Abstract
CARD9 is a cytosolic adaptor in myeloid cells, has a critical role in inflammatory disorders, and provides a protective function against microbial pathogen, especially fungal infection. Recently, CARD9 polymorphisms are of interest, showing a positive correlation with the elevated risk of fungal infection, inflammatory bowel disease, and other autoimmune diseases. Mechanistically, CARD9 polymorphisms impair the activation of RelB, a subunit of non-canonical NF-κB, which lead to the reduced cytokine and chemokine production by innate immune cells. In addition, CARD9 polymorphisms show a defective neutrophil accumulation in infectious sites. Furthermore, CARD9 polymorphisms could alter the composition of the gut microbiome. In this review, we summarize the latest findings of CARD9 polymorphisms with respect to inflammatory diseases.
Keywords: CARD9 polymorphisms, Inflammatory, Infection, Autoimmune diseases
Caspase recruitment domain-containing protein 9 (CARD9) is a cytosolic adaptor protein in myeloid cells, which plays an important role in immunity response. CARD9 can trigger the NF-κB and MAPK signaling pathways, induce the inflammation cytokine cascade, and subsequently protect the host from microbial invasion, especially fungal infection.
Although the functions of CARD9 are widely demonstrated in the last decade, the correlation between CARD9 polymorphism and disease risk is not well understood. Recently, increasing evidence has indicated that CARD9 gene polymorphisms predispose to inflammatory diseases, such as fungal infection and autoimmune. Thereby, this review aims at comprehensively examining all known CARD9 polymorphisms as well as their nature.
CARD9 structure and protein expression
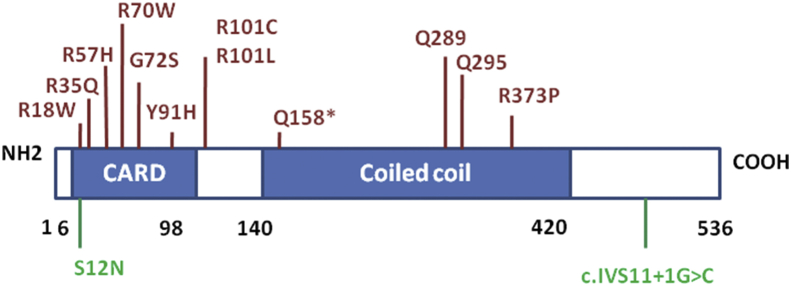
CARD9 is a novel member of the CARD protein family, which is defined by the presence of a characteristic caspase-associated recruitment domain. CARD9 is located on chromosome 9q34.3, and its full-length 2108-bp cDNA encodes a 536-amino acid protein with a predicted molecular mass of 62.3 kDa [1]. CARD9 is structurally similar to the CARMA family members but lacks the C-terminal MAGUK motif, and contains an amino-terminal CARD and a carboxy-terminal coiled-coil domain [Fig. 1] [2]. The N-terminal CARD region of CARD9, comprising of 7–98 amino acids (aa), can interact with CARD motifs from many apoptosis proteins including BCL10 and RIP-associated Ich-1/Ced-3 homologous protein (RAIDD) [3]. The coiled-coil region of 140–420 aa at the C terminus of CARD9 serves as an oligomerization domain [4]. CARD9 expression is detected in a variety of human adult tissues, including the spleen, thymus, liver, placenta, lung, bone marrow, brain, and peripheral blood [4]. CARD9 is abundantly expressed by myeloid cells, particularly antigen presenting cells such as macrophages, dendritic cells and neutrophils.
Fig. 1.
Schematic diagram of the human CARD9 gene and its known pathogenic mutations. Several CARD9 loss-of-function mutations associated with fungal infections are indicated in red. CARD9 mutations associated inflammatory bowel diseases are indicated in green, in which CARD9S12N SNP increase disease risk while CARD9 c.IVS11+1G>C variant has protective function.
CARD9 polymorphisms and fungal infections
CARD9 loss-of-function mutations in patients have unequivocally demonstrated its importance in fungal infections, predominantly localizing to the central nervous system (CNS), subcutaneous tissues, oral mucosa, bone, and abdominal organs [[5], [6], [7], [8], [9]]. The species of pathogenic fungi are found to be Candida species, dark-walled molds, and yeast-like fungi (e.g., Phialophora, Aspergillus, fumigatus and Exophiala), some of them maybe cause lethal infections [[5], [6], [7], [8], [9]]. Although many CARD9-deficient patients have a high susceptibility to fungal infections, there are diverse genetic mutations in CARD9. More than 24 missense and nonsense mutations of CARD9 are reported in the N-terminal CARD and C-terminal coiled-coil domains, as well as the promoter region [Fig. 1] [5,10]. Inactivation of both alleles is necessary for disease occurrence, so CARD9-deficient patient follows an autosomal recessive mode of inheritance.
To date, CARD9 mutations in patients were found to associate with fungal meningitis. A 4-year-old Turkish girl was detected with a homozygous point mutation in exon 6 (c.883C>T) of CARD9, hospitalized for chronic Candida albicans (C. albicans) meningitis [11]. A 13-year-old Asian girl with Candida dubliniensis meningoencephalitis was diagnosed as a result of c.214G>A and c.1118G>C mutations in the CARD9 gene [12]. An 11-year-old girl from USA was reported with C. albicans infection of the CNS, due to c.170G>A CARD9 missense mutation [13]. An 8-year-old girl developed Exophiala dermatitidis pachymeningitis, who was found to have CARD9 R18W, a homozygous c.52C>T missense mutation in exon 2, located in the N-terminal caspase-recruitment domain [14]. The other mutations in CARD9, including homozygous R70W (c.208C>T in exon 3), R35Q (c.104G>A in exon 2), Q289T (c.865C>T in exon 6), Y91H (c.-529T>C), G72S, R373P, and R57H mutation, also presented with severe Candida meningoencephalitis [[15], [16], [17], [18]]. The potential mechanisms maybe attribute to the following several aspects: First, CARD9 gene mutation impaired CARD9 protein expression level and its biological function. CARD9 c.883C>T, c.214G>A and c.1118G>C mutations showed the complete absence of protein level, led to the loss of its function. CARD9 c.170G>A missense mutation maintains the normal expression level of protein, but damages its protein structure and then inhibited its biological functions. Second, CARD9 gene mutation exhibited a defective neutrophil accumulation and significant eosinophil infiltration in the CNS of CARD9-deficient patients [12,13,[15], [16], [17]]. As previously reported, neutrophils played a critical role against systemic C. albicans infection, while eosinophils did not [19]. In the infected cerebrospinal fluid (CSF) of CARD9-deficient patients, CXCL1, CXCL2, CXCL5 and IL-8 known as neutrophil-targeted chemokines were obviously reduced. Conversely, the CSF of patients with the wild-type mature CARD9 protein greatly increased the expression level of IL-1β and CXCL1 neutrophil-recruitment chemokines [20]. Thereby, the phenomenon of CNS-specific neutropenia in CARD9 deficiency patients was caused by a striking lack of neutrophil-targeted chemoattractants.
CARD9 mutation was found in patients with fungal subcutaneous infection. 3 mutations of CARD9, 2 compound heterozygous mutations (c.191192insTGCT and c.472C>T, p.L64fsX59 and p.Q158X) and 1 homozygous frameshift mutation (c.819820insG, p.D274fsX60), were validated in the subcutaneous phaeohyphomycosis caused by Phialophora verrucosa, exhibiting a lack expression of CARD9 protein but sufficient level of CARD9 mRNA [21]. In addition, a homozygous nonsense mutation in CARD9 (Q295X), resulting in a premature termination codon and a loss of CARD9 protein function, gave rise to chronic mucocutaneous candidiasis in a large consanguineous family [8]. A homozygous premature stop codon mutation (Q289∗), homozygous missense mutation (R101C) and homozygous R101L, and compound heterozygous (c.883C>T and c.1118G>C, Q295X and R373P) mutation in CARD9 were identified in patients, resulting in a susceptibility to dermatophytes such as Trichophyton violaceum, Microsporum ferrugineum, and T. rubrum [7,[22], [23], [24], [25], [26]]. The underlying mechanisms that cause susceptibility to fungal subcutaneous infection are not well understood, and could be related to immunodeficiencies in CARD9-deficient patients via impairing the pivotal cytokine production of innate immune cells and differentiation of TH17 cells [21].
Human CARD9 mutation is associated with specific families of pathogenic fungi. CARD9 variant (c.191-192InsTGCT, p.L64fsX59) was identified with a Chinese patient, leading to Corynespora cassiicola infection [27]. C. cassiicola are plant pathogens, which rarely cause human infection [27]. c.1118G>C, c.820_821insG, and p.Glu9Lys (c.25G>A) mutations in CARD9 are linked to porotrichosis infection [28]. c.759dup (p. Lys254fs) mutation in the exon 5 of CARD9 was associated with E. dermatitidis (E dermatitidis) [29]. A synonymous variant c951G>A, A317A and a missense variant c.1138G>C, A380P caused a specific susceptibility to endogenous Candida endophthalmitis and osteomyelitis [30]. Patients with phaeohyphomycosis caused by Exophiala spinifera, Ochroconis musae, Phialophora americana, and C. cassiicola showed CARD9 mutations (c.68C>A and c.819-820insG in exon 2, p.S23X and p.D274fsX60 in exon 6, c.191e192insTGCT and p.L64fsX59 in exon 3) [27,31,32]. Saprochaete capitata infection was associated with p.Q295∗ mutation in CARD9, which disseminated to common bile duct and lymph nodes [33]. The CARD9 c.3G>C, M1I mutation was found to increase susceptibility to extrapulmonary Aspergillus infection in the abdomen and brain [6]. Another CARD9 mutation, Ser12Asn, rs4077515, predisposed patients to idiopathic recurrent vulvovaginal candidiasis [34]. The CARD9 S12N (c.35G>A, rs4077515) polymorphism was identified as a risk factor for the development of candidemia [35]. A homozygous R18W CARD9 mutation in patients was strongly linked to invasive Exophiala infection [14]. However, the unique genetic alteration in CARD9, which is associated with a specific fungal infection, remains to be fully explored.
Vaezi et al reported that fungal infectious diseases were associated with 24 CARD9 mutations, further evaluated the frequency and geographic distribution of CARD9 mutations [10]. Three CARD9 genetic mutations, p.Q289X (c.865C > T), p.Q295X (c.883C > T) and p.D274fsX60 (c.819-820insG), were identified most frequently, which accounted for 25.8%, 17.7%, and 8.1% of the patients, respectively. CARD9 p.Q289X (c.865C > T) and p.Q295X (c.883C > T) mutations were associated with a high risk of candidiasis and dermatophytosis infection [10]. CARD9 p.Q289X (c.865C > T) and p.Q295X (c.865C > T) accounted for 75% and 37.9% of the African and Asian cases, indicating an obviously different geographical distribution [10].
CARD9 polymorphisms and inflammatory bowel disease
Several single nucleotide polymorphisms (SNPs) in the human CARD9 gene are closely associated with inflammatory bowel diseases (IBD). CARD9 rs10870077, rs4077515, and rs10781499, as the predisposing variants, exhibit an increased risk, while c.IVS11+1G>C and rs200735402, as the protective variants, are shown to have a protective effect on IBD [[36], [37], [38], [39], [40], [41], [42]]. CARD9 rs10870077 refers to the intronic substitution of base C (cytosine) for G (guanine) in the CARD9 genetic locus on chromosome 9, which presumably influence the function of CARD9 adaptor protein and thereby modulate the CARD9-dependent inflammatory signaling [36]. CARD9 variant rs4077515 is known to carry an asparagine instead of a serine residue in the CARD domain in position 12 (CARD9S12N). CARD9S12N leads to aberrant activation of NF-κB and inflammatory factors in response to A. fumigates, potentially contributing to intestinal inflammation [37,38]. CARD9 rs10781499, the substitution of A for G at position 139266405, could alter the composition of the gut microbiota, leading to a higher risk of developing IBD [[39], [40], [41]]. Different from the risk SNPs, CARD9 c.IVS11+1G>C with the substitution of G for C at position 1 of exon 11, CARD9 rs200735402 with the substitution of C for T at position 139265120, could provide a protective function in the intestinal immune system during IBD pathogenesis [[42], [43], [44]]. CARD9 c.IVS11+1G>C leads to create a CARD9 protein with a shortened C-terminal tail, so that CARD9 fails to bind TRIM62 for NF-κB activation, and is unable to induce pro-inflammatory cytokines production [45]. Among 500 IBD patients and 1000 unrelated healthy controls, CARD9 rs200735402 showed an OR of 0.09 (95% CI 0.22 to 0.37, P = 5.28 × 10−5) with a functionally protective role in IBD [44].
CARD9, one very prominent IBD susceptibility gene in intestinal homeostasis, is being increasingly understood, which balances interactions between the host immune system and the gut microbiome. CARD9-deficient mice were confirmed to aggregate colitis severity, exhibiting a defect in intestinal epithelial cell restitution and a great body weight loss during IBD recovery [46]. CARD9 deficiency had also reduced the expression levels of inflammatory cytokines (IL-22, IL-6, TNFα, IFNγ etc), impaired the immunity responses of T-Helper 17 and innate lymphoid cell. Furthermore, CARD9 signaling is involved in the composition of the gut microbiome, exhibiting an aberrant fungal microbiome from the C. rodentium and Malassezia restricta [47], bacterial species from the Adlercreutzia genus and Lactobacillus reuteri. Mechanistic studies have demonstrated that the gut microbiome of CARD9−/− mice failed to metabolize tryptophan into aryl hydrocarbon receptor (AHR) ligands, which are critical factors for the production of IL-22 [40,48,49]. IL-22, which binds to its receptor on intestinal epithelial cells (IECs), drives their regenerative proliferation and stimulates the production of antimicrobial peptides against intruding microbes, increases the regenerative proliferation of basal epithelial cells, and regulates glycosylation patterns of epithelial cell-surface molecules [50]. Finally, CARD9 deficiency was found in patients with colitis caused by invasive intestinal infection with Candida glabrata and β-glucan-containing microalgae Prototheca zopfii [17,51].
CARD9 polymorphisms and inflammatory disease
Over the last decade, human CARD9 genetic mutation emerged as a risk factor of prevalent inflammatory disorders, including IgA nephropathy [52], primary immune thrombocytopenia [53], leprosy [54], rheumatoid arthritis [55], intestinal failure [56], ankylosing spondylitis [57], as well as pulmonary tuberculosis [58].
CARD9 rs4077515 in the human genome results from the substitution from guanine (G) to adenine (A) nucleotide, which encodes substitution of asparagine for serine at position 12 (S12N) in the protein CARD9 (CARD9S12N). The CARD9 rs4077515-A allele was correlated with an increased risk of IgA nephropathy [52]. This is probably because this substitution encoded higher expression of CARD9 in immunity cells, which leads to a hyper-reactive immune state. The CARD9 rs4077515 allele C and the genotype CC conferred significantly protective against primary immune thrombocytopenia [53] and ankylosing spondylitis [57] in HLA-B27-negative Iranian patients. One possibility was that this polymorphism decreased the level of CARD9 expression, contributing to the CARD9-IL23 axis for the pathogenesis of inflammatory disorders [53,57]. The CARD9 mutation (rs4077515) was associated with intestinal failure, showing the worse clinical outcomes in patients [56]. One possibility was that CARD9 deficiency is unable to modulate an adequate innate immune response to the invading microbial agents [56]. CARD9 rs59902911, the minor/low frequency T allele, was identified as a genetic risk factor that influences joint damage in rheumatoid arthritis [55]. A rare variant rs149308743 in CARD9 (P = 2.09 × 108, odds ratio [OR] = 4.75) showed involvement in the pathogenesis of leprosy, an ancient infectious disease caused by Mycobacterium leprae [54]. Although CARD9 mutant mice were found to increase the severity of pulmonary tuberculosis [59], CARD9 genetic variants rs4077515, rs10781499 and rs10870077 in patients do not affect the susceptibility and severity of disease [58]. Indeed, lung injures in humans were evaluated by sputum microbiology and chest Xray scores, whereas lung injures in mice were assayed by histopathologic examination and inflammatory factors. As a result, the different methods between mice and human likely led to the disparity results [58].
Potential mechanism for CARD9 polymorphisms
CARD9 is an intracellular adaptor molecule that transmits signals emerging from various microbe-sensing receptors. CARD9 is activated by all SYK-coupled C-type lectin receptors, including Dectin-1, Dectin-2 and Mincle. Furthermore, Toll-like receptors (TLRs) including TLR3 and TLR7, the cytosolic nucleic acid sensors retinoic acid-inducible gene 1, as well as RAD50, utilize CARD9 for signal transduction. CARD9 could couple to B-cell CLL/lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1), forming CARD-CC/BCL10/MALT1 (CBM) signalosomes. Such CBM signaling complexes mediate NF-κB activation, which results in induction of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-12, and IL-23 [60]. What is more, CARD9 is required for the differentiation of T lymphocytes into IL-17 producing T-Helper cells, further mediating the innate immune and adaptive response [46].
Upon these immune receptors activation, CARD9 was phosphorylated at T231 by PKCδ. Subsequently, CARD9 recruits the downstream binding partner, Bcl10, which interacts through its own N-terminal CARD with the N-terminal CARD of CARD9, a CARD–CARD interaction critical for subsequent NF-κB activation. The CARD9-CARD assemblies form a nucleating helical template that directly nucleates Bcl10 polymerization, along with other domains of activated CARD9, then recruits downstream signaling molecules, including MALT1, cIAPs, and TRAF6 that mediate subsequent ubiquitination. Finally, these ubiquitination led to degradation of IκB and activation of IΚΚ, thus allowing NF-κB to translocate to the nucleus and to induce the synthesis of proinflammatory molecules [61].
In addition to N terminus, C terminus of CARD9 also played a key role in CARD9-mediated signaling pathway. TRIM62 was identified as a novel binding partner with the CARD9 C-terminus (aa 416–536), and facilitated CARD9 ubiquitination at residue K125. Ubiquitination of CARD9 K125 recruited BCL10 to form a CBM complex, which activated the canonical NF-κB pathway. Conversely, CARD9 Δ11, a splice variant in which exon 11 of CARD9 C-terminus is deleted, could disrupt the CARD9-TRIM62 interaction, and abrogate CARD9-induced NF-κB signaling [45].
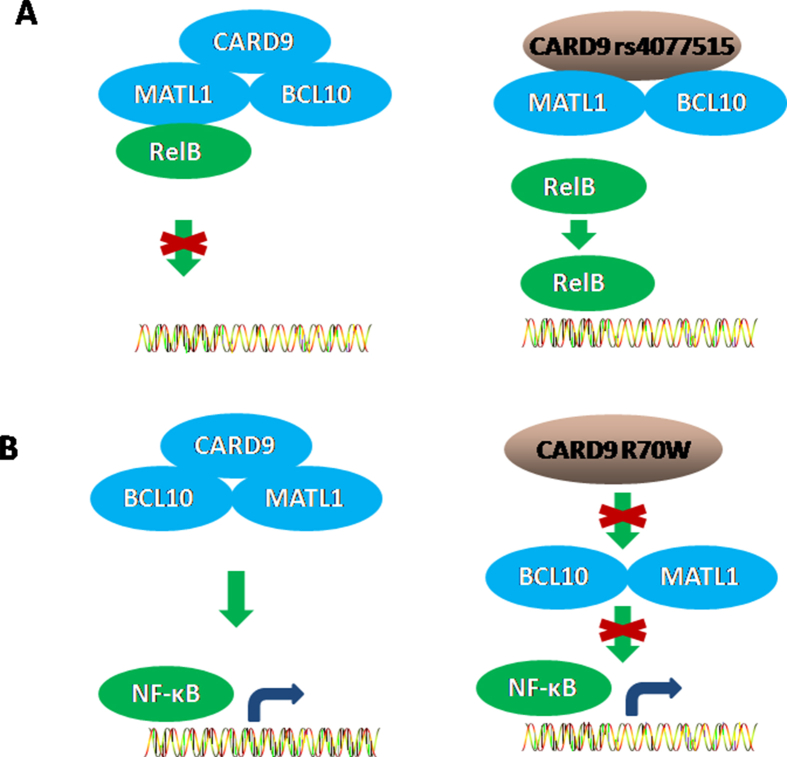
CARD9 rs4077515 in the human genome encodes substitution of asparagine for serine at position 12 (S12N) in CARD9 protein. CARD9S12N did not significantly affect the degradation of the NF-κB inhibitor IκBα or phosphorylation of the kinases Syk, Erk, Jnk. CARD9S12N had no influence on the translocation of classical NF-κB p65 to the nucleus, whereas it could facilitate the nuclear translocation of RelB [Fig. 2 A], a subunit of noncanonical NF-κB. As a result, CARD9S12N could facilitate the activation of NF-κB subunit RelB in macrophages. In addition, the co-localization of CARD9 and RelB was confirmed in RAW267.4 cells, and CARD9S12N disrupted the interaction between CARD9 and RelB. Furthermore, CARD9S12N facilitated the degradation of CARD9 protein in a proteasome-dependent pathway [62]. Different from the above result, CARD9 R70W (c.208C>T) mutation failed to activate NF-κB [Fig. 2 B]. R70W mutation abrogated the ability of CARD9-CARD to mediate filamentous forms of the protein, inhibited the recruitment of downstream BCL10, prevented the formation of CBM signalosomes, eventually failed to activate NF-κB [18]. To our knowledge, these two papers have first provided the direct evidence for CARD9 polymorphism, indicative of its potential molecular mechanisms. To date, the underlying mechanisms that CARD9 polymorphisms impact NF-κB activation are not well understood.
Fig. 2.
Potential mechanism for CARD9 polymorphisms. A: CARD9 rs4077515 impaired its interaction with RelB, which led to RelB translocation into nucleus, and subsequently Th2–mediated allergic responses; B: CARD9 R70W mutation abrogated the binding ability of CARD9-CARD, prevented the formation of CBM, and eventually failed to activate NF-κB.
Conclusion
Although many patients with CARD9 deficiency may share similar clinical presentations, there is a high diversity of CARD9 mutations underlying the condition. Lots of CARD9 genetic mutations are identified in the N-terminal CARD and C-terminal coiled-coil domains, as well as the promoter region. Along with an in-depth understanding of CARD9 gene, de novo variants in CARD9 are consistently reported. Of note, some of CARD9 mutations are found in patients, whereas the same mutations do not cause a similar clinical phenotype in other patients. Therefore, it is still unclear which mutations easily give rise to which clinical phenotypes, whether there is any overlap among patients, whether frequency and geographic distribution of CARD9 mutations are associated with patients. More clinical experiments will be required to confirm the correlation between the unique genotype of CARD9 gene mutations and disease, and provide further investigations into CARD9-dependent inflammatory and immune response, especially in humans. Identifying the impact of CARD9 genetic variation on inflammatory diseases will improve our understanding of the etiology and may ultimately aid future interventions.
Funding
The authors are grateful to the National Natural Science Foundation of China (No: 81960452), Shanghai Municipal Health Commission (No: 201840035), Shanghai Science and Technology Medical Innovation Funds (No: 20Y11911400) for the financial support.
Conflicts of interest
The authors report no conflicts of interest in this work.
Acknowledgements
Our profound admiration and respect go out to the researchers in this field and in our laboratories for their dedication and hard work.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Bertin J., Guo Y., Wang L., Srinivasula S.M., Jacobson M.D., Poyet J.L., et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem. 2000;275:41082–41086. doi: 10.1074/jbc.C000726200. [DOI] [PubMed] [Google Scholar]
- 2.Jiang C., Lin X. Regulation of NF-kappaB by the CARD proteins. Immunol Rev. 2012;246:141–153. doi: 10.1111/j.1600-065X.2012.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth S., Ruland J. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 2013;34:243–250. doi: 10.1016/j.it.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Hara H., Saito T. CARD9 versus CARMA1 in innate and adaptive immunity. Trends Immunol. 2009;30:234–242. doi: 10.1016/j.it.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Drummond R.A., Lionakis M.S. Mechanistic insights into the role of C-type lectin receptor/CARD9 signaling in human antifungal immunity. Front Cell Infect Microbiol. 2016;6:39. doi: 10.3389/fcimb.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieber N., Gazendam R.P., Freeman A.F., Hsu A.P., Collar A.L., Sugui J.A., et al. Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI insight. 2016;1 doi: 10.1172/jci.insight.89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanternier F., Pathan S., Vincent Q.B., Liu L., Cypowyj S., Prando C., et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–1714. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glocker E.O., Hennigs A., Nabavi M., Schaffer A.A., Woellner C., Salzer U., et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavino C., Hamel N., Zeng J.B., Legault C., Guiot M.C., Chankowsky J., et al. Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in French-Canadians. J Allergy Clin Immunol. 2016;137:1178–1188. doi: 10.1016/j.jaci.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Vaezi A., Fakhim H., Abtahian Z., Khodavaisy S., Geramishoar M., Alizadeh A., et al. Frequency and geographic distribution of CARD9 mutations in patients with severe fungal infections. Front Microbiol. 2018;9:2434. doi: 10.3389/fmicb.2018.02434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst M., Gazendam R., Reimnitz D., Sawalle-Belohradsky J., Groll A., Schlegel P.G., et al. Chronic Candida albicans meningitis in a 4-year-old girl with a homozygous mutation in the CARD9 gene (Q295X) Pediatr Infect Dis J. 2015;34:999–1002. doi: 10.1097/INF.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 12.Drewniak A., Gazendam R.P., Tool A.T., van Houdt M., Jansen M.H., van Hamme J.L., et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–2392. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 13.Drummond R.A., Collar A.L., Swamydas M., Rodriguez C.A., Lim J.K., Mendez L.M., et al. CARD9-Dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanternier F., Barbati E., Meinzer U., Liu L., Pedergnana V., Migaud M., et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis. 2015;211:1241–1250. doi: 10.1093/infdis/jiu412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin S., Balligand E., Peeters J., Nassogne M.C., Mondovits B., Loop M., et al. A 7-year-old child with headaches and prolonged fever associated with oral and nail lesions. Open Forum Infect Dis. 2019;6:ofz229. doi: 10.1093/ofid/ofz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cetinkaya P.G., Ayvaz D.C., Karaatmaca B., Gocmen R., Soylemezoglu F., Bainter W., et al. A young girl with severe cerebral fungal infection due to card 9 deficiency. Clin Immunol. 2018;191:21–26. doi: 10.1016/j.clim.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Lanternier F., Mahdaviani S.A., Barbati E., Chaussade H., Koumar Y., Levy R., et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015;135:1558–1568. doi: 10.1016/j.jaci.2014.12.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bruyne M., Hoste L., Bogaert D.J., van den Bossche L., Tavernier S.J., Parthoens E., et al. A CARD9 founder mutation disrupts NF-kappaB signaling by inhibiting BCL10 and MALT1 recruitment and signalosome formation. Front Immunol. 2018;9:2366. doi: 10.3389/fimmu.2018.02366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lionakis M.S. New insights into innate immune control of systemic candidiasis. Med Mycol. 2014;52:555–564. doi: 10.1093/mmy/myu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond R.A., Swamydas M., Oikonomou V., Zhai B., Dambuza I.M., Schaefer B.C., et al. CARD9(+) microglia promote antifungal immunity via IL-1 beta- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20:559–570. doi: 10.1038/s41590-019-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Wang W., Lin Z., Wang X., Li T., Yu J., et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905–908. doi: 10.1016/j.jaci.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Jachiet M., Lanternier F., Rybojad M., Bagot M., Ibrahim L., Casanova J.L., et al. Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Dermatol. 2015;151:192–194. doi: 10.1001/jamadermatol.2014.2154. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Mijiti J., Huang C., Song Y., Wan Z., Li R., et al. Deep dermatophytosis caused by Microsporum ferrugineum in a patient with CARD9 mutations. Br J Dermatol. 2019;181:1093–1095. doi: 10.1111/bjd.18146. [DOI] [PubMed] [Google Scholar]
- 24.Boudghene Stambouli O., Amrani N., Boudghene Stambouli K., Bouali F. Dermatophytic disease with deficit in CARD9: a new case with a brain impairment. J Mycolog Med. 2017;27:250–253. doi: 10.1016/j.mycmed.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Grumach A.S., de Queiroz-Telles F., Migaud M., Lanternier F., Filho N.R., Palma S.M., et al. A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J Clin Immunol. 2015;35:486–490. doi: 10.1007/s10875-015-0170-4. [DOI] [PubMed] [Google Scholar]
- 26.Nazarian R.M., Lilly E., Gavino C., Hamilos D.L., Felsenstein D., Vinh D.C., et al. Novel CARD9 mutation in a patient with chronic invasive dermatophyte infection (tinea profunda) J Cutan Pathol. 2020;47:166–170. doi: 10.1111/cup.13574. [DOI] [PubMed] [Google Scholar]
- 27.Yan X.X., Yu C.P., Fu X.A., Bao F.F., Du D.H., Wang C., et al. CARD9 mutation linked to Corynespora cassiicola infection in a Chinese patient. Br J Dermatol. 2016;174:176–179. doi: 10.1111/bjd.14082. [DOI] [PubMed] [Google Scholar]
- 28.Bao F., Fu X., Yu G., Wang Z., Liu H., Zhang F. CARD9 variants in Chinese patients with sporotrichosis. J Dermatol. 2019;46:e188–e189. doi: 10.1111/1346-8138.14721. [DOI] [PubMed] [Google Scholar]
- 29.Wang C., Xing H., Jiang X., Zeng J., Liu Z., Chen J., et al. Cerebral phaeohyphomycosis caused by Exophiala dermatitidis in a Chinese CARD9-deficient patient: a case report and literature review. Front Neurol. 2019;10:938. doi: 10.3389/fneur.2019.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones N., Garcez T., Newman W., Denning D. Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Zhang R., Wu W., Song Y., Wan Z., Han W., et al. Impaired specific antifungal immunity in CARD9-deficient patients with phaeohyphomycosis. J Invest Dermatol. 2018;138:607–617. doi: 10.1016/j.jid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Huang C., Zhang Y., Song Y., Wan Z., Wang X., Li R. Phaeohyphomycosis caused by Phialophora americana with CARD9 mutation and 20-year literature review in China. Mycoses. 2019;62:908–919. doi: 10.1111/myc.12962. [DOI] [PubMed] [Google Scholar]
- 33.Erman B., Firtina S., Aksoy B.A., Aydogdu S., Genc G.E., Dogan O., et al. Invasive saprochaete capitata infection in a patient with autosomal recessive CARD9 deficiency and a review of the literature. J Clin Immunol. 2020;40:466–474. doi: 10.1007/s10875-020-00759-w. [DOI] [PubMed] [Google Scholar]
- 34.Rosentul D.C., Delsing C.E., Jaeger M., Plantinga T.S., Oosting M., Costantini I., et al. Gene polymorphisms in pattern recognition receptors and susceptibility to idiopathic recurrent vulvovaginal candidiasis. Front Microbiol. 2014;5:483. doi: 10.3389/fmicb.2014.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosentul D.C., Plantinga T.S., Oosting M., Scott W.K., Velez Edwards D.R., Smith P.B., et al. Genetic variation in the dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J Infect Dis. 2011;204:1138–1145. doi: 10.1093/infdis/jir458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhernakova A., Festen E.M., Franke L., Trynka G., van Diemen C.C., Monsuur A.J., et al. Genetic analysis of innate immunity in Crohn's disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82:1202–1210. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y.H., Song G.G. Pathway analysis of a genome-wide association study of ileal Crohn's disease. DNA Cell Biol. 2012;31:1549–1554. doi: 10.1089/dna.2012.1605. [DOI] [PubMed] [Google Scholar]
- 38.Janse M., Lamberts L.E., Franke L., Raychaudhuri S., Ellinghaus E., Muri Boberg K., et al. Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL, and CARD9. Hepatology. 2011;53:1977–1985. doi: 10.1002/hep.24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imhann F., Vich Vila A., Bonder M.J., Fu J., Gevers D., Visschedijk M.C., et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. doi: 10.1136/gutjnl-2016-312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G., et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell S., Borowski K., Espin-Garcia O., Milgrom R., Kabakchiev B., Stempak J., et al. The unsolved link of genetic markers and crohn's disease progression: a north American cohort experience. Inflamm Bowel Dis. 2019;25:1541–1549. doi: 10.1093/ibd/izz016. [DOI] [PubMed] [Google Scholar]
- 42.Rivas M.A., Beaudoin M., Gardet A., Stevens C., Sharma Y., Zhang C.K., et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beaudoin M., Goyette P., Boucher G., Lo K.S., Rivas M.A., Stevens C., et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong S.N., Park C., Park S.J., Lee C.K., Ye B.D., Kim Y.S., et al. Deep resequencing of 131 Crohn's disease associated genes in pooled DNA confirmed three reported variants and identified eight novel variants. Gut. 2016;65:788–796. doi: 10.1136/gutjnl-2014-308617. [DOI] [PubMed] [Google Scholar]
- 45.Cao Z., Conway K.L., Heath R.J., Rush J.S., Leshchiner E.S., Ramirez-Ortiz Z.G., et al. Ubiquitin ligase TRIM62 regulates CARD9-mediated anti-fungal immunity and intestinal inflammation. Immunity. 2015;43:715–726. doi: 10.1016/j.immuni.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokol H., Conway K.L., Zhang M., Choi M., Morin B., Cao Z., et al. Card 9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology. 2013;145:591–601. doi: 10.1053/j.gastro.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Limon J.J., Tang J., Li D., Wolf A.J., Michelsen K.S., Funari V., et al. Malassezia is associated with crohn's disease and exacerbates colitis in mouse models. Cell Host Microbe. 2019;25:377–388. doi: 10.1016/j.chom.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelante T., Lannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Ridler C. IBD: dysbiosis underlies CARD9 risk alleles in colitis. Nat Rev Gastroenterol Hepatol. 2016;13:316. doi: 10.1038/nrgastro.2016.82. [DOI] [PubMed] [Google Scholar]
- 50.Eyerich K., Dimartino V., Cavani A. IL-17 and IL-22 in immunity: driving protection and pathology. Eur J Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 51.Sari S., Dalgic B., Muehlenbachs A., DeLeon-Carnes M., Goldsmith C.S., Ekinci O., et al. Prototheca zopfii colitis in inherited CARD9 deficiency. J Infect Dis. 2018;218:485–489. doi: 10.1093/infdis/jiy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiryluk K., Li Y., Scolari F., Sanna-Cherchi S., Choi M., Verbitsky M., et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheng Z., Li J., Wang Y., Li S., Hou M., Peng J., et al. A CARD9 single-nucleotide polymorphism rs4077515 is associated with reduced susceptibility to and severity of primary immune thrombocytopenia. Ann Hematol. 2019;98:2497–2506. doi: 10.1007/s00277-019-03796-7. [DOI] [PubMed] [Google Scholar]
- 54.Liu H., Wang Z., Li Y., Yu G., Fu X., Wang C., et al. Genome-Wide analysis of protein-coding variants in leprosy. J Invest Dermatol. 2017;137:2544–2551. doi: 10.1016/j.jid.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Arya R., Del Rincon I., Farook V.S., Restrepo J.F., Winnier D.A., Fourcaudot M.J., et al. Genetic variants influencing joint damage in Mexican Americans and European Americans with rheumatoid arthritis. Genet Epidemiol. 2015;39:678–688. doi: 10.1002/gepi.21938. [DOI] [PubMed] [Google Scholar]
- 56.Burghardt K.M., Avinashi V., Kosar C., Xu W., Wales P.W., Avitzur Y., et al. A CARD9 polymorphism is associated with decreased likelihood of persistent conjugated hyperbilirubinemia in intestinal failure. PloS One. 2014;9 doi: 10.1371/journal.pone.0085915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Momenzadeh P., Mahmoudi M., Beigy M., Garshasbi M., Vodjdanian M., Farazmand A., et al. Determination of IL1 R2, ANTXR2, CARD9, and SNAPC4 single nucleotide polymorphisms in Iranian patients with ankylosing spondylitis. Rheumatol Int. 2016;36:429–435. doi: 10.1007/s00296-015-3391-1. [DOI] [PubMed] [Google Scholar]
- 58.Streata I., Weiner J., 3rd, Iannaconne M., McEwen G., Ciontea M.S., Olaru M., et al. The CARD9 polymorphisms rs4077515, rs10870077 and rs10781499 are uncoupled from susceptibility to and severity of pulmonary tuberculosis. PloS One. 2016;11 doi: 10.1371/journal.pone.0163662. :e0165853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dorhoi A., Desel C., Yeremeev V., Pradl L., Brinkmann V., Mollenkopf H.J., et al. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med. 2010;207:777–792. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong X., Chen B., Yang L., Yang Z. Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis. 2018;9:52. doi: 10.1038/s41419-017-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holliday M.J., Witt A., Rodriguez Gama A., Walters B.T., Arthur C.P., Halfmann R., et al. Structures of autoinhibited and polymerized forms of CARD9 reveal mechanisms of CARD9 and CARD11 activation. Nat Commun. 2019;10:3070. doi: 10.1038/s41467-019-10953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X., Xu J.F., Zheng G., Lu H.W., Duan J.L., Rui W., et al. CARD9(S12N) facilitates the production of IL-5 by alveolar macrophages for the induction of type 2 immune responses. Nat Immunol. 2018;19:547–560. doi: 10.1038/s41590-018-0112-4. [DOI] [PubMed] [Google Scholar]