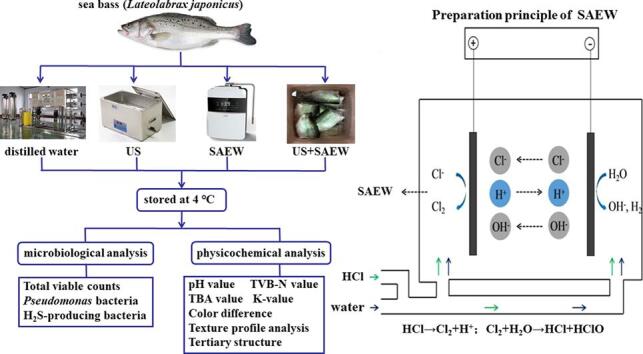
Graphical abstract
Keywords: Ultrasound, Slightly acidic electrolyzed water, Lateolabrax Japonicus, Refrigerated storage
Highlights
-
•
The ultrasound (US) incorporated with slightly acidic electrolyzed water (SAEW) treatment could inhibit the increase of TVC, Pseudomonas bacteria counts and H2S-producing bacteria counts.
-
•
US + SAEW treatment had distinctly effects on inhibiting protein degradation and lipid oxidation, maintaining better texture and sensory scores.
-
•
US + SAEW treatment could increase the shelf life of sea bass for another 4 days during refrigerated storage.
Abstract
A novel technique for sea bass (Lateolabrax Japonicus) fillets by combining ultrasound (US) and slightly acidic electrolyzed water (SAEW) to inactivate bacteria and maintain quality was developed. Samples were treated with distilled water (DW), US, SAEW and ultrasound combined with slightly acidic electrolyzed water (US + SAEW) for 10 min, respectively. The results suggested that US + SAEW treatment could retard the increase of total viable counts (TVC), Pseudomonas bacteria counts and H2S-producing bacteria counts, which also inhibit the rise of total volatile basis nitrogen (TVB-N), thiobarbituric acid (TBA), pH and K value. In addition, compared with SAEW or US treatment alone, US + SAEW treatment had distinctly effects on inhibiting protein degradation and maintaining better sensory scores. Compared with DW group, the shelf life of sea bass treated with US + SAEW was increased for another 4 days. It indicated that the combined treatment of US and SAEW could be used to the preservation of sea bass.
1. Introduction
Sea bass (Lateolabrax japonicas) is one of popular aquatic products because of its low-fat and mild taste. However, sea bass is highly perishable and has a relatively short shelf life during storage [1]. The activities of microorganisms and endogenous enzymes cause the decline of fish freshness, protein degradation and lipid oxidation [2]. Short shelf-life is not conducive to the marketing and distribution of sea bass. Therefore, it is essential to the aquaculture and consumers for prolonging the shelf-life and keeping the quality.
Ultrasound (US) is a high energy frequency (over 16 kHz) sound waves that could not be detected by ear [3], which is used as an environmental-friendly antibacterial method for a long time [4]. S. Knobloch et al. [5] found that the surface microbial community of sea bass could be influenced by close-proximity and continuous ultrasound treatment. The US has an antimicrobial effect on Staphylococcus aureus [6], Pseudomonas fluorescens [7], Listeria monocytogenes and other pathogens [8], which was mainly due to the destruction of cell structure and integrity by ultrasonic cavitation leading to microbial apoptosis [9], [10]. The recent researches showed that ultrasound in combination with SAEW and other physical processing methods could reduce the number of bacteria efficiently [11], [12], [13].
Slightly acidic electrolyzed water (SAEW) has a high concentration of hypochlorous acid (HOCl) and its pH value range is 5.0–6.5 [14]. Compared with other disinfectants, SAEW has the advantage of minimizing the impaction of chlorine residual on human health and safety [15]. It is a potential substitute for anti-microbial detergents and is considered an environmental-friendly disinfection method [14]. The antibacterial activity of SAEW is mainly due to the potential oxidative damage of HOCl to biomolecules [16]. SAEW is combined with other disinfectants or mechanical force in the washing process, which can greatly reduce the microorganisms in food [17], [18]. However, there are few studies on the application of US, SAEW and their combined effect on sea bass (Lateolabrax japonicas).
Therefore, this research aimed to examine the efficacy of US, SAEW and combined treatments for improving the quality of refrigerated sea bass. Microbiological (TVC, Pseudomonas bacteria counts and H2S-producing bacteria counts), physicochemical (pH, TVB-N, TBA, K value, intrinsic fluorescence intensity (IFI), texture profile analysis (TPA), color difference) and sensory attributes were determined to assess the quality of sea bass fillets stored at 4 °C.
2. Materials and methods
2.1. Ultrasound treatment
For US treatment, samples were submerged in a beaker containing 2.0 L distilled water and placed beaker in an ultrasonic bath (KQ-250B, Kunshan Ultrasonic Instrument Co., Kunshan, China) for 10 min. For US + SAEW treatment, distilled water was replaced with the previously prepared SAEW and the other methods were the same as above. The frequency, power and time of ultrasound bath were 20 kHz, 600 W and 10 min respectively. In the whole process, the temperature of the ultrasonic bath was always controlled at about 20 °C by replacing with fresh cold water.
2.2. Preparation of SAEW
SAEW was made by electrolysis of tap water and a dilute hydrochloric acid (6.0%) in the SAEW generator (Intercontinental resources Environmental Science and Technology Co. Ltd., Beijing, China) at 7.0 A and generated with a rate of 2.05 L/min. Before the experiment, the ORP and pH values were measured with a pH/ORP meter (CON60; Trans-Wiggens, Singapore). ACC was determined using a chlorometer (RC-2Z; Kasahara Chemical Instruments Co., Saitama, Japan). SAEW with pH of 6.35 ± 0.04, ORP of 861.6 ± 12.35 mv and ACC of 30.0 ± 1.54 mg/L was prepared for next experiments.
2.3. Sample preparation and treatments
Thirty fresh sea bass (27.5 ± 1.2 cm in length, 500 ± 20 g in weight) were purchased from the local supermarket (Shanghai, China). The live sea bass samples were packed in plastic bags filled with oxygenated water and delivered to the laboratory by foam boxes within 30 min. The head, bone, and skin of sea bass were removed and cut into fillets. The fillets were divided into four groups: (1) samples were immersed in distilled water for 10 min (DW); (2) samples were dipped in the distilled water with ultrasound treatment (20 kHz, 600 W) for 10 min (US); (3) samples were dipped in SAEW for 10 min (SAEW); (4) samples were dipped in SAEW combined with US treatment for 10 min (US + SAEW). Then, they were put in polyethylene bags and stored at 4 °C for further analysis at 2-days interval during 14 days.
2.4. Microbiological enumeration
TVC was measured on Plate Count Agar (PCA) (PCA, HaiBo Biological Technology Co., Ltd, Qingdao, China) incubated at 30 °C for 72 h; Pseudomonas bacteria counts were enumerated after incubation at 25 °C for 48 h on Pseudomonas agar base added with C.F.C supplement (HaiBo Biological Technology Co., Ltd, Qingdao, China); H2S-producing bacteria were incubated by triple sugar iron agar at 25 °C for 3 days. The above analysis was in triplicate and represented as log10 CFU/g.
2.5. Physicochemical analysis
2.5.1. Determination of pH
Chopped fish samples (5 g) and distilled water (45 mL) were stirred well and stood for 30 min [19]. After filtration, the pH values in the supernatant were measured with a digital pH meter (FE20, Mettler Toledo, Shanghai, China).
2.5.2. Determination of total volatile basis nitrogen (TVB-N)
TVB-N values were obtained according to C. Ruiz-Capillas et al. [20] and expressed as mgN/100 g. TVB-N values were observed with a Kjeltec 8400 apparatus (Foss, Sweden).
2.5.3. Determination of thiobarbituric acid (TBA)
TBA value was monitored by evaluating thiobarbituric acid reactive substances (TBARS) according to the procedure of Milijasevic et al. [21].
2.5.4. Determination of K-value
The degree of degradation of ATP could be expressed by the K-value. ATP and its decomposition products, including ADP, AMP, IMP, HxR and Hx were measured by HPLC (1260 LC; Agilent, Palo Alta, CA, USA) equipped with Agilent C18 (5 μm, 4.6 mm × 250 mm) HPLC column and a UV detector. The K-value was calculated using the equation below:
2.5.5. Determination of intrinsic fluorescence intensity (IFI)
Intrinsic fluorescence intensity was measured according to the previous study [22]. The excitation wavelength is 295 nm, the scanning speed is 1200 nm/min, and the intrinsic fluorescence spectrum is obtained in the range of 300 ∼ 400 nm. The width of the excitation and emission slit is 5 nm.
2.5.6. Texture profile analysis (TPA)
TPA was conducted according to the protocol in previous study [23]. Samples of 20 × 20 × 10 mm were taken from the back muscles. After absorbing the surface water, TPA mode was used to measure the hardness, springiness, chewiness and cohesiveness of samples, which were subject to two compression analyses: (1) the probe model is a P/50 flat-bottomed cylindrical probe, the speed before the test is 3 mm/s, the test speed and the return speed after the test are both 1 mm/s. (2) compression interval is 5 s, compression degree is 50%, relaxation time is 5 s.
2.5.7. Color measurements
The color of the samples was measured using the method described by P. Chuesiang et al. [24] The WSC-S colorimeter (Shanghai Precision Instrument Co., Ltd., Shanghai, China) was used to measure the surface color of fish fillets. Before analysis, a standard white and black plate was used to calibrate the instrument. The L*, a*, b* values were analyzed with three parallels for each group. Meaning of L*, a*, and b* was lightness (black ∼ white = 0 ∼ 100 points), redness (a*) or green (-a*), and yellowness (b*) or blueness (-b*), respectively [7].
2.6. Sensory evaluation
Samples were assessed by the quality index method (QIM) following the protocol of ZHU [25]. Briefly, sensory evaluation was conducted by a panel of 10 experienced panelists. Changes in color, odor, texture and overall acceptability were evaluated according to the criteria for sensory evaluation of CHAN et al. [26] with some modification (Table 2 Supplementary File). Sensory scores were divided into three ranges (4.0–5.0 = good quality, 2.0–4.0 = average quality, 1.0–2.0 = unacceptable quality). The final sensory score was the average of quality parameters.
2.7. Statistical analyses
Experimental data were analyzed by Origin program version 9.0. The SPSS 2017 was applied to perform an analysis of variance (ANOVA). Statistical significance was reported as a level of p < 0.05.
3. Results and discussion
3.1. Microbiological analyses
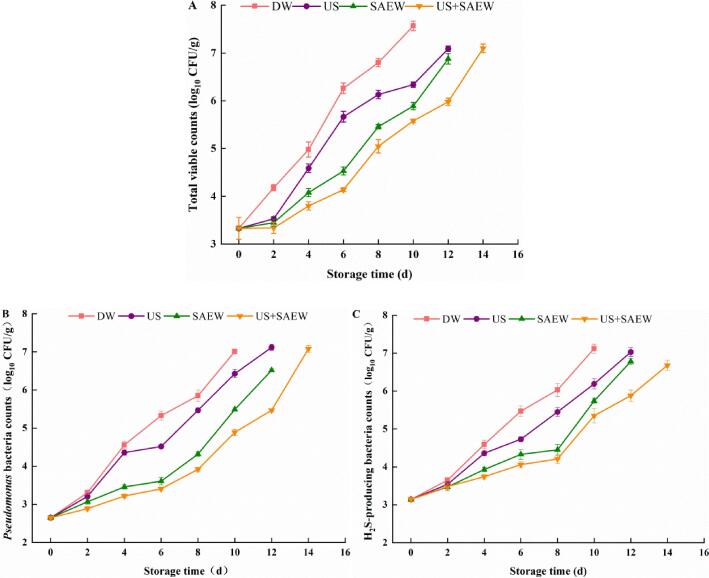
The microbiological changes of DW, US, SAEW and US + SAEW group during storage were presented in Fig. 1. The initial TVC of all groups was 3.33 log10 CFU/g (Fig. 1A). The TVC of samples treated with DW, US, SAEW and US + SAEW reached the acceptable limit of 7.0 log10 CFU/g [27] for marine fish at day 10, 12, 12 and 14, respectively. Compared with the DW group, the TVC of US, SAEW, and US + SAEW groups were reduced by 1.23, 1.68 and 1.99 log10 CFU/g, respectively, indicating that the combined treatment of US and SAEW could result in the decrease of TVC.
Fig. 1.
Effects of different treatments for TVC (A), Pseudomonas bacteria counts (B) and H2S-producing bacteria counts (C) of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water).
The main spoilage microorganisms of marine fish are usually Gram-negative bacteria, especially Pseudomonas and Shewanella (mainly H2S-producing bacteria). During refrigerated storage, the numbers of H2S-producing bacteria and Pseudomonas bacteria were increased in all groups (Fig. 1B and C) and experienced a similar growth trend with TVC. Pseudomonas bacteria counts (Fig. 1B) and H2S-producing bacteria counts (Fig. 1C) were 2.65 log10 CFU/g and 3.14 log10 CFU/g in the initial of storage time, and the number of microorganisms increased in all groups during storage. The Pseudomonas bacteria counts reached 7.0 log10 CFU/g [28] in DW group at day 10, whereas Pseudomonas bacteria counts of US, SAEW and US + SAEW groups were 6.43, 5.49 and 4.89 log10 CFU/g, respectively.
Previous studies showed that US treatment could improve the disinfection ability of SAEW [13]. In addition, US did not weaken the bactericidal effect of SAEW, indicating that US combined with SAEW was an ideal decontamination method [29]. A number of studies had also demonstrated that US + SAEW treatment could reduce the additional targeted pathogens [22], [30], [31]. Due to the high pressure and high temperature generated by the ultrasonic bubbles, US treatment could promote the penetration of cell membranes by chemical oxidants, thereby improving the efficiency of disinfectants [32]. The antibacterial effect of US + SAEW was enhanced, which was possible that SAEW became the liquid medium of US. The cavitation produced by US destroyed the walls of bacteria in a short time and increased the contact area between SAEW and bacteria [6], [33].
3.2. Physicochemical analysis
3.2.1. Changes in pH, TVB-N and TBA
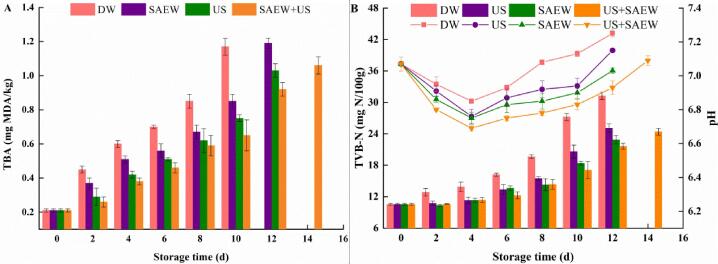
TBA could determine the lipid oxidation of aquatic products. On day 4, the TBA value of DW group was markedly higher than those of treated groups (p < 0.05) (Fig. 2A). On day 8, the TBA value of DW, US, SAEW and US + SAEW groups were 0.85, 0.67, 0.62, and 0.59 mg MDA/kg, respectively. The TBA value of SAEW + US group was increased by 0.47 mg MDA/kg after day 8. The US processing might affect oxidation, free radical formation, and other active substances, and was prone to react with an oxidizing compound [34]. In addition, ultrasound combined with other treatment methods could influence the results of lipid oxidation [35]. Guan et al. [36] observed that the treatment of ultrasonic combined with coffee acid could effectively inhibit the increase of TBA in sea bass. It was reported that ultrasound alone and ultrasound with plasma-activated water treatment could be used to inhibit lipid oxidation of mackerel fillets. Zhao et al. [7] mentioned that US treatment might inactivate prooxidative enzymes to achieve antioxidation in the complex process of lipid oxidation.
Fig. 2.
Effects of different treatments for TBA (A), TVB-N value (column chart) and pH value (line chart) (B) of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water).
Changes in pH value among four groups were shown in Fig. 2B. The pH value of different groups in the initial stage was 7.07 and then decreased in the first 4 days, which may be the result of bacterial fermentation leading to the formation and organic acids accumulated in fish [37]. At the later storage period, the pH value increased owing to volatile base component produced by endogenous enzymes or microorganisms, such as ammonia, trimethylamine, which was consistent with the previous studies [38]. This implied the spoilage of samples. Whereas, the pH values in different groups were increased obviously at different rates, among which the pH value of DW group was the highest, and the pH value of US + SAEW group was the lowest. Hence, the pH values were increased to 7.13, 6.94, 6.90, and 6.83 for DW, US, SAEW and US + SAEW samples at day 10, respectively. The results showed that US + SAEW had an inhibitory effect on spoilage microorganisms, which could slow down the rise of pH and delay the generation of basic nitrogen compounds.
The increase of TVB-N is related to the destruction of bacteria and activities of endogenous enzymes, which is an important indicator for evaluating the quality of seafood. Changes in TVB-N value with different treatments were shown in Fig. 2B.
The TVB-N value of fresh samples was low at the beginning of storage, which indicates the high freshness. TVB-N values in all groups were increased steadily over storage time. However, the growth rate of TVB-N in treated groups was apparently slower than that of DW group (p < 0.05). The TVB-N value of US + SAEW group presented the slowest increasing rate, which was in accordance with pH value and TVC. The TVB-N of DW group rose rapidly to 27.2 mgN/100 g at day 10, yet lower values of 20.6, 18.4, and 16.1 mgN/100 g were observed in US, SAEW, and US + SAEW groups. On the 12th day, the TVB-N value of US + SAEW group was 21.6 mg N/100 g, which was significantly lower than other groups (p < 0.05). According to reports of Gökodlu et al.[39], the TVB-N value of many fish species gradually increases during corruption, and 30.0 mgN/100 g was recommended as the acceptable limit of fish. On the 14th day, the TVB-N value of samples treated with US + SAEW was still below 30.0 mgN/100 g. The combined treatment of US and SAEW could extend the shelf-life of sea bass from 8 to 12 days and inhibit the formation of TVB-N effectively.
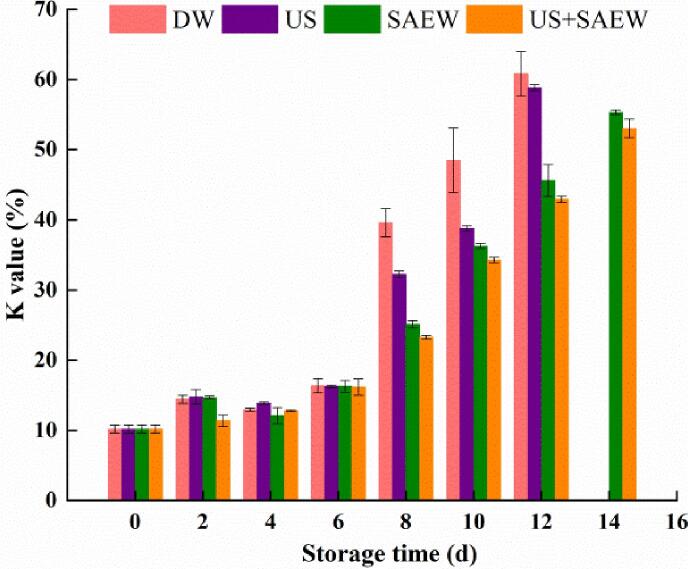
3.2.2. Changes in K-value
K value is generally considered below 20% as fresh fish, 20–50% as secondary freshness, and higher than 60% as inedible [40]. From Fig. 3, with the increase of storage time, K values of different group were increased. The K value of fresh fillets was 10.16%, which suggested that the fish was in good freshness. Before day 8, the K values of all groups were<50.0%. The K-value of DW group increased to 60.8%, which passed the acceptable limit on day 12. At the same time, the K-value of US + SAEW group at day 12 was 42.9%, which was lower than those of other groups significantly (p < 0.05). K value could judge fish freshness with nucleotide decomposition products as indicators. With the extension of storage time, ATP is gradually degraded into HxR and Hx, which eventually leads to the production of spoilage taste in fish [41].
Fig. 3.
Effects of different treatments for K-value of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water).
3.2.3. Changes in tertiary structure of the protein
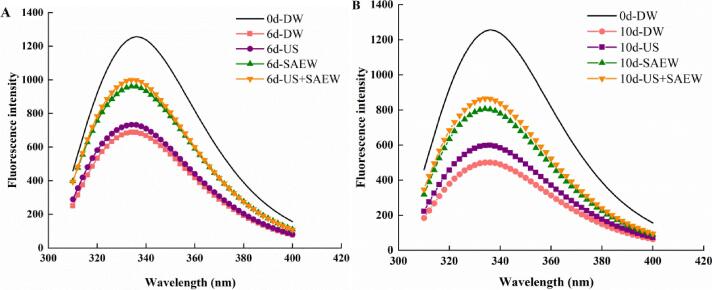
Protein molecule fluorophore will fluoresce under ultraviolet light, called the intrinsic fluorescence of protein [42]. Fluorescence intensity could be used to reflect the magnitude index and the exposure of tryptophan (Trp) in amino acids. And Trp is related to the extent and unfolding of proteins [43]. Since the fluorescence decrease in fluorescence intensity is generally transferred to the inner surface of protein, resulting in fluorescence quenching. Therefore, the changes in endogenous fluorescence can reflect the conformational changes of protein molecules [44].
The results indicated that the fresh sample at day 0 showed the highest fluorescence intensity at 336 nm. The decrease in fluorescence intensity was observed in four groups during storage. The endogenous fluorescence intensity of protein in DW group was decreased sharply throughout the storage period. Meanwhile, samples treated US also showed significant decline in fluorescence intensity after 10 days of storage but the decline was lesser than samples of DW. Especially, samples of US + SAEW showed slowest decline in fluorescence intensity, indicating higher stability of Trp residues in proteins (Fig. 4). Because samples were subjected to ultrasonic treatment in the process of fluorescence quenching, resulting in protein structure folding. In addition, as the intensity of ultrasound treatment increased, the protein would denature and aggregate, and Trp residues could be exposed from the interior of the protein molecules, thereby increasing the fluorescence intensity [45].
Fig. 4.
Effects of different treatments on the intrinsic fluorescence spectrum of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water).
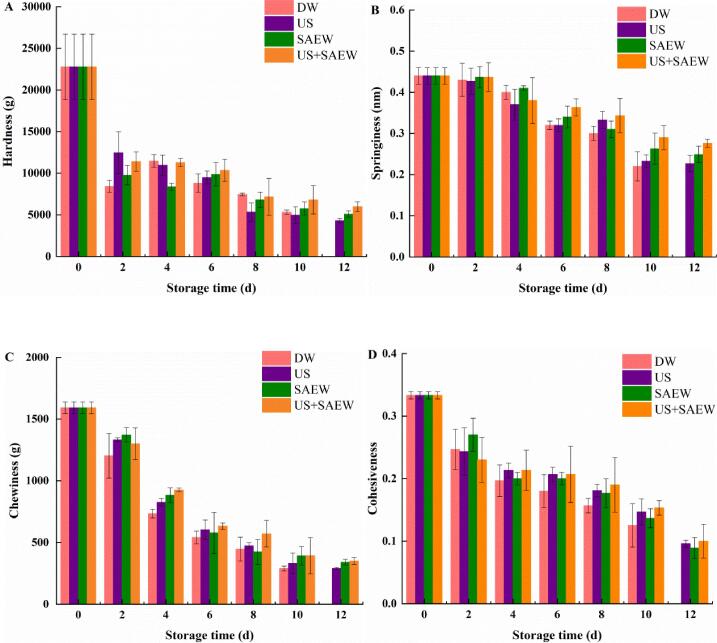
3.2.4. Changes in texture profile analysis
Hardness is an important textural attribute in aquatic products, which indicates the integrity of flesh structure and shows a downward trend during storage (Fig. 5A). The hardness of samples at the first 3 days was maintained in US + SAEW treatment. The significant reductions in hardness value of DW group and other treated groups occurred at day 2 (p < 0.05). Nevertheless, there was no significant difference in each group from day 8 (p > 0.05). The hardness reduction range of US, SAEW and US + SAEW groups was 73.66 ∼ 81.12% during storage.
Fig. 5.
Effects of different treatments on TPA of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water). (A: Hardness, B: Springiness, C: Chewiness, D: Cohesiveness).
Springiness refers to the recovery degree of samples after the external force is applied. The loss of springiness was no significant in all groups during the first 2 days of storage (p > 0.05) (Fig. 5B). After that, the springiness gradually decreased, especially in DW group. It was also worth noting that US + SAEW group had higher springiness than those of other groups, which did not change significantly after 10 days (p > 0.05). The chewiness of samples was changed significantly and showed an overall decreasing trend during refrigerated storage (p < 0.05) (Fig. 5C). Compared with the DW, US, SAEW groups, the US + SAEW group suppressed the decrease in cohesiveness after 8 days of storage (p < 0.05) (Fig. 5D). In the field of cohesiveness, there were no virtual changes among groups, which were in consistent with the previous research [46]. According to the correlation analysis between different parameters, the TPA indexes were significantly correlated with microorganism, TBA, TVB-N, K value and sensory score (p < 0.05) (Table 3 Supplementary File). With the extension of storage time, the protein in samples degraded under the action of microorganisms, and the nitrogen-containing substances increased correspondingly. The deterioration of texture was due to the decomposition of proteins supporting the texture of fish, and the sensory scores of texture became increasingly unacceptable [47].
3.2.5. Changes in color difference
Color difference (L*, a* and b*) was performed on the treated samples and the DW group at day 0, 2, 4, 6, 8 and 10. As the Tab. 1 showed, the changes of L* value in four group fluctuated up and down during storage. In addition, the L* value of DW group was significantly different from the other three groups during storage. And there were no differences in L* value of US, SAEW and US + SAEW groups at day 8, 10, 12. Therefore, US or SAEW treatment had a certain effect on the brightness of fish fillets, but there was no significant difference among three treated groups in the later stage of storage. However, the a* value of US samples decreased faster than those of DW and SAEW groups during storage, illustrating that US treatment would cause the fillets to lose original color. The b* value of fillets in four groups decreased gradually with the increase of storage time. The trend of change in this study was similar to that of A. Jc et al.[48]. Color index (a* and b*) of fish might be associated with denaturation of some heme-proteins and lipid oxidation [49], [50]. Overall, US treatment alone had negative effects on the color of samples, but US + SAEW treatment could delay the color deterioration (see Table 1).
Table 1.
Color difference of Lateolabrax Japonicus with different treatments during refrigerated storage.
| Color difference | Storage time (d) | DW | US | SAEW | US + SAEW |
|---|---|---|---|---|---|
| L* | 0 | 49.50 ± 1.14Aab | 49.50 ± 1.14Aa | 49.50 ± 1.14Aa | 49.50 ± 1.14Aa |
| 2 | 44.84 ± 0.84Cc | 46.23 ± 1.15Ab | 48.12 ± 0.33Ba | 48.23 ± 1.31Aab | |
| 4 | 46.46 ± 0.44Dc | 46.50 ± 0.73Cb | 45.8 ± 2.04bBc | 45.73 ± 1.73Abc | |
| 6 | 48.39 ± 1.44ABb | 43.44 ± 0.03Bc | 44.20 ± 0.28Ac | 44.04 ± 3.73Acd | |
| 8 | 42.01 ± 1.15Ad | 42.41 ± 0.30Bc | 42.07 ± 0.92ABd | 41.97 ± 0.48Bd | |
| 10 | 51.00 ± 0.81Ba | 47.34 ± 2.02Ab | 47.70 ± 0.89Aab | 51.30 ± 1.51Aa | |
| 12 | 49.24 ± 1.21Ab | 50.07 ± 0.76Ab | 55.21 ± 0.91Aa | ||
| 14 | 52.17 ± 0.83a | ||||
| a* | 0 | 0.05 ± 0.13Ac | 0.05 ± 0.13Aab | 0.05 ± 0.13Abc | 0.05 ± 0.13Aab |
| 2 | 0.61 ± 0.44Ab | −0.66 ± 0.93Aab | 0.33 ± 0.53Aab | 0.49 ± 0.32Aa | |
| 4 | 1.23 ± 0.18Aa | −0.37 ± 0.24Aab | 0.73 ± 0.26Aa | 0.00 ± 0.60Aab | |
| 6 | −0.82 ± 0.17Ae | −0.43 ± 0.14Aab | 0.01 ± 0.38Abc | 0.15 ± 0.60Aab | |
| 8 | −0.15 ± 0.15Acd | 0.11 ± 0.48Aa | −0.06 ± 0.52Abc | 0.72 ± 0.11Aa | |
| 10 | −0.37 ± 0.05Ad | −0.90 ± 0.62Bb | −0.37 ± 0.05Cc | −0.40 ± 0.28Bb | |
| 12 | −0.78 ± 0.47Aa | −0.35 ± 0.12Aa | −0.44 ± 0.18Ab | ||
| 14 | −0.48 ± 0.09a | ||||
| b* | 0 | −0.41 ± 0.78Aab | −0.41 ± 0.78Aa | −0.41 ± 0.78Aab | −0.41 ± 0.78Aa |
| 2 | 0.20 ± 0.65Aa | −1.93 ± 0.30Bab | −0.29 ± 0.61Ba | −0.34 ± 1.70Aa | |
| 4 | 0.26 ± 0.58Aa | −2.19 ± 0.91Ab | −0.75 ± 0.22Aab | −1.32 ± 0.71Aab | |
| 6 | −2.69 ± 0.88Ac | −1.57 ± 0.68Bab | −1.21 ± 0.70Aab | −1.57 ± 0.83Bab | |
| 8 | −1.20 ± 0.08Ab | −0.53 ± 0.68Bab | 0.01 ± 1.74Aa | −0.45 ± 0.28Aa | |
| 10 | −2.58 ± 0.30Ac | −1.31 ± 1.45Aab | −2.04 ± 0.39ABb | −2.71 ± 1.22Bb | |
| 12 | −1.41 ± 0.86Aa | −2.21 ± 0.25Ab | −2.57 ± 0.19Ab | ||
| 14 | −2.12 ± 0.30a | ||||
Note: The results are expressed as Means ± S.D., different superscript lowercase letters represent significant differences within groups (p < 0.05), and different superscript uppercase letters represent significant differences between groups (p < 0.05).
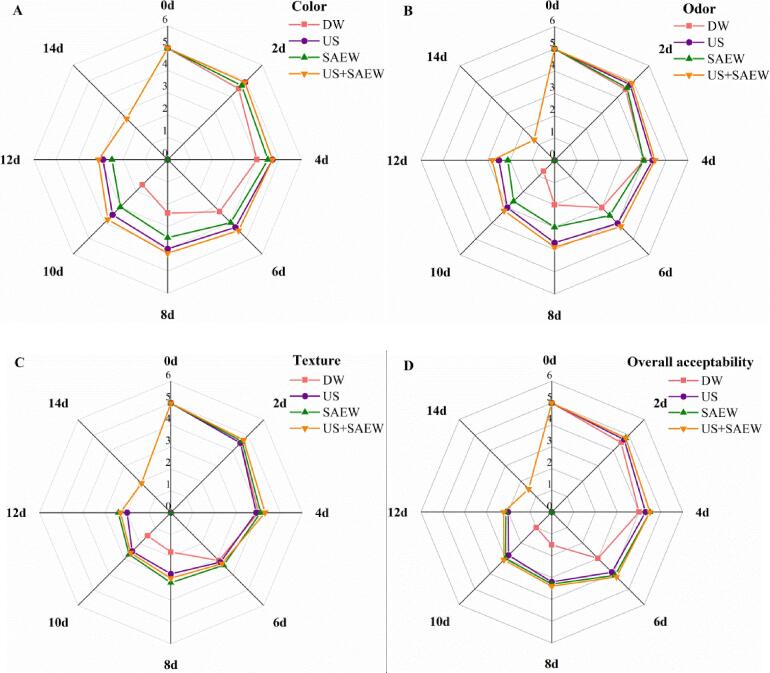
3.3. Sensory evaluation
Sensory evaluation is an important indicator of freshness. The color, odor, and texture and overall acceptability were used to estimate the quality of samples. With the increase of storage time, the sensory scores of different groups were declined significantly. After day 6, the sensory scores of US + SAEW group in color, odor, texture and overall acceptability were significantly higher than DW group (p < 0.05) (Fig. 6A, B, C, D).
Fig. 6.
Effects of different treatments on sensory scores of Lateolabrax Japonicus during refrigerated storage (DW: distilled water, US: ultrasound, SAEW: slightly acidic electrolyzed water, US + SAEW: ultrasound combined with slightly acidic electrolyzed water).
On day 8, the color of DW group was slightly dull, but the fillets of US + SAEW had glossy appearance. The initial glossiness of sea bass displayed a decreasing trend along with the development of greyish appearance. On day 14, color scores of US + SAEW group were still within the acceptable range. The odor scores of DW group declined rapidly and displayed a significantly different score compared to US + SAEW group. DW group had low acceptability scores for odor after 8 days. While samples treated with SAEW and US could maintain acceptable odor quality at day 12. At the end of storage, samples of US group showed strong fishy or amine smell, which could be related to metabolites produced by bacterial activities [51]. This phenomenon suggested that US combined with SAEW could inhibit the growth of off odors producing microorganisms. After day 8, the sensory scores of DW groups in texture were greatly lower than US, SAEW or US + SAEW groups (p < 0.05) (Fig. 6C). Moreover, on day 8, the texture of DW group was inelastic, but the US + SAEW group was still elastic on day 12. But more than that, the US + SAEW group had higher sensory scores in overall acceptability than US or SAEW alone. On day 8, the samples showed different degrees of corruption. And samples of DW group were soft and loose, with strong fishy and amine smell, which was unacceptable from sensory evaluation. On day 12, the sensory scores of all treatment groups in color, odor and overall acceptability were still within the acceptable range.
4. Conclusions
This study introduced that US + SAEW treatment could retain the freshness of refrigerated sea bass. Compared with SAEW or US treatment alone, US + SAEW treatment on sea bass had obvious effect on inhibiting protein degradation and microbial growth, maintaining better texture and sensory scores. This combination treatment could prolong the shelf-life of sea bass for another 4 days at least. The results illustrated that the US treatment enhanced the decontamination ability of SAEW and delayed the deterioration of quality and got the higher sensory score. Therefore, the cooperative treatment of US and SAEW is an effective approach to keep the quality and extend shelf life of sea bass during refrigerated storage.
CRediT authorship contribution statement
Weiqing Lan and Dapeng Zhou designed the experiment, finished the study, collected test data and drafted the original manuscript. Ai Lang reviewed the data interpretation and edited the manuscript. Jing XIE was responsible for project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was financially supported by National Key R&D Program of China (2019YFD0901602), China Agriculture Research System (CARS-47-G26), Ability promotion project of Shanghai Municipal Science and Technology Commission Engineering Center (19DZ2284000).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105854.
Contributor Information
Weiqing Lan, Email: wqlan@shou.edu.cn.
Jing Xie, Email: jxie@shou.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Boyd L.C., Green D.P., Lepors L.A. Quality changes of pond-raised hybrid striped bass during chillpack and refrigerated storage. J. Food Sci. 1992;57(1):59–62. [Google Scholar]
- 2.Böhme K., Fernández-No I.C., Gallardo J.M., Cañas B., Calo-Mata P. Safety assessment of fresh and processed seafood products by MALDI-TOF mass fingerprinting. Food Bioprocess Technol. 2011;4(6):907–918. [Google Scholar]
- 3.Jayasooriya S.D., Bhandari B.R., Torley P., D'Arcy B.R. Effect of high power ultrasound waves on properties of meat: a review. Int. J. Food Prop. 2004;7(2):301–319. [Google Scholar]
- 4.Cao S., Hu Z., Pang B., Wang H., Xie H., Wu F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control. 2010;21(4):529–532. [Google Scholar]
- 5.Knobloch S., Philip J., Ferrari S., Benhaïm D., Bertrand M., Poirier I. The effect of ultrasonic antifouling control on the growth and microbiota of farmed European sea bass (Dicentrarchus labrax) Mar. Pollut. Bull. 2021;164:112072. doi: 10.1016/j.marpolbul.2021.112072. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Ding T., Liao X.Y., Chen S.G., Ye X.Q., Liu D.H. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrason. Sonochem. 2017;38:711–719. doi: 10.1016/j.ultsonch.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y.M., Oliveira M., Burgess C.M., Cropotova J., Rustad T., Sun D.W., Tiwari B.K. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT- Food Sci. Technol. 2021;150 [Google Scholar]
- 8.Baumann A.R., Martin S.E., Feng H. Power ultrasound treatment of Listeria monocytogenes in apple cider. J. Food Prot. 2005;68:2333–2340. doi: 10.4315/0362-028x-68.11.2333. [DOI] [PubMed] [Google Scholar]
- 9.Lin L., Wang X., Li C., Cui H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrason. Sonochem. 2019;59:104751. doi: 10.1016/j.ultsonch.2019.104751. [DOI] [PubMed] [Google Scholar]
- 10.Al Khawli F., Pallarés N., Martí-Quijal F.J., Ferrer E., Barba F.J. Sea bass side streams valorization assisted by ultrasound. LC-MS/MS-IT determination of mycotoxins and evaluation of protein yield, molecular size distribution and antioxidant recovery. Appl. Sci. 2021;11(5):2160. doi: 10.3390/app11052160. [DOI] [Google Scholar]
- 11.Forghani F., Rahman S., Park M.S., Park J.H., Oh D.H. Ultrasonication enhanced low concentration electrolyzed water efficacy on bacteria inactivation and shelf life extension on lettuce. Food Sci. Biotechnol. 2013;22:131–136. [Google Scholar]
- 12.Lee J.H., Chun H.H., Oh D.H., Park J., Won M., Song K.B. Sensory and microbiological qualities of romaine lettuce and kale affected by a combined treatment of aqueous chlorine dioxide and ultraviolet-C, Horticulture. Environ. Biotechnol. 2012;53:387–396. [Google Scholar]
- 13.Luo K., Oh D.-H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016;53:165–171. doi: 10.1016/j.fm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Cai L.Y., Cao A.L., Bai F.L., Li J.R. Effect of ε-polylysine in combination with alginate coating treatment on physicochemical and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) during refrigerated storage. LWT - Food Sci. Technol. 2015;62:1053–1059. [Google Scholar]
- 15.Odunayo O.O., Soottawat B. Nonthermal processes for shelf-life extension of seafoods: a revisit. Comprehensive Rev. Food Sci. Food Safety. 2017 doi: 10.1111/1541-4337.12354. [DOI] [PubMed] [Google Scholar]
- 16.Issa-Zacharia A., Kamitani Y., Miwa N., Muhimbula H., Iwasaki K. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts. Food Control. 2011;22:601–607. [Google Scholar]
- 17.Afari G.K., Hung Y.-C., King C.H., Hu A. Reduction of Escherichia coli O157:H7 and Salmonella Typhimurium DT 104 on fresh produce using an automated washer with near neutral electrolyzed (NEO) water and ultrasound. Food Control. 2016;63:246–254. [Google Scholar]
- 18.Tango C.N., Mansur A.R., Kim G.H., Oh D.H. Synergetic effect of combined fumaric acid and slightly acidic electrolysed water on the inactivation of food-borne pathogens and extending the shelf life of fresh beef. J. Appl. Microbiol. 2015;117:1709–1720. doi: 10.1111/jam.12658. [DOI] [PubMed] [Google Scholar]
- 19.Li T.T., Li J.R., Hu W.Z., Li X.P. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2013;138:821–826. doi: 10.1016/j.foodchem.2012.11.092. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Capillas C., Moral A. Correlation between biochemical and sensory quality indices in hake stored in ice. Food Res. Int. 2001;34:441–447. [Google Scholar]
- 21.Babi M.J., Milan M., Danijela V., Inovi S.J., Vladimir K. Effect of modified atmosphere packaging on the shelf life of chilled common carp (Cyprinus carpio) steaks: chemical and sensory attributes. Czech J. Food Sci. 2018;36 [Google Scholar]
- 22.Forghani F., Oh D.-H. Hurdle enhancement of slightly acidic electrolyzed water antimicrobial efficacy on Chinese cabbage, lettuce, sesame leaf and spinach using ultrasonication and water wash. Food Microbiol. 2013;36:40–45. doi: 10.1016/j.fm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Marquez D.P., Fuenmayor C.A., Mahecha H.S. Effect of chitosan-propolis edible coatings on stability of refrigerated cachama (Piaractus brachypomus) vacuum-packed fish fillets. Packaging Technol. Sci. 2019;32:143–153. [Google Scholar]
- 24.Chuesiang P., Sanguandeekul R., Siripatrawan U. Siripatrawan, Phase inversion temperature-fabricated cinnamon oil nanoemulsion as a natural preservative for prolonging shelf-life of chilled Asian seabass (Lates calcarifer) fillets. LWT- Food Sci. Technol. 2020;125:109122. doi: 10.1016/j.lwt.2020.109122. [DOI] [Google Scholar]
- 25.Zhu Z.W., Ruan Z., Li B.S., Meng M.Y., Zeng Q.X. Quality loss assessment of crisp grass carp (Ctenopharyngodon idellus C. et V) fillets during ice storage. J. Food Process. Preservation. 2013;37:254–261. [Google Scholar]
- 26.Bai C., Xu P., Huang M., Xiong G.Q., Wang J.G., Liao T. Effect of lrradiation combined with composite preservatives on the storage quality of largemouth bass (Micropterus salmoides) Meat Res. 2021;35:50–56. [Google Scholar]
- 27.Wang R., Hu X., Agyekumwaa A.K., Li X., Xiao X., Yu Y. Synergistic effect of kojic acid and tea polyphenols on bacterial inhibition and quality maintenance of refrigerated sea bass (Lateolabrax japonicus) fillets. LWT- Food Sci. Technol. 2021;137:110452. doi: 10.1016/j.lwt.2020.110452. [DOI] [Google Scholar]
- 28.Zhao X., Chen L., Wongmaneepratip W., He Y., Zhao L., Yang H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129581. [DOI] [PubMed] [Google Scholar]
- 29.Ding T., Ge Z., Shi J., Xu Y.T., Jones C.L., Liu D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT - Food Sci. Technol. 2015;60:1195–1199. [Google Scholar]
- 30.Mikš-Krajnik M., James Feng L.X., Bang W.S., Yuk H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control. 2017;74:54–60. [Google Scholar]
- 31.Cichoski A.J., Flores D.R.M., De Menezes C.R., Jacob-Lopes E., Zepka L.Q., Wagner R., Barin J.S., de Moraes Flores É.M., da Cruz Fernandes M., Campagnol P.C.B. Ultrasound and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019;301:27–33. doi: 10.1016/j.ijfoodmicro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Gogate P.R., Kabadi A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009;44:60–72. [Google Scholar]
- 33.Ni Z.P., Li Y.Y., Yu Z.C., Song X.L. Effect of combined treatment of slightly acid electrolytic water and ultrasonic on sterilization of scomberomorus sinensis. Food Technol. 2021;05:123–127. [Google Scholar]
- 34.Soria A.C., Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 2010;21(7):323–331. [Google Scholar]
- 35.de Lima Alves L., Stefanello da Silva M., Martins Flores D.R., Rodrigues Athayde D., Roggia Ruviaro A., da Silva Brum D., Fagundes Batista V.S., de Oliveira Mello R., Ragagnin de Menezes C., Bastianello Campagnol P.C., Wagner R., Smanioto Barin J., Cichoski A.J. Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami. Food Res. Int. 2018;106:363–373. doi: 10.1016/j.foodres.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 36.Guan Y., Lan W.Q., Sun Y.Q., Liu L., Zhou D.P., Jing X. Effects of ultrasonic combined with caffeic acid on the quality of sea bass (Lateolabrax japonicas) during refrigerated storage. Food Sci. 2021:1–10. [Google Scholar]
- 37.Khan M.A., Parrish C.C., Shahidi F. Quality indicators of cultured Newfoundland blue mussels (Mytilus edulis) during storage on ice: microbial growth, pH, lipid oxidation, chemical composition characteristics, and microbial fatty acid contents. J. Agric. Food Chem. 2005;53:7067–7073. doi: 10.1021/jf050082g. [DOI] [PubMed] [Google Scholar]
- 38.Li T.T., Li J.R., Hu W.Z. Changes in microbiological, physicochemical and muscle proteins of post mortem large yellow croaker (Pseudosciaena crocea) Food Control. 2013;34:514–520. [Google Scholar]
- 39.Gökodlu, N., MS, Ö. Ö., N. Erkan, M. S., Physical, Chemical and Sensory Analyses of Freshly Harvested Sardines (Sardina pilchardus) Stored at 4℃, J. Aquat. Food Product Technol. 7 (1998) 5-15.
- 40.Ehira S., Uchiyama H. Freshness-lowering rates of cod and sea bream viewed from changes in bacterial count, total volatile base- and trimethylamine-nitrogen, and ATP related compounds. Bull. Jpn. Soc. Sci. Fish. 1974;40(5):479–487. [Google Scholar]
- 41.Sarmast E., Fallah A.A., Habibian Dehkordi S., Rafieian-Kopaei M. Impact of glazing based on chitosan-gelatin incorporated with Persian lime (Citrus latifolia) peel essential oil on quality of rainbow trout fillets stored at superchilled condition. Int. J. Biol. Macromol. 2019;136:316–323. doi: 10.1016/j.ijbiomac.2019.06.087. [DOI] [PubMed] [Google Scholar]
- 42.Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019;299 doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- 43.Cai L.Y., Zhang W.D., Cao A.L., Cao M.J., Li J.R. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum) Ultrason. Sonochem. 2019;55:96–104. doi: 10.1016/j.ultsonch.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Wang C.Y., Feng D., Zhang M. Physico-chemical and structural properties of four rice bran protein fractions based on the multiple solvent extraction method. Czech J. Food Sci. 2015;33:283–291. [Google Scholar]
- 45.Wang Y.Y., Tayyab Rashid M., Yan J.K., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao F., Ng V.K., Marta M.K., Yang H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol. 2017;10:89–102. [Google Scholar]
- 47.Zhou D.P., Lan W.Q., Mo Y.X., Jun M., Feng H.J., Xie J. Effects of ultrasound pretreatment on the quality and protein characteristics in Japanese sea bass (Lateolabrax japonicas) during refrigeration. Food Ferment. Ind. 2020;17:204–211. [Google Scholar]
- 48.Cropotova J., Tappi S., Genovese J., Rocculi P., Dalla Rosa M., Rustad T. The combined effect of pulsed electric field treatment and brine salting on changes in the oxidative stability of lipids and proteins and color characteristics of sea bass (Dicentrarchus labrax) Heliyon. 2021;7(1):e05947. doi: 10.1016/j.heliyon.2021.e05947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui G.H., Liu W., Feng H.L., Li J., Gao Y.Y. Effects of chitosan combined with nisin treatment on storage quality of large yellow croaker (Pseudosciaena crocea) Food Chem. 2016;203:276–282. doi: 10.1016/j.foodchem.2016.01.122. [DOI] [PubMed] [Google Scholar]
- 50.Cai L.Y., Xu Q., Cao A.L. Effects of different ultrasound–assisted thawing methods on the quality of the sea bass. Sci. Technol. Food Ind. 2020;24:264–271. [Google Scholar]
- 51.J.L. Liu, W.Q. Lan, X.H. Sun, J. Xie, Effects of chitosan grafted phenolic acid coating on microbiological, physicochemical and protein changes of sea bass (Lateolabrax japonicus) during refrigerated storage, J. Food Sci. 85 (2020). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.