gesundheit, Gesellschaft für Gesundheitsmanagement GmbH, and Charlotte Strøm (CS), SharPen (medical writer)
Conceptualisation: SB, LE, MR, JRL, BF, MC, CP, and NF. Literature search: JRL. Data curation: EGS, MS. Formal analysis: EGS. Funding acquisition: The study was funded by ALK. Investigation: SB, JRL, LE, MR, EGS, NF. Methodology: all authors. Project administration: SB, MS. Software: EGS. Supervision: SB, JRL. Visualization: SB. Writing – original draft: CS. Writing – review & editing: CS – along with BF, MC, CP, SB, JRL, LE, MR, and NF. EGS and MS accessed and verified the underlying data provided in the study report and outputs, SB and JRL verified that data in the publication are in line with the study report and outputs.
Data availability statement
Data are owned by the BKK sickness fund, which provides access to anonymised data derived from routinely collected administrative claims data. These data can only be accessed by a permitted 3rd party (Team Gesundheit, Gesellschaft für Gesundheitsmanagemetn GmbH, Essen, Germanny) for research purposes.
Declaration of interests
Dr. Fritzsching reports personal fees (pertaining travelling to study meeting) from ALK during the conduct of the study; and speaker honorarium from Novartis and from Merck Sharp & Dohme, outside the submitted work. Dr. Contoli reports personal fees from Alk-Abello, during the conduct of the study; grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, grants, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees and non-financial support from Zambon, grants from University of Ferrara - Italy, outside the submitted work. Dr. Porsbjerg reports grants from ALK, grants and personal fees from Astra Zeneca, grants and personal fees from GSK, grants and personal fees from Novartis, grants, and personal fees from Chiesi, grants and personal fees from Sanofi, grants and personal fees from TEVA, outside the submitted work. Sarah Buchs reports to be employed at ALK-Abello. Dr. Rask Larsen reports being an employee at ALK. Dr. Elliott has nothing to disclose. Ms. Romano Rodriguez reports she is an ALK employee. Dr. Freemantle reports personal fees from Astrazeneca, personal fees from Ipsen, personal fees from Sanofi Aventis, personal fees from Grifols, personal fees from Novatis, personal fees from Aimmune, personal fees from Vertex, personal fees from MSD, personal fees from Allergan, outside the submitted work.
Acknowledgements
The study was funded by ALK A/S. The authors thank Dr. Michael Schultze, Kantar Health, Munich, and Elena Georgiadou-Schmidt, Team Gesundheit, Gesellschaft für Gesundheitsmanagement GmbH, Essen for support to access data and statistical analysis, and Charlotte Strøm, PhD, for medical writing assistance; services that were financially supported by ALK A/S.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100275.
Appendix. Supplementary materials
References
- 1.Pawankar R.C., Holgate S.T., Lockey R.F., et al. Wisconsin World Allergy Organization; Milwaukee: 2013. WAO white book on allergy 2013 update. GW. [Google Scholar]
- 2.Bousquet J., Hellings P.W., Agache I., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019;143(3):864–879. doi: 10.1016/j.jaci.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 3.GINA Global Strategy for Ashtma Management and Prevention. 2021 [Google Scholar]
- 4.Bousquet J., Van Cauwenberge P., Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5):S147–S334. doi: 10.1067/mai.2001.118891. Suppl. [DOI] [PubMed] [Google Scholar]
- 5.Braido F., Baiardini I., Brandi S., Porcu A., Canonica G.W. Allergic rhinitis and asthma ad hoc survey: clinical and psychological perspectives. Clin Exp Allergy. 2007;37(5):788–793. doi: 10.1111/j.1365-2222.2007.02702.x. [DOI] [PubMed] [Google Scholar]
- 6.Vandenplas O., Dramaix M., Joos G., et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65(10):1290–1297. doi: 10.1111/j.1398-9995.2010.02365.x. [DOI] [PubMed] [Google Scholar]
- 7.Clatworthy J., Price D., Ryan D., Haughney J., Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim Care Respir J. 2009;18(4):300–305. doi: 10.4104/pcrj.2009.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linneberg A., Dam Petersen K., Hahn-Pedersen J., Hammerby E., Serup-Hansen N., Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12. doi: 10.1186/s12948-016-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linneberg A., Henrik Nielsen N., Frolund L., et al. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57(11):1048–1052. doi: 10.1034/j.1398-9995.2002.23664.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozdoganoglu T., Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis. 2012;6(1):11–23. doi: 10.1177/1753465811431975. [DOI] [PubMed] [Google Scholar]
- 11.Larsen J.N., Broge L., Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today. 2016;21(1):26–37. doi: 10.1016/j.drudis.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Roberts G., Pfaar O., Akdis C.A., et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 13.Durham S.R., Emminger W., Kapp A., et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725. doi: 10.1016/j.jaci.2011.12.973. e5. [DOI] [PubMed] [Google Scholar]
- 14.Durham S.R., Walker S.M., Varga E.M., et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 15.Nolte H., Maloney J. The global development and clinical efficacy of sublingual tablet immunotherapy for allergic diseases. Allergol Int. 2018;67(3):301–308. doi: 10.1016/j.alit.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Mosbech H., Deckelmann R., de Blay F., et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134(3):568–575. doi: 10.1016/j.jaci.2014.03.019. e7. [DOI] [PubMed] [Google Scholar]
- 17.Virchow J.C., Backer V., Kuna P., et al. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults With Allergic Asthma: a Randomized Clinical Trial. JAMA. 2016;315(16):1715–1725. doi: 10.1001/jama.2016.3964. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti G., DiBona D., Chu D.K., et al. Allergen immunotherapy: the growing role of observational and randomisedtrial "Real-World Evidence". Allergy. 2021 doi: 10.1111/all.14773. [DOI] [PubMed] [Google Scholar]
- 19.Roche N., Reddel H.K., Agusti A., et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013;1(10):e29–e30. doi: 10.1016/S2213-2600(13)70199-1. [DOI] [PubMed] [Google Scholar]
- 20.Pilny A., Wubker A., Ziebarth N.R. Introducing risk adjustment and free health plan choice in employer-based health insurance: evidence from Germany. J Health Econ. 2017;56:330–351. doi: 10.1016/j.jhealeco.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Busse R., Blumel M., Knieps F., Barnighausen T. Statutory health insurance in Germany: a health system shaped by 135 years of solidarity, self-governance, and competition. Lancet. 2017;390(10097):882–897. doi: 10.1016/S0140-6736(17)31280-1. [DOI] [PubMed] [Google Scholar]
- 22.Biedermann T., Kuna P., Panzner P., et al. The SQ tree SLIT-tablet is highly effective and well tolerated: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2019;143(3):1058–1066. doi: 10.1016/j.jaci.2018.12.1001. e6. [DOI] [PubMed] [Google Scholar]
- 23.Di Bona D., Plaia A., Leto-Barone M.S., La Piana S., Di Lorenzo G. Efficacy of Grass Pollen Allergen Sublingual Immunotherapy Tablets for Seasonal Allergic Rhinoconjunctivitis: a Systematic Review and Meta-analysis. JAMA Intern Med. 2015;175(8):1301–1309. doi: 10.1001/jamainternmed.2015.2840. [DOI] [PubMed] [Google Scholar]
- 24.Radulovic S., Calderon M.A., Wilson D., Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;(12) doi: 10.1002/14651858.CD002893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise S.K., Lin S.Y., Toskala E., et al. International Consensus Statement on Allergy and Rhinology: allergic Rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi: 10.1002/alr.22070. [DOI] [PubMed] [Google Scholar]
- 26.PRENTICE R.L., WILLIAMS B.J., PETERSON A.V. On the regression analysis of multivariate failure time data. Biometrika. 1981;68(2):373–379. [Google Scholar]
- 27.Schmitt J., Wustenberg E., Kuster D., Mucke V., Serup-Hansen N., Tesch F. The moderating role of allergy immunotherapy in asthma progression: results of a population-based cohort study. Allergy. 2020;75(3):596–602. doi: 10.1111/all.14020. [DOI] [PubMed] [Google Scholar]
- 28.Woehlk C., von Bulow A., Kriegbaum M., Backer V., Porsbjerg C. Allergic asthma is associated with increased risk of infections requiring antibiotics. Ann Allergy Asthma Immunol. 2018;120(2):169–176. doi: 10.1016/j.anai.2017.11.015. e1. [DOI] [PubMed] [Google Scholar]
- 29.Halken S., Larenas-Linnemann D., Roberts G., et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–745. doi: 10.1111/pai.12807. [DOI] [PubMed] [Google Scholar]
- 30.Zielen S., Devillier P., Heinrich J., Richter H., Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. 2018;73(1):165–177. doi: 10.1111/all.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt J., Schwarz K., Stadler E., Wustenberg E.G. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136(6):1511–1516. doi: 10.1016/j.jaci.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 32.Wahn U., Bachert C., Heinrich J., Richter H., Zielen S. Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma. Allergy. 2019;74(3):594–604. doi: 10.1111/all.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are owned by the BKK sickness fund, which provides access to anonymised data derived from routinely collected administrative claims data. These data can only be accessed by a permitted 3rd party (Team Gesundheit, Gesellschaft für Gesundheitsmanagemetn GmbH, Essen, Germanny) for research purposes.